Abstract
The synaptic plasticity and memory hypothesis asserts that activity-dependent synaptic plasticity is induced at appropriate synapses during memory formation and is both necessary and sufficient for the encoding and trace storage of the type of memory mediated by the brain area in which it is observed. Criteria for establishing the necessity and sufficiency of such plasticity in mediating trace storage have been identified and are here reviewed in relation to new work using some of the diverse techniques of contemporary neuroscience. Evidence derived using optical imaging, molecular-genetic and optogenetic techniques in conjunction with appropriate behavioural analyses continues to offer support for the idea that changing the strength of connections between neurons is one of the major mechanisms by which engrams are stored in the brain.
Keywords: synaptic plasticity, memory, long-term potentiation, engram, initial consolidation, dopamine
1. Introduction
The idea that changes in the efficacy of synapses within diverse neural circuits could mediate the storage of information acquired during learning has a long history. Theoretical hypotheses about the growth of neuronal connections in the brain and the circumstances in which such growth might take place date back to Ramón y Cajal [1] and, in the mid-twentieth century, to Hebb [2] and Konorski [3]. The first experimental evidence emerged from studies of habituation and sensitization in the marine mollusc Aplysia [4,5]. The discovery of long-term potentiation (LTP) [6] acted as a further stimulus to the concept, not least because LTP was first discovered in an area of the brain—the hippocampal formation—that had been implicated in memory from clinical observations of amnesia [7]. Indeed, the last sentence of Bliss & Lömo's paper raises the question. Noting the possibility that the time-scale of LTP was long enough to be potentially useful for information storage, they go on to conclude in characteristically quizzical fashion:
whether or not the intact animal makes use in real life of a property which has been revealed by synchronous, repetitive volleys to a population of fibres the normal rate and pattern of activity along which are unknown, is another matter. [6, p. 355].
In our quest for understanding ‘mechanisms’ in neuroscience, a focus in research on activity-dependent synaptic plasticity such as LTP and long-term depression (LTD) has been on identifying the causal steps that occur at individual synapses mediating lasting changes in synaptic efficacy in terms of changes in presynaptic transmitter release, alterations in postsynaptic glutamatergic receptors, the action of neuromodulatory transmitters, the signal transduction pathways activated, gene activation and synthesis of new proteins. A contemporary focus is on the endo- and exocytosis of specific sub-types of glutamate receptors, and alterations in the scaffolding molecules that make up the pre- and postsynaptic elements of neuronal connectivity [8]. Recent reviews of the persistence of LTP/LTD collectively point to the importance of translational control of dendritic mRNAs, and that spine dynamics may be remarkably fast to be in register with the relatively immediate functional changes [9–11]. Accompanying papers in this issue discuss similar themes.
However, in our view, no less important is Bliss & Lömo's now 40-year-old question—do animals actually make use of this capacity for change and, if so, how? This systems-level question was initially approached in terms of seeking correlations between learning and synaptic potentiation [12], but the diverse techniques of contemporary neuroscience offer new tools for securing a definitive and causal answer [13,14], and addressing whether such understanding could be a route towards more effective cognition [15]. Here, we review progress in identifying whether and how synaptic plasticity may mediate distinct aspects of learning and memory with focus on the hippocampus, beginning with the generic hypothesis.
2. The synaptic plasticity and memory hypothesis
The synaptic plasticity and memory (SPM) hypothesis is not identified with any one individual scientist, it being an idea that has come forward in various guises over the years (see [16] for review). At its heart is the notion that the memory of prior experience is mediated by the reactivation of ‘traces’ or ‘engrams’ whose basis involves alterations, possibly bidirectional alterations, in synaptic efficacy. The intellectual debt to Hebb, in particular, is very clear but the concept of ‘memory of prior experience’ has to be unpacked with respect to the distinct ways in which it can be interpreted. The most everyday sense of memory is that of an event happening to someone, it somehow being recorded in their brain, and then this person later bringing to mind some representation of that same event. One can think of this loosely as ‘real’ memory in the everyday ‘folk-psychology’ sense of the term. However, prior experience may also induce changes in the nervous system, possibly mediated by synaptic plasticity, that in no sense allow recall of the prior experience that occurred. A runner training for a marathon will gradually build up muscle mass and all manner of changes in his or her body that reflect the experience of training. However, changes in muscle mass in no sense ‘represent’ the actual events that constitute that training, even though they are triggered by it. These two examples of putative ‘memory’ can be thought of as qualitatively distinct ways in which experience drives plasticity, or alternatively, as two ends of a continuum. Huebener & Bonhoeffer [17] take a ‘continuum’ point of view, suggesting that study of the neural mechanisms of ocular dominance or orientation-sensitive plasticity could be a valid route to discovering the neural mechanisms of memory. The fine-tuning of cortical connections in the visual system during the sensitive period of early development is now known to involve bidirectional changes in synaptic efficacy mediated by LTP-like and LTD-like mechanisms, and various tantalizing phenomena potentially relevant to memory have recently been discovered such as ‘savings’ with respect to future plasticity after incomplete experiences during infancy. Specifically, Hofer et al. [18] have demonstrated successful adult cortical plasticity in mice that had a period of eye closure during the sensitive period that was then terminated early on. This phenomenon is reminiscent of the ‘savings’ that can be seen in learning a second language if a person has been exposed, at least for a period, to that language early in life. Huebener and Bonhoeffer's view is that drawing a sharp categorical boundary between ‘real’ memory and the diverse array of experience-dependent changes that occur in the nervous system throughout life is both unnecessary and misleading.
