Abstract
The differential allocation hypothesis predicts increased investment in offspring when females mate with high-quality males. Few studies have tested whether investment varies with mate relatedness, despite evidence that non-additive gene action influences mate and offspring genetic quality. We tested whether female lekking lance-tailed manakins (Chiroxiphia lanceolata) adjust offspring sex and egg volume in response to mate attractiveness (annual reproductive success, ARS), heterozygosity and relatedness. Across 968 offspring, the probability of being male decreased with increasing parental relatedness but not father ARS or heterozygosity. This correlation tended to diminish with increasing lay-date. Across 162 offspring, egg volume correlated negatively with parental relatedness and varied with lay-date, but was unrelated to father ARS or heterozygosity. Offspring sex and egg size were unrelated to maternal age. Comparisons of maternal half-siblings in broods with no mortality produced similar results, indicating differential allocation rather than covariation between female quality and relatedness or sex-specific inbreeding depression in survival. As males suffer greater inbreeding depression, overproducing females after mating with related males may reduce fitness costs of inbreeding in a system with no inbreeding avoidance, while biasing the sex of outbred offspring towards males may maximize fitness via increased mating success of outbred sons.
Keywords: compatibility, differential allocation, inbreeding avoidance, inbreeding depression, maternal effects, relatedness
1. Introduction
Life-history theory predicts that females should adjust investment in their offspring in response to expected fitness returns. Specifically, the differential allocation hypothesis predicts that females should increase investment in offspring after mating with an attractive male of high genetic quality, thereby producing a non-genetic positive correlation between offspring fitness and mate genetic quality [1–3]. In vertebrates, such maternal effects may consist of increasing offspring size, or resources provided to offspring [2,4]. Additionally, in polygynous species with higher variance in male reproductive success, females are predicted to produce more male offspring after mating with a relatively high-quality male (sex allocation [3]). Although numerous studies in birds have found evidence that females increase egg number and size in response to mate attractiveness [4], sex ratio adjustment has been demonstrated in relatively few studies [5–7], and numerous others have found weak or no evidence for adjustment [8–10].
Most studies of differential allocation have considered mate quality in a good genes or Fisherian context, i.e. assuming all females prefer the same males of high additive genetic quality [2,4]. They therefore test the prediction that females increase investment after mating with more ‘attractive’ ornamented males with high mating success [4–6]. Remarkably, few studies have tested whether investment varies with mate genetic compatibility despite the potential for male genetic quality to reflect non-additive gene action, and hence the optimal mate to vary among females (but see [11–14]).
A specific case of compatible mate choice models predicts that mate genetic quality will vary with mate relatedness, given widespread inbreeding depression in fitness in nature [15–17]. If outbred males have higher fitness than inbred males, and because mating success is more variable in males than females, females may gain higher fitness returns from producing male offspring when those offspring are likely to be outbred [3]. Several studies have identified stronger inbreeding depression in male sexual and life-history traits than in female traits [18–21]. Producing female offspring after inbreeding may therefore be less costly than producing male offspring. Consequently, females should invest more in offspring and produce sons rather than daughters when mated to an unrelated male; ‘the outbred son hypothesis’ [13]. Additionally, because more heterozygous males can produce relatively outbred offspring under certain conditions, such as in small or structured populations [22,23], females may also increase investment after mating with a more heterozygous male. Such differential allocation with respect to mate relatedness and offspring genetic diversity could reduce the cost of inbreeding in situations where inbreeding avoidance through mate choice is either not possible (owing to limited availability of unrelated mates, limited dispersal or inability to detect kin) or selected against given the inclusive fitness benefit from inbreeding [24,25]. Indeed, inbreeding avoidance via mate choice is relatively rare in the wild despite the inevitable cost of inbreeding depression [24]. Furthermore, the absence of inbreeding avoidance in combination with inbreeding depression in many systems creates potential selective pressure for mechanisms to lessen fitness costs when inbred offspring are produced, for example via reducing investment in inbred offspring.
We are aware of only three studies that have explicitly tested whether females differentially invest in response to their mate's relatedness in vertebrates, or whether related pairings produce biased sex ratios as predicted by the outbred son hypothesis. Two studies on a population of song sparrows (Melospiza melodia) found no evidence for sex ratio adjustment in response to mate relatedness [13], but that females provisioned offspring more frequently (and offspring consequently grew faster) when less related to their mate [12]. A third study found female zebra finches (Taeniopygia guttata) produced smaller clutches and eggs with lower mass when closely related to their mate [26]. Indirect evidence from two further studies found inbreeding level was higher among male than female offspring [20,27]. It therefore remains widely untested whether females adjust offspring sex or egg size (a commonly used proxy of female investment in birds [4,28]) with respect to mate relatedness [13,26]. Additionally, few studies have considered maternal effects in lekking species (but see [7,29]) despite the inherent utility of this mating system for isolating maternal contributions, as males do not contribute any obvious non-genetic resources to offspring [30].
