Abstract
The molecular processes involved in establishing long-term potentiation (LTP) have been characterized well, but the decay of early and late LTP (E-LTP and L-LTP) is poorly understood. We review recent advances in describing the mechanisms involved in maintaining LTP and homeostatic plasticity. We discuss how these phenomena could relate to processes that might underpin the loss of synaptic potentiation over time, and how they might contribute to the forgetting of short-term and long-term memories. We propose that homeostatic downscaling mediates the loss of E-LTP, and that metaplastic parameters determine the decay rate of L-LTP, while both processes require the activity-dependent removal of postsynaptic GluA2-containing AMPA receptors.
Keywords: long-term potentiation, decay, homeostatic scaling, metaplasticity, forgetting
1. Introduction
Although some forms of long-term potentiation (LTP) can last for weeks and even months [1–4], eventually most forms of LTP decay. Little is known about mechanisms underpinning the gradual, progressive loss of LTP that may return the synapse to its basal state. We will discuss two forms of LTP (early or E-LTP and late or L-LTP), and what is known currently about how these forms of potentiation may persist. We will then address possible mechanisms that may lead to the reduction of synaptic potentiation and that could therefore mediate the decay of E-LTP and L-LTP. Specifically, we suggest that different forms of homeostatic plasticity will control these phenomena: while the fate of E-LTP is tied to synaptic downscaling, metaplasticity regulates the persistence of L-LTP. We will present a simple model to link receptor trafficking, homeostatic plasticity and the loss of E-LTP and L-LTP and will relate these phenomena to sleep and forgetting.
2. A brief characterization of early and late long-term potentiation
Activity- or experience-dependent long-lasting alterations of synaptic efficacy, such as LTP of synaptic strength at the glutamatergic synapses in CA1 neurons in the hippocampus, has been intensely studied as a possible physiological model of memory. In the CA1 subfield of the hippocampus, stimulating Schaffer collaterals in brain slices in vitro or in intact animals in vivo can induce LTP [5–9]. Depending on the stimulation protocols, LTP can temporally and mechanistically be divided into two phases, the early phase of LTP (E-LTP) and the late phase of LTP (L-LTP). E-LTP is often induced with a weaker induction protocol (such as a single tetanic burst), can decay within one to a few hours, and does not depend on the synthesis of new proteins; conversely, L-LTP can be induced with stronger stimulation protocols (such as multiple tetani in quick succession), lasts at least several hours and requires new protein synthesis [5,10–14]. Several findings that accumulated in recent years suggest that E-LTP may be converted into L-LTP via diverse physiological and/or pharmacological means. These mechanisms could be critical for the transformation of short-lasting forms of memory (i.e. short-term memory, STM) into long-term memory (LTM) [13,15,16]. However, how and why E-LTP decays, how this process compares to the mechanisms involved in the decay of L-LTP, and how E-LTP can be converted into L-LTP remain poorly understood.
Under certain conditions, L-LTP can be transformed back into E-LTP (i.e. a decaying LTP) by a stimulus protocol that induces depotentiation (the depression of a previously established long-lasting potentiation [17]), or by pharmacological inhibition of molecules required for maintaining L-LTP [18,19]. It is interesting to note that, as detailed below, both depotentiation or infusing drugs that target molecular pathways involved in LTM maintenance can induce the decay of L-LTP, which can be prevented by inhibiting the internalization of α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate subtype glutamate receptors (AMPARs) [20,21]. Thus, it is important to highlight the dynamic nature of LTP: E-LTP and L-LTP can be converted to each other depending on many factors and manipulations, some of which we will outline in the following sections.
3. Mechanisms of long-term potentiation maintenance
Long-lasting enhancements of synaptic strength, such as LTP, are a central component of memory representations. The formation and stability of LTP depends on the modulation of the number and subunit composition, and thus the properties, of AMPARs. The N-methyl-d-aspartate subtype glutamate receptor (NMDAR) emerged as a core element in regulating AMPAR composition and trafficking. NMDAR activation can lead to an increase in intracellular levels of calcium, which activates several relevant signalling cascades that, to name a few, involve protein kinases (e.g. CaMKII, protein kinase C (PKC), etc.), transcription factors (e.g. CREB, C/EBP), translation initiation factors (e.g. elF4E) and growth factors (e.g. BDNF) [22,23].
The mechanisms involved in establishing changes in potentiation are, however, not sufficient for sustaining these enhancements in synaptic strength [24]. The view that, once established, biologically active processes are not required to maintain these alterations in synaptic efficacy started changing in recent years [25]. Findings implicating the constitutively active atypical PKC isoform M-zeta (PKMζ) in the persistence of L-LTP and several forms of consolidated LTM [18,26,27] gave rise to the antithetic idea that continuously engaged maintenance processes are necessary to preserve changes in synaptic potentiation [21,28].
LTP induction as well as memory formation leads to de novo PKMζ synthesis and increases in PKMζ levels can be detected about 1 h after LTP induction [24,29]. PKMζ could promote its own synthesis, and this could be one of the mechanisms that permit memories to persist over the long term, probably also involving, at least for some forms of memory, epigenetic mechanisms [30–32]. Further support for a central role for PKMζ in LTM maintenance comes from studies showing that overexpressing PKMζ enhances weak LTMs, while overexpressing a dominant-negative PKMζ mutation blocks memory persistence [27]. Transient pharmacological inhibition of PKMζ activity with infusions of the peptide ZIP (zeta inhibitory peptide) impairs LTP and can abolish fully established memories, even remote memories that are several months old [33,34]. Once ZIP is metabolized, the memory loss persists, but new LTMs can be stored again [35].
The role of PKMζ in LTM maintenance has been called into question recently. Findings from preparations in which PKMζ was genetically deleted challenge the exclusive role of PKMζ in supporting LTP and LTM. In these developmental and inducible PKMζ knockout mice, neither LTP nor LTM formation was impaired [19,36]. Importantly, infusions of ZIP abolished LTM in these animals, suggesting that ZIP might be less selective than originally assumed. Indeed, PKMζ and PKCι/λ share almost identical nucleotide sequences; the pseudosubstrate sequence is the same in both, and consequently ZIP will likely inhibit PKMζ as well as PKCι/λ [26]. Together with earlier studies, these results suggest that other PKC/PKM isoforms, such as PKCι/λ, either provide functional compensation when PKMζ is unavailable, or that, along with PKMζ and possibly other proteins, these kinases also might be critically involved in memory maintenance [37,38].
AMPARs that contain the GluA2 subunit (GluA2/AMPARs) form a central component of LTM representations. LTM strength for auditory fear conditioning positively correlates with GluA2 but not GluA1 levels in the basolateral nucleus of the amygdala [21]. Especially, the GluA2 subunit seems critical for regulating postsynaptic expression of AMPARs. Binding motifs critical for activity-dependent AMPAR endocytosis and internalization have been identified on the C-terminal of GluA2, or play a role in endosomal sorting of internalized receptors, promoting either the degradation or extra-synaptic reinsertion of AMPARs [17,39–41]. Several findings suggest that PKMζ might participate in mechanisms that regulate the postsynaptic expression of GluA2/AMPARs. Infusion of PKMζ can enhance postsynaptic AMPAR currents, and, during the maintenance phase of L-LTP, PKMζ appears to promote postsynaptic insertion of GluA2/AMPARs [42]. PKMζ could thus be part of a mechanism that prevents activity-dependent endocytosis of GluA2/AMPARs, which seems to be a central mechanism of LTM maintenance. Clathrin-mediated GluA2-dependent endocytosis of AMPARs can be prevented with the GluA23Y peptide, which comprises a tyrosine-rich binding motif on the carboxyl tail of the GluA2 subunit and thus competitively prevents receptor endocytosis [43,44]. During LTD, the molecule BRAG2 binds to this motif, which eventually leads to the internalization of AMPARs [39]. The loss of LTM and L-LTP typically observed after infusing ZIP to target PKMζ can be prevented when GluA23Y is applied prior to ZIP infusions [21]. These findings suggest that PKMζ participates in regulating AMPAR trafficking by actively restricting synaptic removal of GluA2/AMPARs, which might be a crucial maintenance mechanism for both L-LTP and LTM.
