Abstract
Human–megafauna interaction in the Americas has great scientific and ethical interest because of its implications on Pleistocene extinction. The Arroyo del Vizcaíno site near Sauce, Uruguay has already yielded over 1000 bones belonging to at least 27 individuals, mostly of the giant sloth Lestodon. The assemblage shows some taphonomic features suggestive of human presence, such as a mortality profile dominated by prime adults and little evidence of major fluvial transport. In addition, several bones present deep, asymmetrical, microstriated, sharp and shouldered marks similar to those produced by human stone tools. A few possible lithic elements have also been collected, one of which has the shape of a scraper and micropolish consistent with usage on dry hide. However, the radiocarbon age of the site is unexpectedly old (between 27 and 30 thousand years ago), and thus may be important for understanding the timing of the peopling of America.
Keywords: megafauna, South America, Quaternary, taphonomy, bonebed, peopling
1. Introduction
(a). Background
The South American Pleistocene megafauna [1] includes spectacular, taxonomically unique assemblages of many giant-sized species whose extinction has been attributed to human arrival in the past millennia of the Pleistocene [2]. Here, we account for the megafaunal site of Arroyo del Vizcaíno, which had been preliminarily dated to about 30 thousand years ago (hereafter, ka) [3]. Some fossils were collected when the site was first discovered during a severe drought in January 1997. More rigorous excavations had to wait until permission was obtained from the Comisión del Patrimonio Cultural de la Nación and the weather conditions became favourable. The results of two field seasons, undertaken in late March 2011 and in late January to early February 2012, are also reported here.
(b). Geological setting
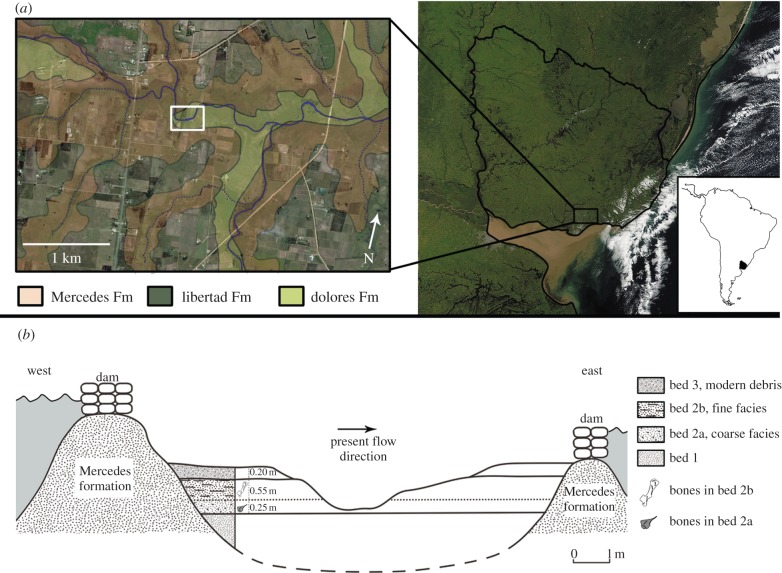
The site (figure 1a) is formed by a streambed in a place where the stream becomes deeper, forming a natural pond on a substrate of a Cretaceous silicified sandstone (Mercedes Fm.) [4]. The sediments were exposed in an area of about 30 m2 on the basin delimited by the Mercedes Fm. (figure 1b). Three beds were identified. Bed 1 is a greenish muddy sand bed that is more than 0.60 m thick and lies unconformably upon the Mercedes Fm. in the central, deeper part of the basin. Bed 2 is a richly fossiliferous layer that is 0.60–0.80 m thick, conformably overlying bed 1 and fining upwards (see electronic supplementary material, figure S3) from a greenish muddy sandy gravel (facies a) to a brownish muddy sand (facies b), with polymictic (K-feldspar-quartz pegmatite, quartz, reworked sandstones) clasts. Igneous clasts are angular to subangular and reworked sandstone clasts are angular to rounded. Two facies can be observed in bed 2, related with an upward shift in coloration from green (facies a) to brown (facies b), which may be due to slightly different sedimentary conditions or perhaps differential postdepositional oxidation events. The morphology of bed 2 is compatible with a fluvial system deposit [5]. Finally, bed 3, which unconformably overlies bed 2, shows variable thickness (0.20–1 m) and is composed of reworked bed 2 sediments mixed with modern stream alluvium, mainly fine sand and clayey silt.