We tested the differential allocation hypothesis by investigating whether female lek-breeding lance-tailed manakins, Chiroxiphia lanceolata, adjust offspring sex and egg size in response to mate genetic quality. We measured: (i) male attractiveness (an indicator of additive genetic quality, quantified as annual reproductive success (ARS) because females choose freely among males and base their choice on individual males rather than display sites [31]), (ii) male heterozygosity (an indicator of both additive genetic quality and his ability to produce outbred offspring), and (iii) male relatedness (an indicator of non-additive genetic quality and the degree to which outbred offspring will be produced). Research on the wire-tailed manakin, Pipra filicauda, has revealed both inbreeding depression in male siring success and no avoidance of mating with relatives [32], and in a separate study we found similar effects in lance-tailed manakins [33]. This system is therefore ideal for testing predictions of the outbred son hypothesis and more general predictions of the differential allocation hypothesis. Patterns of investment with respect to mate genetic quality may be age- or context-dependent [34–36]. Previous work in this population suggested an effect of maternal age on offspring sex in some years [37], and the breeding season spans varying environmental conditions. Therefore, we also tested whether offspring sex and egg size varied with interactions between mate quality and both female age and lay-date.
2. Material and methods
(a). Study system
A wild population of lance-tailed manakins on 46 ha of secondary-growth dry tropical forest on Isla Boca Brava, Chiriquí, Panama (8°12′45″ N, 82°12′54″ W) has been monitored since 2000. Lance-tailed manakins display in an exploded lek with display sites in auditory but not visual contact [38]. Females can be aged exactly if captured prior to their third year while molt limits are visible [39], or banded as nestlings. At each display site, one alpha and usually one beta male cooperate in courtship displays, but social status is determined prior to female visitation and with few exceptions, only alpha males reproduce [40]. Females can produce one to three broods per season. Although females usually lay two eggs per nest, some nests are found with only one egg (approx. 9%). The majority of second and third broods represent re-nesting following nest failure. Males provide no obvious resources to females (other than sperm) and females mate outside their immediate nesting area [40]. For further details of the study system, see [38,41].
(b). Field methods
The population was monitored during the peak breeding season each year (late February until mid-June). Individuals were mistnetted and individually identified with aluminium rings and a unique combination of colour bands. Nests were located by systematically searching areas of forest or observing female behaviour. Each nest was visited approximately every 2 days to monitor lay-date, clutch size, hatching and fledging success and identify the attending female. On day 7 (2000) or on day 1–3 (2001–2012) after hatching, a small (5 µl) blood sample was taken from the brachial vein of each chick. Owing to the naturally high predation rates, one egg from two-egg clutches was artificially incubated to increase the number of genetically sampled nests starting in 2005 and for all nests since 2010 [42]. Chicks from artificially incubated eggs were blood sampled and returned to the focal nest within 3 h of hatching or sacrificed before hatching if that nest failed. In 2011 and 2012, egg length and width were measured to the nearest 0.01 mm at the longest and widest points, and egg volume was calculated as the volume of an ellipsoid (=π × (4/3) × width2 × length).
(c). Molecular analyses
Individuals were genotyped at 20 microsatellite loci [40]. Parentage was assigned using CERVUS v. 3.0 [43]. Mother identity was determined from nest attendance and confirmed genetically, and all adult males alive in that year were considered as potential sires. When no female was observed, the mother was assigned if parent-pair analyses in CERVUS identified maternity with greater than or equal to 95% confidence. Thorough sampling of candidate sires (more than 95% on the main study site in all but the first year) and strict paternity assignment criteria (details in [40]) allowed assignment of both parents for 80% of 1320 sampled offspring from 808 nests (n = 1058 assigned) in 2000–2012 [41]. Ninty-three per cent of sampled offspring (1226) were sexed genetically [37,44] including 216 offspring from 266 eggs sacrificed before hatching or that failed to hatch (see the electronic supplementary material, table S1). Percentages reflect all sampled offspring, not only those genotyped as reported elsewhere [40]. A total of 968 offspring from 597 nests were of known sex, parentage and lay-date, and of these, 756 offspring were from 401 completely sampled broods. Incompletely sampled broods could result from either failure of the nest while one egg was being artificially incubated, partial predation, or amplification failure during genetic sexing.
(d). Male genetic quality
We quantified male attractiveness as the total number of offspring assigned to each male in each year, following DuVal & Kempenaers [40]. We calculated male and offspring heterozygosity as the number of heterozygous loci divided by the number of loci typed at 20 microsatellite loci. Pairwise relatedness of males and females was estimated from these 20 microsatellites using the Queller & Goodnight relatedness index, which ranges from −1 to 1, where 0 is random with respect to the reference population allele frequencies [45].
(e). Statistical analyses
To test whether offspring sex varied with father ARS, father heterozygosity or parental relatedness, we used generalized linear mixed models (GLMMs) run using the R-package lme4, specifying a binomial error structure and logit link function [46]. Offspring sex was coded as the binary-dependent variable (0 = female, 1 = male). We included brood, mother and father identification (ID), and year (2000–2012) as random effects to account for non-independence among offspring in the same brood and produced by the same parents or in the same year [47]. Models were also rerun for brood sex ratio (using the number of male offspring as the numerator and brood size as the denominator) and results were quantitatively similar (not presented). Offspring sex (instead of brood sex ratio) was chosen as the dependent variable for final models as this enabled broods with two sires to be included (13% of two-egg broods with assigned parentage). Final analyses included incompletely sampled broods, as excluding these may produce misleading results [48]; however, analyses were repeated using only those broods where the number of eggs laid equalled offspring sexed and assigned parentage. We also tested whether offspring sex varied with lay-date (mean-centred by year). A restricted dataset of offspring produced by females of known age tested whether offspring sex varied with mother age modelled as a covariate. We also tested for interactions between mother age and father genetic quality, and between lay-date and father genetic quality. Significance from binomial models was assessed using likelihood ratio tests.