However, while accepting the force of this argument, a problem is that while the underlying neural mechanisms of plasticity at glutamatergic synapses may be very similar for changes right across this continuum, the function(s) that activity-dependent synaptic plasticity serves will, in our view, depend critically on the neural circuit in which that plasticity is embedded in a non-monotonic manner. Let us contrast two cases. Pre-synaptic facilitation at the sensory to motor neuron synapse of the abdominal ganglion of Aplysia is thought to realize an increase in synaptic throughput that mediates a behavioural facilitation of responsiveness. A stimulus to the siphon then gives rise to a larger and longer withdrawal of the gill and siphon reflex. Conversely, habituation is associated with a decrease in transmitter release measured using quantal analysis [5]. In these cases, there is an elegant isomorphism between the physiological change and the behavioural change that has been likened to a ‘cellular alphabet of learning’ [19]. A similar principle may also apply to fear conditioning mediated by the amygdala of mammals in which an initially neutral stimulus can, through synaptic potentiation mediated by changes in post-synaptic receptor expression [20], give rise to graded evocation of fear through associative conditioning with a painful or biologically dangerous stimulus (e.g. a predator). The amygdala's efferent connections trigger the various expressions of that fear via downstream connections to the autonomic nervous system [14]. Isomorphism may be a common characteristic of learned reflex systems.
What is less well appreciated is, however, that other neural circuits of memory do not operate on an isomorphism principle. Rather, synaptic potentiation and depression sculpt the possibility of associations between stimuli such that learning, rather than giving rise to larger or smaller responses, enables one stimulus representation to evoke the memory representation of another. This is qualitatively different. For example, a key characteristic of episodic-like memory is what has been called the ‘automatic recording of experience’ [21] whereby the memory of an event is automatically associated with the context in which it happens. Biologically significant events such as reward and punishment are not necessary for making such associations; nor need they enter into them in the conventional sense of Pavlovian conditioning between a conditional stimulus (CS) and a biologically significant unconditioned stimulus (US). Instead, the co-occurrence of events and contexts enables, in the distributed associative machinery of the hippocampal formation operating on Hebb-like principles, an association that ties these two entities together. That is, if you remember an event you will automatically remember the context where it happened. Realizing this kind of association requires distributed temporo-spatial representations of events and contexts that can be discriminated (pattern separation) and sometimes generalized (pattern completion), with multiple events and contexts overlaid in a distributed manner within a common neural circuit [22]. Hebbian synaptic plasticity, possibly coupled to associative synaptic depression, within a neural circuitry that enables distributed associative memory through the pattern of excitatory and inhibitory interconnections will realize this function—albeit subject to the myriad of inhibitory feed-forward and feedback connections that regulate excitability in different compartments of the dendritic tree [23]. The Huebener–Bonhoeffer continuum principle, with its focus on mechanism at the level of individual synapses, does not yet provide an effective framework for thinking about these systems-level issues. In fact, at a systems-level, we are a long way from understanding the detailed manner in which information is represented in temporo-spatial codes, how the interplay of excitatory and inhibitory activation enables encoding and later retrieval, and even the distinct and sometimes sparse representational codes of different sub-regions of the hippocampal formation [24]. However, computational neuroscience techniques are proving invaluable in tackling these issues and a systems approach is essential if the representational issues are to be addressed mechanistically.
Two last points to make about the generic SPM hypothesis concern plasticity itself and new techniques. Research over the 40 years since LTP was discovered has revealed a family of different forms of activity-dependent synaptic plasticity. In addition to NMDA receptor-dependent LTP and LTD, there are forms of synaptic potentiation and depression that are NMDA receptor-independent. Homeostatic plasticity has been discovered and it may play both a normalizing and protective role in neural circuits that might otherwise be risk at seizure through potentiation of too high a proportion of neural afferents [25]. Spike-timing-dependent plasticity [26,27] has been implicated in the learning and expression of, for example, remembered sequences [28]. Second, contemporary neuroscience is characterized by an array of novel technologies including new molecular-genetic technologies, optical imaging in living animals and optogenetic manipulations of individual neurons [29–34] as well as new behavioural paradigms [35–37]. Their use, illustrated below, is offering the opportunity to secure more definitive answers to the validity of the SPM hypothesis.
To conclude, this hypothesis can be stated, in its most general form as follows:
activity-dependent synaptic plasticity is induced at appropriate synapses during memory formation, and is both necessary and sufficient for the encoding and trace storage of the type of memory mediated by the brain area in which that plasticity is observed. [16, p. 650]
This definition is intended to be both inclusive and sufficiently precise for the hypothesis to be falsifiable. We turn now to four critical tests of the hypothesis that have been examined in numerous studies over the past 25 years.
3. Criteria for assessing the synaptic plasticity and memory hypothesis
Martin et al. [16] identified four distinct criteria of assessment and corresponding tests of the SPM hypothesis. The first they called detectability whereby if learning involves activity-dependent synaptic plasticity, it should be possible to detect changes in synaptic efficacy following learning. This is one aspect of a ‘sufficiency’ criterion—that synaptic change occurs during learning—though it falls short of establishing that such a change is actually sufficient. Second, they argued that if some treatment (pharmacological, physiological and molecular-genetic) were to be given prior to learning, the rate of learning should be blocked, enhanced or otherwise altered in a predictable manner if the treatment in question were to alter the induction or expression of synaptic plasticity. They called this the anterograde alteration criterion, a component of ‘necessity’. Third, if learning were to occur and then, after learning, certain retrograde manipulations were made that might affect the expression of earlier changes in synaptic weights, the ability of the neural circuit to reconstruct the appropriate representational pattern should be affected. The experimental subject might then behave as if it had retrieved different information from that which had been learned. This is the retrograde alteration criterion—a second component of necessity. Last, if memory resides in specific distributed patterns of altered synaptic weights, the artificial creation of such a pattern should result in the creation of a ‘false memory’ for an event that did not happen or some aspect of knowledge or skill that had not been taught or trained. This mimicry criterion, the second component of sufficiency and essentially the engineering criterion, is arguably the most demanding.
(a). Detectability
There is now strong evidence that learning can be associated with the induction of changes in synaptic weights in apparently relevant neural circuits. This is the essence of the ‘detectability’ criterion—with critical issues arising over what constitute relevant neural circuits for any particular instance of learning (see [38] for a detailed discussion).
