Abstract
Long-term depression (LTD) reduces the functional strength of excitatory synapses through mechanisms that include the removal of AMPA glutamate receptors from the postsynaptic membrane. LTD induction is also known to result in structural changes at excitatory synapses, including the shrinkage of dendritic spines. Synaptic adhesion molecules are thought to contribute to the development, function and plasticity of neuronal synapses largely through their trans-synaptic adhesions. However, little is known about how synaptic adhesion molecules are altered during LTD. We report here that NGL-3 (netrin-G ligand-3), a postsynaptic adhesion molecule that trans-synaptically interacts with the LAR family of receptor tyrosine phosphatases and intracellularly with the postsynaptic scaffolding protein PSD-95, undergoes a proteolytic cleavage process. NGL-3 cleavage is induced by NMDA treatment in cultured neurons and low-frequency stimulation in brain slices and requires the activities of NMDA glutamate receptors, matrix metalloproteinases (MMPs) and presenilin/γ-secretase. These results suggest that NGL-3 is a novel substrate of MMPs and γ-secretase and that NGL-3 cleavage may regulate synaptic adhesion during LTD.
Keywords: long-term depression, synaptic adhesion molecules, NMDA receptors, metalloproteinase, γ-secretase
1. Introduction
Synaptic adhesion molecules play important roles in the regulation of synaptic development, function and plasticity [1–14]. A large number of synaptic adhesion molecules have recently been identified. These include neuroligins, neurexins, SynCAMs (synaptic cell adhesion molecules), LRRTMs (leucine-rich repeat transmembrane neuronal proteins, NGLs (netrin-G ligands), SALMs (synaptic adhesion-like molecules), netrin-Gs, LAR-RPTPs (leucocyte common antigen-related protein-receptor-type protein tyrosine phosphatases), EphB receptors (EphB class ephrin receptors), GluRδ2 (δ2 glutamate receptor), Cbln1 (cerebellin 1 precursor protein), TrkC (tropomyosin receptor kinase C), Slitrks (SLIT and NTRK-like proteins), MDGAs (MAM domain-containing glycosylphosphatidylinositol anchor proteins), IL1RAPL1 (interleukin-1 receptor accessory protein-like 1) [15–28] and IL1RAcP (interleukin-1 receptor accessory protein) [29].
Synaptic adhesion molecules are thought to contribute to synaptic development largely through their trans-synaptic adhesions, but relatively little is known about how synaptic adhesions are structurally weakened and how this contributes to functional weakening of synapses. It has recently been shown that neuroligin-1 is cleaved in an activity-dependent manner through mechanisms requiring the activation of metalloproteinases MMP-9 (matrix metalloproteinase 9) and ADAM-10 (a disintegrin and metalloproteinase 10); this cleavage leads to both structural and functional weakening of synapses [30,31]. However, it remains unclear whether other synaptic adhesion molecules are similarly regulated. It is also not certain whether long-term depression (LTD), which is accompanied by the shrinkage of dendritic spines, loss of F-actin in spines and separation of pre- and postsynaptic structures [32–34], leads to proteolytic cleavage of synaptic adhesion molecules.
NGLs (netrin-G ligands) are a family of postsynaptic adhesion molecules with three known members: NGL-1, NGL-2 and NGL-3 [9]. The C-terminal tails of NGLs interact with the postsynaptic scaffolding protein PSD-95 [19], suggesting that these interactions promote the recruitment of PSD-95-associated receptors and signalling molecules to the sites of presynaptic release [35]. Extracellular domains of NGL-1 and NGL-2 interact with netrin-G1 and netrin-G2, respectively [18,19]; these latter molecules are axonally enriched glycosylphosphatidyl inositol (GPI)-anchored adhesion proteins [36–39]. NGL-3 interacts with members of the LAR family of receptor tyrosine phosphatases (LAR, PTPδ and PTPσ) [20,21,40], which are critically involved in regulating presynaptic development and function [41]. More recently, LAR family proteins have been shown to interact with diverse postsynaptic adhesion molecules, including TrkC, Slitrks, IL1RAPL1 and IL1RAcP [20–22,25–29] and have emerged as novel organizers of presynaptic development, although a postsynaptic role in synapse development and maintenance has also been suggested [42].
Matrix metalloproteinases (MMPs) are a group of zinc-dependent endopeptidases known to process extracellular matrix components and cell surface proteins. In the nervous system, MMPs are involved in various brain functions and dysfunctions, including brain development, synaptogenesis, synaptic plasticity, learning and memory, neuron–glia interactions, neuronal injury and neurological and neuropsychiatric disorders [43–54]. Of the numerous MMPs expressed in the nervous system, MMP-9 has been extensively characterized and shown to regulate late-phase long-term potentiation (LTP), dendritic spine morphology and learning and memory, through mechanisms including integrin signalling and NMDA (N-methyl-d-aspartate) receptor trafficking and function [55–65]. MMP-9, however, does not regulate early-phase LTP, presynaptic release, NMDA receptor-dependent LTD or metabotropic glutamate receptor (mGluR)-dependent LTD. Although a study using general MMP inhibitors has suggested a role for MMPs in the regulation of LTD [66], the regulation of LTD by MMPs, unlike MMP-dependent regulation of LTP, is not well understood. In addition, it remains unclear which substrate proteins mediate MMP-dependent regulation of LTP and LTD, although a recent study has identified subunits of NMDA receptors, which directly regulate synaptic plasticity [67], as novel substrates of MMP-7 [68]. Notably, many substrates of MMPs, including cadherins, ephrins, Eph receptors, β-dystroglycan and neuroligin-1, are SynCAMs [30,31,69–76]. Given the increasing recognition that synaptic adhesion molecules are involved in the regulation of synaptic plasticity [77–81], it is possible that MMP-dependent cleavage of synaptic adhesion molecules could contribute to regulation of synaptic plasticity.
The presenilin/γ-secretase complex is a group of membrane-embedded proteinases that is composed of four proteins, including presenilin, nicastrin, Aph-1 and Pen-2 [82]. Well-known substrates of γ-secretase include the amyloid β-protein precursor (APP) and Notch, which have been implicated in Alzheimer's disease and brain development, respectively. A large number of γ-secretase substrates have recently been identified and the number is now approaching approximately 90 [83–85]. Notably, many γ-secretase substrates are synaptic surface proteins [83–85], including N-cadherin [86], ErbB4 [87,88], nectin-1α [89], syndecan-1/2/3 [85,90], GluR3 (GluA3) [91], ephrinB1/2 [92–94], EphA4/B2 [95,96], LAR [97], neurexin-1/3β [98,99] and neuroligin-1 [30,31]. This suggests that γ-secretase could act on these proteins to regulate synapse structure and function.
In this study, we tested whether NGL-3, used as a model adhesion molecule, undergoes a series of proteolytic cleavages in a neuronal activity-dependent manner. We found that LTD-inducing chemical and electrical stimuli caused proteolytic cleavage of NGL-3 in a manner requiring the activation of NMDA receptors, MMPs and γ-secretase. These results suggest that NGL-3 cleavage may regulate synaptic structure and function during LTD.
2. Results
(a). NMDA treatment of cultured neurons induces NGL-3 cleavage
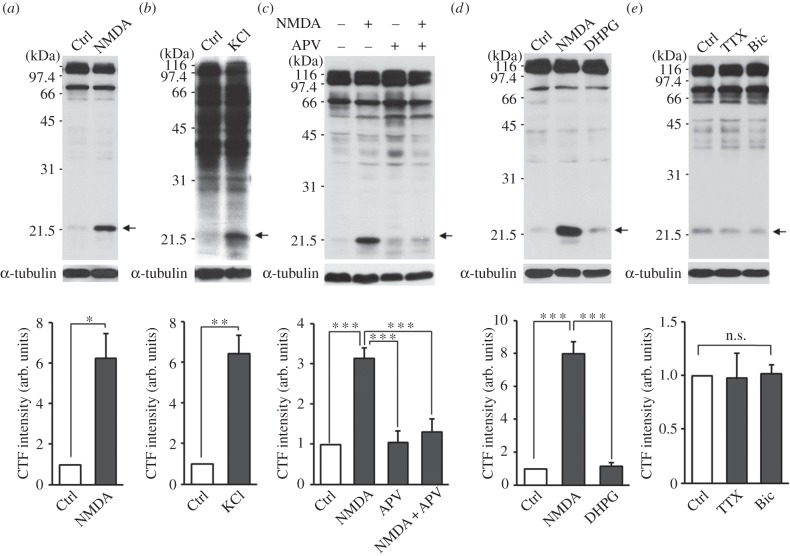
To test whether LTD induction leads to the cleavage of NGL-3, we first treated cultured hippocampal neurons with NMDA (20 μM, 3 min), which is known to induce chemical LTD in brain slices [100] and a long-lasting decrease in surface levels of AMPA receptors in cultured neurons [101,102]. Immunoblot analyses showed that NMDA treatment of cultured neurons increased the levels of an NGL-3 fragment (approx. 22 kDa) recognized by an NGL-3 antibody raised against the cytoplasmic region of NGL-3 (amino acids 622–690; #1948) (figure 1a). Three other antibodies raised against the cytoplasmic region of NGL-3 recognized the same 22 kDa band (see electronic supplementary material, figure S1), suggesting the authenticity of the band as NGL-3 C-terminal fragments (hereafter termed NGL-3-CTFs). The small levels of NGL-3-CTFs before NMDA treatment suggest that NGL-3 cleavage occurs under basal conditions. A faint band beneath the NGL-3-CTF band is not likely to be an alternative or subsequent cleavage product, because it is not recognized by other NGL-3 antibodies (see electronic supplementary material, figure S1).
