Abstract
Sexual conflict over mating can result in sex-specific morphologies and behaviours that allow each sex to exert control over the outcome of reproduction. Genital traits, in particular, are often directly involved in conflict interactions. Via genital manipulation, we experimentally investigated whether genital traits in red-sided garter snakes influence copulation duration and formation of a copulatory plug. The hemipenes of male red-sided garter snakes have a large basal spine that inserts into the female cloaca during mating. We ablated the spine and found that males were still capable of copulation but copulation duration was much shorter and copulatory plugs were smaller than those produced by intact males. We also anaesthetized the female cloacal region and found that anaesthetized females copulated longer than control females, suggesting that female cloacal and vaginal contractions play a role in controlling copulation duration. Both results, combined with known aspects of the breeding biology of red-sided garter snakes, strongly support the idea that sexual conflict is involved in mating interactions in this species. Our results demonstrate the complex interactions among male and female traits generated by coevolutionary processes in a wild population. Such complexity highlights the importance of simultaneous examination of male and female traits.
Keywords: copulation duration, copulatory plugs, hemipene, sexual selection, genitalia
1. Introduction
Sexual conflict results when the evolutionary interests of males and females are not optimized simultaneously during mating, fertilization and/or parental care [1–3]. During mating, conflict often occurs when the ideal copulation duration is different for males and females [1,4–6]. For females, copulation times beyond those required for optimal sperm transfer may be detrimental owing to decreased ability to choose other mates as fathers of her offspring [7–9], increased risk of injury and predation, and/or decreased feeding rates [10]. Therefore, females are expected to favour shorter copulations especially if sperm transfer occurs quickly [11]. For males, however, longer copulations can serve not only to transfer more sperm, but also to mate-guard the female, thus males are expected to typically favour longer copulations than females. When males transfer other substances during copulation, for example a copulatory plug, conflict is likely to be more intense if these secretions negatively affect female fitness but positively affect male fitness [5,12,13]. Male genital morphology has been shown to be associated with sexual conflict during copulation in many taxa [14–20].
Sexual conflict over mating is a prominent feature of the mating system of red-sided garter snakes (Thamnophis sirtalis parietalis) [21,22]. In this species, the sex ratio at spring emergence is strongly male-biased. Several dozen males will congregate around a newly emerged female forming ‘mating balls’ in which males court her and attempt copulation [23,24]. When the female is ready to mate, she gapes her cloaca so copulation can occur. Mating is effectively random with respect to male size in the large aggregations of males (6–24) a female encounters as she emerges [25,26]. Females, therefore, do not seem able to choose larger, fitter males prior to copulation (garter snakes exhibit indeterminate growth, thus large males are successful at foraging and evading predators [27,28]). It also seems unlikely that females use features of the hemipene to assess mates during copulation because small males with small hemipenes are just as successful as large males with large hemipenes [29]. Females may also be forced to copulate if males can elicit a cloacal gaping response owing to oxygen deprivation in the female using caudocephalic waves during courtship [30].
Sexual conflict may be further intensified by the gelatinous copulatory plug that a male deposits within the female's cloaca. The plug delays female remating [31], prevents female ejection of sperm from unwanted males, prevents sperm leakage and acts as a spermatophore from which sperm are liberated over the course of 2 days [32]. These features of the red-sided garter snake mating system suggest that sexual conflict over copulation duration, rather than cryptic female choice (CFC), is an important selective pressure operating during the female's first mating in the den [22]. As the female moves away from the den, the plug from her first mating dissolves and she may mate again in the smaller, less dense aggregations in small aspen groves surrounding the den where larger males are more prevalent and successful [23–25]. Thus, female choice may be important when females move away from the den into the aspen groves.
Copulation duration and plug deposition can be affected by female behavioural strategies. In a related species, the checkered garter snake (Thamnophis marcianus), copulatory plug formation is prevented when females perform axial rotations (body rolls) that terminate copulations early [33]. This body-roll behaviour also leads to significantly shorter copulation durations in the plains garter snake (Thamnophis radix) [34]. Thus, copulation duration may be important for plug formation and female body-roll behaviour may indicate sexual conflict over mating [34].
In addition to body rolls, female genital musculature may also influence plug formation and copulation duration. The cranial pouch of the cloacal urodaeum of females (henceforth vaginal pouch) is highly muscularized [35,36]. The ease with which a male intromits his hemipene and the period he maintains intromission could depend on the muscular contractions of the vaginal pouch [32]. Therefore, copulation duration and plug deposition are likely both mediated by female behaviour and by interactions between female and male genitalia during copulation.
Male genital morphology has been shown to be associated with sexual conflict during copulation in many taxa [14–18]. In North American natricine snakes, taxa with bilobed hemipenes have longer copulation durations than those with cylindrical hemipenes [34]. Male hemipenes in T. sirtalis are relatively simple cylindrical structures [34], with a ring of keratinized spines and a large sharp basal spine located on the lateral aspect of the everted hemipenes that may also be associated with conflict over copulation duration (figure 1). The basal spine is the largest hemipenial spine and the first to make contact with the female cloaca at intromission, and has been hypothesized to act as a grappling hook that allows the male to anchor and evert his hemipene into the female cloaca [37]. However, the adaptive significance of the basal spine has yet to be tested experimentally.
Figure 1.
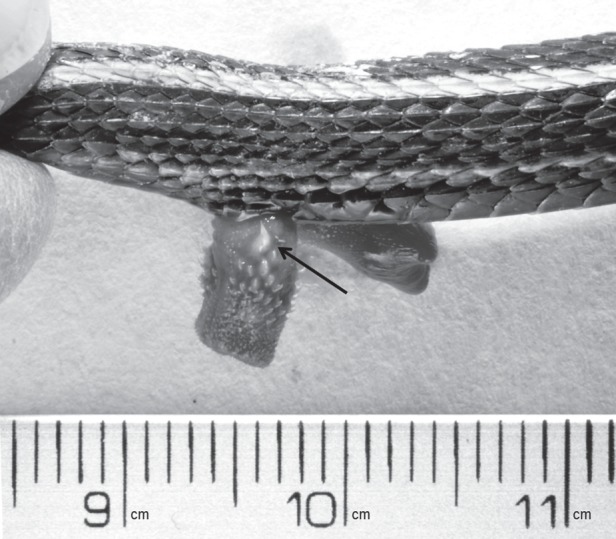
A photo of the basal spine on the right hemipene of Thamnophis sirtalis parietalis. The arrow indicates the basal spine.
