Abstract
Interspecific brood parasitism represents a prime example of the coevolutionary arms race where each party has evolved strategies in response to the other. Here, we investigated whether common cuckoos (Cuculus canorus) actively select nests within a host population to match the egg appearance of a particular host clutch. To achieve this goal, we quantified the degree of egg matching using the avian vision modelling approach. Randomization tests revealed that cuckoo eggs in naturally parasitized nests showed lower chromatic contrast to host eggs than those assigned randomly to other nests with egg-laying date similar to naturally parasitized clutches. Moreover, egg matching in terms of chromaticity was better in naturally parasitized nests than it would be in the nests of the nearest active non-parasitized neighbour. However, there was no indication of matching in achromatic spectral characteristics whatsoever. Thus, our results clearly indicate that cuckoos select certain host nests to increase matching of their own eggs with host clutches, but only in chromatic characteristics. Our results suggest that the ability of cuckoos to actively choose host nests based on the eggshell appearance imposes a strong selection pressure on host egg recognition.
Keywords: brood parasitism, cuckoo, egg coloration, egg mimicry, great reed warbler
1. Introduction
Mutual interactions among various animal taxa can be considered as an important selective force affecting evolutionary diversity [1]. Such reciprocity in relations is typical also of obligate brood parasitism, a life strategy used by some arachnids, insects, fishes and birds [2–5]. Obligate brood parasites are notorious for abandoning their parental duties to other species, the hosts. Because of the costs incurred on the side of the hosts, the brood parasitic breeding strategy has led to the evolution of host defences, which, in turn, have selected for more intricate counteradaptations in the brood parasite. Such an escalating arms race between brood parasites and their hosts thus represents an outstanding textbook example of coevolution [6]. This reciprocal relationship can fundamentally shape life histories, morphologies, physiologies and behaviours of both brood parasites and their hosts and influence trajectories and outcomes of their subsequent coevolutionary interactions [7]. In birds, about 1% of species have adopted the brood parasitic lifestyle [5]. The most striking adaptations by which avian brood parasites attempt to evade host defences are those related to the resemblance of host eggs, i.e. mimicry—the phenomenon that has fascinated researchers since Baldamus [8].
From the adaptive perspective, it should be beneficial for an individual brood parasite to produce progeny that will tend to exploit the same host species and evolve better tuned egg mimicry. This may consequently lead to the evolution of host-specific lineages, also called gentes [9–11], where females often lay eggs of a certain type to mimic the eggs of a particular host species [12,13]. In this respect, there is a wide variety of host specificity across different brood parasitic systems, ranging from an opportunistic host use in a majority of parasitic Molothrus cowbirds [14] to strict host specificity associated with host–parasite co-speciation in African indigobirds (Vidua spp.) [15].
Brood parasitism by the common cuckoo (Cuculus canorus, hereafter cuckoo) is a system where the parasite interacts with its hosts via egg phenotype matching [16]. This is especially apparent in the evolution of various cuckoo host-specific races, which have highly polymorphic eggs that resemble the egg appearance of the preferred host species [12,17–19]. However, a considerable potential for egg phenotype matching also exists within each cuckoo gens. While cuckoo eggs sometimes closely match the appearance of the host clutch, on other occasions, they often show rather imperfect mimicry [20]. Nonetheless, the degree of egg matching is crucial for the breeding success of the brood parasite, as a good mimicry impedes effective egg discrimination by the hosts [21–24].
The cuckoo female searches for suitable nests by observing host nest-building activity from a close vantage point [5]. Then, she follows the status of several host nests to correctly time her egg laying (for details, see [25]). During the host egg-laying period, the parasitic female often visits the nests before the parasitism act [26–29], which gives her the possibility of choosing a fitting host clutch. Indeed, tracking of radio-tagged cuckoo females showed that they are capable of finding almost all nests in their territories, but eventually choose only some of them for parasitism [29]. Hence, the brood parasite could adopt the strategy of fine-tuned egg matching and select preferentially those host nests with eggs more similar to their own. This interesting hypothesis was investigated only recently, but with ambiguous results. Avilés et al. [30] and Cherry et al. [31] showed that cuckoo eggs were more similar to host clutches in naturally parasitized nests than in non-parasitized nests. However, these studies characterized the degree of egg matching by principal component analysis (PCA) based on reflectance spectrophotometry data with no respect to avian colour vision. However, Antonov et al. [32] used an avian vision physiological model [33,34], but did not confirm this hypothesis. In this study [32], however, the authors only compared cuckoo egg mimicry in naturally parasitized clutches and the nearest non-parasitized conspecific neighbour despite the fact that cuckoo females may find many nests in the broader neighbourhood, but parasitize only some of them [28,29]. The last two studies [24,35] compared matching of different egg morphs assessed by human evaluation and reported equivocal results.
In our study, we compensated for the methodological artefacts of the previous studies. To quantify egg mimicry more objectively, we used the method of physiological modelling of avian colour vision implemented in the program Avicol [36], which calculates chromatic and achromatic contrasts between two colours (see §2). We did not only compare the mimicry of cuckoo eggs in naturally parasitized great reed warbler nests and their nearest non-parasitized conspecific neighbours, but we assessed cuckoo egg matching also in other host clutches that were suitable for parasitism in terms of timing. Moreover, we used a randomization approach [37] for statistical analysis, which is a very appropriate tool for the simulation of host nest choice by the brood parasite, providing intuitively interpretable results. By using these methods, we investigated whether cuckoo females target nests non-randomly within a population of a major host, the great reed warbler (Acrocephalus arundinaceus), to better match host clutches enhancing thereby the probability of egg acceptance. More specifically, our prediction was that cuckoo eggs in naturally parasitized nests will match the appearance of host eggs better than they would do by chance.