The earliest attempts to detect changes in synaptic weight in association with specific experiences revealed changes in synaptic strength and the magnitude of population spikes in the hippocampal formation in association with exposure of animals, normally in isolation, to a complex social living environment [39]. It later transpired that these may, at least in part, be associated with alterations in brain temperature rather than exploration-associated changes in synaptic weights [40]. A later study, with suitable calibration for temperature, did reveal transitory changes in excitatory postsynaptic potentials (EPSPs) associated with novelty exposure, but these rapidly decayed to baseline [41].
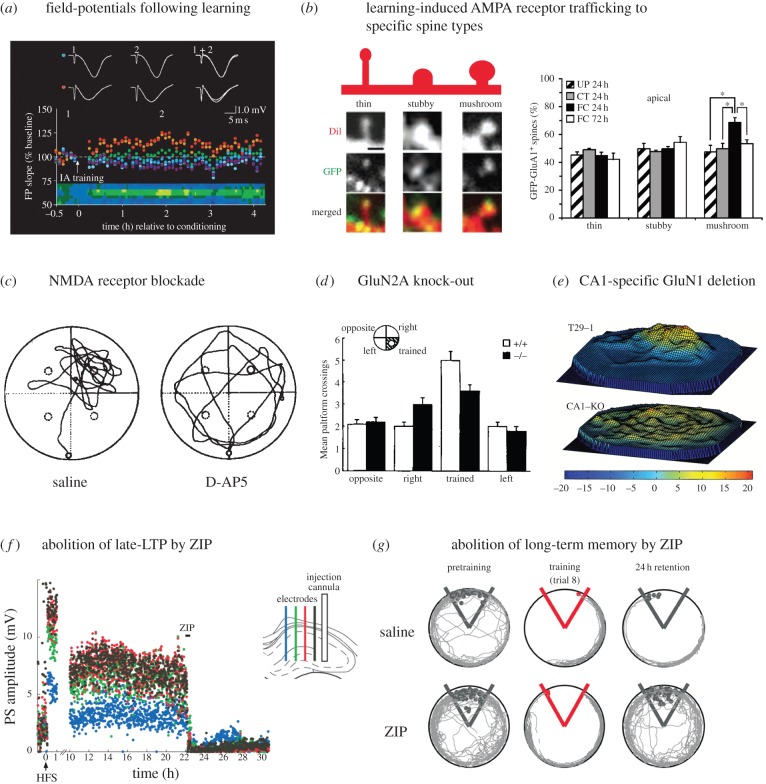
Bear's group reconsidered the issue of changes in the hippocampus associated with learning using an inhibitory avoidance paradigm and the use of multiple recording electrodes [42]. The supposition was that a system with significant storage capacity would not be expected to show global changes across a high proportion of neurons when just a single task is learned. In keeping with this intuition, stable increases in synaptic weights were seen at some recording sites but not others (figure 1a). Subsequent induction of LTP was impaired only at the recording sites where potentiation was observed following learning. In addition, alterations in AMPA receptor phosphorylation and trafficking akin to those observed after LTP were seen in a biochemical assay. Learning-induced enhancement in synaptic strength within the hippocampus has since been observed electrophysiologically in several other tasks that engage the hippocampus, including trace eyeblink conditioning [49] and novel object recognition [50]. In the light of Whitlock et al.'s [42] results, it is nonetheless surprising that these later studies did not require use of multiple electrode arrays.
Figure 1.
Illustrative findings relevant to the established criteria for assessing the SPM hypothesis. (a,b) Detectability. Field-potentials are increased on some but not all electrodes of a multi-electrode array in area CA1 following inhibitory avoidance learning (Adapted with permission from Whitlock et al. [42] © AAAS) (a). AMPA receptor trafficking detected optically using a GFP label in association with learning, with GluA1 targeted specifically at mature, mushroom-shaped spines (Adapted with permission from Matsuo et al. [43] © AAAS) (b). (c–e) Anterograde alterations. Pharmacological blockade of NMDA receptors in rats with chronic infusion of D-AP5 impairs spatial learning (Adapted with permission from Morris et al. [44]) (c). Genetic knock-out of GluN2A in mice also impairs spatial learning in the watermaze (Adapted with permission from Sakimura et al. [45] © Macmillan Publishers Ltd) (d). CA1 pyramidal cell-specific knockout of GluN1 in mice also impairs selective searching in the watermaze (Adapted with permission from Tsien et al. [46,47] © Elsevier) (e). (f,g) Retrograde alterations. Successful abolition by ZIP of long-lasting LTP 22 h after its initial induction (f). Corresponding abolition of long-term place-memory on a rotating platform by ZIP (Adapted with permission from Pastalkova et al. [48] © AAAS) (g).
While detection of learning-induced synaptic potentiation through recording of electrically evoked field potentials in behaving animals provides a relatively unambiguous tool for validating the detectability criterion, it is conceivable that most learning-induced changes in synaptic strength are sparsely distributed and therefore hard to detect with field recordings. Additionally, synaptic and/or homeostatic depression may occur concomitantly in a spatially overlapping set of synapses, further reducing the possibility of observing a net enhancement of the evoked field response. Therefore, the use of various molecular and structural hallmarks normally associated with LTP as markers for learning-induced potentiation is a more robust way of determining whether synaptic potentiation has taken place.
