Abstract
The study sought to determine the prevalence and impact of pain in a nationally representative sample of older adults in the United States (US). Data from the 2011 National Health and Aging Trends Study were analyzed. In-person interviews were conducted in 7,601 adults ages ≥65 years. The response rate was 71.0% and all analyses were weighted to account for the sampling design. The overall prevalence of bothersome pain in the last month was 52.9%, afflicting 18.7 million older adults in the US. Pain did not vary across age groups (P=0.21) and this pattern remained unchanged when accounting for cognitive performance, dementia, proxy-responses, and residential care living status. Pain prevalence was higher in women and in older adults with obesity, musculoskeletal conditions, and depressive symptoms (P<0.001). The majority (74.9%) of older adults with pain endorsed multiple sites of pain. Several measures of physical capacity, including grip strength and lower extremity physical performance, were associated with pain and multisite pain. For example, self-reported inability to walk 3 blocks was 72% higher in participants with than without pain [adjusted Prevalence Ratio=1.72 (95% Confidence Interval: 1.56–1.90)]. Participants with 1, 2, 3, and >4 sites of pain had gait speeds that were 0.01, 0.03, 0.05, and 0.08 meters per second slower, respectively, than older adults without pain, adjusting for disease burden and other confounders (P<0.001). In summary, bothersome pain in the last month was reported by half of the older adult population of the US in 2011 and was strongly associated with decreased physical function.
Keywords: pain, aging, geriatric assessment, locomotor activity, prevalence study
Introduction
Population aging is occurring in nearly every country of the world [48]. Not only are the number and proportion of older adults increasing globally, but the older adult population itself is getting older as well. Gains in life expectancy at older ages have fueled the rapid growth of the oldest-old segment of the population, although it is unclear whether improvements in functional status of older adults have kept pace [14; 20; 21; 52]. Considering that disability in late-life is a major predictor of medical and social service need, investigating risk factors for functional decline is a major public health priority.
Pain is one of the most widely cited symptoms underlying disability among older adults [18; 34; 40]. For instance, in a population-based cohort of moderately-to-severely disabled women, pain was the most commonly endorsed cause of disability in basic activities of daily living (ADLs) (e.g., bathing), instrumental ADLs (e.g., housework), and mobility function (e.g., walking a quarter of a mile) [34]. Although these findings have been observed in other community-based studies of older adults, the epidemiology of pain in older adults is not well established. For example, the overall prevalence of pain estimated in previous studies ranges considerably from 24% to 72% [2; 3; 5; 13; 26; 27; 51; 63]. Further, the age pattern of pain is not well characterized as some studies suggest an increased prevalence with advancing age while others report a flat or decreasing prevalence [28]. Much of the variance in prevalence estimates can be attributed to inadequate sampling of the oldest-old in the community and in residential care settings, and to differences in survey methods and case definitions. The effects of dementia status and cognitive function of respondents on prevalence estimates is unclear and the role of proxy respondents has not been investigated. In addition to the uncertainties in pain prevalence among older adults, the impact of pain has primarily been assessed with self-reported functional outcomes. Relatively few studies have examined the impact of pain using objective, physical performance measures that can capture a wide range of function and that are now used in geriatric patient assessment.
We sought to determine the prevalence and impact of pain in a large, nationally representative sample of older adults in the United States (US). Specifically, the aims of the current study were to (1) determine the overall prevalence of pain according to demographic and health characteristics; (2) determine the prevalence of pain at specific anatomic sites and the total number of pain sites according to age and sex; (3) evaluate the effects of cognitive function, dementia status, residential care status and proxy-respondents on pain reporting and the age-to-pain relationship; and (4) assess the impact of overall pain and multisite pain on grip strength, gait speed and lower extremity physical performance as well as on self-reported function. Considering that the numbers of older adults with multiple chronic conditions are large and will continue to grow, there is a critical need to assess the burden of pain in the older adult population.
Methods
Study Population
The National Health and Aging Trends Study (NHATS) was designed to investigate multiple aspects of functioning in later life and is funded by the US National Institute on Aging, National Institutes of Health [29]. In 2011, a stratified, multistage sampling design was used to enroll 8,245 adults ages 65 and older into the study. The sampling response rate was 71% (8,245/11,637) and the sample, which was drawn from the Medicare enrollment file, represents Medicare beneficiaries living in the contiguous US. Medicare is the national health care insurance program that is used by 96% of all older adults in the US. Written informed consent was obtained from all study participants or their proxy-respondents, and the study protocol was approved by the Johns Hopkins University Institutional Review Board.
In-person interviews, including cognitive and physical performance assessments, were completed by trained survey research staff in the homes of study participants living in the community or in residential care facilities (e.g., retirement or assistive living communities), but not in participants who were residing in nursing homes and who were not expected to return to their original home residence. Therefore, 468 (5.7%) nursing home residents were excluded from the data analysis. Persons in all other residential care settings are represented in the study sample (weights for those with sample person interviews, n=353, were adjusted to represent those who were not interviewed, n=168) [41]. Also excluded were persons missing data on the pain measures (n=5). The final analytic sample size of the current study was 7,601, which is representative of 35.3 million older adults residing in the US. Participants that were excluded from the current study were older (P <0.001) and more likely to be female (P <0.001) than those included.
Measures
During the interview, participants were asked, “In the last month, have you been bothered by pain?” For participants who were too sick and/or unable to communicate (n=579, 7.6%), proxy-respondents were asked, “In the last month, has he/she been bothered by pain?” Those who responded “yes” were asked to report where they had pain in the last month by looking at a card with the following anatomic sites listed: back, hips, knees, legs, feet, hands, wrists, arms, shoulders, stomach, head, and neck. Participants could also specify other sites that were not listed, but this information was not analyzed. Each of the listed anatomic sites as well as the total number of sites were examined. The side of the body where pain occurred was not recorded and therefore pain in the right and left hip, for example, would be counted as a single pain site.
Several measures of physical capacity that represent the building blocks of daily function were included in the study [19; 22]. The ability to do the following 6 pairs of activities in the last month was assessed by self-report: (1) walk 6 blocks (about ½ mile)/walk 3 blocks; (2) walk up 20 stairs/walk up 10 stairs; (3) lift and carry 20 pounds/lift and carry 10 pounds; (4) kneel down without holding on to anyone or anything/bend over without holding on to anyone or anything; (5) put a heavy object on a shelf overhead/reach up over head; and (6) open a sealed jar using hands only/grasp small objects. For each pair, the first activity is generally more challenging than the second; therefore, respondents who were able to do the more difficult activity were not asked about the second, easier activity and were assumed to be able to do it. Those who reported “no” or “don’t know” to the first activity, were asked the second. A composite score of self-reported physical capacity was computed by summing the total number of activities the respondent reported they were able to do. Scores ranged 0 to 12 with higher values indicating greater physical capacity.
