Abstract
Prolonged and severe stress leads to cognitive deficits, but facilitates emotional behaviour. Little is known about the synaptic basis for this contrast. Here, we report that in rats subjected to chronic immobilization stress, long-term potentiation (LTP) and NMDA receptor (NMDAR)-mediated synaptic responses are enhanced in principal neurons of the lateral amygdala, a brain area involved in fear memory formation. This is accompanied by electrophysiological and morphological changes consistent with the formation of ‘silent synapses’, containing only NMDARs. In parallel, chronic stress also reduces synaptic inhibition. Together, these synaptic changes would enable amygdalar neurons to undergo further experience-dependent modifications, leading to stronger fear memories. Consistent with this prediction, stressed animals exhibit enhanced conditioned fear. Hence, stress may leave its mark in the amygdala by generating new synapses with greater capacity for plasticity, thereby creating an ideal neuronal substrate for affective disorders. These findings also highlight the unique features of stress-induced plasticity in the amygdala that are strikingly different from the stress-induced impairment of structure and function in the hippocampus.
Keywords: long-term potentiation, neural plasticity, emotion, NMDA receptors, silent synapses, dendritic spines
1. Introduction
Chronic stress triggers a wide spectrum of behavioural abnormalities [1,2]. Interestingly, some of the key symptoms of stress disorders display contrasting behavioural manifestations. This difference is strikingly evident in the effects of stress on the output of two brain areas critically involved in learning and memory—the hippocampus and amygdala. There is considerable evidence showing how repeated stress impairs hippocampal function at multiple levels of neural organization [1–4]. At the cellular level, various animal models of stress cause dendritic atrophy and suppress hippocampal long-term synaptic potentiation (LTP) mediated by the NMDA (N-methyl-d-aspartate) subtype of glutamate receptors (NMDARs) [1,5,6]. Impaired NMDAR-dependent LTP and dendritic atrophy, in turn, are believed to be key factors contributing to chronic stress-induced deficits in hippocampal learning and memory [3,7–9]. Strikingly, NMDAR-dependent LTP in the lateral amygdala (LA) also plays a pivotal role in forms of emotional memory such as classical fear conditioning [10,11]. Why then does repeated stress facilitate amygdala-dependent fear learning [12–16], when previous studies report stress-induced suppression of NMDAR-dependent LTP in the hippocampus [1,5,6]? The goal of this study is to investigate the synaptic basis for this contrast in the amygdala.
2. Material and methods
(a). Experimental animals
Male Wistar rats, 60–65 days old, were used for the chronic immobilization stress protocol. All animals were housed in groups of two or three with ad libitum access to food and water, unless specified otherwise in the stress protocols. Control animals, which were age matched with the stress-treated animals, were housed in separate cages. Animals were maintained in a temperature-controlled room, with a 14 L : 10 D cycle (lights on at 06.00). All procedures related to maintenance and experimentation were approved by the Institutional Animal Ethics Committee, National Centre for Biological Sciences (NCBS), Bangalore, India. Rats in the experimental group were subjected to chronic immobilization stress for 10 consecutive days as described earlier [17,18]. This stress paradigm consisted of complete immobilization (2 h per day, 10.00–noon) in rodent immobilization bags without access to either food or water. Control animals were not subjected to any type of stress.
(b). Slice preparation
Animals were deeply anaesthetized with halothane 1 day after the 10 day stress protocol, decapitated, and the brains were removed quickly and transferred to ice-cold artificial cerebrospinal fluid (aCSF) containing (in mM): NaCl, 115; KCl, 3.3; CaCl2, 2; MgCl2, 1; NaHCO3, 25.5; NaH2PO4, 1.05; glucose, 25 (pH 7.4; equilibrated with 95% O2/5% CO2). Coronal slices (400 µm), containing the amygdala, were cut in aCSF (4°C) using a Vibratome-1000 Plus (Technical Products International, St Louis, MO, USA) and then transferred to a storage chamber containing aCSF (room temperature; equilibrated with 95% O2/5% CO2), where they were allowed to recover for at least 1 h before being transferred to a submerged recording chamber at room temperature, attached to an upright microscope (Olympus BX50WI, Melville, NY, USA).
(c). Electrophysiology
Whole-cell patch-clamp recordings, from excitatory principal neurons in the dorsal part of the LA (figure 1b), were obtained under IR-DIC visualization (BX50WI, Olympus, USA) with an EPC-9 amplifier (HEKA Elektronik, Lambrecht, Germany). To this end, we selected neurons possessing large somata, which are typical of spiny excitatory principal neurons in the LA [19,20]. Whole-cell pipettes (3–6 MΩ) for current-clamp recordings were filled with (in mM): K-gluconate, 130; KCl, 5; MgCl2, 2; Mg-ATP, 2; Na-GTP, 0.3; HEPES, 10; EGTA, 0.6 (pH 7.3). For voltage-clamp experiments patch pipettes contained (in mM): CsOH, 120; d-gluconic acid, 120; CsCl, 10; HEPES, 10; NaCl, 8; QX-314, 5; Mg-ATP, 2; GTP, 0.3; EGTA 0.2 (pH 7.3). All membrane potentials were corrected for junction potential. Only cells with membrane potentials more negative than −60 mV and action potentials that exceeded 0 mV were included in this study. Further, series resistance (Rs) was tested and recordings were not used if they changed by more than 10% from beginning to end or if the Rs exceeded 20 MΩ.
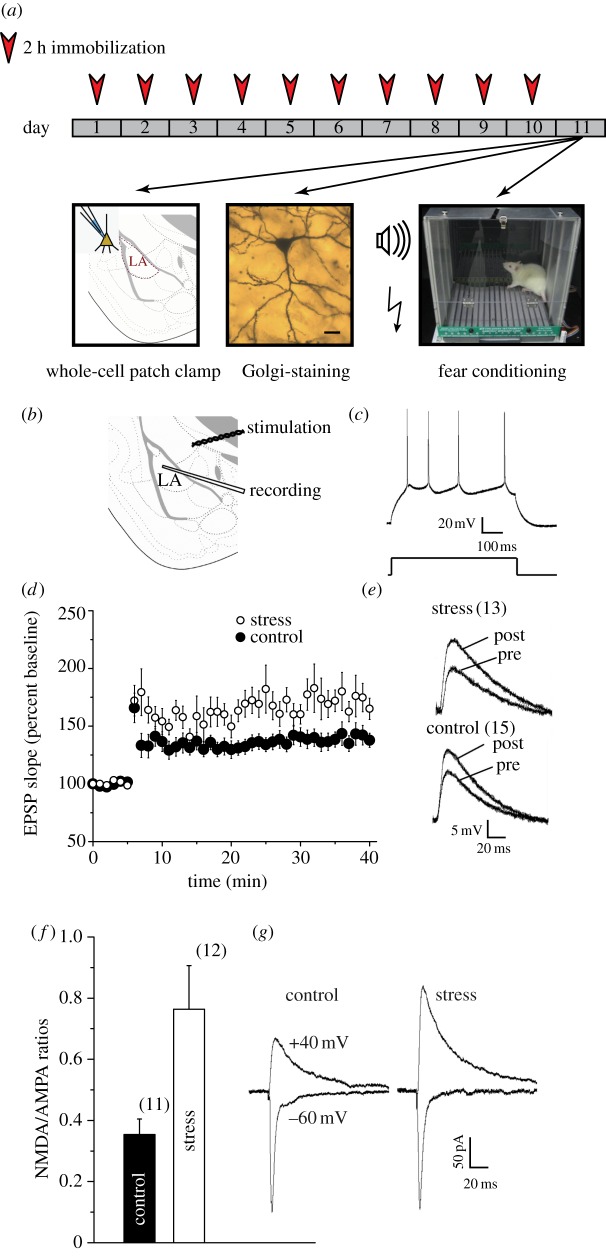
Figure 1.
