Abstract
It is well established that Zif268/Egr1, a member of the Egr family of transcription factors, is critical for the consolidation of several forms of memory; however, it is as yet uncertain whether increasing expression of Zif268 in neurons can facilitate memory formation. Here, we used an inducible transgenic mouse model to specifically induce Zif268 overexpression in forebrain neurons and examined the effect on recognition memory and hippocampal synaptic transmission and plasticity. We found that Zif268 overexpression during the establishment of memory for objects did not change the ability to form a long-term memory of objects, but enhanced the capacity to form a long-term memory of the spatial location of objects. This enhancement was paralleled by increased long-term potentiation in the dentate gyrus of the hippocampus and by increased activity-dependent expression of Zif268 and selected Zif268 target genes. These results provide novel evidence that transcriptional mechanisms engaging Zif268 contribute to determining the strength of newly encoded memories.
Keywords: recognition memory, long-term potentiation, dentate gyrus, transcription factor, conditional mutant mouse, Egr1
1. Introduction
There has nearly been a century of interest in the idea that encoding and storage of information in the brain rely on changes in the efficacy of synaptic connections within the neural networks that are activated during a learning experience, a process required to form a stored memory trace available for recall. The prevailing model for cellular consolidation underlying the laying down of memory suggests that a sequence of events including receptor activation, synapse-to-nuclear signalling and the activation of selective gene programmes and subsequent synthesis of proteins is a key mechanism underlying the enduring modification of neural networks required for the stability of memories. One critical step in this process is the activation of a class of immediate early genes (IEGs) encoding inducible transcription factors that interact with promoter regulatory elements on a host of downstream late-response genes to regulate their expression. Zif268/Egr1 is one such IEG encoding a zinc finger transcription factor of the Egr family that plays a crucial role in the maintenance of hippocampal long-term potentiation (LTP) and the consolidation of several forms of memories [1]. Studies in Zif268 knockout mice highlighted a particular sensitivity to Zif268-deficiency of hippocampal-dependent spatial learning and spatial recognition memory [2,3]. Consistent with this, Zif268-deficiency was also found to impair the formation of stable hippocampal place cell representations of novel environments [4]. Zif268 mRNA and protein are also rapidly induced in association with LTP and in defined brain structures following learning or recall of several types of memory (see [5,6] for reviews) and this can lead to a functional increase in Zif268 protein binding to its DNA consensus Egr response element (ERE) [7]. Using a gain-of-function strategy in transgenic mice, a recent study reported that enhanced neuronal expression of Zif268 can slow down extinction of conditioned taste aversion [8]. As resistance to extinction can be taken as a sign of stronger memory formed during initial training, this raises the question of whether Zif268 overexpression can directly facilitate the formation of long-term memory.
In the current study, we therefore examined whether Zif268 overexpression can enhance the capacity for forming long-term memory in a task that does not require an explicit reinforcer. To this end, we assessed memory performance of transgenic forebrain-specific Zif268 overexpressing mice and control littermates in object and object–place recognition memory tasks, allowing us to evaluate the impact of graded spatial demand in the same paradigm. Novel object recognition memory engages the perirhinal cortex as well as the hippocampus to varying degrees depending on the experimental conditions of the task, while object–place recognition memory is more strongly dependent on hippocampal functions [3, 9–12]. Spatial exploration of objects is associated with synaptic potentiation in the hippocampus [13] and increased Zif268 expression in the dentate gyrus of the hippocampus [14]. Whereas Zif268 knockout mice are impaired in both object and object–place recognition memory, heterozygous mice carrying half the complement of Zif268 are impaired in spatial, but not object recognition memory, suggesting increasing dependence on Zif268 activity as the explicit spatial demand of the task increases [1,15]. We therefore predicted that Zif268 overexpression might be a prevalent aid to memory of the spatial location of objects. Because Zif268 deficiency impairs LTP in the dentate gyrus of the hippocampus [1], we also examined whether Zif268 overexpression would enhance dentate gyrus LTP.
2. Zif268 overexpressing mice
We used inducible transgenic Zif268 overexpressing mice based on the tetracycline-controlled transactivator system (rtTA2(S)-M2), carrying a forebrain-specific CaMKIIα promoter-rtTA2 transgene and a transgene carrying a bitetO-promoter fused to a LacZ reporter gene and a Zif268 open reading frame as described previously [8]. Experiments were performed blind to the genotype and in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and the French National Committee (87/848). Transgenic and control wild-type (WT) littermate mice (6–12 months old) were maintained under constant temperature and lighting conditions (22°C, 12 L : 12 D cycle) and received ad libitum doxycycline (Dox)-supplemented food prepared daily (6 mg per 100 g of wet food, West-Ward Pharmaceuticals or a generous gift from Mark Nelson, Paratek Pharmaceuticals) for at least 8 days before and then throughout the duration of the experiments to induce Zif268 overexpression. Because of the limited number of double transgenic mice available, females were used for behavioural experiments (all tested the same day) and males were used for electrophysiological measures (one per day). To confirm that there was no sex-dependent effect due to differences in Zif268 expression, we measured the level of Zif268 and targets of Zif268 in females and males. Basal levels of Zif268 (F3,21 = 1.53; p = 0.235), PSMB9 (F3,21 = 0.51; p = 0.241) and synapsin II (F3,21 = 1.51; p = 0.241) were similar in wild-type and Zif268-overexpressing females (n = 15) and males (n = 10).