Figure 1.
NGL-3 cleavage requires NMDAR activation, but not mGluR activation or chronic changes in neuronal activity. (a) NMDA treatment of cultured neurons induces NGL-3 cleavage. Rat hippocampal neurons at days in vitro (DIV) 18–21 were stimutated with NMDA (20 µM, 3 min), followed by direct lysis of the neurons in SDS-PAGE sample buffer and immunoblotting with NGL-3 antibodies (#1948). For quantitative analyses, the intensity of the 22 kDa band (indicated by an arrow) was normalized to that of α-tubulin and compared with other normalized intensities. The bar graphs represent mean ± s.e.m.; n = 3, *p < 0.05, Student's t-test. (b) KCl-dependent depolarization induces NGL-3 cleavage. Rat hippocampal neurons at DIV 18–21 were stimutated with KCl (50 mM, 5 min) followed by immunoblotting; n = 4, **p < 0.01, Student's t-test. (c) NMDA receptor activation is required for NMDA-induced NGL-3 cleavage. Rat hippocampal neurons pretreated with APV (NMDA receptor antagonist; 50 µM, 30 min) were stimulated with NMDA (20 µM, 3 min) and analysed by immunoblotting; n = 5, ***p < 0.001, one-way ANOVA. (d) Activation of NMDA receptors, but not mGluRs, leads to NGL-3 cleavage. Rat hippocampal neurons at DIV 18–21 were stimutated with NMDA (20 µM, 3 min) or DHPG (group I mGluR agonist; 50 µM, 30 min); n = 3, ***p < 0.001, one-way ANOVA. (e) Chronic inhibition or activation of cultured neurons does not induce NGL-3 cleavage. Rat hippocampal neurons at DIV 18–21 were stimutated with tetrodotoxin (TTX; 1 µM for 48 h) or bicuculline (Bic; 10 µM for 36 h); n = 3, n.s., not significant, one-way ANOVA. arb. units, arbitrary units; Ctrl, control.
l-Glutamate treatment (50 μM, 1 min) of cultured hippocampal neurons, which has been used to induce internalization of AMPA receptors in cultured neurons [103], induced the same NGL-3 cleavage (see electronic supplementary material, figure S2a), similar to the results of the NMDA treatment. In addition, KCl treatment (50 mM, 5 min) induced NGL-3 cleavage (figure 1b), suggesting that general depolarization of neurons can induce NGL-3 cleavage.
(b). NGL-3 cleavage requires NMDA receptor activity but not mGluR activity or chronic changes in neuronal activity
NMDA-induced cleavage of NGL-3 may involve the activation of NMDA receptors. Indeed, incubation of cultured neurons with APV (d-2-amino-5-phosphovalerate, 50 μM), an antagonist of NMDA receptors, 30 min before and during NMDA treatment (20 μM, 3 min) blocked NGL-3 cleavage (figure 1c). In addition, APV also inhibited glutamate- and KCl-induced NGL-3 cleavage (see electronic supplementary material, figure S2a,b).
By contrast, incubation of cultured neurons with DHPG ((RS)-3,5-dihydroxyphenylglycine, 50 μM, 30 min), an agonist of group I mGluRs (mGluR1/5) known to induce mGluR-dependent LTD (mGluR-LTD) in brain slices [104–107] and AMPA receptor internalization in cultured neurons [108], had no effect on NGL-3 cleavage, suggesting that mGluR activation does not induce NGL-3 cleavage (figure 1d). In addition, blocking neuronal network activity with tetrodotoxin (1 μM, 48 h) or enhancing network activity with bicuculline (10 μM, 36 h) had no effect on NGL-3 cleavage (figure 1e). These results suggest that neither mGluR activation nor chronic modulation of neuronal activity induces NGL-3 cleavage.
(c). Long-term depression-inducing low-frequency stimulation causes NGL-3 cleavage in brain slices
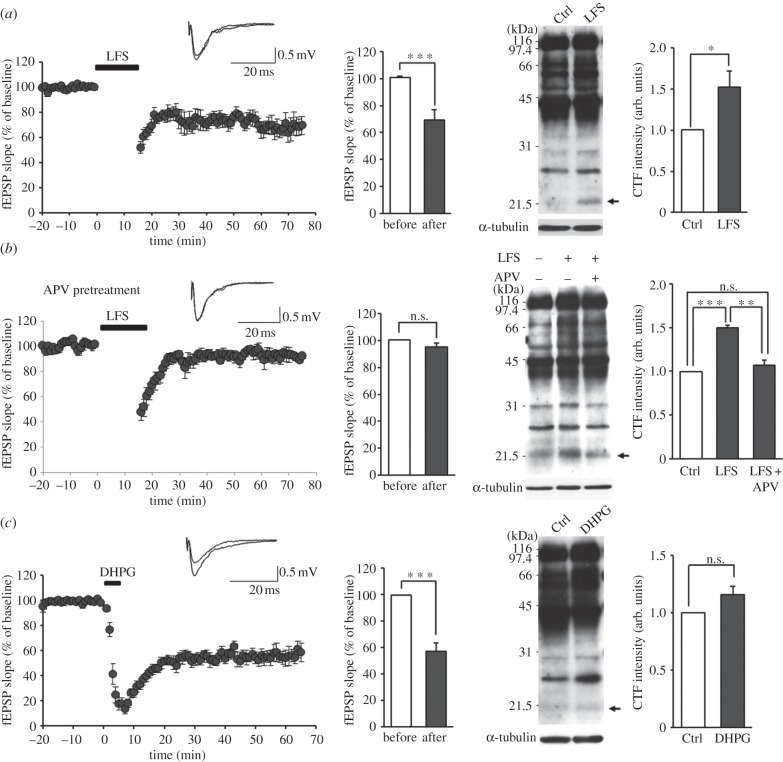
We next tested whether LTD-inducing electrical stimulation in brain slices causes NGL-3 cleavage. To accomplish this, we stimulated the Schaffer collateral pathway in mouse hippocampal slices (three weeks) by low-frequency stimulation (LFS, 1 Hz, 900 pulses), a stimulation paradigm known to cause NMDA receptor-dependent LTD in rat and mouse hippocampal slices [109,110], followed by confirmation of LTD induction by electrophysiological measurements, and immunoblotting analysis of hippocampal lysates. We found that LFS-LTD induced a significant increase in NGL-3 cleavage (figure 2a), similar to the results of chemical LTD induction in cultured neurons (figure 1). The presence of NGL-3-CTFs in hippocampal slices before LFS-LTD indicates that NGL-3 cleavage occurs under basal conditions, similar to the results from cultured neurons. Incubation of brain slices with APV (50 μM) blocked LFS-induced NGL-3 cleavage (figure 2b), suggesting that NMDA receptor activation is required. In contrast to LFS-LTD, inducing mGluR-LTD by incubation of hippocampal slices with DHPG (50 μM, 5 min) had no effect on NGL-3 cleavage (figure 2c), similar to the results of DHPG stimulation in cultured neurons (figure 1d). A similar NGL-3 cleavage could also be observed in rat slices stimulated by LFS (data not shown).
Figure 2.
LTD-inducing low-frequency stimulation induces NGL-3 cleavage in hippocampal slices. (a) Low-frequency stimulation in slices induces NGL-3 cleavage. The Schaffer collateral pathway of acute mouse hippocampal slices (3–4 weeks old) was stimulated by low-frequency stimuli (LFS, 1 Hz, 15 min), followed by immunoblot analysis of hippocampal lysates using NGL-3 antibodies (#2020). The induction of LFS-LTD was confirmed by comparing the average slope of fEPSPs (field excitatory postsynaptic potentials) before stimulation with that during the last 5 min of recording. The top panel shows sample EPSPs. The bar graphs represent mean ± s.e.m; n = 4 slices from three mice, *p < 0.05, ***p < 0.001, Student's t-test. (b) NMDA receptor activation is required for LFS-induced cleavage of NGL-3-CTF. Acute mouse hippocampal slices (3–4 weeks old) were incubated with APV (NMDA receptor antagonist; 50 µM) throughout the experiment starting from 20 min before LFS, followed by immunoblot analysis of hippocampal lysates. The blockade of LFS-LTD was confirmed by comparing the average slope of fEPSPs before stimulation with that during the last 5 min of recording; n = 3 slices from two mice, **p < 0.01, ***p < 0.001, n.s., not significant, Student's t-test and one-way ANOVA. (c) DHPG treatment in slices does not induce NGL-3 cleavage. Acute mouse hippocampal slices (3–4 weeks old) were stimulated with DHPG (50 µM, 5 min), followed by immunoblot analysis of hippocampal lysates. The induction of mGluR-LTD by DHPG was confirmed by comparing the average slope of fEPSPs before stimulation with that during the last 5 min of recording. The top panel shows sample EPSPs; n = 6 slices from four mice, ***p < 0.001, n.s., not significant, Student's t-test. Ctrl, unstimulated control slices.