One way to test adaptive hypotheses of sexual conflict during mating is to manipulate both females and males such that the putative adaptations for control over conflict are rendered ineffective [1,38–41]. Here, we conducted experiments in red-sided garter snakes during the female's first mating at the den where sexual conflict is most likely to be important. We hypothesized that the basal spine allows the male's hemipene to maintain position during copulation despite female body rolls or vaginal contractions used to terminate copulation. To test this hypothesis, we ablated the basal spine of the male hemipenes to determine whether males could initiate and maintain intromission. We predicted that, if sexual conflict over copulation duration and plug size occurs in this system, spine-ablated males would be able to copulate, but for shorter periods and produce smaller copulatory plugs. Alternatively, if the basal spine is required for normal hemipene eversion as hypothesized by Pisani [37], then spine-ablated males should not be able to copulate at all. Female garter snakes perform body rolls to terminate copulations, but we also hypothesized that they use muscles of the vaginal pouch to mediate copulation duration and plug deposition. To test this hypothesis, we held females at intromission to prevent body rolls that may terminate copulation immediately. These females were either injected with saline or a local anaesthetic in the cloaca and vaginal pouch region to reduce female control over the course of copulation. We predicted that males would be able to deposit larger plugs in anaesthetized females and these females would be subjected to longer copulations even though they could perform body rolls after intromission because they would not be able to use their cloacal and vaginal structures to terminate copulations.
2. Material and methods
(a). Study species
Thamnophis sirtalis parietalis in Manitoba exhibit a condensed period of mating (four to six weeks) during the spring when they emerge from limestone hibernacula. Males emerge earlier than females and remain at the den sites longer, resulting in a strongly male-biased sex ratio [42].
All experiments were carried out on individuals collected from Inwood, Manitoba, Canada (50°31.58′ N, 97°29.71′ W). All individuals used in the mating trials were identified with a letter written in permanent marker (Sharpie) on a 0.25 × 0.25 cm piece of 3M, Nexcare adhesive tape affixed to their head. All animals were housed in nylon arenas (1 × 1 × 1 M) and were provided water ad libitum until mating trials. For the trials, newly emerged, unmated females (typically still cold and slow) were collected as they emerged from the ground.
(b). Experiment 1: treated males mating in arenas with untreated females
We collected 42 actively courting males directly from the den and placed them in circular nylon mating arenas (45 cm diameter × 75 cm tall). We standardized male experience by allowing all males to mate once in a larger arena (1 × 1 × 1 M) on the same day prior to assigning them to experimental groups. During these initial mating trials, we introduced two unmated females to the arena until copulation commenced. The mating pair was then gently moved to a smaller enclosure where copulation could be closely monitored to record copulation duration (±10 s). We also recorded which hemipene was used during copulation as it may correlate with plug mass [29].
After mating, we measured and weighed, and assigned randomly to one of two groups: ablated or control. All males were lightly anaesthetized with 0.0015 µl of 0.5% methohexital sodium per gram body mass administered subcutaneously at the juncture between the dorsal and ventral scales approximately 4 cm from the head of the snake [43]. Ten minutes after anaesthesia, each male received two 10 µl bilateral, subcutaneous injections of the local anaesthetic 2% Lidocaine HCl. The injection sites were both between the first and second dorsal scale rows, four subcaudal scales caudal to the cloaca. Five to 10 min after Lidocaine injections, both hemipenes were everted manually exposing the basal spines, which were then cut close to its base with corneoscleral scissors from both hemipenes of the experimental males. Clipping the spine resulted in very little or no bleeding. Control males were treated in the same way except that the basal spine was touched but not clipped with the scissors. Snakes were then placed in a 20 gal (75.7 l) aquarium situated on a heating pad (30°C) and monitored every 10 min until righting reflex returned [43]. Males of this species regain courtship behaviour quickly after invasive surgeries and show no deficit in courtship ability [44], and all the males engaged in courtship during the experimental matings.
Males were housed indoors in two small circular arenas (45 cm diameter × 75 cm tall) for 4 days, given water ad libitum and then were taken back to the den site and placed together in a nylon arena (1 × 1 × 1 M). Two newly emerged unmated females were introduced into the arena and observed until copulation occurred. If a male achieved intromission, we timed copulation duration in the same way as in the first mating. We recorded copulation duration even in situations where a female rolled her body to dislodge a male prior to depositing a copulatory plug. If the pair remained in copulo for more than a few seconds, we gently removed the pair to an empty arena. In order to keep the number of courting males constant, we introduced untreated males with tape placed over their cloaca to prevent copulation while still allowing courtship. We continued introducing newly emerged unmated females and untreated males until the end of the day. Soon after the termination of copulation, we removed the copulatory plug using a blunt probe [32,45] and measured plug mass (±0.01 g). We also visually inspected females for the presence of semen inside the cloaca. All trials were conducted in a single day and 25 s matings were recorded (14 ablated versus 11 control males).
(c). Statistical analysis
Copulation duration was analysed using two-way repeated measures ANOVA in SigmaPlot v. 11.0 with treatment and male mating number as factors. Logistic regression could not be used to test for the effect of copulation duration on plug deposition as there was complete separation in the data (plugs were produced in all copulations over, and none under, 400 s). Average copulation duration ranges from 480 to 1200 s in this species (reviewed in [34]). We set a cut-off (300 s) to obtain a test of independence of the effect of copulation duration on plug deposition using χ2 with Yates continuity correction. We then used the same test to determine whether treatments differed significantly in the number of copulations above and below the 300 s threshold.
In those matings that produced a plug, the plug mass data were normally distributed with equal variance, so we fit these models using generalized linear models (Gaussian distribution; identity link function) in R v. 2.15.3, [46]. Previous studies have demonstrated the effects of male and female size and hemipene (left or right) on plug mass [29]. We employed multi-model selection using the Akaike's information criterion corrected for small sample sizes (AICc) on regression models (R: MuMIn package [47]). AICc allowed us to identify a set of model parameters that best fit our data while adding penalties for extra parameters [48–50]. Our initial candidate model included the following parameters: treatment, male and female size (snout-to-vent length, svl), hemipene, copulation duration as main effects, and male size by female size and treatment by copulation duration interactions. We hoped to use the best model (lowest AICc value) to describe observed data; however, the top-ranked model included only the intercept. Instead, we identified the only significant model-averaged parameter, which was the interaction between copulation duration and treatment (p = 0.0405: electronic supplementary material, table S1). Thus, we constructed a model that included the main effects of treatment and copulation duration and their interaction. The analysis (ANCOVA) and graphing of this model was conducted in XLSTAT v. 2012.6.02.