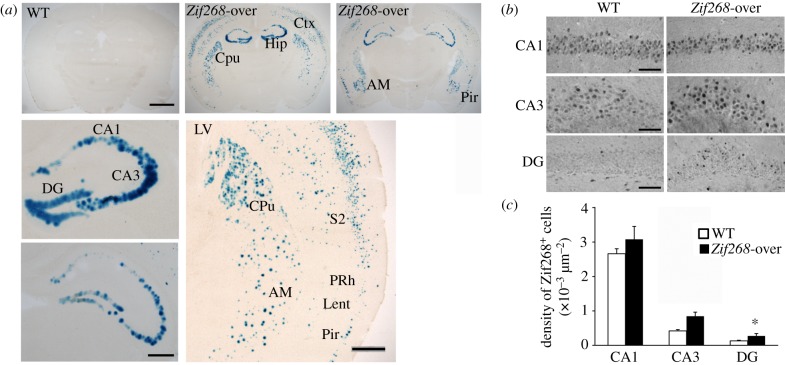
With this protocol of Dox supplementation, analyses of β-galactosidase staining on brain sections [16] showed intense labelling in neocortex, hippocampus, medial caudate putamen, amygdala and piriform cortex of Zif268-overexpressing mice compared with WT mice on Dox (figure 1a). In allocortical regions such as the perirhinal and entorhinal cortices, labelling was sparse compared with the neocortical area (figure 1a). To examine expression of Zif268, we measured the density of Zif268 expressing cells in hippocampal subfields by immunofluorescence using a rabbit anti-Zif268 primary antibody (1/1000; Santa Cruz Biotechnology, CA, USA). Zif268 positive cells were automatically counted under an Olympus BX60 microscope, coupled to a mapping software (Mercator Pro; Explora Nova, La Rochelle, France), in CA1, CA3 and dentate gyrus cell layers of the hippocampus (two sections per animal, inter-section intervals 280 µm) to estimate cell density (number of Zif268-positive cells per µm2). The volume of each layer was obtained from five sections (inter-section interval 280 µm) and the total number of neurons in each layer was estimated using NeuN immunohistochemistry (mouse anti-NeuN antibody, 1/2000, and goat anti-mouse antibody conjugated to Alexa 647; Molecular Probes, Eugene, OR, USA) and conventional unbiased stereological quantification methods, as described previously [17].
Figure 1.
Zif268 overexpression in the brain of the transgenic mice. (a) Coronal brain sections of Dox-induced expression of ß-galactosidase in WT and Zif268-overexpressing mice. Note the strong expression of lacZ in CA1, CA3 and dentate gyrus (DG) areas of the hippocampus (Hip), in neocortex (Ctx), medial caudate putamen (CPu), amygdala (AM) and piriform cortex (Pir). Bottom right image shows sparse lacZ expression in perirhinal (PRh) and lateral entorhinal (Lent) cortices. S2, somatosensory cortex; Pir, piriform cortex; LV, lateral ventricle. Scale bars, top: 2000 μm, bottom: 500 μm. (b) Photomicrographs showing higher Zif268 staining in CA1, CA3 and DG of the hippocampus in Zif268-overexpressing mice. Scale bar, 50 μm. (c) The density of Zif268+ cells was significantly increased in the DG of Zif268-overexpressing mice compared with WT mice. *p < 0.05. (Online version in colour.)
After Dox treatment, volumes of hippocampal subfields were indistinguishable between WT and Zif268 overexpressing mice (CA1: p > 0.05; CA3: p > 0.05; dentate gyrus (DG): p > 0.05; non-parametric Mann–Whitney comparisons, data not shown). Quantification of the number of neurons using NeuN labelling also revealed no differences between genotypes (CA1: p > 0.05; CA3: p > 0.05; DG: p > 0.05; data not shown), suggesting that gross hippocampal anatomy was not affected in the transgenic mice. Analysis of basal levels of Zif268 expression revealed a moderate (figure 1b), but non-significant increase in the density of Zif268-positive cells in CA1 and CA3 of Zif268-overexpressing mice (n = 5) compared with WT (n = 4) mice (CA1: Mann–Whitney p > 0.05; CA3: p > 0.05; figure 1b). In the dentate gyrus, however, a region where, in contrast to CA1 and CA3, basal expression is very low, there was a significant 2.07-fold increase in Zif268-positive cells in zif268-overexpressing mice (DG: p < 0.05; figure 1b,c). This was confirmed by quantifying the number of Zif268-positive nuclei in relation to the total number of neurons. In the dentate gyrus, there was a near twofold increase in the proportion of Zif268-expressing neurons (WT: 1.24 ± 0.15%; Zif268-overexpressing mice (Zif268-over): 2.40 ± 0.81%; p < 0.05), whereas in CA1/CA3, the proportion of Zif268-expressing neurons was much higher, but with a smaller and statistically non-significant increase in Zif268-overexpressing mice (WT: 60.01 ± 3.62%; Zif268-over: 69.47 ± 8.79%, in CA1; WT: 14.09 ± 1.49%; Zif268-over: 24.76 ± 4.52% in CA3). In all, these results show that, at the basal state, there is a substantial increase in Zif268 expressing neurons in the dentate gyrus in our transgenic mice under Dox treatment, and only a moderate increase in hippocampal pyramidal neurons. This, in comparison with β-galactosidase staining, suggests a relatively rapid turnover of Zif268 proteins in this region in the transgenic mice.
3. Zif268 overexpression facilitates memory of the spatial location of objects
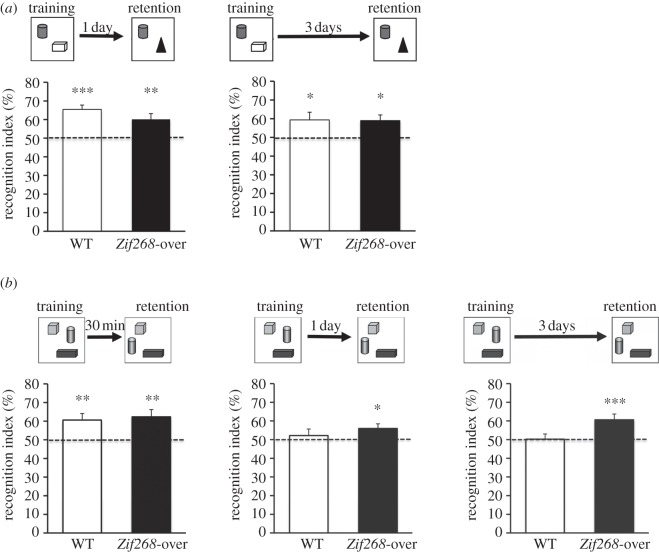
To determine the effect of Zif268 overexpression on recognition memory, we first evaluated performance in an object recognition memory task using standardized procedures as previously described [1,15]. Briefly, the experimental apparatus was a black plastic square open field arena (45 × 45 × 30 cm) with wood shavings on the floor and a cue card placed at a fixed location on the top of one of the walls of the open field to facilitate spatial mapping. It was located in a room with dim lighting and constant background noise, with a video camera mounted above the test apparatus that relayed to a video tracking system. General activity and exploratory behaviour of the animals were automatically recorded using ANY-maze software (Stoelting Co., USA), and object exploration was manually scored. The criteria for exploration were based strictly on active exploration, where the mouse had both forelimbs within a circle of 4 cm around an object, head oriented towards it or touching it with its nose. Mice were handled (twice a day for 2 days) and familiarized to the empty open field (2 days) prior to the experiment. They were then allowed to explore two different objects made of wooden pieces or assembled interlocking plastic Lego pieces of different shapes and colours, for two 10-min sessions with a 10-min interval. Following a 1- or 3-day delay, one of the familiar objects was replaced with a novel object and the time spent exploring the familiar and novel objects was measured and compared with chance level. The objects and their spatial arrangement in the open field were chosen in a pseudorandom order and were counterbalanced among individuals and genotypes.