2. Material and methods
(a). Study area and field measurements
The study was carried out in two fishpond systems between Mutěnice (48°54′ N, 17°02′ E) and Hodonín (48°51′ N, 17°07′ E), Czech Republic, from 5 May to 23 June 2009. We systematically searched for great reed warbler nests in littoral vegetation surrounding the fishponds. A majority of them were found during nest building, and were checked daily until clutch completion. During these checks, each newly laid egg was numbered using a felt tip pen to allow its identification in the laying sequence. If a cuckoo egg was found in a nest, then the nest was considered as parasitized. The total sample used in analyses comprised 61 clutches (39 non-parasitized, 19 parasitized and three of uncertain parasitism status).
Each parasitized clutch included in the analyses was spectrophotometrically measured immediately after a cuckoo egg was detected. The remaining eggs from these clutches, as well as the non-parasitized clutches and those with uncertain status, were measured on the day after clutch completion. If the nest was parasitized twice, then we used only the first cuckoo egg in our analyses (n = 4). We measured spectral reflectance of each egg in the range of 300–700 nm using a spectrophotometer (USB2000, Ocean Optics) under standard light conditions. To prevent nest desertion during the measurements, we temporarily exchanged host clutch with four to five great reed warbler eggs from abandoned nests. For measurements, we divided each egg into three regions across the longitudinal axis and took three measurements from each region (each covering ca 1 mm2). We avoided the egg poles to eliminate a possible measurement error owing to marked curvature of eggshell surface. We also avoided extremely dark spots because they had very low reflectance and their measurements could influence mean reflectance values calculated per the whole egg surface.
During the measurements, the illuminant was a deuterium and halogen light source (DT-Mini-GS, Ocean Optics). The light was transferred to the eggshells through a quartz optic fibre (QR400-7-UV/VIS-BX, Ocean Optics), and was reflected at an angle of 45° to the surface. Data from the spectrophotometer were loaded into OOIBase 32 (Ocean Optics) software. The measurements were relative and referred to a standard white reference (WS-2, Ocean Optics) and to darkness. Reference and dark calibration were made prior to the measurement of each clutch.
(b). Quantification of egg mimicry
As the shape of a reflectance curve need not necessarily correspond to how the signal is processed by the receiver, we analysed the reflectance data using models of avian vision [24,38]. Specifically, we used physiological models [33,34] implemented in Avicol v. 6 [36] that reproduce bird retinal functioning and that account for nest luminosity and bird sensitivity to estimate chromatic and achromatic contrasts between the parasitic and host eggs. These models integrated information about ambient light conditions, the reflectance spectra of cuckoo and great reed warbler eggs, published information for single- and double-cone photoreceptor spectral sensitivities, photoreceptor noise and the transmission properties of avian eyes to get biologically reliable colour-matching estimates [39]. Ambient light values at nests of a typical open nester such as the great reed warbler were taken from Avilés et al. [40]. Sensitivity of single-cone photoreceptors was used to calculate chromatic contrasts and sensitivity of double cones to calculate achromatic contrasts [41]. Spectral sensitivity has never been measured in the cuckoo, but most bird species belong to one of two main groups differing particularly in spectral sensitivity of UV cones—UVS (ultraviolet-sensitive) and VS (violet sensitive) group [42]. Therefore, we used data published in TetraColourSpace [43] for two representatives of each group, the blue tit (Cyanistes caeruleus) for UVS [44] and the Indian peafowl (Pavo cristatus) for VS type of colour vision [45] and conducted all statistical analyses for both types of colour vision separately. We determined the relative proportions of the different single-cone types in the retina according to available data (blue tit: ultraviolet-sensitive (UVS) single cones = 1, short-wavelength-sensitive (SWS) single cones = 1.92, medium-wavelength-sensitive (MWS) single cones = 2.68 and long-wavelength-sensitive (LWS) single cones = 2.70, derived from [44]; peafowl: UVS = 1, SWS = 1.9, MWS = 2.2 and LWS = 2.1, derived from [46]). For the high-intensity noise, we used a Weber fraction value of 0.05 in both models.
The Vorobyev–Osorio model calculates chromatic (difference in hue) and achromatic (difference in brightness) contrasts between cuckoo and great reed warbler eggs in just noticeable differences. Essentially, cuckoo eggs that appear similar to a host clutch have smaller values of both contrasts than those with poor mimicry. For further details of contrasts calculations, see [33,34,36].
(c). Statistical analyses
Cuckoos frequently parasitize nests at the beginning of host egg laying [5] and eat up one to three host eggs before or during the parasitism event [28]. Therefore, earlier-laid host eggs may be removed preferentially in comparison with the later-laid eggs, which are usually paler [47,48]. Moreover, cuckoos could non-randomly predate on host eggs of a particular type. This could make the parasitized clutches distinct from the non-parasitized ones and influence our results. Therefore, we tested whether the naturally parasitized (n = 19) and non-parasitized (n = 39) host clutches differ in various characteristics of colour, such as hue, brightness and saturation. Specifically, we defined hue as relative photon catches of all four blue tit cone types involved in chromatic discrimination and brightness as blue tit double-cone photon catches [41,49]. Saturation was estimated as the distance of the point from the achromatic centre of blue tit colour space [43]. For analysis of spectral data, we used R package Pavo [50,51]. All comparisons were conducted using Wilcoxon tests, because the data did not comply with normality.