(d). Experiment 2: treated females mating in the natural den with untreated males
Twenty-four unmated females were collected 1 day before mating trials. Females were housed overnight in a nylon arena (1 × 1 × 1 M) and were provided water ad libitum. The next day, females were returned to Inwood quarry for assisted mating trials. Females randomly assigned to the local anaesthesia treatment (N = 12) received two 30 µl bilateral injections of 0.5% Marcaine (Bupivacaine HCl) directly lateral to the cloaca between the first and second dorsal scale rows approximately 30 min prior to mating trials [51,52]. Control females (N = 12) received injections of saline instead of Marcaine in the same locations. Immediately after the injections, females were placed in small arenas (45 cm diameter × 75 cm tall) and after 30 min females were placed in a natural aggregation of males within the center of the den. We had determined in experiment no. 1 that female body rolls could dislodge a male and prevent the hemipene from anchoring to the cloaca at intromission. Because we were interested in the female's ability to affect copulation duration via cloacal and/or vaginal control, with the thumb and index finger we held each female approximately 1 cm caudal of the cloaca while males courted her, and then used a blunt probe to lift the ventral scale that covers the opening to her cloaca as soon as a male aligned with the female [53]. Females from both treatments were handled the same. These assisted matings resulted in males everting a hemipene into the female's cloaca after only 1–10 s, so the females were restrained for a very short interval. We released the female once intromission occurred, and the pair then copulated naturally. Therefore, females could roll during copulation after intromission and we noted that some females from each treatment rolled during copulation. When intromission occurred, a stopwatch was started immediately. After 1 min of copulation, the pair was gently moved from the large natural aggregation to a small circular arena (45 cm diameter × 75 cm tall) where they were constantly observed until copulation terminated and the duration was recorded (±10 s). Once copulation was completed, the copulatory plug was removed and plug mass was measured (±0.01 g).
(e). Statistical analysis
The copulation duration and plug mass (matings that produced a plug) satisfied normality and equal variance assumptions so we fit these models using generalized linear models (Gaussian distribution; identity link function) in R. We employed multi-model AICc selection as described in experiment 1. Our initial candidate model included the following parameters: treatment, male and female size (svl), hemipene as main effects variables and male by female size interaction. The top-ranked model of plug mass included treatment, female size (svl) and copulation duration as main effects, and the interaction of copulation duration with treatment. We plotted the plug mass model using the standardized residuals of plug mass given female size to better illustrate the effect of the interaction between treatment and copulation duration on plug mass (figure 5). The analysis and graphing of the best models were conducted in XLSTAT v. 2012.6.02 and/or Sigmaplot v. 11.
Figure 5.
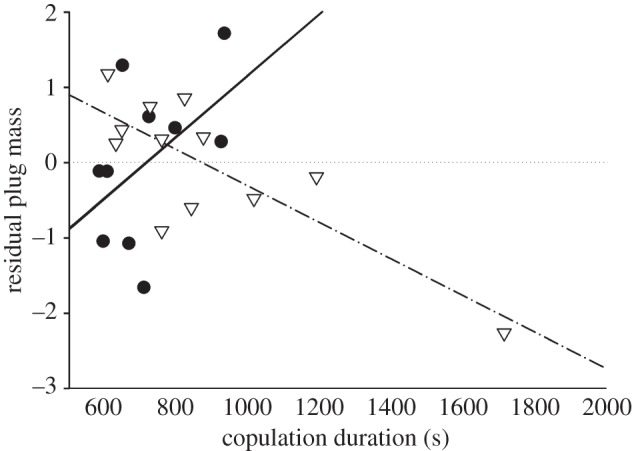
Standardized residuals of plug mass given female snout-to-vent length plotted as a function of copulation duration. This analysis only includes those matings that produced a plug. There are separate linear regressions for each treatment to illustrate the significant interaction between treatment and copulation duration (Marcaine: open triangles, dashed regression line, R2 = 0.641, p = 0.002; saline control: filled circles, solid regression line, R2 = 0.239, p = 0.152).
3. Results
(a). Experiment 1: spine-ablated males
(i). Copulation duration
As predicted, copulation duration was significantly shorter in spine-ablated males than control males, but not different in the first copulation pre-treatment (figure 2). Spine-ablated males (n = 14): copulation duration at first mating (pre-ablation;  ), 855 ± 57 s; at second mating (post-ablation) 249 ± 73 s. Control males (n = 11): copulation duration at first mating
), 855 ± 57 s; at second mating (post-ablation) 249 ± 73 s. Control males (n = 11): copulation duration at first mating  , 819 ± 68 s; at second mating 643 ± 90 s. Eight of the 14 ablated males copulated for less than 1 min and failed to deposit a copulatory plug. Visual inspection of the female cloaca revealed that five of these males did not inseminate the female, but three males did. When spine-ablated males achieved copulation, their hemipene shafts were much further outside the female cloaca compared with matings by intact males, and the hemipene was held in place by the crown of smaller spines in the middle of the hemipene (figure 1).
, 819 ± 68 s; at second mating 643 ± 90 s. Eight of the 14 ablated males copulated for less than 1 min and failed to deposit a copulatory plug. Visual inspection of the female cloaca revealed that five of these males did not inseminate the female, but three males did. When spine-ablated males achieved copulation, their hemipene shafts were much further outside the female cloaca compared with matings by intact males, and the hemipene was held in place by the crown of smaller spines in the middle of the hemipene (figure 1).
Figure 2.
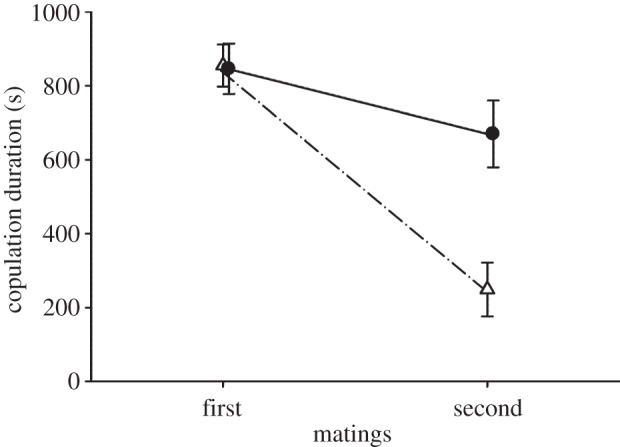
(a) Spine-ablated males (open triangles): copulation duration at first mating (pre-ablation) 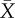 (s.e.m.), 855 s (57 s); at second mating (post-ablation) 249 s (73 s). (b) Control males (filled circles): copulation duration at first mating
(s.e.m.), 855 s (57 s); at second mating (post-ablation) 249 s (73 s). (b) Control males (filled circles): copulation duration at first mating 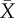 (s.e.m.), 819 s (68 s); at second mating 643 s (90 s).
