Abstract
Several sacoglossan sea slugs (Plakobranchoidea) feed upon plastids of large unicellular algae. Four species—called long-term retention (LtR) species—are known to sequester ingested plastids within specialized cells of the digestive gland. There, the stolen plastids (kleptoplasts) remain photosynthetically active for several months, during which time LtR species can survive without additional food uptake. Kleptoplast longevity has long been puzzling, because the slugs do not sequester algal nuclei that could support photosystem maintenance. It is widely assumed that the slugs survive starvation by means of kleptoplast photosynthesis, yet direct evidence to support that view is lacking. We show that two LtR plakobranchids, Elysia timida and Plakobranchus ocellatus, incorporate 14CO2 into acid-stable products 60- and 64-fold more rapidly in the light than in the dark, respectively. Despite this light-dependent CO2 fixation ability, light is, surprisingly, not essential for the slugs to survive starvation. LtR animals survived several months of starvation (i) in complete darkness and (ii) in the light in the presence of the photosynthesis inhibitor monolinuron, all while not losing weight faster than the control animals. Contrary to current views, sacoglossan kleptoplasts seem to be slowly digested food reserves, not a source of solar power.
Keywords: Gastropoda, Sacoglossa, Kleptoplasty, Elysia, photoautotroph, photosynthetic slugs
1. Introduction
Symbioses between animals or heterotrophic protist and algae are fairly common in nature [1]; prominent examples include the zoochlorellae of Hydra viridis [2,3] or dinoflagellates (zooxanthellae) of corals [4] and the many different species of algae found in ciliates [5]. A more curious kind of symbiosis is found among the sacoglossan molluscs (marine slugs) from the Plakobranchoidea. These animals establish a symbiosis with only a part of their algal partner: the plastid. Nearly 150 species of plakobranchoids have been described to date, and similar to all sacoglossans they feed upon algae by sucking the cytoplasm out of the large, syncytial algal cells upon which they feed. While most sacoglossan species simply digest the plastids, plakobranchoidean species (termed plakobranchids for convenience) exhibit a delayed digestion, and four species—Elysia chlorotica, Elysia timida, Elysia crispata and Plakobranchus ocellatus—retain the ingested plastids, the kleptoplasts, in their digestive gland for several months [6–10]. This gland fills most of the animal's body and gives them a distinctive green colour (figure 1), which is why they are sometimes called ‘leaves that crawl’ [6] or ‘solar-powered slugs’ [9]. Because these four species can maintain plastids with functional photosystems for several months, they are designated as long-term retention (LtR) species in contrast to those plakobranchids that are only able to maintain functional plastids for up to two weeks, and which are hence termed short-term retention (StR) species [7]. In E. timida, the undigested plastids remain ultrastructurally intact and photosynthetically active, as determined by photosystem fluorescence, for more than two months [7,8]. Having acquired a load of plastids, the animals can be kept in the laboratory, in the light, for months without additional food [7–10].
Figure 1.
Overview of phylogenetic relationships of Plakobranchoidea (Sacoglossa). Blue rectangles indicate photosynthetic activity over at least two weeks of starvation (based on pulse–amplitude–modulation (PAM) measurements), whereas grey squares indicate species that immediately digest plastids. Slugs showing photosynthetic activities over two months are highlighted in green and the respective pictures of the species and food alga are provided on the right. Photo of E. chlorotica with permission of M. Rumpho, and that of Vaucheria litorea with permission of C. F. Carter. Phylogeny based on Wägele et al. [8].
How LtR plakobranchids maintain their kleptoplasts for such long periods of time has been the subject of much speculation and considerable recent research. The predicted proteome of Arabidopsis plastids ranges from 1000 to approximately 3500 proteins [11], but plastid genomes only encode for 60 (higher plants) to 200 (red algae) protein-coding genes in the organelle's DNA [12]. The remaining plastid proteins (more than 90%) are encoded in the nucleus, synthesized as precursor proteins on cytosolic ribosomes and imported from the cytosol through the plastid-specific protein translocon machinery (reviewed in [13–15]). Because plakobranchids do not sequester algal nuclei, which can however be ingested for a short time during feeding [16], and because some proteins in higher plant chloroplasts can have turnover rates on the order of 30–120 min [17–19], it has been widely assumed that sequestered plastids of plakobranchids also require imported proteins to remain photosynthetically active. The most popular theory for the source of those assumedly essential genes has been lateral gene transfer (LGT) from the algae to the slug, and some PCR-based reports provided evidence in favour of that view, for example involving the gene for the light-harvesting complex protein LHC [10].
The by far most prominent report for putative involvement of LGT in sacoglossans concerns a sequence for the manganese cluster-stabilizing protein PsbO of photosystem II in E. chlorotica [20]. The PCR amplification products for PsbO obtained from E. chlorotica were identical in sequence to those from Vaucheria, including a canonical bipartite targeting signal [21,22] that directs the PsbO precursor across the four membranes that surround the plastid in Vaucheria. However, the Vaucheria plastids that are sequestered in E. chlorotica are only surrounded by two membranes; the outer two are removed during sequestration [23]. As a consequence, were the E. chlorotica PsbO precursor protein [20] really expressed, the gene product would enter the secretory pathway, and thus be excreted from the cell because of its intact and highly conserved signal peptide, rather than being targeted to the remaining inner two membranes of the sequestered Vaucheria plastid [8]. This aspect of targeting renders the case for LGT of PsbO in E. chlorotica very problematic and raises the question: is there LGT from algae to slugs in LtR plakobranchids, or not?