To test the egg-matching hypothesis, we used a randomization test [37]. Because the evidence for exclusive territory defence of cuckoo females is equivocal [52–56] and because home-range sizes of female cuckoos vary widely between 33 and 217 ha in our study area [57], we randomized the occurrence of parasitic eggs in host clutches across the whole host population while accounting for similar timing of egg laying. Accordingly, we assigned to each cuckoo egg the host clutches that were in the laying phase on the day when the focal cuckoo egg was laid, including the naturally parasitized clutches. The laying phase was defined as a 6-day interval from the day when the first host egg was laid, because cuckoos in our population parasitize host nests most frequently during this period. As a result, between four to sixteen host clutches were assigned to each cuckoo egg, and each measured host clutch (n = 61) was used at least once. We then calculated both chromatic and achromatic contrasts between each parasitic egg (n = 19) and all the assigned host clutches, whereby we obtained a total of 203 chromatic and achromatic contrasts. Further, we randomly selected one contrast belonging to each cuckoo egg and calculated a mean value of these 19 contrasts. This procedure was repeated 9999 times. Finally, we sorted these 9999 mean contrasts by their values and also included the mean contrast from 19 naturally parasitized nests. According to the egg-matching hypothesis, cuckoo eggs should exhibit lower contrasts to host eggs in the naturally parasitized clutches than cuckoo eggs in randomly assigned host clutches. To explore this scenario, we calculated the proportion of all simulated mean contrasts that were lower than the mean contrast of naturally parasitized nests. This proportion represents the significance level of the randomization test [37].
Additionally, we conducted pair-wise comparisons (t-tests) of both chromatic and achromatic contrasts of the naturally parasitized clutches with the nearest active non-parasitized clutches available for the cuckoo on the day of parasitism on the focal nest (n = 15). The mean distance between the nearest neighbour nests was 438.9±343.4 m (range: 20–1068 m). All statistical calculations were performed in R v. 2.15 [51].
3. Results
There were no differences in parasitized and non-parasitized host clutches in terms of eggshell colour characteristics, such as hue, brightness and saturation (all p-values > 0.19 for UVS and VS types of colour vision), and thus these differences could not influence the results of our randomization analyses.
Randomization tests revealed that cuckoos did not lay their eggs into host nests haphazardly, but matched host clutches in chromatic spectral characteristics (figure 1). Significant p-values (p = 0.0032 for UVS, p = 0.0142 for VS) showed that only in 32 and 142 cases of 9999 runs, respectively, was the mean chromatic contrast from 19 randomly simulated clutches lower than the mean chromatic contrast calculated from the naturally parasitized clutches. On the other hand, there was no indication of achromatic matching (randomization tests: p = 0.703 for UVS and p = 0.666 for VS). Similarly, the comparisons between the naturally parasitized nests and their nearest non-parasitized neighbours with similar timing showed a significant difference in chromatic contrast (t = −3.24, p = 0.006 for UVS; t = −2.40, p = 0.030 for VS), but not in achromatic contrast (t = −0.21, p = 0.83 for UVS; t = −0.24, p = 0.82 for VS; figure 2). Both types of analyses give compelling evidence that the cuckoos actively select host nests for parasitism in order to match the appearance of host clutches in chromatic characteristics.
Figure 1.
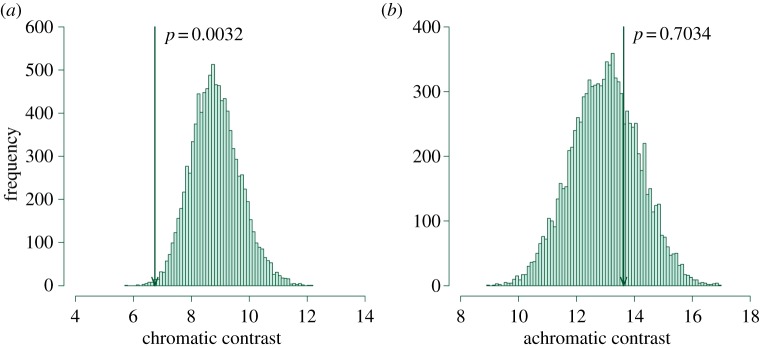
Histograms of 9999 averaged (a) chromatic and (b) achromatic contrasts between cuckoo eggs (n = 19) and all host clutches (n = 61) with a similar egg-laying date obtained from the randomization procedure. Arrows denote positions of values of mean contrasts between cuckoo eggs and host clutches in naturally parasitized nests (p-values given). Only results for UVS bird colour vision presented. (Online version in colour.)
Figure 2.
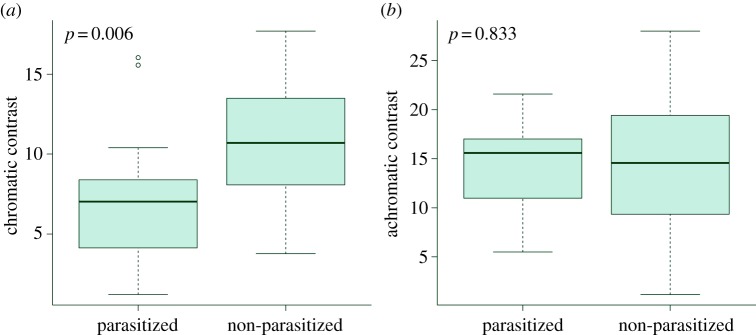
(a) Chromatic and (b) achromatic contrasts between cuckoo and host eggs in naturally parasitized nests and nearest non-parasitized active nests with a similar egg-laying date (n = 15, p-values given). Only results for UVS bird colour vision presented. (Online version in colour.)
