Abstract
Memories can be easily distorted, and a lack of relevant animal models has largely hindered our understanding of false-memory formation. Here, we first identified a population of cells in the dentate gyrus (DG) of the hippocampus that bear the engrams for a specific context; these cells were naturally activated during the encoding phase of fear conditioning and their artificial reactivation using optogenetics in an unrelated context was sufficient for inducing the fear memory specific to the conditioned context. In a further study, DG or CA1 neurons activated by exposure to a particular context were labelled with channelrhodopsin-2 (ChR2). These neurons were later optically reactivated during fear conditioning in a different context. The DG experimental group showed increased freezing in the original context in which a foot shock was never delivered. The recall of this false memory was context specific, activated similar downstream regions engaged during natural fear-memory recall, and was also capable of driving an active fear response. Together, our data demonstrate that by substituting a natural conditioned stimulus with optogenetically reactivated DG cells that bear contextual memory engrams, it is possible to incept an internally and behaviourally represented false fear memory.
Keywords: memory engram, false memory, optogenetics, hippocampus
1. Introduction
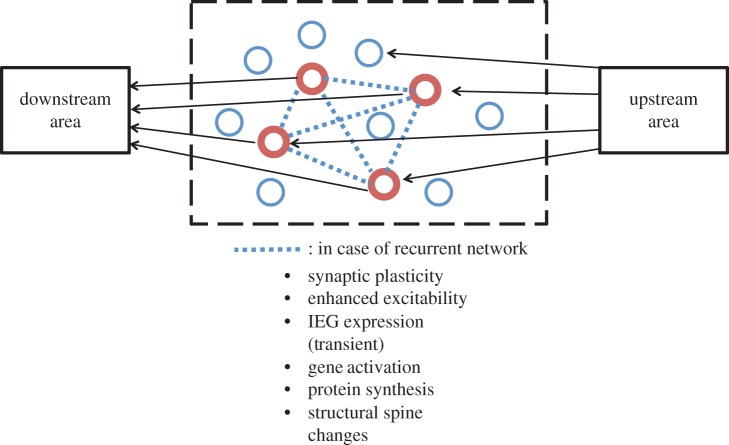
Hebb's [1] pioneering conceptualization of synaptic plasticity in 1949 followed by Bliss & Lomo's [2] discovery of long-term potentiation (LTP) has provided the principal framework with which neuroscientists have pursued the neural mechanisms subserving learning and memory. Additional mechanisms, including alterations in membrane excitability and activation of immediately early genes (IEGs) at the whole single-cell level, as well as biochemical and structural alterations of dendritic spines have been recognized [3]. In neuropsychology, Semon [4] put forward the ‘engram’ theory of memory in the early twentieth century, which in current terms could be roughly stated as: when a memory is formed, a subpopulation of neurons will be excited and stay excited latently for the storage of the memory information (engram). When part of the total information at the time of storage is subsequently available, it will re-excite the engram for recall. Figure 1 presents a conceptual diagram of an engram-bearing neuronal population that incorporates neurobiological mechanisms including LTP.
Figure 1.
Conceptual diagram of engram-bearing neuronal subpopulation. Engram theory of memory posits that when a memory of a certain experience is formed, a subpopulation of neurons, including their synapses, are activated and undergo enduring though primarily latent physical and chemical changes. These changes, referred to as the ‘engram’ of that memory, are induced by a patterned activity of the cells in the upstream area. The memory engram-bearing cells (red) may be mutually connected (dotted line) or independent. The changes that occurred in these cells include synaptic plasticity including LTP and LTD, enhanced excitability of whole single cells, transient activation of IEGs and other selected genes, new rounds of protein synthesis, and structural changes (both growth and contraction) of dendritic spines. When part of the total information at the time of storage is subsequently available, it may re-excite the engram-bearing cells and memory recall may ensue.
A recent study showed that a selective post-training ablation of an IEG (i.e. CREB)-rich cell population in the amygdala that was activated during a fear-memory task results in a loss of that fear memory [5]. These data are consistent with the engram theory, but the final test of any hypothesis concerning memory engrams must be a mimicry experiment, in which apparent memory is generated (expressed) artificially without the usual requirement for sensory experience [6]. We recently reported such an experiment. In this article, we first provide a brief summary of this work. As the main body of this article, we then proceed to our more recent work on a mouse model of false memory that was made possible by the identification of memory engram-bearing cells.