Figure 1.
Arroyo del Vizcaíno site: (a) geographical location near Sauce, Departamento de Canelones, Uruguay (34°37′3′ S, 56°2′33′ W); (b) geological setting. (Online version in colour.)
Bed 2 is the main source of the remains studied here, although some reworked elements are found in bed 3.
2. Material and methods
(a). Geology and age
Standard descriptive methods were followed for the geological setting [6]. Seven additional samples [3], including purified and non-purified bone collagen and wood [7], were radiocarbon dated. See the electronic supplementary material for further details.
(b). Taphonomy
All the specimens were identified and counted. The biostratinomical characterization of the vertebrate assemblage follows [8]. The number of specimens (NISP), minimum number of anatomical units (MAU), minimum number of individuals (MNI) and minimum number of elements (MNE) were calculated. Weathering and evidence for transport of the bones were assessed through macroscopical analyses. Unless otherwise stated, counts and percentages refer to a total with glyptodont scutes excluded. Bones were cleaned following the usual procedures [9]. Statistics follow Sokal & Rohlf [10].
(c). Surface modifications of bones
Bones were examined under low magnification using a hand lens to determine the presence of surface modifications and were preliminarily classified to distinguish trampling from possible anthropogenic marks [11]. Further analysis of selected marks was carried out with light microscopy under magnifications of 20×, 30× and 45×. Several pictures of the marks at different focal depths were taken and a complete in-focus image was made. A three-dimensional model of each mark accurately representing the micromorphology of the modifications was constructed. At least four perpendicular sections of the grooves were obtained for each mark. The cross-sectional profiles were then digitized and measured [12,13]. Five morphological attributes were measured [13], including opening angle, angle of the tool impact index, shoulder height index, depth of cut and floor radius. Other, larger bone modifications were macroscopically analysed through digital and plastic models. Lithic material was analysed with the usual techniques [14]. See the electronic supplementary material for further details.
3. Results and discussion
(a). Age
Based on nine samples of bone collagen and wood from bed 2b, the site age is between 27 ± 0.45 and 30.1 ± 0.6 14C ka (see electronic supplementary material, table S1). Except for the rib URU 0496, the other eight dates obtained are statistically the same (p > 0.05) and their pooled average is 29 ± 0.106 14C ka, between 32.298 and 31.219 cal ka (0.941).
(b). Assemblage characterization
The site is a bonebed with numerous, mostly megafaunal, fossil remains. Only a small portion has yet been collected, resulting in 1145 catalogue entries, with NISP = 1095, or 779 excluding glyptodont scutes. Unless otherwise stated, this number (779) will be used hereafter to make appropriate comparisons and calculate percentages. The giant ground sloth Lestodon armatus is the most abundant of the taxa represented (94% of the identifiable specimens, NISP = 732, MNI = 17). A small number of elements of two other extinct giant sloths (Glossotherium robustum and Mylodon darwinii), three glyptodonts (Glyptodon cf. clavipes, Panochthus tuberculatus and Doedicurus clavicaudatus), one notoungulate (Toxodon platensis), one fossil horse (Hippidion principale), one proboscidean (the gomphothere Stegomastodon sp.), one adult deer (Cervidae indet.) and one sabretoothed felid (Smilodon populator) were also collected.
Only a small number of bones have juvenile features: two differently sized right femora, one left femur, a fragmentary hyoid, two mandibular fragments, a rib, a sacrum, two vertebrae, two tibiae, two caniniforms assigned to L. armatus, one femur of a glyptodont, an incompletely worn deciduous tooth of the gomphothere and the four horse bones, which amounts to 20 identified specimens (2.6%) belonging to at least five (mostly subadult) juvenile individuals. Owing to the presence of osteoarthritis, 58 bones (7.4%) have been identified as belonging to at least two old individuals. The remaining 90% of the elements belong to adult, prime individuals, yielding a prime-dominated mortality profile. This profile is unlike those found in either attritionally, catastrophically or accidentally accumulated assemblages [15]. By contrast, the age profile is similar to that seen in kill sites, in which a selection of the strongest individuals by human hunters with appropriate technology (long-distance weaponry) and cooperative ambush strategies is implied.