4. Discussion
Similar to Avilés et al. [30] and Cherry et al. [31], we found that cuckoo females probably use egg appearance as an important cue to select host nests. Although our methodological approach was different from that used in these two studies (see §2), it is interesting that we obtained quite similar results. Within a host population, cuckoo females parasitized clutches with lower chromatic contrast than their own eggs but did not use the information about the differences in achromatic eggshell characteristics. Low importance of the achromatic contrast in the great reed warbler could be explained by the nest light environment hypothesis [58–60]. This hypothesis proposes that visual signals perceived by the receiver are significantly affected by the amount of ambient light. Dim light conditions allow only scotopic or mesopic vision, and perception of colour signals is therefore strongly limited [61]. In these situations, discrimination based on achromatic contrast may be favoured [40,62,63]. However, the great reed warbler is a typical open nester, and the amount of light inside its nests most likely allows photopic vision and good discrimination of colours [61]. In such conditions, chromatic differences between two spectra are more perceptible than under poor light conditions [64]. Therefore, egg discrimination under such light conditions may favour chromatic rather than achromatic visual signals [33,39]. Indeed, some studies confirmed that egg-rejection behaviour in bright light conditions depends primarily on chromatic rather than on achromatic contrast [23,24,60]. Such host behaviour may create a selection pressure on the cuckoo, which can gain an advantage if it chooses the correct host clutches on the basis of the chromatic signal. And this is what we found in our study population—cuckoo females selected host clutches similar to their own eggs in chromatic features.
In contrast to the results of Avilés et al. [30], Cherry et al. [31] and the present study, Antonov et al. [32] did not support the egg-matching hypothesis in the closely related marsh warbler (Acrocephalus palustris). The authors argued that selection of better matching host egg phenotypes probably cannot exist in a host–parasite system where host inter-clutch variation is continuous, overall low or moderate. However, these traits also partly apply to great reed warbler eggs [65–67]. The discrepancy in results could be explained by different light conditions in the nesting habitats of these two warbler species. The vegetation surrounding marsh warbler nests is generally denser than in the pure reed beds used by the great reed warblers [68,69] and probably provides a different light environment, not only in terms of the quantity but also the quality of the light [70]. However, it is not apparent whether this difference in nest light environment is sufficient to explain the differing results of the cited studies. In addition, it is also possible that the denser nesting vegetation of the marsh warbler makes the nest search more difficult for the cuckoos and prevents them from finding enough nests from which to make their selection, and the differences in nest availability between the two warbler species at the two study sites could also play a role. While Antonov et al. [32] claimed that the marsh warbler shows a secretive nesting behaviour and low nest density, our data on the great reed warbler demonstrate that during the peak breeding season the cuckoos have ample opportunities to choose [71].
It is interesting that our results were consistent for both main types of avian colour vision. These two groups differ primarily in the spectral sensitivity of their ultra-short-wavelength cones (UVS or VS type). The other three types of cones are relatively conservative in all bird species with certain variability in SWS cones [42,72]. Unfortunately, it is not yet known for certain whether the common cuckoo belongs to the UVS or the VS group (although some indirect evidence suggests the VS group in cuckoos; see [73,74]). However, we conducted our analyses using both types of avian colour vision, thus we are confident that any errors associated with this ‘cone sensitivity problem’ did not affect our results.
It must be pointed out, however, that our findings could be potentially biased owing to quick and thus unobserved rejections of poorly matching parasitic eggs in nests of uncertain parasitism status (n = 3). To be sure that this was not the case, we performed new randomizations where we added three artificially created (simulated) parasitized clutches with average chromatic contrasts to the original sample size. Detailed description of methods of these additional simulations is summarized in the electronic supplementary material. The results of these simulations showed that the addition of three simulated parasitized clutches did not affect the results of our original analysis (p = 0.0173 and 0.0311 for UVS and VS, respectively). Moreover, the cuckoo is not only a brood parasite but it can also partially predate on host clutches without parasitizing them [75]. In addition, Moksnes et al. [28], who videotaped nests of the reed warbler (Acrocephalus scirpaceus) during egg laying, recorded cuckoo visits in 20 nests; however, only 14 of them were parasitized. The remaining six nests were only partially depredated by the cuckoo. If we apply this ratio to our data (19 parasitized nests and three nests with uncertain status), then it would suggest that some (if not all) of these three nests were most probably only partially depredated and not parasitized.
An interesting and important question is whether cuckoos really profit from the host selection in our study area. Our recent study revealed that the great reed warblers recognize parasitic eggs based on the chromatic contrast, but only in well illuminated nests [60]. However, the chromatic contrast alone (calculated for UVS type of cones) did not differ between rejecters (n = 16) and acceptors (n = 23; Wilcoxon test, W = 217, p = 0.36, data from 2009 and 2010). This may be because cuckoos match the appearance of the host clutches, thus reducing the contrast perceived by the hosts. We suggest that cuckoo parasitizing host clutches similar to its own eggs in chromatic aspects may even get below the discrimination threshold of its host. By reaching the host's acceptance threshold, the brood parasite may effectively escape host rejection of parasitic eggs and thereby increase its reproductive success.
If the egg-matching scenario is true and the cuckoo females preferentially select the best-matching host nests, then they should know what their own eggs look like. A number of studies have been published on the mechanisms of own egg recognition in cuckoo hosts [76–83] and some of them suggest that birds possess an internal template of their own eggs [81–83]. So, one may expect that cuckoos may exhibit such abilities as well. Owing to the elusive lifestyle of the cuckoo, however, the direct mechanism of how cuckoo females know the appearance of their own eggs remains enigmatic. Similar to the hosts, the memory template of their own eggs may comprise an inherited and a learned component acquired during the first egg laying [79]. The idea mentioned by Antonov et al. [32] that the first cuckoo egg is laid somewhere in isolation seems highly unlikely [5]. Instead, cuckoo females are probably able to remember the appearance of their own eggs and compare this self-referent phenotype with the appearance of host clutches they have visited prior to laying. However, to the best of our knowledge, the cuckoo has never been observed watching its egg during the very short parasitism events.