To address this, Wägele et al. [8] sequenced expressed sequence tags (ESTs) from the LtR species E. timida and P. ocellatus and found no evidence in either species for the expression of any genes of demonstrably green algal nuclear provenance. Similar results for E. chlorotica were subsequently obtained, with no transcripts for PsbO or any other Vaucheria-derived nuclear genes identified [24], leading to the conclusion that, contrary to earlier claims, LGT probably does not underpin photosynthetic activity of sequestered plastids in E. chlorotica after all. However, Pierce et al. [25] reported that among the 100 million E. chlorotica transcripts that they sequenced, about 100 reads might indicate LGT in E. chlorotica, although only one pointed to an essential function in photosynthesis (a light-harvesting complex protein). But photosynthesis requires the expression of thousands of nuclear genes [11,26], not 100. Moreover, transcripts for photosynthetic functions are generally abundant: for example, the small subunit of RuBisCO and LHC together constitute approximately 20% of all transcripts in Arabidopsis leaves [27]. The 100 genes that Pierce et al. [25] found comprise 0.000001% of the mRNA each, or 0.0001% of the total, so even if those 100 genes are LGTs, they cannot underpin a photosynthetic lifestyle. While Pierce et al. [25] interpret those 100/100 000 000 reads as evidence of LGT from alga to mollusc, we would interpret that same data to indicate that their sequencing substrate was 99.9999% free of contaminating algal nucleic acids.
The very expectation that some sacoglossans have undergone LGT stems from the inference that plastids require many proteins in order to support a photosynthetic lifestyle. As the genes for the proteins are missing, the next question is: how strong is the evidence that the slugs depend upon photosynthesis to begin with? The main evidence supporting the view that plakobranchids are photosynthetic (in the sense of being photoautotrophic) comes from earlier studies and is of two main types. First, Trench and co-workers [28,29] showed that E. viridis incorporates 14C from 14CO2. A number of other studies also reported the incorporation of 14C from 14CO2 in plakobranchids that sequester plastids [30–33], but animals can also incorporate CO2 via carboxylation reactions. The second line of evidence for plakobranchids being photosynthetic comes from the observation that once the plastids have been incorporated into the digestive gland, LtR species can survive for months in the absence of additional food [7,10,23,24,34,35]. Such plakobranchids are said to be ‘starved’ and are typically cultivated in the light [8,9].
However, a subtlety of such experiments that is not immediately evident to the observer (who is understandably fascinated by the sight of plastid-bearing slugs), but that has been pointed out in earlier work [36–40], is that starved animals become smaller as starvation progresses. Starved animals also tend to lose their green colour with time, getting pale as starvation progresses [37,38,41]. Here, we take a step back in the study of ‘photosynthetic slugs’—as many, including ourselves, have called them in the past—by re-inspecting the role of light. We test the light dependence of 14CO2 incorporation into acid-stable compounds in E. timida and P. ocellatus, the long-term starvation survival of plastid-bearing slugs in light versus dark, and the effect of the photosynthesis inhibitor monolinuron on the ability of P. ocellatus to survive starvation in the light. Surprisingly, photosynthesis was not essential for the slugs to survive months of starvation, which explains the lack of gene transfer from alga to animal in these species and, more importantly, calls for a general rethinking of the ‘photosynthetic slug’ story.
2. Results and discussion
The relationship between sacoglossans that perform LtR of their sequestered plastids is now widely reported in the literature as an example of acquired photoautotrophy in animals [9,24,42], typically leading to questions of how many and what kinds of genes have been transferred to support this photoautotrophic lifestyle [20]. Critical of that view, we recently tested the gene transfer hypothesis in sacoglossans that perform LtR and found no evidence for the expression of any genes of demonstrably green algal nuclear provenance to support plastid longevity in two of the four known LtR species, E. timida and P. ocellatus [8]. That eyebrow-raising result prompted us to further re-inspect the degree to which plakobranchid sacoglossans exhibiting LtR depend on photosynthesis in the first place.
(a). Elysia timida and Plakobranchus ocellatus display light-dependent CO2 fixation
Previous studies on several plastid-bearing sea slugs have shown that green animals can fix 14CO2 [29,32,33,43–45]. However, there are also exchange reactions and carboxylation steps in animal metabolism that would allow 14CO2 to be incorporated into animal tissue in a light-independent manner. For example, propionate is a main primary source of reduced carbon in many animals; it is absorbed from the gut, where it is released from ingested food by the gut microbial flora. Propionate is channelled into metabolism as propionyl-CoA, which is then carboxylated to methylmalonyl-CoA and rearranged in a vitamin B12-dependent reaction to the citric acid cycle intermediate succinyl-CoA and then succinate, which can be used either for biosynthetic (amino acids, haem, etc.) or for energetic purposes [45]. Thus, via succinate, 14CO2 can be incorporated into animal tissue, but in a light-independent manner. Furthermore, the fixation rates reported so far vary substantially between different species studied [23,32,33,43–45].