AMPA receptor insertion into the postsynaptic membrane is a hallmark of NMDA receptor-dependent synaptic potentiation [51,52]. Several studies used modified AMPA receptors to monitor AMPA receptor trafficking in the hippocampus after a learning session. In a clever set of experiments, Matsuo et al. [43] fused GluR1 (GluA1) subunit of AMPA receptors to green fluorescent protein (GFP). Synthesis of those receptors was regulated using a tetracycline-controlled transcriptional activation system and was also dependent on neural activity (via an immediate early gene (IEG) c-Fos promoter). The results showed a significant increase in GFP-positive spines on CA1 pyramidal neurons after contextual fear conditioning as well as after exposure to the context or footshock alone (figure 1b). Critically, when spines were sorted according to their type, contextual learning resulted in a relative increase in GFP-positive mushroom spines, when compared to the two control conditions. This provides strong evidence that learning results in enhanced AMPA receptor trafficking at mature, stable synapses in the hippocampus (for review of the role of mushroom-type spines in memory, see [53]). Similarly, Mitsushima et al. [54] used ‘electrophysiologically tagged’ AMPA receptors [55] to monitor synaptic AMPA receptor delivery in CA1 pyramidal cells. Incorporation of these receptors into functional synapses could later be detected in brain slices via intracellular recordings through detection of an increased level of inward rectification. The authors have found significant increase in insertion of GluA1-containing AMPA receptors into functional synapses following inhibitory avoidance learning—a phenomenon consistent with enhancement of synaptic strength. In the light of an estimate of the number of amygdala neurons required for learned fear expression [20], the distributed nature of hippocampal circuitry suggests that these changes in rectification may also have been expressed in a subset of neurons.
(b). Anterograde alterations
Fulfilling the detectability criterion has been important, but it remains a correlation and there is no obvious way of establishing a causal link between the physiological and behavioural changes. The logic of the ‘anterograde alteration’ approach is to make some prescribed manipulation that is known to affect the induction or expression of synaptic plasticity and see whether doing so affects learning or memory, or vice versa.
This approach began to be influential following pharmacological studies which showed that blocking NMDA receptors—in the forebrain or locally in specific brain circuits—impaired spatial learning in a watermaze (figure 1c, [44]). This work was followed up by a range of dose–response, neurochemical and other behavioural protocols that collectively established that the dose–response profile of the extracellular concentration of D-AP5 with respect to impairment of learning exactly matched that for blockade of the induction of LTP, and that impairments of olfactory and spatial learning, and fear-conditioning, all followed intracerebral infusions of NMDA receptor antagonists [56,57]. Pharmacological studies have the advantage that it is relatively easy to develop a protocol that can contrast initial learning with later memory retrieval—and this established that NMDA receptor-dependent plasticity is generally required at the initial induction of a new memory, but neither for storage nor later retrieval [58–61].
Occlusion is a useful analytical design in biomedical science and has recently been deployed to examine whether prior spatial learning in a watermaze can occlude the subsequent induction of LTP. This is not generally observed with the strong high-frequency tetanization protocols often used to investigate LTP itself, but Habib et al. [62] have shown that it can occlude the induction of LTP in area CA1 following brief trains of low-frequency stimulation and does so in a time-dependent manner. Conversely, strong tetanization of the perforant path using multiple stimulation sites at the level of the angular bundle can saturate LTP in the dentate gyrus (DG) and occlude subsequent spatial learning [63].
One series of experiments has investigated the differential impact of NMDA receptor blockade on first versus second learning of a related task, revealing the initially surprising result that the learning of a second similar task may sometimes proceed normally in the presence of D-AP5 [64,65]. Such a finding represented a challenge to the supposition that NMDA receptor-dependent synaptic plasticity is always necessary for learning. The reasons that this sparing occurs have not been completely worked out, but are likely to reflect the combination of two major factors. The first is that any given learning task generally consists of a number of components (declarative and procedural) and that some of these generalize from one task to another—resulting in substantial ‘savings’ in learning the second task. However, careful task design can lead to protocols that, irrespective of how many times new learning takes place, sensitivity to NMDA receptor blockade is retained. In the case of hippocampus-dependent learning, these are episodic-like memory tasks such as delayed matching-to-place [59] in which the animals are trained and tested repeatedly using a within-subjects design. The second issue is that drug diffusion through a brain area is often incomplete such that sub-areas of a given circuit which are less affected by the drug may be used for second learning that are not used for the first [66]. If pharmacological studies have the advantage of ‘reversibility’, they have the major difficulty that it is extremely difficult to maintain drug concentrations at a steady-state over time or to ensure effective distribution of a drug within a brain area or network without affecting another ostensibly independent region [67].
Molecular-genetic studies, by contrast, enable a specific molecule to be knocked out. In second and third generation transgenic animals, this is done in a region-, cell- and even temporal-specific manner. In the early 1990s, a new technology for investigating the role of synaptic plasticity in learning and memory emerged from this approach and, specifically, via gene ‘knockout’ studies. The possibility of knocking out the NMDA receptor was naturally considered, but it was soon shown that a standard homologous recombination knockout of NR1 subunit (GluN1) displayed abnormal development (e.g. in barrel cortex) and died soon after birth [68]. The first successful NMDA receptor knockout study related to learning was conducted by Mishina's group [45], who showed that deletion of NR2A (GluN2A) affected learning in the watermaze (figure 1d) much as Morris's group had shown earlier with D-AP5 [44] (but see [69] for an apparent failure to replicate). However, the big step forward was the importation into neuroscience of Cre-Lox technology by Tonegawa's group in 1996 [46].
A series of ingenious studies using specific lines of mice showed, first, that it was possible to knock out the GluN1 subunit of the NMDA receptor specifically in area CA1 of the hippocampus by cross-breeding a line of mice expressing Cre downstream of the αCaMKII promoter with a separate line in which GluN1 coding sequence was flanked by LoxP sites [47]. These mice display abnormal place fields in CA1 [70] and are impaired in learning the watermaze [47] (figure 1e). A later study confirmed that the GluN1 knockout was relatively restricted to CA1 and subiculum, but presented evidence that it may be more widespread after two months of age [71]. Using a kainate receptor (GluK4) promoter rather than αCaMKII, this group has gone on to use this same molecular-genetic approach to dissect differential functions of NMDA receptors in distinct parts of hippocampal circuitry. For example, one study provided evidence that NMDA receptors within area CA3 were essential at memory encoding to enable pattern completion at the time of memory retrieval in a watermaze surrounding by specific sets of cues and another for what is sometimes called ‘one-shot’ learning [72,73]. This approach to using gene-targeting was recently extended with another promoter to include a selective deficit in pattern separation when GluN1 is deleted in the DG [74].
