Abstract
The silkmoth Bombyx mori is the main producer of silk worldwide and has furthermore become a model organism in biological research, especially concerning chemical communication. However, the impact domestication might have had on the silkmoth's olfactory sense has not yet been investigated. Here, we show that the pheromone detection system in B. mori males when compared with their wild ancestors Bombyx mandarina seems to have been preserved, while the perception of environmental odorants in both sexes of domesticated silkmoths has been degraded. In females, this physiological impairment was mirrored by a clear reduction in olfactory sensillum numbers. Neurophysiological experiments with hybrids between wild and domesticated silkmoths suggest that the female W sex chromosome, so far known to have the sole function of determining femaleness, might be involved in the detection of environmental odorants. Moreover, the coding of odorants in the brain, which is usually similar among closely related moths, differs strikingly between B. mori and B. mandarina females. These results indicate that domestication has had a strong impact on odour detection and processing in the olfactory model species B. mori.
Keywords: domestication, Bombyx mori, Bombyx mandarina, olfactory sensilla, olfactory coding, W chromosome
1. Introduction
About 5000 years ago, humans discovered the ability of moth larvae to spin silk threads and began to breed these moths to optimize silk production. This artificial selection led to the evolution of the domesticated silkmoth, Bombyx mori. The putative precursor of B. mori is Bombyx mandarina, an extant silkmoth of East Asia [1]. In the course of domestication, several phenotypic characteristics changed, e.g. colour (figure 1a) and size of the body; the latter caused the moths to lose the ability to fly. However, wild and domesticated silkmoths are still similar to each other in several aspects: the larvae of both species are specialized on leaves from mulberry trees, unlike the adult moths of both species, which have no mouthparts and do not feed. Furthermore, both species rely on the same sex pheromone [3], which means that wild and domesticated silkmoths can be cross-bred and produce fertile offspring [4].
Figure 1.
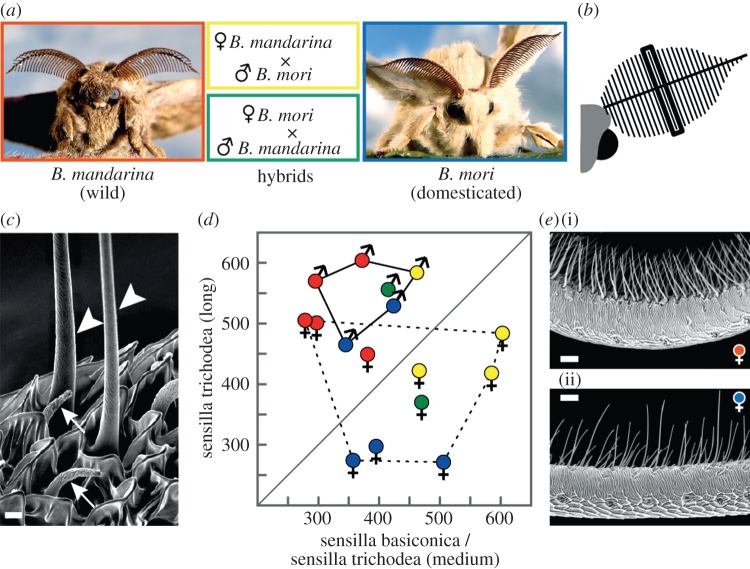
Antennal morphology is preserved in domesticated males but not in females. (a) Wild silkmoths B. mandarina, domesticated silkmoths B. mori and hybrids of both were studied. (b) Schematic of a silkmoth's head with its left antenna; the bold line represents the antennal stem; thin lines perpendicular to the stem denote branches. The black frame marks the middle branch where olfactory sensilla were counted. (c) Close-up view of long trichoids (arrowheads) and sensilla basiconica (arrows) of a female B. mori; scale bar, 2 µm. (d) Frequency of olfactory sensilla on the middle antennal branch of males (solid line) and females (dotted line); size ranges of medium-length trichoids and sensilla basiconica broadly overlap [2], so the numbers of these two sensillum types were pooled. The middle antennal branch was chosen because sensillum numbers and their ratios are constant in the middle region of the antenna of B. mori, irrespective of the size of the animal [2]. Symbols represent data from individual silkmoths; the diagonal separates animals that have more long trichoids than sensilla basiconica and medium-length trichoids (above the diagonal) from animals with the reverse sensillum ratio (below the diagonal). The sensillum ratios for B. mori are in accordance with ratios from data based on an estimation of sensillum numbers of the whole antenna [2]. (e) Detail of a middle antennal branch of a B. mandarina female (i) and a B. mori female (ii); scale bar, 20 µm.
Over the past decades, B. mori has become a model organism in research [5], primarily because it was the first Lepidopteran insect whose genome was fully sequenced [6,7]. Furthermore, early neurophysiological work with B. mori (e.g. the discovery of the first insect sex pheromone [8] and the first neurophysiological measurements of an insect antenna [9]) led to substantial progress in understanding chemical communication in insects. However, domesticated animals typically differ from their progenitors regarding appearance, behaviour and physiology [10,11], and several studies demonstrated a clear effect of domestication on olfaction-related genes. Laboratory strains of the nematode Caenorhabditis elegans, for example, changed their reproductive behaviour owing to a mutation of the worm's pheromone receptor genes [12], and the loss of heterozygosity in the domesticated camel is especially notable in the olfactory receptor gene family [13]. Because of B. mori's significance in olfactory research, we investigated possible anatomical and functional domestication effects on the silkmoth's olfactory system, both on the sensory periphery and at the first olfactory processing centre in the brain.