The main conclusion of this study is that cuckoos do not lay eggs haphazardly in a host population, but match the appearance of host clutches with respect to chromatic contrast. By doing so, they may effectively reduce the chance of egg rejection by the host and thus enhance their reproductive success. However, it is currently difficult to explain how the cuckoo can learn the appearance of its own eggs. Therefore, we highly encourage others to test this appealing idea in future studies.
Acknowledgements
We thank Miroslav Čapek, Klára Morongová and Zuzana Šebelíková for their assistance in the field and Lukáš Kratochvíl for his constructive comments. We are obliged to the management of the Fish Farm Hodonín and local conservation authorities for permission to conduct the fieldwork. The experiments comply with the current laws and ethical guidelines of the Czech Republic. M.H., M.Š., V.J. and P.P. designed the research; M.H., M.P., V.J. and P.P. performed the fieldwork; M.Š. analysed the data; M.H., M.Š., V.J., P.P. and M.P. wrote the paper; M.H. supported the research.
Data accessibility
Data are deposited in the Dryad repository: doi:10.5061/dryad.5m400.
Funding statement
The study was supported by the Grant Agency of the Academy of Sciences of the Czech Republic (grant no. IAA600930903) and partly by the project of the Grant Agency of the Czech Republic (grant no. P506/12/2404) and the Institutional Research Plan (RVO: 68081766).
References
- 1.Yoder JB, Nuismer SL. 2010. When does coevolution promote diversification? Am. Nat. 176, 802–817 (doi:10.1086/657048) [DOI] [PubMed] [Google Scholar]
- 2.Boulton AM, Polis GA. 2002. Brood parasitism among spiders: interactions between salticids and Diguetia mojavea. Ecology 83, 282–287 (doi:10.2307/2680138) [Google Scholar]
- 3.Cervo R, Macinai V, Dechigi F, Turillazzi S. 2004. Fast growth of immature brood in a social parasite wasp: a convergent evolution between avian and insect cuckoos. Am. Nat. 164, 814–820 (doi:10.1086/425987) [DOI] [PubMed] [Google Scholar]
- 4.Sato T. 1986. A brood parasitic catfish of mouthbrooding cichlid fishes in Lake Tanganyika. Nature 323, 58–59 (doi:10.1038/323058a0) [DOI] [PubMed] [Google Scholar]
- 5.Davies NB. 2000. Cuckoos, cowbirds and other cheats. London, UK: T & AD Poyser [Google Scholar]
- 6.Rothstein SI. 1990. A model system for coevolution: avian brood parasitism. Annu. Rev. Ecol. Syst. 21, 481–508 (doi:10.1146/annurev.ecolsys.21.1.481) [Google Scholar]
- 7.Feeney WE, Welbergen JA, Langmore NE. 2012. The frontline of avian brood parasite–host coevolution. Anim. Behav. 84, 3–12 (doi:10.1016/j.anbehav.2012.04.011) [Google Scholar]
- 8.Baldamus E. 1853. Neue Beiträge zur Fortpflanzungsgeschichte des europäischen Kuckucks, Cuculus canorus. Naumannia 3, 307–325 [Google Scholar]
- 9.Brooke MD, Davies NB. 1988. Egg mimicry by cuckoos Cuculus canorus in relation to discrimination by hosts. Nature 335, 630–632 (doi:10.1038/335630a0) [Google Scholar]
- 10.Gibbs HL, Sorenson MD, Marchetti K, Brooke MD, Davies NB, Nakamura H. 2000. Genetic evidence for female host-specific races of the common cuckoo. Nature 407, 183–186 (doi:10.1038/35025058) [DOI] [PubMed] [Google Scholar]
- 11.Fossøy F, Antonov A, Moksnes A, Røskaft E, Vikan JR, Møller AP, Shykoff JA, Stokke BG. 2011. Genetic differentiation among sympatric cuckoo host races: males matter. Proc. R. Soc. B 278, 1639–1645 (doi:10.1098/rspb.2010.2090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moksnes A, Røskaft E. 1995. Egg-morphs and host preference in the common cuckoo (Cuculus canorus): an analysis of cuckoo and host eggs from European museum collections. J. Zool. 236, 625–648 (doi:10.1111/j.1469-7998.1995.tb02736.x) [Google Scholar]
- 13.Starling M, Heinsohn R, Cockburn A, Langmore NE. 2006. Cryptic gentes revealed in pallid cuckoos Cuculus pallidus using reflectance spectrophotometry. Proc. R. Soc. B 273, 1929–1934 (doi:10.1098/rspb.2006.3490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorenson MD, Payne RB. 2002. Molecular genetic perspectives on avian brood parasitism. Integr. Comp. Biol. 42, 388–400 (doi:10.1093/icb/42.2.388) [DOI] [PubMed] [Google Scholar]
- 15.Sorenson MD, Sefc KM, Payne RB. 2003. Speciation by host switch in brood parasitic indigobirds. Nature 424, 928–931 (doi:10.1038/nature01863) [DOI] [PubMed] [Google Scholar]