Exploratory behaviour during habituation to the open field was similar in WT and Zif268-overexpressing mice (total distance travelled: genotype: Student's t-test, t = 1.7, p > 0.05). Similarly, during the acquisition phase, mice of the two genotypes spent similar lengths of time exploring the two sample objects (WT: 134.7 s, Zif268-over: 173.9 s; t = 1.2, p > 0.05; data not shown), indicating no discernible effect of Zif268 overexpression on locomotor activity or novelty-seeking behaviour. When retention was tested 1 day or 3 days after training with different sets of objects, both WT and Zif268-overexpressing mice explored the novel object preferentially (figure 2a). Statistical analyses showed that the time spent exploring the novel object was significantly above chance levels in both groups and for both retention delays, with no difference between genotypes (WT 1 day: t = 6.6, p < 0.0001, n = 13; Zif268-over 1 day: t = 3.3, p < 0.01, n = 9; WT 3 days: t = 2.3, p < 0.05, n = 12; Zif268-over 3 days: t = 3.0, p < 0.05; n = 9).
Figure 2.
Recognition memory in Zif268-overexpressing mice. (a) Schematic of the object recognition memory paradigm and retention performance of WT and Zif268-overexpressing (Zif268-over) mice 1 and 3 days after training. The histograms represent the recognition index expressed as the per cent time spent exploring the novel object over the total time of objects exploration. Both WT and Zif268-overexpressing mice spent significantly more time exploring the novel object at the 1 day (n = 13 and n = 9, respectively) and 3 days (n = 12 and n = 9, respectively) retention delays. (b) Schematic of the object–place recognition memory paradigm and retention performance of WT and Zif268-overexpressing mice 30 min, 1 and 3 days after training. Both WT (n = 11) and Zif268-overexpressing mice (n = 9) showed preferential exploration of the displaced object at the 30 min delay (left histograms). WT mice no longer showed a preference for the displaced object at the 1 day (n = 20) or the 3 days (n = 19) retention tests with the training protocol used in this study, whereas Zif268-overexpressing mice still spent significantly more time exploring the displaced object at both the 1 day (n = 16) and 3 days (n = 16) retention delays, indicating that Zif268 overexpression facilitates the formation of long-term object–place recognition memory. The horizontal line represents equal exploration of the familiar and novel (a) or displaced (b) objects. *p < 0.05, **p < 0.01, ***p < 0.005 compared with chance.
We then assessed the performance of WT and Zif268-overexpressing mice in an object–place recognition memory task following procedures described previously [15,18]. In this task, three different objects were present during training (consisting of two 10-min sessions with a 10-min interval) and during the test conducted 30 min, 1 day or 3 days after training, the spatial position of one of the objects was changed, in a counterbalanced manner among individuals, to a new spatial location. The time spent exploring the displaced and non-displaced objects was measured and compared with chance level (50%, averaging the time spent exploring the two non-displaced objects). During the retention test 30 min after training, mice of both genotypes explored the displaced object above chance levels (WT: t = 3.0, p < 0.01, n = 11; Zif268-over: t = 3.2, p < 0.01, n = 9; figure 2b), indicating similar level of short-term (30 min) object–place memory. WT mice showed no evidence for long-term object–place recognition memory, indicated by a similar level of exploration of the displaced and non-displaced objects, both at 1 day or 3 days post-training (WT 1 day: t = 0.6, p > 0.05, n = 20; WT 3 days: t = 0.1, p > 0.05, n = 19; figure 2b). Thus, with this more demanding task and a relatively short exposure protocol during acquisition, WT mice can form short-term but not long-term memory of the spatial location of objects, as previously observed with a similar training regime [18]. By contrast, Zif268-overexpressing mice still explored the displaced object significantly above chance, both 1 day and 3 days after training (Zif268-over 1 day: t = 2.4, p < 0.05, n = 16; Zif268-over 3 days: t = 3.4, p < 0.005, n = 16; figure 2b), indicating that Zif268 overexpression results in enhanced object–place long-term recognition memory.
4. Zif268 overexpression enhances dentate gyrus long-term potentiation
Next, we examined the effect of Zif268 overexpression on synaptic transmission and LTP at the medial perforant path (MPP) to dentate granule cell synapses in vivo. Stimulation procedures and electrophysiological recordings were performed as previously described [19]. Briefly, WT and Zif268-overexpressing mice (n = 5–6 per group) were anaesthetized with a mixture of oxygen (Air Liquid Santé, Bonneuil-sur-Marne, France) and isoflurane (CSP Translab, Cournon, France) during surgery and throughout recordings. Animals, held in a stereotactic frame and maintained at a constant body temperature of 37.0 ± 0.5°C, were implanted with concentric bipolar stimulating electrodes in the perforant path (3 mm lateral to lambda, depth approximately 1.5 mm from brain surface) and a borosilicate glass micropipette recording electrode containing a silver wire immersed in saline lowered into the dendritic layer of the ipsilateral dentate gyrus (2 mm posterior to bregma, 1.6 mm lateral, approximately 1.5 mm from brain surface). After surgery, low-frequency baseline stimuli (60 μs monophasic pulses, 0.033 Hz) were delivered to evoke a population field excitatory postsynaptic potential (fEPSP), stored for off-line analysis of the maximum slope of the rising phase of the fEPSP as described previously [1,19]. Analyses were performed using one- and two-way analyses of variance (ANOVA) with genotypes as between factor and intensity and time as within factors. Statistical significance was set at p < 0.05. Significant main effects were further analysed by post hoc comparisons of means using Student–Newman–Keuls tests.
After a stable response was established, input–output (I/O) curves were generated using a range of stimulus intensities (0–600 μA; three responses for each intensity). I/O curves in both WT (n = 6) and Zif268-overexpressing mice (n = 5) showed a typical increase in the fEPSP with increasing stimulus strength (figure 3a). The slope of the fEPSP increased significantly with intensity for all mice (F15,135 = 40.47, p < 0.0001), and although the I/O curve in Zif268-overexpressing mice was slightly above that of the WT mice (figure 3a), no significant difference was observed (genotype effect: F1,135 = 0.45, p > 0.05; genotype × intensity interaction: F15,135 = 1.01, p > 0.05), indicating normal basal synaptic transmission in the transgenic mice.