Chronic stress enhances long-term potentiation (LTP) at thalamic inputs to projection neurons in the lateral amygdala (LA). (a) Schematic of experimental protocol: animals were subjected to 2 h of immobilization, for 10 consecutive days. On the 11th day, animals either underwent fear conditioning, or were sacrificed for Golgi staining or for slice electrophysiology. (b) Placement of recording and stimulating electrodes in a coronal slice of the amygdala. (c) Current-clamp recordings of accommodating action potential firing (top) from a typical LA principal neuron in response to depolarizing current injection (bottom, 600 ms, 0.1 nA). (d) Significantly (*p < 0.05, Student's t-test) greater LTP was induced in stress neurons (open circles, n = 13 cells, from 13 rats) compared with control neurons (filled circles, n = 15 cells, from 15 rats). Summary graphs depict the time course of LTP induced by 30 Hz tetanus (100 pulses per train at 30 Hz, two trains delivered 10 s apart). (e) Superimposed sample traces, from control (bottom) and stress (top) neurons, showing individual EPSPs immediately before (pre) and 35 min after (post) induction of LTP. (f) Summary graph showing significant increase in mean NMDAR/AMPAR-EPSC ratio values in stress neurons (n = 10 cells, 10 rats) compared with controls (n = 11 cells, 11 rats). (g) Traces (averages of 10 recorded responses) of sample AMPAR (−60 mV) and NMDAR (+40 mV) EPSCs from control (left) and stress (right) neurons; traces were selected to obtain matching AMPAR-EPSCs at −60 mV. Error bars are±s.e.m. (Online version in colour.)
Electrical stimulation (100 µs pulse duration; 500 µs for excitatory postsynaptic potential (EPSP)–inhibitory postsynaptic potential (IPSP) sequence experiments) was delivered through bipolar platinum/iridium electrodes (Frederick Haer & Co., Bowdoin, ME, USA) to internal capsule fibres in the ventral striatum just medial to the dorsal LA (figure 1b). This stimulation configuration has been shown to activate auditory thalamic afferents to the LA [19]. Data were acquired at 4 KHz, filtered at 2.9 KHz and analysed with Pulse 8.65, PulseFit 8.65 (Heka Elektronik) and Igor Pro (Wave Metrics, Portland, OR, USA).
For LTP experiments, baseline synaptic responses were monitored at 0.33 Hz. After obtaining a stable baseline, LTP was induced by applying a 30 Hz tetanus protocol (100 stimuli at 30 Hz, given twice with a 20 s interval) that has been established in previous studies [11]. For each cell, the stimulation intensity for LTP induction was the same as that used to elicit the pre-tetanus baseline. To evaluate the magnitude of LTP achieved in any given neuron, the average initial slope of the EPSP recorded during the last 5 min of the recording session (35–40 min post-tetanus) was compared with the pre-tetanus average baseline. Comparison of the percentages of LTP between groups (control versus stress) was tested with a two-tailed, independent Student's t-test.
To calculate the ratio of NMDAR-mediated to AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor (AMPAR)-mediated excitatory postsynaptic currents (EPSCs) [20], we first measured the peak amplitude of evoked AMPAR-EPSCs (100–200 pA) recorded at −60 mV, following which the AMPA–kainate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μM) was washed in for 20 min and NMDAR-EPSCs were recorded at +40 mV, and the peak amplitude was measured. In addition, 75 μM picrotoxin and 100 μM nifedipine (to block L-type Ca2+ channels) were present during all voltage-clamp recordings of NMDAR-EPSCs at +40 mV.
Cells were held at −70 mV for recording spontaneous IPSCs (sIPSCs), which were pharmacologically isolated by adding CNQX (10 μM) and the NMDAR antagonist d-2-amino-5-phosphonovalerate (D-AP5, 33 μM) to the recording solution. Application of the gamma-aminobutyric acid (GABA)A receptor antagonist bicuculline (10 μM) completely blocked the sIPSCs. The Mini Analysis Program (Synaptosoft, Fort Lee, NJ, USA) was used to analyse spontaneous synaptic activity, and the threshold for detection of events by the program was set at 10 pA. The average frequency, inter-event interval and amplitude for sIPSCs in any given neuron were calculated from the total number of synaptic events that were captured for the first 600 s of recording from that neuron. Statistical comparisons of sIPSC frequency and amplitude were made using ‘n’ as number of cells with Student's t-test. Further statistical comparisons of the inter-event interval and amplitude of synaptic currents were made using cumulative probability analysis, with statistical significance determined by the Kolmogorov–Smirnov non-parametric two-sample test (p < 0.05 was considered significant).
For EPSP–IPSP sequence experiments, a 2 s sequence was recorded while holding the cells between −55 and −58 mV. Stimulus intensity was adjusted to get an EPSP amplitude of approximately 7–10 mV while obtaining a clean disynaptic EPSP–IPSP sequence for 25 traces. The cell was then voltage clamped at Ereversal for GABAA (−75 to 77 mV) for another 25 traces. The EPSP found at Ereversal was scaled to the slope of the EPSP in the EPSP–IPSP sequence, and subtracted to obtain a pure IPSP. This IPSP was then compared between stress and control animals.