Physical performance was also assessed during the home interview. Grip strength was measured in kilograms (kg) by having participants squeeze a dynamometer as hard as they could. The maximum recorded strength from 2 trials was analyzed. Lower extremity function was assessed with the Short Physical Performance Battery (SPPB), which is a widely used summary measure that incorporates standing balance, gait speed, and ability to rise from a chair [23; 24]. For the balance component, participants were asked to stand and maintain their feet in side-by-side, semi-tandem (heel of one foot beside the big toe of the other foot), and tandem (heel of one foot in front of and touching the other foot) positions for 10 seconds each. The more difficult balance tests were not given when a participant was unable to hold an easier test for the full 10 seconds. Gait speed was assessed by having participants walk at their usual pace over a 3 meter course from a standing start. Participants were allowed to use a cane (n=185) or a walker (n=214) if necessary; the assessment and scoring protocols remained the same regardless of whether a walking aide was used. The faster of 2 timed trials was analyzed. Finally, participants were asked to rise from a chair and return to the seated position 5 times as quickly as possible while keeping their arms folded over their chest. The time to complete the 5 chair rises was recorded. All 3 components of the SPPB were scored from 0 to 4, with 0 indicating the inability to complete the test and 4 indicating the highest level of performance. Participants who were able to complete the walking and chair-rise tasks were each scored 1 to 4 based on quartile cut-points from normative data on community-dwelling older adults [24]. The following scores were assigned for the balance component: 0 if participants were unable to hold the side-by-side position for 10 seconds, 1 if participants could only hold the side-by-side standing position for 10 seconds; 2 if they could hold a semi-tandem position for 10 seconds, but were unable to hold a full-tandem position for more than 2 seconds; 3 if they could stand in a fulltandem position for 3 to 9 seconds; and 4 if they could stand in a full-tandem position for 10 seconds. The composite SPPB score is the sum of the balance, walking, and chair-rise subscores and ranges from 0 to 12 possible points with higher values reflecting better lower extremity function. In addition to grip strength and SPPB scores, gait speed was examined in meters per second (m/s) as a separate variable given its salience in daily function and clinical use. All 3 measures are powerful predictors of various adverse outcomes in older adults, including hospitalization, disability and mortality [12; 61].
Cognitive function was assessed using tests of verbal recall (i.e, memory) and orientation [29]. Ten words (common nouns) were read out loud to participants, who were then immediately asked to recall as many words as possible. After an approximately 5-minute delay during which participants answered other survey questions, they were asked again to recall as many of the 10 words as possible. Orientation to the day, date, month, and year was also assessed. The numbers of correct answers to the 3 tests were summed into a composite cognitive performance score, ranging from 0 to 24 possible points (10 points for immediate recall, 10 points for delayed recall, and 4 points for orientation).
A wide range of demographic and health status variables were also collected. Participants were asked to self-identify their race and ethnicity. Socioeconomic position was assessed in terms of the highest grade of education completed. A standard set of questions was used to determine smoking status (i.e., never, former, or current smoker). Body mass index (BMI) was calculated using measured height and weight (BMI = weight in kilograms divided by height in meters squared). Obesity was defined by BMI >30.0 kg/m2. Medical conditions were assessed by asking participants if a doctor has ever told them that they had any of the following: a heart attack or myocardial infarction; high blood pressure or hypertension; arthritis (including osteoarthritis or rheumatoid arthritis); osteoporosis or thinning of the bones; diabetes; lung diseases, such as emphysema, asthma, or chronic bronchitis; a stroke; dementia or Alzheimer’s disease; cancer; a broken or fractured hip (since age 50). Two screening questions from the Patient Health Questionnaire-2 (PHQ-2) were used to assess depressive symptoms (PHQ-2 score >3) [30].
Data Analysis
All analyses were weighted to account for unequal probabilities of selection into the NHATS sample as well as to obtain estimates of pain prevalence and standard errors. Analytic sample weights account for differential selection of subgroups (e.g., oversampling of black individuals and oldest-old adults) and adjust for non-response. Variance estimates [95% confidence intervals (CI)] were calculated using a Taylor series linearization that incorporated the complex sample design of the survey. Data management and statistical analysis were performed with Stata/SE version 12.1 (Stata Corp., College Station, Texas).
Adjusted Wald statistics were used to evaluate differences in pain prevalence estimates across demographic and health characteristics (Tables 1 & 2). Poisson regression was used to estimate prevalence ratios (PR) and 95% CI for comparison of site-specific pain in women versus men (Table 3), to assess the effects of dementia, cognitive function, residential care status, and type of interview respondent on pain reporting (Table 4), and to model the association of pain with the ability to carry out specific, individual functional tasks (Table 5). Logistic regression models were not fitted because odds ratios can be overestimated when the study outcomes are common [59]. Linear regression was used to model the association of pain with grip strength, gait speed, SPPB scores, and self-reported physical capacity composite scores (Table 6). All of the analyses with the physical capacity outcomes (reported in Tables 5 and 6) adjusted for the participants’ demographic and health characteristics that might confound the pain-to-physical capacity relationship. Not only were individual conditions, such as arthritis and depressive symptoms, specified in the model but also the total number of medical conditions was included to account for the potential confounding effect of multimorbidity, overall disease burden. These adjustments provide a more conservative test of the associations of interest. Larger effect sizes were observed in models adjusted for only demographic characteristics (these results are available upon request).
Table 1.
Prevalence of pain according to demographic and health characteristics in adults 65 years and older, United States: National Health and Aging Trends Study, 2011
| Characteristic | No. in the United States with pain |
% Prevalence of pain (95% CI) |
|---|---|---|
| Total in the older adult population | 18,666,000 | 52.9 (51.5–54.3) |
| Age | ||
| 65–69 years | 5,157,000 | 52.3 (49.5–55.1) |
| 70–74 years | 4.566,000 | 51.9 (49.3–54.4) |
| 75–79 years | 3,618,000 | 53.8 (50.8–56.8) |
| 80–84 years | 2,690,000 | 51.9 (49.5–54.2) |
| 85–89 years | 1,795,000 | 56.0 (53.1–58.8) |
| ≥90 years | 840,000 | 56.0 (50.9–61.0) |
| Sex | ||
| Men | 7,137,000 | 46.7 (44.5–48.8) |
| Women | 11,528,000 | 57.7 (56.0–59.5) |
| Race/Ethnicity | ||
| Non-Hispanic White | 15,022,000 | 52.9 (51.4–54.4) |
| Non-Hispanic Black | 1,592,000 | 55.7 (52.4–58.9) |
| Hispanic | 1,265,000 | 53.2 (46.6–59.7) |
| Other | 616,000 | 50.2 (44.8–55.5) |
| Education | ||
| <9 years | 2,053,000 | 56.9 (52.2–61.5) |
| 9–11 years | 2,275,000 | 57.3 (54.0–60.6) |
| High school graduate | 5,180,000 | 53.8 (51.6–56.1) |
| Some college/vocational | 5,057,000 | 55.4 (52.9–57.9) |
| College graduate | 2,145,000 | 47.2 (43.2–51.2) |
| Masters or professional degree | 1,777,000 | 45.0 (39.9–50.1) |
| Smoking history | ||
| Never smoked | 8,613,000 | 51.7 (49.7–53.6) |
| Former smoker | 8,529,000 | 54.8 (52.7–57.0) |
| Current smoker | 1,501,000 | 50.3 (45.7–54.8) |
| Obesity | 5,983,000 | 63.1 (60.2–65.8) |
| Medical conditions | ||
| Arthritis | 13,047,000 | 68.9 (67.1–70.6) |
| Osteoporosis | 4,987,000 | 66.9 (64.4–69.3) |
| Hip fracture | 976,000 | 67.2 (62.3–71.7) |
| Cancer | 5,231,000 | 57.4 (54.8–60.0) |
| Myocardial infarction | 3,041,000 | 61.4 (58.6–64.2) |
| Diabetes | 5,173,000 | 61.5 (59.2–63.8) |
| Hypertension | 12,875,000 | 57.2 (55.6–58.8) |
| Stroke | 2,161,000 | 61.1 (56.3–65.7) |
| Dementia | 1,014,000 | 65.6 (61.4–69.5) |
| Depressive symptoms | 3,514,000 | 68.9 (65.5–72.1) |
| Total No. of medical conditions | ||
| 0 | 813,000 | 25.0 (20.8–29.7) |
| 1 | 2,544,000 | 38.1 (35.4–40.8) |
| 2 | 4,329,000 | 48.1 (45.7–50.5) |
| 3 | 4,650,000 | 60.4 (57.7–63.0) |
| ≥4 | 6,330,000 | 73.4 (71.2–75.5) |
Table 2.