Figure 3.
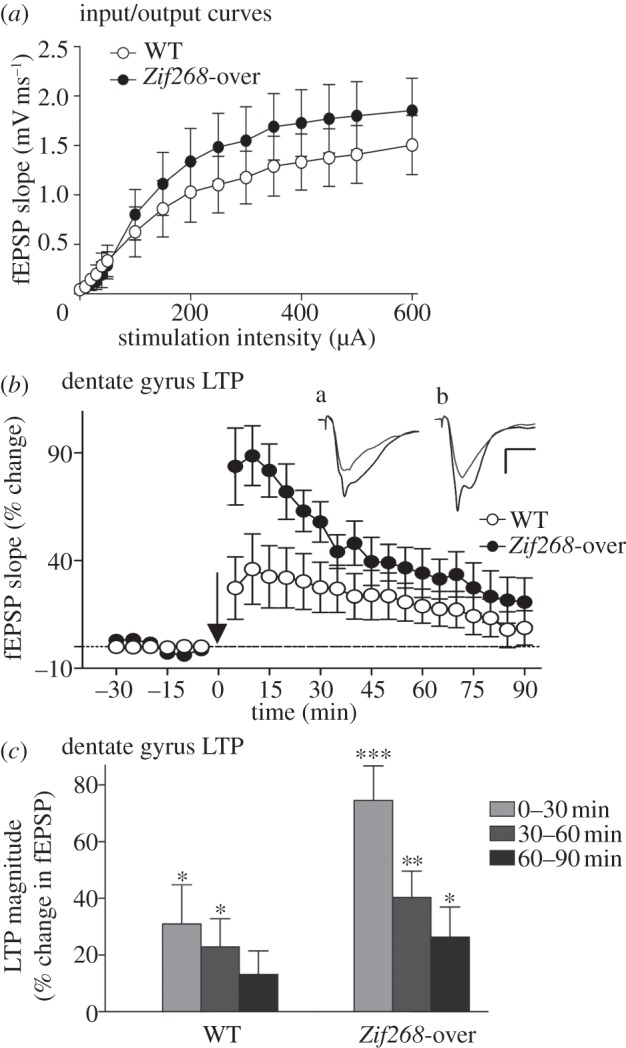
Synaptic transmission and plasticity in the dentate gyrus of Zif268-overexpressing mice. (a) The graph plots stimulus–response relationships at MPP–granule cell synapses using a range of stimulation intensities (0–600 µA) in anaesthetized WT and Zif268-overexpressing mice (Zif268-over). Each data point is an average of fEPSP slope values from three responses (abscissa in the log scale). No significant change in the I/O relationship was observed between WT (open circles, n = 6) and Zif268-overexpressing mice (filled circles, n = 5). (b,c) Dentate gyrus LTP in WT and Zif268-overexpressing mice. (b) Time course of LTP induced at MPP–granule cell synapses (WT: open circles, n = 6; Zif268-over: filled circles, n = 5). Responses were recorded for 30 min before and 90 min after the tetanus (arrow) to the MPP. Each data point is an average of 10 consecutive responses recorded over 5 min. All mice showed significant potentiation of fEPSP slope after tetanic stimulation. Inserts are sample waveforms from a WT (a) and a Zif268-overexpressing mouse (b) recorded before (grey line) and 10 min after tetanic stimulation (black line). Scale bars, 1.5 mV; 10 ms. (c) Per cent potentiation of the fEPSP over three 30 min periods post-tetanus. LTP in Zif268-overexpressing mice was of a higher magnitude and lasted longer than in WT mice. Significant difference from baseline: *p < 0.05; **p < 0.01, ***p < 0.005.
We then examined LTP in the same mice. To this end, test stimuli were delivered at 0.033 Hz for 30 min at an intensity to evoke an fEPSP slope at 50% of maximum, to establish a baseline. A tetanus was then delivered to the MPP, which consisted of six series of six trains of six stimuli at 400 Hz, 200 ms between trains, 20 s between series [1,19]. Pulse-width was doubled during the tetanus. After the tetanus, low-frequency stimulation was resumed for 90 min. The stimulus intensity used in both groups of mice was comparable between genotypes (WT: 235 ± 49 μA; Zif268-over: 218 ± 47 μA; genotype effect: F1,9 = 0.058; p > 0.05), as was the mean fEPSP slope values during baseline (WT: 1.14 ± 0.14 mV ms−1; Zif268-over: 0.79 ± 0.16 mV ms−1; genotype effect: F1,9 = 2.875; p > 0.05). Tetanic stimulation of the MPP resulted in a robust, long-lasting potentiation of the fEPSP slope in both groups (figure 3b). Immediately after the tetanus, however, the magnitude of potentiation of the fEPSP measured during the first 5 min was significantly enhanced in Zif268-overexpressing mice compared with WT mice (WT: 27.26 ± 14.53%; Zif268-over: 83.71 ± 17.84%; genotype effect: F1,9 = 6.16, p < 0.05). Potentiation of the fEPSP remained above that of WT mice for the duration of recording, although the magnitude of LTP decreased progressively for both genotypes (time effect: F17,153 = 21.26, p < 0.0001; genotype×time interaction: F17,153 = 5.53, p < 0.0001; figure 3b). Analysis of the averaged magnitude of fEPSP potentiation across successive 30 min periods post-tetanus (figure 3c) revealed significant effects of genotype (F1,36 = 7.57, p < 0.01) and time (F3,36 = 10.75, p < 0.0001). In WT mice, potentiation of the fEPSP was significantly above baseline for up to 60 min (0–30 min: F1,10 = 4.96, p < 0.05; 30–60 min: F1,10 = 5.33, p < 0.05), but not thereafter, whereas it remained significantly above baseline for the duration of recording in Zif268-overexpressing mice (0–30 min: F1,8 = 37.28, p < 0.0005; 30–60 min: F1,8 = 18.66, p < 0.005; 60–90 min: F1,8 = 6.15, p < 0.05). Thus, these results indicate that Zif268 overexpression does not change basal synaptic transmission but increases the magnitude and prolongs the duration of LTP in the dentate gyrus in vivo.