For measuring, NMDA–AMPA ratios of mEPSCs, cells were voltage clamped at −40 mV in the absence of extracellular Mg2+ and in 50 µM glycine (in addition to standard aCSF). External solution also contained 0.5 µM tetrodotoxin (Alomone Labs, Israel), 75 µM picrotoxin, 10 µM bicuculline methiodide. Internal solution contained (in mM): d-gluconic acid, 125; CsOH, 125; CsCl, 10; NaCl, 4; Mg-ATP, 2; GTP, 0.3; HEPES, 10; EGTA, 0.2 (pH set to 7.3 with CsOH). After 5 min of recording, aCSF containing 1 mM Mg2+ and 50 µM D-AP5 was then washed in for 1 min before events were collected again. Fifty randomly picked events in each condition were selected with a detection threshold of 15 pA, aligned by rise times and averaged. The NMDA + AMPA trace was scaled to the peak amplitude of AMPA.
CNQX and D-AP5 were obtained from Tocris (Balwin, MO, USA), or from Sigma (St. Louis, MO, USA) and QX-314 was obtained from Alomone Labs. All other drugs were from Sigma. Experimental values are expressed as mean ± s.e.m., and sample size, n, represents the number of cells. All statistical comparisons were carried out with paired Student's t-test (unless stated otherwise), and p < 0.05 was considered significant.
(d). Golgi staining and spine-density analysis
One day after completion of stress, i.e. on day 11, animals were anaesthetized using halothane, decapitated immediately, and the whole brain was submerged in Golgi–Cox fixative [21]. Golgi–Cox fixative is composed of 5% potassium dichromate, 5% mercuric chloride and 4% potassium chloride solutions in double distilled water. The three solutions were sequentially mixed and diluted. Emergent precipitate was dissolved with sodium chloride.
The brains remained in Golgi–Cox fixative for a period of 8–10 weeks at room temperature, after which the brains were processed in nitrocellulose blocks, and 120 µm thick coronal sections were obtained using a rotary microtome (Jung RM 2055; Leica, Rueil-Malmaison, France). Sections were collected serially, dehydrated in absolute alcohol, cleared in xylene and then coverslipped. Slides were coded prior to quantitative analysis. The experimenter was blind to the code, which was broken only after the morphological analysis was completed.
The basolateral amygdala neurons selected for analysis were restricted to bregma −2.0 to −3.2 mm. A criterion to select a neuron was chosen as described earlier [18], and analysis was performed using the NeuroLucida image analysis system (Micro-BrightField, Williston, VT, USA) attached to an Olympus BX61 microscope (100×, 1.3 N.A., Olympus BX61; Olympus, Shinjuku-Ku, Tokyo, Japan); all protrusions, irrespective of their morphological characteristics, were counted as spines. For the purpose of this study, dendrites directly originating from the cell soma of pyramidal cells were classified as main shaft and those originating from the main shaft were classified as primary dendrites. Spines were counted along an 80 µm region of primary apical dendrite emerging from the main shaft. Values are reported as mean ± s.e.m. Statistical significance was calculated using Student's t-test and n refers to the number of dendrites used for spine-density analysis.
(e). Fear conditioning protocol
Fear conditioning was done 1 day after the end of the 10 day stress protocol. Conditioning and tone testing were conducted in two distinct chambers. For conditioning, rats were placed in a conditioning chamber (context A, model E10-11R, Coulbourn Instruments, USA) with a metal grid floor. Context A was dimly illuminated by a single house light. Testing took place in a home-cage-like chamber (context B) with additional transparent Plexiglas walls that was brightly illuminated with white light. Only during the testing session, foam with peppermint odour was present on the floor tray kept under the home cage. Both conditioning and testing chambers were placed within a sound-attenuating cubicle (model E10-24, Coulbourn Instruments). The apparatus was cleaned with 70% ethyl alcohol after each use. A video camera was used to videotape behaviour.
Twenty-four hours before conditioning, rats were habituated for 15 min to both contexts A and B (see figure 4a). On the day of conditioning, following a 3 min acclimation period to the conditioning chamber, rats received five pairings of a 20 s tone conditioned stimulus (CS) (5 kHz, 70 dB) that co-terminated with a foot-shock unconditioned stimulus (US) (0.5 s, 0.5 mA). Following conditioning, all rats were returned to their home cages. Long-term memory for the tone was evaluated the following day (i.e. 24 h after conditioning). Rats were placed in context B and presented with three tones (30 s, 5 kHz, 70 dB, ITI = 120 s, varied between 90 and 150 s). Fear memory was measured from the videotape by scoring manually the amount of time during each tone presentation that rats engaged in freezing behaviour, defined as a lack of all other movements except respiration.
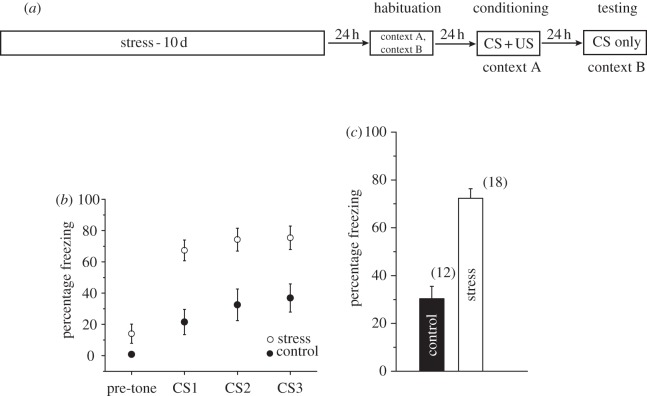
Figure 4.
Chronic stress enhances the expression of auditory fear conditioning. (a) Outline of general behavioural procedures. (b) Mean percentage freezing in control (n = 12) and stress (n = 18) rats in the testing chamber (context B) before tone presentation (‘pre-tone’), which is followed by the three tones (CS1, CS2, CS3). (c) Percentage freezing averaged for all three tones (mean) in control (n = 12) and stress (n = 18) rats showing a significant enhancement in the expression of fear memory after chronic stress. Error bars are ± s.e.m.
3. Results
We first examined the electrophysiological impact of chronic immobilization stress (2 h per day for 10 consecutive days) on principal neurons of the LA. To this end, we obtained whole-cell current-clamp recordings in coronal brain slices (figure 1b) prepared from unstressed control (‘control’) and stress-treated male rats (‘stress’) 24 h after the end of the 10-day chronic stress protocol. As reported earlier [11,20,22], these LA principal neurons show spike frequency adaptation upon injection of depolarizing current (figure 1c). In view of previous reports on stress-induced suppression of NMDAR-dependent LTP in the hippocampus, we first examined the impact of stress on a well-established form of LTP at thalamic inputs (figure 1b) to the LA that also requires NMDAR activation. Strikingly, compared with LTP in control animals (139 ± 5% of pre-tetanus baseline, n = 15 slices; figure 1d,e), LTP was significantly enhanced in stress-treated animals (174 ± 11%, n = 13). Thus, these results provide direct evidence that, in contrast to the hippocampus, LTP at excitatory synaptic inputs from thalamic afferents to LA is facilitated by chronic exposure to stress.