(d). NGL-3 cleavage requires matrix metalloproteinase activity
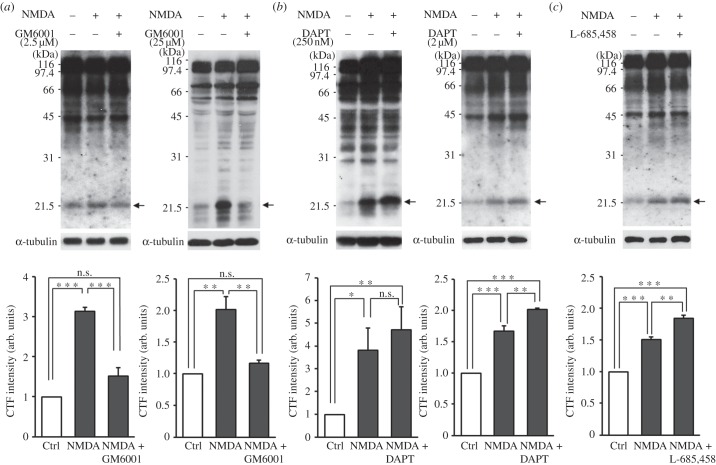
NMDA receptor activation often leads to the activation of metalloproteinases [30,31,111]. We thus tested whether NMDA-induced NGL-3 cleavage requires MMP activity. Inhibition of MMPs with two different concentrations (2.5 and 25 μM) of GM6001, a broad-spectrum MMP inhibitor [112,113], for 30 min before and during NMDA treatment blocked NGL-3 cleavage in cultured neurons (figure 3a), suggesting that NGL-3 cleavage induced by NMDA receptor activation requires activation of MMPs.
Figure 3.
NGL-3 cleavage is blocked by inhibition of MMPs and presenilin/γ-secretase. (a) MMP inhibition blocks NMDA-induced NGL-3 cleavage. Rat hippocampal neurons at DIV 18–21 were incubated with GM6001 (2.5 and 25 µM, 30 min) before and during NMDA stimulation (20 µM, 3 min), followed by immunoblotting. The bar graphs represent mean ± s.e.m; n = 4; **p < 0.01, ***p < 0.001, n.s., not significant, one-way ANOVA. (b) γ-Secretase inhibition by DAPT blocks NGL-3 cleavage, leading to an increase in the levels of NGL-3-CTFs. Hippocampal neurons pretreated with the γ-secretase inhibitor DAPT (250 nM, 2 h ; 2 µM, 3 h) were stimulated with NMDA (20 µM, 3 min); n = 5, *p < 0.05, **p < 0.01, ***p < 0.001, n.s., not significant, one-way ANOVA. (c) γ-Secretase inhibition by L-685,458 blocks NGL-3 cleavage, leading to an increase in the levels of NGL-3-CTFs. Hippocampal neurons pretreated with the γ-secretase inhibitor L-685,458 (1 µM, 30 min) were stimulated with NMDA (20 µM, 3 min); n = 3, **p < 0.01, ***p < 0.001, one-way ANOVA.
(e). NGL-3 cleavage requires γ-secretase activity
MMP cleavage of surface membrane proteins is usually followed by subsequent cleavage of their CTFs by γ-secretase [82]. To test whether this also occurs for NGL-3, we treated cultured hippocampal neurons with DAPT (N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester), a γ-secretase inhibitor, at two different concentrations (250 nM and 2 μM) for 2–3 h before and during NMDA treatment. The levels of NGL-3-CTFs in cultured neurons induced by NMDA treatment combined with DAPT inhibition (2 μM) were higher than those induced by NMDA alone (figure 3b). We obtained a similar result by using L-685,458 (1 μM), another γ-secretase inhibitor (figure 3c). These results suggest that NGL-3-CTFs can be further cleaved by γ-secretase.
3. Discussion
Our data indicate that LTD-inducing stimuli promote proteolytic cleavage of NGL-3 in a manner that requires the activation of NMDA receptors, MMPs and presenilin/γ-secretase. These results suggest that (i) NGL-3 is a novel substrate of MMPs and γ-secretase, (ii) LTD induction promotes NGL-3 processing and (iii) NGL-3 may regulate excitatory synapse structure and function during LTD.
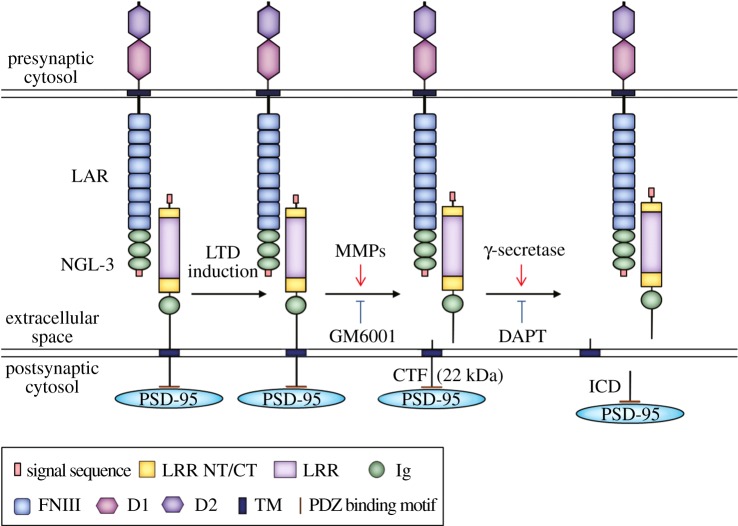
What might be the consequences of NGL-3 cleavage by MMPs and γ-secretase? One straightforward possibility would be the weakening of NGL-3-mediated trans-synaptic adhesion at excitatory synapses that undergo LTD (figure 4). In addition, γ-secretase-mediated cleavage of NGL-3-CTFs, the step following MMP cleavage, would further remove the C-terminal tail from NGL-3, destabilizing the interaction between NGL-3 and PSD-95 (figure 4).
Figure 4.
A schematic diagram showing that NGL-3 cleavage at the postsynaptic surface occurs in a manner that requires the activation of NMDA receptors, MMPs and γ-secretase, and may involve the destabilization of presynaptic and cytoplasmic binding partners of NGL-3. Cleavage of full-length NGL-3 is induced by LTD-inducing stimuli. MMPs (inhibited by the broad-spectrum MMP inhibitor GM6001) are thought to mediate the initial LTD-induced NGL-3 cleavage and generation of NGL-3-CTFs (22 kDa). γ-Secretase (inhibited by DAPT) may further cleave NGL-3-CTFs to generate NGL-3-ICDs, although they were not detectable in our immunoblots. Presynaptic LAR family receptor tyrosine phosphatases, which trans-synaptically interact with NGL-3, may undergo removal from the presynaptic site. Cytoplasmically, the postsynaptic scaffolding protein PSD-95, which binds the cytoplasmic tail of NGL-3, may lose its connection with NGL-3, contributing to destabilization of the synapse during LTD. LRRNT, leucine-rich repeat N-terminal domain; LRRCT, leucine-rich repeat C-terminal domain; LRR, leucine-rich repeats; Ig, immunoglobulin domain; TM, transmemrbane domain; PDZ binding motif, PDZ domain-binding motif; FNIII, fibronectin type III domain; D1 and D2, tyrosine phosphatase domains.
NGL-3 trans-synaptically interacts with LAR family receptor protein tyrosine phosphatases (LAR, PTPδ and PTPσ) [20,21]. Therefore, NGL-3 cleavage may cause the removal of LARs from synapses, similar to the removal of presynaptic neurexins observed at the site of neuroligin-1 cleavage [30]. Functionally, LAR is a well-known regulator of presynaptic development and function [41,114–116]. Synaptic removal of LAR induced by NGL-3 cleavage is thus likely to exert a significant influence on presynaptic structure and function. Notably, LAR can be processed by ADAM-17/TACE (an α-secretase) and γ-secretase [97,117,118]. It is conceivable that NGL-3 and LAR may undergo simultaneous proteolytic cleavages by specific MMPs/ADAMs during LTD, although we could not test LTD-induced LAR cleavage in this study owing to a lack of suitable LAR antibodies.
Our results are reminiscent of the recently reported activity-dependent cleavage of neuroligin-1 mediated by MMP-9/ADAM-10 and γ-secretase [30,31]. Neurexin-1β and neurexin-3β, which interact with neuroligins, have also been shown to be substrates of γ-secretase [98,99]. The complex of neuroligins and neurexins has been clearly demonstrated to regulate diverse aspects of synapse development and function [2–4,13]. Therefore, these previous observations taken together with the results of this study support the notion that the cleavage of synaptic adhesion molecules by MMPs/ADAMs and γ-secretase regulates diverse aspects of synapse structure and function. One rather unique finding of the current study is that LTD-inducing low-frequency stimulation in slices leads to NGL-3 cleavage. It thus may be worth testing whether neuroligin-1 undergoes a similar LTD-induced cleavage and whether neuroligin-1 and NGL-3 act in a synergistic manner during LTD.