Another group led by Seeburg and Sakmann, with behavioural studies led by Rawlins, have also used ‘knockout’ technology to investigate the role of glutamate receptors in learning and memory. They observed that whole brain deletion of GluA1 can cause deficits in LTP at CA3–CA1 synapses but, importantly, a behavioural dissociation between impaired spatial working-memory alongside intact reference-memory [75,76]. However, the LTP deficit in these mice may have been overestimated in the original study [77]. Nevertheless, that the deficit in spatial working memory can be rescued by transgenic expression of GluA1 on the knockout background is important [78]. This group has recently shown that GluA1 knockout impairs short-term spatial habituation but, surprisingly, enhances long-term spatial habituation [79]. These findings raise the possibility that the spatial working memory deficit in GluA1 knockout mice might be indirect, and reflect only the impairment in non-associative short-term habituation [80].
More recently, they have turned their attention to NMDA receptors using a cell-type and region-specific strategy, and observed that selective deletion of GluN1 in DG also causes the behavioural dissociation between spatial reference and spatial working memories [81]. A very recent paper using a new line of mice in which GluN1 is deleted in both CA1 and the DG again shows the relative sparing of spatial reference memory in the watermaze, but suggests that deficits can be observed if a beacon task is used which maximizes the opportunity for navigational interference, particularly when a path has to be inhibited [82]. The lesson from all these studies is that the specific type of memory being investigated has to be considered carefully with respect to the brain area targeted—a key argument of §2.
In addition to the glutamate receptor knockout studies described above, a wide range of genetically modified mice having mutations in different components of the postsynaptic machinery and downstream signalling molecules have been created. The now extensive list of mutated genes includes those coding for PSD scaffolding proteins [83], kinases and phosphatases [84–86], motor proteins [87,88], regulators for epigenetic mechanisms [89], translational regulators [9,90] and immediate early genes [91]. Interpreting the underlying complexity is difficult as some of these mutations have effects on the nervous system that go beyond changes specific to synaptic plasticity. In addition, simple monotonic changes in the expression or magnitude of synaptic potentiation concomitant to parallel changes in learning and memory are not always seen. While this may to some undermine the simplest versions of the SPM hypothesis, the vast majority of studies in which changes in synaptic plasticity are observed also show changes in memory in the mutant animals.
(c). Retrograde alterations
If synaptic weights are indeed the core substrate of hippocampal memory traces, alteration of the spatial distribution of synaptic efficacy across neurons and their dendrites within the hippocampus should interfere with memories of past events. Such ‘retrograde alteration’ can be achieved either by erasure of any learning-induced synaptic changes or by artificial induction of additional synaptic potentiation soon after memory encoding. The latter would effectively scramble the pattern of synaptic weights and thus render it behaviourally meaningless. As an example of this approach, Brun et al. [92] tetanized the perforant pathway input to the DG of rats trained on a water maze reference memory task. As expected, induction of hippocampal LTP in vivo after 5 days of training resulted in a profound deficit in memory retrieval—an effect blocked by intraperitoneal administration of 3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid. By contrast, rats that received control stimulation of the same pathway showed normal memory of the hidden platform, indicating that NMDA receptor-dependent potentiation of hippocampal synapses interfered with the activated memory trace. If recently induced hippocampal LTP is followed by trains of low-frequency stimulation, enhanced field potentials often return to the pre-LTP baseline, effectively erasing the effects of the tetanus [93]. Though this phenomenon has, to date, only been used to depotentiate artificially induced LTP, it could in theory be used to reverse learning-induced changes at hippocampal synapses, as discussed in our previous review [38].
Sacktor's group have pursued the idea that a particular form of protein kinase C (PKC) may be involved in memory. They first showed that infusion of a peptide blocker of a constitutively active but atypical form of PKC (PKM-ζ) called ZIP could block LTP maintenance in vivo and abolish well-established place avoidance memory (figure 1f,g) [48]. These landmark findings relevant to retrograde alteration of synaptic efficacy were followed by papers implicating the role of PKM-ζ in maintenance of synaptic plasticity and memory in a variety of brain areas [94]. It therefore came as a surprise that PKM-ζ knockout mice are reported to have no deficits in plasticity and memory, while maintaining sensitivity to ZIP treatment [95,96]. Although these findings call the specificity of ZIP into question and, with it, the role of PKM-ζ as the quintessential ‘memory molecule’, they do not undermine the fact that a drug that causes a post-learning reversal of synaptic enhancement is associated with a complete erasure of a recently encoded memory (see Sacktor and co-workers' paper [97] in this issue). Recent work by Migues and Hardt is consistent with this association in demonstrating that PKM-ζ also maintains long-term memory for the location of recently explored objects in the rat hippocampus [98] and does so by regulating the trafficking of GluA2-containing AMPA receptors with memory strength positively correlated with post-synaptic GluA2 levels [99].
Some evidence points to another kinase, CaMKII, as a key player in LTP maintenance [86] in addition to its widely established role in LTP induction. Redondo & Morris [100] have implicated CaMKII as being on the pathway to the setting of synaptic tags—one essential step for lasting memory. Other work has shown that blocking the interaction between CaMKII and NMDA receptors with a CN21 peptide reverses hippocampal LTP in vitro [101], though it is yet to be demonstrated whether the same peptide interferes with maintenance of hippocampal memories. In line with the role of the CaMKII–NMDA receptor complex in maintenance of hippocampal plasticity and memory, ‘knockin’ mice with the NMDA receptor GluN2B subunit that is incapable of forming this complex show a deficit in consolidation of spatial reference memory [102]. The same animals also show a reduction of LTP magnitude on the Schaffer collateral/commissural-CA1 pathway, but we are not aware of any published data regarding a possible impairment of LTP maintenance in these animals.
(d). Mimicry
The creation of an artificial engram using a putative mechanism of memory formation would be a particularly stringent test of the SPM hypothesis. The test in question would be to artificially introduce changes in synaptic weights in a distributed pattern and show that this results in a predictable display of ‘memory’ for something that in practice had either not happened or had happened earlier and then had been demonstrably forgotten. There is a pleasing irony here, for studies of false memory by neuropsychologists are generally seen as studies of how memory fails; for the neurobiologist, the possibility of artificially creating a false memory represents an intriguing experimental opportunity.