5. Zif268 overexpression increases activity-dependent expression of ZIF268 and target genes
LTP is associated with the activation of several intracellular signalling pathways, some of which direct rapid and transient activation of transcription factors, among them Zif268, leading to the expression of specific gene programmes. We therefore examined whether and to what extent LTP in Zif268-overexpressing mice would lead to enhanced activation of selected Zif268 target proteins. For this, the dentate gyri ipsilateral and contralateral to the site of LTP induction were removed 3 h after LTP induction in WT (n = 5) and Zif268-overexpressing (n = 5) mice, a time point associated with activation of several LTP-associated genes [20,21]. We selected two downstream targets of Zif268, synapsin II and the proteasome 20S β-subunit PSMB9 (LMP2), a subunit of the proteasome belonging to the multisubunit catalytic core of the proteolytic machinery [22], to examine their expression following LTP. Both genes possess Zif268 binding sites on their promoter regions [23–25]. Dentate gyrus tissue was processed for western blotting as described previously [26], using anti-synapsin II (1/15000 in TBST BSA 5%, Abcam, France) and anti-PSMB9 (1/1000 in TBST BSA 5%, Abcam, France) antibodies. We also examined Zif268 expression using anti-Zif268 antibodies (1/1000 in TBST BSA 5%, Cell Signalling, Ozyme, France). Protein values were normalized first to actin and then calculated as a per cent change from the contraleral side of the Zif268 WT mice.
Overall analysis of variance (ANOVA) showed a significant difference in the expression of Zif268 between groups (F3,16 = 6.19; p < 0.01; figure 4a). In the control, non-stimulated dentate gyrus contralateral to the site of LTP induction, there was no significant difference between genotypes (Fisher's post hoc p > 0.05). This probably reflects the impracticality of western blotting from whole dentate gyrus tissue to detect small increases in the number of Zif268-positive neurons in this region, compared with quantification of immunolabelled neurons. Following LTP, there was a small but not significant increase in expression of Zif268 in WT mice (LTP versus control side, Fisher's post hoc p > 0.05; figure 4a) as expected at this relatively late time point [7]. By contrast, in Zif268-overexpressing mice, the increase in Zif268 expression 3 h following LTP reached a high level, significantly above that of the control, non-stimulated sides of the WT and Zif268-overexpressing mice (Zif268-over: LTP versus control side, p < 0.05; Zif268-over LTP side versus WT control side, p < 0.05; figure 4a). Overall, while our Zif268-overexpressing mouse line shows a significant but moderate increase in the number of Zif268-expressing dentate gyrus neurons at basal levels (figure 1), a major feature is an enhanced capacity for activity-dependent expression of Zif268.
Figure 4.
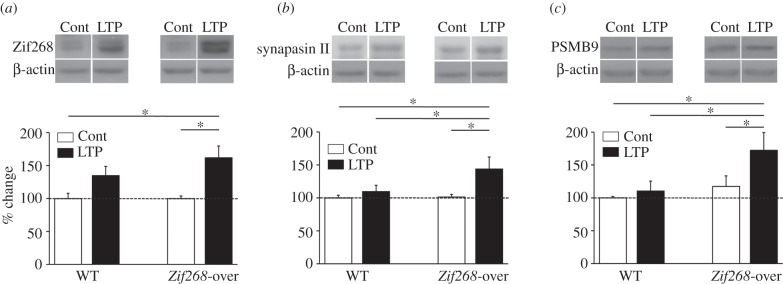
LTP-induced expression of Zif268, synapsin II and the proteasome 20S β-subunit PSMB9 in Zif268-overexpressing mice. (a) Zif268, (b) synapsin II and (c) PSMB9 protein expression was measured by western blotting from the dentate gyrus taken 3 h after induction of LTP (LTP side) and from the contralateral (non-stimulated) side (Cont). Data for each protein and genotype are normalized to the reference protein β-actin and expressed as a per cent change relative to protein levels in the control side of WT mice. There was a trend towards an increase in Zif268, but not synapsin II or PSMB9 expression after LTP in WT mice, and a higher and significant increase of all three proteins after LTP in Zif268-overexpressing mice (Zif268-over). Sample western blots of each protein and actin are represented above the histograms. *p < 0.05.
Analyses of synapsin II and PSMB9 expression revealed significant differences between genotypes for both proteins (F3,16 = 3.68; p < 0.05 and F3,16 = 3.43; p < 0.05, respectively). In WT mice, there were no significant changes in synapsin II or PSMB9 protein levels after induction of LTP (WT mice: LTP versus control side, Fisher's post hoc p > 0.05 for each protein; figure 4b,c). However, in Zif268-overexpressing mice, which displayed large overexpression of Zif268 after LTP, LTP resulted in a significant increase in both synapsin II and PSMB9 expression (Zif268-over: LTP versus control side, Fisher's post hoc p < 0.05 for each protein; figure 4b,c), suggesting that Zif268 overexpression can boost regulation of target genes in an activity-dependent manner.
6. Discussion
The evidence to date suggests that rapid regulation of the expression of IEGs encoding inducible, regulatory transcription factors is a key mechanism of the genomic response underlying synaptic plasticity and the modification of neural networks required for the stabilization of memories. In neurons activated by learning, these transcriptional events are thought to mediate the activation of selective gene programmes and subsequent synthesis of proteins, leading to stable functional and structural remodelling of the activated networks, so that the memory can later be reactivated upon recall. Over the last decades, novel insights have been gained in identifying key transcriptional regulators that can control the genomic response of synaptically activated neurons. Here, as an example of this approach, we focused on one such activity-dependent transcription factor, Zif268, known to be implicated in neuronal plasticity and memory formation. Whereas targeted deletion of the Zif268 gene results in profound impairments of several forms of long-term memory, including a fundamental memory ability, recognition memory [1,2,15,27], here we report that overexpression of Zif268 in forebrain neurons in mice is sufficient to augment the mice's ability to form a long-lasting spatial recognition memory. These findings, together with the demonstration that Zif268 overexpression can slow down extinction of conditioned taste aversion [8], clearly add to the growing evidence that Zif268 brain expression is an important factor for establishing stable long-lasting memories.