2. Material and methods
(a). Animals
Eggs and pupae of the wild silkmoth B. mandarina stem from moths that were collected in the area of Sakado, Japan, and have been bred in the laboratory since 1982. Pupae of the domesticated silkmoth B. mori (hybrid strain Kinshu (Japanese) × Showa (Chinese)) were purchased from Aseptic Sericulture System Laboratory (Kyoto, Japan). Another B. mori strain (p50) was additionally used in some of the electro-antennogram (EAG) recordings. Crossings between B. mori (Kinshu × Showa) and B. mandarina were done in our laboratory; pairings between B. mori females and B. mandarina males were easily obtained because of the immobility of the domesticated females. Wild females, in contrast, refused to mate with domesticated males, and therefore had to be mated by forced hand-pairing [14]. Larvae were fed mulberry leaves (Morus alba) or artificial diet containing dried mulberry leaves. Pupae were kept in a climate chamber (25–26°C, 75% relative humidity, 16 L : 8 D rhythm). One to two days after emergence, moths were transferred to a refrigerator (4°C) until they were used for EAG recordings. For morphological investigations of the antennae, moths were stored at −20°C.
(b). Scanning electron microscopy and counting of olfactory sensilla
Both parts of the middle branch of an antenna were clipped close to the antennal stem and mounted upright on a holder covered with adhesive tape. The samples were sputter coated with gold prior to examination with a scanning electron microscope (LEO 1450 VP, Zeiss). Photographs were taken at 500× magnification from different views (12–45 pictures per branch, depending on how much the branch was bent and twisted) to determine the total number of olfactory sensilla located at the respective branch.
(c). Electro-antennogram recordings
One antenna of a silkmoth was excised at the base, the distal three to four branches were cut and the antenna was mounted between two glass capillary electrodes filled with physiological saline solution [15]. The amplified EAG signal was captured and processed via an IDAC-4 interface (Syntech) and analysed with the software EAGPro v. 2.0 (Syntech). The antennal preparation was placed in a charcoal-filtered and humidified air stream (0.3 l min−1), which was delivered via a thermoplastic tube (PEEK, 5 mm diameter). The outlet of this tube was about 10 mm apart from the inner, sensilla-bearing side of the basket-shaped antenna. Two glass pipettes were inserted through small holes in the tube (20 mm from the end of the tube). One pipette was empty and added clean air to the continuous airstream (0.1 l min−1). This airstream could be switched to the second pipette, which contained a filter paper soaked with the pheromone or odorant (see below). The EAG signal was automatically recorded at 100 Hz for 2 s before the onset of the stimulus (duration: 0.5 s) and 8 s after stimulus-onset (Stimulus Controller CS-55, Syntech).
(d). Sex pheromones
Bombykol (Pherobank) and its aldehyde bombykal (synthesized from bombykol via oxidation with pyridinium dichromate and purified by silica gel column chromatography) were diluted in hexane and tested at two concentrations. Based on pilot experiments, we used a concentration of bombykol (60 ng, 600 ng) that was threefold higher than that of bombykal (20 ng, 200 ng) because males were less sensitive to bombykol. This result is in accordance with single sensillum recordings from long trichoids of B. mori [16] and with recordings from Xenopus oocytes expressing the pheromone receptors of B. mori [17]. The purity of the pheromones and their concentrations were confirmed with GC–FID (gas chromatography with flame ionization detection). Six microlitres of the diluted pheromones were applied to a circular piece of filter paper (diameter: 12 mm); 6 µl of hexane served as a control stimulus. Filter papers were inserted into glass pipettes and were renewed after three experiments. Antennae were first stimulated with the low pheromone concentration, and then with the high pheromone concentration. This procedure was repeated in each animal with bombykol and bombykal. Half of the animals was tested first with bombykol; the other half was tested first with bombykal. Interstimulus interval was 1 min.
(e). Environmental odorants
We tested 14 monomolecular odorants that typically occur in the headspace of leaves and flowers. The volatiles belong to five chemical classes: (i) aromatics: methyl salicylate (purity: 99%), phenyl acetaldehyde (90%); (ii) terpenes: geraniol (96%), (±)-linalool (97%), β-caryophyllene (98.5%); (iii) alcohols: 1-hexanol (99.9%), 1-octanol (99%), 1-nonanol (99.5%); (iv) aldehydes: hexanal (98%), octanal (99%), nonanal (95%) and (v) ketones: 2-hexanone (99.5%), 2-octanone (99.5%), 2-nonanone (99.5%). The odorants were diluted in mineral oil to a concentration of 1 : 103 (v/v). This corresponded to a stimulus load of 4.9–7.3 µg, depending on the density of the compound. Six microlitres of the diluted odorants were applied onto a circular piece of filter paper (diameter: 12 mm); 6 µl of mineral oil served as a control stimulus. Filter papers were inserted into glass pipettes and were renewed every day. Interstimulus interval was 1 min, and the order of compounds was constant (cf. figure 2).
Figure 2.