We may be getting very close to a true demonstration of mimicry in some brain structures with unambiguously defined CS and US inputs, most notably the amygdala in which learning follows Pavlovian principles (for review, see [14]). It is postulated that associative fear learning occurs through Hebbian LTP at synapses onto cells in lateral amygdala (LA). An association between CS and US occurs when cortical/thalamic projections carrying information about the CS fire coincidently with US-associated depolarization of postsynaptic LA cells. LeDoux's group demonstrated this using optogenetic stimulation of LA neurons at the time of tone delivery, which resulted in the formation of an artificial fear memory without the need for a foot shock [103].
The distributed-associative network of the hippocampus, on the other hand, makes designing a potential mimicry experiment much more difficult—and also brings out some of the key issues discussed in §2. One way of approaching the issue is to use learning to create a pattern, allow the memory to fade and be forgotten but hopefully not the molecular markers of its earlier existence. An analogy might be to the act of bringing back to life a ghost town in the wild west of America. But how might this resurrection of memory be achieved?
In recent years, optogenetics was developed as a way to control the activity of spatially and genetically defined populations of neurons with millisecond precision [31]. Coupling optogenetics to advanced temporal gene expression control systems enables one to tag a population of neurons active during a specific event and subsequently reactivate them at will. Tonegawa's group have used tetracycline-controlled transcriptional activation system to selectively express channelrhodopsin (ChR2) in those neurons of DG that were active during the encoding phase of a contextual fear conditioning task [104]. Crucially, tetracycline transactivator (tTA) was placed under the control of c-Fos promoter and ChR2 was controlled by the tetracycline-responsive element (TRE). As long as the mice were kept on doxycycline (Dox), expression of ChR2 was blocked by preventing tTA from binding to the TRE site. When taken off Dox on the day of critical memory encoding, ChR2 expression could be induced in DG neurons in an activity-dependent manner. Having expressed ChR2 in DG neurons that were active during engram formation, these investigators were able to reactivate the engram with light in a different context. Reactivation of the memory trace resulted in a robust increase in freezing behaviour—an observation consistent with fear memory recall. In a related approach, Mayford's group have described their use of the DREADD system to generate a synthetic memory trace [105]. Powerful and striking as these two landmark studies are, we believe that to fulfil the mimicry criterion of the SPM hypothesis, it is critical to move from the level of neuronal assemblies down to the level of synapses.
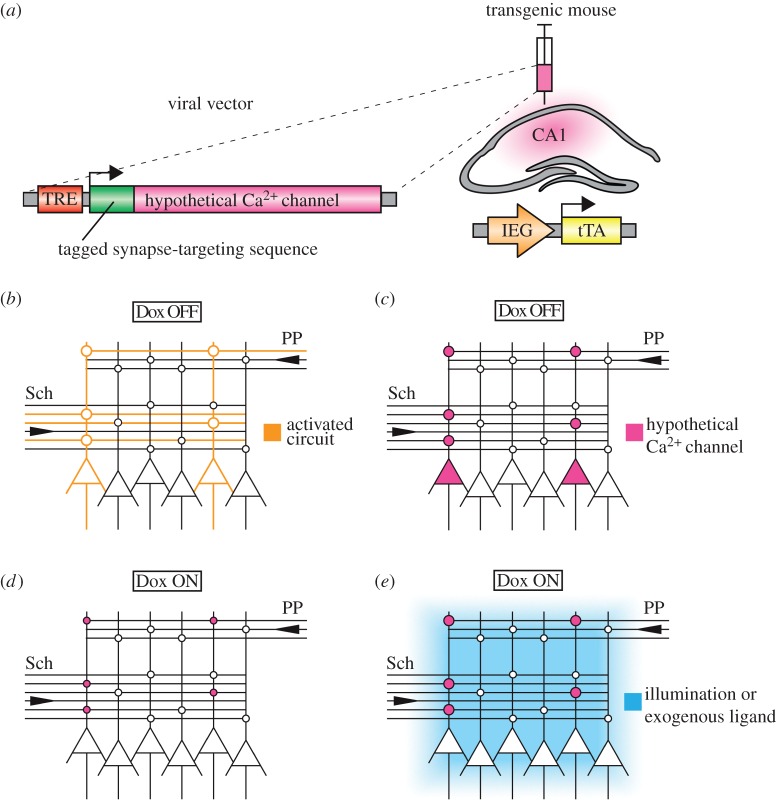
As suggested previously by Neves et al. [106], an experiment testing whether hippocampal NMDA receptor-dependent plasticity is sufficient for episodic memory could involve a hypothetical light or exogenous ligand-activated Ca2+ channel. As Ca2+ influx into the spine may be sufficient to induce early-LTP [107], activation of this Ca2+ channel once it has made its way to the spine should result in synapse-specific potentiation. Though a remotely controlled ion channel selective for Ca2+ has not yet been described, it is worth noting that some new ChR2 variants show enhanced Ca2+ conductivity [108] and it would be interesting to see whether activation of these channels within a dendritic spine could lead to LTP without the need for presynaptic activation.
In a ‘dream experiment’, the hypothetical Ca2+ channel would be expressed in all hippocampal neurons postsynaptic to the set of synapses under investigation, its transcription would be tTA-regulated and IEG-dependent, and the channel protein itself would contain a targeting sequence that directs it to recently potentiated (tagged) spines in the same way these synapses recognize plasticity-related products (PRPs) as outlined by the synaptic tagging and capture hypothesis [100,109,110] (figure 2a). Though we are beginning to unravel how PRPs like Homer-1a/Vesl-1S and Arc/Arg 3.1 are transported around the neuron [111,112], the precise mechanisms of PRP capture by tagged synapses remain elusive and will have to be uncovered before an experiment like this can be carried out.
Figure 2.
