Abstract
We have previously shown that when over-expressed in neurons, green fluorescent protein (GFP) tagged GluA1 (GluA1-GFP) delivery into synapses is dependent on plasticity. A recent study suggests that GluA1 over-expression leads to its incorporation into the synapse, in the absence of additional long-term potentiation-like manipulations. It is possible that a GFP tag was responsible for the difference. Using rectification index as a measure of synaptic delivery of GluA1, we found no difference in the synaptic delivery of GluA1-GFP versus untagged GluA1. We recently published a study showing that while D-APV blocks NMDAr-dependent long-term depression (LTD), MK-801 and 7-chloro kynurenate (7CK) fail to block LTD. We propose a metabotropic function for the NMDA receptor in LTD induction. In contrast to our observations, recent unpublished data suggest that the above antagonists are equally effective in blocking LTD. We noticed different methodology in their study. Here, we show that their methodology has complex effects on synaptic transmission. Therefore, it is not possible to conclude that 7CK is effective in blocking LTD from their type of experiment.
Keywords: AMPA receptors, long-term potentiation, long-term depression
1. Introduction
We will take this opportunity to address two issues that have been raised with respect to studies published from the Malinow laboratory. The first is with regard to the trafficking of GluA1 to synapses; the second is with regard to the role of NMDA receptors in long-term depression (LTD).
We have previously published studies indicating that when over-expressed in neurons, green fluorescent protein (GFP) tagged GluA1 (GluA1-GFP) fails to go into synapses [1]; however, GluA1-GFP can be added to synapses by long-term potentiation (LTP) [2], experience [3], constitutively active CaMKII [2] or over-expressed PSD-95 [3]. An independent group studied the association fibre input to the CA3 neurons and found that over-expression of GluA1-GFP only went into synapses with LTP stimulus [4]. Other groups have examined the subunit-specific nature of AMPA receptor incorporation during chemically induced LTP (chemLTP) [5–7]. One group found that chemLTP produced a selective incorporation of GluA1 onto the neuronal surface [8]. These studies are consistent with a model in which newly synthesized GluA1 is restricted away from synapses and only upon LTP-like stimuli is this receptor stably added to synapses. A recent study from the Nicoll laboratory showed data indicating that GluA1 over-expression led to its incorporation into the synapse, in the absence of additional LTP-like manipulations [9]. The authors suggested that the GFP tag could account for the differences observed in GluA1 trafficking. We have sought to test this possibility.
A second issue we have recently addressed is the role of the NMDA receptor in LTD. We recently published a study in which we show that while D-APV blocks LTD, MK-801 and 7-chloro kynurenate (7CK) fail to block LTD [10]. We propose that ligand-dependent ion-flux-independent signalling by the NMDA receptor is responsible for LTD. After presenting this work at meetings, an investigator indicated that experiments carried out in his laboratory showed block of LTD by MK-801 and 7CK (R. Malenka 2012, personal communication). However, we noticed that the methodology in our study and their study was different. In our study, we maintained the drugs in the bathing solution before, during and after low-frequency conditioning (LFS). In their study, they added the drugs briefly before conditioning and washed them out soon after conditioning. We sought to investigate any possible differences in methodology that could account for the different results.
2. Results: GluA1 trafficking
To examine the potential difference in trafficking to synapses between GluA1 with and without a GFP tag, we generated four double promoter Sindbis viruses that express the following:
virus (1) promoter 1: GluA1-GFP; promoter 2: empty;
virus (2) promoter 1: GluA1; promoter 2: GFP;
virus (3) promoter 1: GluA2-GFP; promoter 2: empty;
virus (4) promoter 1: GluA2; promoter 2: GFP.