(s.e.m.), 819 s (68 s); at second mating 643 s (90 s).
(ii). Copulatory plug mass
Only six of 14 spine-ablated males deposited a plug, compared with eight of 11 control males. The likelihood of plug deposition was significantly greater for copulations lasting more than or equal to 5 min than those less than 5 min (14/16 copulations more than 5 min produced a plug, versus 0/9 copulations less than 5 min; χ2 test of independence with Yates’ continuity correction: χ2 = 14.52, p = 0.00014). Copulations of spine-ablated males were more likely to be below the 5 min threshold (8/14 versus 1/10 for the control group; χ2 = 3.94, p = 0.026).
Copulatory plug mass was affected by both copulation duration and treatment. Plug mass increased with copulation duration in spine-ablated males but not in control males; this relationship was primarily driven by the small plugs made by males that copulated for very short periods (figure 3). Because the top-ranked model using the AICc model selection procedure was the intercept only model, we built a model based on the only significant model-averaged parameter, which was the interaction between copulation duration and treatment. We chose the model that included the main effects and the interaction between them (AICc = −56.8, Δi = 4.15; R2 = 0.445). In this model, both copulation duration and treatment are significant predictors of plug mass (see electronic supplementary material, table S1).
Figure 3.
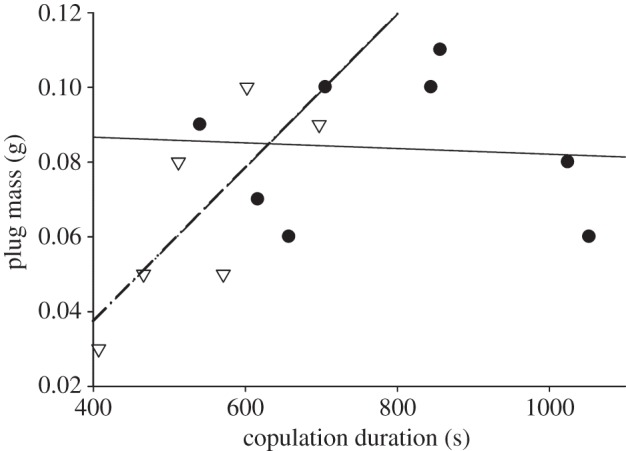
Copulatory plug mass as a function of copulatory duration and treatment. There are separate regressions for each treatment to illustrate the significant interaction between treatment and copulation duration on plug mass (spine-ablated males: open triangles, dashed regression line, R2 = 0.602, p = 0.070; control males: solid circles, solid regression line R2 = 0.006, p = 0.861). Plug mass increases with copulatory duration in ablated males but not in control males.
(b). Experiment 2: anaesthetized females
(i). Copulation duration
Females whose cloaca and vagina were anaesthetized had longer copulation durations than control females. The top-ranked model for the copulation duration data only included ‘Treatment’ as a factor (AICc = 348.4, Δi = 0). When female body rolls were prevented at intromission, and their cloacal and vaginal tissues were anaesthetized (Marcaine treated), copulation duration was significantly longer in anaesthetized (M) than saline-injected (S) females (figure 4; t-test: t22 = 2.374, p = 0.027. Group, 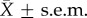 : M, 908 ± 96 s; S, 609 ± 82 s). Five females rolled during copulation (two M-treated and three saline-injected), rolling did not shorten copulation duration in this experiment (ANOVA with rolls nested within treatments, F3,20 = 2.246, p = 0.114), but we may not have the statistical power to detect the effect of rolls on copulation duration.
: M, 908 ± 96 s; S, 609 ± 82 s). Five females rolled during copulation (two M-treated and three saline-injected), rolling did not shorten copulation duration in this experiment (ANOVA with rolls nested within treatments, F3,20 = 2.246, p = 0.114), but we may not have the statistical power to detect the effect of rolls on copulation duration.
Figure 4.
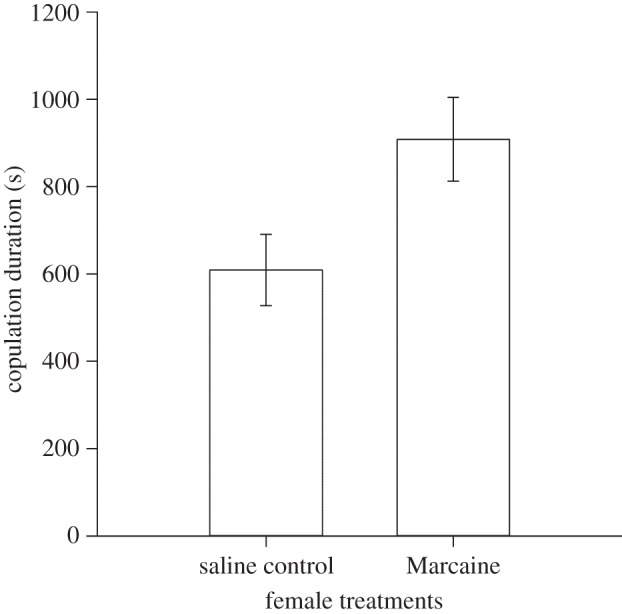
Copulation duration as a function of female treatment in locally anaesthetized females (Marcaine treated) versus saline-injected (control) females. Copulation was much longer in Marcaine-treated females. Bars in the graph represent the s.e.
(ii). Copulatory plug mass
The best fit model for plug mass included treatment, female size (svl) and copulation duration as main effects and the interaction of copulation duration with treatment (AICc = −97.0, Δi = 0, R2 = 0.434), all of which were highly significant (see electronic supplementary material, table S2). Overall, larger females received larger plugs, and the treatments had opposite effects on plug mass. After controlling for the effect of female body size (svl), Marcaine-treated females received smaller plugs as copulation duration increased. In the control females, after controlling for the effect of female body size (svl), longer copulation durations yielded larger plugs (figure 5). The longest copulation duration for the Marcaine-treated females is within the observed range for copulation durations in this species [29,31,34,45,53].