NGL-3 cleavage induced by LTD-inducing stimuli requires MMP activation. Because MMPs have been implicated in the regulation of synaptic plasticity including LTP and LTD [55–62,66], our data suggest that NGL-3 may be one of the downstream effectors that mediate MMP-dependent regulation of LTD. With regard to specific MMPs that may mediate NGL-3 cleavage, MMP-9 is unlikely to be involved because LTD is unaffected in MMP-9-deficient mice [58] and in slices with specific inhibition of MMP-9 [66]. Other possible MMPs include MMP-3 and MMP-7, which have been shown to regulate NMDA receptor function, synaptic plasticity, dendritic spine morphology, and learning and memory [62,68,119–126]. GM6001 used in this study is a broad-spectrum inhibitor that acts on both MMPs and ADAMs [112,113]. Therefore, ADAMs may also act on NGL-3, similar to the cleavage of neuroligin-1 by both MMP-9 and ADAM-10 [30,31]. However, ADAM-17 (also known as TACE) is unlikely to process NGL-3 because it is known to mainly regulate mGluR1/5-dependent LTD [127], and DHPG stimulation in cultured neurons or mGluR-LTD in brain slices does not induce NGL-3 cleavage (figures 1d and 2c).
NGL-3 cleavage requires γ-secretase activity. γ-Secretase has been shown to enhance LTD through the production of amyloid-β and endocytosis of NMDA receptors [128–130]. It is thus possible that NGL-3 also contributes to γ-secretase-dependent regulation of LTD. In addition, γ-secretase acts on synaptic adhesion molecules, including neurexins, neuroligins, cadherins, nectins, syndecans, LARs, ephrins and Eph receptors [83–85], and many of these molecules regulate synaptic plasticity [77,78,131–136]. Thus, the results of our study, taken together with these previous observations, corroborate the notion that γ-secretase regulates synaptic adhesion and plasticity through activity-dependent cleavage of synaptic adhesion molecules.
γ-Secretase action is usually preceded by the MMP-mediated cleavage of membrane proteins into N- and C-terminal fragments (NTFs and CTFs) [82–85]. CTFs are further processed by γ-secretase to generate intracellular domains (ICDs), which are known to regulate intracellular signalling in the cytoplasm or nucleus by, for instance, interacting with transcriptional regulators. Alternatively, ICDs are degraded by the proteasome for protein turnover. In this study, we could not observe detectable levels of NGL-3-ICDs. This suggests that NGL-3-ICDs may be labile and degraded by the proteasome, similar to the case of ICDs from neuroligin-1 [31]. However, this does not exclude the possibility that NGL-3-ICDs have some cytoplasmic functions. Notably, ICDs derived from NGL-3-interacting LAR translocate to the nucleus and regulate β-catenin-dependent gene expression [97].
A member of the LAR family, PTPδ (encoded by the PTPRD gene), has been associated with attention deficit/hyperactivity disorder (ADHD) [137] and restless leg syndrome [138], a disorder often comorbid with ADHD [139], autism spectrum disorder [140] and bipolar disorder [141]. This suggests the possibility that abnormalities in the trans-synaptic interactions between LAR family proteins and their postsynaptic partners including NGL-3 may contribute to the development of these disorders.
In summary, our data suggest that induction of LTD in neurons leads to proteolytic cleavage of NGL-3 in a manner requiring the activation of NMDA receptors, MMPs and γ-secretase. This cleavage may lead to the weakening of NGL-3-dependent trans-synaptic adhesion at excitatory synapses and contribute to structural and functional weakening of excitatory synapses during LTD.
4. Material and methods
(a). Antibodies and animals
Guinea pig polyclonal NGL-3 antibodies (#2020 and #2021) were raised in this study using synthetic peptides mimicking the last 30 amino acid residues of NGL-3 (CGAKGPGLNSIHEPLLFKSCGSKENVQETQI). Rabbit polyclonal pan-NGL (#1583; against last 15 amino acid residues of NGL-2; CIIQTHTKDKVQETQI) [17] and rabbit polyclonal NGL-3 antibodies (#1948; against GST-NGL-3 amino acids 622–690) [21] have been described. α-Tubulin antibody was purchased from Sigma. Experiments on animals were performed in accordance with the guidelines of the Animal Welfare Committee of KAIST, Korea.
(b). Quantification of immunoblot results
NGL-3-CTF bands were quantified by normalizing the integrated intensities of the 22 kDa bands to those of tubulin bands, and comparing these normalized values from treated cultured neurons or stimulated brain slices with those from untreated control neurons or slices.
(c). Primary rat hippocampal neuron culture and drug treatment
Cultured hippocampal neurons were prepared from embryonic day 18 Sprague-Dawley rat brains. Dissociated neurons on poly-l-lysine coated (1 mg ml−1) coverslips were placed in neurobasal medium supplemented with B27 (Invitrogen), 0.5 mM l-glutamate and penicillin–streptomycin. For activation, cultured neurons at days in vitro (DIV) 18–21 were treated with NMDA (Sigma; 20 µM, 3 min), KCl (Sigma; 50 mM, 5 min) or l-glutamate (Sigma; 50 µM, 30 min). For induction of NMDA receptor-dependent LTD or mGluR-LTD, cultured neurons were treated with NMDA (20 µM, 3 min) and DHPG (Tocris; 50 μM, 30 min), respectively, and returned to normal conditioning medium. For the blockade of NMDA receptors, cultured neurons were pretreated with APV (Tocris; d-2-amino-5-phosphovalerate; 50 µM, 30 min), followed by stimulation with NMDA (20 µM, 3 min) in the presence of APV or with l-glutamate (50 µM, 1 min) or KCl (30 mM, 1 min) in the absence of APV. For chronic neuronal inhibition or activation, neurons were treated with tetrodotoxin (Tocris; TTX; 1 µM, 48 h) or with bicuculline (Tocris; Bic; 10 µM, 36 h). For blockade of metalloproteinase and γ-secretase activity, neurons were pretreated with GM6001 (Enzo Life Sciences; 2.5 and 25 µM, 30 min), DAPT (Sigma; 250 nM, 2 h; 2 µM, 3 h) and L-685,458 (Calbiochem; 1 µM, 30 min) before and during NMDA stimulation (20 µM, 3 min).
(d). Electrophysiology
C57BL/6 background wild-type mice of both sexes at the age of postnatal day 21–28 were used for electrophysiological experiments. Sagittal hippocampal slices (400 μm thick) were prepared using a vibratome (Leica VT1200) in ice-cold dissection buffer containing (in mM) 212 sucrose, 25 NaHCO3, 5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 3.5 MgSO4, 10 d-glucose, 1.25 l-ascorbic acid and 2 Na-pyruvate bubbled with 95% O2/5% CO2. The slices were recovered at 32°C for 1 h in normal artificial cerebrospinal fluid (ACSF) (in mM: 125 NaCl, 25 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 2.5 CaCl2, 1.3 MgCl2, 10 d-glucose) and thereafter maintained at room temperature. Extracellular field recordings were performed to monitor LTD induction. Stimulation and recording pipettes were pulled from borosilicate glass capillaries (Harvard Apparatus) using a micropipette electrode puller (Narishege). Field excitatory postsynaptic potentials (fEPSPs) were recorded in the stratum radiatum of the hippocampal CA1 using pipettes filled with ACSF (1 MΩ). The Schaffer collateral pathway was stimulated every 20 s with pipettes filled with ACSF (0.3–0.5 MΩ). The stimulation intensity was adjusted to yield a half-maximal response, and three successive responses were averaged and expressed relative to the normalized baseline. After a stable baseline was established, NMDA receptor-dependent LTD and mGluR-LTD were induced by low-frequency stimulation (1 Hz, 15 min) and applying DHPG (50 µM, 5 min), respectively. To inhibit NMDA receptor-dependent LTD, APV (50 µM) was bath-applied throughout the recordings starting from 20 min before LFS, as reported previously [110]. Recordings were made by using MultiClamp 700B amplifier (Molecular Devices) under visual control with differential interference contrast illumination in an upright microscope (BX50WI; Olympus). Data were acquired by Clampex 10.2 (Molecular Devices) and analysed by Clampfit 10 (Molecular Devices). Picrotoxin (100 µM) was added during both LTD experiments. Immediately after electrophysiological recordings (60 min after LFS), mouse brain slices were homogenized in buffered sucrose (0.32 M sucrose, 4 mM HEPES, 1 mM MgCl2, 0.5 mM CaCl2, pH 7.3) with freshly added protease inhibitors for immunoblot analyses.
Funding statement
This study was supported by the Institute for Basic Science (IBS).