Organotypic hippocampal slices were prepared and infected with each one of these viruses. Recombinant proteins were allowed to express for 16–24 h. Infected slices were placed in a recording chamber and whole-cell recordings were obtained from cells displaying GFP fluorescence. Internal solution contained Cs+. Excitatory transmission was evoked by placement of stimulating electrodes in stratum radiatum; perfusate contained 100 µM picrotoxin and 100 µM D-APV to isolate AMPA receptor-mediated transmission. Synaptic responses (n = 100–200) were obtained at holding potentials of −60, +40 and 0 mV. Rectification index (RI) for each recording was obtained by measuring the average synaptic current at each holding potential and calculating
where IX indicates synaptic current at holding potential X mV.
Our findings are summarized in figure 1. We found no significant difference among the RI values for synaptic transmission onto neurons expressing GluA1 tagged with GFP, untagged GluA1 coexpressed with GFP and non-infected neurons. As we have previously reported [1], we did see a significant difference in the RI values for synaptic transmission onto neurons expressing GluA1 (tagged or untagged) and GluA2 (tagged or untagged). We conclude that the difference in the trafficking behaviour for GluA1 described in the Granger study [9] and studies from this laboratory cannot be explained by a GFP tag on the receptor.
Figure 1.
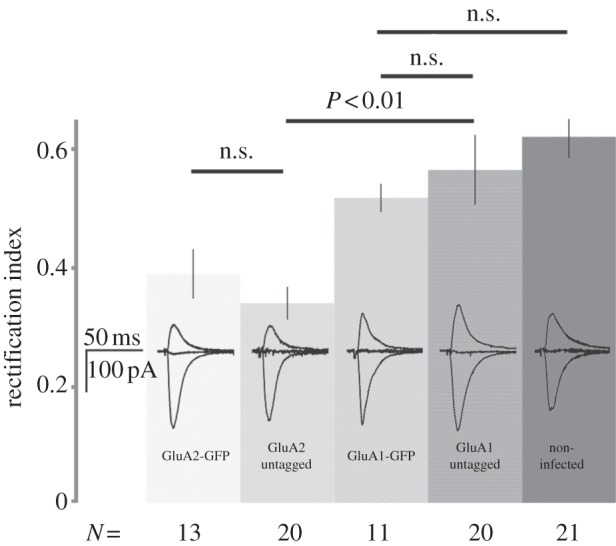
Bar graph indicating RI (see text for definition) for synaptic transmission onto neurons expressing different constructs (indicated on bars). Number of recordings indicated below each bar. p-values (Student's t-test) indicated above bars; n.s., non-significant. Traces display recordings made at −60, 0 and +40 mV from neurons expressing the indicated constructs.
3. Results: 7-chloro kynurenate block of LTD
We focused on the use of 7CK since this is not a use-dependent blocker and thus avoids issues related to the efficacy of NMDA receptor blockade. First, we tested if we could reproduce our own previous findings by examining LTD in acute hippocampal slices that were perfused before, during and after conditioning in bathing solution containing 100 µM 7CK. As shown in figure 2, LTD remained intact in these conditions. We next examined conditions in which 7CK was reported to block LTD. One difference in our study was that we monitored a control pathway in addition to the conditioned pathway. In addition, the other group used 10 µM 7CK (rather than 100 µM in our experiments; 10 µM 7CK did effectively block NMDA currents; figure 3b, inset). Following a baseline period of 13 min, 10 µM 7CK was added to the bathing solution and 7 min of continued baseline transmission displayed no changes. At this point, a low-frequency stimulus (3 Hz, 7 min, as reported by the investigator) was delivered to the conditioned pathway. After conditioning, transmission was monitored on the control and conditioned pathway. 7CK was washed from the bathing solution following conditioning. As reported by the investigator, the test pathway did return to baseline levels (in fact, transmission returned to slightly higher levels than baseline transmission). Surprisingly, transmission in the control pathway returned to a level that was significantly potentiated relative to the baseline period and relative to the test pathway. Stated another way, following LFS in the presence of 7CK, the conditioned pathway remained significantly depressed relative to a control pathway.
Figure 2.