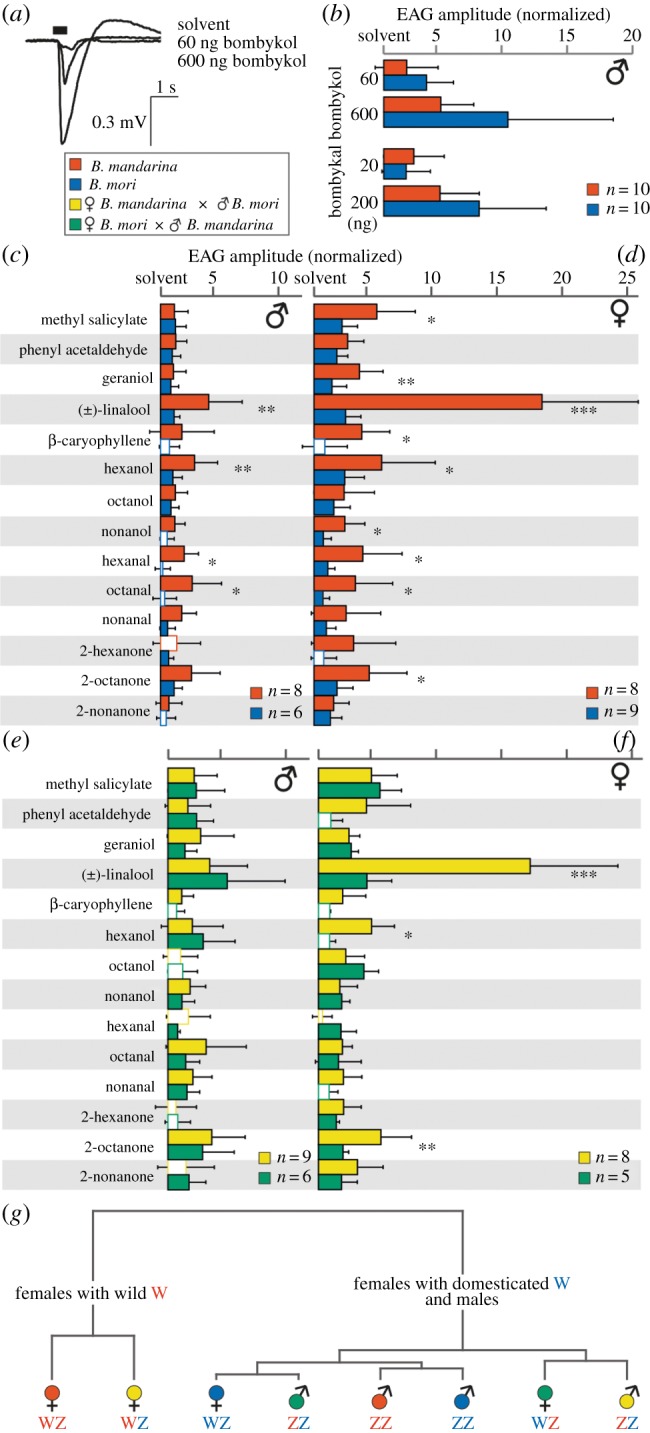
Plant responses but not pheromone responses are affected by domestication. (a) Example EAG recordings from the clipped antennae of a B. mandarina male; stimulus duration (black horizontal bar) was 0.5 s. (b) Pheromone responses are shown as a multiple of the response to the solvent hexane (see the electronic supplementary material, data file). Solvent responses were similar between the species; B. mandarina: 0.218 mV ± 0.202 mV, B. mori: 0.219 mV ± 0.124 mV; p = 0.99, t-test. Pheromone concentrations were chosen to elicit similar amplitudes for both compounds [16]. Bars show mean normalized EAG amplitudes; error bars represent s.d. All pheromone responses are different from responses to the solvent (p < 0.05, paired t-test). Values for wild and domesticated silkmoths did not differ (p > 0.05, t-test). (c–f) Electro-antennogram recordings after stimulation with 14 environmental odorants. (c) Responses of wild and domesticated males, (d) wild and domesticated females, (e) hybrid males, (f) hybrid females are shown as a multiple of the response to the solvent (see the electronic supplementary material, data file). Odorants were diluted in mineral oil (1 : 103, 4.9–7.3 µg on filter paper); stimulus duration was 0.5 s. Open bars represent values that were not different from the solvent response (p > 0.05, paired t-test). Responses to the solvent were similar between species; B. mandarina male: 0.046 mV ± 0.029 mV, B. mori male: 0.046 mV ± 0.008 mV; p = 0.98; B. mandarina female: 0.031 mV ± 0.010 mV, B. mori female: 0.035 mV ± 0.020 mV; p = 0.56, t-test. For each odorant, asterisks mark significant differences between species; *p < 0.05, **p < 0.01, ***p < 0.001, t-test. (g) Dendrogram of silkmoths according to their mean EAG amplitudes; hierarchical cluster analysis using Ward's minimum variance method. The origin of the sex chromosomes is shown next to the symbol of the respective species (red, from wild silkmoths; blue, from domesticated silkmoths).
(f). Analysis of electro-antennogram recordings
The median of the values 1 s prior to stimulus-onset was subtracted from each value. Then, the maximal deflection from this baseline, i.e. the EAG amplitude, was determined. Amplitudes evoked by stimulation with pheromones or odorants, respectively, were normalized to the median amplitude evoked by the solvent, i.e. results are presented as multiples of the median solvent control in the respective animal.
(g). Morphology of antennal lobes
Antennal lobes were stained with Lucifer yellow [18] and pictures of brain sections were taken with a laser-scanning microscope (Zeiss LSM 510 Meta). Antennal lobes were scanned at a 1024 × 1024 pixel resolution in the x–y direction and 1 µm in the z-direction using an argon laser (488 µm) with a 10× water immersion objective. The outline of one antennal lobe per animal was traced at 5 µm steps, its surface was generated and the volume was calculated in Amira v. 4.1.1.