We routinely use memories as guides for cognition and behaviour [6–9]. Memory, however, can often be unreliable because it is not a carbon copy but rather a reconstruction of the past [10–14]. The prevalence of false memories permeates our day-to-day lives. Moreover, false memories also play a pivotal role in social and legal settings and have often resulted in grave and costly consequences. For instance, in a famous case, a jury convicted Ronald Cotton of assaulting Jennifer Thompson based on her faulty personal testimony that she genuinely believed to be true. Cotton was sentenced to two lifetimes in jail. A decade later, new DNA evidence became available and proved Cotton's innocence. Strikingly, one study reports that about 75% of the first 251 people exonerated by DNA evidence were victims of faulty eyewitness testimony (for reviews, see [10,11]). Cognitive studies in humans have reported robust activity in the hippocampus during the recall of both false and genuine memories [15]. However, human studies using behavioural and fMRI techniques have not been able to delineate the mechanistic relationship between genuine and false memories. To resolve these issues, we have investigated these two types of memories at the memory engram level.
2. Identification of contextual memory engrams in the dentate gyrus (see [16] for complete text and methods)
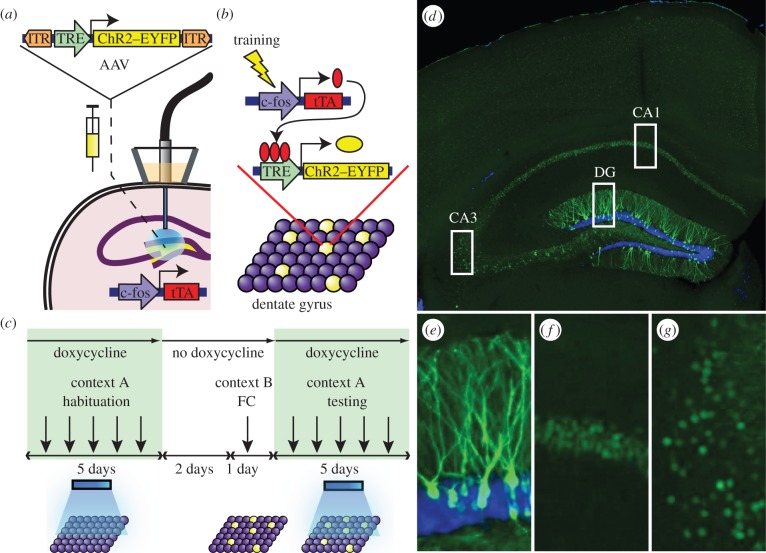
To label and reactivate a subpopulation of dentate gyrus (DG) cells active during the encoding of a memory, we targeted the DG of c-fos–tTA transgenic mice [17] with a TRE-ChR2-EYFP virus and an optical fibre implant (figure 2a). This approach couples the promoter of c-fos, an IEG often used as a marker of recent neuronal activity [18,19], to the tetracycline transactivator (tTA), a key component of the doxycycline (Dox) system for inducible expression of a gene of interest [20]. In our system, the absence of Dox permits c-fos-promoter-driven tTA to bind to its target tetracycline-responsive element (TRE) site, which in turn drives channelrhodopsin-2 (ChR2)–enhanced yellow fluorescent protein (EYFP) expression in neurons active during this defined period. These neurons can then be reactivated by light stimulation during testing (figure 2b,c). In the presence of Dox, however, tTA is blocked from binding to TRE and neurons active during this Dox-on period remains unlabelled by ChR2–EYFP.
Figure 2.
Basic experimental protocols and selective labelling of the DG cells by ChR2–EYFP. (a) The c-fos–tTA mouse was injected with AAV9-TRE-ChR2-EYFP and implanted with an optical fibre targeting the DG. (b) When off Dox, training induces the expression of c-fos–tTA, which binds to TRE and drives the expression of ChR2–EYFP, labelling a subpopulation of activated cells (yellow) in the DG. (c) Basic experimental scheme. Mice were habituated in context A with light stimulation while on Dox for 5 days, then taken off Dox for 2 days and fear conditioned (FC) in context B. Mice were put back on Dox and tested for 5 days in context A with light stimulation. (d) Representative image showing the expression of ChR2–EYFP in a mouse that was taken off Dox for 2 days and underwent fear conditioning training. An image of each rectangular area in (d) is magnified showing (e) DG, (f) CA1 and (g) CA3. The green signal from ChR2–EYFP in the DG spreads throughout granule cells, including dendrites (e), while the green signal confined to the nuclei in CA1 and CA3 is owing to shEGFP expression from the c-fos–shEGFP construct of the transgenic mouse (f,g). Blue is the nuclear marker DAPI.