We investigated the ability of E. timida bearing Acetabularia plastids to fix 14CO2 in the absence and presence of light. In total, we used 24 slugs for three individual experiments. We analysed the light-dependent incorporation of [14C]-labelled CO2 after 2 min, 1 and 2 h. Four slugs were used for every time point and kept either in the light or in the dark. Afterwards, incorporation of labelled carbon was measured. We note that adult slugs lacking plastids cannot be used as a control here, because individuals of these sacoglossan species do not develop into adults unless they feed, at the larval stage, upon their specific algae, and because plastid-bearing adults die before they can be starved to the stage of lacking plastids altogether. After 2 min incubation with [14C]-labelled CO2, incorporation in the light was slightly higher than that in the dark (0.05 nmol in the light versus 0.04 nmol in the dark). After 1 h, slugs in the light showed incorporation 23-fold higher than slugs in the dark (6.73 nmol incorporated 14CO2 in the light and 0.30 nmol in the dark). In the light, 14CO2 incorporation after 2 h was 60 times greater than for slugs kept in the dark (28.1 versus 0.46 nmol 14CO2; figure 2). Thus, we can confirm that Acetabularia plastids in E. timida fix CO2 in a light-dependent manner, as has been reported for other plakobranchids [29,32,33,43–45]. That CO2 fixation is almost abolished in the dark demonstrates that light-independent carbon fixation reactions, although they can occur in slug metabolism, are overshadowed by light-dependent CO2 fixation in sequestered plastids.
Figure 2.
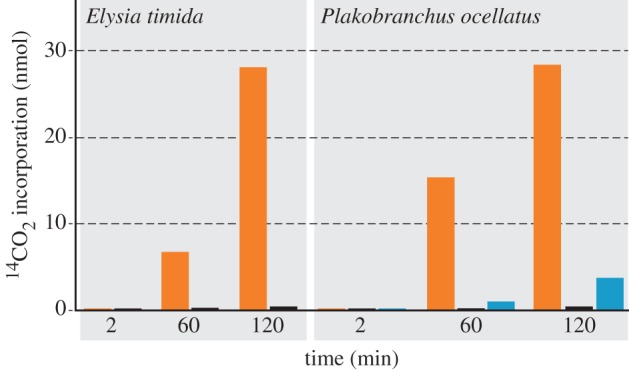
Light-dependent incorporation of 14CO2 by E. timida and P. ocellatus. CO2 incorporation in E. timida is almost completely blocked when the slugs were kept in the dark. In P. ocellatus, we additionally blocked photosynthesis using monolinuron, which led to an 87% decrease of CO2 incorporation. Owing to the size difference, four E. timida specimens (always representing an equal amount of weight) and only one P. ocellatus were used for each individual time point measured. Only for the 120 min values of P. ocellatus, the mean of two individual measurements is shown (values (nmol per incubation) of these 120 min incubations of P. ocellatus were D: 0.42 and 0.47; L: 24.5 and 32.2; M: 3.14 and 4.35); for all others a single measurement was carried out. Orange, light; black, dark; blue, light + monolinuron.
For P. ocellatus, we obtained similar results (figure 2), showing further that in comparison to the untreated slugs, the incorporation of 14CO2 in the monolinuron-treated samples was 87% lower after 120 min, indicating that photosynthesis in the slugs is inhibited by the drug. Previous studies reported 14C in a variety of slug metabolites [29,33,43–45], but whether the label stems from photosynthate exported from intact plastids or simply from decomposing plastids is not known. That is, it is possible that sequestered plastids do not export reduced carbon, but are simply digested, a possibility that is supported by microscopic observations suggesting that kleptoplasts accumulate substrate under starvation conditions, rather than secreting it [46,47]. Notwithstanding many studies in the literature addressing the nature of plastid–slug metabolite interactions, it seemed that the more crucial question was whether light-dependent CO2 fixation was essential for survival of the animals grown without algal food.
(b). Blocking photosynthesis affects neither survival rate nor weight decrease during starvation
Earlier work on the LtR species E. timida and P. ocellatus delivered conflicting results with respect to the role of light during starvation. Some studies indicated that specimens starved in the dark lost weight faster and had a higher death rate than those starved in the light, from which it was concluded that photosynthesis is important for the survival of these LtR slugs [36–38]. However, in those experiments some slugs survived just fine in the dark, and conversely some kept in the light died. In the starvation experiments performed on E. timida [36], the survival rate was monitored for only three aquaria, which were chosen apparently randomly from a total of nine, and the exact survival rate across all aquaria was not reported. While Yamamoto et al. [40] also reported a higher death rate for those slugs kept in the dark, they noted that the higher dark death rate could be attributable to water fouling, as the survival rate even for those kept in the light was very low. From our experience, it is crucial to keep each experimental animal in a separate repository, while at the same time regularly monitoring water quality. In all of our experiments, only one animal (one E. timida kept at 40 µmol quanta m−2 s−1) died on day 23 of starvation.