(h). Calcium imaging
(i). Moths
Wild and domesticated silkmoth females and females from three hawkmoth species (Manduca sexta, Acherontia atropos and Smerinthus ocellata) were dissected as described [19]. A fluorescent calcium indicator (Calcium Green-2 AM, Invitrogen) was dissolved in physiological saline solution [15] with 6% Pluronic F-127 (Invitrogen) to a concentration of 30 μmol. The exposed brain was incubated with 20 μl of this solution for 90 min at 4°C. The dye accumulates by olfactory sensory neurons that contribute largely to the neural circuits of a glomerulus but that are shown to actively expel dye molecules [20], and also by glial cells that tightly surround each glomerulus and retain the fluorescent dye. Glial cells respond with an influx of calcium upon odorant stimulation of olfactory sensory neurons projecting to the respective glomerulus [21], thus leading to an increase in fluorescence at the site and reflecting the activity of olfactory sensory neurons.
(ii). Vinegar flies
In Drosophila melanogaster females, we genetically expressed a calcium-sensitive protein (instead of bath-applying a calcium-sensitive dye as in moths). To do so, we used the standard GAL4-UAS system and expressed the calcium sensor GCamp v. 3.0 in olfactory sensory neurons using the specific transgenic Orco driver line. Flies were dissected for optical imaging as described [22].
(iii). Optical imaging
The imaging set-up was controlled by the software Tillvision v. 4.0 (Till Photonics) and consisted of a CCD camera (Sensicam Qe) mounted to an upright microscope (Olympus BX51WI) with a water immersion objective (Olympus, 10×/0.30 for moths, 20×/0.95 for flies). Calcium Green-2 and GCamp v. 3.0 were excited at 475 nm (500 nm SP; xenon arc lamp, Polychrome V, Till Photonics) and fluorescence was detected at 490/515 nm (DCLP/LP). Fourfold symmetrical binning resulted in an image size of 344 × 260 pixels with a pixel corresponding to 2.5 × 2.5 μm (moths) or 1.25 × 1.25 μm (flies). Six microlitres of the diluted odorants were applied to a circular piece of filter paper (12 mm diameter). Filter papers were inserted into glass pipettes and were renewed every day. The immobilized insect was placed under the microscope. A glass tube was directed to one antenna (5 mm diameter; ending 10–15 mm from the tip of the antenna), delivering a constant stream of clean, moistened air (1 l min−1). Two glass pipettes were inserted through small holes in the tube. An empty pipette (inserted 5.5 cm from the end of the tube) added clean air to the continuous airstream (0.1 l min−1). This airstream could automatically be switched (Stimulus Controller CS-55, Syntech) to the second pipette (inserted 3.5 cm from end of tube), which contained an odorant-laden filter paper. Each odorant stimulation experiment lasted 10 s and was recorded with a sampling rate of 4 Hz using the following protocol: 2 s clean airstream, 2 s odorant airstream and 6 s clean airstream. The same odorants as in EAG recordings were used and presented at intervals of at least 1 min between stimulations. The sequence of stimulations was changed from animal to animal. Additionally, three stimulations with the solvent were carried out at the beginning, in the middle and at the end of the sequence of odorant stimulations.
(iv). Analysis of optical imaging data
The imaging data were processed with custom-written software (IDL) to enhance the signal-to-noise ratio, as described recently [19]. Increased neural activity upon odorant stimulation led to spatially restricted spots of increased fluorescence in the antennal lobe. In the centre of each activity spot, the average increase in fluorescence over background fluorescence was determined in a small area according to the size of a glomerulus. The maximum value at this spot was calculated and in each individual, values were normalized to the maximal response [19].
(v). Comparing neural activation patterns
To compare odour-evoked activity patterns across animals from different species, we first calculated the similarity between those patterns in each individual. As we stimulated the animals with 14 odorants, a pairwise correlation of activation patterns led to 91 correlation coefficients per animal (single missing values were supplemented with the median values of the respective species). With this dataset, we performed principal component analyses and hierarchical cluster analyses to reveal possible similarities between individuals from different species.
3. Results
We first checked for differences in the fine structure of the antennae, and identified and counted the olfactory sensilla located at a branch in the middle of the feathered antenna (figure 1b). The sensillum types described for B. mori [2] were also found on the antenna of B. mandarina and the hybrids (figure 1c): sensilla trichodea (medium-length and long), sensilla basiconica and sensilla coeloconica. As the numbers of sensilla coeloconica were low and similar across species (mean number/middle branch/species: 19–29), we focused our analysis on the other sensillum types, which are located in a narrow array along the branches.
In males, long trichoids were the most frequent sensillum type, and occurred in comparable numbers across wild, domesticated and hybrid species (figure 1d, solid line). In females, in contrast, we observed a clear difference in sensillum ratios between individuals from different species (figure 1d, dotted line): in wild females, long trichoids were the most abundant type, whereas in domesticated females, medium-length trichiods and sensilla basiconica were more common than long trichiods (figure 1e). Hybrid females combined characteristics from both parent species: they had many long trichoids but a yet higher number of medium-length trichoids and sensilla basiconica.
Next, we were interested in the possible neurophysiological consequences of these anatomical results. We therefore exposed the antennae to different olfactory stimuli and performed EAG recordings (figure 2a). Long trichoids of males house neurons that mainly detect the sex pheromone bombykol (produced by wild and domesticated females) and its aldehyde bombykal (produced by females of related species and in traces by domesticated females) [3,16]. In both wild and domesticated males, bombykol and bombykal evoked odorant-specific EAG amplitudes in a concentration-dependent manner. The response levels were comparable between wild and domesticated males (figure 2b), indicating that pheromone communication has not been affected in the course of domestication. The detection of environmental odorants, in contrast, seems to have been degraded, as several plant-associated compounds, e.g. (±)-linalool and hexanol, elicited higher antennal responses in wild males than in domesticated males (figure 2c).