Taken together, these findings lend strong support to the notion that long-lasting changes in synaptic efficacy, which most probably form the neural basis of LTM, require continuous, active maintenance in order not to perish, as transient disruption of key components of this maintenance mechanism swiftly abolish established L-LTP and many forms of fully consolidated, even very remote LTM.
4. Mechanisms of long-term potentiation loss
It has been suggested that LTP decay may reflect the effects of protein turnover or the activity of phosphatases that target proteins required for LTP maintenance, reversing the effects of LTP induction that are required to maintain synaptic potentiation [10,45–47]. On the other hand, some studies suggest that LTP decay may involve NMDAR activation, which could trigger mechanisms that overlap with signalling pathways involved in long-term depression (LTD) and depotentiation [15,20,48–51]. In other words, it may be constitutive processes that actively cause the decay of LTP, and perhaps the loss of LTM [28].
One of the earliest findings suggesting that the decline of LTP over time may depend on processes involving glutamate receptor activation comes from a study involving chronic systemic application of the competitive NMDAR antagonist CPP [15]. LTP was induced with a theta burst protocol at synapses of the perforant path in freely moving animals. Daily systemic administration of CPP was started either 1 h or 2 days after LTP induction, and in both cases the NMDAR antagonist prevented LTP decay. Studies in slices obtained from juvenile rats showing that blocking NMDARs with the competitive antagonist AP5 prevents the decay of E-LTP suggest that similar mechanisms are involved in the decay of both L-LTP and E-LTP [52]. Similarly, systemic daily injections of CPP after radial arm maze training prevented forgetting of spatial memory that occurred over 5 days [15]. These findings suggest that LTP decay, like LTP induction, requires activation of NMDARs, and that both processes are well-regulated forms of synaptic plasticity. Furthermore, it seems that a similar active process may be involved in some forms of forgetting of LTM.
Both depotentiation and LTD lead to a reduction of synaptic potentiation, and both require NMDAR activation [53,54]. These two physiological phenomena are thus obvious candidates for mechanisms that may mediate LTP decay over time. In accordance with the role of AMPARs in persistence of L-LTP and LTM, both LTD and depotentiation lead to reduced AMPAR levels at postsynaptic sites [17,20,39,55]. For slice preparations, successful depotentiation of LTP has mostly been reported when homosynaptic low-frequency stimulation was applied shortly after LTP induction [56], which would suggest that depotentiation may be of limited use as a possible mechanism for the gradual decay of L-LTP over longer time periods. However, recent studies addressing the neurobiological mechanisms underpinning extinction, i.e. the suppression, or attenuation of a previously acquired conditioned response, suggests that depotentiation can occur long after long-lasting synaptic enhancements were induced [20,49].
In these studies, animals were submitted to extinction training, in which they learn that a conditioned stimulus (tone) that originally had been paired with a reinforcer (electric footshock) no longer predicts its occurrence. This way, animals learn to no longer express the conditioned response (freezing) upon presentation of the conditioned stimulus. This absence of a response following extinction training is often classified as a form of forgetting because the acquired behaviour is not expressed anymore. Earlier studies have demonstrated that fear conditioning goes along with an increase of synaptic potentiation in the lateral amygdala, and that during extinction training these enhancements are reversed [57]. Similarly, inducing depotentiation with low-frequency stimulation in the lateral amygdala after fear-potentiated startle training can reduce the fear response in a later test [58].
Extinction and depotentiation have been strongly linked more recently [20]. In this study, animals were sacrificed 3 days after auditory fear conditioning, then slices were prepared for ex vivo depotentiation of lateral amygdala synapses that had been strengthened during fear conditioning. Paired-pulse low-frequency stimulation of the potentiated pathways in the lateral amygdala reduced the synaptic responses, and this effect required activation of GluN2B-containing NMDARs, as it was blocked by AP5 [20] as well as Ro25-6981, a highly selective GluN2B-specific antagonist [49]. After auditory fear conditioning, GluA2 levels increased more so than GluA1 expression in the lateral amygdala, and depotentiation reduced the amount of GluA2 and GluA1 in synaptosomes prepared from lateral amygdala tissue samples down to the level found in naive animals [20]. Importantly, competitively preventing clathrin-dependent GluA2-mediated endocytosis of postsynaptic AMPARs in the lateral amygdala with the peptide GluA23Y prevented depotentiation and extinction [20]. Depotentiation thus affected postsynaptic AMPAR expression in this model, and their reduction accompanied the absence of the conditioned response, a principal relation that has been replicated for the loss of conditioned fear following ZIP infusions to block PKMζ in the basolateral amygdala [21].
Despite these demonstrations, it is not yet clear whether processes akin to LTD or depotentiation give rise to the reduction of potentiation observed after extinction, as both mechanisms have been implicated in this behavioural phenomenon [20,48]. Both LTD and depotentiation will lead to a reduction of postsynaptic AMPAR expression, and the molecular pathways involved seem to converge on GluA2-mediated synaptic removal of AMPARs [20,39,43,44,48]. In the light of the finding that blocking NMDARs prevents LTP decay [15], it seems that NMDAR activation will be required to activate the pathways that promote GluA2-dependent receptor internalization.
Several potential pathways have been described that could be involved in this phenomenon [39,55,59]. NMDAR activation following low-frequency stimulation to induce LTD leads to the rise of intracelluar Ca2+ levels, which activates the phosphatases calcineurin and PP1. These then dephosphorylate PIP5Kγ661. Dephosphorylated, PIP5Kγ661 can associate with AP2 at postsynaptic sites, and this produces PI(4,5)P2, which promotes AMPAR endocytosis through the recruitment of AP2 to postsynaptic endocytic areas [55,60]. The dephosphorylation of PIP5Kγ661 appears as the key step in initiating AMPAR endocytosis following NMDAR activation, and PIP5Kγ661 could thus be the target of various pathways leading to the synaptic removal of AMPARs.
The molecule BRAG2 emerged as a key component involved in the final steps of synaptic AMPAR removal. BRAG2 could represent another common endpoint element of different pathways that promote AMPAR internalization, because its role in reducing synaptic AMPAR expression does not depend on the induction pathway [39]. BRAG2 interacts with the tyrosine-rich 3Y sequence on the C-terminal of the GluA2 subunit, and this interaction, which amplifies the catalytic activity of BRAG2, promotes the formation of clathrin-coated vesicles, thus permitting receptor endocytosis. The peptide GluA23Y seems to prevent GluA2/AMPAR endocytosis by restricting the binding of BRAG2 to GluA2 [39]. Importantly, the phosphorylation state of the 3Y motif regulates this process, such that, depending on the particular form of LTD, AMPAR endocytosis during the expression of LTD can be promoted by either phosphorylation [43,61] or dephosphorylation [39] of Tyr876 of the 3Y sequence. It thus seems that the future of synaptic potentiation, i.e. whether it decays or persists, may critically depend on processes that affect phosphorylation and dephosphorylation of the 3Y sequence of GluA2.