We used linear mixed models (LMMs) to test whether egg volume varied with father ARS, father heterozygosity, parental relatedness, mother age and nest lay-date. We included random effects of mother ID, father ID, brood ID and year (2011 or 2012). Two additional LMMs were run to test whether egg size varied with offspring hatching or fledging success. We also tested for interactions between father genetic quality and lay-date but not female age owing to small sample size. Egg volume was divided by 10 000 for analyses to aid interpretation of model output. We used the ‘pvals.fnc’ function in the R package LanguageR [49] to calculate p-values based on a Markov chain Monte Carlo (MCMC) sample of 10 000 simulations (not available for binomial models).
All analyses were run using R v. 2.15.1 [50]. For all analyses, separate models were run for each fixed effect, but results remained quantitatively similar in multivariate models. For all analyses, nonlinear relationships were explored using generalized additive mixed models (GAMMs) run in the R-package mgcv including smooth functions for each predictor [51]; but these models were retained as final models only where significance of nonlinear effects reached p < 0.10.
3. Results
(a). Offspring sex
Across 13 years (2000–2012), 49% of sexed offspring (597 of 1226) were male, which did not differ from 0.5 (exact binomial test p = 0.38, 95% confidence interval = 0.46, 0.52) as previously reported using data from 2000 to 2006 ([37]; electronic supplementary material, table S1). Brood sex ratio did not differ between completely versus incompletely sampled broods, or between complete one- versus two-egg broods, suggesting these factors did not generate a sex bias in our data (see the electronic supplementary material, table S1 and figure S1).
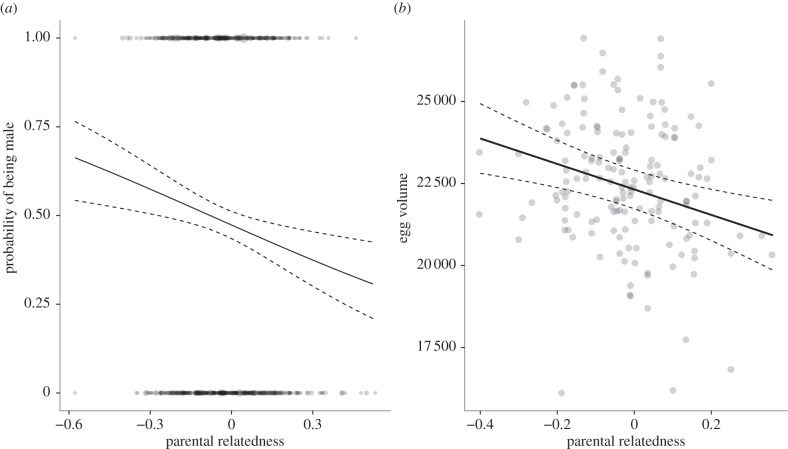
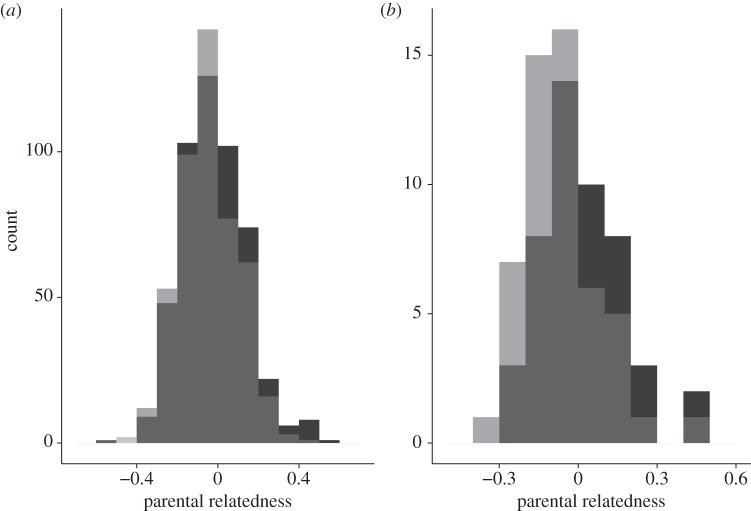
The probability of an offspring being male was significantly negatively correlated with parental relatedness (estimate = −1.35 ± 0.44, χ2 = 9.39, p < 0.01, n = 968 offspring from 597 broods produced over 13 years by 256 females and 110 males; figures 1 and 2). This correlation remained significant when we restricted analyses to 100 offspring from 50 broods of mixed paternity, thereby controlling for female quality (and environmental conditions) by comparing maternal half-siblings that differed in parental relatedness (estimate = −3.15 ± 1.48, χ2 = 5.00, p = 0.03; figure 2). As offspring heterozygosity is inherently negatively correlated with parental relatedness, the probability that an offspring was male also tended to be positively correlated with the offspring's own heterozygosity (estimate = 1.21 ± 0.66, χ2 = 3.38, p = 0.07), such that male offspring were more heterozygous. By contrast, offspring sex was unrelated to either father ARS (estimate = 0.01 ± 0.01, χ2 = 0.45, p = 0.50) or father heterozygosity (estimate = 0.57 ± 0.66, χ2 = 0.71, p = 0.40).
Figure 1.