Because silkmoth females are not able to smell their own sex pheromone [3], we tested the antennae of females only with environmental odorants (figure 2d). Most of these compounds evoked higher EAG amplitudes in wild females; responses towards (±)-linalool, for example, were on average five to six times stronger in B. mandarina than in B. mori. To control for a potential strain-specific effect, we tested females of another B. mori strain (p50), a standard strain in genetic research [5]. The responses evoked in p50 females were similar to those in females of the strain Kinshu × Showa, which is often used in olfactory research [18,23] and which we used throughout our study, indicating that the observed domestication effects in females were not specific to the particular B. mori strain used (see the electronic supplementary material, figure S1).
As we found significant neurophysiological differences between wild and domesticated silkmoths after stimulation with environmental odorants, we also performed these experiments with hybrids between the species. Hybrid males of both possible origins (mother-mandarina/father-mori and mother-mori/father-mandarina) responded similarly to each other (figure 2e). In females, however, we observed a clear difference between the two hybrids. Remarkably, the response profiles of the hybrid females resembled the profiles of their respective mothers: mother-mandarina hybrids reacted like wild females, especially regarding their high EAG amplitudes after stimulation with (±)-linalool, whereas mother-mori hybrids reacted like domesticated females (figure 2d,f). In Bombyx, as in all butterflies and moths, males are the homogametic sex (sex chromosomes: ZZ), whereas females are heterogametic (WZ). To clarify how the observed phenotypes are linked to sex chromosomes, we performed a cluster analysis of mean EAG amplitudes across sexes and species (figure 2g). The analysis revealed that mother-mandarina hybrid females group together with B. mandarina females, whereas the other types cluster densely in a second group. Only mother-mandarina hybrid females possess the ‘wild’ female sex chromosome (W) of B. mandarina females, whereas mother-mori hybrid females have the ‘domesticated’ W chromosome of B. mori females. Therefore, the observed differences in EAG-response profiles between wild and domesticated females seem to be linked to the W chromosome. Wild and domesticated male sex chromosomes (Z), however, are found in both clusters and seem to have no dominant influence in this context.
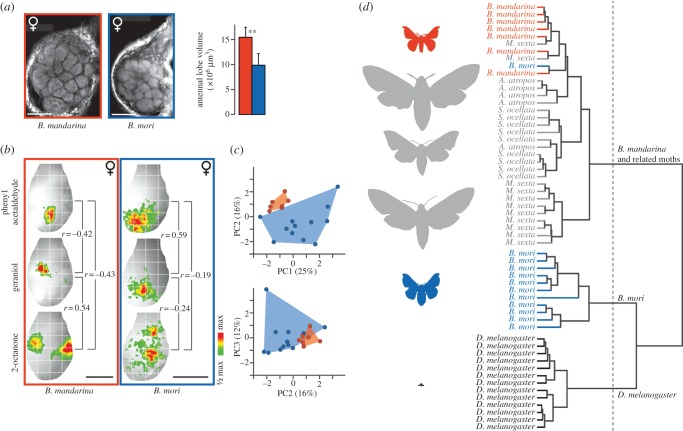
We next asked whether the effects of domestication can also be found in the first processing centre of the brain, the antennal lobe. Domesticated species often have smaller brains, probably a consequence of the less complex environment that exists under domestication [11]. As domestication effects were most pronounced in females, we measured the volume of an antennal lobe in five wild and five domesticated females, respectively. We found that the antennal lobe was about one-third larger in wild females than in domesticated females (figure 3a). Next, we compared odour-evoked activity patterns in the antennal lobe between wild and domesticated females by successively stimulating the antennae with 14 environmental odorants (the same odorants as in figure 2c–f). All tested odorants evoked neural activity patterns that were visualized by calcium imaging (examples in figure 3b). We found similarities between both species (e.g. a low correlation between patterns for phenyl acetaldehyde and 2-octanone), but also odorants that elicited dissimilar patterns in B. mandarina and overlapping patterns in B. mori (e.g. phenyl acetaldehyde and geraniol), and vice versa (e.g. geraniol and 2-octanone). A principal component analysis based on all possible pairwise comparisons between the odour-evoked neural activity patterns revealed a significant difference in olfactory coding between B. mandarina and B. mori females (figure 3c). Moreover, B. mori females mapped further apart from each other than did B. mandarina females, i.e. the intraspecific variability was higher in domesticated silkmoths than in wild silkmoths (standard deviation of 91 correlation coefficients in B. mori: 0.39 ± 0.11, in B. mandarina: 0.27 ± 0.09, p < 0.0001, paired t-test).
Figure 3.
Odour-evoked neural activity patterns in the brain are altered in domesticated females. (a) Frontal view of the left antennal lobe; the antennal nerve enters from the top right corner; scale bar, 100 µm. Mean antennal lobe volumes differed between wild (n = 5) and domesticated (n = 5) females; error bars represent s.d.; **p < 0.01, t-test; (b) Example results of calcium imaging recordings after a 2 s odour stimulation. The odour-evoked increase of fluorescence is colour-coded (see scale) and superimposed onto the view of the left antennal lobe; the entrance of the antennal nerve points upwards; r, correlation coefficient between neural activity patterns; scale bar, 200 µm; the white checked grid was added for better orientation. (c) Distribution of individual wild (n = 7) and domesticated (n = 12) females based on their neural responses evoked by 14 environmental odorants (same as in figure 2c–f). Correlations between all possible pairwise combinations of odorants (n = 91 comparisons) were used for this principle component analysis. The first three principal components (PCs) are shown; values in parentheses depict the proportion of the observed variance in the data that was explained by PC1, PC2 and PC3. Neural response similarities differ between wild and domesticated females (p = 0.005, ANOSIM). (d) Dendrogram of individual females (n = 57) from six insect species based on results from calcium imaging recordings (n = 91 correlations per animal; see the electronic supplementary material, data file); hierarchical cluster analysis using Ward's method; dotted line, automatic truncation. Silhouettes of the insects are drawn proportionally.