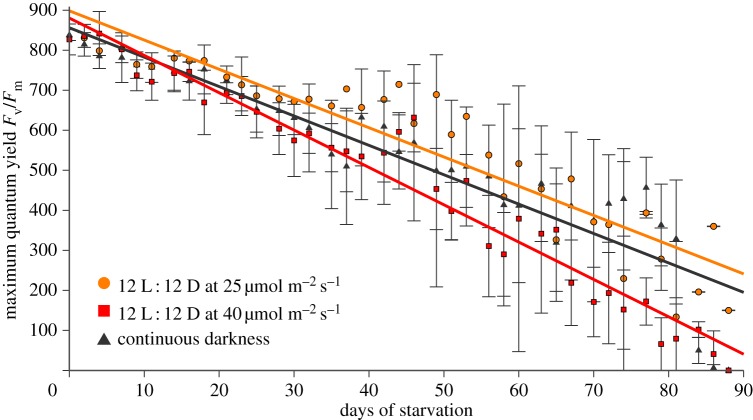
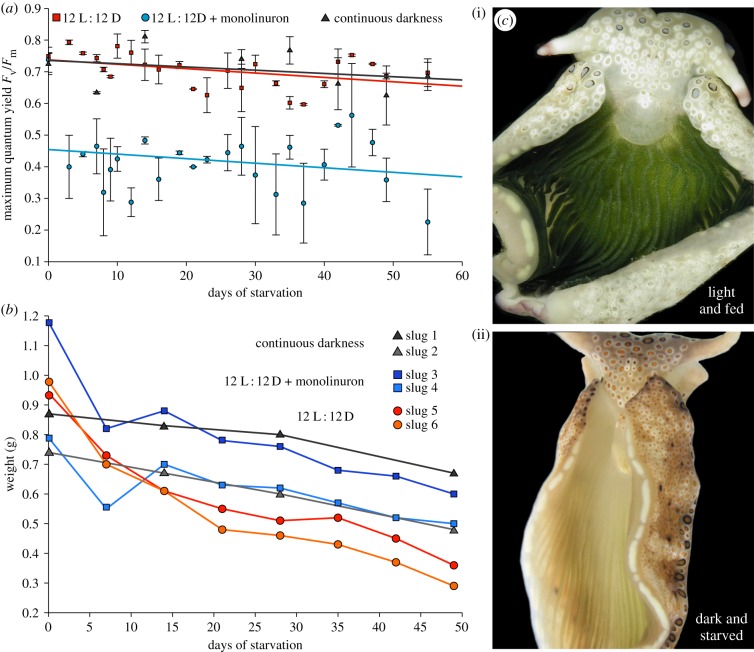
Recent results reported for P. ocellatus indicate that in the wild, the contribution of photosynthesis by sequestered plastids to the animal's carbon uptake is very minor, and raised the question of whether photosynthesis in kleptoplasts contributes significantly to nutrition during starvation [48]. To readdress the role of light, we blocked photosynthesis in two ways: first by simply culturing slugs in the dark and second by inhibiting photosynthesis with monolinuron. We kept six specimens of E. timida individually in total darkness over a time course of almost three months. Based on pulse–amplitude–modulation (PAM) fluorescence, the maximum quantum yield was better for the dark-kept animals than for those kept in the light (40 µmol quanta m−2 s−1; figure 3). Plakobranchus ocellatus animals were weighed and their plastid photosynthetic capacity measured through PAM. Using 2 µg ml−1 of monolinuron, slugs cultivated at 40 µmol quanta m−2 s−1 revealed an average inhibition of photosynthesis by 42%, as determined by PAM-measurements (figure 4a). Yet, animals cultivated in the presence of monolinuron survived just as well as the control set over the 55 days analysed. Importantly, the control slugs, the monolinuron-treated slugs and those kept in the dark, all showed approximately the same degree of weight loss on day 49 when the experiment was ended (figure 4b).
Figure 3.
PAM measurements of E. timida. The maximum quantum yields of slugs kept in the dark (black) were compared to slugs kept under low- (orange) and high-light (red) conditions. Those kept under high light show the strongest decrease over the three months measured, whereas the linear regression of those kept in the dark runs in parallel to that of those kept under low-light conditions. Six specimens were used for each condition tested. The error bars present the standard deviation.
Figure 4.
Influence of photosynthesis inhibition on P. ocellatus. (a) PAM measurements of monolinuron-treated slugs in a 12 L : 12 D cycle (25 µmol quanta m−2 s−1; blue) in comparison to those kept in the dark (black) and under at a 12 L : 12 D cycle (25 µmol quanta m−2 s−1; red). Two specimens were used for each condition tested and the error bars present the standard deviation. (b) Weight measurements of the P. ocellatus specimens shown in (a). (c) Exemplary images of P. ocellatus specimens. Image (i) shows a slug kept in the light and which was regularly fed, hence best representing natural conditions. Image (ii) shows a slug after 55 days of starvation in the dark.
In our hands, both LtR plakobranchids studied survived equally well in the dark as they did in the light. In fact, for Plakobranchus, comparing the individual regressions of weight loss with each other demonstrated that those kept in the dark lost weight the slowest, albeit only slightly slower than those kept in the light, which lost weight the fastest. Weight loss measurements for E. timida were unreliable because these animals are small (less than 100 mg each) and handling proved difficult. However, the results clearly indicate that photosynthesis as a core carbon source cannot be essential for slug survival in these two LtR sacoglossan species, because survival was not light-dependent. It remains a possibility that the slugs require specific compounds synthesized in plastids for proper development, for example the synthesis of pyrone-containing proprionates [49], in particular because Ireland & Scheuer [30] suggested that a significant part of the fixed CO2 might be dedicated to the biosynthesis of pyrone-containing proprionates. But as far as basic nutrition goes, the slugs are apparently not ‘solar powered’ at all. That readily explains why no algal genes essential for photosynthesis are expressed by slug nuclei: the gene products are not required and this is why algal nuclear genes are not required to support kleptoplast longevity [8,24]. Accordingly, recent nuclear genome data of egg DNA of E. chlorotica uncovered no evidence for putatively transferred algal genes [50]. Once a nuclear genome becomes available, it will need to provide evidence that genes of algal origin occur at the same copy number in the assembly as a single copy plakobranchid gene.