Interestingly, in previous experiments we showed that half the complement of Zif268 in heterozygous mutant mice is not sufficient to allow the formation of a memory for the spatial location of objects as heterozygous mice are impaired in this task, however it is sufficient to form a memory of the nature of the objects [15]. Mirroring this, overexpression of Zif268 leads to enhancement of the more demanding memory for the spatial location of objects, but not to improvement of object memory over and above that observed in WT mice. As discussed in detail previously, object recognition memory engages the perirhinal cortex [12] and the hippocampus with varying degree of requirement depending on the experimental conditions of the task, in particular the complexity and richness of the spatial context during objects exploration and their consequences on the ability to form spatial configurations, associative relationships between items and objects–scene relationships [3, 9–11]. The formation of object memory also recruits several of the same cellular and molecular mechanisms in both structures (reviewed in [3,12]). Our β-galactosidase expression results, however, suggest moderate, if any, overexpression of Zif268 in the perirhinal cortex. This may explain the apparent absence of enhancement of novel object recognition memory in our experimental conditions. However, it remains possible that the absence of improvement of object recognition memory, at least within the limit of 3 days after the mice first encountered the objects, is simply due to a ceiling effect, the control mice displaying robust long-term object memory at this delay. Alternatively, it is also possible that object memory is less sensitive to variations in Zif268 expression, as exemplified by the absence of deficit in this task in Zif268 heterozygous mice [1].
Spatial recognition memory places higher demands on hippocampal processing of information and Zif268 heterozygous mice display as much impairment in spatial memory as Zif268 homozygous mutant mice, while they are less impaired in tasks that do not place a high demand on hippocampal function [1], suggesting hippocampal functions are highly dependent on Zif268 gene expression dosage. In support of this idea, it has been shown in contextual fear conditioning that partial knockdown (by approx. 66%) of Zif268 by injection of antisense oligodesoxynucleotides in the hippocampus is insufficient to affect consolidation of contextual fear memory, but impairs its reconsolidation after recall [28], a memory process that is more vulnerable to interfering treatments than initial consolidation (see [29] for a review). The ubiquitin–proteasome system has also been involved in reconsolidation processes and during updating of memory content [30,31]. Furthermore, in a context pre-exposure facilitation paradigm, Zif268 downregulation was shown to prevent updating of the hippocampal memory content when new information present at recall is linked to the retrieved memory [31]. These findings, together with the present results with Zif268 overexpression, highlight the importance of Zif268 gene expression dosage in determining the strength of memory in relation to task difficulty and cognitive demand.
Our results using conditional mice overexpressing Zif268 in several structures of the forebrain cannot address precisely the issue of structure-specificity. However, the above findings all suggest a high sensitivity of hippocampal functions to Zif268 gene expression dosage and thus lead to the proposal that in spatial/contextual and relational memory tasks, hippocampal Zif268 expression levels become increasingly vital for the hippocampal component of the memory trace. In this brain area, in particular in the dentate gyrus, the extent of Zif268 expression after LTP correlates with the persistence of LTP [32,33] and LTP in Zif268 knockout mice cannot be maintained over 24 h, a phenotype found in both homozygous and heterozygous Zif268 mutant mice [1]. Mirroring this, we now report that dentate gyrus LTP, but not basal synaptic transmission, is enhanced under conditions of Zif268 overexpression, although in this case even the induction phase of LTP was enhanced, suggesting that Zif268 overexpression may modify expression of as yet unknown molecular/cellular synaptic components involved in induction of LTP. In the continuing debate about the role of LTP mechanisms in memory, these findings provide an important complement to the suggestion that synaptic changes brought about by LTP and during memory consolidation and storage share, at least in part, common underlying molecular mechanisms.
Mechanistically, exploration of objects in an arena is associated with the slow development of NMDA receptor-dependent synaptic potentiation in the hippocampus that can be occluded by prior induction of LTP, resulting in recognition memory deficits [13]. At the molecular level, several canonical cell-signalling cascades are activated (reviewed in [3,34]), including the MAP kinase cascade [26] known to be instrumental for LTP-induced regulation of Zif268 [35]. Further, expression of Zif268 is rapidly induced in the dentate gyrus of the hippocampus following spatial exploration of objects [14]. Here, we also found enhanced or prolonged LTP-induced expression both of Zif268 itself and of two Zif268 target genes in Zif268-overexpressing mice, suggesting that increasing the levels of Zif268 increases the capacity for activity-dependent regulation of downstream gene programmes. Synapsin II contributes to regulation of transmitter release whereas PSMB9 is part of the ubiquitin–proteasome system. Although we observed a significant upregulation of these two target proteins and of Zif268 in overexpressing mice 3 h following the induction of LTP, this is not the case in WT mice. It is possible that the time post-LTP is not the optimal window in which to detect significant changes in expression of these proteins in WT mice and that, as for Zif268 itself, the observed changes in synapsin II and PSMB9 expression reflect a prolonged wave of expression following LTP in Zif268-overexpressing mice. In the absence of evidence clearly documenting the time course of regulation of these proteins following induction of LTP, we cannot rule out, however, the possibility that they may be functionally regulated by alternative signalling pathways when Zif268 is overexpressed. Although the precise mechanisms by which Zif268 overexpression facilitates LTP and long-term spatial recognition memory are unknown, the increased neuronal capacity to regulate Zif268 downstream gene programmes may be one mechanism underlying the enhancement of LTP and the facilitation of the formation of a long-term recognition memory. Although several potential Zif268 target genes bearing ERE consensus sequences on their promoter regions have been suggested [23,25,36], characterizing the selective gene programmes controlled by Zif268 in relation to learning and memory remains a challenge for future research.
There is also accumulating evidence that transcriptional changes underlying long-term memory are supported by epigenetic modifications across diverse brain regions, including DNA methylation and posttranslational modifications of histone tails such as phosphorylation, acetylation and methylation [37]. Several findings suggest an important role of Zif268 in experience-dependent epigenetic mechanisms that underlie memory. For example, regulation of histone methylation at the Zif268 promoter can facilitate contextual fear memory [38], histone H4 acetylation at the Zif268 promoter correlates with Zif268 expression in the hippocampus [39] and histone acetylation critically modulates object recognition memory consolidation [40]. Furthermore, exploration of objects was recently shown to trigger rapid phosphorylation, acetylation and methylation of histones at the Zif268 promoter in the hippocampus and the prefrontal cortex [41]. Blocking these epigenetic marks at the Zif268 promoter impairs recognition memory, while their enhancement by intensive training or by transgenic intervention favours recognition memory [41]. Finally, Zif268 can mediate epigenetic programming via DNA methylation and histone posttranslational modifications to influence downstream gene regulation [42,43]. Down-regulation of Zif268 has been observed in ageing or certain diseases leading to cognitive deficits [44–46]. Selective intervention on these epigenetic mechanisms at the Zif268 promoter to enhance its expression could be one promising tool for attempting to rescue certain cognitive deficiencies.