To put our results with wild and domesticated silkmoths in an evolutionary context, we analogously tested three hawk moth species [19] that belong to the same superfamily as the Bombyx species. In addition, the vinegar fly, D. melanogaster, was included as an outgroup. Pairwise comparisons of odour-evoked neural activity patterns in the antennal lobe of the tested 57 female insects were used in a cluster analysis that revealed three distinct groups: B. mandarina together with the hawk moths, B. mori and D. melanogaster (figure 3d). Hence, the neural representation of environmental odorants is similar across wild silkmoths and their relatives, but is clearly different from the neural patterns found in the domesticated silkmoth. In addition, the intraspecific variability of neural activation patterns in B. mori was significantly higher than that in B. mandarina, the three hawk moth species and the vinegar fly (see the electronic supplementary material, figure S2).
Is the change in the coding of odorants, like the change at the sensory level, linked to the W chromosome? To answer this question, we performed imaging experiments with mother-mandarina hybrids and included the results in our cluster analysis. A link to the W chromosome would have led to a close grouping of hybrid females with B. mandarina females. The hybrid animals, however, were scattered among both wild and domesticated silkmoths (see the electronic supplementary material, figure S3), indicating that the W chromosome had no significant influence on domestication effects found at the level of the antennal lobe.
4. Discussion
We showed that domestication altered the perception of environmental odorants in both sexes of silkmoths, while the pheromone detection system of the males was preserved. Why did domestication act differentially on these two olfactory subsystems? The female-produced sex pheromone bombykol guides flying wild silkmoths to their mates, a function that has become obsolete under domesticated breeding conditions. However, bombykol also triggers the males’ mating behaviour. The ability to reliably detect bombykol is therefore still crucial for the mating success of domesticated males, and might have been under strong stabilizing selection and, consequently, unaffected by domestication.
It has been shown that the activation of pheromone processing neurons is enhanced in the presence of host plant odorants [24]. A high sensitivity towards environmental odorants might therefore also enhance a wild male's possibility of finding a female. For domesticated males, however, mate localization in a natural environment is not necessary. Accordingly, domesticated males were less sensitive than wild males to several of the compounds, indicating that host plant detection, in contrast to pheromone detection, is altered by domestication.
We found that long trichoids were the most abundant sensilla in B. mandarina females. Correspondingly, they were very sensitive to (±)-linalool, a ubiquitous plant and flower volatile described as the best ligand for neurons housed in the long trichoids of B. mori females [25]. The domesticated females’ response to (±)-linalool, however, was clearly decreased when compared with their wild ancestors, as was the number of long trichoids on their antennae. A comparable decrease in the number of long trichoids is described for oriental fruit moth males after rearing the moths in the laboratory for only 20 years [26], indicating that in B. mori the loss of these sensilla might have also happened early in the course of domestication. Taken together, our results suggest that domesticated silkmoths have a reduced sensitivity towards environmental odorants, possibly as a result of the absence of selection pressures during domestication.
Experiments with hybrids between wild and domesticated silkmoths were done to reveal the genetic basis of the domestication effects. The B. mori Z chromosome houses many functional genes controlling different phenotypes [5]. A significant contribution of the Z chromosome to the observed domestication effect in females, however, seems unlikely because mother-mori hybrid females exhibited a ‘domesticated’ odour response profile even though they possess a ‘wild’ Z chromosome. On the other hand, mother-mandarina hybrid females resembled B. mandarina females, although they have a ‘domesticated’ Z chromosome. As these two female groups (mother-mandarina hybrid females and B. mandarina females) have Z chromosomes of different origins but share the same ‘wild’ W chromosome, one might hypothesize that genes located on the W chromosome might be responsible for the observed domestication effects, i.e. the low EAG responses in B. mori females and in mother-mori hybrid females.
A recent whole genome comparison between wild and domesticated silkmoths identified 354 putative domestication genes [27]. Olfaction-related genes, such as olfactory receptors and olfactory binding proteins, however, were not included in the group of potential domestication genes (SilkDB at http://silkworm.genomics.org.cn). Moreover, the W chromosome harbours only a single, female-determining gene [28], but high numbers of transposable elements [4,14]. In vinegar flies, we find a similar situation regarding the male sex chromosome (Y): it contains mainly transposable elements and only a few protein-coding genes [29]. However, the fly's Y chromosome controls the expression of up to 1000 genes via Y-linked regulatory elements, and thereby determines various phenotypes [30]. Accordingly, our results suggest that the silkmoths’ W chromosome might affect the expression of genes that control, for example, the number of olfactory sensilla on the female antenna. However, we cannot exclude more complex genetic mechanisms that might also explain our findings.