Hypothetical experiment testing the prediction that LTP at a given set of synapses is sufficient for engram formation. (a) Activity-dependent expression of hypothetical Ca2+ channel in hippocampal CA1 area. The IEG promoter-driven tTA transgenic animal is injected with a viral vector in which the hypothetical light- or exogenous ligand-activated Ca2+ channel with tagged synapse-targeting sequence is expressed in an activity-dependent and Dox-regulated manner. (b) Memory encoding in CA1. A specific pattern of activation of afferent fibres results in Hebbian LTP at a fraction of synapses onto CA1 pyramidal cells. (c) Synaptic tagging and capture. In absence of Dox, strong encoding results in formation of synaptic tags and synthesis of not only PRPs but also the hypothetical Ca2+ channel. The channel protein is then distributed around the neuron and captured by the tagged synapses. (d) Trace decay. The animal is put back on Dox. Synaptic potentiation at hypothetical Ca2+ channel-targeted synapses decays with time. (e) LTP reinstatement. Activation of the Ca2+ channel (either by illumination of the target area or infusion of the exogenous ligand) should result in LTP at synapses tagged during the critical encoding session. A successful mimicry experiment would involve a subsequent demonstration of retrieval of the reinstated memory trace. Sch, Schaffer collaterals; PP, perforant path.
The toolkit described above could in principle be used to study any IEG-expressing population of neurons in the hippocampus and beyond. The experiment outlined below focuses on principal neurons in the hippocampal area CA1 (figure 2b). The hypothetical channel would be expressed selectively at tagged synapses at the time of encoding (figure 2c), and these steps followed by forgetting and loss of the underlying memory trace (figure 2d). The question then is whether the trace could be brought back (figure 2e). Specifically, experimental animals would be trained in the presence of Dox on a one-shot spatial memory task in which the location of the goal—either a hidden platform in the watermaze [59], or the availability of food in a sandwell in the event arena [37]—changes every day. On a specific day, animals would be taken off Dox and subjected to strong memory encoding (figure 2b), which should induce IEG expression (and thus the Ca2+ channel) in hippocampal neurons (figure 2c). Animals would then be put back on Dox and the ‘tagged’ memory trace would be allowed to decay or would be overwritten by extensive training (figure 2d). The animals would then need to be tested to be confident that forgetting has occurred. The experimental question is then addressed by attempting to reintroduce this specific memory. This would be attempted optogenetically or pharmacogenetically with the effect that the only subset of synapses that would be potentiated would be those expressing the hypothetical Ca2+ channel and therefore those that were tagged during the critical memory encoding phase (figure 2e). If LTP at a specific set of hippocampal synapses is sufficient to construct a meaningful memory trace, re-inducing LTP via activation of this hypothetical channel should mimic weak memory of the correct location of the goal on the ‘tagged’ day.
One potential obstacle of such experimental set-up is protein turnover. Endocytosis and degradation of the hypothetical Ca2+ channel would lead to its progressive elimination from spines. Since by that time the synaptic tag would be long gone, selective redelivery of the channel protein to the spines of interest would be impossible. As mechanisms of protein degradation are being increasingly understood [113], it might be possible to engineer the channel to resist various forms of cellular ‘clean-up’ and thus stay in the spine for a time period relevant to our proposed experiment.
4. The logical connection between the persistence of synaptic plasticity and the persistence of memory
A somewhat ironic feature of ‘LTP’ is its name. Synaptic potentiation outlasts the events of its induction and it can in exceptional circumstances be seen to last over a year [114], but in general LTP decays back to baseline within a few hours. Barnes used the differential rate of decay of LTP as a function of ageing in her pioneering studies of the relationship between synaptic plasticity and learning [12,115]. Other lasting forms of synaptic plasticity have also been observed, such as stimulus-selective response potentiation, which take some hours to be expressed but are then persistent over time [116]. This instability of induction and persistence is problematic for the view that LTP-like changes in synaptic efficacy occur immediately and go on to mediate really lasting memory, albeit a puzzle that is complicated by the dual existence of both ‘cellular consolidation’ and the separate ‘systems-consolidation’ that enables one brain region to serve a time-limited role in bringing about lasting storage elsewhere in the brain (and most likely in the neocortex).
A systems perspective on this problem involves recognizing the interplay between cellular consolidation triggered within hippocampal neurons at the time of learning and systems consolidation between hippocampus and neocortex that comes into operation after learning (and is thought by many to involve sleep). New data at a systems level have emerged from the fMRI studies of LTP by Canals et al. [117]. They have shown that the induction of LTP in the hippocampal formation, and specifically the DG, is characterized by a larger BOLD signal at the monosynaptic site of potentiation but also by the appearance of a detectable change in BOLD in other circuits, including the prefrontal lobe. This indicates that potentiation at one level may somehow be projected to or otherwise affect other levels of the neural circuit mediating memory processing. Preliminary data secured by Canals’ group indicate that similar remote changes in neural activation occur following induction of LTP in CA1. The reason(s) for this systems aspect of LTP induction remain to be investigated, but could include an alteration in the excitation–inhibition balance following some types of LTP that is detectable through observation of a change in the coupling of excitatory post-synaptic potentials to population spike generation in field-potential recordings. The work of Canals and co-workers (see [118] in this issue) discusses LTP-induced alterations in feed-forward disinhibition.
A separate cellular consolidation possibility is that early-LTP decays precisely in order to re-set hippocampal circuits back to a level whereby they can most effectively process new information at a later time—not least the same day. This gets round the need for lasting potentiation within the hippocampus, though it is of course still necessary in the neocortex, but also raises the issue of the timeframe for which information must be retained by cellular consolidation for the systems-consolidation aspect to work effectively.