(a) Predicted relationship between parental relatedness and the probability of an offspring being male across 968 offspring from 597 broods, and (b) egg volume (mm3) across 162 eggs from 108 broods. Dashed lines represent 95% confidence intervals based on fixed effects only. Raw data was shown by points scaled by sample size. Full models included random effects of brood, mother and father ID and year.
Figure 2.
Distribution of parental relatedness values for (a) 968 male (pale grey) and female (dark grey) offspring from 597 broods and (b) 100 male and female offspring from 50 mixed paternity broods. Values represented by both sexes are shown in mid-grey.
Offspring sex did not vary with the main effect of lay-date (estimate = 0.00 ± 0.00, χ2 = 0.11, p = 0.74). However, we identified a marginally significant interaction between lay-date and parental relatedness (estimate = 0.03 ± 0.02, χ2 = 2.85, p = 0.09); the correlation between offspring sex and parental relatedness tended to lessen as the season progressed (see the electronic supplementary material, figure S2). We did not identify any interactions between lay-date and either the offspring's own heterozygosity, father heterozygosity or father ARS (see the electronic supplementary material, table S2).
Across a restricted dataset of known-age females (with age classes 7–12 pooled owing to small sample sizes in older age classes), offspring sex was not correlated with mother age (estimate = −0.05 ± 0.10, χ2 = 0.22, p = 0.64, n = 146 offspring from 95 broods, produced by 44 males and 45 females), nor did we detect any interaction effects (see the electronic supplementary material, table S2). Using only those broods that were completely sampled gave qualitatively similar results for all models (data not presented).
(b). Egg size
Egg volume decreased with increasing parental relatedness (estimate = −0.39 ± 0.12, MCMCp = 0.01, n = 162 eggs from 108 broods across 2 years produced by 76 females and 26 males; figure 1). Despite the inherent correlation between offspring heterozygosity and parental relatedness, egg volume was not correlated with the offspring's own heterozygosity (estimate = 0.11 ± 0.12, MCMCp = 0.29), nor was it correlated with father ARS (estimate = 0.00 ± 0.00, MCMCp = 0.55), or father heterozygosity (estimate = 0.09 ± 0.17, MCMCp = 0.49).
We found no strong evidence that differences in egg size were related to environmental effects or mother age. Egg volume was not correlated with the linear expressions of lay-date (estimate = 0.00 ± 0.00, MCMCp = 0.54) but a GAMM suggested a quadratic relationship with lay-date (p = 0.04; 5% of deviance explained; electronic supplementary material, figure S3). No interactions between any measure of male genetic quality and lay-date were identified (see the electronic supplementary material, table S3). Egg volume did not vary with offspring sex (estimate = −0.01 ± 0.02, MCMCp = 0.97, n = 160 offspring from 108 broods produced by 76 females and 26 males). Across a restricted dataset of known-age females, egg volume was not correlated with a mother's age (age: estimate = 0.03 ± 0.02, MCMCp = 0.11, n = 49 offspring from 32 broods produced by 24 known-age females and 14 males, pooling females more than 6 years old because of low sample size in advanced age categories). An egg's volume was also unrelated to whether the offspring it contained later hatched (estimate = −0.03 ± 0.04, MCMCp = 0.82, n = 128 offspring from 86 broods 65 females and 24 males) or fledged (estimate = 0.0 ± 0.03, MCMCp = 0.85, n = 106 offspring from 69 broods 58 females and 23 males).
4. Discussion
We show that in lekking lance-tailed manakins, a species with strong sexual selection but no obvious inbreeding avoidance, offspring sex and egg volume reflect parental relatedness, suggesting that females adjust maternal investment in ways that minimize the negative effects of inbreeding depression for offspring. When females mated with less related males they were more likely to produce male offspring and larger eggs. Such apparent differential allocation may be adaptive in this polygynous species as less related mates produce more outbred offspring and males suffer stronger inbreeding depression in reproductive success than females [33]. Adaptive adjustment of primary sex ratio in birds remains controversial [8,9] although it has been demonstrated in several notable studies [5,6,52]. Sex allocation may be more common (and/or easier to detect) in species with small clutch sizes, for example lance-tailed manakins [35,52].
While our results are consistent with differential allocation in relation to mate relatedness, they could result from other processes. Firstly, a correlation between offspring sex and parental relatedness could represent sampling bias resulting from biased mortality between conception and sampling, for example if inbred male offspring and/or outbred female offspring die before sampling. This effect is unlikely to explain our results as we sampled offspring at an early stage (see Material and methods), included offspring from unhatched eggs, and sexed a high percentage (93%) of all sampled offspring. However, because our analyses necessarily excluded offspring that could not be assigned parentage and those that died before sampling, we did not strictly measure primary sex ratio. Nevertheless, results were similar across broods with no mortality before sampling, including 50 two-egg broods of maternal half-siblings that differed in parental relatedness, suggesting primary sex allocation rather than sex-specific inbreeding depression in early survival. Finally, there is no indication that offspring early survival varies with parental relatedness or offspring sex in this system [33]. In any case, biased survival between conception and independence theoretically represents secondary sex allocation [3,10]. Although we do not have data on any differential allocation post-laying that could alter survival to sampling, when we repeated analyses of offspring sex and parental relatedness across only those offspring that fledged, effect sizes were stronger despite smaller sample size (see the electronic supplementary material, figure S4). Stronger correlations across fledged offspring suggest that females may alter post-hatch investment in offspring with respect to mate relatedness and offspring sex, but this remains to be robustly tested. Overall, our results may therefore reflect both primary sex allocation, and potentially, secondary allocation or biased survival post-hatch.