References
- 1.Johnson-Venkatesh EM, Umemori H. 2010. Secreted factors as synaptic organizers. Eur. J. Neurosci. 32, 181–190. ( 10.1111/j.1460-9568.2010.07338.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sudhof TC. 2008. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455, 903–911. ( 10.1038/nature07456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siddiqui TJ, Craig AM. 2011. Synaptic organizing complexes. Curr. Opin. Neurobiol. 21, 132–143. ( 10.1016/j.conb.2010.08.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen K, Scheiffele P. 2010. Genetics and cell biology of building specific synapse connectivity. Annu. Rev. Neurosci. 33, 473–507. ( 10.1146/annurev.neuro.051508.135302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biederer T, Stagi M. 2008. Signaling by synaptogenic molecules. Curr. Opin. Neurobiol. 18, 261–269. ( 10.1016/j.conb.2008.07.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalva MB, McClelland AC, Kayser MS. 2007. Cell adhesion molecules: signalling functions at the synapse. Nat. Rev. Neurosci. 8, 206–220. ( 10.1038/nrn2075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brose N. 2009. Synaptogenic proteins and synaptic organizers: ‘many hands make light work’. Neuron 61, 650–652. ( 10.1016/j.neuron.2009.02.014) [DOI] [PubMed] [Google Scholar]
- 8.Tallafuss A, Constable JR, Washbourne P. 2010. Organization of central synapses by adhesion molecules. Eur. J. Neurosci. 32, 198–206. ( 10.1111/j.1460-9568.2010.07340.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woo J, Kwon SK, Kim E. 2009. The NGL family of leucine-rich repeat-containing synaptic adhesion molecules. Mol. Cell Neurosci. 42, 1–10. ( 10.1016/j.mcn.2009.05.008) [DOI] [PubMed] [Google Scholar]
- 10.Williams ME, de Wit J, Ghosh A. 2010. Molecular mechanisms of synaptic specificity in developing neural circuits. Neuron 68, 9–18. ( 10.1016/j.neuron.2010.09.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuzaki M. 2010. Synapse formation and maintenance by C1q family proteins: a new class of secreted synapse organizers. Eur. J. Neurosci. 32, 191–197. ( 10.1111/j.1460-9568.2010.07346.x) [DOI] [PubMed] [Google Scholar]
- 12.Ko J. 2012. The leucine-rich repeat superfamily of synaptic adhesion molecules: LRRTMs and Slitrks. Mol. Cells 34, 335–340. ( 10.1007/s10059-012-0113-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krueger DD, Tuffy LP, Papadopoulos T, Brose N. 2012. The role of neurexins and neuroligins in the formation, maturation, and function of vertebrate synapses. Curr. Opin. Neurobiol. 22, 412–422. ( 10.1016/j.conb.2012.02.012) [DOI] [PubMed] [Google Scholar]
- 14.Missler M, Sudhof TC, Biederer T. 2012. Synaptic cell adhesion. Cold Spring Harb. Perspect. Biol. 4, a005694 ( 10.1101/cshperspect.a005694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Wit J, et al. 2009. LRRTM2 interacts with Neurexin1 and regulates excitatory synapse formation. Neuron 64, 799–806. ( 10.1016/j.neuron.2009.12.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko J, Fuccillo MV, Malenka RC, Sudhof TC. 2009. LRRTM2 functions as a neurexin ligand in promoting excitatory synapse formation. Neuron 64, 791–798. ( 10.1016/j.neuron.2009.12.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siddiqui TJ, Pancaroglu R, Kang Y, Rooyakkers A, Craig AM. 2010. LRRTMs and neuroligins bind neurexins with a differential code to cooperate in glutamate synapse development. J. Neurosci. 30, 7495–7506. ( 10.1523/JNEUROSCI.0470-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin JC, Ho WH, Gurney A, Rosenthal A. 2003. The netrin-G1 ligand NGL-1 promotes the outgrowth of thalamocortical axons. Nat. Neurosci. 6, 1270–1276. ( 10.1038/nn1148) [DOI] [PubMed] [Google Scholar]
- 19.Kim S, et al. 2006. NGL family PSD-95-interacting adhesion molecules regulate excitatory synapse formation. Nat. Neurosci. 9, 1294–1301. ( 10.1038/nn1763) [DOI] [PubMed] [Google Scholar]
- 20.Kwon SK, Woo J, Kim SY, Kim H, Kim E. 2010. Trans-synaptic adhesions between netrin-G ligand-3 (NGL-3) and receptor tyrosine phosphatases LAR, protein-tyrosine phosphatase δ (PTPδ), and PTPσ via specific domains regulate excitatory synapse formation. J. Biol. Chem. 285, 13 966–13 978. ( 10.1074/jbc.M109.061127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woo J, Kwon SK, Choi S, Kim S, Lee J, Dunah AW, Sheng M, Kim E. 2009. Trans-synaptic adhesion between NGL-3 and LAR regulates the formation of excitatory synapses. Nat. Neurosci. 12, 428–437. ( 10.1038/nn.2279) [DOI] [PubMed] [Google Scholar]
- 22.Takahashi H, Arstikaitis P, Prasad T, Bartlett TE, Wang YT, Murphy TH, Craig AM. 2011. Postsynaptic TrkC and presynaptic PTPσ function as a bidirectional excitatory synaptic organizing complex. Neuron 69, 287–303. ( 10.1016/j.neuron.2010.12.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuda K, et al. 2010. Cbln1 is a ligand for an orphan glutamate receptor δ2, a bidirectional synapse organizer. Science 328, 363–368. ( 10.1126/science.1185152) [DOI] [PubMed] [Google Scholar]
- 24.Uemura T, Lee SJ, Yasumura M, Takeuchi T, Yoshida T, Ra M, Taguchi R, Sakimura K, Mishina M. 2010. Trans-synaptic interaction of GluRδ2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell 141, 1068–1079. ( 10.1016/j.cell.2010.04.035) [DOI] [PubMed] [Google Scholar]
- 25.Takahashi H, et al. 2012. Selective control of inhibitory synapse development by Slitrk3-PTPδ trans-synaptic interaction. Nat. Neurosci. 15, 389–398. ( 10.1038/nn.3040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yim YS, Kwon Y, Nam J, Yoon HI, Lee K, Kim DG, Kim E, Kim CH, Ko J. 2013. Slitrks control excitatory and inhibitory synapse formation with LAR receptor protein tyrosine phosphatases. Proc. Natl Acad. Sci. USA 110, 4057–4062. ( 10.1073/pnas.1209881110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valnegri P, Montrasio C, Brambilla D, Ko J, Passafaro M, Sala C. 2011. The X-linked intellectual disability protein IL1RAPL1 regulates excitatory synapse formation by binding PTPδ and RhoGAP2. Hum. Mol. Genet. 20, 4797–4809. ( 10.1093/hmg/ddr418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida T, Yasumura M, Uemura T, Lee SJ, Ra M, Taguchi R, Iwakura Y, Mishina M. 2011. IL-1 receptor accessory protein-like 1 associated with mental retardation and autism mediates synapse formation by trans-synaptic interaction with protein tyrosine phosphatase δ. J. Neurosci. 31, 13 485–13 499. ( 10.1523/JNEUROSCI.2136-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshida T, Shiroshima T, Lee SJ, Yasumura M, Uemura T, Chen X, Iwakura Y, Mishina M. 2012. Interleukin-1 receptor accessory protein organizes neuronal synaptogenesis as a cell adhesion molecule. J. Neurosci. 32, 2588–2600. ( 10.1523/JNEUROSCI.4637-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peixoto RT, Kunz PA, Kwon H, Mabb AM, Sabatini BL, Philpot BD, Ehlers M. 2012. Transsynaptic signaling by activity-dependent cleavage of neuroligin-1. Neuron 76, 396–409. ( 10.1016/j.neuron.2012.07.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki K, et al. 2012. Activity-dependent proteolytic cleavage of neuroligin-1. Neuron 76, 410–422. ( 10.1016/j.neuron.2012.10.003) [DOI] [PubMed] [Google Scholar]
- 32.Tada T, Sheng M. 2006. Molecular mechanisms of dendritic spine morphogenesis. Curr. Opin. Neurobiol. 16, 95–101. ( 10.1016/j.conb.2005.12.001) [DOI] [PubMed] [Google Scholar]
- 33.Bastrikova N, Gardner GA, Reece JM, Jeromin A, Dudek SM. 2008. Synapse elimination accompanies functional plasticity in hippocampal neurons. Proc. Natl Acad. Sci. USA 105, 3123–3127. ( 10.1073/pnas.0800027105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halpain S, Hipolito A, Saffer L. 1998. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J. Neurosci. 18, 9835–9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han K, Kim E. 2008. Synaptic adhesion molecules and PSD-95. Prog. Neurobiol. 84, 263–283. ( 10.1016/j.pneurobio.2007.10.011) [DOI] [PubMed] [Google Scholar]
- 36.Nakashiba T, Ikeda T, Nishimura S, Tashiro K, Honjo T, Culotti JG, Itohara S. 2000. Netrin-G1: a novel glycosyl phosphatidylinositol-linked mammalian netrin that is functionally divergent from classical netrins. J. Neurosci. 20, 6540–6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakashiba T, Nishimura S, Ikeda T, Itohara S. 2002. Complementary expression and neurite outgrowth activity of netrin-G subfamily members. Mech. Dev. 111, 47–60. ( 10.1016/S0925-4773(01)00600-1) [DOI] [PubMed] [Google Scholar]