The plastids of these LtR species remain capable of photosynthesis, as the PAM and 14CO2 incorporation results show (figures 3, 4 and 2, respectively), but the observation that light has no detectable effect on animal survival or weight loss during starvation indicates that whatever the plastids do, they do not have a life-extending effect on the animals whose survival does not depend upon plastid photosynthetic activity over the three-month period that we analysed. Photosynthesis in sequestered plastids of LtR species might be important for plastid longevity, but this has yet to be shown. Our experiments measured the survival of the slugs, not the survival of the plastids directly, although the data in figure 4a show that plastids maintained in the dark are, by the measure of PAM fluorescence, just as viable as those maintained under 12 L : 12 D. Thus, the plastids of the LtR species P. ocellatus and E. timida appear to be a source of stored food, and although similar experiments have yet to be reported for the other two LtR species—E. chlorotica and E. crispata—the implication from our findings is that light is probably not required for long-term survival during starvation of those species either.
If plastid fitness had a direct impact on slug fitness, then blocking photosynthesis, whether through light deprivation or monolinuron, should influence the weight and survival rate of the animals. Yet, those Plakobranchus specimens kept in the dark or treated with monolinuron lost weight at the same rate as the starving control specimens (figure 4b) and the E. timida slugs kept in the dark appeared as healthy as the control set, too. Furthermore, the linear regression of the maximum quantum yield of slugs kept in the dark (figure 3) and those treated with monolinuron (figure 4a) runs parallel to those of the control animals, rather than declining more rapidly over time as a proxy of reduced and essential plastid contribution to nutrition. Klochkova et al. [39] recently observed that specimens of the StR species Elysia nigrocapitata survived for five months without performing photosynthesis, during which time the starved animals dramatically lost weight. Notably, E. nigrocapitata animals go from 3 cm in length to 3 mm during starvation, but reversibly, if provided with food [39].
3. Conclusion
It has been established that LGT is not involved in kleptoplast maintenance following starvation either in slugs [8,24] or in Foraminifera [51]. The present findings go a step further by showing that sacoglossan slugs survive for months with the help of kleptoplasts, but without the help of photosynthesis. While the plastids are photosynthetically active, they do not confer a photoautotrophic lifestyle upon the slugs. It rather appears that the slugs sequester their plastids not directly as a source of photosynthetic capabilities, but as a source of stored food reserves, whose nutritional value does not depend on light subsequent to sequestration. Plastid longevity in LtR sacoglossan slugs remains an interesting phenomenon, but the present results prompt a shift in emphasis from viewing the kleptoplasts as green solar panels towards viewing them as green food reserves.
4. Experimental procedures
Elysia timida individuals were collected in Banyuls-sur-Mer (France) between July and September 2012 and transferred to Bonn (Germany). Specimens were kept with food algae in Petri dishes with artificial seawater (Tropic Marin) at 20°C and water changed every 2 days. For acclimation to laboratory conditions, the slugs were illuminated at 25 µmol quanta m−2 s−1 and a 12 L : 12 D cycle under a ‘daylight lamp’ (Androv Medical, model AND1206-CH) for 6 days. Then six individuals of E. timida were separately starved in Petri dishes under 25 and 40 µmol quanta m−2 s−1 under a 12 L : 12 D cycle and under complete darkness for a maximum of 88 days. Analyses of photosynthetic activity were performed with a pulse–amplitude–modulated fluorometer (Diving PAM, Walz, Germany) by measuring the maximum quantum yield of chlorophyll a fluorescence in photosystem II. Specimens kept under light conditions were dark acclimated for 15 min prior to the measurement.
Plakobranchus ocellatus individuals were collected in the Philippines in November 2012 and transferred to Bonn (Germany). Two aquaria were set up with 20 l artificial seawater (Tropic Marin) at 22°C with two specimens of P. ocellatus, respectively, each in individual fishnets. Additionally, two specimens were placed in an aquarium in individual fishnets in 10 l artificial seawater at 22°C. One-third of the water in every aquarium was changed weekly and the best water quality established through the use of an internal filter (Eheim, Germany). To one 20 l aquarium, 2 µg 3-(4-Chlorophenyl)-1-Methoxy-1-Methylurea (monolinuron) ml−1 seawater was added. This and the 10 l aquarium were illuminated at 25 µmol quanta m−2 s−1 under a 12 L : 12 D. The second 20 l aquarium was kept in the dark. All specimens were starved for 55 days and afterwards fixed in 4% formaldehyde for further analysis not addressed here. PAM-measurements were taken using a Diving PAM (Walz, Germany). Weights of all six specimens were measured on days 0, 14, 28 and 49 of the experiment by placing the slugs on a spoon, gently removing all remaining water with a paper towel and placing them into a preset water container on a scale. Measurements were taken three times and mean values determined. Data of each trial were pooled. For each individual, the weights were scaled to a maximum of 1 and the linear regression calculated. With −0.0059, ‘dark’ had the lowest slope followed by the monolinuron-treated slugs with −0.0067 and the control set with −0.0114. Using a Tukey test, we tested pairwise whether the slopes of the linear regression lines were equal (H0). The resulting q-values with a significance level of 0.05 showed a significant difference between ‘dark’ and ‘normal’ conditions, while the remaining slopes did not significantly differ (p-values: control to monolinuron-treated ≥ 0.0567; monolinuron-treated to darkness ≥ 0.9193; and darkness to control ≥ 0.023).