4. Discussion
We found evidence that genital features play a role in sexual conflict interactions over copulation duration and copulatory plug deposition in red-sided garter snake matings. Our experiments revealed that both removal of the hemipene basal hook and local anaesthetization of the female cloaca and vaginal pouch while preventing females from rolling during intromission significantly affected both copulation duration and size of the copulatory plug.
(a). Spine-ablation
As predicted, copulation duration decreased when the hemipenial basal spine was ablated. The decrease was mostly driven by matings of less than 1 min that were typically terminated by female body rolls. These results support our initial hypothesis that the basal spine allows males to gain and maintain intromission despite female behaviours that shorten copulation. Overall, short copulations were less likely to produce copulatory plugs. As predicted, spine-ablated males produced smaller copulatory plugs, and this relationship was primarily owing to very small plugs produced during short copulations.
Although the basal spine helps males maintain intromission despite female body rolls, the spine is not required for males to evert their hemipenes inside the female as suggested by Pisani [37]. This is unsurprising given that the basal spine is absent in non-natricine snakes, which have functional hemipenes [54–58]. However, the spine allowed a better fit between male and female genitalia, because spine-ablated males often appeared to be farther outside the female cloaca (CR Friesen 2009, 2011, & PLR Brennan, 2011, personal observation). Full eversion and inflation of the hemipene takes place a few seconds after copulation begins (CR Friesen 2009, 2011, PLR Brennan, 2011 & RT Mason 1999–2011, personal observation), but males begin inseminating females almost immediately upon eversion and prior to plug formation [11,31]. Males who were unable to make a plug, may therefore still gain some fertilizations, but with the shortened copulation duration in this species versus other snakes [31,59], the plug became necessary to prevent sperm leakage [32] and a potential source of sexual conflict.
Traits involved in sexual conflict may have originally evolved as a result of male–male competition [60]. Male–male competition in red-sided garter snakes is manifested in ‘tail wrestling’ rather than body-combat, and males constantly push their tails in between a female and any other courting male [61]. Many natricine snakes, all of which have basal hemipene spines, mate in aggregations, and have no overt male combat [62]. Having a basal spine may help to quickly initiate copulation during tail wrestling, but also to prevent competitors from interrupting intromission when multiple males’ tails are proximate to the female's cloaca. The evolution of the basal spine allows males to gain more control over copulation duration, forcing females to evolve some counter trait to regain some control, leading to sexually antagonistic coevolution.
(b). Anaesthetized females
Anaesthetization of the female cloaca and vaginal pouch increased copulation duration as we had predicted. In this experiment, we restrained all females (treated and control) during the initiation of copulation to minimize female body rolls during intromission, but females could roll after the hemipene was anchored in place, but this did not affect copulation duration in this study. Thus the increased copulation duration in anaesthetized females was then likely due to relaxation of the musculature of the vaginal pouch and cloaca. This suggests that these structures, in addition to body rolls, are involved in female control of copulation duration. Interestingly, average copulation duration has been reported to be about 840–1176 s [29,34], and in our first experiment, with unassisted mating, copulation duration was 840 ± 43 s. However, in our second experiment, when we prevented females from rolling, copulation duration of control females was much shorter (609 ± 82 s). It is possible that control females used their vaginal and cloacal tissues to make copulations much shorter than if we had allowed normal unassisted copulations to occur because they were prevented from rejecting unwanted males during intromission.
The effect of the interaction between treatment and copulation duration on copulatory plug mass was complex. We predicted that longer copulations would result in increased plug mass and, therefore, that Marcaine-treated females would have larger plugs deposited within them. However, plug mass was smaller than expected in Marcaine-treated females after accounting for female body size, and this deficit increased with copulation duration. This could mean that the cloacal and vaginal tissues provide a surface against which males can pack their copulatory plug material and that anaesthesia relaxes the tissues so that this packing of material is less efficient such that it takes longer to deposit the plug. The size and density of the copulatory plug may depend on those same muscular contractions of the vaginal pouch owing to a change in volume of the pouch and/or time in copulo. Anecdotally, in experiment 1, we observed that most of the plugs produced by spine-ablated males were less formed, less dense and extruded around the hemipene. Those plugs produced during control mating were more solid and compact, perhaps because the tip of the hemipene was further from the vaginal pouch walls, but we did not quantify this variable. Further anecdotal evidence that the vaginal pouch musculature limits plug size comes from a field observation in which a male deposited a plug within the rectum of a female (CR Friesen, PLR Brennan & RT Mason, 2011, personal observation). The plug deposited in the rectum was very large and not densely packed, possibly because the female could not squeeze the rectum to limit space.
It can be very difficult to distinguish between sexual conflict and CFC hypotheses of genital evolution [41], and here we hypothesized that sexual conflict rather than CFC explains the role of the basal spine in increasing copulation duration. Under the CFC hypothesis, the basal spine could be a stimulatory trait to the female. If this were the case, then spine ablation would result in shorter copulation duration because females rejected males that failed to properly stimulate them rather than because males could not remain attached when females were rolling or actively using their vaginal muscles to eject the male as proposed by sexual conflict. According to CFC, when females were anaesthetized (i.e. experimentally ‘blind’ [41]), copulation duration should have been shorter because females could not sense the males’ stimulation and would be more likely to reject them [63], contrary to our findings. The basal spine may cause physical harm to the female genitalia as females often bleed during and after copulation, likely as a result of the basal spine penetrating the cloacal mucosa (CR Friesen 2006–2011 & PLR Brennan, 2011, personal observation). Damage to the female by the basal spine during mating suggests conflict during mating rather than a stimulatory function hypothesized by CFC. Furthermore, the epithelial tissue of the female cloaca in which the basal spine embeds is thickly stratified and keratinized [64], which may be a female counter-adaptation to minimize damage owing to sexual conflict as has evolved in bedbugs with traumatic insemination [65]. Even if males with bigger, more damaging spines achieved longer copulations, this would not represent evidence of a process of female choice, but rather an expected outcome of conflict interactions [60]. We cannot, however, rule out that males may expect resistance and interpreted the anaesthetized females as weakened and unfit leading them to reduce allocation of their ejaculate accordingly, if males mate multiply during the breeding season [66]. Male mate choice is often under-appreciated [67]. Male garter snakes have been demonstrated to preferentially court larger, more fecund females [62,68–72]. There is evidence that males allocate more plug material but not more sperm to larger females [45] and males are significantly less likely to deposit a plug after two matings [32]. Ejaculates can be costly to produce [73,74], and plug production appears to be energetically expensive for male red-sided garter snakes [75]. Given these facts, male ejaculate adjustment with respect to female size and condition deserves further investigation in this species. In this study, we measured copulatory plug mass and copulation duration, but other variables related to plug formation may be important in conflict interactions. For example, the optimal rate at which the plug dissolves would be expected to be higher for females than males but the optimal rate of dissolution may vary with male quality. Females might benefit from the evolution of female enzymes that breakdown the plug matrix to coevolve with the protein composition of the plug, which would allow them to mate sooner after mating within the den, where mating is random with respect to male size. In conclusion, we have documented that manipulation of genital traits changes copulation duration and plug size in a manner mostly consistent with predictions of sexual conflict over mating using a new and tractable model system. These are wild populations of animals so although they are manipulated, they exhibit typical mating behaviours which are difficult to observe in other species of vertebrates. This provides a novel vertebrate model system that is especially suited for manipulation and controlled experiments.