- 38.Yin Y, Miner JH, Sanes JR. 2002. Laminets: laminin- and netrin-related genes expressed in distinct neuronal subsets. Mol. Cell Neurosci. 19, 344–358. ( 10.1006/mcne.2001.1089) [DOI] [PubMed] [Google Scholar]
- 39.Nishimura-Akiyoshi S, Niimi K, Nakashiba T, Itohara S. 2007. Axonal netrin-Gs transneuronally determine lamina-specific subdendritic segments. Proc. Natl Acad. Sci. USA 104, 14 801–14 806. ( 10.1073/pnas.0706919104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linhoff MW, Lauren J, Cassidy RM, Dobie FA, Takahashi H, Nygaard HB, Airaksinen MS, Strittmatter SM, Craig AM. 2009. An unbiased expression screen for synaptogenic proteins identifies the LRRTM protein family as synaptic organizers. Neuron 61, 734–749. ( 10.1016/j.neuron.2009.01.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stryker E, Johnson KG. 2007. LAR, liprin α and the regulation of active zone morphogenesis. J. Cell Sci. 120, 3723–3728. ( 10.1242/jcs.03491) [DOI] [PubMed] [Google Scholar]
- 42.Dunah AW, Hueske E, Wyszynski M, Hoogenraad CC, Jaworski J, Pak DT, Simonetta A, Liu G, Sheng M. 2005. LAR receptor protein tyrosine phosphatases in the development and maintenance of excitatory synapses. Nat. Neurosci. 8, 458–467. [DOI] [PubMed] [Google Scholar]
- 43.Yong VW, Zabad RK, Agrawal S, Goncalves Dasilva A, Metz LM. 2007. Elevation of matrix metalloproteinases (MMPs) in multiple sclerosis and impact of immunomodulators. J. Neurol. Sci. 259, 79–84. ( 10.1016/j.jns.2006.11.021) [DOI] [PubMed] [Google Scholar]
- 44.Ethell IM, Ethell DW. 2007. Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. J. Neurosci. Res. 85, 2813–2823. ( 10.1002/jnr.21273) [DOI] [PubMed] [Google Scholar]
- 45.Agrawal SM, Lau L, Yong VW. 2008. MMPs in the central nervous system: where the good guys go bad. Semin. Cell Dev. Biol. 19, 42–51. ( 10.1016/j.semcdb.2007.06.003) [DOI] [PubMed] [Google Scholar]
- 46.Huntley GW. 2012. Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat. Rev. Neurosci. 13, 743–757. ( 10.1038/nrn3320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frischknecht R, Gundelfinger ED. 2012. The brain's extracellular matrix and its role in synaptic plasticity. Adv. Exp. Med. Biol. 970, 153–171. ( 10.1007/978-3-7091-0932-8_7) [DOI] [PubMed] [Google Scholar]
- 48.Gundelfinger ED, Frischknecht R, Choquet D, Heine M. 2010. Converting juvenile into adult plasticity: a role for the brain's extracellular matrix. Eur. J. Neurosci. 31, 2156–2165. ( 10.1111/j.1460-9568.2010.07253.x) [DOI] [PubMed] [Google Scholar]
- 49.Dityatev A, Rusakov DA. 2011. Molecular signals of plasticity at the tetrapartite synapse. Curr. Opin. Neurobiol. 21, 353–359. ( 10.1016/j.conb.2010.12.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dityatev A, Schachner M, Sonderegger P. 2010. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat. Rev. Neurosci. 11, 735–746. ( 10.1038/nrn2898) [DOI] [PubMed] [Google Scholar]
- 51.Milward EA, Fitzsimmons C, Szklarczyk A, Conant K. 2007. The matrix metalloproteinases and CNS plasticity: an overview. J. Neuroimmunol. 187, 9–19. ( 10.1016/j.jneuroim.2007.04.010) [DOI] [PubMed] [Google Scholar]
- 52.Rivera S, Khrestchatisky M, Kaczmarek L, Rosenberg GA, Jaworski DM. 2010. Metzincin proteases and their inhibitors: foes or friends in nervous system physiology? J. Neurosci. 30, 15 337–15 357. ( 10.1523/JNEUROSCI.3467-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mizoguchi H, Yamada K, Nabeshima T. 2011. Matrix metalloproteinases contribute to neuronal dysfunction in animal models of drug dependence, Alzheimer's disease, and epilepsy. Biochem. Res. Int. 2011, 681385 ( 10.1155/2011/681385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wright JW, Harding JW. 2009. Contributions of matrix metalloproteinases to neural plasticity, habituation, associative learning and drug addiction. Neural Plasticity 2009, 579382 ( 10.1155/2009/579382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang XB, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW. 2008. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc. Natl Acad. Sci. USA 105, 19 520–19 525. ( 10.1073/pnas.0807248105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagy V, Bozdagi O, Huntley GW. 2007. The extracellular protease matrix metalloproteinase-9 is activated by inhibitory avoidance learning and required for long-term memory. Learn. Mem. 14, 655–664. ( 10.1101/lm.678307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bozdagi O, Nagy V, Kwei KT, Huntley GW. 2007. In vivo roles for matrix metalloproteinase-9 in mature hippocampal synaptic physiology and plasticity. J. Neurophysiol. 98, 334–344. ( 10.1152/jn.00202.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagy V, et al. 2006. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J. Neurosci. 26, 1923–1934. ( 10.1523/JNEUROSCI.4359-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Michaluk P, Mikasova L, Groc L, Frischknecht R, Choquet D, Kaczmarek L. 2009. Matrix metalloproteinase-9 controls NMDA receptor surface diffusion through integrin β1 signaling. J. Neurosci. 29, 6007–6012. ( 10.1523/JNEUROSCI.5346-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gorkiewicz T, Szczuraszek K, Wyrembek P, Michaluk P, Kaczmarek L, Mozrzymas JW. 2010. Matrix metalloproteinase-9 reversibly affects the time course of NMDA-induced currents in cultured rat hippocampal neurons. Hippocampus 20, 1105–1108. ( 10.1002/hipo.20736) [DOI] [PubMed] [Google Scholar]
- 61.Fragkouli A, Papatheodoropoulos C, Georgopoulos S, Stamatakis A, Stylianopoulou F, Tsilibary EC, Tzinia AK. 2012. Enhanced neuronal plasticity and elevated endogenous sAPPalpha levels in mice over-expressing MMP9. J. Neurochem. 121, 239–251. ( 10.1111/j.1471-4159.2011.07637.x) [DOI] [PubMed] [Google Scholar]
- 62.Meighan SE, Meighan PC, Choudhury P, Davis CJ, Olson ML, Zornes PA, Wright JW, Harding JW. 2006. Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J. Neurochem. 96, 1227–1241. ( 10.1111/j.1471-4159.2005.03565.x) [DOI] [PubMed] [Google Scholar]
- 63.Dziembowska M, Wlodarczyk J. 2012. MMP9: a novel function in synaptic plasticity. Int. J. Biochem. Cell Biol. 44, 709–713. ( 10.1016/j.biocel.2012.01.023) [DOI] [PubMed] [Google Scholar]
- 64.Michaluk P, et al. 2011. Influence of matrix metalloproteinase MMP-9 on dendritic spine morphology. J. Cell Sci. 124, 3369–3380. ( 10.1242/jcs.090852) [DOI] [PubMed] [Google Scholar]
- 65.Wilczynski GM, et al. 2008. Important role of matrix metalloproteinase 9 in epileptogenesis. J. Cell Biol. 180, 1021–1035. ( 10.1083/jcb.200708213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meighan PC, Meighan SE, Davis CJ, Wright JW, Harding JW. 2007. Effects of matrix metalloproteinase inhibition on short- and long-term plasticity of Schaffer collateral/CA1 synapses. J. Neurochem. 102, 2085–2096. ( 10.1111/j.1471-4159.2007.04682.x) [DOI] [PubMed] [Google Scholar]
- 67.Bliss TV, Collingridge GL. 1993. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39. ( 10.1038/361031a0) [DOI] [PubMed] [Google Scholar]
- 68.Szklarczyk A, et al. 2008. MMP-7 cleaves the NR1 NMDA receptor subunit and modifies NMDA receptor function. FASEB J. 22, 3757–3767. ( 10.1096/fj.07-101402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brakeman PR, Lanahan AA, OB R, Roche K, Barnes CA, Huganir RL, Worley PF. 1997. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature 386, 284–288. ( 10.1038/386284a0) [DOI] [PubMed] [Google Scholar]
- 70.Lin KT, Sloniowski S, Ethell DW, Ethell IM. 2008. Ephrin-B2-induced cleavage of EphB2 receptor is mediated by matrix metalloproteinases to trigger cell repulsion. J. Biol. Chem. 283, 28 969–28 979. ( 10.1074/jbc.M804401200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Monea S, Jordan BA, Srivastava S, DeSouza S, Ziff EB. 2006. Membrane localization of membrane type 5 matrix metalloproteinase by AMPA receptor binding protein and cleavage of cadherins. J. Neurosci. 26, 2300–2312. ( 10.1523/JNEUROSCI.3521-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Conant K, Lim ST, Randall B, Maguire-Zeiss KA. 2012. Matrix metalloproteinase dependent cleavage of cell adhesion molecules in the pathogenesis of CNS dysfunction with HIV and methamphetamine. Curr. HIV Res. 10, 384–391. ( 10.2174/157016212802138733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Conant K, Lonskaya I, Szklarczyk A, Krall C, Steiner J, Maguire-Zeiss K, Lim ST. 2011. Methamphetamine-associated cleavage of the synaptic adhesion molecule intercellular adhesion molecule-5. J. Neurochem. 118, 521–532. ( 10.1111/j.1471-4159.2010.07153.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Michaluk P, Kolodziej L, Mioduszewska B, Wilczynski GM, Dzwonek J, Jaworski J, Gorecki DC, Ottersen OP, Kaczmarek L. 2007. β-dystroglycan as a target for MMP-9, in response to enhanced neuronal activity. J. Biol. Chem. 282, 16 036–16 041. ( 10.1074/jbc.M700641200) [DOI] [PubMed] [Google Scholar]