Elysia timida used for incubations with [14C]-labelled CO2 were collected in the Mediterranean Sea (Banyuls-sur-Mer, France) in October 2012 and transferred to Düsseldorf (Germany). They were maintained for six weeks at 15°C and 33 µmol quanta m−2 s−1 in 12 l-aquaria containing 25 specimens, artificial seawater (37 g l−1 hw-Marinemix professional (Wiegandt, Germany)) and Acetabularia acetabulum as food source. They were starved for several days before labelling. To test the light-driven incorporation of CO2 by Elysia, the slugs were incubated in 1.2 ml artificial seawater supplemented with 0.32 mM [14C]-NaHCO2 (18 µCi per incubation, NEN-radiochemicals, MA, USA). For each measurement, four slugs were incubated together in a transparent plastic 1.5 ml tube. Plakobranchus ocellatus individuals used for incubations with [14C]-labelled CO2 were collected in the Philippines in April 2013 and transferred to Bonn (Germany). To test the light-driven incorporation of CO2, they were incubated in 5 ml artificial seawater supplemented with 0.16 mM [14C]NaHCO2 (36 µCi per incubation). Each measurement contained one single organism in an 8 ml glass tube. The measurements for time point 120 were carried out twice and the mean values are shown in figure 2. All incubations of both species were performed at room temperature either in the dark or illuminated (72 µmol quanta m−2 s−1). The incubations lasted 2 min, 1 or 2 h, and afterwards the slugs were separated from the radioactive incubation medium, rinsed five times with seawater, and then homogenized in a small glass teflon Potter-Elvehjem tissue grinder in 1 ml (Elysia) or 3 ml H2O (Plakobranchus). The homogenates of Elysia were removed and the Potter tube was rinsed twice with 1 ml H2O. These 3 ml, containing the homogenized slugs, were acidified with 150 µl 1M HCl and the open vial was then shaken overnight to remove all the substrate, that is (labelled) carbon dioxide. Afterwards, incorporation of labelled carbon atoms by the Elysia slugs was determined in a scintillation counter after the addition of 12 ml LUMA-Gel scintillation cocktail (LUMAC, The Netherlands). To the homogenates of Plakobranchus 3 ml H2O and 300 µl 1M HCl were added, while the rest of the method to measure acid-stable incorporation of carbon dioxide was the same as for Elysia.
Acknowledgement
We thank Margarete Stracke and Valérie Schmitt for collecting and cultivating animals and algae.
Funding statement
This study was financially supported by an ERC grant to W.F.M. and DFG grants to H.W. and S.B.G.
References
- 1.Venn AA, Loram JE, Douglas AE. 2008. Photosynthetic symbioses in animals. J. Exp. Bot. 59, 1069–1080 (doi:10.1093/jxb/erm328) [DOI] [PubMed] [Google Scholar]
- 2.Bosch TCG. 2012. What Hydra has to say about the role and origin of symbiotic interactions. Biol. Bull. 223, 78–84 [DOI] [PubMed] [Google Scholar]
- 3.Kovacević G, Franjević D, Jelencić B, Kalafatić M. 2010. Isolation and cultivation of endosymbiotic algae from green Hydra and phylogenetic analysis of 18S rDNA sequences. Folia Biol. 58, 135–143 (doi:10.3409/fb58_1-2.135-143) [PubMed] [Google Scholar]
- 4.Archibald JM. 2009. The puzzle of plastid evolution. Curr. Biol. 19, R81–R88 (doi:10.1016/j.cub.2008.11.067) [DOI] [PubMed] [Google Scholar]
- 5.Johnson MD. 2011. Acquired phototrophy in ciliates: a review of cellular interactions and structural adaptations. J. Eukaryot. Microbiol. 58, 185–195 (doi:10.1111/j.1550-7408.2011.00545.x) [DOI] [PubMed] [Google Scholar]
- 6.Trench RK. 1975. Of ‘leaves that crawl’, functional chloroplasts in animal cells. Symp. Soc. Exp. Biol. 29, 229–265 [PubMed] [Google Scholar]
- 7.Händeler K, Grzymbowski Y, Krug JP, Wägele H. 2009. Functional chloroplasts in metazoan cells: a unique evolutionary strategy in animal life. Front. Zool. 6, 28 (doi:10.1186/1742-9994-6-28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wägele H, et al. 2011. Transcriptomic evidence that longevity of acquired plastids in the photosynthetic slugs Elysia timida and Plakobranchus ocellatus does not entail lateral transfer of algal nuclear genes. Mol. Biol. Evol. 28, 699–706 (doi:10.1093/molbev/msq239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rumpho ME, Summer EJ, Manhart JR. 2000. Solar-powered sea slugs. Mollusc/algal chloroplast symbiosis. Plant Physiol. 123, 29–38 (doi:10.1104/pp.123.1.29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pierce SK, Curtis NE, Hanten JJ, Boerner SL, Schwartz JA. 2007. Transfer, integration and expression of functional nuclear genes between multicellular species. Symbiosis 43, 57–64 [Google Scholar]