- 16.Clayton DH, Moore J. 1997. Host–parasite evolution: general principles and avian models. Oxford, UK: Oxford University Press [Google Scholar]
- 17.Honza M, Moksnes A, Røskaft E, Stokke BG. 2001. How are different common cuckoo Cuculus canorus egg morphs maintained? An evaluation of different hypotheses. Ardea 89, 341–352 [Google Scholar]
- 18.Stoddard MC, Stevens M. 2010. Pattern mimicry of host eggs by the common cuckoo, as seen through a bird's eye. Proc. R. Soc. B 277, 1387–1393 (doi:10.1098/rspb.2009.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoddard MC, Stevens M. 2011. Avian vision and the evolution of egg color mimicry in the common cuckoo. Evolution 65, 2004–2013 (doi:10.1111/j.1558-5646.2011.01262.x) [DOI] [PubMed] [Google Scholar]
- 20.Grim T. 2002. Why is mimicry in cuckoo eggs sometimes so poor? J. Avian Biol. 33, 302–305 (doi:10.1034/j.1600-048X.2002.330312.x) [Google Scholar]
- 21.Procházka P, Honza M. 2003. Do common whitethroats (Sylvia communis) discriminate against alien eggs? J. Ornithol. 144, 354–363 (doi:10.1007/bf02465635) [Google Scholar]
- 22.Avilés JM, et al. 2011. The common cuckoo Cuculus canorus is not locally adapted to its reed warbler Acrocephalus scirpaceus host. J. Evol. Biol. 24, 314–325 (doi:10.1111/j.1420-9101.2010.02168.x) [DOI] [PubMed] [Google Scholar]
- 23.Avilés JM, Vikan JR, Fossøy F, Antonov A, Moksnes A, Røskaft E, Stokke BG. 2010. Avian colour perception predicts behavioural responses to experimental brood parasitism in chaffinches. J. Evol. Biol. 23, 293–301 (doi:10.1111/j.1420-9101.2009.01898.x) [DOI] [PubMed] [Google Scholar]
- 24.Spottiswoode CN, Stevens M. 2010. Visual modelling shows that avian host parents use multiple cues in rejecting parasitic eggs. Proc. Natl Acad. Sci. USA 107, 8672–8676 (doi:10.1073/pnas.0910486107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honza M, Taborsky B, Taborsky M, Teuschl Y, Vogl W, Moksnes A, Røskaft E. 2002. Behaviour of female common cuckoos, Cuculus canorus, in the vicinity of host nests before and during laying: a radiotelemetry study. Anim. Behav. 64, 861–868 (doi:10.1006/anbe.2002.1969) [Google Scholar]
- 26.Wyllie I. 1981. The cuckoo. London, UK: Batsford [Google Scholar]
- 27.Gärtner K. 1981. Das Wegnehmen von Wirtsvogeleiern durch den Kuckuck Cuculus canorus. Ornithologische Mitteilungen 33, 115–131 [Google Scholar]
- 28.Moksnes A, Røskaft E, Hagen LG, Honza M, Mork C, Olsen PH. 2000. Common cuckoo Cuculus canorus and host behaviour at reed warbler Acrocephalus scirpaceus nests. Ibis 142, 247–258 (doi:10.1111/j.1474-919X.2000.tb04864.x) [Google Scholar]
- 29.Nakamura H, Miyazawa Y, Kashiwagi K. 2005. Behavior of radio-tracked common cuckoo females during the breeding season in Japan. Ornithol. Sci. 4, 31–41 (doi:10.2326/osj.4.31) [Google Scholar]
- 30.Avilés JM, Stokke BG, Moksnes A, Røskaft E, Åsmul M, Møller AP. 2006. Rapid increase in cuckoo egg matching in a recently parasitized reed warbler population. J. Evol. Biol. 19, 1901–1910 (doi:10.1111/j.1420-9101.2006.01166.x) [DOI] [PubMed] [Google Scholar]
- 31.Cherry MI, Bennett ATD, Moskát C. 2007. Do cuckoos choose nests of great reed warblers on the basis of host egg appearance? J. Evol. Biol. 20, 1218–1222 (doi:10.1111/j.1420-9101.2007.01308.x) [DOI] [PubMed] [Google Scholar]
- 32.Antonov A, Stokke BG, Fossøy F, Ranke PS, Liang W, Yang CC, Moksnes A, Shykoff J, Røskaft E. 2012. Are cuckoos maximizing egg mimicry by selecting host individuals with better matching egg phenotypes? PLoS ONE 7 e31704 (doi:10.1371/journal.pone.0031704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vorobyev M, Osorio D. 1998. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. Lond. B 265, 351–358 (doi:10.1098/rspb.1998.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vorobyev M, Osorio D, Bennett ATD, Marshall NJ, Cuthill IC. 1998. Tetrachromacy, oil droplets and bird plumage colours. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 183, 621–633 (doi:10.1007/s003590050286) [DOI] [PubMed] [Google Scholar]
- 35.Yang CC, et al. 2010. Coevolution in action: disruptive selection on egg colour in an avian brood parasite and its host. PLoS ONE 5, e10816 (doi:10.1371/journal.pone.0010816) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomez D. 2006. AVICOL: a program to analyse spectrometric data, vol. 6 See http://sites.google.com/site/avicolprogram.