What are the synaptic changes that could potentially contribute to this enhanced LTP? Stimulation of thalamic afferents to LA activates both postsynaptic AMPARs and NMDARs [20]. We therefore compared the relative contribution of AMPARs and NMDARs with thalamic EPSCs recorded in LA principal neurons 24 h after the end of the chronic stress protocol. To this end, we used a metric established earlier in hippocampal neurons in vitro—we quantified the ratio of NMDAR-mediated EPSCs (NMDAR-EPSCs) to those mediated by AMPARs (AMPAR-EPSCs) [20] (§2c). This assay also offers the important advantage that it is independent of the number of synapses activated and therefore independent of the variability between individual slices in terms of the anatomical distribution of afferents or positioning of stimulating electrodes. Using this measure, we found that rats exposed to chronic stress exhibit more than twice the ratio of NMDAR to AMPAR-EPSCs compared with control rats (figure 1f,g).
The findings presented so far show that stress increases the ratio of NMDAR to AMPAR-mediated synaptic currents, NMDAR-dependent LTP, and overall NMDAR levels in the LA. Taken together, these synaptic changes are reminiscent of earlier observations on the development of so-called silent synapses in the hippocampus and cortex [23,24]. Such synapses containing only NMDARs and little or no AMPARs, despite being functionally silent at resting membrane potentials, provide a ready substrate for the creation of new, functional synapses through the insertion of AMPARs. If stress indeed generates more silent synapses in the LA, then it would increase NMDAR-mediated synaptic responses and also enhance the magnitude of LTP, as seen in the results presented here. Therefore, we examined this possibility in greater detail using electrophysiological assays for silent synapses that were previously established in area CA1 of the hippocampus. To this end, we first compared the coefficient of variation (CV) of the AMPAR-EPSCs and NMDAR-EPSCs in the same LA cell. The CV, which measures the trial-to-trial variability of synaptic responses, varies inversely with quantal content. In other words, the larger the number of synapses contributing to the EPSC being measured, the lower its CV. If an evoked EPSC consists of both functional and silent synapses, then the CV of the evoked NMDAR-EPSC would be smaller than that of the corresponding AMPAR-EPSC. Because the CV of the EPSC is inversely related to the number of synapses contributing to it, this would imply an increase in the number of NMDAR-containing synapses. Therefore, we hypothesized that if the stress-induced increase in NMDAR-EPSC in LA neurons is due to an increase in silent synapses, we should observe a decrease in the within-cell ratio of the CV of NMDAR-EPSCs to CV of AMPAR-EPSCs. In agreement with this prediction, the ratio of CVs was indeed significantly smaller in LA neurons from stressed animals compared with unstressed ones (figure 2a). Moreover, this reduction in the ratio was caused by a decrease in the CV of NMDAR-EPSCs in the stress-treated neurons, and not an increase in the CV of AMPAR-EPSCs (figure 2b,c).
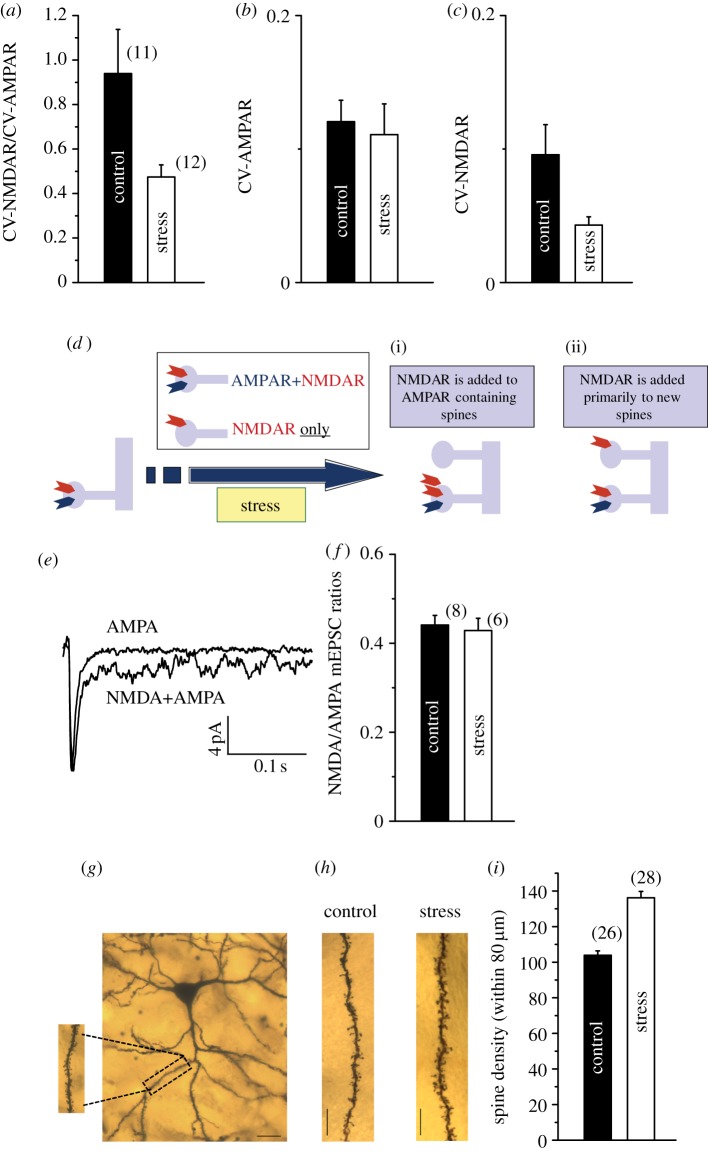
Figure 2.
Chronic stress leads to the formation of silent synapses on LA principal neurons. (a) Mean ratios of CV of NMDAR-EPSCs to CV of AMPAR-EPSCs in control (n = 11 cells, from 11 rats) and stress (n = 12 cells, from 12 rats) neurons. (b) There was difference between mean CV of AMPAR-EPSCs in control (n = 11) and stress (n = 12) neurons. (c) There was a significant reduction in mean CV of NMDAR-EPSCs in stress (n = 12) neurons compared with control (n = 11) neurons. (d) Schematic depiction of two possible scenarios that can both give rise to an increase in NMDAR-EPSCs owing to stress. First, the larger evoked NMDAR-EPSCs could be the result of new NMDARs added to spine synapses that originally contained AMPARs, i.e. pre-existing functional synapses (i). Second, enhanced NMDAR/AMPAR ratios may be caused by the addition of NMDARs, not to pre-existing AMPAR-containing synapses, but to new synapses, thereby creating NMDAR-only or silent synapses (ii). (e) Representative electrophysiological trace showing an average mEPSC with both NMDAR- and AMPAR-dependent components, and AMPAR-dependent component alone (average of 50 traces). (f) Summary of results: NMDA/AMPA ratios of mEPSCs were not different between control and stress animals. n = 8 cells from 5 rats for control, and n = 6 cells from 4 rats for stress. (g) Low-power photomicrograph of a Golgi stain-impregnated pyramidal neuron in the LA (scale bar, 10 μm). (inset) High-resolution image of spines on an apical dendrite from the same neuron. (h) Photomicrographs of representative segments of primary dendritic branches from control (left) and stress (right) neurons, demonstrating an increase in the number of spines (scale bar, 10 μm). (i) Mean values for spine-density (calculated as the average number of spines per 80 μm of primary branches) on pyramidal LA neurons from control (n = 26) and stress (n = 28) neurons. Error bars are ± s.e.m. (Online version in colour.)