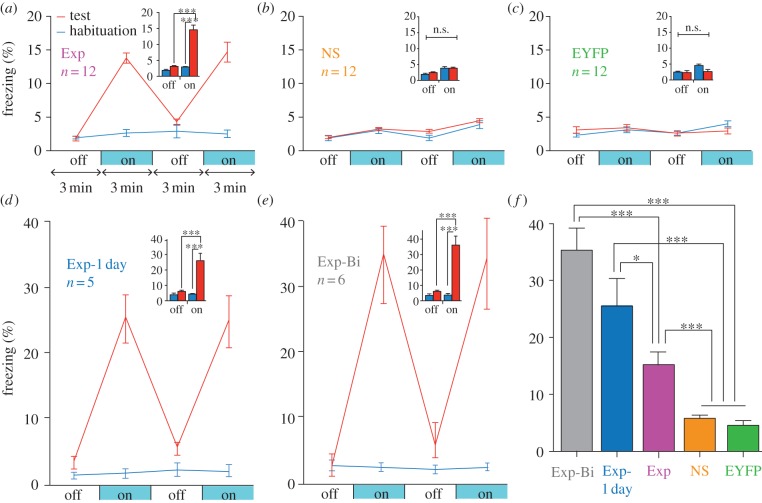
We first tested whether the reactivation of a population of DG neurons active during the encoding phase of a fear memory was sufficient for the reinstatement of that memory. The experimental group (Exp) consisted of c-fos–tTA mice injected with TRE-ChR2-EYFP and implanted with an optical fibre targeting the dorsal DG (figure 2a). Mice were kept on Dox and underwent a habituation period to record their basal level of freezing in one context (context A) during which they received both light-off and light-on epochs. Next, they were taken off Dox and underwent fear conditioning in a distinct chamber (context B) in which a tone was paired with shock. The mice were then subjected to testing sessions with light-off and light-on epochs in context A while being back on Dox to prevent any subsequent labelling of active DG cells (figure 2c–g). During the habituation sessions, the Exp mice showed very little freezing during either light-off or light-on epochs. By contrast, after fear conditioning—during which putative fear memory engram-bearing DG cells were labelled with ChR2–EYFP—freezing levels during light-on epochs were higher compared with light-off epochs, which indicated light-induced fear-memory recall (figure 3a). A group of mice (NS group) that went through exactly the same procedures as the experimental group except that no shock was delivered during the training session did not freeze above background levels when the light was shone during the post-training session (figure 3b). Another group of mice (EYFP group) that went through the same experimental protocol except that the virus had no ChR2 gene also showed no augmented post-training freezing upon the light delivery (figure 3c). On the other hand, the post-training freezing levels were increased even more than those of the experimental group when the Dox-off training period was reduced to 1 day (Exp-1 day group, figure 3d), presumably because non-specific environmental stimuli, which compete with the specific context stimuli, were reduced. The level of freezing was increased further when the manipulation was performed bilaterally (Exp-Bi group, figure 3e).
Figure 3.
Optical stimulation of engram-bearing cells induces post-training freezing. (a) c-fos–tTA mice injected with AAV9-TRE-ChR2-EYFP and trained with fear conditioning (Exp group) show elevated freezing during 3 min light-on epochs. Freezing for each epoch represents 5-day average. Freezing levels for the two light-off and light-on epochs are further averaged in the inset. (n = 12, F1,22 = 37.98, ***p < 0.001). (b) Mice trained similar to (a) but without foot shock (NS group) do not show increased light-induced freezing (n = 12). (c) Mice injected with AAV9-TRE-EYFP and trained with fear conditioning (EYFP group) do not show increased light-induced freezing (n = 12). (d) Mice trained similar to (a) but kept off Dox for 1 day before fear conditioning training (Exp-1 day group) showed greater freezing during test light-on epochs compared with Exp group (n = 5, F1,8 = 38.26, ***p < 0.001). (e) Mice trained similar to (a) but bilaterally injected with AAV9-TRE-ChR2-EYFP and implanted with optical fibres (Exp-Bi group) showed even higher levels of freezing during test light-on epochs (n = 6, F1,10 = 85.14, ***p < 0.001). (f) Summary of freezing levels of the five groups during test light-on epochs (F4,42 = 37.62, *p < 0.05; ***p < 0.001).