- 37.Manly BFJ. 1997. Monte Carlo methods. In Randomization, bootstrap, and Monte Carlo methods in biology, pp. 69–78 London, UK: Chapman and Hall [Google Scholar]
- 38.Cassey P, Grim T, Honza M, Hauber ME. 2008. The modelling of avian visual perception model predicts behavioural responses to foreign egg colours. Biol. Lett. 4, 515–517 (doi:10.1098/rsbl.2008.0279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Avilés JM. 2008. Egg colour mimicry in the common cuckoo Cuculus canorus as revealed by modelling host retinal function. Proc. R. Soc. B 275, 2345–2352 (doi:10.1098/rspb.2008.0720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Avilés JM, Pérez-Contreras T, Navarro C, Soler JJ. 2008. Dark nests and conspicuousness in color patterns of nestlings of altricial birds. Am. Nat. 171, 327–338 (doi:10.1086/527493) [DOI] [PubMed] [Google Scholar]
- 41.Osorio D, Vorobyev M. 2005. Photoreceptor spectral sensitivities in terrestrial animals: adaptations for luminance and colour vision. Proc. R. Soc. B 272, 1745–1752 (doi:10.1098/rspb.2005.3156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ödeen A, Håstad O. 2003. Complex distribution of avian color vision systems revealed by sequencing the SWS1 opsin from total DNA. Mol. Biol. Evol. 20, 855–861 (doi:10.1093/molbev/msg108) [DOI] [PubMed] [Google Scholar]
- 43.Stoddard MC, Prum RO. 2008. Evolution of avian plumage color in a tetrahedral color space: a phylogenetic analysis of new world buntings. Am. Nat. 171, 755–776 (doi:10.1086/587526) [DOI] [PubMed] [Google Scholar]
- 44.Hart NT, Partridge JC, Cuthill IC, Bennett ATD. 2000. Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine bird: the blue tit (Parus caeruelus L.) and the blackbird (Turdus merula L.). J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 186, 375–387 (doi:10.1007/s003590050437) [DOI] [PubMed] [Google Scholar]
- 45.Hart NS. 2002. Vision in the peafowl (Aves: Pavo cristatus). J. Exp. Biol. 205, 3925–3935 [DOI] [PubMed] [Google Scholar]
- 46.Hart NS. 2001. Variations in cone photoreceptor abundance and the visual ecology of birds. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 187, 685–697 (doi:10.1007/s00359-001-0240-3) [DOI] [PubMed] [Google Scholar]
- 47.Moreno J. 2005. Evidence for the signaling function of egg color in the pied flycatcher Ficedula hypoleuca. Behav. Ecol. 16, 931–937 (doi:10.1093/beheco/ari072) [Google Scholar]
- 48.López de Hierro MDG, De Neve L. 2010. Pigment limitation and female reproductive characteristics influence egg shell spottiness and ground colour variation in the house sparrow (Passer domesticus). J. Ornithol. 151, 833–840 (doi:10.1007/s10336-010-0520-1) [Google Scholar]
- 49.Endler JA, Mielke PW. 2005. Comparing color patterns as birds see them. Biol. J. Linn. Soc. 86, 405–431 (doi:10.1111/j.1095-8312.2005.00540.x) [Google Scholar]
- 50.Maia R, Eliason CHM, Bitton PP, Doucet SM, Shawkey MD. 2013. pavo: an R package for the analysis, visualization and organization of spectral data. Methods Ecol. Evol. 4, 906–913 (doi:10.1111/2041-210X.12069) [Google Scholar]
- 51.R Development Core Team 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 52.Molnár B. 1944. The cuckoo in the Hungarian Plain. Aquila 51, 100–112 [Google Scholar]
- 53.Dröscher LM. 1988. A study on radio-tracking of the European cuckoo (Cuculus canorus canorus). Proc. 100th Int. Deutsche Ornithologen-Gesellschaft Meeting. Deutsche Ornithologen-Gesellschaft 187–193 [Google Scholar]
- 54.Nakamura H, Miyazawa Y. 1997. Movements, space use and social organisation radio-tracked common cuckoos during the breeding season in Japan. Jpn. J. Ornithol. 46, 23–54 (doi:10.3838/jjo.46.23) [Google Scholar]
- 55.Moskát C, Honza M. 2002. European cuckoo Cuculus canorus parasitism and host's rejection behaviour in a heavily parasitized great reed warbler Acrocephalus arundinaceus population. Ibis 144, 614–622 (doi:10.1046/j.1474-919X.2002.00085.x) [Google Scholar]
- 56.Moskát C, Hauber ME, Avilés JM, Bán M, Hargitai R, Honza M. 2009. Increased host tolerance of multiple cuckoo eggs leads to higher fledging success of the brood parasite. Anim. Behav. 77, 1281–1290 (doi:10.1016/j.anbehav.2009.01.030) [Google Scholar]
- 57.Vogl W, Taborsky M, Taborsky B, Teuschl Y, Honza M. 2002. Cuckoo females preferentially use specific habitats when searching for host nests. Anim. Behav. 64, 843–850 (doi:10.1006/anbe.2002.1967) [Google Scholar]
- 58.Endler JA. 1993. The color of light in forests and its implication. Ecol. Monogr. 63, 1–27 (doi:10.2307/2937121) [Google Scholar]
- 59.Cherry MI, Bennett ATD. 2001. Egg colour matching in an African cuckoo, as revealed by ultraviolet-visible reflectance spectrophotometry. Proc. R. Soc. Lond. B 268, 5565–5571 (doi:10.1098/rspb.2001.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Honza M, Procházka P, Morongová K, Čapek M, Jelínek V. 2011. Do nest light conditions affect rejection of parasitic eggs? A test of the light environment hypothesis. Ethology 117, 539–546 (doi:10.1111/j.1439-0310.2011.01900.x) [Google Scholar]
- 61.Węgrzyn E, Leniowski K, Rykowska I, Wasiak W. 2011. Is UV and blue-green egg colouration a signal in cavity-nesting birds? Ethol. Ecol. Evol. 23, 121–139 (doi:10.1080/03949370.2011.554882) [Google Scholar]