While the above findings are consistent with the formation of silent synapses, the analysis based on measurements of evoked EPSCs cannot distinguish between two possible scenarios (figure 2d) that can both give rise to an increase in NMDAR-EPSCs owing to stress. In one scenario, the larger evoked NMDAR-EPSCs could be the result of new NMDARs added to spine synapses that originally contained AMPARs, i.e. pre-existing functional synapses (figure 2d(i)). The second possibility involves enhanced NMDAR/AMPAR ratios caused by the addition of NMDARs, not to pre-existing AMPAR-containing synapses, but to new synapses, thereby creating NMDAR-only or silent synapses (figure 2d(ii)). In the measurements of synaptic currents evoked through electrical stimulation of afferents, both these scenarios would lead to an increase in the ratio of NMDAR-EPSCs to AMPAR-EPSCs, although only one would involve silent synapses. Therefore, to distinguish between these two possibilities without the confounding effects of evoked responses, we quantified changes in the relative number of AMPA and NMDA receptors at functional AMPAR-containing synapses by recording dual-component spontaneous miniature synaptic currents (mEPSCs) in LA principal neurons [25]. We first monitored the dual-component mEPSCs (figure 2e) by voltage-clamping LA neurons at −40 mV in the absence of extracellular magnesium and in the presence of glycine (i.e. conditions allowing for contributions from both AMPA and NMDA receptors). Following this, we continued to record mEPSCs in the same cell held at −70 mV in the presence of the NMDAR antagonist D-AP5, thereby isolating mEPSCs mediated by AMPARs alone (figure 2e). Taken together, this provided a measure of the average amplitudes of mEPSCs mediated by both AMPARs and NMDARs in the same LA cell and allowed a comparison of their ratios in stressed and control animals (see §2c). We reasoned that if stress indeed generated silent synapses, then the amplitude ratio of mEPSCs mediated by AMPARs and NMDARs in stressed LA cells will not differ from their unstressed counterparts, because the new NMDARs would not be added to pre-existing AMPAR-containing functional synapses (figure 2d(ii)). Alternatively, the new NMDARs could be added to pre-existing AMPAR-containing synapses (figure 2d(i)), thereby increasing the amplitude ratio of mEPSCs mediated by NMDARs over AMPARs in stressed LA cells. We found that the ratio of the amplitudes of the mEPSCs mediated by NMDARs and AMPARs was unaltered in LA neurons from stressed animals (mEPSC NMDA/AMPA ratio, figure 2f). Because the NMDAR-mediated component is not enhanced in relation to that of AMPARs at synapses containing AMPARs, the hypothesis that stress leads to the creation of silent synapses gains further support (figure 2d(ii)).
The above analysis of spontaneous dual-component synaptic currents suggests that the stress-induced generation of silent synapses, and the consequent increase in NMDAR/AMPAR ratios, is not due to changes in pre-existing AMPAR-containing synapses. This is also in agreement with results from the analysis of CV of evoked EPSCs—the reduction in the ratio of CVs for NMDAR-EPSCs to AMPAR-EPSCs was not due to an increase in the CV of the AMPAR-EPSC, i.e. changes in AMPAR synapses. Together, these findings suggest that exposure to chronic stress leads to the creation of new silent synapses. As a final test of this hypothesis, we investigated whether the density of dendritic spines, the post-synaptic site of excitatory glutamatergic transmission, was increased in LA neurons from chronically stressed animals. Morphometric analysis of Golgi-impregnated principal neurons in the LA (figure 2g) of stressed and control animals (see §2d) shows that chronic stress leads to a significant increase in spine density on apical dendrites (figure 2h,i). This morphological change lends further support to the hypothesis that chronic stress leads to the generation of new silent synapses in the amygdala. These silent synapses, in turn, provide an ideal substrate for enhanced synaptic plasticity, thereby providing an explanation for the increase in magnitude of LTP caused by chronic stress (figure 1).