Once AMPARs are internalized, they will either be reinserted back into the plasma membrane or undergo lyosomal or proteasomal degradation. PICK1, which seems to play a central role in LTD [62,63], is one of the core elements in the process that determines the fate of internalized AMPARs. The influx of Ca2+ following NMDAR activation changes the configuration of PICK1, which promotes, in conjunction with PKC-mediated phosphorylation of Ser880 on GluA2, the binding of PICK1 to internalized GluA2/AMPARs. This then interferes with the reinsertion of GluA2/AMPARs into the extra-synaptic membrane, and thus contributes to the loss of synaptic potentiation [59,64].
Whether LTP decays or persists may thus depend critically on NMDAR activation. These receptors have been implicated as key elements of homeostatic processes, i.e. metaplasticity and synaptic scaling, that control synaptic potentiation dynamics.
5. Homeostatic processes and decay of synaptic potentiation
Out of the many possible mechanisms involved in homeostatic regulation of synaptic potentiation, two seem more relevant to the question why E-LTP and L-LTP may decay over time. Given the temporal dynamics, synaptic downscaling, operating on the time scale of hours (and sometimes days), appears as a possible process involved in the decay of E-LTP. The loss of L-LTP that sets in days after LTP induction rather might be controlled by metaplastic mechanisms. Importantly, both synaptic downscaling and metaplastic downregulation lead to reductions of synaptic potentiation and thus will be likely to involve activity-dependent synaptic removal of GluA2-containing AMPARs, engaging some of the molecular pathways discussed briefly above.
Homeostatic synaptic downscaling can be observed after persistently enhanced synaptic stimulation. Heightened synaptic activity can increase intracellular Ca2+ levels and affect trafficking of GluA2/AMPARs [65]. Although the term itself implies that downscaling might be a heterosynaptic phenomenon affecting all synapses of a neuron, a recent elegant study demonstrates that downscaling might also operate in an input-specific, i.e. homosynaptic manner. Using an optogenetic approach to persistently increase presynaptic glutamate release at only specific synapses, downscaling was found only at stimulated terminals, but not at unstimulated neighbouring synapses [66]. This synapse-specific synaptic downscaling led after 15 min of stimulation to reduced postsynaptic surface expression of GluA1- and GluA2/3-containing AMPARs at the stimulated synapses. This effect required NMDAR but not AMPAR activation [65,66]. Importantly, the ubiquitin–proteasome system was recruited for AMPAR downregulation (see also [67]), but activation of calcineurin or CaMKII was not required [66]. Synaptic downscaling further does not involve PICK1 to regulate postsynaptic AMPAR levels [59,68]. Taken together, these characteristics seem to suggest that synaptic downscaling may not involve LTD-like processes, but that both LTD and synaptic downscaling may share some initial steps in pathways that soon diverge; it also seems likely that they will converge again for receptor endocytosis. The receptors internalized during synaptic downscaling seem to undergo proteosomal degradation, instead of intracellular retention. Downscaling following enhanced synaptic stimulation leads to expression of the immediate early gene Homer1a, which engages an mGluR1-activation-dependent pathway that leads to the dephosphorylation of tyrosine on GluA2 [69]. As discussed above, BRAG2-mediated GluA2 internalization requires dephosphorylation of Tyr876 [39], and thus it is likely that downscaling, like LTD and depotentiation, will engage similar mechanism to internalize AMPARs.
The observation that neuronal activation history can affect the direction of future plasticity in visual cortex gave rise to the concept of metaplasticity, a theory about the ‘plasticity of plasticity’ [70,71]. According to this model, a moving modification threshold determines the direction of synaptic plasticity, in that, depending on its current setting, the same amount of synaptic stimulation can induce processes that increase or decrease synaptic potentiation, such as LTP, LTD and depotentiation. For example, prolonged stimulation can set the modification threshold to a point at which the very stimulation frequencies that had induced LTP will no longer increase synaptic potentiation, but might in fact cause synaptic depression [72,73]. This way, runaway potentiation or reversal of potentiation due to feed-forward mechanisms inherent to LTP and LTD can be averted, in order to prevent eventual synaptic saturation or silencing [74]. On the other hand, the threshold might be set such that stimulation prevents most forms of synaptic plasticity, and thus ‘locks’ the synapse at a certain level of potentiation, which could be a mechanism to preserve critically important memories [75].
NMDA receptors have emerged as major regulators of metaplastic processes. These receptors are the main source of postsynaptic Ca2+ influx in neurons. The magnitude of Ca2+ change upon NMDAR activation determines the direction of changes in synaptic potentiation, in that small rises in Ca2+ promote LTD, while higher influx of Ca2+ leads to LTP. NMDARs are heterotetramers and are found in the mammalian forebrain in different configurations, of which GluN1–GluN2A and GluN1–GluN2B are the most prevalent. Owing to their slower decay kinetics (e.g. 1 ms application of glutamate results in about six times longer channel currents for GluN1–GluN2B than for GluN1–GluN2A receptors) [76], GluN2B-containing NMDARs enable a greater inward flow of Ca2+ than those containing the GluN2A subunit [77,78]. Subunit composition also determines the binding affinity for glutamate, which is higher for GluN2B than for GluN2A-containing NMDARs [79].
Thus, GluN2B could determine the direction of synaptic plasticity to a greater extent than GluN2A, and the proportion of postsynaptic (and possibly extra-synaptic) GluN2B/NMDARs could contribute significantly to a synapse's metaplastic threshold, determining the future development of a synaptic connection. For example, prolonged strong synaptic activity or very strong behavioural training leads to an overall reduction of GluN2B, in that relatively more GluN2A than GluN2B-containing NMDARs will be expressed at postsynaptic sites [80–83]. Similarly, persistently increasing the relative number of GluN2A relative to GluN2B receptors by genetically overexpressing GluN2A-containing NMDARs in the forebrain of rats effectively abolished one form of LTD [84].
The overall quicker deactivation of the NMDAR population due to a higher proportion of GluN2A-containing receptors will make Hebbian learning less likely because of the shortened period for coincidence detection. In order to further increase synaptic strength at these synapses, stronger stimulation will thus be required than before because the Ca2+ influx upon NMDAR activation has been reduced owing to the relative drop in GluN2B- compared to GluN2A-containing NMDARs. At the same time, stimulation intensities that previously induced LTP or that previously had no effect on synaptic potentiation now may induce LTD [85]. Thus, the speed of LTP decay might depend on the proportion of GluN2B to GluN2A receptors at postsynaptic sites. For example, their ratio could moderate the probability that synaptic connections change and thus prevent, for example, the loss of ‘critical’ or ‘strong’ memories. Imprinting memories are a good example of such acquired knowledge that lasts a lifetime. Imprinting memory in chicks can be acquired only during a brief critical period, which correlates with GluN2B expression in the core region of the hyperpallium densocellulare, one of the brain regions critical for imprinting memory. Here, imprinting increases GluN2B expression, but at the end of the critical period the proportion of GluN2A-containing receptors is higher than the proportion of GluN2B/NMDARs [86]. This may protect these essential memories from future modification and may reduce or eliminate the impact of constitutive decay processes.