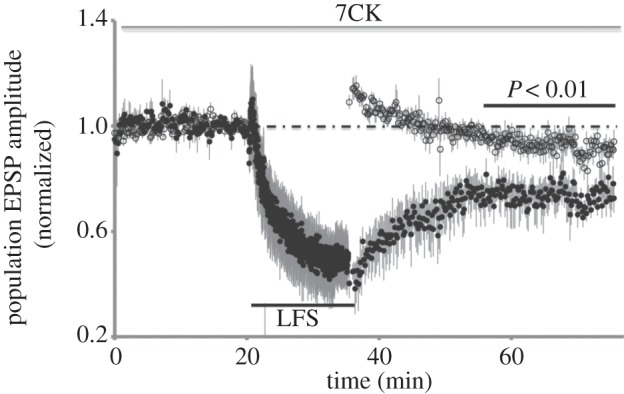
Evoked synaptic transmission plotted versus time for experiments (n = 5) in which 100 µM 7CK was maintained in the bath before, during and after LFS (15 min, 1 Hz, where indicated) delivered to test pathway (filled circles). Control pathway (open circles) received no stimulation during LFS. p-value (paired Student's t-test) comparing averaged transmission in test and control pathways at indicated period.
Figure 3.
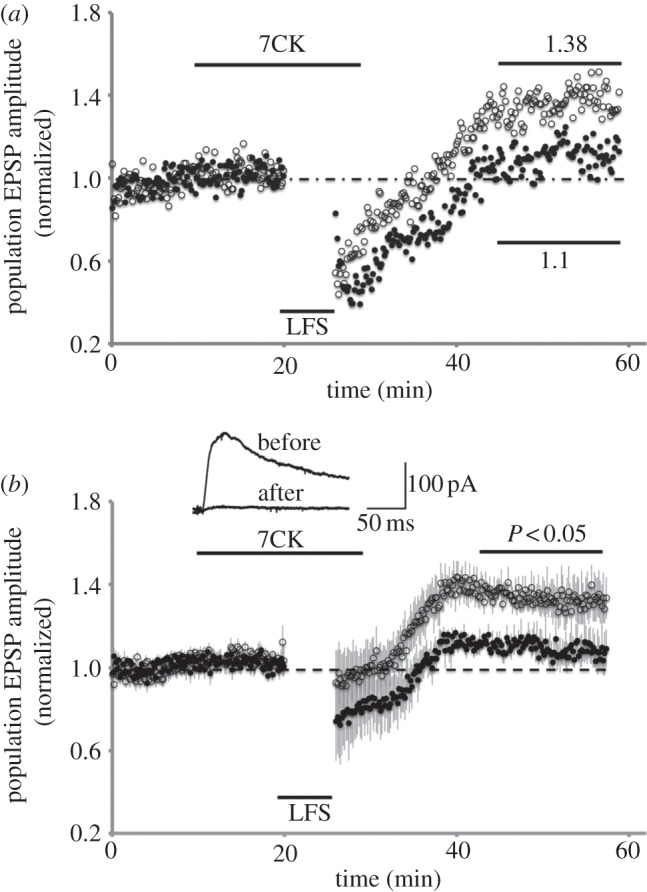
Evoked synaptic transmission plotted versus time for individual (a) and average of four (b) experiments in which 10 µM 7CK was maintained in the bath only during LFS (7 min, 3 Hz, where indicated) delivered to test pathway (filled circles). Control pathway (open circles) received no stimulation during LFS. Average transmission relative to baseline period indicated in (a). P-value (paired Student's t-test) comparing averaged transmission in test and control pathways indicated in (b). Inset displays whole-cell recording of evoked transmission onto CA1 neuron with holding potential at +40 mV, before and after bath application of 10 µM 7CK.