Prevalence of pain in specific anatomic sites, by age group among adults 65 years and older, United States: National Health and Aging Trends Study, 2011
| Pain site | Total (N=7,601) % |
65–69
years (n=1,407) % |
70–74
years (n=1,578) % |
75–79
years (n=1,511) % |
80–84
years (n=1,505) % |
85–89
years (n=951) % |
≥90
years (n=649) % |
|---|---|---|---|---|---|---|---|
| Back | 30.3 | 30.4 | 28.7 | 30.5 | 31.7 | 30.9 | 32.0 |
| Knee | 24.8 | 25.0 | 22.9 | 26.7 | 23.3 | 26.0 | 28.5 |
| Shoulder | 19.9 | 20.9 | 19.4 | 18.2 | 19.6 | 20.4 | 22.4 |
| Hip | 17.7 | 17.5 | 17.8 | 17.4 | 17.9 | 17.9 | 19.1 |
| Foot | 17.7 | 18.5 | 17.9 | 16.1 | 18.3 | 15.4 | 17.2 |
| Hand | 16.8 | 18.6 | 16.3 | 14.6 | 16.4 | 18.0 | 16.1 |
| Neck | 16.0 | 18.0 | 16.3 | 14.9 | 15.2 | 14.6 | 11.6 |
| Head | 9.7 | 10.5 | 9.5 | 9.3 | 9.7 | 9.2 | 7.5 |
| Wrist | 9.8 | 10.9 | 10.2 | 8.6 | 8.8 | 10.4 | 9.0 |
| Leg | 4.0 | 3.2 | 4.1 | 4.5 | 3.7 | 5.6 | 4.4 |
| Stomach | 1.4 | 1.4 | 1.2 | 0.8 | 2.0 | 1.8 | 2.2 |
| Arm | 1.2 | 1.2 | 1.2 | 1.1 | 1.0 | 1.3 | 2.2 |
| Total No. of sites | |||||||
| 0 | 47.1 | 47.7 | 48.1 | 46.2 | 48.1 | 44.1 | 44.0 |
| 1 | 13.3 | 13.0 | 13.3 | 14.0 | 11.9 | 15.1 | 14.4 |
| 2 | 12.0 | 11.9 | 10.4 | 13.1 | 12.9 | 12.7 | 13.4 |
| 3 | 9.2 | 8.1 | 9.9 | 9.8 | 8.6 | 10.1 | 8.9 |
| ≥4 | 18.4 | 19.4 | 18.4 | 16.9 | 18.6 | 18.1 | 19.4 |
Table 3.
Prevalence of pain in specific anatomic sites, by sex among adults 65 years and older, United States: National Health and Aging Trends Study, 2011
| Pain site | Men (N=3,168) % |
Women (N=4,433) % |
Prevalence Ratio (95% CI) Women : Men |
|---|---|---|---|
| Back | 24.7 | 34.6 | 1.40 (1.29–1.51) |
| Knee | 19.8 | 28.7 | 1.45 (1.31–1.60) |
| Shoulder | 16.9 | 22.2 | 1.31 (1.16–1.49) |
| Hip | 14.3 | 20.3 | 1.42 (1.26–1.61) |
| Foot | 14.4 | 20.3 | 1.41 (1.22–1.64) |
| Hand | 12.3 | 20.2 | 1.64 (1.43–1.89) |
| Neck | 12.3 | 18.8 | 1.53 (1.35–1.74) |
| Head | 6.1 | 12.4 | 2.04 (1.66–2.50) |
| Wrist | 7.6 | 11.5 | 1.52 (1.28–1.79) |
| Leg | 3.6 | 4.3 | 1.18 (0.93–1.51) |
| Stomach | 1.1 | 1.6 | 1.42 (0.90–2.22) |
| Arm | 1.0 | 1.4 | 1.42 (0.88–2.29) |
| Total No. of sites | |||
| 0 | 53.3 | 42.3 | 0.79 (0.75–0.84) |
| 1 | 14.6 | 12.4 | 0.85 (0.75–0.97) |
| 2 | 10.5 | 13.2 | 1.25 (1.10–1.43) |
| 3 | 8.2 | 9.9 | 1.20 (1.01–1.43) |
| ≥4 | 13.4 | 22.3 | 1.67 (1.46–1.91) |
CI=Confidence Interval
Table 4.
Association of residential care status, type of interview respondent, cognitive performance, and dementia with pain in adults 65 years and older, United States: National Health and Aging Trends Study, 2011
| Model 1 PR (95% CI) |
Model 2 PR (95% CI) |
Model 3 PR (95% CI) |
Model 4 PR (95% CI) |
Model 5 PR (95% CI) |
|
|---|---|---|---|---|---|
| Residential care status | |||||
| Community | 1.00 | 1.00 | |||
| Residential care resident | 1.06 (0.96–1.18) | 1.00 (0.88–1.12) | |||
| Type of interview respondent | |||||
| Sampled person (self-respondent) | 1.00 | 1.00 | |||
| Proxy respondent | 1.11 (1.02–1.22) | 1.04 (0.94–1.15) | |||
| Cognitive performance | |||||
| Per 1 SD increment | 0.96 (0.94–0.99) | 0.98 (0.95–1.01) | |||
| Dementia | |||||
| No | 1.00 | 1.00 | |||
| Yes | 1.23 (1.15–1.32) | 1.19 (1.09–1.29) |
PR=Prevalence Ratio; CI=Confidence Interval; SD=Standard Deviation
All models were adjusted for age and sex; Model 5 included age, sex, residential care status, interview respondent type, cognitive performance, and dementia
Table 5.