If metaplastic processes could play a role in establishing normal, i.e. constitutive forgetting rates, they may also play a role in pathological forgetting, as seen, for example, in Alzheimer's disease (AD). The early stages of AD, which are characterized by mild cognitive dysfunctions, such as increased forgetfulness, could reflect to some degree altered metaplastic processes. Changes in metaplasticity may be associated with altered GluN2B expression, as suggested by a pharmacological rat model of AD in which chronic systemic administration of AlCl3 in combination with a single amyloid-β (Aβ) injection into the lateral ventricle was used to establish AD pathology (i.e. increased soluble amyloid expression and apoptosis in hippocampus and disease-typical behavioural deficits) [87]. Compared to control animals, the AD rats presented with increased levels of GluN2B in the hippocampus, while GluN2A expression was not affected. This synaptic configuration of NMDAR subtypes could set a metaplastic state that makes it more likely that certain stimulation frequencies induce LTD than LTP. This can have two consequences. First, it might become more difficult to form lasting memories, i.e. convert E-LTP into L-LTP. Second, alterations in the proportion of GluN2B to GluN2A found in AD might promote the decay of established L-LTP, thus increasing the forgetting rate of LTM.
Both possible outcomes have been reported in animal AD models. Given conditions that normally block LTD induction (i.e. when NMDAR currents are inhibited by the antagonist AP5), Aβ overexpression in hippocampal slices permits the induction of LTD. In order to block NMDAR-dependent LTD induction in this preparation, higher concentrations of a more potent NMDAR antagonist (D-AP5) are necessary [88]. Changes in the expression of certain NMDAR subtypes are, however, only one of several possible mechanisms that might contribute to the behavioural and cellular phenotype of AD. For example, Aβ also leads to higher glutamate levels, possible due to compromised reuptake mechanisms, and excess glutamate could diffuse out of the synaptic cleft, thus activating extrasynaptic NMDARs, which could further promote induction of LTD [53,88]. The other possible consequence of altered NMDAR subunit expression in AD, i.e. accelerated forgetting, was found in the PDAPP mouse model of AD. When trained to meet the same performance criteria as wild-type control mice, PDAPP mice forget the location of a hidden platform in the watermaze task much quicker than their wild-type counterparts [89]. It is, however, not yet clear whether stronger forgetfulness in this model reflects quicker memory decay due to metaplastic conditions that favour LTD (or depotentiation), or whether memories are lost at higher rates because changes in NMDAR expression promote other computational deficits, such as reduced pattern separation in the hippocampus, which could make memory loss by interference more likely [28,89,90].
6. A simplified, preliminary model of long-term potentiation decay
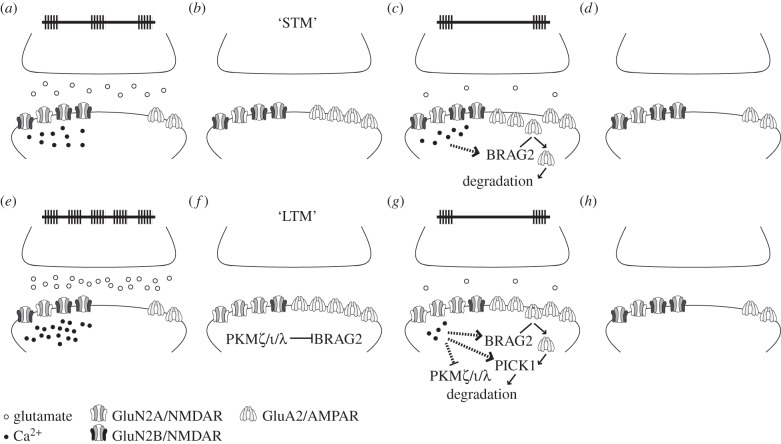
Taken together, the findings discussed so far suggest to us the following simplified model to account for the decay of E-LTP and L-LTP, in which we at the present time do not consider epigenetic contributions, although they will most likely be involved (figure 1). Weak stimulation protocols typically lead to E-LTP, a form of LTP that decays over the course of some hours. In terms of memory systems or memory types, E-LTP might thus be the physiological phenomenon that could correspond to STM. Unlike L-LTP, which might correspond to LTM, E-LTP does not engage cellular mechanisms typically associated with synaptic consolidation [24,26]. Homeostatic downscaling mechanisms that engage after synaptic stimulation will lead to the loss of postsynaptic AMPARs, involving BRAG2-mediated synaptic removal of GluA2, as outlined above. Although the role of NMDARs in this downscaling process is not entirely clear, findings showing that the decay of E-LTP can be prevented by blocking NMDARs suggest a possible involvement of NMDAR activation [47,52,91].
Figure 1.
Simplified model to account for E-LTP and L-LTP decay. (a–d) Weak stimulation protocols induce a short-lasting form of LTP (E-LTP) that decays within hours after induction, owing to homeostatic downscaling. (b) After induction, increases in postsynaptic AMPAR expression reflect increased synaptic potentiation, which might correspond to the concept of STM. (c) Over time, stimulation-induced (or spontaneous) glutamate release activates NMDARs, which leads to Ca2+ influx that stimulates pathways leading to AMPAR endocytosis and degradation. (d) Eventually, these processes can return the synapse to its basal state. (e–h) Strong stimulation protocols induce a long-lasting form of LTP (L-LTP). Its stability depends on metaplastic parameters that are partly reflected by the relative amount of synaptic GluN2A and GluN2B. (f) This type of stimulation leads to the synthesis and synaptic recruitment of PKMζ and possibly other isoforms, such as PKCι/λ, which attenuates AMPAR endocytosis. The thus preserved increased synaptic potentiation may correspond to LTM. (g) Glutamate release (either spontaneous or due to very weak stimulation typically used to read out LTP amplitude) can promote the reversal of synaptic changes that stabilize L-LTP, such as degradation of PKC/ι/λ and receptor internalization. The speed of L-LTP decay depends thus on the relative amount of GluN2B/NMDARs, in that, for example, downregulation of GluN2B expression promotes memory persistence. (h) Over time, L-LTP decay can return the synapse to its basal state.
If the stimulation and resulting potentiation was sufficient to engage molecular mechanisms leading to the development of L-LTP (such as synthesis and recruitment of PKMζ and other proteins), homeostatic downscaling will not cause a loss of LTP but instead adjust it such that the relative potentiation gain will be preserved. Thus, the synthesis of PKMζ or other relevant molecules involved in the maintenance of synaptic potentiation will counteract homeostatic downregulation, ultimately preventing postsynaptic removal of GluA2/AMAPRs and promoting the establishing of L-LTP. Regulating postsynaptic GluA2/AMPAR expression presents therefore the core mechanism to prevent E-LTP decay and to establish and maintain L-LTP. The pathways involved in this process could thus represent the molecular substrate of the synaptic tag that marks a synapse for L-LTP, as described in the synaptic tagging and capture hypothesis [11]. The presence of mechanisms that promote persistently increased postsynaptic expression of GluA2/AMPARs might play a central component of the synaptic tag, and a possible reason why weak stimulation leads to decaying E-LTP might be that homeostatic downscaling removes the synaptic tag, a process that requires internalization and proteasomal degradation of GluA2/AMPARs.