An additional alternative explanation for our results is covariation between female (or environmental) quality and mate relatedness; for example, if more heterozygous females, experienced females or those nesting under better conditions both produce male-biased offspring (and larger eggs) and also tend to mate with less related mates. However, results were robust when we restricted analyses to maternal half-siblings that differed in parental relatedness. Furthermore, neither offspring sex (GLMM estimate = 0.23 ± 0.61, χ2 = 0.14, p = 0.71) nor egg volume (estimate = 0.10 ± 0.20, MCMCp = 0.25) varied with female heterozygosity (or age, see Results) while there is no correlation between mate relatedness and lay-date (a correlate of nesting success [33]). Therefore, we did not identify any variable that could cause covariation and it appears likely that our results reflect differential sex allocation.
We also identified a significant correlation between egg volume and parental relatedness, suggesting increased investment in eggs produced by mating with less related males. However, because egg size was known for only five broods of mixed paternity, we were unable to robustly exclude covariation between female quality/condition and parental relatedness in this instance (although egg size showed the same negative tendency with parental relatedness across 10 offspring from five mixed paternity broods; LMM estimate = −0.58 ± 0.27, MCMCp = 0.06). In the zebra finch, smaller eggs were produced when parents were more closely related [26] and in lekking black grouse (Tetrao tetrix), offspring growth rate was negatively correlated with parental kinship which potentially reflected increased investment in offspring produced by less related mates, although this latter result could also result from inbreeding depression in offspring growth [53]. Differential allocation with respect to parental relatedness may therefore be more widespread than previously appreciated.
Although egg size can have a profound impact on offspring morphology and growth rate [28], the benefits of differential allocation in egg size in this study remained unclear. Offspring from larger eggs were no more likely to hatch or fledge (although the sample of offspring that failed owing to factors other than depredation was relatively small across this 2 year dataset). Instead, offspring from larger eggs may benefit in later life. Indeed, natal conditions can have major impact on later life survival and reproduction, and such differences may be sex-specific [54,55]. The relationship of egg size with the offspring's fitness in terms of adult survival or reproductive success has rarely been tested in birds but such analyses are essential to measure the overall fitness benefits of maternal investment [28].
We predicted that female investment would vary with age owing to changing fitness returns or condition [3,56]. However, no correlation between offspring sex and female age was detected, despite previous work that reported a quadratic relationship between brood sex ratio and female age in 2 years in this population (2005–2006, in an analysis that included females of unknown but advanced age [37]). These different conclusions suggest effects are only apparent across an expanded age range (beyond 7) or previous results represent age-independent trends [37]. We also found no evidence that egg volume varied with mother age. Importantly, this suggests that apparent differential allocation with respect to mate relatedness did not result from covariation between offspring sex or egg size and female age. Our results did however suggest a weak quadratic relationship between egg volume and lay-date (see the electronic supplementary material, figure S3). Body condition shows an inverse quadratic relationship with capture date in this population, and the middle of the breeding season usually coincides with the end of the dry season in Panama (E. H. DuVal 2000-2013, unpublished data). Small egg sizes in the middle of the season therefore probably reflect generally challenging environmental conditions.
Although, on average, offspring were more likely to be male when parents were less related, a weak interaction between parental relatedness and lay-date suggests that as the season progressed the negative correlation between the probability that an offspring was male and parental relatedness tended to weaken (but did not become positive; electronic supplementary material, figure S2). If sons are more costly to raise (as generally predicted), females may only benefit from differential allocation with respect to mate genetic quality early in the season, when nesting success is especially high in this population [33]. Indeed, all females are predicted to invest similarly by the end of the breeding season, regardless of mate quality [36]. Overall, however, evidence for context-dependent differential allocation in this study was weak.
A number of studies report stronger inbreeding depression in male than female reproductive success [18–21], and there are similar trends in lance-tailed manakins [33]. If females can alter the fitness outcome of mating with a related male via non-genetic maternal effects, this may explain why some systems (including this one) do not show inbreeding avoidance despite inbreeding depression. Inbreeding levels in this population are not especially high; mean mate relatedness of mated pairs was 0, and 95% confidence intervals ranged between −0.26 and 0.21. Relatively related mates were therefore related at a level slightly less than that of half-siblings (0.25). Maternal effects such as those reported here may be more common in populations with high variance in inbreeding among mated pairs and hence high power to detect inbreeding effects, as is common in insular populations with reduced dispersal or polygynous species with high reproductive skew [24,32].
We suggest that this study system illustrates mate choice and investment for both male additive and non-additive genetic value via a hierarchical process [15,57]. In this population, more heterozygous males are more successful and produce offspring that are more likely to survive until fledging [33]. Although maternal effects could amplify any potential genetic benefit from such mate choice for heterozygosity, we found no evidence that investment varied with father heterozygosity. Heterozygous males may provide a genetic benefit to all females owing to their additive genetic value and/or ability to produce outbred offspring [15,22]. Females may instead invest in offspring relative to their relatedness to heterozygous males and genetic diversity of resultant chicks. Although more heterozygous males are less related, on average, to their mates and produce more outbred offspring, this relationship is highly variable; choosing heterozygous males sometimes produces pairings between related males and females, and hence relatively inbred offspring. Females may therefore alter the long-term fitness consequences of their choice post-copulation, reducing the detrimental fitness effect of producing (and reproductive investment in) relatively inbred offspring by producing smaller eggs and more female offspring which are generally considered cheaper to produce, and increasing the fitness benefit from outbred offspring by producing large eggs and biasing their sex towards males.