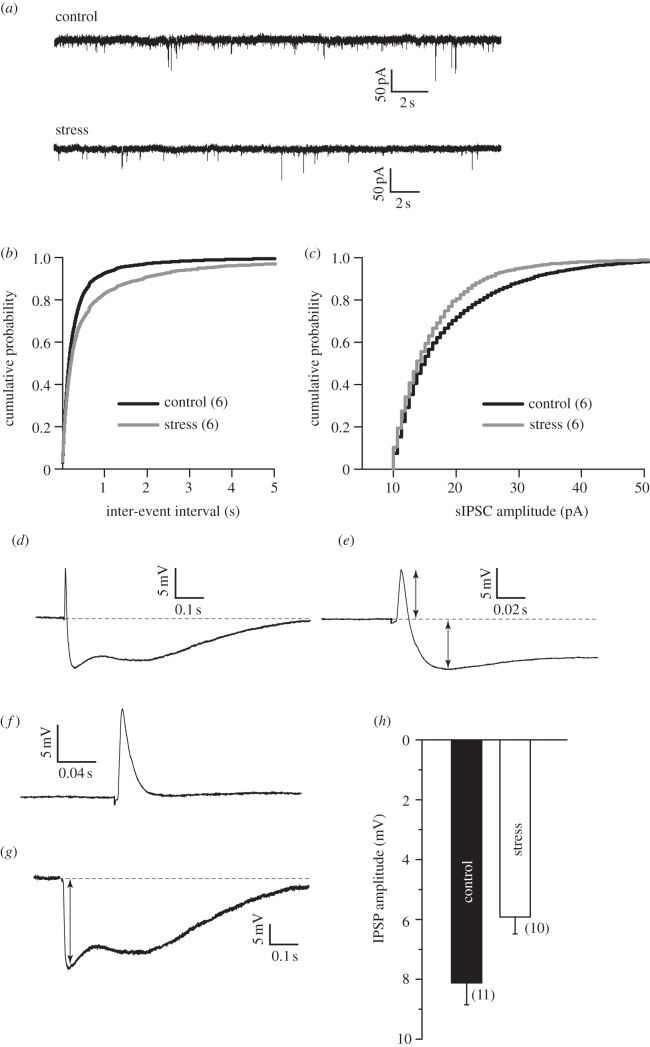
While these electrophysiological and morphological changes at excitatory synapses in themselves are likely to have a significant impact on amygdalar function, there is growing evidence highlighting the importance of GABA inhibition in gating and modulating the output of the amygdala. It has been shown that GABA receptor-mediated inhibition is a potent regulator of plasticity at excitatory glutamatergic synapses in LA [26,27]. Further, anxiolytic drugs act by enhancing inhibitory tone in the basolateral amygdala [26,28]. Chronic stress, on the other hand, potentiates anxiety-like behaviour in rats [12,17,18,29]. Hence, we next examined the impact of the same 10 day chronic stress on sIPSCs mediated by GABAA receptors using voltage-clamp recordings from LA projection neurons (figure 3a). We observed a significant decrease in the mean frequency of sIPSCs after stress (figure 3a). This reduction in sIPSC frequency was also reflected in a significant shift in the cumulative frequency distribution to longer inter-event intervals in stressed neurons (figure 3b), and was accompanied by smaller sIPSC amplitudes in stressed neurons (figure 3c). Because spontaneous currents were measured from LA neurons without stimulation of any afferent pathway, the reduction in sIPSC amplitude and frequency is a measure of the global inhibitory synaptic input impinging on the recorded LA principal neuron. Is this reduction in the overall inhibitory tone in the LA also reflected at thalamic–LA synapses that exhibit enhanced LTP after stress? To address this question, we recorded evoked EPSP–IPSP sequences in LA principal neurons from control and stressed animals. Thalamic fibres into the LA were stimulated in the absence of pharmacological blockers of GABAergic inhibition, resulting in an EPSP at the excitatory neurons, followed by a disynaptic IPSP (figure 3d,e). The stimulation intensity was modified until EPSPs with peak amplitude of approximately 10 mV were obtained (figure 3f). The EPSPs were not different between neurons from stress and control rats. The IPSP that followed, however, was significantly reduced in stressed animals (figure 3g,h). Together, these data show that chronic stress also causes a significant reduction in inhibitory drive at thalamic inputs to LA principal neurons. Overall, therefore, we observe a significant shift in the balance between synaptic excitation over inhibition after chronic stress.
Figure 3.
(a) Voltage-clamp recordings (VHOLD = −70 mV) of spontaneous IPSCs from representative control (top) and stress (bottom) neurons. (b,c) Chronic stress decreases mean sIPSC frequency and causes a rightward shift in the cumulative probability plot of sIPSC inter-event intervals (b) in stress neurons (n = 6 cells in each group, from six rats each) compared with controls (p < 0.001, Kolmogorov–Smirnov test). (c) Chronic stress also decreases the mean sIPSC amplitude (n = 6 cells in each group) and causes a leftward shift in the cumulative probability plot of sIPSC amplitudes in stress neurons compared with controls (p < 0.001, Kolmogorov–Smirnov test). (d) EPSP–IPSP sequences obtained by thalamic stimulation. (e) Inset of above trace, showing EPSP and IPSP distinctly. (f) EPSP alone obtained when cell was held at GABAA reversal potential. (g) EPSP was scaled and subtracted from EPSP–IPSP sequence, resulting in an IPSP alone. (h) Summary of results: no difference in EPSP, but IPSP is significantly lower in cells from stress animals. n = 11 cells from six rats for control, and 10 cells from five rats for stress group. Error bars are ± s.e.m.
Modulation of excitatory and inhibitory synaptic transmission in the LA has a profound impact on fear learning. In particular, NMDAR-dependent LTP at thalamic inputs to the LA has been shown to play an important role in fear memory formation [10,11]. Therefore, we reasoned that the generation of silent synapses and consequent facilitation of NMDAR-dependent LTP, coupled with a reduction in GABAergic inhibition, should enhance fear learning following chronic stress. Accordingly, we measured the behavioural impact of chronic stress by subjecting rats to an auditory fear conditioning protocol, which involved five pairings of a tone (CS) and foot-shock (US) (figure 4a; see §2e). The stressed animals showed no significant difference from control animals in their levels of freezing during this training session (data not shown). This suggests that the stressed animals acquired fear conditioning at the same rate as control animals and reached comparable levels of freezing at the end of the training session. Twenty-four hours later, the rats were presented with three CSs in a separate context. Strikingly, stressed animals exhibited significantly higher freezing compared with control animals consistently across all three presentations of the CS (figure 4b). Thus, conditioned freezing in rats with prior exposure to chronic stress was significantly enhanced relative to control rats (figure 4c). Together, these behavioural findings indicate that the synaptic changes triggered by chronic stress in the LA enhance long-term consolidation of fear memory.
4. Discussion
Our results indicate that some of the contrasting effects of stress observed at the behavioural level may also be reflected at the synaptic level. Specifically, experimental observations reported here provide evidence for stress-induced enhancement of NMDAR-dependent LTP in the LA, which is in sharp contrast to stress-induced impairment of hippocampal LTP [1,6,7]. Our findings also suggest that prolonged stress leaves its mark in the LA by tilting the balance in favour of greater excitation, evident as greater NMDAR-mediated synaptic responses and reduced GABAergic inhibitory tone. This creates permissive conditions that enable LA neurons to undergo NMDAR-mediated synaptic plasticity, the consequences of which may be manifested in several ways. First, this increase in NMDAR currents provides a synaptic substrate that is primed to undergo further strengthening through NMDAR-dependent LTP, a prediction confirmed in this study, as well as earlier findings [30–33]. Second, this also implies that any behavioural output that relies on NMDAR-dependent LTP in the LA would also be amplified by chronic stress. Consistent with this prediction, prior exposure to stress has been shown to facilitate various forms of classical fear conditioning in rats [12,14,16,31]. Importantly, local infusion of NMDAR antagonists into the basolateral amygdala has been reported to prevent these facilitatory effects of stress [34]. Further, infusion of GABAA antagonists into the basolateral amygdala has been shown to elicit anxiogenic effects, which are also blocked by NMDAR antagonists [28,29]. Third, the LA is believed to be a site of long-term consolidation of fear memories, and therefore presents an attractive locus for more enduring structural encoding of aversive experiences [10]. Accumulating evidence shows that various models of repeated (6 h per day for 21 days) and chronic (2 h per day for 10 days) stress lead to spine formation and dendritic growth in the basolateral amygdala [17,18,30,35–37]. Because these forms of morphological plasticity are mediated by NMDARs in other areas of the brain, the increase in NMDAR function reported here could also contribute to stress-induced structural plasticity in the amygdala. While enhancement in NMDAR-mediated synaptic currents and LTP, along with a shift in balance towards glutamatergic excitation over GABAergic inhibition, are all well-positioned to strengthen the formation of fear memories, neuromodulatory and neuroendocrine mechanisms are also likely to play a significant role [7,15,35,38–41].