Because L-LTP persistence might involve a positive feedback loop (e.g. it has been suggested that the postsynaptic accumulation of GluA2 promotes stable levels of PKMζ [18,21,26]), the interesting possibility arises that interfering with GluA2 internalization after E-LTP induction might transform E-LTP that would otherwise be lost into long-lasting L-LTP. This might artificially set a ‘synaptic tag’ and it will promote the development of L-LTP. Once L-LTP has been established, metaplastic processes will regulate its persistence. These will depend on NMDAR signalling, such that the proportion of GluN2B to GluN2A receptors, i.e. the metaplastic ‘modification threshold’, will affect the direction of synaptic potentiation following synaptic stimulation. Thus, L-LTP induced with strong stimulation protocols will lead to a modification threshold setting that promotes memory persistence because probe stimulation will not raise Ca2+ to levels required for engaging the pathways involved in GluA2-dependent AMPAR endocytosis. Also, stimulation that normally would induce LTD or depotentiation will be less effective owing to the relative lack of GluN2B-containing NMDARs [48,49,53,92–94]. Our model predicts that there should be forms of L-LTP that will persist forever [45]. This prediction, which is rather hard to test empirically, finds support from demonstrations that L-LTP can last for up to five weeks [1,95], and, in one study, for even 1 year [2]. If this form of synaptic plasticity models forms of LTM, then such exceptionally long-lasting changes in synaptic potentiation may not be surprising.
These types of processes have recently been proposed to be involved in the loss of LTM [28]. According to this model, a dedicated decay process operates in the brain that systematically removes memories. This process might operate predominantly during sleep [96] and involves activity-dependent removal of GluA2-containing AMPARs [28]. In line with our suggestion regarding the decay of L-LTP, during sleep or certain sleep phases spontaneous presynaptic releases of glutamate can activate postsynaptic NMDARs [97], and, depending on the amount of GluN2A and GluN2B expressed at a particular synapse, this may lead to Ca2+ influx dynamics engaging pathways that induce depotentiation or LTD. LTD leads to a persistent reduction of PKMζ [98], which is likely to be involved in decreasing the amount of postsynaptic GluA2/AMPARs, [21] thus reducing synaptic potentiation.
Our model assigns a central role to the NMDA receptor in determining the direction of future synaptic potentiation. These receptors provide the major source of Ca2+ influx that drives synaptic plasticity, but GluA1-lacking, Ca2+-permeable AMPARs also contribute to changes in intracellular Ca2+ levels. It has been speculated that these receptors participate both in homeostatic scaling as well as in metaplasticity [99–101]. The characterization of their role in homeostatic processes currently remains preliminary, and we therefore did not consider them in our simple model.
It is likely that the weak stimuli typically applied in LTP preparations to read out current synaptic potentiation, which are often of the same intensity as stimuli applied to induce depotentiation [47], ‘simulate’ the sleep-dependent decay process found in animals and thus promote the loss of L-LTP. That is, the test pulse used in studies to measure synaptic potentiation might promote the decay of LTP and thus artificially increase the rate at which potentiation is lost over time.
7. Conclusion
It is a principle of biology that to each process of synthesis there exists a complementary process of degradation. Proteins do not simply fall apart at some point, but are disassembled by processes as complex and well organized as those that once built them (R. Davis 2013, personal communication). Well-organized processes lead to cell death during apoptosis. The enzymatic action of phosphatases removes phosphate groups from phosphorylated proteins. In contrast to what seems the norm, however, memory is not understood as a dyadic system that comprises processes of formation as well as dedicated and constitutive processes of elimination. As a consequence, while memory consolidation has been intensively studied, the neurobiology of forgetting is still in its infancy.
It is hard to imagine a phenomenon of biological disintegration that does not require energy and organization. Yet, it is widely believed that forgetting is either a passive process, or that it is the result of a glitch or failure in memory formation, or that it is a side-effect of other memory processes that somehow interfere with existing memory representations. We propose that neural networks retain only a fraction of the vast number of memories that were originally formed and consolidated because most will not withstand the continuously present force of active forgetting. Memory constitutes a state of higher organization, and preserving it requires energy. Contrary to intuitive beliefs, it seems that the natural tendency of memory systems is to forget, not to preserve.
LTP and LTD have prevailed as reasonably good models for memory phenomena, and the processes that determine how stimulation-induced synaptic potentiation dynamically changes over time suggest possible mechanisms that could regulate memory persistence and memory loss. We propose that stimulation-induced synaptic enhancements, such as E-LTP and L-LTP, can be reversed over time by active processes that eliminate synaptic potentiation. These processes promote the removal of AMPARs from postsynaptic densities and are under the control of homeostatic plasticity mechanisms that regulate the probability and thus speed of LTP decay. Tonic inhibition of such homeostatic negative feedback mechanism by newly synthesized proteins may be a critical mechanism responsible for preventing the decay of E-LTP. Metaplastic processes that regulate whether stimulation maintains, increases or reduces synaptic potentiation control the decay of L-LTP.
Acknowledgements
O.H., K.N. and Y.T.W. thank CIHR for support. K.N. thanks NSERC for the Steacie Fellowship. Y.T.W. thanks The Holder of Heart and Stroke Foundation of British Columbia and the Yukon Chair in stroke research for support.
References
- 1.Trepel C, Racine RJ. 1998. Long-term potentiation in the neocortex of the adult, freely moving rat. Cereb. Cortex 8, 719–729 (doi:10.1093/cercor/8.8.719) [DOI] [PubMed] [Google Scholar]
- 2.Abraham WC, Logan B, Greenwood JM, Dragunow M. 2002. Induction and experience-dependent consolidation of stable long-term potentiation lasting months in the hippocampus. J. Neurosci. 22, 9626–9634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abraham WC, Mason-Parker SE, Williams J, Dragunow M. 1995. Analysis of the decremental nature of LTP in the dentate gyrus. Brain Res. Mol. Brain Res. 30, 367–372 (doi:10.1016/0169-328X(95)00026-O) [DOI] [PubMed] [Google Scholar]
- 4.Barnes CA. 1979. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 93, 74–104 (doi:10.1037/h0077579) [DOI] [PubMed] [Google Scholar]