Acknowledgements
The authors are grateful to Mark L. Nelson for the generous gift of doxycycline.
Funding statement
This work was supported by the Centre National de la Recherche Scientifique (CNRS, France), University Paris-Sud (France), by a grant from the Agence Nationale de la Recherche (ANR-06-NEURO-020-01) to S.L. and by a fellowship from Neuropôle de Recherche Francilien (NeRF IF-08-1699/R) to E.M.
References
- 1.Jones MW, et al. 2001. A requirement for the immediate early gene Zif268 in the expression of late LTP and the consolidation of long-term memories. Nat. Neurosci. 4, 289–296. ( 10.1038/85138) [DOI] [PubMed] [Google Scholar]
- 2.Bozon B, Kelly A, Josselyn SA, Silva AJ, Davis S, Laroche S. 2003. MAPK, CREB and zif268 are all required for the consolidation of recognition memory. Phil. Trans. R. Soc. Lond. B 358, 805–814. ( 10.1098/rstb.2002.1224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis S, Renaudineau S, Poirier R, Poucet B, Save E, Laroche S. 2010. The formation and stability of recognition memory: what happens upon recall? Front. Behav. Neurosci. 4, 177 ( 10.3389/fnbeh.2010.00177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Renaudineau S, Poucet B, Laroche S, Davis S, Save E. 2009. Impaired long term stability of CA1 place cell representation in mice lacking the transcription factor zif268/egr1. Proc. Natl Acad. Sci. USA 106, 11 771–11 775. ( 10.1073/pnas.0900484106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis S, Bozon B, Laroche S. 2003. How necessary is the activation of the immediate early gene zif268 in synaptic plasticity and learning? Behav. Brain Res. 142, 17–30. ( 10.1016/S0166-4328(02)00421-7) [DOI] [PubMed] [Google Scholar]
- 6.Knapska E, Kaczmarek L. 2004. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Progr. Neurobiol. 74, 183–211. ( 10.1016/j.pneurobio.2004.05.007) [DOI] [PubMed] [Google Scholar]
- 7.Cheval H, Chagneau C, Levasseur G, Veyrac A, Faucon-Biguet N, Laroche S, Davis S. 2012. Distinctive features of Egr transcription factor regulation and DNA binding activity in CA1 of the hippocampus in synaptic plasticity and consolidation and reconsolidation of fear memory. Hippocampus 22, 631–642. ( 10.1002/hipo.20926) [DOI] [PubMed] [Google Scholar]
- 8.Baumgartel K, Genoux D, Welzl H, Tweedie-Cullen RY, Koshibu K, Livingstone-Zatchej M, Mamie C, Mansuy IM. 2008. Control of the establishment of aversive memory by calcineurin and Zif268. Nat. Neurosci. 11, 572–578. ( 10.1038/nn.2113) [DOI] [PubMed] [Google Scholar]
- 9.Brown MW, Aggleton JP. 2001. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat. Rev. Neurosci. 2, 51–61. ( 10.1038/35049064) [DOI] [PubMed] [Google Scholar]
- 10.Jenkins TA, Amin E, Pearce JM, Brown MW, Aggleton JP. 2004. Novel spatial arrangements of familiar visual stimuli promote activity in the rat hippocampal formation but not the parahippocampal cortices: a c-fos expression study. Neuroscience 124, 43–52. ( 10.1016/j.neuroscience.2003.11.024) [DOI] [PubMed] [Google Scholar]
- 11.Eichenbaum H, Yonelinas AR, Ranganath C. 2007. The medial temporal lobe and recognition memory. Annu. Rev. Neurosci. 30, 123–152. ( 10.1146/annurev.neuro.30.051606.094328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winters BD, Saksida LM, Bussey TJ. 2008. Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci. Biobehav. Rev. 32, 1055–1070. ( 10.1016/j.neubiorev.2008.04.004) [DOI] [PubMed] [Google Scholar]
- 13.Clarke JR, Cammarota M, Gruart A, Izquierdo I, Delgado-García JM. 2010. Plastic modifications induced by object recognition memory processing. Proc. Natl Acad. Sci. USA 107, 2652–2657. ( 10.1073/pnas.0915059107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soulé J, Penke Z, Alme MN, Kanhema T, Laroche S, Bramham CR. 2008. Object–place recognition learning triggers rapid induction of plasticity-related immediate early genes and synaptic proteins in the rat dentate gyrus. Neural Plasticity 2008, 1–12. ( 10.1155/2008/269097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bozon B, Davis S, Laroche S. 2002. Regulated transcription of the immediate early gene Zif268: mechanisms and gene dosage-dependent function in synaptic plasticity and memory formation. Hippocampus 12, 570–577. ( 10.1002/hipo.10100) [DOI] [PubMed] [Google Scholar]
- 16.Michalon A, Koshibu K, Baumgartel K, Spirig DH, Mansuy IM. 2005. Inducible and neuron-specific gene expression in the adult mouse brain with the rtTA2S-M2 system. Genesis 43, 205–212. ( 10.1002/gene.20175) [DOI] [PubMed] [Google Scholar]
- 17.Veyrac A, Reibel S, Sacquet A, Mutin M, Camdessanche JP, Kolattukudy P, Honnorat J, Jourdan F. 2011. CRMP5 regulates generation and survival of newborn neurons in olfactory and hippocampal neurogenic areas. PLoS ONE 6, e23721 ( 10.1371/journal.pone.0023721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poirier R, Cheval H, Mailhes C, Charnay P, Davis S, Laroche S. 2007. Paradoxical role of an Egr transcription factor family member, Egr2/Krox20, in learning and memory. Front. Behav. Neurosci. 1, 6 ( 10.3389/neuro.08.006.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Errington ML, Bliss TVP, Morris RJ, Laroche S, Davis S. 1997. Long-term potentiation in awake mutant mice. Nature 387, 666–667. ( 10.1038/42625) [DOI] [PubMed] [Google Scholar]
- 20.Ryan MM, Ryan B, Kyrke-Smith M, Logan B, Tate WP, Abraham WC, Williams JM. 2012. Temporal profiling of gene networks associated with the late phase of long-term potentiation in vivo. PLoS ONE 7, e40538 ( 10.1371/journal.pone.0040538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas KL, Laroche S, Errington ML, Bliss TVP, Hunt SP. 1994. Spatial and temporal changes in signal transduction pathways during LTP. Neuron 13, 737–745. ( 10.1016/0896-6273(94)90040-X) [DOI] [PubMed] [Google Scholar]