The overall results (figure 3f) suggest that DG cells that express endogenous c-Fos during training, and therefore become labelled by ChR2–EYFP, define an active neural population that is sufficient for memory recall upon subsequent reactivation [16].
3. Inception of a false memory (see [21] for complete text and methods)
Having identified contextual engram-bearing cells in the DG, we went on to test whether an artificial conditioned (CS)–unconditioned stimulus (US) association—what we refer to as a false memory—could be formed. We first took virus-infected and fibre-implanted animals off Dox to open a time window for labelling cells activated by the exploration of a novel context (context A) with ChR2–mCherry. The animals were then immediately put back on Dox to prevent any further labelling. The next day, we fear-conditioned this group in a distinct context (context B) while optically reactivating the cells labelled in context A. In the following 2 days, we tested the animals’ fear memory in either the original context A or a novel context C. If the light-reactivated cells labelled in context A can produce a functional CS during fear conditioning in context B, then the animals should express a false fear memory by freezing in context A, but not in context C.
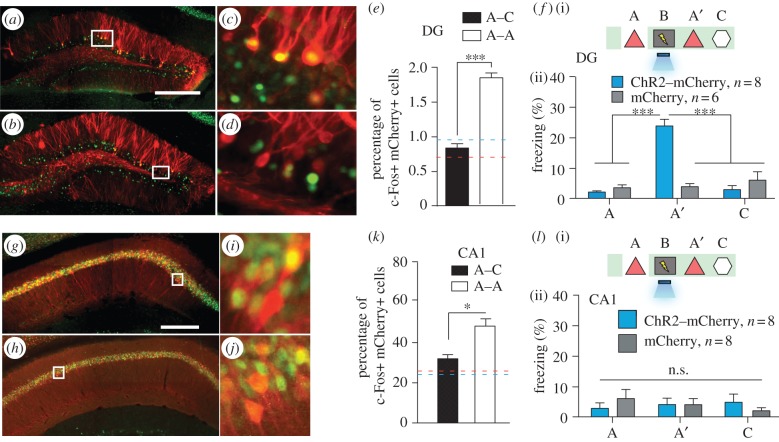
Prior to conducting the behavioural experiments, we confirmed that distinct populations of cells in the DG represent contexts A and C (figure 4a–e), enabling the manipulation of context-specific memories at the level of defined neural populations. When DG cells activated by the exposure to context A were reactivated with light during fear conditioning in a distinct context B, the animals subsequently froze in context A at levels significantly higher than the background levels (figure 4f). Freezing in context C did not differ from background levels (figure 4f). This increased freezing in context A was not due to generalization because a control group expressing only mCherry that underwent the exact same training protocol did not show the same effect (figure 4f). These results indicate that artificial reactivation of DG cells initially activated by exposure to a particular context (context A) can serve as a functional CS during fear conditioning in a distinct context (context B) and results in the formation of a false memory [21].
Figure 4.