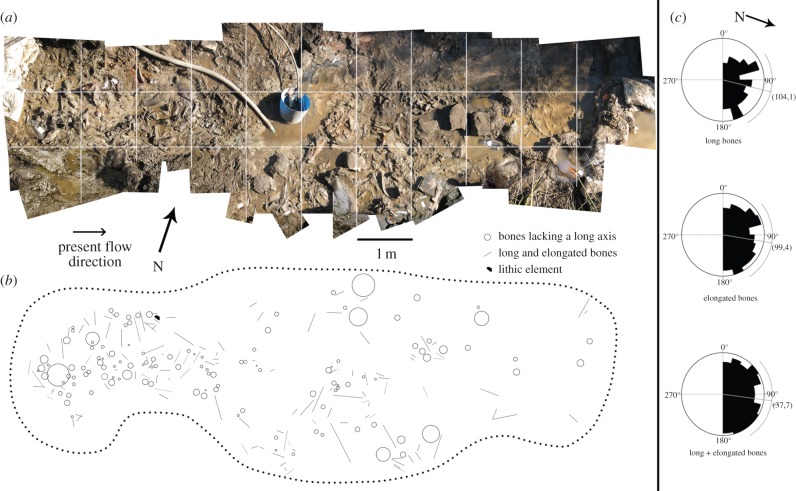
Among the identified specimens, limb bones and girdles (35.7%) predominate, followed by vertebrae (33.9%), rib portions (15.7%), cranial fragments (9.2%), and bones of manus and pes (5.3%). The proportions found resemble those of the surface assemblage in Amboseli [16], African hominid sites [17], carnivore dens [18] and San hunter–gatherer camps [17] (figure 3a). The representation of all anatomical regions despite their hydraulic transportability potential [19] suggests the accumulation being mostly autochthonous, with little evidence for major hydraulic transport, although slight outgoing hydraulic transport might have removed some of the lighter elements. Limb and elongated bones are randomly oriented (figure 2), compatible with no major sorting of the bones owing to fluvial transport [20] and suggesting biogenic agency [8]. This, in turn, is congruent with the sedimentological evidence of low current speed. The teeth : vertebrae ratio of the sloth elements (as corrected for making allowance of the appropriate numbers in the individuals of the clade, 18 and 34, respectively) is 0.76. This resembles a trampled surface accumulation and is unlike a sorting by transport, in which heavier, durable elements are over-represented [16]. However, one-fifth of the bones show some signs of transport and must have had a different taphonomic history. In addition, between one-fifth and one-quarter of the bones collected exhibit several features indicative of transport, and are slightly more weathered [21] and trampled than the majority of the recovered sample (see electronic supplementary material, figure S6). Those specimens show an orangish colour when wet and tend to come from the lower part of bed 2 (facies a). The representation of the anatomical units (%MAU) of L. armatus (figure 3b) resembles those in kill sites associated with gourmet consumption [23].
Figure 3.