- 5.Bliss TV, Collingridge GL. 1993. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39 (doi:10.1038/361031a0) [DOI] [PubMed] [Google Scholar]
- 6.Osten P, et al. 1998. The AMPA receptor GluR2 C terminus can mediate a reversible, ATP-dependent interaction with NSF and α- and β-SNAPs. Neuron 21, 99–110 (doi:10.1016/S0896-6273(00)80518-8) [DOI] [PubMed] [Google Scholar]
- 7.Milner B, Squire LR, Kandel ER. 1998. Cognitive neuroscience and the study of memory. Neuron 20, 445–468 (doi:10.1016/S0896-6273(00)80987-3) [DOI] [PubMed] [Google Scholar]
- 8.Malenka RC, Nicoll RA. 1999. Long-term potentiation—a decade of progress? Science 285, 1870–1874 (doi:10.1126/science.285.5435.1870) [DOI] [PubMed] [Google Scholar]
- 9.Collingridge GL, Isaac JTR, Wang YT. 2004. Receptor trafficking and synaptic plasticity. Nat. Rev. Neurosci. 5, 952–962 (doi:10.1038/nrn1556) [DOI] [PubMed] [Google Scholar]
- 10.Huang EP. 1998. Synaptic plasticity: going through phases with LTP. Curr. Biol. 8, R350–R352 (doi:10.1016/S0960-9822(98)70219-2) [DOI] [PubMed] [Google Scholar]
- 11.Redondo RL, Morris RGM. 2011. Making memories last: the synaptic tagging and capture hypothesis. Nat. Rev. Neurosci. 12, 17–30 (doi:10.1038/nrn2963) [DOI] [PubMed] [Google Scholar]
- 12.Redondo RL, Okuno H, Spooner PA, Frenguelli BG, Bito H, Morris RGM. 2010. Synaptic tagging and capture: differential role of distinct calcium/calmodulin kinases in protein synthesis-dependent long-term potentiation. J. Neurosci. 30, 4981–4989 (doi:10.1523/JNEUROSCI.3140-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen PV, Abel T, Kandel ER. 1994. Requirement of a critical period of transcription for induction of a late phase of LTP. Science 265, 1104–1107 (doi:10.1126/science.8066450) [DOI] [PubMed] [Google Scholar]
- 14.Bolshakov VY, Golan H, Kandel ER, Siegelbaum SA. 1997. Recruitment of new sites of synaptic transmission during the cAMP-dependent late phase of LTP at CA3-CA1 synapses in the hippocampus. Neuron 19, 635–651 (doi:10.1016/S0896-6273(00)80377-3) [DOI] [PubMed] [Google Scholar]
- 15.Villarreal DM, Do V, Haddad E, Derrick BE. 2002. NMDA receptor antagonists sustain LTP and spatial memory: active processes mediate LTP decay. Nat. Neurosci. 5, 48–52 (doi:10.1038/nn776) [DOI] [PubMed] [Google Scholar]
- 16.Lu Y, Ji Y, Ganesan S, Schloesser R, Martinowich K, Sun M, Mei F, Chao MV, Lu B. 2011. TrkB as a potential synaptic and behavioral tag. J. Neurosci. 31, 11 762–11 771 (doi:10.1523/JNEUROSCI.2707-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collingridge GL, Peineau S, Howland JG, Wang YT. 2010. Long-term depression in the CNS. Nat. Rev. Neurosci. 11, 459–473 (doi:10.1038/nrn2867) [DOI] [PubMed] [Google Scholar]
- 18.Sacktor TC. 2011. How does PKMζ maintain long-term memory? Nat. Rev. Neurosci. 12, 9–15 (doi:10.1038/nrn2949) [DOI] [PubMed] [Google Scholar]
- 19.Volk LJ, Bachman JL, Johnson R, Yu Y, Huganir RL. 2013. PKM-ζ is not required for hippocampal synaptic plasticity, learning and memory. Nature 493, 420–423 (doi:10.1038/nature11802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, et al. 2007. Amygdala depotentiation and fear extinction. Proc. Natl Acad. Sci. USA 104, 20 955–20 960 (doi:10.1073/pnas.0710548105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, Nader K. 2010. PKMζ maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat. Neurosci. 13, 630–634 (doi:10.1038/nn.2531) [DOI] [PubMed] [Google Scholar]
- 22.Tsien JZ, Huerta PT, Tonegawa S. 1996. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 87, 1327–1338 (doi:10.1016/S0092-8674(00)81827-9) [DOI] [PubMed] [Google Scholar]
- 23.Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. 2000. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat. Neurosci. 3, 238–244 (doi:10.1038/72945) [DOI] [PubMed] [Google Scholar]
- 24.Ling DSF, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, Sacktor TC. 2002. Protein kinase Mζ is necessary and sufficient for LTP maintenance. Nat. Neurosci. 5, 295–296 (doi:10.1038/nn829) [DOI] [PubMed] [Google Scholar]
- 25.Kandel ER. 2001. The molecular biology of memory storage: a dialogue between genes and synapses. Science 294, 1030–1038 (doi:10.1126/science.1067020) [DOI] [PubMed] [Google Scholar]
- 26.Sacktor TC. 2012. Memory maintenance by PKMζ—an evolutionary perspective. Mol. Brain Res. 5, 31 (doi:10.1186/1756-6606-5-31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shema R, Haramati S, Ron S, Hazvi S, Chen A, Sacktor TC, Dudai Y. 2011. Enhancement of consolidated long-term memory by overexpression of protein kinase Mζ in the neocortex. Science 331, 1207–1210 (doi:10.1126/science.1200215) [DOI] [PubMed] [Google Scholar]
- 28.Hardt O, Nader K, Nadel L. 2013. Decay happens: the role of active forgetting in memory. Trends Cogn. Sci. 17, 109–118 (doi:10.1016/j.tics.2013.01.001) [DOI] [PubMed] [Google Scholar]
- 29.Kelly MT, Crary JF, Sacktor TC. 2007. Regulation of protein kinase Mζ synthesis by multiple kinases in long-term potentiation. J. Neurosci. 27, 3439–3444 (doi:10.1523/JNEUROSCI.5612-06.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westmark PR, Westmark CJ, Wang S, Levenson J, O'Riordan KJ, Burger C, Malter JS. 2010. Pin1 and PKMζ sequentially control dendritic protein synthesis. Sci. Signal. 3, ra18 (doi:10.1126/scisignal.2000451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zovkic IB, Guzman-Karlsson MC, Sweatt JD. 2013. Epigenetic regulation of memory formation and maintenance. Learn. Mem. 20, 61–74 (doi:10.1101/lm.026575.112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landry CD, Kandel ER, Rajasethupathy P. 2013. New mechanisms in memory storage: piRNAs and epigenetics. Trends Neurosci. 36, 535–542 (doi:10.1016/j.tins.2013.05.004) [DOI] [PubMed] [Google Scholar]
- 33.Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. 2006. Storage of spatial information by the maintenance mechanism of LTP. Science 313, 1141–1144 (doi:10.1126/science.1128657) [DOI] [PubMed] [Google Scholar]
- 34.Serrano P, et al. 2008. PKMζ maintains spatial, instrumental, and classically conditioned long-term memories. PLoS Biol. 6, e318 (doi:10.1371/journal.pbio.0060318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sacktor TC. 2008. Progress in brain research. Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 36.Lee AM, et al. 2013. Prkcz null mice show normal learning and memory. Nature 493, 416–419 (doi:10.1038/nature11803) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez EM, Dunham EE, Martin GS. 2009. Atypical protein kinase C activity is required for extracellular matrix degradation and invasion by Src-transformed cells. J. Cell. Physiol. 221, 171–182 (doi:10.1002/jcp.21841) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frankland PW, Josselyn SA. 2013. Neuroscience: memory and the single molecule. Nature 493, 312–313 (doi:10.1038/nature11850) [DOI] [PubMed] [Google Scholar]
- 39.Scholz R, Berberich S, Rathgeber L, Kolleker A, Köhr G, Kornau H-C. 2010. AMPA receptor signaling through BRAG2 and Arf6 critical for long-term synaptic depression. Neuron 66, 768–780 (doi:10.1016/j.neuron.2010.05.003) [DOI] [PubMed] [Google Scholar]