Inception of a false contextual fear memory. (a–e) c-fos–tTA mice injected with AAV9-TRE-ChR2-mCherry in the DG were taken off Dox and exposed to context A to label the activated cells with mCherry (red), then put back on Dox and exposed to the same context A (a,c) or a novel context C (b,d) 24 h later to let activated cells express c-Fos (green). Representative images of the DG from these animals are shown in (a–d), and the quantifications from the dorsal or ventral blades of the DG are shown in (e) (n = 4 subjects each; ***p < 0.001, unpaired Student's t-test). Blue and red dashed lines indicate the chance level of overlap for A–A and A–C groups, respectively, which is calculated by multiplying observed percentage of ChR2–mCherry-single-positive and c-Fos-single-positive cells. (f)(i) Training and testing scheme of animals injected with AAV9-TRE-ChR2-mCherry or AAV9-TRE-mCherry. The light green shading indicates the presence of Dox in the diet during corresponding stages of the scheme. Prime (′) indicates the second exposure to a given context. The yellow lightning symbol and blue shower symbol indicate foot shocks and blue light delivery, respectively. (ii) Animals’ freezing levels in context A before fear conditioning, and in context A and C after fear conditioning (n = 8 for ChR2–mCherry group and n = 6 for mCherry group; ***p < 0.001, two-way analysis of variance (ANOVA) with repeated measures followed by Bonferroni posthoc test). (g–k) Animals underwent the same protocol as in (a–e), except the virus injection was targeted to CA1. Representative images of CA1 from these animals are shown in (g–j), and the quantifications are shown in (k) (n = 4 subjects each; *p = 0.009, unpaired Student's t-test). (l) Same as (f), except the viral injection and implants were targeted to CA1 (n = 8 for ChR2–mCherry and mCherry groups; n.s., not significant, two-way ANOVA with repeated measures followed by Bonferroni posthoc test). Scale bar in (a,g) 250 μm.
4. No false-memory inception by CA1 engram manipulation
It is possible that not all c-Fos-expressing brain regions are sufficient to elicit the recall of a CS. Indeed, the hippocampus processes mnemonic information by shifting the combined activity of subsets of cells within defined subregions in response to discrete episodes [19,22]. Given that each subregion differentially contributes to an overall memory, we investigated whether a false memory could be formed by applying the same parameters and manipulations to CA1 as we did to the DG. Similar to the DG (figure 4a–e), the overlap was significantly lower across contexts (A and C) compared with a re-exposure to the same context (A and A) in CA1 (figure 4g–k). However, the degree of overlap for the two contexts was much greater in CA1 (30%) than in the DG (approx. 0.4%; figure 4e,k). Notably, exposure to a single context (A or C) consistently labelled approximately 50% of CA1 cells compared with approximately 6% of DG cells. When we labelled CA1 cells activated in context A and reactivated these cells with light during fear conditioning in context B, no increase in freezing was observed in the experimental group expressing ChR2–mCherry compared with the mCherry-only control group in either context A or context C (figure 4l). These data suggest that applying the same behavioural and stimulation parameters as we did in the DG does not result in the inception of a false memory in CA1.
5. Competition between false and genuine memory
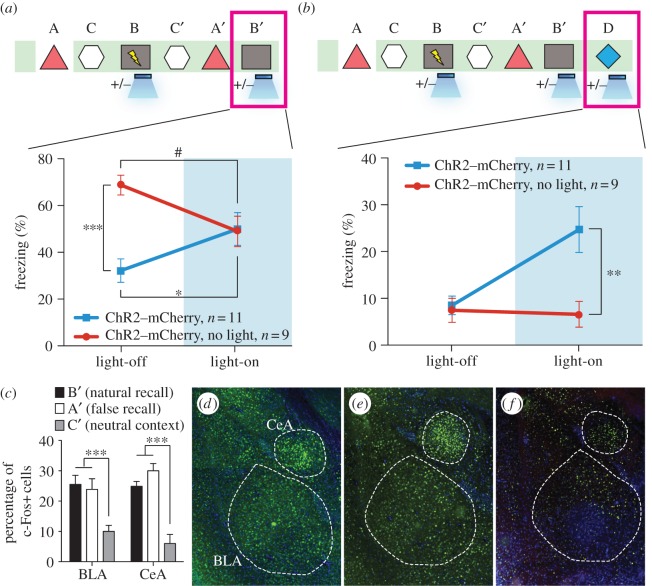
According to classical learning theory, the simultaneous acquisition of two CSs can sometimes be a competitive phenomenon such that a memory for a single CS is acquired optimally when it is presented alone, whereas the presentation of two simultaneous CSs can lead to an overall decrement in behavioural output [23]. In our experiments, it is possible that the light-activated DG cells encoding context A interfered with the acquisition or expression of the genuine fear memory for context B. Indeed, upon re-exposure to context B, the experimental group froze significantly less than the group that did not receive light during fear conditioning or the group expressing mCherry alone (figure 5a). During light-on epochs in the context B test, freezing increased in the experimental group and decreased in the group that did not receive light during fear conditioning (figure 5a). We conducted similar experiments with mice in which the manipulation was targeted to the CA1 region and found no differences in the experimental or control groups during either light-off or light-on epochs of the context B test.