Recent theoretical work predicted sex allocation weakens sexual selection over time by eroding female preferences for male traits; therefore, species with elaborate sexual displays, for example lekking species, are predicted to show little control over offspring sex ratio [58,59]. Our results are particularly interesting in light of this prediction, as they suggest that systems with strong sexual selection can nevertheless exhibit sex allocation, and that mate choice for heterozygosity and differential investment by relatedness may allow for the persistence of both this phenomenon and directional female mate preferences [22].
Acknowledgements
We thank all fieldworkers over the 13 years of this study, and F. Koehler and E.Y. Pinzon for field site access.
The study was conducted under the supervision of the Florida State University IACUC (protocol no. 0718 and 1101).
Data accessibility
Microsatellite sequences: GenBank accession nos. AY752868.1–AY75276.1, EF105316.1–EF105326.1. All other data are accessible in the dryad repository doi: 10.5061/dryad.vj3gm.
Funding statement
This work and the long-term project were supported by NSF (IOS-0843334) and Florida State University.
References
- 1.Burley N. 1986. Sexual selection for aesthetic traits in species with biparental care. Am. Nat. 127, 415–445 (doi:10.1086/284493) [Google Scholar]
- 2.Sheldon BC. 2000. Differential allocation: tests, mechanisms and implications . Trends Ecol. Evol. 15, 397–402 (doi:10.1016/S0169-5347(00)01953-4) [DOI] [PubMed] [Google Scholar]
- 3.Trivers RL, Willard DE. 1973. Natural selection of parental ability to vary the sex ratio of offspring . Science 179, 90–92 (doi:10.1126/science.179.4068.90) [DOI] [PubMed] [Google Scholar]
- 4.Horváthová T, Nakagawa S, Uller T. 2012. Strategic female reproductive investment in response to male attractiveness in birds . Proc. R. Soc. B 279, 163–170 (doi:10.1098/rspb.2011.0663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellegren H, Gustafsson L, Sheldon BC. 1996. Sex ratio adjustment in relation to paternal attractiveness in a wild bird population . Proc. Natl Acad. Sci. USA 93, 11 723–11 728 (doi:10.1073/pnas.93.21.11723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheldon BC, Andersson S, Griffith SC, Örnborg J, Sendecka J. 1999. Ultraviolet colour variation influences blue tit sex ratios . Nature 402, 874–877 (doi:10.1038/47239) [Google Scholar]
- 7.Pike TW, Petrie M. 2005. Offspring sex ratio is related to paternal train elaboration and yolk corticosterone in peafowl . Biol. Lett. 1, 204–207 (doi:10.1098/rsbl.2005.0295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ewen JG, Cassey P, Moller AP. 2004. Facultative primary sex ratio variation: a lack of evidence in birds? Proc. R. Soc. Lond. B 271, 1277–1282 (doi:10.1098/rspb.2004.2735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassey P, Ewen JG, Møller AP. 2006. Revised evidence for facultative sex ratio adjustment in birds: a correction . Proc. R. Soc. B 273, 3129–3130 (doi:10.1098/rspb.2006.3628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postma E, Heinrich F, Koller U, Sardell RJ, Reid JM, Arcese P, Keller LF. 2011. Disentangling the effect of genes, the environment and chance on sex ratio variation in a wild bird population . Proc. R. Soc. B 278, 2996–3002 (doi:10.1098/rspb.2010.2763) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pryke SR, Griffith SC. 2009. Genetic incompatibility drives sex allocation and maternal investment in a polymorphic finch . Science 323, 1605–1607 (doi:10.1126/science.1168928) [DOI] [PubMed] [Google Scholar]
- 12.Potvin DA, MacDougall-Shackleton EA. 2009. Parental investment amplifies effects of genetic complementarity on growth rates in song sparrows, Melospiza melodia . Anim. Behav. 78, 943–948 (doi:10.1016/j.anbehav.2009.07.023) [Google Scholar]
- 13.Potvin DA, MacDougall-Shackleton EA. 2010. Paternal song complexity predicts offspring sex ratios close to fledging, but not hatching, in song sparrows . Wilson J. Ornithol. 122, 146–152 (doi:10.1676/09-069.1) [Google Scholar]
- 14.Pryke SR, Griffith SC. 2010. Maternal adjustment of parental effort in relation to mate compatibility affects offspring development . Behav. Ecol. 21, 226–232 (doi:10.1093/beheco/arp180) [Google Scholar]
- 15.Mays HL, Hill GE. 2004. Choosing mates: good genes versus genes that are a good fit . Trends Ecol. Evol. 19, 554–559 (doi:10.1016/j.tree.2004.07.018) [DOI] [PubMed] [Google Scholar]
- 16.Keller LF, Waller DM. 2002. Inbreeding effects in wild populations . Trends Ecol. Evol. 17, 230–241 (doi:10.1016/S0169-5347(02)02489-8) [Google Scholar]