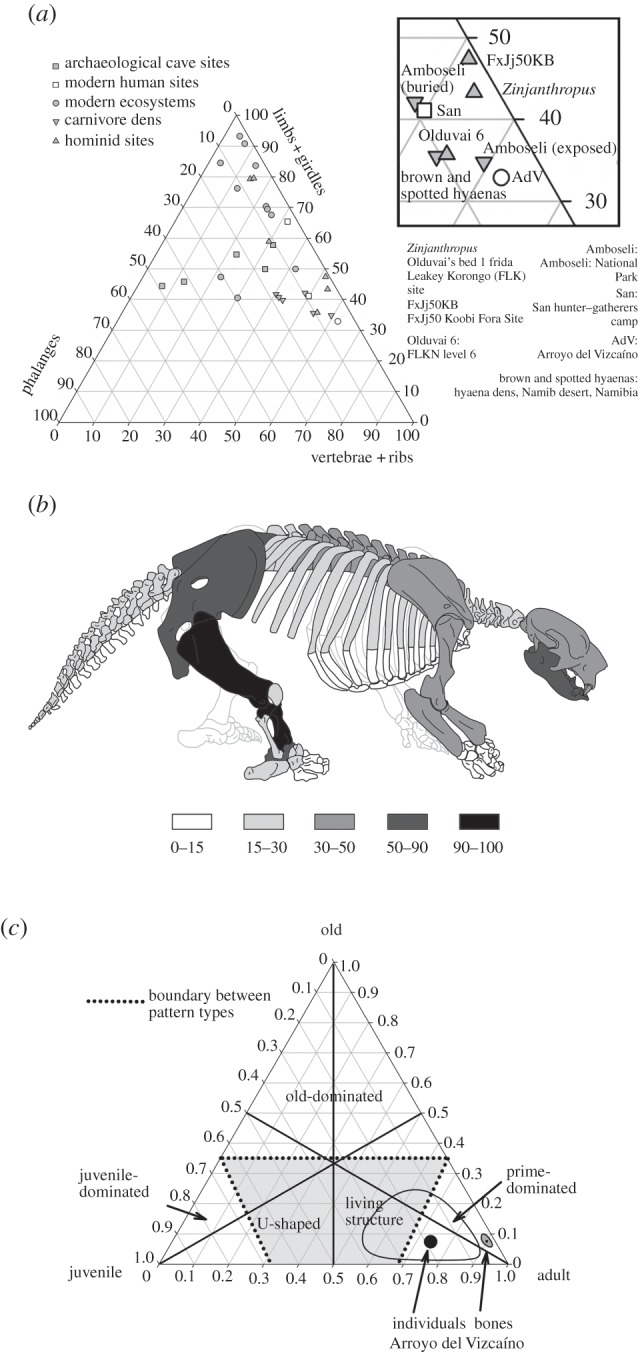
Frequency of the bones collected in Arroyo del Vizcaíno: (a) proportion of anatomical regions found in biogenic sites with detail of the ternary diagram near the position of the Arroyo del Vizcaíno (AdV) site recalculated and redrawn after [22]; (b) percentage of minimum anatomical units of ground sloth bones; (c) proportion of bones and individuals in the mortality profile.
Figure 2.
Panoramic view and orientation of the bones: (a) the bonebed showing the 1 m grid used to reference collected elements: (b) schematic of the bones to show their orientation; (c) rose diagrams showing orientation of the bones. (Online version in colour.)
In summary, fluvial agency can be ruled out as the main source of the accumulation. This conclusion is also supported by the general state of preservation of the bones, which mostly display little abrasion and only a slight polish. However, the existence of more than one population of bones cannot be discarded or corroborated with the data currently available and will require finer stratigraphic control in future excavations. In any case, physical preservation is rather homogeneous in the vast majority of the elements, indicating fast burial, perhaps as a single event [24], which is congruent with the obtained dates.
Because major long-distance fluvial transport can be excluded as the main origin of the accumulation, the fossil association must have involved external factors. Among such processes, natural trap or other catastrophic sources can also be excluded [25], as they do not show the inverted U-shaped age distribution found here (figure 3c).
(c). Surface modification of bones
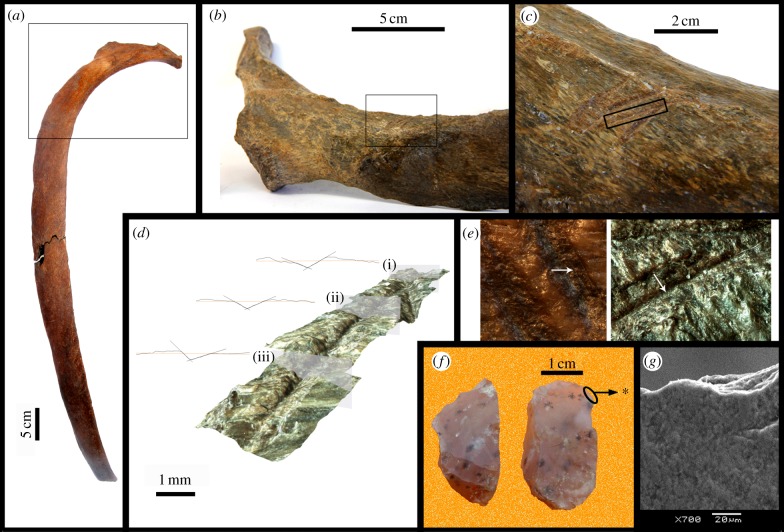
No carnivore tooth marks were identified. Nearly 59% of the bones collected show modifications with features identifiable as trampling marks [26], with over one-third of the bones exhibiting trampling abrasion on more than 25% of their surface area (see electronic supplementary material, figure S7). Furthermore, 40 elements (nearly 5% of the identified specimens, a percentage similar to the proportion in human sites [23]) show marks that have macroscopical features consistent with human agency (figure 4; electronic supplementary material, figures S8–S13). Ten of the bones that showed little or no trampling abrasion in the studied area of the anthropogenic-style marks were examined under greater magnifications. A total of 15 marks were analysed from the selected bones. Congruent with previous preliminary findings [27], most of these marks show microscopical features described in cuts made by human stone tools, such as shoulder effects, Herzian cones and even microstriations [12] (figure 4a–e; electronic supplementary material, table S6), the latter being very rarely preserved in prehistoric material [11,26]. The mean value of the opening angle was 112.5 ± 12.1°. For the angle of tool impact (ATI) index, the mean value was 0.089 ± 0.058, indicating that the object that made the mark was on average oriented at a consistent angle relative to the bone surface and not perpendicular to it.