Consistent with earlier findings [18,30,35–37], we also find that chronic immobilization stress increases the number of dendritic spines in the amygdala. However, the limitations posed by the Golgi staining method did not allow us to carry out a more detailed analysis of potential changes in spine volume and size. Thus, future studies will be needed to investigate how stress brings about ultrastructural changes at the level of individual synapses on principal neurons of the LA. Moreover, all of the behavioural, electrophysiological and morphological analyses in this study were carried out 24 h after the end of chronic stress. However, a growing body of evidence indicates that the morphological effects of chronic stress in the hippocampus and amygdala differ not only in polarity, but also in terms of their temporal persistence. For instance, exposure to the 10-day chronic stress used here elicits dendritic hypertrophy in basoleteral amygdala principal neurons that lasts till at least 21 days after the termination of stress [42]. Hippocampal CA3 atrophy, on the other hand, is reversible within the same period of post-stress recovery [12,42]. Further, CA3 synapse loss caused by 21-day restraint stress can be reversed following water maze training [43]. More recent findings based on simultaneous in vivo recordings from the amygdala and hippocampus in awake, behaving rats also highlight the contrasting spatio-temporal dynamics of neural activity across the two areas before, during, and up to 10 days after chronic stress [44]. Thus, future studies will be needed to investigate if stress-induced generation of silent synapses and enhancement in LTP lasts beyond the duration of the stress.
In conclusion, in this study, stress appears to have caused a form of plasticity, through the generation of ‘silent synapses’, which may push these synapses to a state where they are more likely to potentiate in response to a given pattern of synaptic activation during experience-dependent modifications within the amygdalar circuitry. This propensity for greater synaptic potentiation may distinguish normal from pathological fear. Therefore, it is possible that exposure to severe or prolonged stress renders the LA network hyper-responsive to subsequent emotional experiences, thereby triggering affective symptoms such as abnormally high fear and anxiety observed in stress-related psychiatric disorders.
Acknowledgements
We are grateful to Andreas Lüthi for helpful advice and discussions.
Funding statement
This research was supported by funds from NCBS and an International Senior Research Fellowship from the Wellcome Trust, UK.
References
- 1.McEwen BS. 1999. Stress and hippocampal plasticity. Annu. Rev. Neurosci. 22, 105–122. ( 10.1146/annurev.neuro.22.1.105) [DOI] [PubMed] [Google Scholar]
- 2.Sapolsky RM. 2003. Stress and plasticity in the limbic system. Neurochem. Res. 28, 1735–1742. ( 10.1023/A:1026021307833) [DOI] [PubMed] [Google Scholar]
- 3.Sandi C, Pinelo-Nava MT. 2007. Stress and memory: behavioral effects and neurobiological mechanisms. Neural Plast. 2007, 78970 ( 10.1155/2007/78970) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandi C. 2004. Stress, cognitive impairment and cell adhesion molecules. Nat. Rev. Neurosci. 5, 917–930. ( 10.1038/nrn1555) [DOI] [PubMed] [Google Scholar]
- 5.Stewart MG, et al. 2005. Stress suppresses and learning induces plasticity in CA3 of rat hippocampus: a three-dimensional ultrastructural study of thorny excrescences and their postsynaptic densities. Neuroscience 131, 43–54. ( 10.1016/j.neuroscience.2004.10.031) [DOI] [PubMed] [Google Scholar]
- 6.Kim JJ, Diamond DM. 2002. The stressed hippocampus, synaptic plasticity and lost memories. Nat. Rev. Neurosci. 3, 453–462. ( 10.1038/nrn849) [DOI] [PubMed] [Google Scholar]
- 7.Popoli M, Yan Z, McEwen BS, Sanacora G. 2012. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 13, 22–37. ( 10.1038/nrn3138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conrad CD, Galea LA, Kuroda Y, McEwen BS. 1996. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav. Neurosci. 110, 1321–1334. ( 10.1037/0735-7044.110.6.1321) [DOI] [PubMed] [Google Scholar]
- 9.Luine V, Villegas M, Martinez C, McEwen BS. 1994. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 639, 167–170. ( 10.1016/0006-8993(94)91778-7) [DOI] [PubMed] [Google Scholar]
- 10.Ledoux JE. 2000. Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184. ( 10.1146/annurev.neuro.23.1.155) [DOI] [PubMed] [Google Scholar]
- 11.Bauer EP, Schafe GE, LeDoux JE. 2002. NMDA receptors and L-type voltage-gated calcium channels contribute to long-term potentiation and different components of fear memory formation in the lateral amygdala. J. Neurosci. 22, 5239–5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conrad CD, LeDoux JE, Magariños AM, McEwen BS. 1999. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav. Neurosci. 113, 902–913. ( 10.1037/0735-7044.113.5.902) [DOI] [PubMed] [Google Scholar]
- 13.Cordero MI, Venero C, Kruyt ND, Sandi C. 2003. Prior exposure to a single stress session facilitates subsequent contextual fear conditioning in rats. Horm. Behav. 44, 338–345. ( 10.1016/S0018-506X(03)00160-0) [DOI] [PubMed] [Google Scholar]
- 14.Rau V, DeCola JP, Fanselow MS. 2005. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci. Biobehav. Rev. 29, 1207–1223. ( 10.1016/j.neubiorev.2005.04.010) [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues SM, LeDoux JE, Sapolsky RM. 2009. The influence of stress hormones on fear circuitry. Annu. Rev. Neurosci. 32, 289–313. ( 10.1146/annurev.neuro.051508.135620) [DOI] [PubMed] [Google Scholar]
- 16.Shors TJ, Weiss C, Thompson RF. 1992. Stress-induced facilitation of classical conditioning. Science (New York, NY) 257, 537–539. ( 10.1126/science.1636089) [DOI] [PubMed] [Google Scholar]
- 17.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. 2002. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 22, 6810–6818. (doi:20026655) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. 2005. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc. Natl Acad. Sci. USA 102, 9371–9376. ( 10.1073/pnas.0504011102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisskopf MG, LeDoux JE. 1999. Distinct populations of NMDA receptors at subcortical and cortical inputs to principal cells of the lateral amygdala. J. Neurophysiol. 81, 930–934. [DOI] [PubMed] [Google Scholar]