Association of pain and number of pain sites with self-reported inability to independently carry out functional tasks without use of assistive devices in adults 65 years and older, United States: National Health and Aging Trends Study, 2011
| Walk 3 blocks (N=7,132) PR (95% CI) |
Climb 10
stairs (N=7,123) PR (95% CI) |
Carry 10
pounds (N=7,145) PR (95% CI) |
Bend over (N=7,153) PR (95% CI) |
Reach overhead (N=7,149) PR (95% CI) |
Grasp small
objects (N=7,161) PR (95% CI) |
|
|---|---|---|---|---|---|---|
| Pain status | ||||||
| No pain | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Pain | 1.72 (1.56–1.90) | 1.79 (1.60–2.01) | 1.73 (1.50–2.00) | 1.66 (1.46–1.89) | 1.80 (1.54–2.09) | 1.70 (1.29–2.24) |
| No. of pain sites | ||||||
| 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1 | 1.41 (1.24–1.59) | 1.32 (1.13–1.54) | 1.40 (1.15–1.71) | 1.26 (1.07–1.49) | 1.24 (0.97–1.58) | 0.94 (0.54–1.64) |
| 2 | 1.57 (1.38–1.78) | 1.60 (1.36–1.89) | 1.48 (1.23–1.78) | 1.51 (1.24–1.83) | 1.33 (1.06–1.68) | 0.89 (0.53–1.48) |
| 3 | 1.81 (1.59–2.06) | 1.90 (1.57–2.31) | 1.89 (1.57–2.28) | 1.73 (1.49–2.00) | 1.84 (1.52–2.23) | 1.48 (1.01–2.18) |
| ≥4 | 2.05 (1.83–2.30) | 2.28 (2.00–2.61) | 2.11 (1.78–2.50) | 2.11 (1.82–2.45) | 2.59 (2.15–3.12) | 2.90 (2.07–4.07) |
PR=Prevalence Ratio; CI=Confidence Interval
All 12 models were adjusted for age, sex, race/ethnicity, education, smoking history, body mass index, obesity, depressive symptoms, dementia, arthritis, osteoporosis, hip fracture, cancer, chronic lung disease, myocardial infarction, diabetes, hypertension, stroke, and total number of medical conditions
Table 6.
Association of pain and number of pain sites with grip strength, gait speed, the Short Physical Performance Battery, and a self-reported physical capacity scale in adults 65 years and older, United States: National Health and Aging Trends Study, 2011
| Grip strength in kilograms (N=5,941) β (95% CI) |
Gait speed
in meters/second (N=5,926) β (95% CI) |
Short Physical Performance Battery (N=6,274) β (95% CI) |
Self-reported physical capacity (N=7,061) β (95% CI) |
|
|---|---|---|---|---|
| Pain status | ||||
| No pain | Ref. | Ref. | Ref. | Ref. |
| Pain | −0.65 (−1.05, −0.24) | −0.04 (−0.05, −0.02) | −0.64 (−0.81, −0.47) | −1.12 (−1.29, −0.95) |
| No. of pain sites | ||||
| 0 | Ref. | Ref. | Ref. | Ref. |
| 1 | −0.41 (−1.02, 0.20) | −0.01 (−0.03, 0.01) | −0.15 (−0.36, 0.07) | −0.46 (−0.65, 0.27) |
| 2 | −0.24 (−0.81, 0.34) | −0.03 (−0.05, −0.01) | −0.51 (−0.80, −0.22) | −0.72 (−0.96, −0.47) |
| 3 | −0.81 (−1.60, −0.01) | −0.05 (−0.08, −0.02) | −0.78 (−1.09, −0.47) | −1.29 (−1.57, −1.01) |
| ≥4 | −1.35 (−2.04, −0.65) | −0.08 (−0.10, −0.06) | −1.25 (−1.52, −0.99) | −2.16 (−2.46, −1.87) |
β= unstandardized coefficient; CI=Confidence Interval
Each of the 8 models were adjusted for age, sex, race/ethnicity, education, smoking history, body mass index, obesity, depressive symptoms, dementia, arthritis, osteoporosis, hip fracture, cancer, chronic lung disease, myocardial infarction, diabetes, hypertension, stroke, and total number of medical conditions
Results
The overall prevalence of bothersome pain in the last month was 52.9% (95% CI: 51.5–54.3%), afflicting 18.7 million older adults in the US. Table 1 shows the distribution of pain according to major demographic and health characteristics. Notably, there was no difference in pain prevalence across age groups (P = 0.21). Women had a higher burden of pain than men (P<0.001). Pain reporting did not vary by race/ethnicity (P = 0.40), but there was a clear decrease in pain prevalence with higher levels of education (P <0.001). Smoking history was not associated with pain. As expected, pain prevalence was substantially higher in older adults with obesity and musculoskeletal conditions, including arthritis, osteoporosis and hip fracture, as well as in those with depressive symptoms. Importantly, pain increased considerably with greater multimorbidity.
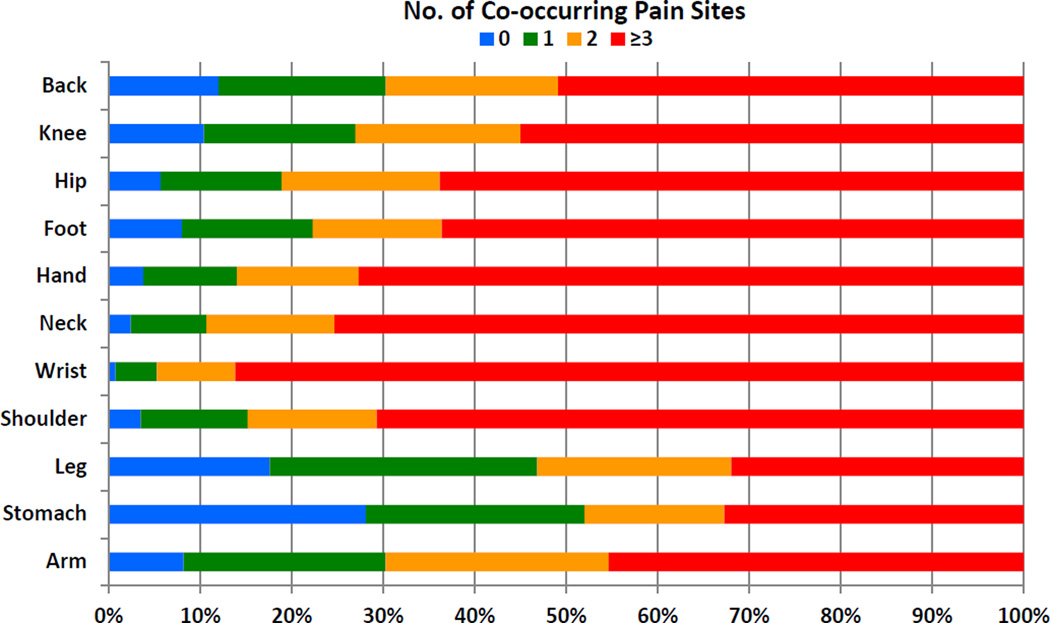
Table 2 shows the prevalence of site-specific pain in the total population and in each age group. Nearly a third of older adults reported back pain, while a quarter had knee pain. Shoulder, hip, foot, hand, and neck were the next most common set of pain sites; stomach and arm were the least common. It is noteworthy that the majority (74.9%) of older adults with pain had multiple sites of pain, and approximately a fifth of all older adults (6.5 million) reported 4 or more sites of pain (Table 2). Indeed, Figure 1 illustrates that pain at any one site is usually accompanied by pain in other sites, typically at 3 or more locations. Among those with pain, 88.9% reported pain in the lower body (back, hips, knees, legs, or feet), while two-thirds (65.8%) reported pain in the upper body regions (head, neck, shoulders, arms, wrists, hands, or stomach). The age-stratified results shown in Table 2 indicate that with the exception of the neck, pain did not vary by age at any of the other sites (all Ps >0.10). The prevalence of neck pain decreased with advancing age (P = 0.02). The distributions of site specific pain and multisite pain stratified by sex are presented in Table 3. Women had a higher prevalence of pain at each anatomic site and a greater total number of pain sites in comparison to men.