- 40.Kim CH, Chung HJ, Lee HK, Huganir RL. 2001. Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc. Natl Acad. Sci. USA 98, 11 725–11 730 (doi:10.1073/pnas.211132798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang YT, Linden DJ. 2000. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron 25, 635–647 (doi:10.1016/S0896-6273(00)81066-1) [DOI] [PubMed] [Google Scholar]
- 42.Yao Y, Kelly MT, Sajikumar S, Serrano P, Tian D, Bergold PJ, Frey JU, Sacktor TC. 2008. PKM maintains late long-term potentiation by N-ethylmaleimide-sensitive factor/GluR2-dependent trafficking of postsynaptic AMPA receptors. J. Neurosci. 28, 7820–7827 (doi:10.1523/JNEUROSCI.0223-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmadian G, et al. 2004. Tyrosine phosphorylation of GluR2 is required for insulin-stimulated AMPA receptor endocytosis and LTD. EMBO J. 23, 1040–1050 (doi:10.1038/sj.emboj.7600126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brebner K, Wong TP, Liu L, Liu Y, Campsall P, Gray S, Phelps L, Phillips AG, Wang YT. 2005. Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science 310, 1340–1343 (doi:10.1126/science.1116894) [DOI] [PubMed] [Google Scholar]
- 45.Abraham WC, Williams JM. 2003. Properties and mechanisms of LTP maintenance. Neuroscientist 9, 463–474 (doi:10.1177/1073858403259119) [DOI] [PubMed] [Google Scholar]
- 46.Genoux DD, Haditsch UU, Knobloch MM, Michalon AA, Storm DD, Mansuy IMI. 2002. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature 418, 970–975 (doi:10.1038/nature00928) [DOI] [PubMed] [Google Scholar]
- 47.Huang CC, Liang YC, Hsu KS. 2001. Characterization of the mechanism underlying the reversal of long term potentiation by low frequency stimulation at hippocampal CA1 synapses. J. Biol. Chem. 276, 48 108–48 117 [DOI] [PubMed] [Google Scholar]
- 48.Dalton GL, Wang YT, Floresco SB, Phillips AG. 2008. Disruption of AMPA receptor endocytosis impairs the extinction, but not acquisition of learned fear. Neuropsychopharmacology 33, 2416–2426 (doi:10.1038/sj.npp.1301642) [DOI] [PubMed] [Google Scholar]
- 49.Park S, Lee S, Kim J, Choi S. 2012. Ex vivo depotentiation of conditioning-induced potentiation at thalamic input synapses onto the lateral amygdala requires GluN2B-containing NMDA receptors. Neurosci. Lett. 530, 121–126 (doi:10.1016/j.neulet.2012.10.011) [DOI] [PubMed] [Google Scholar]
- 50.Cazakoff BN, Howland JG. 2011. AMPA receptor endocytosis in rat perirhinal cortex underlies retrieval of object memory. Learn. Mem. 18, 688–692 (doi:10.1101/lm.2312711) [DOI] [PubMed] [Google Scholar]
- 51.Lee H-K, Kirkwood A. 2011. AMPA receptor regulation during synaptic plasticity in hippocampus and neocortex. Semin. Cell Dev. Biol. 22, 514–520 (doi:10.1016/j.semcdb.2011.06.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao MY, Niu YP, Wigström H. 1996. Activity-dependent decay of early LTP revealed by dual EPSP recording in hippocampal slices from young rats. Eur. J. Neurosci. 8, 1916–1923 (doi:10.1111/j.1460-9568.1996.tb01335.x) [DOI] [PubMed] [Google Scholar]
- 53.Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnár E, Collingridge GL, Bashir ZI. 2004. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J. Neurosci. 24, 7821–7828 (doi:10.1523/JNEUROSCI.1697-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lüscher C, Malenka RC. 2012. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harbor Perspect. Biol. 4, pii: a005710 (doi:10.1101/cshperspect.a005710) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Unoki T, et al. 2012. NMDA receptor-mediated PIP5K activation to produce PI(4,5)P2 is essential for AMPA receptor endocytosis during LTD. Neuron 73, 135–148 (doi:10.1016/j.neuron.2011.09.034) [DOI] [PubMed] [Google Scholar]
- 56.Bliss T, Collingridge G, Morris RGM. 2007. Synaptic plasticity in the hippocampus. In The hippocampus book (eds Andersen P, Morris RGM, Amaral D, Bliss T, O'Keefe J.), pp. 343–368 Oxford, UK: Oxford University Press [Google Scholar]
- 57.Quirk GJ, Repa C, LeDoux JE. 1995. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron 15, 1029–1039 (doi:10.1016/0896-6273(95)90092-6) [DOI] [PubMed] [Google Scholar]
- 58.Lin C-H, Lee C-C, Gean P-W. 2003. Involvement of a calcineurin cascade in amygdala depotentiation and quenching of fear memory. Mol. Pharmacol. 63, 44–52 (doi:10.1124/mol.63.1.44) [DOI] [PubMed] [Google Scholar]
- 59.Citri A, Bhattacharyya S, Ma C, Morishita W, Fang S, Rizo J, Malenka RC. 2010. Calcium binding to PICK1 is essential for the intracellular retention of AMPA receptors underlying long-term depression. J. Neurosci. 30, 16 437–16 452 (doi:10.1523/JNEUROSCI.4478-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakano-Kobayashi A, et al. 2007. Role of activation of PIP5Kgamma661 by AP-2 complex in synaptic vesicle endocytosis. EMBO J. 26, 1105–1116 (doi:10.1038/sj.emboj.7601573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hayashi T, Huganir RL. 2004. Tyrosine phosphorylation and regulation of the AMPA receptor by SRC family tyrosine kinases. J. Neurosci. 24, 6152–6160 (doi:10.1523/JNEUROSCI.0799-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steinberg JP, et al. 2006. Targeted in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron 49, 845–860 (doi:10.1016/j.neuron.2006.02.025) [DOI] [PubMed] [Google Scholar]
- 63.Volk L, Kim C-H, Takamiya K, Yu Y, Huganir RL. 2010. Developmental regulation of protein interacting with C kinase 1 (PICK1) function in hippocampal synaptic plasticity and learning. Proc. Natl Acad. Sci. USA 107, 21 784–21 789 (doi:10.1073/pnas.1016103107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. 2000. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J. Neurosci. 20, 7258–7267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goold CP, Nicoll RA. 2010. Single-cell optogenetic excitation drives homeostatic synaptic depression. Neuron 68, 512–528 (doi:10.1016/j.neuron.2010.09.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hou Q, Gilbert J, Man H-Y. 2011. Homeostatic regulation of AMPA receptor trafficking and degradation by light-controlled single-synaptic activation. Neuron 72, 806–818 (doi:10.1016/j.neuron.2011.10.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fu AKY, Hung K-W, Fu W-Y, Shen C, Chen Y, Xia J, Lai K-O, Ip NY. 2011. APC(Cdh1) mediates EphA4-dependent downregulation of AMPA receptors in homeostatic plasticity. Nat. Neurosci. 14, 181–189 (doi:10.1038/nn.2715) [DOI] [PubMed] [Google Scholar]
- 68.Anggono V, Clem RL, Huganir RL. 2011. PICK1 loss of function occludes homeostatic synaptic scaling. J. Neurosci. 31, 2188–2196 (doi:10.1523/JNEUROSCI.5633-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu J-H, et al. 2010. Homeostatic scaling requires group I mGluR activation mediated by Homer1a. Neuron 68, 1128–1142 (doi:10.1016/j.neuron.2010.11.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abraham WC, Bear MF. 1996. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 19, 126–130 (doi:10.1016/S0166-2236(96)80018-X) [DOI] [PubMed] [Google Scholar]