- 75.Bajor M, Michaluk P, Gulyassy P, Kekesi AK, Juhasz G, Kaczmarek L. 2012. Synaptic cell adhesion molecule-2 and collapsin response mediator protein-2 are novel members of the matrix metalloproteinase-9 degradome. J. Neurochem. 122, 775–788. ( 10.1111/j.1471-4159.2012.07829.x) [DOI] [PubMed] [Google Scholar]
- 76.Conant K, Wang Y, Szklarczyk A, Dudak A, Mattson MP, Lim ST. 2010. Matrix metalloproteinase-dependent shedding of intercellular adhesion molecule-5 occurs with long-term potentiation. Neuroscience 166, 508–521. ( 10.1016/j.neuroscience.2009.12.061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tai CY, Kim SA, Schuman EM. 2008. Cadherins and synaptic plasticity. Curr. Opin. Cell Biol. 20, 567–575. ( 10.1016/j.ceb.2008.06.003) [DOI] [PubMed] [Google Scholar]
- 78.Lai KO, Ip NY. 2009. Synapse development and plasticity: roles of ephrin/Eph receptor signaling. Curr. Opin. Neurobiol. 19, 275–283. ( 10.1016/j.conb.2009.04.009) [DOI] [PubMed] [Google Scholar]
- 79.Robbins EM, Krupp AJ, Perez de Arce K, Ghosh AK, Fogel AI, Boucard A, Südhof TC, Stein V, Biederer T. 2010. SynCAM 1 adhesion dynamically regulates synapse number and impacts plasticity and learning. Neuron 68, 894–906. ( 10.1016/j.neuron.2010.11.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dityatev A, Bukalo O, Schachner M. 2008. Modulation of synaptic transmission and plasticity by cell adhesion and repulsion molecules. Neuron Glia Biol. 4, 197–209. ( 10.1017/S1740925X09990111) [DOI] [PubMed] [Google Scholar]
- 81.Bliss T, Errington M, Fransen E, Godfraind JM, Kauer JA, Kooy RF, Maness PF, Furley AJW. 2000. Long-term potentiation in mice lacking the neural cell adhesion molecule L1. Curr. Biol. 10, 1607–1610. ( 10.1016/S0960-9822(00)00865-4) [DOI] [PubMed] [Google Scholar]
- 82.Wolfe MS. 2009. γ-Secretase in biology and medicine. Semin. Cell Dev. Biol. 20, 219–224. ( 10.1016/j.semcdb.2008.12.011) [DOI] [PubMed] [Google Scholar]
- 83.Kopan R, Ilagan MX. 2004. γ-secretase: proteasome of the membrane? Nat. Rev. Mol. Cell Biol. 5, 499–504. ( 10.1038/nrm1406) [DOI] [PubMed] [Google Scholar]
- 84.Haapasalo A, Kovacs DM. 2011. The many substrates of presenilin/γ-secretase. J. Alzheimer's Dis. 25, 3–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hemming ML, Elias JE, Gygi SP, Selkoe DJ. 2008. Proteomic profiling of γ-secretase substrates and mapping of substrate requirements. PLoS Biol. 6, e257 ( 10.1371/journal.pbio.0060257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marambaud P, Wen PH, Dutt A, Shioi J, Takashima A, Siman R, Robakis NK. 2003. A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell 114, 635–645. ( 10.1016/j.cell.2003.08.008) [DOI] [PubMed] [Google Scholar]
- 87.Ni CY, Murphy MP, Golde TE, Carpenter G. 2001. γ-Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science 294, 2179–2181. ( 10.1126/science.1065412) [DOI] [PubMed] [Google Scholar]
- 88.Ni CY, Yuan H, Carpenter G. 2003. Role of the ErbB-4 carboxyl terminus in γ-secretase cleavage. J. Biol. Chem. 278, 4561–4565. ( 10.1074/jbc.M210504200) [DOI] [PubMed] [Google Scholar]
- 89.Kim DY, Ingano LA, Kovacs DM. 2002. Nectin-1α, an immunoglobulin-like receptor involved in the formation of synapses, is a substrate for presenilin/γ-secretase-like cleavage. J. Biol. Chem. 277, 49 976–49 981. ( 10.1074/jbc.M210179200) [DOI] [PubMed] [Google Scholar]
- 90.Schulz JG, Annaert W, Vandekerckhove J, Zimmermann P, De Strooper B, David G. 2003. Syndecan 3 intramembrane proteolysis is presenilin/γ-secretase-dependent and modulates cytosolic signaling. J. Biol. Chem. 278, 48 651–48 657. ( 10.1074/jbc.M308424200) [DOI] [PubMed] [Google Scholar]
- 91.Meyer EL, Strutz N, Gahring LC, Rogers SW. 2003. Glutamate receptor subunit 3 is modified by site-specific limited proteolysis including cleavage by γ-secretase. J. Biol. Chem. 278, 23 786–23 796. ( 10.1074/jbc.M301360200) [DOI] [PubMed] [Google Scholar]
- 92.Waschbusch D, Born S, Niediek V, Kirchgessner N, Tamboli IY, Walter J, Merkel R, Hoffmann B. 2009. Presenilin 1 affects focal adhesion site formation and cell force generation via c-Src transcriptional and posttranslational regulation. J. Biol. Chem. 284, 10 138–10 149. ( 10.1074/jbc.M806825200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tomita T, Tanaka S, Morohashi Y, Iwatsubo T. 2006. Presenilin-dependent intramembrane cleavage of ephrin-B1. Mol. Neurodegener. 1, 2 ( 10.1186/1750-1326-1-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Georgakopoulos A, Litterst C, Ghersi E, Baki L, Xu C, Serban G, Robakis NK. 2006. Metalloproteinase/Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation and signaling. EMBO J. 25, 1242–1252. ( 10.1038/sj.emboj.7601031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Inoue E, et al. 2009. Synaptic activity prompts γ-secretase-mediated cleavage of EphA4 and dendritic spine formation. J. Cell Biol. 185, 551–564. ( 10.1083/jcb.200809151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Litterst C, Georgakopoulos A, Shioi J, Ghersi E, Wisniewski T, Wang R, Ludwig A, Robakis NK. 2007. Ligand binding and calcium influx induce distinct ectodomain/γ-secretase-processing pathways of EphB2 receptor. J. Biol. Chem. 282, 16 155–16 163. ( 10.1074/jbc.M611449200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Haapasalo A, Kim DY, Carey BW, Turunen MK, Pettingell WH, Kovacs DM. 2007. Presenilin/γ-secretase-mediated cleavage regulates association of leukocyte-common antigen-related (LAR) receptor tyrosine phosphatase with β-catenin. J. Biol. Chem. 282, 9063–9072. ( 10.1074/jbc.M611324200) [DOI] [PubMed] [Google Scholar]
- 98.Bot N, Schweizer C, Ben Halima S, Fraering PC. 2011. Processing of the synaptic cell adhesion molecule neurexin-3β by Alzheimer disease α- and γ-secretases. J. Biol. Chem. 286, 2762–2773. ( 10.1074/jbc.M110.142521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saura CA, Servian-Morilla E, Scholl FG. 2011. Presenilin/γ-secretase regulates neurexin processing at synapses. PLoS ONE 6, e19430 ( 10.1371/journal.pone.0019430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee HK, Kameyama K, Huganir RL, Bear MF. 1998. NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron 21, 1151–1162. ( 10.1016/S0896-6273(00)80632-7) [DOI] [PubMed] [Google Scholar]
- 101.Snyder EM, Colledge M, Crozier RA, Chen WS, Scott JD, Bear MF. 2005. Role for A kinase-anchoring proteins (AKAPS) in glutamate receptor trafficking and long term synaptic depression. J. Biol. Chem. 280, 16 962–16 968. ( 10.1074/jbc.M409693200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oh MC, Derkach VA, Guire ES, Soderling TR. 2006. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J. Biol. Chem. 281, 752–758. ( 10.1074/jbc.M509677200) [DOI] [PubMed] [Google Scholar]
- 103.Carroll RC, Beattie EC, Xia H, Luscher C, Altschuler Y, Nicoll RA, Malenka RC, von Zastrow M. 1999. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc. Natl Acad. Sci. USA 96, 14 112–14 117. ( 10.1073/pnas.96.24.14112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Camodeca N, Breakwell NA, Rowan MJ, Anwyl R. 1999. Induction of LTD by activation of group I mGluR in the dentate gyrus in vitro. Neuropharmacology 38, 1597–1606. ( 10.1016/S0028-3908(99)00093-3) [DOI] [PubMed] [Google Scholar]
- 105.Fitzjohn SM, Kingston AE, Lodge D, Collingridge GL. 1999. DHPG-induced LTD in area CA1 of juvenile rat hippocampus; characterisation and sensitivity to novel mGlu receptor antagonists. Neuropharmacology 38, 1577–1583. ( 10.1016/S0028-3908(99)00123-9) [DOI] [PubMed] [Google Scholar]
- 106.Huber KM, Kayser MS, Bear MF. 2000. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science 288, 1254–1257. ( 10.1126/science.288.5469.1254) [DOI] [PubMed] [Google Scholar]