- 62.Langmore NE, Stevens M, Maurer G, Kilner RM. 2009. Are dark cuckoo eggs cryptic in host nests? Anim. Behav. 78, 461–468 (doi:10.1016/j.anbehav.2009.06.003) [Google Scholar]
- 63.Antonov A, Avilés JM, Stokke BG, Spasova V, Vikan JR, Moksnes A, Yang CC, Liang W, Røskaft E. 2011. Egg discrimination in an open nesting passerine under dim light conditions. Ethology 117, 1128–1137 (doi:10.1111/j.1439-0310.2011.01969.x) [Google Scholar]
- 64.Schaefer HM, Schaefer V, Vorobyev M. 2007. Are fruit colours adapted to consumer vision and birds equally efficient in detecting colourful signals? Am. Nat. 169, S159–S169 (doi:10.1086/510097) [DOI] [PubMed] [Google Scholar]
- 65.Moskát C, Szentpéteri J, Barta Z. 2002. Adaptations by great reed warblers to brood parasitism: a comparison of populations in sympatry and allopatry with the common cuckoo. Behaviour 139, 1313–1329 (doi:10.1163/156853902321104181) [Google Scholar]
- 66.Antonov A, Stokke BG, Vikan JR, Fossøy F, Ranke PS, Røskaft E, Moksnes A, Møller AP, Shykoff JA. 2010. Egg phenotype differentiation in sympatric cuckoo Cuculus canorus gentes. J. Evol. Biol. 23, 1170–1182 (doi:10.1111/j.1420-9101.2010.01982.x) [DOI] [PubMed] [Google Scholar]
- 67.Honza M, Procházka P, Požgayová M. 2012. Within- and between-season repeatability of eggshell colouration in the great reed warbler Acrocephalus arundinaceus. J. Avian Biol. 43, 91–96 (doi:10.1111/j.1600-048X.2011.05392.x) [Google Scholar]
- 68.Leisler B. 1991. Acrocephalus arundinaceus – Drosselrohrsänger. In Handbuch der Vögel Mitteleuropas, vol. 12, pp. 486–539 Wiesbaden, Germany: Glutz von Blotzheim, UN & Bauer KM [Google Scholar]
- 69.Schulze-Hagen K. 1991. Acrocephalus palustris – Sumpfrohrsänger. In Handbuch der Vögel Mitteleuropas, vol. 12, pp. 377–433 Wiesbaden, Germany: Glutz von Blotzheim UN & Bauer KM [Google Scholar]
- 70.Honkavaara J, Koivula M, Korpimäki E, Siitari H, Viitala J. 2002. Ultraviolet vision and foraging in terrestrial vertebrates. Oikos 98, 505–511 (doi:10.1034/j.1600-0706.2002.980315.x) [Google Scholar]
- 71.Jelínek V, Procházka P, Požgayová M, Honza M. 2013. Common cuckoos Cuculus canorus change their nest-searching strategy according to the number of available host nests. Ibis (doi:10.1111/ibi.12093) [Google Scholar]
- 72.Hart NS, Hunt DM. 2007. Avian visual pigments: characteristics, spectral tuning, and evolution. Am. Nat. 169, S7–S26 (doi:10.1086/510141) [DOI] [PubMed] [Google Scholar]
- 73.Mullen P, Pohland G. 2008. Studies on UV reflection in feathers of some 1000 bird species: are UV peaks in feathers correlated with violet-sensitive and ultraviolet-sensitive cones? Ibis 150, 59–68 (doi:10.1111/j.1474-919X.2007.00736.x) [Google Scholar]
- 74.Aidala Z, et al. 2012. Ultraviolet visual sensitivity in three avian lineages: paleognaths, parrots, and passerines. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 198, 495–510 (doi:10.1007/s00359-012-0724-3) [DOI] [PubMed] [Google Scholar]
- 75.Schulze-Hagen K. 1992. Parasitierung und Brutverluste durch Kuckuck (Cuculus canorus) bei Teich- und Sumpfrohrsänger (Acrocephalus scirpaceus, A. palustris) in Mittel- und Westeuropa. J. Ornithol. 133, 237–249 (doi:10.1007/BF01645635) [Google Scholar]
- 76.Lotem A, Nakamura H, Zahavi A. 1992. Rejection of cuckoo eggs in relation to host age: a possible evolutionary equilibrium. Behav. Ecol. 3, 128–132 (doi:10.1093/beheco/3.2.128) [Google Scholar]
- 77.Moskát C, Hauber ME. 2007. Conflict between egg recognition and egg rejection decisions in common cuckoo (Cuculus canorus) hosts. Anim. Cogn. 10, 377–386 (doi:10.1007/s10071-007-0071-x) [DOI] [PubMed] [Google Scholar]
- 78.Moskát C, Székely T, Cuthill IC, Kisbenedek T. 2008. Hosts’ response to parasitic eggs: which cues elicit hosts’ egg discrimination? Ethology 114, 186–194 (doi:10.1111/j.1439-0310.2007.01456.x) [Google Scholar]
- 79.Moskát C, Bán M, Székely T, Komdeur J, Lucassen RWG, van Boheemen LA, Hauber ME. 2010. Discordancy or template-based recognition? Dissecting the cognitive basis of the rejection of foreign eggs in hosts of avian brood parasites. J. Exp. Biol. 213, 1976–1983 (doi:10.1242/jeb.040394) [DOI] [PubMed] [Google Scholar]
- 80.Soler M, Ruiz-Castellano C, Carra LG, Ontanilla J, Martín-Galvez D. 2013. Do first-time breeding females imprint on their own eggs? Proc. R. Soc. B 280 20122518 (doi:10.1098/rspb.2012.2518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hauber ME, Sherman PW. 2001. Self-referent phenotype matching: theoretical considerations and empirical evidence. Trends Neurosci. 24, 609–616 (doi:10.1016/s0166-2236(00)01916-0) [DOI] [PubMed] [Google Scholar]
- 82.Hauber ME, Moskát C, Bán M. 2006. Experimental shift in hosts’ acceptance threshold of inaccurate-mimic brood parasite eggs. Biol. Lett. 2, 177–180 (doi:10.1098/rsbl.2005.0438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bán M, Moskát C, Barta Z, Hauber ME. 2013. Simultaneous viewing of own and parasitic eggs is not required for egg rejection by a cuckoo host. Behav. Ecol. 24, 1014–1021 (doi:10.1093/beheco/art004) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are deposited in the Dryad repository: doi:10.5061/dryad.5m400.