- 71.Bienenstock EL, Cooper LN, Munro PW. 1982. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J. Neurosci. 2, 32–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang YY, Colino A, Selig DK, Malenka RC. 1992. The influence of prior synaptic activity on the induction of long-term potentiation. Science 255, 730–733 (doi:10.1126/science.1346729) [DOI] [PubMed] [Google Scholar]
- 73.Wang H, Wagner JJ. 1999. Priming-induced shift in synaptic plasticity in the rat hippocampus. J. Neurophysiol. 82, 2024–2028 [DOI] [PubMed] [Google Scholar]
- 74.Abbott LF, Nelson SB. 2000. Synaptic plasticity: taming the beast. Nat. Neurosci. 3, 1178–1183 (doi:10.1038/81453) [DOI] [PubMed] [Google Scholar]
- 75.Finnie PSB, Nader K. 2012. The role of metaplasticity mechanisms in regulating memory destabilization and reconsolidation. Neurosci. Biobehav. Rev. 36, 1667–1707 (doi:10.1016/j.neubiorev.2012.03.008) [DOI] [PubMed] [Google Scholar]
- 76.Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR. 1998. Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J. Neurophysiol. 79, 555–566 [DOI] [PubMed] [Google Scholar]
- 77.Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. 1994. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature 368, 144–147 (doi:10.1038/368144a0) [DOI] [PubMed] [Google Scholar]
- 78.Sobczyk A, Scheuss V, Svoboda K. 2005. NMDA receptor subunit-dependent [Ca2+] signaling in individual hippocampal dendritic spines. J. Neurosci. 25, 6037–6046 (doi:10.1523/JNEUROSCI.1221-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cull-Candy S, Brickley S, Farrant M. 2001. NMDA receptor subunits: diversity, development and disease. Curr. Opin. Neurobiol. 11, 327–335 (doi:10.1016/S0959-4388(00)00215-4) [DOI] [PubMed] [Google Scholar]
- 80.Philpot BD, Cho KKA, Bear MF. 2007. Obligatory role of NR2A for metaplasticity in visual cortex. Neuron 53, 495–502 (doi:10.1016/j.neuron.2007.01.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zinebi F, Xie J, Liu J, Russell RT, Gallagher JP, McKernan MG, Shinnick-Gallagher P. 2003. NMDA currents and receptor protein are downregulated in the amygdala during maintenance of fear memory. J. Neurosci. 23, 10 283–10 291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Quinlan EM, Lebel D, Brosh I, Barkai E. 2004. A molecular mechanism for stabilization of learning-induced synaptic modifications. Neuron 41, 185–192 (doi:10.1016/S0896-6273(03)00874-2) [DOI] [PubMed] [Google Scholar]
- 83.Wang S-H, de Oliveira Alvares L, Nader K. 2009. Cellular and systems mechanisms of memory strength as a constraint on auditory fear reconsolidation. Nat. Neurosci. 12, 905–912 (doi:10.1038/nn.2350) [DOI] [PubMed] [Google Scholar]
- 84.Cui Z, Feng R, Jacobs S, Duan Y, Wang H, Cao X, Tsien JZ. 2013. Increased NR2A:NR2B ratio compresses long-term depression range and constrains long-term memory. Sci. Rep. 3, 1036 (doi:10.1038/srep01036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kirkwood A, Lee HK, Bear MF. 1995. Co-regulation of long-term potentiation and experience-dependent synaptic plasticity in visual cortex by age and experience. Nature 375, 328–331 (doi:10.1038/375328a0) [DOI] [PubMed] [Google Scholar]
- 86.Nakamori T, Maekawa F, Sato K, Tanaka K, Ohki-Hamazaki H. 2013. Neural basis of imprinting behavior in chicks. Dev. Growth Differ. 55, 198–206 (doi:10.1111/dgd.12028) [DOI] [PubMed] [Google Scholar]
- 87.Liu Z, Lv C, Zhao W, Song Y, Pei D, Xu T. 2012. NR2B-containing NMDA receptors expression and their relationship to apoptosis in hippocampus of Alzheimer's disease-like rats. Neurochem. Res. 37, 1420–1427 (doi:10.1007/s11064-012-0726-0) [DOI] [PubMed] [Google Scholar]
- 88.Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. 2009. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron 62, 788–801 (doi:10.1016/j.neuron.2009.05.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Daumas S, Sandin J, Chen KS, Kobayashi D, Tulloch J, Martin SJ, Games D, Morris RGM. 2008. Faster forgetting contributes to impaired spatial memory in the PDAPP mouse: deficit in memory retrieval associated with increased sensitivity to interference? Learn. Mem. 15, 625–632 (doi:10.1101/lm.990208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Frankland PW, Köhler S, Josselyn SA. 2013. Hippocampal neurogenesis and forgetting. Trends Neurosci. 36, 497–503 (doi:10.1016/j.tins.2013.05.002) [DOI] [PubMed] [Google Scholar]
- 91.Dozmorov M, Niu Y-P, Xu H-P, Xiao M-Y, Li R, Sandberg M, Wigström H. 2003. Active decay of composite excitatory postsynaptic potentials in hippocampal slices from young rats. Brain Res. 973, 44–55 (doi:10.1016/S0006-8993(03)02536-8) [DOI] [PubMed] [Google Scholar]
- 92.Fox CJ, Russell KI, Wang YT, Christie BR. 2006. Contribution of NR2A and NR2B NMDA subunits to bidirectional synaptic plasticity in the hippocampus in vivo. Hippocampus 16, 907–915 (doi:10.1002/hipo.20230) [DOI] [PubMed] [Google Scholar]
- 93.Yu SY, Wu DC, Zhan RZ. 2010. GluN2B subunits of the NMDA receptor contribute to the AMPA receptor internalization during long-term depression in the lateral amygdala of juvenile rats. Neuroscience 171, 1102–1108 (doi:10.1016/j.neuroscience.2010.09.038) [DOI] [PubMed] [Google Scholar]
- 94.Dalton GL, Ma LM, Phillips AG, Floresco SB. 2011. Blockade of NMDA GluN2B receptors selectively impairs behavioral flexibility but not initial discrimination learning. Psychopharmacology 216, 525–535 (doi:10.1007/s00213-011-2246-z) [DOI] [PubMed] [Google Scholar]
- 95.Staubli U, Lynch G. 1987. Stable hippocampal long-term potentiation elicited by ‘theta’ pattern stimulation. Brain Res. 435, 227–234 (doi:10.1016/0006-8993(87)91605-2) [DOI] [PubMed] [Google Scholar]
- 96.Tononi G, Cirelli C. 2003. Sleep and synaptic homeostasis: a hypothesis. Brain Res. Bull. 62, 143–150 (doi:10.1016/j.brainresbull.2003.09.004) [DOI] [PubMed] [Google Scholar]
- 97.Kavalali ET, Chung C, Khvotchev M, Leitz J, Nosyreva E, Raingo J, Ramirez DMO. 2011. Spontaneous neurotransmission: an independent pathway for neuronal signaling? Physiology 26, 45–53 (doi:10.1152/physiol.00040.2010) [DOI] [PubMed] [Google Scholar]
- 98.Hrabetova S, Sacktor TC. 2001. Transient translocation of conventional protein kinase C isoforms and persistent downregulation of atypical protein kinase Mζ in long-term depression. Mol. Brain Res. 95, 146–152 (doi:10.1016/S0169-328X(01)00185-1) [DOI] [PubMed] [Google Scholar]
- 99.Shepherd JD. 2012. Memory, plasticity and sleep—a role for calcium permeable AMPA receptors? Front. Mol. Neurosci. 5, 49 (doi:10.3389/fnmol.2012.00049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee H-K. 2012. Ca-permeable AMPA receptors in homeostatic synaptic plasticity. Front. Mol. Neurosci. 5, 17 (doi:10.3389/fnmol.2012.00017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Man H-Y. 2011. GluA2-lacking, calcium-permeable AMPA receptors–inducers of plasticity? Curr. Opin. Neurobiol. 21, 291–298 (doi:10.1016/j.conb.2011.01.001) [DOI] [PMC free article] [PubMed] [Google Scholar]