Figure 1.
Percentage of co-occurring pain sites among those with the index pain site
The effects of residential care status (versus community-dwelling), type of interview respondent, cognitive function, and dementia status on pain reporting also were examined. All of these results are shown in Table 4 and adjusted for age and sex. There was no association of residential care with pain (Model 1). However, pain prevalence was 11% higher in reports given by proxy-respondents compared with self-respondents (Model 2). The primary reasons reported for using proxy-respondents were because the sample person had dementia, was too ill, and/or had speech or hearing impairment. Better cognitive performance was associated with a decreased probability of pain (Model 3). Older adults with a doctor’s diagnosis of dementia were 23% more likely to report pain than those without dementia (Model 4). When including residential care status, type of interview respondent, cognitive function, and dementia status in the same model (along with age and sex), only dementia status remained significantly associated with pain (Model 5). Interestingly, the association of age with pain remained non-significant (P = 0.69) when adjusting for these factors. That is, pain prevalence remained similar across age groups. Moreover, excluding study participants with dementia did not alter the age-to-pain relationship (P = 0.87), nor did further exclusion of proxy-respondents (P = 0.99).
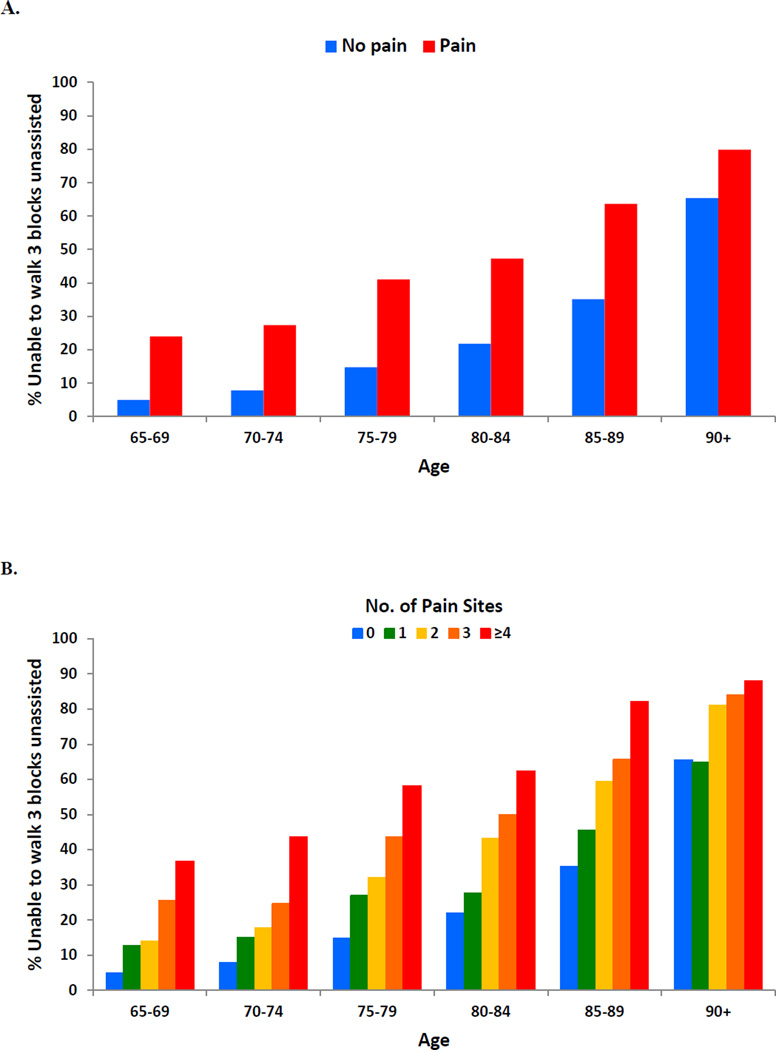
Although there was no age-related pattern to pain reporting among older adults, age is a well-established risk factor for mobility disability as illustrated in Figure 2A. Among older adults without pain, the inability to walk 3 blocks (approximately a quarter mile) increased with advancing age. However, within each age group, older adults with pain had a substantially higher prevalence of mobility disability than those without pain, suggesting that pain is an accelerator of functional decline. For example, the level of mobility disability in 65–69 years old adults with pain (23.9%) is not reached in the non-pain population until ages 80–84 (21.8%). Figure 2B illustrates this effect of pain as well and further shows the impact of multisite pain on mobility disability. The multivariable results examining the association of overall pain and multisite pain on the inability to walk 3 blocks and on 5 other functional tasks are provided in Table 5. For each task, prevalence of disability was 70–80% higher in older adults with pain than in those without pain, adjusting for several demographic and behavioral factors, individual medical conditions, and the total number of medical conditions. In addition, multisite pain had a clear graded effect whereby those with a greater number of pain sites had significantly increased probability of disability (P for linear trend <0.001 for each functional task). Similarly, Table 6 shows that older adults with pain and multiple sites of pain had significantly weaker grip strength, slower gait speed, and poorer overall lower extremity function as measured by the SPPB. Similar disabling effects were observed with the self-reported physical capacity composite measure.
Figure 2.
Parts A–B. Percentage of older adults unable to independently walk 3 blocks according to pain status (A) and number of pain sites (B), by age group
Discussion
In this large, nationally representative sample, “bothersome pain” in the last month was reported by half of the community-dwelling older adult population of the US in 2011 and was strongly associated with decreased physical function. Pain was more common in women and in persons with lower levels of education, chronic musculoskeletal conditions, and greater multimorbidity. There was no variation in pain prevalence across age groups, even when accounting for factors that might affect pain reporting in the oldest-old segments of the population, such as cognitive function and proxy-respondents. The current study provides a comprehensive analysis of contemporary data that reveals a high burden of pain in the older adult population, spanning from the youngest old to the oldest old.
The impact of pain in older adults is substantial. While a number of studies have examined pain with physical function outcomes [9; 13; 17; 36; 40; 42; 43; 50; 51; 53; 57; 58; 62; 63], the current study assessed a broad range of upper and lower extremity function tasks that are considered by many older adults and health care providers as clinically relevant for maintaining independent living. The inability to do some of the most fundamental tasks that underlie daily function was 70–80% more common in older adults with pain than in those without pain. These effects were even more pronounced in those with pain in multiple sites. Indeed, older adults with pain in 3 or 4 or more locations had clinically significant decrements in function. For example, a small but meaningful change and substantial change in usual gait speed in older adults has been estimated using distributional and anchoring methods to range 0.03–0.05 m/s and 0.08–0.10 m/s, respectively [31; 46]. Further, a 0.1 m/s decline in gait speed and 1-point decline in the SPPB over a year were previously shown to be associated with about a 2-fold increased 5-year mortality risk [47]. In the current study, participants with 1, 2, 3, and >4 pain sites had gait speeds that were 0.01, 0.03, 0.05, and 0.08 m/s slower, respectively, than older adults without pain, adjusting for disease burden and other potential confounders (Table 6). Similar clinically significant, large effects were observed with the SPPB. These findings from the NHATS are in line with other, smaller studies of community-dwelling older adults showing that multisite or widespread pain is associated with falls, poorer physical performance, and the onset and progression of disability [1; 9; 17; 33; 35; 36; 43]. Given that 40% of older adults in the current study had multisite pain (74.9% of those who reported pain), there is an urgent need to develop interventions to prevent functional decline in this large, vulnerable population.