Procedures performed on animals were approved by Yale (IACUC 2009-11290) and Oregon State University (IACUC 2009-11 ACUP-3738), and the research was conducted under permit from Manitoba Conservation (WB1240).
Acknowledgements
We thank Dave Roberts (Manitoba Department of Natural Resources) for logistical support, the residents of Chatfield (especially the Johnson Family) for encouragement and two anonymous reviewers and S. L. Eddy for insightful and helpful comments on a previous version of this manuscript. Any opinions, findings and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Funding statement
This material is based in part upon work supported by the National Science Foundation (IOS-0920344, IOS- 0620125, IOS-1011727).
References
- 1.Arnqvist G, Rowe L. 2005. Sexual conflict. Princeton, NJ: Princeton University Press [Google Scholar]
- 2.Trivers RL. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man (ed. Campbell B.), pp. 136–179 Chicago, IL: Aldine Publishing Company [Google Scholar]
- 3.Parker GA. 1979. Sexual selection and sexual conflict. In Sexual selection and reproductive competition in insects B2: sexual selection and reproductive competition in insects (eds Blum MS, Blum NA.), pp. 123–163 New York, NY: Academic Press [Google Scholar]
- 4.Chapman T, Arnqvist G, Bangham J, Rowe L. 2003. Sexual conflict. Trends Ecol. Evol. 18, 41–47 (doi:10.1016/S0169-5347(02)00004-6) [Google Scholar]
- 5.Mazzi D, Kesäniemi J, Hoikkala A, Klappert K. 2009. Sexual conflict over the duration of copulation in Drosophila montana: why is longer better? BMC Evol. Biol. 9, 132 (doi:10.1186/1471-2148-9-132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersson MB. 1994. Sexual selection, p. 599 Princeton, NJ: Princeton University Press [Google Scholar]
- 7.Pilastro A, Mandelli M, Gasparini C, Dadda M, Bisazza A. 2007. Copulation duration, insemination efficiency and male attractiveness in guppies. Anim. Behav. 74, 321–328 (doi:10.1016/j.anbehav.2006.09.016) [Google Scholar]
- 8.Thornhill R. 1983. Cryptic female choice and its implications in the scorpionfly Harpobittacus nigriceps. Am. Nat. 122, 765–788 (doi:10.1086/284170) [Google Scholar]
- 9.Simmons L. 1987. Sperm competition as a mechanism of female choice in the field cricket, Gryllus bimaculatus. Behav. Ecol. Sociobiol. 21, 197–202 (doi:10.1007/BF00303211) [Google Scholar]
- 10.Daly M. 1978. The cost of mating. Am. Nat. 112, 771–774 (doi:10.1086/283319) [Google Scholar]
- 11.Wilmes AJ, Siegel DS, Aldridge RD. 2011. Premature sperm ejaculation in captive African brown house snake Lamprophis fuliginosus. Afr. J. Herpetol. 60, 177–180 (doi:10.1080/21564574.2011.608384) [Google Scholar]
- 12.Simmons LW. 2001. Sperm competition and its evolutionary consequences in the insects. Princeton, NJ: Princeton University Press [Google Scholar]
- 13.Stockley P. 1997. Sexual conflict resulting from adaptations to sperm competition. Trends Ecol. Evol. 12, 154–159 (doi:10.1016/S0169-5347(97)01000-8) [DOI] [PubMed] [Google Scholar]
- 14.House CM, Simmons LW. 2003. Genital morphology and fertilization success in the dung beetle Onthophagus taurus: an example of sexually selected male genitalia. Proc. R. Soc. Lond. B 270, 447–455 (doi:10.1098/rspb.2002.2266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brennan PL, Prum RO, McCracken KG, Sorenson MD, Wilson RE, Birkhead TR. 2007. Coevolution of male and female genital morphology in waterfowl. PLoS ONE 2, e418 (doi:10.1371/journal.pone.0000418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adler M. 2010. Sexual conflict in waterfowl: why do females resist extrapair copulations? Behav. Ecol. 21, 182–192 (doi:10.1093/beheco/arp160) [Google Scholar]
- 17.Brennan PLR, Clark CJ, Prum RO. 2010. Explosive eversion and functional morphology of the duck penis supports sexual conflict in waterfowl genitalia. Proc. R. Soc. B 277, 1309–1314 (doi:10.1098/rspb.2009.2139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotzy C, Arnqvist G. 2009. Sperm competition favors harmful males in seed beetles. Curr. Biol. 19, 404–407 (doi:10.1016/j.cub.2009.01.045) [DOI] [PubMed] [Google Scholar]
- 19.Yassin A, Orgogozo V. 2013. Coevolution between male and female genitalia in the Drosophila melanogaster species subgroup. PLoS ONE 8, e57158 (doi:10.1371/journal.pone.0057158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans JP, van Lieshout E, Gasparini C. 2013. Quantitative genetic insights into the coevolutionary dynamics of male and female genitalia. Proc. R. Soc. B 280, 20130749 (doi:10.1098/rspb.2013.0749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shine R, O'Connor D, Mason RT. 2000. Sexual conflict in the snake den. Behav. Ecol. Sociobiol. 48, 392–401 (doi:10.1007/s002650000255) [Google Scholar]
- 22.Shine R. 2004. Mechanisms and consequences of sexual conflict in garter snakes (Thamnophis sirtalis, Colubridae). Behav. Ecol. 15, 654–660 (doi:10.1093/beheco/arh058) [Google Scholar]
- 23.Shine R, Elphick MJ, Harlow PS, Moore IT, LeMaster MP, Mason RT. 2001. Movements, mating, and dispersal of red-sided gartersnakes (Thamnophis sirtalis parietalis) from a communal den in Manitoba. Copeia 2001, 82–91 (doi:10.1643/0045-8511(2001)001[0082:MMADOR]2.0.CO;2) [Google Scholar]
- 24.Shine R, Langkilde T, Wall M, Mason RT. 2006. Temporal dynamics of emergence and dispersal of garter snakes from a communal den in Manitoba. Wildl. Res. 33, 103–111 (doi:10.1071/WR05030) [Google Scholar]
- 25.Shine R, Olsson MM, Moore I, LeMaster MP, Greene M, Mason RT. 2000. Body size enhances mating success in male gartersnakes. Anim. Behav. 59, F4–F11 (doi:10.1006/anbe.1999.1338) [DOI] [PubMed] [Google Scholar]