Figure 4.
Bone surface modifications: (a) rib CAV 451; (b) detail of the proximal region; (c) detail of the cut marks: (d) three-dimensional reconstruction of the boxed mark indicated in (c), indicating where sections (i), (ii) and (iii) were taken (section profiles show the two slopes and unaffected bone surface); (e) portion of that cut mark displaying microstriations (arrow) preserved at the base of the groove (right), and parallel set of microstriations (arrow) preserved on a cut mark in CAV 453 showing a V-shaped section (left); (f) lithic element with features characteristic of a scraper, showing an area (*) that differentially reflects the incidental light; (g) SEM photography showing micropolish in area *.
The shoulders are 42 μm high on average (higher than in many experimental marks [12]), with mean shoulder height (SH) index value of 0.256 ± 0.251, similar to the published values for a tool held at 45° in regard to the bone surface [12]. The mean value for the depth of cut was 0.191 ± 0.070 µm. The floor radius shows a mean value of 0.161 ± 0.073 µm (figure 4a–d). These features and values resemble those found in fossil bones cut with flint tools [13,28,29], including in xenarthrans [30].
Most marks are observed in the anatomical regions that would be expected to have been produced by humans, for instance in the proximal portions of three ribs, trochlear notch of an ulna, hyoid, lingual side of the lower jaw, etc. [11,31]. Remarkably, at least one of the bones has marks located in a very concave surface, the groove on the distal epiphysis of a tibia (where the tendon of the flexor hallucis longus muscle runs), which renders it nearly impossible to have been caused by trampling [26]. Indentations are discussed in the electronic supplementary material.
All individuals with body masses not much above one tonne (juvenile L. armatus, other ground sloths, adult and juvenile glyptodont, toxodont, juvenile gomphothere, juvenile horse, deer, sabretooth) are represented by few bones (61, or 8%), whereas the four-tonne adult individuals of L. armatus constitute over 92% (718 specimens) of the identified specimens. San hunter–gatherers have been reported [17] to schlep entire carcasses with masses in excess of several hundred kilograms to more permanent camps, whereas the largest individuals are processed in situ. A similar pattern has been observed in archaeological kill sites [23]. Stalking by human hunters is performed in places in which the animals transit frequently [15], such as on the way to a body of water, which might help explain the observed extent of the trampling.