Consistent with other studies, women in the current study were more likely to report overall pain and to endorse more sites of pain than men. This sex difference is believed to reflect a complex interplay between biological, psychological and social factors that underlie pain reporting [64]. It is also conceivable that sex differences in pain reporting among older adults reflect an antagonistic feedback loop whereby pain exacerbates disability and vice versa. The higher burden of disability in older women is driven by a combination of higher incidence of disability, lower rates of recovery, and higher probability of survival with disability when compared with older men [25; 37]. Whether the known sex differences in the dynamics of disability in older adults also apply to pain merits further investigation.
In addition to sex differences, there was a clear socioeconomic gradient in pain reporting. Older adults with lower levels of education were more likely to report pain than those with higher levels of education. This likely reflects the cumulative effects of social disadvantage on disease burden over the life course as well as the persistent effects of occupational, work-related injury [32; 38]. Interestingly, there were no major differences in pain reporting by race/ethnicity. Future research should examine whether the impact of pain varies by race/ethnicity and socioeconomic position.
The large number of older adults participating in the NHATS, including 1,600 individuals ages 85 and older, permitted stable estimation of pain prevalence in the oldest old segments of the US population. The results of this study and others indicate that the prevalence of pain in late life does not change with advancing age, including pain in specific anatomic locations (except the neck) [13; 50; 63]. However, it was unclear from prior investigations whether the flat age-pattern to pain reporting was an artifact of cognitive impairment, lack of proxy respondents for those unable to self-report pain, and non-coverage of older adults living in residential care communities or nursing homes. Although some clinical studies in older adults suggest that pain reporting is lower in cognitively impaired persons [39; 45], a recent community-based study showed no difference in pain reporting between cognitively intact and impaired older adults and that the impact of pain on mood and function was similar in both populations [56; 57]. Another study showed that older adults with chronic low back pain have decreased neuropsychological performance compared to controls without pain [66]. In the current study, poorer cognitive performance (on tests of memory and orientation) and self-reported doctor’s diagnosis of dementia were associated with pain, but the age-to-pain relationship remained unchanged adjusting for dementia and cognitive performance as well as proxy-response and residential care status.
The role of medications and polypharmacy in pain reporting among older adults was not examined in the current study, but merits investigation. Research on the relationship of age with pain severity is also warranted as previous work has shown that the prevalence of severe back pain increases with advancing age but prevalence of minor or less severe back pain decreases after the sixth decade of life [16]. Additional research is needed to estimate the effect of pain in nursing home residents on national pain prevalence estimates, although it is unlikely to strongly influence the age-to-pain relationship as a relatively small proportion of the older adult population resides in nursing homes.
As with many national surveys, a limitation of the NHATS is that it did not have a detailed pain assessment that included information on pain severity, duration, or direct measures of interference. Nonetheless, the pain questionnaire item used has good face validity and a 1-month recall period of pain is reasonably reliable [8; 49; 65]. Further, the bothersome pain item was strongly related to depressive symptoms, chronic musculoskeletal conditions, and function, providing some evidence of criterion validity. Location of pain was also assessed and multisite pain was strongly associated with functional outcomes. Interestingly, a study comparing the effects of multisite pain and pain intensity (as measured by the Brief Pain Inventory [10; 11]) in 600 community-dwelling older adults showed that multisite pain and greater pain intensity were each associated with decreased SPPB scores in separate models; however, when both pain variables were entered simultaneously, only multisite pain remained significantly associated with the SPPB [17]. Additional epidemiologic studies in large samples are needed to investigate the characteristics of multisite pain in older adults, including information on medications and pain treatment which, unfortunately, were not available in the current study. A clear strength of the current study is the generalizability of the results as the sampling methods used provided a national sample of community-dwelling older adults, reducing the potential for selection bias [15]. In contrast to many other studies that use postal questionnaires, the mode of data collection was in-person interviewing and the use of proxy-respondents reduced case underascertainment that can bias prevalence estimates of pain [26]. Finally, there was comprehensive assessment of health status and function, including tests of cognitive and physical performance, which permitted a robust analysis on the impact of pain with adjustment for numerous potential confounders.
In summary, pain is a common condition in older adults that is associated with clinically significant decrements in function. The major insights gained from this unique, nationally-representative study are (1) bothersome pain afflicts half of community-dwelling older adults in the US and three-fourths of them have pain in more than one location (multisite pain); (2) pain reporting did not vary by age, even when accounting for dementia, cognitive performance, proxy-response, and residential care status; and (3) bothersome pain, particularly pain in multiple locations, was associated with decreased grip strength, gait speed, and overall lower extremity physical performance as well as decreased self-reported physical capacity. Considering that pain is often undertreated in the older adult population [44; 60], the findings of the current study underscore the need for public health action, including additional epidemiologic research on other geriatric outcomes such as falls and frailty [4; 6; 35; 36; 54].
Summary.
Bothersome pain afflicts half of the community-dwelling US older adult population and is associated significant reduction in physical function, particularly in those with multisite pain.
Acknowledgements
The National Health and Aging Trends Study (NHATS) is sponsored by the National Institute on Aging (grant number NIA U01AG032947) through a cooperative agreement with the Johns Hopkins Bloomberg School of Public Health. The National Institute on Aging had no role in the design and conduct of the current study; management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors thank Professor Judith Kasper of the Johns Hopkins Bloomberg School of Public Health for providing comments on a draft of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: The authors have no conflicts of interest to declare.