Figure 5.
The false- and genuine-fear memories interact with each other and both recruit the amygdala. (a) Animals that underwent the behavioural protocol shown in figure 2g were re-exposed to context B and the freezing levels were examined both in the absence and presence of light stimulation (n = 11 for ChR2–mCherry group and n = 9 for ChR2–mCherry, no light group; *p = 0.027; ***p < 0.001; #p = 0.034, two-way ANOVA followed by Bonferroni posthoc test). (b) Animals that underwent the behavioural protocol shown in (a) were placed in a novel context D and the freezing levels were examined both in the absence and presence of light stimulation (n = 11 for ChR2–mCherry group and n = 9 for ChR2–mCherry, no light group; **p = 0.007, two-way ANOVA followed by Bonferroni posthoc test). (c) Three groups of mice underwent the training shown in (a) and were sacrificed after testing in either context B (natural recall), A (false recall) or C (neutral context). The percentage of c-Fos-positive cells was calculated for each group in BLA and CeA (n = 6 subjects each; ***p < 0.001). Representative images for natural recall, false recall or neutral context are shown in (d), (e) and (f), respectively.
6. The false memory is a real memory
We could now probe the behavioural relevance of the DG cells that were artificially associated with an aversive event of high valence (e.g. US). If an artificial CS–US association was generated using our experimental parameters, then activation of the CS should now be sufficient to elicit the associated behavioural output (i.e. freezing). To test whether a false fear memory can be artificially recalled by light-reactivation of the corresponding DG cells in a similar way as a genuine fear memory can be light-activated [16], we examined fear-memory recall in experimental and control groups of mice in a distinct context (context D) by administering light-off and light-on epochs (figure 5b). All groups exhibited background levels of freezing during light-off epochs. The experimental group, however, froze at significantly higher levels (approx. 25%) during light-on epochs. This light-induced freezing in context D was not observed in control animals that underwent the same behavioural schedule but did not receive light during fear conditioning in context B, in animals expressing mCherry alone, or in animals in which CA1 was manipulated instead (figure 5b).
To map the downstream brain areas involved during false-memory recall, in separate groups of animals we also performed histological analyses measuring c-Fos expression during three sessions: the false-memory recall test in context A, the context B natural fear-memory recall session and the context C control session. We predicted that the recall of a false memory engages neural substrates known to underlie genuine fear-memory recall, and therefore quantified the levels of c-Fos expression in the basolateral amygdala (BLA) and the central amygdala (CeA) [24–29]. Indeed, false- and genuine-memory recall sessions elicited a significant increase in c-Fos-positive cells in the BLA and CeA compared with a control group exploring a neutral context (figure 5c–f). These results indicated that similar circuit mechanisms underlie false memory as they do genuine-memory recall.
7. Discussion
Our data indicate that hippocampal DG cells activated previously during context exploration can subsequently serve as a functional CS in a fear-conditioning paradigm when artificially reactivated during the delivery of a US. The consequence is the encoding of an artificial associative fear memory to the CS that was not naturally available at the time of the US delivery [30].
Memory is reconstructive in nature; the act of recalling a memory renders it labile and highly susceptible to modification [10–14]. In humans, memory distortions and illusions occur frequently. These modifications often result from the incorporation of misinformation into memory from external sources [10,11,13,14]. Interestingly, cognitive studies in humans have reported robust activity in the hippocampus during the recall of both false and genuine memories [15]. However, human studies using behavioural and fMRI techniques have not been able to delineate the hippocampal subregions and circuits that are responsible for generating false memories. To help to resolve these issues, our experiments provide an animal model in which false and genuine memories can be investigated at the memory engram level [31]. We propose that optical reactivation of cells that were naturally activated during the formation of a contextual memory induced the retrieval of that memory and, more importantly, the retrieved memory became associated with an event of high valence (i.e. a foot shock) to form a new but false memory that never had its component experiences naturally linked. Thus, the experimental group of animals showed increased freezing in a context in which they were never shocked (context A). Although our design for the formation and expression of a false memory was for a laboratory setting, and the retrieval of the contextual memory during conditioning occurred by artificial means (i.e. light), we suggest that the formation of at least some false memories may occur in natural settings by internally driven retrieval of previous experiences and their association with external stimuli of high valence.