(d). Lithic material
Although during the fieldwork no systematic effort was made to collect lithic material and only a part of this site has been exhaustively explored, a few lithic elements were found to have seemingly anthropogenic features, although such elements are scarce, as is usual in South American Pleistocene archaeological sites [31]. These include flakes made of silicified sandstone (see the electronic supplementary material), but their grain size prevented further microscopical analysis. However, a small piece of translucid silcrete has features compatible with a scraper (figure 4f,g) [32]. This piece was found in bed 2 in very close association with several bones (figure 2b). It is a pseudo-pyramidal nucleus with dimensions 29.6 × 14.5 × 15.5 mm. Both proximal and distal borders are short and convex. They are unifacially retouched and asymmetrically bevelled at intermediate angles (70° in the proximal border and 60° in the distal one). The proximal border presents marginal semicircular retouching, and the distal border shows deep retouching of irregularly parallel morphology. Using a scanning electron microscope (SEM) at 700–4300×, an area was identified (asterisk in figure 4f) extending along a large portion of the distal edge of the largest face that reflects the incidental electrons differently from the central parts of that face. That area is rather dull and coarse, with circular microdepressions that are darker than the rest of the surface (see the electronic supplementary material, figure S15), covering the high zones as well as the low ones, and is accompanied by noteworthy edge rounding. These features are consistent with those observed in a second-stage micropolish, as produced by working on dry hide [33]. Micropolish is the only feature observable with optical microscopy that is produced by the use of a tool and not by natural or accidental causes, and thus it is a diagnostic indicator of human agency even in the absence of any other kind of evidence [33]. Both retouched edges show a nearly continuous pattern of microflaking of different size and morphology.
4. Final remarks
The taphonomy of the site, intrinsically important as a large bonebed, is suggestive as having accumulated as the result of biogenic (particularly human) agency. The presence of surface modifications on the bones and the possible scraper are consistent with this interpretation. However, besides some rather controversial claims in both South [34–36] and North [37–41] America, current evidence for humans arriving in the New World (excluding Beringia) before the Last Glacial Maximum (as indicated by the dates at Arroyo del Vizcaíno) is equivocal. Thus, the age of the site and the scarcity of formal tools urge caution in interpretation. In any case, we argue that the Arroyo del Vizcaíno site deserves to be included in the agenda of early American peopling, either as a not foreseeable discovery [37] or as an example of natural processes mimicking human presence. As such, further detailed study of the site is warranted to resolve these issues.
Acknowledgements
We are indebted to the following people and institutions for their help: Eileen Armstrong, Alfonso Arribas, Anna K. Behrensmeyer, Martín Batallés, Silvia Bello, Batallón 14, Cristina Bertoni, Karen Borrazzo, Luis Borrero, Juan Cabrera, Reynaldo Castilla, Gabriela Costoya, Comisión Sectorial de Investigación Científica (CSIC) of the Universidad de la República, Eva Fariña, González family, Signe Haakonsson, IMFIA, Intendencia de Canelones, Emily Lindsey, Dimila Mothé, Gustavo Politis, Ximena Martínez Blanco, Rubens Ottonello and Municipio de Sauce, Elena Pareja, Santiago Patiño, William Rey, Jorge and Elena Rizzo, Valeria Rodríguez, Raymond R. Rogers, Ana Elisa Röhrdanz, Andrea Sánchez, Montevideo, Uruguay: Valetto family, Jorge Wagensberg, Marcelo Arístides Zárate and two anonymous reviewers. R.A.F. wishes to acknowledge the indirect encouragement by Beatriz Aguirre-Urreta, Ana Báez, Ismar Carvalho and Rodolfo Manceñido, who regarded this project as too ‘ambitious’.
References
- 1.Fariña RA, Vizcaíno SF, De Iuliis G. 2013. Megafauna: giant beasts of Pleistocene South America. Bloomington, IN: Indiana University Press [Google Scholar]
- 2.Barnosky AD, Lindsey EL. 2010. Timing of quaternary megafaunal extinction in South America in relation to human arrival and climate change. Quat. Int. 217, 10–29 (doi:10.1016/j.quaint.2009.11.017) [Google Scholar]
- 3.Fariña RA, Castilla R. 2007. Earliest evidence for human-megafauna interaction in the Americas. In Human and faunal relationships reviewed: an archaeozoological approach (eds Corona-M E, Arroyo-Cabrales J.), pp. 31–34 Oxford, UK: Archaeopress [Google Scholar]
- 4.Spoturno J, Oyhantçabal P, Aubet N, Cazaux S, Morales E.2004. Carta Geológica y Memoria Explicativa a Escala 1/100.000 del Departamento de Canelones, CONICYT. Proyecto 6019. Leticia Tejera, the Fondo Clemente Estable.