- 107.Palmer MJ, Irving AJ, Seabrook GR, Jane DE, Collingridge GL. 1997. The group I mGlu receptor agonist DHPG induces a novel form of LTD in the CA1 region of the hippocampus. Neuropharmacology 36, 1517–1532. ( 10.1016/S0028-3908(97)00181-0) [DOI] [PubMed] [Google Scholar]
- 108.Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. 2001. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat. Neurosci. 4, 1079–1085. ( 10.1038/nn746) [DOI] [PubMed] [Google Scholar]
- 109.Dudek SM, Bear MF. 1992. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc. Natl Acad. Sci. USA 89, 4363–4367. ( 10.1073/pnas.89.10.4363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Milner AJ, Cummings DM, Spencer JP, Murphy KP. 2004. Bi-directional plasticity and age-dependent long-term depression at mouse CA3-CA1 hippocampal synapses. Neurosci. Lett. 367, 1–5. ( 10.1016/j.neulet.2004.04.056) [DOI] [PubMed] [Google Scholar]
- 111.Tian L, et al. 2007. Activation of NMDA receptors promotes dendritic spine development through MMP-mediated ICAM-5 cleavage. J. Cell Biol. 178, 687–700. ( 10.1083/jcb.200612097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Grobelny D, Poncz L, Galardy RE. 1992. Inhibition of human skin fibroblast collagenase, thermolysin, and Pseudomonas aeruginosa elastase by peptide hydroxamic acids. Biochemistry 31, 7152–7154. ( 10.1021/bi00146a017) [DOI] [PubMed] [Google Scholar]
- 113.Schultz GS, et al. 1992. Treatment of alkali-injured rabbit corneas with a synthetic inhibitor of matrix metalloproteinases. Investig. Ophthalmol. Vis. Sci. 33, 3325–3331. [PubMed] [Google Scholar]
- 114.Johnson KG, Van Vactor D. 2003. Receptor protein tyrosine phosphatases in nervous system development. Physiol. Rev. 83, 1–24. [DOI] [PubMed] [Google Scholar]
- 115.Gundelfinger ED, Fejtova A. 2012. Molecular organization and plasticity of the cytomatrix at the active zone. Curr. Opin. Neurobiol. 22, 423–430. ( 10.1016/j.conb.2011.10.005) [DOI] [PubMed] [Google Scholar]
- 116.Jin Y, Garner CC. 2008. Molecular mechanisms of presynaptic differentiation. Annu. Rev. Cell Dev. Biol. 24, 237–262. ( 10.1146/annurev.cellbio.23.090506.123417) [DOI] [PubMed] [Google Scholar]
- 117.Ruhe JE, Streit S, Hart S, Ullrich A. 2006. EGFR signaling leads to downregulation of PTP-LAR via TACE-mediated proteolytic processing. Cell Signal. 18, 1515–1527. ( 10.1016/j.cellsig.2005.12.003) [DOI] [PubMed] [Google Scholar]
- 118.Aicher B, Lerch MM, Muller T, Schilling J, Ullrich A. 1997. Cellular redistribution of protein tyrosine phosphatases LAR and PTPσ by inducible proteolytic processing. J. Cell Biol. 138, 681–696. ( 10.1083/jcb.138.3.681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bilousova TV, Rusakov DA, Ethell DW, Ethell IM. 2006. Matrix metalloproteinase-7 disrupts dendritic spines in hippocampal neurons through NMDA receptor activation. J. Neurochem. 97, 44–56. ( 10.1111/j.1471-4159.2006.03701.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wright JW, Meighan PC, Brown TE, Wiediger RV, Sorg BA, Harding JW. 2009. Habituation-induced neural plasticity in the hippocampus and prefrontal cortex mediated by MMP-3. Behav. Brain Res. 203, 27–34. ( 10.1016/j.bbr.2009.04.014) [DOI] [PubMed] [Google Scholar]
- 121.Wright JW, Meighan SE, Murphy ES, Holtfreter KL, Davis CJ, Olson ML, Benoist C, Muhunthan K, Harding J. 2006. Habituation of the head-shake response induces changes in brain matrix metalloproteinases-3 (MMP-3) and -9. Behav. Brain Res. 174, 78–85. ( 10.1016/j.bbr.2006.07.006) [DOI] [PubMed] [Google Scholar]
- 122.Van Hove I, Lemmens K, Van de Velde S, Verslegers M, Moons L. 2012. Matrix metalloproteinase-3 in the central nervous system: a look on the bright side. J. Neurochem. 123, 203–216. ( 10.1111/j.1471-4159.2012.07900.x) [DOI] [PubMed] [Google Scholar]
- 123.Pauly T, Ratliff M, Pietrowski E, Neugebauer R, Schlicksupp A, Kirsch J, Kuhse J, Koch K-W. 2008. Activity-dependent shedding of the NMDA receptor glycine binding site by matrix metalloproteinase 3: a PUTATIVE mechanism of postsynaptic plasticity. PLoS ONE 3, e2681 ( 10.1371/journal.pone.0002681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Olson ML, Meighan PC, Brown TE, Asay AL, Benoist CC, Harding JW, Wright JW. 2008. Hippocampal MMP-3 elevation is associated with passive avoidance conditioning. Regul. Pept. 146, 19–25. ( 10.1016/j.regpep.2007.07.004) [DOI] [PubMed] [Google Scholar]
- 125.Wright JW, Brown TE, Harding JW. 2007. Inhibition of hippocampal matrix metalloproteinase-3 and -9 disrupts spatial memory. Neural Plasticity 2007, 73813 ( 10.1155/2007/73813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Szklarczyk A, Conant K, Owens DF, Ravin R, McKay RD, Gerfen C. 2007. Matrix metalloproteinase-7 modulates synaptic vesicle recycling and induces atrophy of neuronal synapses. Neuroscience 149, 87–98. ( 10.1016/j.neuroscience.2007.07.032) [DOI] [PubMed] [Google Scholar]
- 127.Cho RW, et al. 2008. mGluR1/5-dependent long-term depression requires the regulated ectodomain cleavage of neuronal pentraxin NPR by TACE. Neuron 57, 858–871. ( 10.1016/j.neuron.2008.01.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. 2009. Soluble oligomers of amyloid β protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron 62, 788–801. ( 10.1016/j.neuron.2009.05.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shankar GM, et al. 2008. Amyloid-β protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat. Med. 14, 837–842. ( 10.1038/nm1782) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Snyder EM, et al. 2005. Regulation of NMDA receptor trafficking by amyloid-β. Nat. Neurosci. 8, 1051–1058. ( 10.1038/nn1503) [DOI] [PubMed] [Google Scholar]
- 131.Kim J, et al. 2008. Neuroligin-1 is required for normal expression of LTP and associative fear memory in the amygdala of adult animals. Proc. Natl Acad. Sci. USA 105, 9087–9092. ( 10.1073/pnas.0803448105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jung SY, et al. 2010. Input-specific synaptic plasticity in the amygdala is regulated by neuroligin-1 via postsynaptic NMDA receptors. Proc. Natl Acad. Sci. USA 107, 4710–4715. ( 10.1073/pnas.1001084107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shipman SL, Schnell E, Hirai T, Chen BS, Roche KW, Nicoll RA. 2011. Functional dependence of neuroligin on a new non-PDZ intracellular domain. Nat. Neurosci. 14, 718–726. ( 10.1038/nn.2825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dahlhaus R, Hines RM, Eadie BD, Kannangara TS, Hines DJ, Brown CE, Christie BR, El-Husseini A. 2010. Overexpression of the cell adhesion protein neuroligin-1 induces learning deficits and impairs synaptic plasticity by altering the ratio of excitation to inhibition in the hippocampus. Hippocampus 20, 305–322. ( 10.1002/hipo.20630) [DOI] [PubMed] [Google Scholar]
- 135.Kaksonen M, Pavlov I, Voikar V, Lauri SE, Hienola A, Riekki R, Lakso M, Taira T, Rauvala H. 2002. Syndecan-3-deficient mice exhibit enhanced LTP and impaired hippocampus-dependent memory. Mol. Cell Neurosci. 21, 158–172. ( 10.1006/mcne.2002.1167) [DOI] [PubMed] [Google Scholar]
- 136.Uetani N, Kato K, Ogura H, Mizuno K, Kawano K, Mikoshiba K, Yakura H, Asano M, Iwakura Y. 2000. Impaired learning with enhanced hippocampal long-term potentiation in PTPδ-deficient mice. EMBO J. 19, 2775–2785. ( 10.1093/emboj/19.12.2775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Elia J, et al. 2010. Rare structural variants found in attention-deficit hyperactivity disorder are preferentially associated with neurodevelopmental genes. Mol. Psychiatry 15, 637–646. ( 10.1038/mp.2009.57) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schormair B, et al. 2008. PTPRD (protein tyrosine phosphatase receptor type δ) is associated with restless legs syndrome. Nat. Genet. 40, 946–948. ( 10.1038/ng.190) [DOI] [PubMed] [Google Scholar]
- 139.Cortese S, Konofal E, Lecendreux M, Arnulf I, Mouren MC, Darra F, Dalla Bernardina B. 2005. Restless legs syndrome and attention-deficit/hyperactivity disorder: a review of the literature. Sleep 28, 1007–1013. [DOI] [PubMed] [Google Scholar]
- 140.Pinto D, et al. 2010. Functional impact of global rare copy number variation in autism spectrum disorders. Nature 466, 368–372. ( 10.1038/nature09146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Malhotra D, et al. 2011. High frequencies of de novo CNVs in bipolar disorder and schizophrenia. Neuron 72, 951–963. ( 10.1016/j.neuron.2011.11.007) [DOI] [PMC free article] [PubMed] [Google Scholar]