A previous study applied a similar experimental protocol with pharmacosynthetic methods and failed to see increased freezing upon re-exposure to either context A or context B. Instead, they observed a synthetic memory that could only be retrieved by the combination of both contexts A and B [32]. A key difference in their system is that the c-Fos-expressing cells in the entire forebrain were labelled and reactivated over an extended period by a synthetic ligand. Moreover, the discrepancy observed here suggests two methodological caveats: the spatial and millisecond precision of region-specific optogenetic manipulations, when compared to forebrain-wide pharmacogenetic perturbations that last several minutes, perhaps more reliably recapitulates the endogenous neural activity required for behavioural expression of a memory; and perhaps not all c-Fos-expressing brain regions are sufficient to elicit the recall of a CS. We propose that activating neurons in much wider spatial and temporal domains may favour the formation of a synthetic memory, which may not be easily retrievable by the cues associated with each individual memory. By contrast, activating neurons in a more spatially (only small populations of DG cells) and temporally restricted manner (only a few minutes during light stimulation) may favour the formation of two distinct (false and genuine) memories as observed in our case. In line with this hypothesis, when we manipulated CA1 cells by the same procedures as the ones used for DG cells, we could not incept a false memory (i.e. freezing in context A). In CA1, the overlap of the cell populations activated by consecutive exposures to a pair of contexts is much greater than that in the DG. We hypothesize that our negative CA1 behavioural data could be a result of contextual engrams relying less on a population code and increasingly on a temporal code as they travel through the trisynaptic circuit [8,9,22].
Together, our findings provide a foundation for an experimental bridge between the traditions of rodent behavioural neuroscience and human cognitive neuroscience in the study of memory by illuminating the underlying circuits supporting internally generated representations and their contribution to the formation of false memories.
Acknowledgements
We thank P. Lin, J. Suh, M. Pignatelli, R. L. Redondo, T. J. Ryan, S. Huang, M. Serock, J. Zhou, D. S. Roy and A. Mockett for help with the experiments, J. Z. Young and K. L. Mulroy for comments and discussions on the manuscript, and all the members of the Tonegawa lab for their support.
Funding statement
This work is supported by NIH grant nos. R01-MH078821, P50-MH58880 to S.T. and RIKEN Brain Science Institute.
References
- 1.Hebb DO. 1949. The organization of behavior. New York, NY: Wiley & Sons [Google Scholar]
- 2.Bliss TVP, Lomo T. 1973. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 232, 331–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kandel ER. 2001. The molecular biology of memory storage: a dialogue between genes and synapses. Science 294, 1030–1038 (doi:10.1126/science.1067020) [DOI] [PubMed] [Google Scholar]
- 4.Semon RW. 1921. The mneme. London, UK: G. Allen & Unwin Ltd [Google Scholar]
- 5.Han JH, et al. 2009. Selective erasure of a fear memory. Science 323, 1492–1496 (doi:10.1126/science.1164139) [DOI] [PubMed] [Google Scholar]
- 6.Martin SJ, Morris RG. 2002. New life in an old idea: the synaptic plasticity and memory hypothesis revisited. Hippocampus 12, 609–636 (doi:10.1002/hipo.10107) [DOI] [PubMed] [Google Scholar]
- 7.Eichenbaum H. 2004. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron 44, 109–120 (doi:10.1016/j.neuron.2004.08.028) [DOI] [PubMed] [Google Scholar]
- 8.MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. 2011. Hippocampal ‘time cells’ bridge the gap in memory for discontiguous events. Neuron 71, 737–749 (doi:10.1016/j.neuron.2011.07.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buzsaki G, Moser EI. 2013. Memory, navigation, and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci. 16, 130–138 (doi:10.1038/nn.3304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loftus EF. 2003. Our changeable memories: legal and practical implications. Nat. Rev. Neurosci. 4, 231–234 (doi:10.1038/nrn1054) [DOI] [PubMed] [Google Scholar]