- 5.Nichols G. 2009. Sedimentology and stratigraphy. New York, NY: Wiley-Blackwell [Google Scholar]
- 6.Tucker M. 1982. The field description of sedimentary rocks. Geological Society of London Handbook Series, no. 2 Maidenhead, UK: Open University Press [Google Scholar]
- 7.Longin R. 1971. New method of collagen extraction for radiocarbon dating. Nature 230, 241–242 (doi:10.1038/230241a0) [DOI] [PubMed] [Google Scholar]
- 8.Behrensmeyer AK. 1991. Terrestrial vertebrate accumulations. In Taphonomy: releasing the data locked in the fossil record (eds Allison P, Briggs DEG.), pp. 291–335 New York, NY: Plenum [Google Scholar]
- 9.May P, Reser P, Leiggi P. 1994. Macrovertebrate preparation. In Vertebrate paleontological techniques (eds Leiggi P, May P.), pp. 113–153 Cambridge, UK: Cambridge University Press [Google Scholar]
- 10.Sokal RR, Rohlf FJ. 1981. Biometry, 2nd edn San Francisco, CA: Freeman [Google Scholar]
- 11.Domínguez-Rodrigo M, De Juana S, Galán AB, Rodríguez M. 2009. A new protocol to differentiate trampling marks from butchery cut marks. J. Archaeol. Sci. 36, 2643–2654 (doi:10.1016/j.jas.2009.07.017) [Google Scholar]
- 12.Bello SM, Soligo C. 2008. A new method for the quantitative analysis of cutmark micromorphology. J. Archaeol. Sci. 35, 1542–1552 (doi:10.1016/j.jas.2007.10.018) [Google Scholar]
- 13.Bello SM, Parfitt SA, Stringer C. 2009. Quantitative micromorphological analyses of cut marks produced by ancient and modern handaxes. J. Archaeol. Sci. 36, 1869–1880 (doi:10.1016/j.jas.2009.04.014) [Google Scholar]
- 14.Collins MB. 1975. Lithic technology as a means of Processual inference. In Lithic technology: making and using stone tools (ed. Swanson E.), pp. 15–34 The Hague, The Netherlands: Mouton [Google Scholar]
- 15.Stiner M. 1990. The use of mortality patterns in archaeological studies of hominid predatory adaptations. J. Anthropol. Archaeol. 9, 305–351 (doi:10.1016/0278-4165(90)90010-B) [Google Scholar]
- 16.Behrensmeyer AK, Dechant-Boaz DE. 1980. The recent bones of Amboseli Park, Kenya. In Fossils in the making (eds Behrensmeyer AK, Hill A.), pp. 72–93 Chicago, IL: Chicago University Press [Google Scholar]
- 17.Bunn HT. 1986. Patterns of skeletal representation and hominid subsistence activities at Olduvai Gorge, Tanzania and Koobi Fora, Kenya. J. Hum. Evol. 15, 673–690 (doi:10.1016/S0047-2484(86)80004-5) [Google Scholar]
- 18.Richardson PRK. 1980. Carnivore damage to antelope bones and its archaeological implications. Palaeontol. Afr. 23, 109–125 [Google Scholar]
- 19.Voorhies MR. 1969. Taphonomy and population dynamics of an early Pliocene vertebrate fauna, Knox County, Nebraska. Contrib. Geol. 1, 1–69 [Google Scholar]
- 20.Kreutzer LA. 1988. Megafaunal butchering at Lubbock Lake, Texas: a taphonomic reanalysis. Quat. Res. 30, 221–231 (doi:10.1016/0033-5894(88)90026-9) [Google Scholar]
- 21.Behrensmeyer AK. 1978. Taphonomic and ecologic information from bone weathering. Paleobiology 4, 150–162 [Google Scholar]
- 22.Arribas A, Palmqvist P. 1998. Taphonomy and paleoecology of an assemblage of large mammals: hyaenid activity in the lower Pleistocene site at Venta Micena (Orce, Guadix-Baza Basin, Granada, Spain). Geobios 31, 3–47 (doi:10.1016/S0016-6995(98)80056-9) [Google Scholar]
- 23.Meltzer DJ. 2006. Folsom: new archaeological investigations of a classic Paleoindian bison kill. Berkeley, CA: University of California Press [Google Scholar]