References
- 1.Al Snih S, Markides KS, Ray L, Goodwin JS. Impact of pain on disability among older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2001;56(7):M400–M404. doi: 10.1093/gerona/56.7.m400. [DOI] [PubMed] [Google Scholar]
- 2.Andersson HI, Ejlertsson G, Leden I, Rosenberg C. Chronic pain in a geographically defined general population: studies of differences in age, gender, social class, and pain localization. Clin J Pain. 1993;9(3):174–182. doi: 10.1097/00002508-199309000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Bassols A, Bosch F, Campillo M, Canellas M, Banos JE. An epidemiological comparison of pain complaints in the general population of Catalonia (Spain) Pain. 1999;83(1):9–16. doi: 10.1016/s0304-3959(99)00069-x. [DOI] [PubMed] [Google Scholar]
- 4.Blyth FM, Cumming R, Mitchell P, Wang JJ. Pain and falls in older people. Eur J Pain. 2007;11(5):564–571. doi: 10.1016/j.ejpain.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Blyth FM, March LM, Brnabic AJ, Jorm LR, Williamson M, Cousins MJ. Chronic pain in Australia: a prevalence study. Pain. 2001;89(2–3):127–134. doi: 10.1016/s0304-3959(00)00355-9. [DOI] [PubMed] [Google Scholar]
- 6.Blyth FM, Rochat S, Cumming RG, Creasey H, Handelsman DJ, Le Couteur DG, Naganathan V, Sambrook PN, Seibel MJ, Waite LM. Pain, frailty and comorbidity on older men: the CHAMP Study. Pain. 2008;140(1):224–230. doi: 10.1016/j.pain.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Brattberg G, Parker MG, Thorslund M. The prevalence of pain among the oldest old in Sweden. Pain. 1996;67(1):29–34. doi: 10.1016/0304-3959(96)03047-3. [DOI] [PubMed] [Google Scholar]
- 8.Broderick JE, Schwartz JE, Vikingstad G, Pribbernow M, Grossman S, Stone AA. The accuracy of pain and fatigue items across different reporting periods. Pain. 2008;139(1):146–157. doi: 10.1016/j.pain.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryant LL, Grigsby J, Swenson C, Scarbro S, Baxter J. Chronic pain increases the risk of decreasing physical performance in older adults: the San Luis Valley Health and Aging Study. J Gerontol B Psychol Sci Soc Sci. 2007;62(9):989–996. doi: 10.1093/gerona/62.9.989. [DOI] [PubMed] [Google Scholar]
- 10.Cleeland CS, Nakamura Y, Mendoza TR, Edwards KR, Douglas J, Serlin RC. Dimensions of the impact of cancer pain in a four country sample: new information from multidimensional scaling. Pain. 1996;67(2–3):267–273. doi: 10.1016/0304-3959(96)03131-4. [DOI] [PubMed] [Google Scholar]
- 11.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 12.Cooper R, Kuh D, Hardy R. Mortality Review Group; FALCon and HALCyon Study Teams. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467. doi: 10.1136/bmj.c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Covinsky KE, Lindquist K, Dunlop DD, Yelin E. Pain, functional limitations, and aging. J Am Geriatr Soc. 2009;57(9):1556–1561. doi: 10.1111/j.1532-5415.2009.02388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crimmins EM, Beltran-Sanchez H. Mortality and morbidity trends: is there compression of morbidity? J Gerontol B Psychol Sci Soc Sci. 2011;66(1):75–86. doi: 10.1093/geronb/gbq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crombie IK, Davies HT. Selection bias in pain research. Pain. 1998;74(1):1–3. doi: 10.1016/s0304-3959(97)03364-2. [DOI] [PubMed] [Google Scholar]
- 16.Dionne CE, Dunn KM, Croft PR. Does back pain really decrease with increasing age? Age Ageing. 2006;35(3):229–234. doi: 10.1093/ageing/afj055. [DOI] [PubMed] [Google Scholar]
- 17.Eggermont LH, Bean JF, Guralnik JM, Leveille SG. Comparing pain severity versus pain location in the MOBILIZE Boston study: chronic pain and lower extremity function. J Gerontol A Biol Sci Med Sci. 2009;64(7):763–770. doi: 10.1093/gerona/glp016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ettinger WH, Jr., Fried LP, Harris T, Shemanski L, Schulz R, Robbins J. Self-reported causes of physical disability in older people: the Cardiovascular Health Study. CHS Collaborative Research Group. J Am Geriatr Soc. 1994;42(10):1035–1044. doi: 10.1111/j.1532-5415.1994.tb06206.x. [DOI] [PubMed] [Google Scholar]
- 19.Freedman VA, Kasper JD, Cornman JC, Agree EM, Bandeen-Roche K, Mor V, Spillman BC, Wallace R, Wolf DA. Validation of new measures of disability and functioning in the National Health and Aging Trends Study. J Gerontol A Biol Sci Med Sci. 2011;66(9):1013–1021. doi: 10.1093/gerona/glr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freedman VA, Spillman BC, Andreski PM, Cornman JC, Crimmins EM, Kramarow E, Lubitz J, Martin LG, Merkin SS, Schoeni RF, Seeman TE, Waidmann TA. Trends in late-life activity limitations in the United States: an update from five national surveys. Demography. 2013;50(2):661–671. doi: 10.1007/s13524-012-0167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuller-Thomson E, Yu B, Nuru-Jeter A, Guralnik JM, Minkler M. Basic ADL disability and functional limitation rates among older Americans from 2000–2005: the end of the decline? J Gerontol A Biol Sci Med Sci. 2009;64(12):1333–1336. doi: 10.1093/gerona/glp130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guralnik JM, Ferrucci L. Assessing the building blocks of function: utilizing measures of functional limitation. Am J Prev Med. 2003;25(3 Suppl 2):112–121. doi: 10.1016/s0749-3797(03)00174-0. [DOI] [PubMed] [Google Scholar]
- 23.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 25.Hardy SE, Allore HG, Guo Z, Gill TM. Explaining the effect of gender on functional transitions in older persons. Gerontol. 2008;54(2):79–86. doi: 10.1159/000115004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helme RD, Gibson SJ. The epidemiology of pain in elderly people. Clin Geriatr Med. 2001;17(3):417–431. doi: 10.1016/s0749-0690(05)70078-1. v. [DOI] [PubMed] [Google Scholar]
- 27.Jakobsson U, Klevsgard R, Westergren A, Hallberg IR. Old people in pain: a comparative study. J Pain Symptom Manage. 2003;26(1):625–636. doi: 10.1016/s0885-3924(03)00145-3. [DOI] [PubMed] [Google Scholar]
- 28.Jones GT, Macfarlane GJ. Epidemiology of pain in older persons. In: Gibson SJ, Weiner DK, editors. Pain in Older Persons. Seattle: IASP Press; 2005. pp. 3–22. [Google Scholar]
- 29.Kasper JD, Freedman VA. National Health and Aging Trends Study Round 1 Users Guide. Baltimore: Johns Hopkins University School of Public Health; 2012. [Google Scholar]
- 30.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 31.Kwon S, Perera S, Pahor M, Katula JA, King AC, Groessl EJ, Studenski SA. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study) J Nutr Health Aging. 2009;13(6):538–544. doi: 10.1007/s12603-009-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lacey RJ, Belcher J, Croft PR. Does life course socio-economic position influence chronic disabling pain in older adults? A general population study. Eur J Public Health. 2012 doi: 10.1093/eurpub/cks056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leveille SG, Bean J, Ngo L, McMullen W, Guralnik JM. The pathway from musculoskeletal pain to mobility difficulty in older disabled women. Pain. 2007;128(1–2):69–77. doi: 10.1016/j.pain.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leveille SG, Fried L, Guralnik JM. Disabling symptoms: what do older women report? J Gen Intern Med. 2002;17(10):766–773. doi: 10.1046/j.1525-1497.2002.20229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leveille SG, Jones RN, Kiely DK, Hausdorff JM, Shmerling RH, Guralnik JM, Kiel DP, Lipsitz LA, Bean JF. Chronic musculoskeletal pain and the occurrence of falls in an older population. JAMA. 2009;302(20):2214–2221. doi: 10.1001/jama.2009.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leveille SG, Ling S, Hochberg MC, Resnick HE, Bandeen-Roche KJ, Won A, Guralnik JM. Widespread musculoskeletal pain and the progression of disability in older disabled women. Ann Intern Med. 2001;135(12):1038–1046. doi: 10.7326/0003-4819-135-12-200112180-00007. [DOI] [PubMed] [Google Scholar]