- 22.Kloetzel PM. 2001. Antigen processing by the proteasome. Nat. Rev. Mol. Cell. Biol. 2, 179–187. ( 10.1038/35056572) [DOI] [PubMed] [Google Scholar]
- 23.James AB, Conway AM, Morris BJ. 2005. Genomic profiling of the neuronal target genes of the plasticity-related transcription factor Zif268. J. Neurochem. 95, 796–810. ( 10.1111/j.1471-4159.2005.03400.x) [DOI] [PubMed] [Google Scholar]
- 24.James AB, Conway AM, Morris BJ. 2006. Regulation of the neuronal proteasome by Zif268 (Egr1). J. Neurosci. 26, 1624–1634. ( 10.1523/JNEUROSCI.4199-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersohn D, Schoch S, Brinkmann DR, Thiel G. 1995. The human synapsin II gene promoter. Possible role for the transcription factor zif268/egr-1, polyoma enhancer activator 3, and AP2. J. Biol. Chem. 270, 24 361–24 369. [DOI] [PubMed] [Google Scholar]
- 26.Kelly A, Laroche S, Davis S. 2003. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J. Neurosci. 12, 5354–5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bozon B, Davis S, Laroche S. 2003. A requirement for the immediate early gene zif268 in reconsolidation of recognition memory after retrieval. Neuron 40, 695–701. ( 10.1016/S0896-6273(03)00674-3) [DOI] [PubMed] [Google Scholar]
- 28.Lee JL, Everitt BJ, Thomas KL. 2004. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science 304, 839–843. ( 10.1126/science.1095760) [DOI] [PubMed] [Google Scholar]
- 29.Besnard A, Caboche J, Laroche S. 2012. Reconsolidation of memory: a decade of debate. Progr. Neurobiol. 99, 61–80. ( 10.1016/j.pneurobio.2012.07.002) [DOI] [PubMed] [Google Scholar]
- 30.Lee SH, et al. 2008. Synaptic protein degradation underlies destabilization of retrieved fear memory. Science 319, 1253–1256. ( 10.1126/science.1150541) [DOI] [PubMed] [Google Scholar]
- 31.Lee JL. 2010. Memory reconsolidation mediates the updating of hippocampal memory content. Front. Behav. Neurosci. 4, 168 ( 10.3389/fnbeh.2010.00168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abraham WC, Mason SE, Demmer J, Williams JM, Richardson CL, Tate WP, Lawlor PA, Dragunow M. 1993. Correlations between immediate early gene induction and the persistence of long-term potentiation. Neuroscience 56, 717–727. ( 10.1016/0306-4522(93)90369-Q) [DOI] [PubMed] [Google Scholar]
- 33.Richardson CL, Tate WP, Mason SE, Lawlor PA, Dragunow M, Abraham WC. 1992. Correlation between the induction of an immediate early gene, zif/268, and long-term potentiation in the dentate gyrus. Brain Res. 580, 147–154. ( 10.1016/0006-8993(92)90938-6) [DOI] [PubMed] [Google Scholar]
- 34.Dere E, Huston JP, De Souza Silva MA. 2002. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci. Biobehav. Rev. 31, 673–704. ( 10.1016/j.neubiorev.2007.01.005) [DOI] [PubMed] [Google Scholar]
- 35.Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S. 2000. The MAPK/ERK cascade targets both elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J. Neurosci. 20, 4563–4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baumgartel K, Tweedie-Cullen RY, Grossmann J, Gehrig P, Livingstone-Zatchej M, Mansuy IM. 2009. Changes in the proteome after neuronal Zif/268 overexpression. J. Prot. Res. 8, 3298–3316. ( 10.1021/pr801000r) [DOI] [PubMed] [Google Scholar]
- 37.Zovkic IB, Guzman-Karlsson MC, Sweatt JD. 2013. Epigenetic regulation of memory formation and maintenance. Learn. Mem. 20, 61–74. ( 10.1101/lm.026575.112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta S, Kim SY, Artis S, Molfese DL, Schumacher A, Sweatt JD, Paylor RE, Lubin FD. 2010. Histone methylation regulates memory formation. J. Neurosci. 30, 3589–3599. ( 10.1523/JNEUROSCI.3732-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie L, Korkmaz KS, Braun K, Bock J. 2013. Early life stress-induced histone acetylations correlate with activation of the synaptic plasticity genes Arc and Egr1 in the mouse hippocampus. J. Neurochem. 125, 457–464. ( 10.1111/jnc.12210) [DOI] [PubMed] [Google Scholar]
- 40.Zhao Z, Fan L, Fortress AM, Boulware MI, Frick KM. 2012. Hippocampal histone acetylation regulates object recognition and the estradiol-induced enhancement of object recognition. J. Neurosci. 32, 2344–2351. ( 10.1523/JNEUROSCI.5819-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gräff J, Woldemichael BT, Berchtold D, Dewarrat G, Mansuy IM. 2012. Dynamic histone marks in the hippocampus and cortex facilitate memory consolidation. Nat. Commun. 3, 991 ( 10.1038/ncomms1997) [DOI] [PubMed] [Google Scholar]
- 42.Weaver IC, D'Alessio AC, Brown SE, Hellstrom IC, Dymov S, Sharma S, Szyf M, Meaney MJ. 2007. The transcription factor nerve growth factor-inducible protein A mediates epigenetic programming: altering epigenetic marks by immediate-early genes. J. Neurosci. 27, 1756–1768. ( 10.1523/JNEUROSCI.4164-06.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiong F, Xiao D, Zhang L. 2012. Norepinephrine causes epigenetic repression of PKCɛ gene in rodent hearts by activating Nox1-dependent reactive oxygen species production. FASEB J. 26, 2753–2763. ( 10.1096/fj.11-199422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. 2003. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J. Neurosci. 23, 3807–3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dickey CA, Loring JF, Montgomery J, Gordon MN, Eastman PS, Morgan D. 2003. Selectively reduced expression of synaptic plasticity-related genes in amyloid precursor protein+presenilin-1 transgenic mice. J. Neurosci. 23, 5219–5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gersten M, Alirezaei M, Marcondes MC, Flynn C, Ravasi T, Ideker T, Fox HS. 2009. An integrated systems analysis implicates EGR1 downregulation in simian immunodeficiency virus encephalitis-induced neural dysfunction. J. Neurosci. 29, 12 467–12 476. ( 10.1523/JNEUROSCI.3180-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]