- 26.Joy JE, Crews D. 1988. Male mating success in red-sided garter snakes: size is not important. Anim. Behav. 36, 1839–1841 (doi:10.1016/S0003-3472(88)80126-X) [Google Scholar]
- 27.Bronikowski AM, Arnold SJ. 1999. The evolutionary ecology of life history variation in the garter snake Thamnophis elegans. Ecology 80, 2314–2325 [DOI] [PubMed] [Google Scholar]
- 28.Bronikowski AM. 2000. Experimental evidence for the adaptive evolution of growth rate in the garter snake Thamnophis elegans. Evol. Int. J. Org. Evol. 54, 1760–1767 [DOI] [PubMed] [Google Scholar]
- 29.Shine R, Olsson MM, LeMaster MP, Moore IT, Mason RT. 2000. Are snakes right-handed? Asymmetry in hemipenis size and usage in gartersnakes (Thamnophis sirtalis). Behav. Ecol. 11, 411–415 (doi:10.1093/beheco/11.4.411) [Google Scholar]
- 30.Shine R, Langkilde T, Mason RT. 2003. Cryptic forcible insemination: male snakes exploit female physiology, anatomy and behavior to obtain coercive matings. Am. Nat. 162, 653–667 (doi:10.1086/378749) [DOI] [PubMed] [Google Scholar]
- 31.Shine R, Olsson MM, Mason RT. 2000. Chastity belts in gartersnakes: the functional significance of mating plugs. Biol. J. Linn. Soc. 70, 377–390 (doi:10.1111/j.1095-8312.2000.tb01229.x) [Google Scholar]
- 32.Friesen CR, Shine R, Krohmer RW, Mason RT. 2013. Not just a chastity belt: the functional significance of mating plugs in garter snakes, revisited. Biol. J. Linn. Soc. 109, 893–907 (doi:10.1111/bij.12089) [Google Scholar]
- 33.Perry-Richardson JJ, Schofield CW, Ford NB. 1990. Courtship of the garter snake, Thamnophis marcianus, with a description of a female behavior for coitus interruption. J. Herpetol. 24, 76–78 (doi:10.2307/1564292) [Google Scholar]
- 34.King RB, Jadin RC, Grue M, Walley HD. 2009. Behavioural correlates with hemipenis morphology in New World natricine snakes. Biol. J. Linn. Soc. 98, 110–120 (doi:10.1111/j.1095-8312.2009.01270.x) [Google Scholar]
- 35.Blackburn DG. 1998. Structure, function, and evolution of the oviducts of squamate reptiles, with special reference to viviparity and placentation. J. Exp. Zool. 282, 560–617 (doi:10.1002/(SICI)1097-010X(199811/12)282:4/5<560::AID-JEZ10>3.3.CO;2-A) [PubMed] [Google Scholar]
- 36.Siegel DS, Miralles A, Trauth SE, Aldridge RD. 2011. The phylogenetic distribution and morphological variation of the ‘pouch'in female snakes. Acta Zool. 93, 400–408 (doi:10.1111/j.1463-6395.2011.00514.x) [Google Scholar]
- 37.Pisani GR. 1976. Comments on the courtship and mating mechanics of Thamnophis (Reptilia, Serpentes, Colubridae). J. Herpetol. 10, 139–142 (doi:10.2307/1562795) [Google Scholar]
- 38.Takami Y. 2003. Experimental analysis of the effect of genital morphology on insemination success in the ground beetle Carabus insulicola (Coleoptera Carabidae). Ethol. Ecol. Evol. 15, 51–61 (doi:10.1080/08927014.2003.9522690) [Google Scholar]
- 39.Rodríguez V, Windsor D, Eberhard W. 2004. Tortoise beetle genitalia and demonstrations of a sexually selected advantage for flagellum length in Chelymorpha alternans (Chrysomelidae, Cassidini, Stolaini). New Dev. Biol. Chrysomelidae 2004, 739–748 [Google Scholar]
- 40.Polak M, Rashed A. 2010. Microscale laser surgery reveals adaptive function of male intromittent genitalia. Proc. R. Soc. B 277, 1371–1376 (doi:10.1098/rspb.2009.1720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eberhard WG. 2011. Experiments with genitalia: a commentary. Trends Ecol. Evol. 26, 17–21 (doi:10.1016/j.tree.2010.10.009) [DOI] [PubMed] [Google Scholar]
- 42.Gregory PT. 1974. Patterns of spring emergence of the red-sided garter snake (Thamnophis sirtalis parietalis) in the Interlake region of Manitoba. Can. J. Zool. 52, 1063–1069 (doi:10.1139/z74-141) [Google Scholar]
- 43.Preston DL, Mosley CAE, Mason RT. 2010. Sources of variability in recovery time from methohexital sodium anesthesia in snakes. Copeia 2010, 496–501 (doi:10.1643/CP-09-091) [Google Scholar]
- 44.Nelson RJ, Mason RT, Krohmer RW, Crews D. 1987. Pinealectomy blocks vernal courtship behavior in red-sided garter snakes. Physiol. Behav. 39, 231–233 (doi:10.1016/0031-9384(87)90014-X) [DOI] [PubMed] [Google Scholar]
- 45.Friesen CR, Squire MK, Mason RT. In press. Intra-populational variation of ejaculate traits and sperm depletion in red-sided garter snakes. J. Zool. [Google Scholar]
- 46.R-Core-Team 2013. R: a language and environment for statistical computing, 2.15.3 edn Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 47.Barton K. 2013. MuMIn: multi-model inference. R package 1.9.0 edn Vienna, Austria: CRANR-project.org [Google Scholar]
- 48.Johnson JB, Omland KS. 2004. Model selection in ecology and evolution. Trends Ecol. Evol. 19, 101–108 (doi:10.1016/j.tree.2003.10.013) [DOI] [PubMed] [Google Scholar]
- 49.Anderson DR. 2008. Model based inference in the life sciences: a primer on evidence. New York, NY: Springerverlag [Google Scholar]
- 50.Eddy SL, Kiemnec-Tyburczy KM, Uyeda JC, Houck LD. 2012. The influence of sequential male courtship behaviors on courtship success and duration in a terrestrial salamander, Plethodon shermani. Ethology 118, 1240–1250 (doi:10.1111/eth.12031) [Google Scholar]
- 51.Mendonça M, Crews D. 2001. Control of attractivity and receptivity in female red-sided garter snakes. Horm. Behav. 40, 43–50 (doi:10.1006/hbeh.2001.1665) [DOI] [PubMed] [Google Scholar]
- 52.Mendonça MT, Crews D. 1990. Mating-induced ovarian recrudescence in the red-sided garter snake. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 166, 629–632 (doi:10.1007/BF00240012) [DOI] [PubMed] [Google Scholar]
- 53.Friesen CR, Kerns A, Mason R. Submitted. Sperm competition and mate-order effects in red-sided garter snakes.