- 11.Schacter DL, Loftus EF. 2013. Memory and law: what can cognitive neuroscience contribute? Nat. Neurosci. 16, 119–123 (doi:10.1038/nn.3294) [DOI] [PubMed] [Google Scholar]
- 12.Nader K, Schafe GE, LeDoux JE. 2000. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406, 722–726 (doi:10.1038/35021052) [DOI] [PubMed] [Google Scholar]
- 13.Bartlett FC. 1932. Remembering: a study in experimental and social psychology. Cambridge: Cambridge University Press [Google Scholar]
- 14.Roediger HL, McDermott KB. 1995. Creating false memories: remembering words not presented in lists. J. Exp. Psychol. Learn. 24, 803–814 (doi:10.1037/0278-7393.21.4.803) [Google Scholar]
- 15.Cabeza R, Rao SM, Wagner AD, Mayer AR, Schacter DL. 2001. Can medial temporal lobe regions distinguish true from false? An event-related functional MRI study of veridical and illusory recognition memory. Proc. Natl Acad. Sci. USA 98, 4805–4810 (doi:10.1073/pnas.081082698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S. 2012. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385 (doi:10.1038/nature11028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reijmers LG, Perkins BL, Matsuo N, Mayford M. 2007. Localization of a stable neural correlate of associative memory. Science 317, 1230–1233 (doi:10.1126/science.1143839) [DOI] [PubMed] [Google Scholar]
- 18.Kubik S, Miyashita T, Guzowski JF. 2007. Using immediate-early genes to map hippocampal subregional functions. Learn. Mem. 14, 758–770 (doi:10.1101/lm.698107) [DOI] [PubMed] [Google Scholar]
- 19.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. 1999. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat. Neurosci. 2, 1120–1124 (doi:10.1038/16046) [DOI] [PubMed] [Google Scholar]
- 20.Shockett PE, Schatz DG. 1996. Diverse strategies for tetracycline-regulated inducible gene expression. Proc. Natl Acad. Sci. USA 93, 5173–5176 (doi:10.1073/pnas.93.11.5173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramirez S, et al. 2011. Creating a false memory in the hippocampus. Science 341, 387–391 (doi:10.1126/science.1239073) [DOI] [PubMed] [Google Scholar]
- 22.Leutgeb JK, Leutgeb S, Moser MB, Moser EI. 2007. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science 315, 961–966 (doi:10.1126/science.1135801) [DOI] [PubMed] [Google Scholar]
- 23.Brandon SE, Vogel EH, Wagner AR. 2010. A componential view of configural cues in generalization and discrimination in Pavlovian conditioning. Behav. Brain Res. 110, 67–72 (doi:10.1016/S0166-4328(99)00185-0) [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J, Neve R, Poirazi P, Silva AJ. 2009. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat. Neurosci. 12, 1438–1443 (doi:10.1038/nn.2405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogan M, Staubli U, LeDoux J. 1997. Fear conditioning induces associative long-term potentiation in the amygdala. Nature 390, 604–607 (doi:10.1038/37601) [DOI] [PubMed] [Google Scholar]
- 26.Johansen JP, Hamanaka H, Monfils MH, Behnia R, Deisseroth K, Blair HT, LeDoux JE. 2010. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proc. Natl Acad. Sci. USA 107, 12 692–12 697 (doi:10.1073/pnas.1002418107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maren S, Quirk GJ. 2004. Neuronal signaling of fear memory. Nat. Rev. Neurosci. 5, 844–852 (doi:10.1038/nrn1535) [DOI] [PubMed] [Google Scholar]
- 28.Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B. 2013. Experience-dependent modification of a central amygdala fear circuit. Nat. Neurosci. 16, 332–339 (doi:10.1038/nn.3322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ciocchi S, et al. 2010. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468, 277–282 (doi:10.1038/nature09559) [DOI] [PubMed] [Google Scholar]
- 30.Choi GB, et al. 2011. Driving opposing behaviors with ensembles of piriform neurons. Cell 146, 1004–1015 (doi:10.1016/j.cell.2011.07.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McTighe SM, Cowell RA, Winters BD, Bussey TJ, Saksida LM. 2010. Paradoxical false memory for objects after brain damage. Science 330, 1408–1410 (doi:10.1126/science.1194780) [DOI] [PubMed] [Google Scholar]
- 32.Garner AR, Rowland DC, Hwang SY, Baumgaertel K, Roth BL, Kentros C, Mayford M. 2012. Generation of a synthetic memory trace. Science 335, 1513–1516 (doi:10.1126/science.1214985) [DOI] [PMC free article] [PubMed] [Google Scholar]