- 17.Szulkin M, Garant D, McCleery RH, Sheldon BC. 2007. Inbreeding depression along a life-history continuum in the great tit . J. Evol. Biol. 20, 1531–1543 (doi:10.1111/j.1420-9101.2007.01325.x) [DOI] [PubMed] [Google Scholar]
- 18.Keller LF, Reid JM, Arcese P. 2008. Testing evolutionary models of senescence in a natural population: age and inbreeding effects on fitness components in song sparrows. Proc. R. Soc. B 275, 597–604 (doi:10.1098/rspb.2007.0961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enders L, Nunney L. 2010. Sex-specific effects of inbreeding in wild-caught Drosophila melanogaster under benign and stressful conditions. J. Evol. Biol. 23, 2309–2323 (doi:10.1111/j.1420-9101.2010.02085.x) [DOI] [PubMed] [Google Scholar]
- 20.Brekke P, Bennett PM, Wang J, Pettorelli N, Ewen JG. 2010. Sensitive males: inbreeding depression in an endangered bird. Proc. R. Soc. B 277, 3677–3684 (doi:10.1098/rspb.2010.1144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simmons LW. 2011. Inbreeding depression in the competitive fertilization success of male crickets . J. Evol. Biol. 24, 415–421 (doi:10.1111/j.1420-9101.2010.02179.x) [DOI] [PubMed] [Google Scholar]
- 22.Fromhage L, Kokko H, Reid JM. 2009. Evolution of mate choice for genome-wide heterozygosity . Evolution 63, 684–694 (doi:10.1111/j.1558-5646.2008.00575.x) [DOI] [PubMed] [Google Scholar]
- 23.Reid JM, Keller LF. 2010. Correlated inbreeding among relatives: occurrence, magnitude, and implications . Evolution 64, 973–985 (doi:10.1111/j.1558-5646.2009.00865.x) [DOI] [PubMed] [Google Scholar]
- 24.Szulkin M, Stopher KV, Pemberton JM, Reid JM. 2013. Inbreeding avoidance, tolerance, or preference in animals? Trends Ecol. Evol. 28, 205–211 (doi:10.1016/j.tree.2012.10.016) [DOI] [PubMed] [Google Scholar]
- 25.Kokko H, Ots I. 2006. When not to avoid inbreeding . Evolution 60, 467–475 (doi:10.1554/05-613.1) [PubMed] [Google Scholar]
- 26.Arct A, Rutkowska J, Martyka R, Drobniak SM, Cichoń M. 2010. Kin recognition and adjustment of reproductive effort in zebra finches . Biol. Lett. 6, 762–764 (doi:10.1098/rsbl.2010.0417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carranza J, Pérez-González J, Mateos C, Fernández-García J. 2009. Parents’ genetic dissimilarity and offspring sex in a polygynous mammal . Mol. Ecol. 18, 4964–4973 (doi:10.1111/j.1365-294X.2009.04401) [DOI] [PubMed] [Google Scholar]
- 28.Krist M. 2011. Egg size and offspring quality: a meta-analysis in birds . Biol. Rev. 86, 692–716 (doi:10.1111/j.1469-185X.2010.00166.x) [DOI] [PubMed] [Google Scholar]
- 29.Loyau A, Lacroix F. 2010. Watching sexy displays improves hatching success and offspring growth through maternal allocation . Proc. R. Soc. B 277, 3453–3460 (doi:10.1098/rspb.2010.0473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Höglund J, Alatalo RV. 1995. Leks. Princeton, NJ: Princeton University Press [Google Scholar]
- 31.DuVal EH. 2013. Female mate fidelity in a lek mating system and its implications for the evolution of cooperative lekking behaviour . Am. Nat. 181, 213–222 (doi:10.1086/668830) [DOI] [PubMed] [Google Scholar]
- 32.Ryder T, Tori W, Blake J, Loiselle B, Parker P. 2010. Mate choice for genetic quality: a test of the heterozygosity and compatibility hypotheses in a lek-breeding bird . Behav. Ecol. 21, 203–210 (doi:10.1093/beheco/arp176) [Google Scholar]
- 33.Sardell RJ, Kempenaers B, DuVal EH. In press. Female mating preferences and offspring survival: testing hypotheses on the genetic basis of mate choice in a wild lekking bird. Mol. Ecol. [DOI] [PubMed] [Google Scholar]
- 34.Bluhm CK, Gowaty PA. 2004. Reproductive compensation for offspring viability deficits by female mallards, Anas platyrhynchos . Anim. Behav. 68, 985–992 (doi:10.1016/j.anbehav.2004.01.012) [Google Scholar]
- 35.Addison B, Kitaysky AS, Hipfner JM. 2008. Sex allocation in a monomorphic seabird with a single-egg clutch: test of the environment, mate quality, and female condition hypotheses . Behav. Ecol. Sociobiol. 63, 135–141 (doi:10.1007/s00265-008-0643-z) [Google Scholar]
- 36.Harris WE, Uller T. 2009. Reproductive investment when mate quality varies: differential allocation versus reproductive compensation . Phil. Trans. R. Soc. B 364, 1039–1048 (doi:10.1098/rstb.2008.0299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laucht S, DuVal EH, Kempenaers B. 2008. Maternal correlates of brood sex ratio variation in the lekking lance-tailed manakin Chiroxiphia lanceolata . J. Avian Biol. 39, 198–205 (doi:10.1111/j.2008.0908-8857.04165.x) [Google Scholar]