The influence of domestication was not limited to the peripheral olfactory system of the silkmoths; it was also found in the first processing centre of the brain, the antennal lobe. The neural representation of environmental odorants in the brain of domesticated females differed strikingly from the patterns in wild females and in females of related moth species. Although olfactory coding is basically given by the molecular properties of the perceived odorants [31], specific patterns exist that are related to phylogenetic groups [20]. In B. mori females, however, the olfactory code was much more variable from individual to individual than in the five other insect species investigated and was thus less reliable. Ever since B. mori has been domesticated, it has been provided with appropriate oviposition substrates. Hence, changes in the neural coding of environmental odorants in domesticated silkmoth females could occur because they had no impact on the survival of offspring.
Experiments with mother-mandarina hybrids showed that the W chromosome was not involved in domestication effects found in the antennal lobe of the female. Thus, the amplitude of antennal responses (linked to the W chromosome) and the representation of odours in the brain (not linked to the W chromosome) seem to be controlled by genes located on different chromosomes.
In conclusion, our study revealed that domestication had a considerable effect on the olfactory system of the silkmoth, especially concerning the detection and processing of environmental odorants in the female. As it is possible to genetically label specific neurons in the silkmoth's brain [32], it has been proposed that this method can be used to unravel the principles of the processing of olfactory information [18]. However, as the odour-evoked neural activation patterns in the brain of B. mori females were highly variable and differed significantly from those in the brain of closely related moth species, conclusions drawn from experiments with domesticated females have to take these facts into consideration.
Acknowledgements
We thank Yutaka Banno for the B. mori strain p50; Renate Kaiser and Sandor Nietzsche for help with the scanning electron microscope; Jerrit Weissflog for synthesizing bombykal; Kerstin Weniger for help with the purity check of bombykol and bombykal; Ronald Grandy for programming data analysing tools for EAG and imaging recordings; Christine Missbach, Regina Stieber and Christoph Brütting for help with the antennal lobe histology; Julia van Beesel and Silke Trautheim for help with the imaging of D. melanogaster; Yuki Sugimoto for help with the imaging of Bombyx spp.; Ewald Grosse-Wilde for comments on the manuscript and Emily Wheeler for editorial assistance.
Funding statement
This research was supported by the Max Planck Society, the German Federal Ministry of Education and Research (BMBF) and the MEXT/JSPS KAKENHI grants, Japan.
References
- 1.Sun W, Yu HS, Shen YH, Banno Y, Xiang ZH, Zhang Z. 2012. Phylogeny and evolutionary history of the silkworm. Sci. China Life Sci. 55, 483–496 (doi:10.1007/s11427-012-4334-7) [DOI] [PubMed] [Google Scholar]
- 2.Steinbrecht RA. 1970. Morphometric studies on the antenna of the silk moth, Bombyx mori L.: number and distribution of the olfactory sensilla. Z. Morphol. Tiere 68, 93–126 [Google Scholar]
- 3.Daimon T, Fujii T, Yokoyama T, Katsuma S, Shinoda T, Shimada T, Ishikawa Y. 2012. Reinvestigation of the sex pheromone of the wild silkmoth Bombyx mandarina: the effects of bombykal and bombykyl acetate. J. Chem. Ecol. 38, 1031–1035 (doi:10.1007/s10886-012-0164-0) [DOI] [PubMed] [Google Scholar]
- 4.Abe H, Mita K, Yasukochi Y, Oshiki T, Shimada T. 2005. Retrotransposable elements on the W chromosome of the silkworm, Bombyx mori. Cytogenet. Genome Res. 110, 144–151 (doi:10.1159/000084946) [DOI] [PubMed] [Google Scholar]
- 5.Goldsmith MR, Shimada T, Abe H. 2005. The genetics and genomics of the silkworm, Bombyx mori. Annu. Rev. Entomol. 50, 71–100 (doi:10.1146/annurev.ento.50.071803.130456) [DOI] [PubMed] [Google Scholar]
- 6.International Silkworm Genome Consortium 2008. The genome of a lepidopteran model insect, the silkworm Bombyx mori. Insect Biochem. Mol. Biol. 38, 1036–1045 (doi:10.1016/j.ibmb.2008.11.004) [DOI] [PubMed] [Google Scholar]
- 7.Xia QY, et al. 2004. A draft sequence for the genome of the domesticated silkworm (Bombyx mori). Science 306, 1937–1940 (doi:10.1126/science.1102210) [DOI] [PubMed] [Google Scholar]
- 8.Butenandt A. 1955. Ueber die Wirkstoffe des Insektenreiches. II. Zur Kenntnis der Sexuallockstoffe. Nat. wiss. Rundsch. Stuttg. 8, 457–464 [Google Scholar]
- 9.Schneider D, Hecker E. 1956. Zur Elektrophysiologie der Antenne des Seidenspinners Bombyx mori bei Reizung mit angereicherten Extrakten des Sexuallockstoffes. Z. Naturforsch. B 11, 121–124 [Google Scholar]
- 10.Darwin C. 1868. The variation of animals and plants under domestication. London, UK: John Murray [Google Scholar]