- 37.Leveille SG, Penninx BW, Melzer D, Izmirlian G, Guralnik JM. Sex differences in the prevalence of mobility disability in old age: the dynamics of incidence, recovery, and mortality. J Gerontol B Psychol Sci Soc Sci. 2000;55(1):S41–S50. doi: 10.1093/geronb/55.1.s41. [DOI] [PubMed] [Google Scholar]
- 38.Macfarlane GJ, Norrie G, Atherton K, Power C, Jones GT. The influence of socioeconomic status on the reporting of regional and widespread musculoskeletal pain: results from the 1958 British Birth Cohort Study. Ann Rheum Dis. 2009;68(10):1591–1595. doi: 10.1136/ard.2008.093088. [DOI] [PubMed] [Google Scholar]
- 39.Mantyselka P, Hartikainen S, Louhivuori-Laako K, Sulkava R. Effects of dementia on perceived daily pain in home-dwelling elderly people: a population-based study. Age Ageing. 2004;33(5):496–499. doi: 10.1093/ageing/afh165. [DOI] [PubMed] [Google Scholar]
- 40.Melzer D, Gardener E, Guralnik JM. Mobility disability in the middle-aged: cross-sectional associations in the English Longitudinal Study of Ageing. Age Ageing. 2005;34(6):594–602. doi: 10.1093/ageing/afi188. [DOI] [PubMed] [Google Scholar]
- 41.Montaquila J, Freedman VA, Spillman B, Kasper JD. NHATS Technical Paper #2. Baltimore: Johns Hopkins University School of Public Health; 2012. National Health and Aging Trends Study Development of Round 1 Survey Weights. [Google Scholar]
- 42.Mottram S, Peat G, Thomas E, Wilkie R, Croft P. Patterns of pain and mobility limitation in older people: cross-sectional findings from a population survey of 18,497 adults aged 50 years and over. Qual Life Res. 2008;17(4):529–539. doi: 10.1007/s11136-008-9324-7. [DOI] [PubMed] [Google Scholar]
- 43.Muller S, Thomas E, Peat G. The effect of changes in lower limb pain on the rate of progression of locomotor disability in middle and old age: evidence from the NorStOP cohort with 6-year follow-up. Pain. 2012;153(5):952–959. doi: 10.1016/j.pain.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pahor M, Guralnik JM, Wan JY, Ferrucci L, Penninx BW, Lyles A, Ling S, Fried LP. Lower body osteoarticular pain and dose of analgesic medications in older disabled women: the Women's Health and Aging Study. Am J Public Health. 1999;89(6):930–934. doi: 10.2105/ajph.89.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parmelee PA, Smith B, Katz IR. Pain complaints and cognitive status among elderly institution residents. J Am Geriatr Soc. 1993;41(5):517–522. doi: 10.1111/j.1532-5415.1993.tb01888.x. [DOI] [PubMed] [Google Scholar]
- 46.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 47.Perera S, Studenski S, Chandler JM, Guralnik JM. Magnitude and patterns of decline in health and function in 1 year affect subsequent 5-year survival. J Gerontol A Biol Sci Med Sci. 2005;60(7):894–900. doi: 10.1093/gerona/60.7.894. [DOI] [PubMed] [Google Scholar]
- 48.Population Division, Department of Economic and Social Affairs. World Population Ageing, 2009. New York: United Nations; 2009. [Google Scholar]
- 49.Salovey P, Smith AF, Turk DC, Jobe JB, Willis GB. The Accuracy of Memory for Pain -Not So Bad Most of the Time. Am Pain Soc J. 1993;2(3):184–191. [Google Scholar]
- 50.Scudds RJ, Ostbye T. Pain and pain-related interference with function in older Canadians: the Canadian Study of Health and Aging. Disabil Rehabil. 2001;23(15):654–664. doi: 10.1080/09638280110043942. [DOI] [PubMed] [Google Scholar]
- 51.Scudds RJ, Robertson JM. Pain factors associated with physical disability in a sample of community-dwelling senior citizens. J Gerontol B Psychol Sci Soc Sci. 2000;55(7):M393–M399. doi: 10.1093/gerona/55.7.m393. [DOI] [PubMed] [Google Scholar]
- 52.Seeman TE, Merkin SS, Crimmins EM, Karlamangla AS. Disability trends among older Americans: National Health And Nutrition Examination Surveys, 1988–1994 and 1999–2004. Am J Public Health. 2010;100(1):100–107. doi: 10.2105/AJPH.2008.157388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shah RC, Buchman AS, Boyle PA, Leurgans SE, Wilson RS, Andersson GB, Bennett DA. Musculoskeletal pain is associated with incident mobility disability in community-dwelling elders. J Gerontol A Biol Sci Med Sci. 2011;66(1):82–88. doi: 10.1093/gerona/glq187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shega JW, Dale W, Andrew M, Paice J, Rockwood K, Weiner DK. Persistent pain and frailty: a case for homeostenosis. J Am Geriatr Soc. 2012;60(1):113–117. doi: 10.1111/j.1532-5415.2011.03769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shega JW, Ersek M, Herr K, Paice JA, Rockwood K, Weiner DK, Dale W. The multidimensional experience of noncancer pain: does cognitive status matter? Pain Med. 2010;11(11):1680–1687. doi: 10.1111/j.1526-4637.2010.00987.x. [DOI] [PubMed] [Google Scholar]
- 56.Shega JW, Paice JA, Rockwood K, Dale W. Is the presence of mild to moderate cognitive impairment associated with self-report of non-cancer pain? A cross-sectional analysis of a large population-based study. J Pain Symptom Manage. 2010;39(4):734–742. doi: 10.1016/j.jpainsymman.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shega JW, Weiner DK, Paice JA, Bilir SP, Rockwood K, Herr K, Ersek M, Emanuel L, Dale W. The association between noncancer pain, cognitive impairment, and functional disability: an analysis of the Canadian Study of Health and Aging. J Gerontol A Biol Sci Med Sci. 2010;65(8):880–886. doi: 10.1093/gerona/glq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soldato M, Liperoti R, Landi F, Finne-Sovery H, Carpenter I, Fialova D, Bernabei R, Onder G. Non malignant daily pain and risk of disability among older adults in home care in Europe. Pain. 2007;129(3):304–310. doi: 10.1016/j.pain.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 59.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 60.Stewart C, Leveille SG, Shmerling RH, Samelson EJ, Bean JF, Schofield P. Management of Persistent Pain in Older Adults: The MOBILIZE Boston Study. J Am Geriatr Soc. 2012;60(11):2081–2086. doi: 10.1111/j.1532-5415.2012.04197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thomas E, Mottram S, Peat G, Wilkie R, Croft P. The effect of age on the onset of pain interference in a general population of older adults: prospective findings from the North Staffordshire Osteoarthritis Project (NorStOP) Pain. 2007;129(1–2):21–27. doi: 10.1016/j.pain.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 63.Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP) Pain. 2004;110(1–2):361–368. doi: 10.1016/j.pain.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 64.Turk DC, Okifuji A. Psychological factors in chronic pain: evolution and revolution. J Consult Clin Psychol. 2002;70(3):678–690. doi: 10.1037//0022-006x.70.3.678. [DOI] [PubMed] [Google Scholar]
- 65.Von Korff M. Assessment of chronic pain in epidemiological and health services research. In: Turk DC, Melzack R, editors. Handbook of pain assessment. New York: Guilford Press; 2011. pp. 455–471. [Google Scholar]
- 66.Weiner DK, Rudy TE, Morrow L, Slaboda J, Lieber S. The relationship between pain, neuropsychological performance, and physical function in community-dwelling older adults with chronic low back pain. Pain Med. 2006;7(1):60–70. doi: 10.1111/j.1526-4637.2006.00091.x. [DOI] [PubMed] [Google Scholar]