- 20.Mahanty NK, Sah P. 1999. Excitatory synaptic inputs to pyramidal neurons of the lateral amygdala. Eur. J. Neurosci. 11, 1217–1222. ( 10.1046/j.1460-9568.1999.00528.x) [DOI] [PubMed] [Google Scholar]
- 21.Bennur S, Shankaranarayana Rao BS, Pawlak R, Strickland S, McEwen BS, Chattarji S. 2007. Stress-induced spine loss in the medial amygdala is mediated by tissue-plasminogen activator. Neuroscience 144, 8–16. ( 10.1016/j.neuroscience.2006.08.075) [DOI] [PubMed] [Google Scholar]
- 22.Bissière S, Humeau Y, Lüthi A. 2003. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat. Neurosci. 6, 587–592. ( 10.1038/nn1058) [DOI] [PubMed] [Google Scholar]
- 23.Kullmann DM. 1994. Amplitude fluctuations of dual-component EPSCs in hippocampal pyramidal cells: implications for long-term potentiation. Neuron 12, 1111–1120. ( 10.1016/0896-6273(94)90318-2) [DOI] [PubMed] [Google Scholar]
- 24.Isaac JTR, Nicoll RA, Malenka RC. 1995. Evidence for silent synapses: implications for the expression of LTP. Neuron 15, 427–434. ( 10.1016/0896-6273(95)90046-2) [DOI] [PubMed] [Google Scholar]
- 25.Lüthi A, Schwyzer L, Mateos JM, Gähwiler BH, McKinney RA. 2001. NMDA receptor activation limits the number of synaptic connections during hippocampal development. Nat. Neurosci. 4, 1102–1107. ( 10.1038/nn744) [DOI] [PubMed] [Google Scholar]
- 26.Davis M, Rainnie D, Cassell M. 1994. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 17, 208–214. ( 10.1016/0166-2236(94)90106-6) [DOI] [PubMed] [Google Scholar]
- 27.Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Lüthi A. 2009. Amygdala inhibitory circuits and the control of fear memory. Neuron 62, 757–771. ( 10.1016/j.neuron.2009.05.026) [DOI] [PubMed] [Google Scholar]
- 28.File SE. 2000. The amygdala: anxiety and benzodiazepines. In The amygdala: a functional analysis (ed. Aggleton JP.), pp. 195–212. Oxford, UK: Oxford University Press. [Google Scholar]
- 29.Adamec RE, Burton P, Shallow T, Budgell J. 1998. NMDA receptors mediate lasting increases in anxiety-like behavior produced by the stress of predator exposure: implications for anxiety associated with posttraumatic stress disorder. Physiol. Behav. 65, 723–737. ( 10.1016/S0031-9384(98)00226-1) [DOI] [PubMed] [Google Scholar]
- 30.Padival M, Quinette D, Rosenkranz JA. 2013. Effects of repeated stress on excitatory drive of basal amygdala neurons in vivo. Neuropsychopharmacology 38, 1748–1762. ( 10.1038/npp.2013.74) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodríguez Manzanares PA, Isoardi NA, Carrer HF, Molina VA. 2005. Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. J. Neurosci. 25, 8725–8734. ( 10.1523/JNEUROSCI.2260-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vouimba R-M, Yaniv D, Diamond D, Richter-Levin G. 2004. Effects of inescapable stress on LTP in the amygdala versus the dentate gyrus of freely behaving rats. Eur. J. Neurosci. 19, 1887–1894. ( 10.1111/j.1460-9568.2004.03294.x) [DOI] [PubMed] [Google Scholar]
- 33.Vouimba R-M, Muñoz C, Diamond DM. 2006. Differential effects of predator stress and the antidepressant tianeptine on physiological plasticity in the hippocampus and basolateral amygdala. Stress (Amsterdam, Netherlands) 9, 29–40. ( 10.1080/10253890600610973) [DOI] [PubMed] [Google Scholar]
- 34.Shors TJ, Mathew PR. In press NMDA receptor antagonism in the lateral/basolateral but not central nucleus of the amygdala prevents the induction of facilitated learning in response to stress. Learn. Mem. (Cold Spring Harbor, NY) 5, 220–230. [PMC free article] [PubMed] [Google Scholar]
- 35.Roozendaal B, McEwen BS, Chattarji S. 2009. Stress, memory and the amygdala. Nat. Rev. Neurosci. 10, 423–433. ( 10.1038/nrn2651) [DOI] [PubMed] [Google Scholar]
- 36.Hill MN, et al. 2013. Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure. Mol. Psychiatry 18, 1125–1135. ( 10.1038/mp.2012.90) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson SA, Wang J-F, Sun X, McEwen BS, Chattarji S, Young LT. 2009. Lithium treatment prevents stress-induced dendritic remodeling in the rodent amygdala. Neuroscience 163, 34–39. ( 10.1016/j.neuroscience.2009.06.005) [DOI] [PubMed] [Google Scholar]
- 38.Tully K, Li Y, Tsvetkov E, Bolshakov VY. 2007. Norepinephrine enables the induction of associative long-term potentiation at thalamo-amygdala synapses. Proc. Natl Acad. Sci. USA 104, 14 146–14 150. ( 10.1073/pnas.0704621104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campolongo P, Roozendaal B, Trezza V, Hauer D, Schelling G, McGaugh JL, Cuomo V. 2009. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc. Natl Acad. Sci. USA 106, 4888–4893. ( 10.1073/pnas.0900835106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quirarte GL, Roozendaal B, McGaugh JL. 1997. Glucocorticoid enhancement of memory storage involves noradrenergic activation in the basolateral amygdala. Proc. Natl Acad. Sci. USA 94, 14 048–14 053. ( 10.1073/pnas.94.25.14048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duvarci S, Paré D. 2007. Glucocorticoids enhance the excitability of principal basolateral amygdala neurons. J. Neurosci. 27, 4482–4491. ( 10.1523/JNEUROSCI.0680-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vyas A, Pillai AG, Chattarji S. 2004. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behaviour. Neuroscience 128, 667–673. ( 10.1016/j.neuroscience.2004.07.013) [DOI] [PubMed] [Google Scholar]
- 43.Sandi C, Davies HA, Cordero MI, Rodriguez JJ, Popov VI, Stewart MG. 2003. Rapid reversal of stress induced loss of synapses in CA3 of rat hippocampus following water maze training. Eur. J. Neurosci. 17, 2447–2456. ( 10.1046/j.1460-9568.2003.02675.x) [DOI] [PubMed] [Google Scholar]
- 44.Ghosh S, Laxmi TR, Chattarji S. 2013. Functional connectivity from the amygdala to the hippocampus grows stronger after stress. J. Neurosci. 33, 7234–7244. ( 10.1523/JNEUROSCI.0638-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]