- 38.DuVal EH. 2007. Cooperative display and lekking behavior of the lance-tailed manakin (Chiroxiphia lanceolata) . Auk 124, 1168–1185 (doi:10.1642/0004-8038) [Google Scholar]
- 39.Ryder TB, Durães R. 2005. It's not easy being green: using molt and morphological criteria to age and sex green-plumage manakins (Aves: Pipridae) . Ornitol. Neotrop. 16, 481–491 (doi:10.1642/0004-8038(2007)124) [Google Scholar]
- 40.DuVal EH, Kempenaers B. 2008. Sexual selection in a lekking bird: the relative opportunity for selection by female choice and male competition. Proc. R. Soc. B 275, 1995–2003 (doi:10.1098/rspb.2008.0151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DuVal EH. 2012. Variation in annual and lifetime reproductive success of lance-tailed manakins: alpha experience mitigates effects of senescence on siring success. Proc. R. Soc. B 279, 1551–1559 (doi:10.1098/rspb.2011.1840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tori WP, Ryder TB, Durães R, Hidalgo JR, Loiselle BA, Blake JG. 2006. Obtaining offspring genetic material: a new method for species with high nest predation rates . Condor 108, 948–952 (doi:10.1650/0010-5422) [Google Scholar]
- 43.Kalinowski ST, Taper ML, Marshall TC. 2007. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment . Mol. Ecol. 16, 1099–1106 (doi:10.1111/j.1365-294x.2007.03089.x) [DOI] [PubMed] [Google Scholar]
- 44.Griffiths R, Double MC, Orr K, Dawson RJG. 1998. A DNA test to sex most birds . Mol. Ecol. 7, 1071–1075 (doi:10.1046/j.1365-294x.1998.00389.x) [DOI] [PubMed] [Google Scholar]
- 45.Queller DC, Goodnight KF. 1989. Estimating relatedness using genetic markers . Evolution 43, 258–275 (doi:10.2307/2409206) [DOI] [PubMed] [Google Scholar]
- 46.Bates D, Maechler M, Bolker B. 2012. Linear mixed-effects models using S4 classes. See http://cran.r-project.org/web/packages/lme4/index.html.
- 47.Krackow S, Tkadlec E. 2001. Analysis of brood sex ratios: implications of offspring clustering . Behav. Ecol. Sociobiol. 50, 293–301 (doi:10.1007/s002650100366) [Google Scholar]
- 48.Krackow S, Neuhäuser M. 2008. Insights from complete-incomplete brood sex-ratio disparity . Behav. Ecol. Sociobiol. 62, 469–477 (doi:10.1007/s00265-007-0466-3) [Google Scholar]
- 49.Baayen RH. 2008. LanguageR: data sets and functions with ‘analyzing linguistic data: a practical introduction to statistics’. R package v. 0.953. See http://cran.r-project.org/src/contrib/Archive/languageR/. [Google Scholar]
- 50.R Development Core Team 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 51.Wood SN. 2011. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models . J. R. Stat. Soc. B 73, 3–36 (doi:10.1111/j.1467-9868.2010.00749.x) [Google Scholar]
- 52.Komdeur J, Daan S, Tinbergen J, Mateman C. 1997. Extreme adaptive modification in sex ratio of the Seychelles warbler's eggs . Nature 385, 522–525 (doi:10.1038/385522a0) [Google Scholar]
- 53.Soulsbury CD, Alatalo RV, Lebigre C, Rokka K, Siitari H. 2011. Age-dependent inbreeding risk and offspring fitness costs in female black grouse . Biol. Lett. 7, 853–855 (doi:10.1098/rsbl.2011.0379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilkin TA, Sheldon BC. 2009. Sex differences in the persistence of natal environmental effects on life histories . Curr. Biol. 19, 1998–2002 (doi:10.1016/j.cub.2009.09.065) [DOI] [PubMed] [Google Scholar]
- 55.Van De Pol M, Bruinzeel LW, Heg D, Van Der Jeugd HP, Verhulst S. 2006. A silver spoon for a golden future: long-term effects of natal origin on fitness prospects of oystercatchers (Haematopus ostralegus) . J. Anim. Ecol. 75, 616–626 (doi:10.1111/j.1365-2656.2006.01079.x) [DOI] [PubMed] [Google Scholar]
- 56.Blank JL, Nolan V. 1983. Offspring sex ratio in red-winged blackbirds is dependent on maternal age . Proc. Natl Acad. Sci. USA 80, 6141–6145 (doi:10.1073/pnas.80.19.6141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Colegrave N, Kotiaho JS, Tomkins JL. 2002. Mate choice or polyandry: reconciling genetic compatibility and good genes sexual selection . Evol. Ecol. Res. 4, 911–917 [Google Scholar]
- 58.Fawcett TW, Kuijper B, Weissing FJ, Pen I. 2011. Sex-ratio control erodes sexual selection, revealing evolutionary feedback from adaptive plasticity . Proc. Natl Acad. Sci. USA 108, 15 925–15 930 (doi:10.1073/pnas.1105721108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Booksmythe I, Schwanz LE, Kokko H. 2013. The complex interplay of sex allocation and sexual selection . Evolution 67, 673–678 (doi:10.1111/evo.12003) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Microsatellite sequences: GenBank accession nos. AY752868.1–AY75276.1, EF105316.1–EF105326.1. All other data are accessible in the dryad repository doi: 10.5061/dryad.vj3gm.