- 54.Cliburn JW. 1975. The hemipenis of Pituophis melanoleucus. J. Herpetol. 9, 254–255 (doi:10.2307/1563054) [Google Scholar]
- 55.Mao SH, Yin FY, Guo YW. 1984. The hemipenes of common Taiwanese venomous snakes. Herpetologica 40, 406–410 [Google Scholar]
- 56.Branch WR. 1981. Hemipenes of the Madagascan boas Acrantophis and Sanzinia, with a review of hemipeneal morphology in the Boinae. J. Herpetol. 15, 91–99 (doi:10.2307/1563651) [Google Scholar]
- 57.Keogh JS. 1999. Evolutionary implications of hemipenial morphology in the terrestrial Australian elapid snakes. Zool. J. Linn. Soc. 125, 239–278 (doi:10.1111/j.1096-3642.1999.tb00592.x) [Google Scholar]
- 58.Dowling HG. 1967. Hemipenes and other characters in colubrid classifications. Herpetologica 23, 139–142 [Google Scholar]
- 59.Olsson M, Madsen T. 1998. Sexual selection and sperm competition in reptiles. In Sperm competition and sexual selection (eds Birkhead TR, Møller AP.), pp. 503–578 San Diego, CA: Academic Press [Google Scholar]
- 60.Brennan PL, Prum RO. 2012. The limits of sexual conflict in the narrow sense: new insights from waterfowl biology. Phil. Trans. R. Soc. B 367, 2324–2338 (doi:10.1098/rstb.2011.0284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shine R. 1999. Why do male snakes have longer tails than females? Proc. R. Soc. Lond. B 266, 2147–2151 (doi:10.1098/rspb.1999.0901) [Google Scholar]
- 62.Shine R, Mason RT. 2001. Courting male garter snakes (Thamnophis sirtalis parietalis) use multiple cues to identify potential mates. Behav. Ecol. Sociobiol. 49, 465–473 (doi:10.1007/s002650100334) [Google Scholar]
- 63.Briceño RD, Eberhard WG. 2009. Experimental demonstration of possible cryptic female choice on male tsetse fly genitalia. J. Insect Physiol. 55, 989–996 (doi:10.1016/j.jinsphys.2009.07.001) [DOI] [PubMed] [Google Scholar]
- 64.Siegel DS, Miralles A, Chabarria RE, Aldridge RD. 2011. Female reproductive anatomy: cloaca, oviduct, and sperm storage. In Reproductive biology and phylogeny of snakes (eds Aldridge RD, Sever DM.), pp. 347–409 Enfield, NH: Science Publishers; Marketed and distributed by CRC Press [Google Scholar]
- 65.Morrow EH. 2003. Costly traumatic insemination and a female counter-adaptation in bed bugs. Proc. R. Soc. Lond. B 270, 2377–2381 (doi:10.1098/rspb.2003.2514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wedell N, Gage MJG, Parker GA. 2002. Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 17, 313–320 (doi:10.1016/S0169-5347(02)02533-8) [Google Scholar]
- 67.Bonduriansky R. 2001. The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol. Rev. Camb. Philos. Soc. 76, 305–339 (doi:10.1017/S1464793101005693) [DOI] [PubMed] [Google Scholar]
- 68.Hawley AWL, Aleksiuk M. 1976. Sexual receptivity in the female red-sided garter snake (Thamnophis sirtalis parietalis). Copeia 1976, 401–404 (doi:10.2307/1443979) [Google Scholar]
- 69.Joy JE, Crews D. 1985. Social dynamics of group courtship behavior in male red-sided garter snakes (Thamnophis sirtalis parietalis). J. Comp. Psychol. 99, 145–149 (doi:10.1037/0735-7036.99.2.145) [PubMed] [Google Scholar]
- 70.Shine R, LeMaster MP, Moore IT, Olsson MM, Mason RT. 2001. Bumpus in the snake den: effects of sex, size, and body condition on mortality of red-sided garter snakes. Evolution 55, 598–604 (doi:10.1554/0014-3820(2001)055[0598:BITSDE]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 71.LeMaster MP, Mason RT. 2002. Variation in a female sexual attractiveness pheromone controls male mate choice in garter snakes. J. Chem. Ecol. 28, 1269–1285 (doi:10.1023/A:1016294003641) [DOI] [PubMed] [Google Scholar]
- 72.Shine R, Webb JK, Lane A, Mason RT. 2006. Flexible mate choice: a male snake's preference for larger females is modified by the sizes of females encountered. Anim. Behav. 71, 203–209 (doi:10.1016/j.anbehav.2005.04.005) [Google Scholar]
- 73.Cameron E, Day T, Rowe L. 2007. Sperm competition and the evolution of ejaculate composition. Am. Nat. 169, E158–E172 (doi:10.1086/516718) [DOI] [PubMed] [Google Scholar]
- 74.Thomsen R, Soltis J, Matsubara M, Matsubayashi K, Onuma M, Takenaka O. 2006. How costly are ejaculates for Japanese macaques? Primates 2006, 272–274 (doi:10.1007/s10329-005-0171-7) [DOI] [PubMed] [Google Scholar]
- 75.Friesen CR, Powers DR, Copenhaver PE, Mason RT. In preparation. Energetic costs associated with courtship and mating.