- 11.van Wijk KJ, Baginsky S. 2011. Plastid proteomics in higher plants: current state and future goals. Plant Physiol. 155, 1578–1588 (doi:10.1104/pp.111.172932) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Timmis JN, Ayliffe MA, Huang CY, Martin W. 2004. Endosymbiotic gene transfer: organelle genomes forge eukaryotic chromosomes. Nat. Rev. Genet. 5, 123–135 (doi:10.1038/nrg1271) [DOI] [PubMed] [Google Scholar]
- 13.Gould SB, Waller RF, McFadden GI. 2008. Plastid evolution. Annu. Rev. Plant Biol. 59, 491–517 (doi:10.1146/annurev.arplant.59.032607.092915) [DOI] [PubMed] [Google Scholar]
- 14.Strittmatter P, Soll J, Bölter B. 2010. The chloroplast protein import machinery: a review. Methods Mol. Biol. 619, 307–321 (doi:10.1007/978-1-60327-412-8_18) [DOI] [PubMed] [Google Scholar]
- 15.Shi LX, Theg SM. 2013. The chloroplast protein import system: from algae to trees. Biochim. Biophys. Acta 1833, 314–331 (doi:10.1016/j.bbamcr.2012.10.002) [DOI] [PubMed] [Google Scholar]
- 16.Mujer CV, Andrews DL, Manhart JR, Pierce SK, Rumpho ME. 1996. Chloroplast genes are expressed during intracellular symbiotic association of Vaucheria litorea plastids with the sea slug Elysia chlorotica. Proc. Natl Acad. Sci. USA 93, 12 333–12 338 (doi:10.1073/pnas.93.22.12333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenberg BM, Gaba V, Mattoo AK, Edelman M. 1987. Identification of a primary in vivo degradation product of the rapidly-turning-over 32 kd protein of photosystem II. EMBO J. 6, 2865–2869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godde D, Schmitz H, Weidner M. 1991. Turnover of the D-1 reaction center polypeptide from photosystem-II in intact spruce needles and spinach leaves. Z. Naturforsch. 46, 245–251 [Google Scholar]
- 19.Sundby C, McCaffery S, Anderson JM. 1993. Turnover of the photosystem-II D1 protein in higher-plants under photoinhibitory and nonphotoinhibitory irradiance. J. Biol. Chem. 268, 25 476–25 482 [PubMed] [Google Scholar]
- 20.Rumpho ME, Worful J, Lee J, Kannan K, Tyler MS, Bhattacharya D, Moustafa A, Manhart JR. 2008. Horizontal gene transfer of the algal nuclear gene psbO to the photosynthetic sea slug Elysia chlorotica. Proc. Natl Acad. Sci. USA 105, 17 867–17 871 (doi:10.1073/pnas.0804968105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gruber A, Vugrinec S, Hempel F, Gould SB, Maier UG, Kroth PG. 2007. Protein targeting into complex diatom plastids: functional characterisation of a specific targeting motif. Plant Mol. Biol. 64, 519–530 (doi:10.1007/s11103-007-9171-x) [DOI] [PubMed] [Google Scholar]
- 22.Gould SB. 2008. Ariadne's thread: guiding a protein across five membranes in cryptophytes. J. Phycol. 44, 23–26 (doi:10.1111/j.1529-8817.2007.00437.x) [DOI] [PubMed] [Google Scholar]
- 23.Rumpho ME, Summer EJ, Green BJ, Fox TC, Manhart JR. 2001. Mollusc/algal chloroplast symbiosis: how can isolated chloroplasts continue to function for months in the cytosol of a sea slug in the absence of an algal nucleus? Zoology 104, 303–312 (doi:10.1078/0944-2006-00036) [DOI] [PubMed] [Google Scholar]
- 24.Rumpho ME, Pelletreau KN, Moustafa A, Bhattacharya D. 2011. The making of a photosynthetic animal. J. Exp. Biol. 214, 303–311 (doi:10.1242/jeb.046540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierce SK, Fang X, Schwartz JA, Jiang X, Zhao W, Curtis NE, Kocot KM, Yang B, Wang J. 2012. Transcriptomic evidence for the expression of horizontally transferred algal nuclear genes in the photosynthetic sea slug Elysia chlorotica. Mol. Biol. Evol. 29, 1545–1556 (doi:10.1093/molbev/msr316) [DOI] [PubMed] [Google Scholar]
- 26.Richly E, Dietzmann A, Biehl A, Kurth J, Laloi C, Apel K, Salamini F, Leister D. 2003. Covariations in the nuclear chloroplast transcriptome reveal a regulatory master-switch. EMBO Rep. 4, 491–498 (doi:10.1038/sj.embor.embor828) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhalero R, et al. 2003. Gene expression in autumn leaves. Plant Physiol. 131, 430–442 (doi:10.1104/pp.012732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trench RK, Greene RW, Bystrom BG. 1969. Chloroplasts as functional organelles in animal tissue. J. Cell Biol. 42, 404–417 (doi:10.1083/jcb.42.2.404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trench RK. 1973. Further studies on the mucopolysaccharide secreted by the pedal gland of the marine slug Tridachia crispata (Opisthobranchia, Sacoglossa). Bull. Mar. Sci. 23, 299–312 [Google Scholar]
- 30.Ireland C, Scheuer PJ. 1979. Photosynthetic marine mollusks: in vivo 14C incorporation into metabolites of the sacoglossan Placobranchus ocellatus. Science 205, 922–923 (doi:10.1126/science.205.4409.922) [DOI] [PubMed] [Google Scholar]
- 31.Hinde R. 1978. The metabolism of photosynthetically fixed carbon by isolated chloroplasts from Codium fragile (Chlorophyta: Siphonales) and by Elysia viridis (Mollusca: Sacoglossa). Biol. J. Linn. Soc. 10, 329–342 (doi:10.1111/j.1095-8312.1978.tb00019.x) [Google Scholar]