- 24.Lubinski PM. 2011. Comments on evidence for hunting of pronghorn herds in prehistory. Northwest Sci. 85, 68–70 (doi:10.3955/046.085.0107) [Google Scholar]
- 25.Badgley C. 1986. Counting individuals in mammalian fossil assemblages from fluvial environments. Palaios 1, 328–338 (doi:10.2307/3514695) [Google Scholar]
- 26.Behrensmeyer AK, Gordon KD, Yanagi GT. 1986. Trampling as a cause of bone surface damage and pseudo-cutmarks. Nature 319, 768–771 (doi:10.1038/319768a0) [Google Scholar]
- 27.Arribas A, Palmqvist P, Pérez-Claros JA, Castilla R, Vizcaíno SF, Fariña RA. 2001. New evidence on the interaction between humans and megafauna in South America. Publ. Semin. Paleontol. Zaragoza 5, 228–238 [Google Scholar]
- 28.Walker PL, Long JC. 1977. An experimental study of the morphological characteristics of tool marks. Am. Antiq. 32, 605–616 (doi:10.2307/278934) [Google Scholar]
- 29.Walker PL. 1978. Butchering and stone tool function. Am. Antiq. 43, 710–715 (doi:10.2307/279502) [Google Scholar]
- 30.Redmond BG, McDonald HG, Greenfield HJ, Burr ML. 2012. New evidence for Late Pleistocene human exploitation of Jefferson's Ground Sloth (Megalonyx jeffersonii) from northern Ohio, USA. World Archaeol. 44, 75–101 (doi:10.1080/00438243.2012.647576) [Google Scholar]
- 31.Politis G, Messineo PG. 2008. The Campo Laborde site: new evidence for the Holocene survival of Pleistocene megafauna in the Argentine Pampas. Quat. Int. 191, 98–114 (doi:10.1016/j.quaint.2007.12.003) [Google Scholar]
- 32.Orquera LA, Piana EL. 1986. Normas para la descripción de objetos arqueológicos de piedra tallada. Contribución Científica no. 1, p. 108 Ushuaia, Argentina: CADIC [Google Scholar]
- 33.Mansur ME, Lasa A. 2005. Diversidad Artefactual vs. especialización funcional. Análisis del IV componente de túnel I (Tierra del Fuego, Argentina). Magallania 33, 69–91 (doi:10.4067/S0718-22442005000200006) [Google Scholar]
- 34.Mithen S. 2003. After the ice: a global human history 20,000–5000 BC. London, UK: Orion Books [Google Scholar]
- 35.Guidon N, Delibrias G. 1986. Carbon-14 dates point to man in the Americas 32,000 years ago. Nature 321, 769–771 (doi:10.1038/321769a0) [Google Scholar]
- 36.Lahaye C, et al. 2013. Human occupation in South America by 20,000 BC: the Toca da Tira Peia Site, Piauí, Brazil. J. Archaeol. Sci. 40, 2840–2847 (doi:10.1016/j.jas.2013.02.019) [Google Scholar]
- 37.Pitblado BL. 2011. A tale of two migrations: reconciling recent biological and archaeological evidence for the Pleistocene peopling of the Americas. J. Archaeol. Res. 19, 327–375 (doi:10.1007/s10814-011-9049-y) [Google Scholar]
- 38.Feathers JK, Rhodes EJ, Huot S, Mcavoy JM. 2003. Luminescence dating of sand deposits related to late Pleistocene human occupation at the Cactus hill site, Virginia, United States. Quat. Geochronol. 1, 166–187 [Google Scholar]
- 39.Goebel T, Waters MR, O'Rourke DH. 2008. The Late Pleistocene dispersal of modern humans in the Americas. Science 319, 1497–1502 (doi:10.1126/science.1153569) [DOI] [PubMed] [Google Scholar]
- 40.Waters MR, et al. 2011. The Buttermilk Creek Complex and the origins of clovis at the Debra L. Friedkin Site, Texas. Science 331, 1599–1603 (doi:10.1126/science.1201855) [DOI] [PubMed] [Google Scholar]
- 41.Morrow JE, Fiedel SJ, Johnson DL, Kornfeld M, Rutledge M, Wood WR. 2013. Pre-Clovis in Texas? A critical assessment of the ‘Buttermilk Creek Complex’. J. Archaeol. Sci. 39, 3677e3682 (doi:10.1016/j.jas.2012.05.018) [Google Scholar]