- 11.Diamond J. 2002. Evolution, consequences and future of plant and animal domestication. Nature 418, 700–707 (doi:10.1038/nature01019) [DOI] [PubMed] [Google Scholar]
- 12.McGrath PT, Xu YF, Ailion M, Garrison JL, Butcher RA, Bargmann CI. 2011. Parallel evolution of domesticated Caenorhabditis species targets pheromone receptor genes. Nature 477, 321–325 (doi:10.1038/nature10378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jirimutu WZ, et al. 2012. Genome sequences of wild and domestic bactrian camels. Nat. Commun. 3, 1202 (doi:10.1038/ncomms2192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abe H, Sugasaki T, Terada T, Kanehara M, Ohbayashi F, Shimada T, Kawai S, Mita K, Oshiki T. 2002. Nested retrotransposons on the W chromosome of the wild silkworm Bombyx mandarina. Insect Mol. Biol. 11, 307–314 (doi:10.1046/j.1365-2583.2002.00339.x) [DOI] [PubMed] [Google Scholar]
- 15.Christensen TA, Hildebrand JG. 1987. Male-specific, sex pheromone-selective projection neurons in the antennal lobes of the moth Manduca sexta. J. Comp. Physiol. A 160, 553–569 (doi:10.1007/BF00611929) [DOI] [PubMed] [Google Scholar]
- 16.Kaissling KE, Kasang G. 1978. A new pheromone of the silkworm moth Bombyx mori. Naturwissenschaften 65, 382–384 (doi:10.1007/bf00439702) [Google Scholar]
- 17.Nakagawa T, Sakurai T, Nishioka T, Touhara K. 2005. Insect sex-pheromone signals mediated by specific combinations of olfactory receptors. Science 307, 1638–1642 (doi:10.1126/science.1106267) [DOI] [PubMed] [Google Scholar]
- 18.Kazawa T, Namiki S, Fukushima R, Terada M, Soo K, Kanzaki R. 2009. Constancy and variability of glomerular organization in the antennal lobe of the silkmoth. Cell Tissue Res. 336, 119–136 (doi:10.1007/s00441-009-0756-3) [DOI] [PubMed] [Google Scholar]
- 19.Bisch-Knaden S, Carlsson MA, Sugimoto Y, Schubert M, Missbach C, Sachse S, Hansson BS. 2012. Olfactory coding in five moth species from two families. J. Exp. Biol. 215, 1542–1551 (doi:10.1242/jeb.068064) [DOI] [PubMed] [Google Scholar]
- 20.Manzini I, Schild D. 2003. Multidrug resistance transporters in the olfactory receptor neurons of Xenopus laevis tadpoles. J. Physiol. 546, 375–385 (doi:10.1113/jphysiol.2002.033175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heil JE, Oland LA, Lohr C. 2007. Acetylcholine-mediated axon-glia signaling in the developing insect olfactory system. Eur. J. Neurosci. 26, 1227–1241 (doi:10.1111/j.1460-9568.2007.05756.x) [DOI] [PubMed] [Google Scholar]
- 22.Stokl J, Strutz A, Dafni A, Svatos A, Doubsky J, Knaden M, Sachse S, Hansson BS, Stensmyr MC. 2010. A deceptive pollination system targeting drosophilids through olfactory mimicry of yeast. Curr. Biol. 20, 1846–1852 (doi:10.1016/j.cub.2010.09.033) [DOI] [PubMed] [Google Scholar]
- 23.Tanaka K, Uda Y, Ono Y, Nakagawa T, Suwa M, Yamaoka R, Touhara K. 2009. Highly selective tuning of a silkworm olfactory receptor to a key mulberry leaf volatile. Curr. Biol. 19, 881–890 (doi:10.1016/j.cub.2009.04.035) [DOI] [PubMed] [Google Scholar]
- 24.Namiki S, Iwabuchi S, Kanzaki R. 2008. Representation of a mixture of pheromone and host plant odor by antennal lobe projection neurons of the silkmoth Bombyx mori. J. Comp. Physiol. A 194, 501–515 (doi:10.1007/s00359-008-0325-3) [DOI] [PubMed] [Google Scholar]
- 25.Heinbockel T, Kaissling KE. 1996. Variability of olfactory receptor neuron responses of female silkmoths (Bombyx mori L) to benzoic acid and (+/−)-linalool. J. Insect Physiol. 42, 565–578 (doi:10.1016/0022-1910(95)00133-6) [Google Scholar]
- 26.Nagy BAL, George JA. 1981. Differences in the numbers of sensilla trichodea between reared and wild adults of the Oriental fruit moth, Grapholitha molesta (Lepidoptera, Tortricidae). Proc. Entomol. Soc. Ont. 112, 67–72 [Google Scholar]
- 27.Xia QY, et al. 2009. Complete resequencing of 40 genomes reveals domestication events and genes in silkworm (Bombyx). Science 326, 433–436 (doi:10.1126/science.1176620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abe H, et al. 2008. Identification of the female-determining region of the W chromosome in Bombyx mori. Genetica 133, 269–282 (doi:10.1007/s10709-007-9210-1) [DOI] [PubMed] [Google Scholar]
- 29.Piergentili R. 2010. Multiple roles of the Y chromosome in the biology of Drosophila melanogaster. Sci. World J. 10, 1749–1767 (doi:10.1100/tsw.2010.168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemos B, Araripe LO, Hartl DL. 2008. Polymorphic Y chromosomes harbor cryptic variation with manifold functional consequences. Science 319, 91–93 (doi:10.1126/science.1148861) [DOI] [PubMed] [Google Scholar]
- 31.Haddad R, Khan R, Takahashi YK, Mori K, Harel D, Sobel N. 2008. A metric for odorant comparison. Nat. Methods 5, 425–429 (doi:10.1038/nmeth.1197) [DOI] [PubMed] [Google Scholar]
- 32.Yamagata T, Sakurai T, Uchino K, Sezutsu H, Tamura T, Kanzaki R. 2008. GFP labeling of neurosecretory cells with the GAL4/UAS system in the silkmoth brain enables selective intracellular staining of neurons. Zool. Sci. 25, 509–516 (doi:10.2108/zsj.25.509) [DOI] [PubMed] [Google Scholar]