- 32.Kremer BP, Schmitz K. 1976. Aspects of 14CO2-fixation by endosymbiotic rhodoplasts in the marine Opisthobranchiate Hermaea bifida. Mar. Biol. 34, 313–316 (doi:10.1007/BF00398124) [Google Scholar]
- 33.Green RW, Muscatine L. 1972. Symbiosis in saccoglossan opisthobranchs: phostosythetic products of animal–chloroplast associations. Mar. Biol. 14, 253–259 [Google Scholar]
- 34.Middlebrooks ML, Pierce SK, Bell SS. 2011. Foraging behavior under starvation conditions is altered via photosynthesis by the marine gastropod, Elysia clarki. PLoS ONE 6, e22162 (doi:10.1371/journal.pone.0022162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christa G, Westcott L, Schäberle TF, Koenig G, Wägele H. 2013. What remains after 2 months of starvation? Analysis of sequestered algae in a photosynthetic slug, Plakobranchus ocellatus (Sacoglossa, Opisthobranchia), by barcoding. Planta 237, 559–572 (doi:10.1007/s00425-012-1788-6) [DOI] [PubMed] [Google Scholar]
- 36.Giménez Casalduero F, Muniain C. 2008. The role of kleptoplasts in the survival rates of Elysia timida (Risso 1818): (Sacoglossa: Opisthobranchia) during periods of food shortage. J. Exp. Mar. Biol. Ecol. 357, 181–187 (doi:10.1016/j.jembe.2008.01.020) [Google Scholar]
- 37.Hinde R, Smith DC. 1972. Persistence of functional chloroplasts in Elysia viridis (Opisthobranchia, Sacoglossa). Nature 239, 30–31 (doi:10.1038/239041a0) [DOI] [PubMed] [Google Scholar]
- 38.Hinde R, Smith DC. 1975. The role of photosynthesis in the nutrition of the mollusc Elysia viridis. Biol. J. Linn. Soc. 7, 161–171 (doi:10.1111/j.1095-8312.1975.tb00738.x) [Google Scholar]
- 39.Klochkova TA, Han JW, Chah KH, Kim RW, Kim JH, Kim KY, Kim GH. 2013. Morphology, molecular phylogeny and photosynthetic activity of the sacoglossan mollusc, Elysia nigrocapitata, from Korea. Mar. Biol. 160, 155–168 (doi:10.1007/s00227-012-2074-7) [Google Scholar]
- 40.Yamamoto S, Hirano YM, Hirano YJ, Trowbridge CD, Akimoto A, Sakai A, Yusa Y. 2013. Effects of photosynthesis on the survival and weight retention of two kleptoplastic sacoglossan opisthobranchs. J. Mar. Biol. Ass. UK 1, 1–7 (doi:10.1017/S0025315412000628) [Google Scholar]
- 41.Curtis NE, Massey SE, Pierce SK. 2006. The symbiotic chloroplasts in the sacoglossan Elysia clarki are from several algal species. Invert. Biol. 125, 336–345 (doi:10.1111/j.1744-7410.2006.00065.x) [Google Scholar]
- 42.Weber APM, Osteryoung KW. 2010. From endosymbiosis to synthetic photosynthetic life. Plant Physiol. 154, 593–597 (doi:10.1104/pp.110.161216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark KB, Jensen KR, Stirts HM, Fermin C. 1981. Chloroplast symbiosis in a non-Elysiid mollusc, Costasiella lilianae Marcus (Hermaeidae: Ascoglossa (= Sacoglossa): effects of temperature, light intensity, and starvation on carbon fixation rate. Biol. Bull. 160, 42–54 (doi:10.2307/1540899) [Google Scholar]
- 44.Greene RW. 1970. Symbiosis in sacoglossan opisthobranchs: functional capacity of symbiotic chloroplasts. Mar. Biol. 7, 138–142 (doi:10.1007/BF00354917) [Google Scholar]
- 45.Marín A, Ros J. 1989. The chloroplast–animal association in four Iberian sacoglossan opisthobranchs: Elysia timida, Elysia translucens, Thuridilla hopei, and Bosellia mimetica. Sci. Mar. 53, 429–440 [Google Scholar]
- 46.Hawes CR, Cobb AH. 1980. The effects of starvation on the symbiotic chloroplasts in Elysia viridis: a fine structural study. New Phytol. 84, 375–379 (doi:10.1111/j.1469-8137.1980.tb04437.x) [Google Scholar]
- 47.Evertsen J, Johnsen G. 2009. In vivo and in vitro differences in chloroplast functionality in the two north Atlantic sacoglossans (Gastropoda, Opisthobranchia) Placida dendritica and Elysia viridis. Mar. Biol. 156, 847–859 (doi:10.1007/s00227-009-1128-y) [Google Scholar]
- 48.Maeda T, et al. 2012. Algivore or phototroph? Plakobranchus ocellatus (Gastropoda) continuously acquires kleptoplasts and nutrition from multiple algal species in nature. PLoS ONE 7, e42024 (doi:10.1371/journal.pone.0042024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cutignano A, Cimino G, Villani G, Fontana A. 2009. Shaping the polypropionate biosynthesis in the solar-powered mollusc Elysia viridis. Chembiochem 10, 315–322 (doi:10.1002/cbic.200800531) [DOI] [PubMed] [Google Scholar]
- 50.Bhattacharya D, Pelletreau KN, Price DC, Sarver KE, Rumpho ME. 2013. Genome analysis of Elysia chlorotica egg DNA provides no evidence for horizontal gene transfer into the germ line of this kleptoplastic mollusc. Mol. Biol. Evol. 30, 1843–1853 (doi:10.1093/molbev/mst084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pillet L, Pawlowski J. 2013. Transcriptome analysis of foraminiferan Elphidium margaritaceum questions the role of gene transfer in kleptoplastidy. Mol. Biol. Evol. 30, 66–69 (doi:10.1093/molbev/mss226) [DOI] [PubMed] [Google Scholar]