4. Discussion
In this study, we address two issues that have been raised regarding our recent publications. The first issue concerns the trafficking of recombinant GluA1 receptors to synapses in organotypic slices. We have previously reported that expression of GluA1-GFP could not be detected at synapses in the absence of an LTP stimulus [1]. Granger et al. [9] found that over-expression of GluA1 lacking a GFP tag could be detected at synapses in the absence of an LTP stimulus. As one possibility, the authors suggested that the GFP tag could account for the difference. In this study, we are unable to demonstrate an effect of the GFP tag: GluA1 with and without a GFP tag fail to be detected at synapses following their expression in organotypic slices. Possible explanations for their finding that over-expressed GluA1 can reach synapses may be that the level of neuronal activity in their slices is higher than in our slices, and that this is sufficient to induce plasticity. While they tested this possibility by incubating in high Mg2+, which reduces spontaneous activity, it may be more definitive to incubate slices in D-APV to block activity-driven plasticity.
We recently reported that 7CK fails to block LTD [10]. The block of LTD by 7CK observed by another group (R. Malenka 2012, personal communication) may be owing to differences in the details of the experimental design. In our view, washing 7CK in and out of solution in combination with delivery of LFS has complex effects on synaptic transmission. We believe that it is not possible to conclude from this type of experiment that 7CK is effective in blocking LTD. To determine the exact mechanisms underlying the potentiation of transmission seen in the control pathway in this experiment will require additional studies. We believe that maintaining inhibitors before, during and after conditioning, and recording of a test and control pathway, allows interpretation of results which suggest that ion flux through the NMDA receptor is not required for LTD induction.
5. Methods
Preparation of organotypic slices and viruses, and whole-cell recording methods were as previously described [1]. Preparation of acute brain slices and field recording methods were as described in [10]. Drugs were obtained from Tocris Bioscience.
References
- 1.Shi S, Hayashi Y, Esteban JA, Malinow R. 2001. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105, 331–343 (doi:10.1016/S0092-8674(01)00321-X) [DOI] [PubMed] [Google Scholar]
- 2.Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. 2000. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287, 2262–2267 (doi:10.1126/science.287.5461.2262) [DOI] [PubMed] [Google Scholar]
- 3.Ehrlich I, Malinow R. 2004. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J. Neurosci. 24, 916–927 (doi:10.1523/JNEUROSCI.4733-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kakegawa W, Tsuzuki K, Yoshida Y, Kameyama K, Ozawa S. 2004. Input- and subunit-specific AMPA receptor trafficking underlying long-term potentiation at hippocampal CA3 synapses. Eur. J. Neurosci. 20, 101–110 (doi:10.1111/j.1460-9568.2004.03461.x) [DOI] [PubMed] [Google Scholar]
- 5.Liao D, Scannevin RH, Huganir R. 2001. Activation of silent synapses by rapid activity-dependent synaptic recruitment of AMPA receptors. J. Neurosci. 21, 6008–6017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. 2001. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron 29, 243–254 (doi:10.1016/S0896-6273(01)00194-5) [DOI] [PubMed] [Google Scholar]
- 7.Oh MC, Derkach VA, Guire ES, Soderling TR. 2006. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J. Biol. Chem. 281, 752–758 (doi:10.1074/jbc.M509677200) [DOI] [PubMed] [Google Scholar]
- 8.Appleby VJ, Correa SA, Duckworth JK, Nash JE, Noel J, Fitzjohn SM, Collingridge GL, Molnar E. 2011. LTP in hippocampal neurons is associated with a CaMKII-mediated increase in GluA1 surface expression. J. Neurochem. 116, 530–543 (doi:10.1111/j.1471-4159.2010.07133.x) [DOI] [PubMed] [Google Scholar]
- 9.Granger AJ, Shi Y, Lu W, Cerpas M, Nicoll RA. 2013. LTP requires a reserve pool of glutamate receptors independent of subunit type. Nature 493, 495–500 (doi:10.1038/nature11775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nabavi S, Kessels HW, Alfonso S, Aow J, Fox R, Malinow R. 2013. Metabotropic NMDA receptor function is required for NMDA receptor-dependent long-term depression. Proc. Natl Acad. Sci. USA 110, 4027–4032 (doi:10.1073/pnas.1219454110) [DOI] [PMC free article] [PubMed] [Google Scholar]