Central to addressing this issue is the work of the Magdeburg group of Matthies and Frey that established the separate existence of early- and late-forms of LTP, the latter being defined as protein synthesis-dependent. Their work provided the first experimental evidence suggesting that neuromodulators, especially dopamine (DA), play a significant role in gating of plasticity and memory persistence. In vivo and in vitro electrophysiological studies have revealed a specific role for DA in control of temporal persistence of LTP [119–122]. Pharmacological manipulations of DA receptors also indicate that DA is required for the persistence of memories in the hippocampus [123–125]. DA receptor activation can lead to enhanced somatic and dendritic protein synthesis essential for the establishment of lasting plasticity and memory [126,127]. This function is mediated by protein kinase A, extracellular-signal regulated kinase (ERK) 1/2, CaMKIV and cAMP response element-binding protein CREB [128–131].
The midbrain dopaminergic neurons of the ventral tegmental area (VTA) project to the hippocampal formation [132,133] and are thought to release DA under circumstances of novelty or surprise [134,135]. In addition, a recent paper suggests that noradrenergic (NA) terminals of the locus coeruleus (LC) might have a role in DA transmission in the hippocampus [136]. The necessity of DA-dependent mechanisms within hippocampal neurons in cellular consolidation implies that cellular consolidation depends not just on the intrahippocampal, cellular processes, but also on the action of systems-level components. Therefore, we propose that ‘cellular consolidation’ is renamed ‘initial consolidation’.
There are many lines of evidence suggesting that the persistence of memory is determined largely by neural activity that occurs at the time of memory encoding. However, the synaptic tagging and capture hypothesis of protein synthesis-dependent LTP developed by Frey & Morris [109,110] offers the intriguing but distinct perspective that the persistence of memory is also dependent on independent neural activity afferent to the same pool of neurons mediating LTP that occurs before or after memory traces are encoded. According to this hypothesis, the local setting of ‘synaptic tags’ at activated glutamatergic synapses during memory encoding can be dissociated from DA-dependent synthesis and distribution of PRPs that is induced by surrounding events. PRPs are then captured by synaptic tags in order to stabilize synaptic change—a process that is critical for initial consolidation.
Until recently, a challenge for the synaptic tagging and capture hypothesis has been to assess its relevance to real memory. Considering that exploration of a novel environment probably activates VTA DA neurons to release DA in the hippocampus and thus cause upregulation of IEGs such as Arc and Homer-1a [137,138], the synaptic tagging and capture hypothesis predicts that unrelated novelty exploration before or after memory encoding should enhance the persistence of a recently encoded memory [139]. This prediction was first confirmed using a hippocampus-dependent inhibitory avoidance task in rats [140]. Moncada and Viola showed that weak memory could be consolidated into long-term memory by unrelated exploration of a novel environment. This novelty-induced memory persistence was blocked by intra-hippocampal injection of DA D1/D5 receptor antagonist and β-adrenergic receptors antagonist [140,141] or by inhibition of induction or expression of CA1 LTD [142]. Complementary results have been obtained using different learning tasks including taste memory, spatial object recognition, contextual fear conditioning and spatial memory [37,143,144].
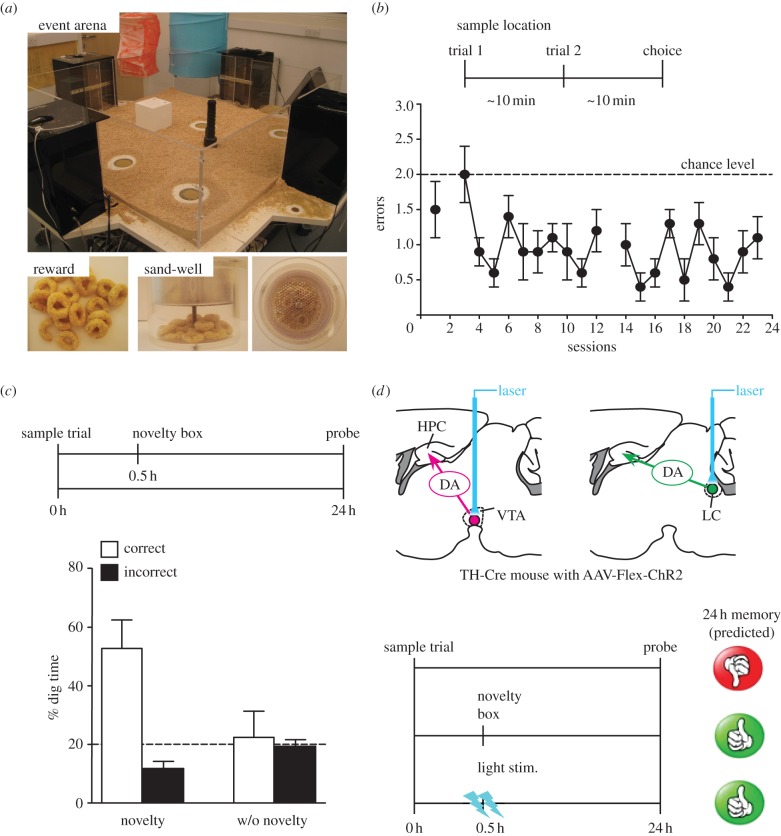
Our laboratory has developed a realistic everyday appetitive paradigm for rats in order to establish whether unrelated novel experiences can facilitate the persistence of reward-motivated spatial memory [37]. We have expanded our analysis to different systems domains and, for this purpose, have recently altered the protocol for the spatial memory task in the event arena and made it suitable for mice (figure 3a). The object of switching the task to mice is to enable genetically modified animals to be tested. The tyrosine hydroxylase-Cre knockin mice with C57BL/6 genetic background [145] were trained on this one-shot spatial memory task over four weeks. The mice have to learn a different location of food each day and, with five sandwells to choose from, performance increases quite rapidly from the chance level of two errors on the daily choice trial to a consistent pattern of less than one error per day (figure 3b). A series of post-learning tests, of which we here show only one, established that: (i) persistence of one-shot memory depends on reward magnitude, lasting only 1 h with two pellets but 24 h with eight pellets; (ii) 5-min exploration of an open field with a variable novel floor substrate 30 min after weak two-pellet encoding successfully transformed 1-h memory into 24-h memory (figure 3c) and (iii) pharmacological interruption of DA D1/D5 receptors, but not β-adrenergic receptors in hippocampus during novelty exploration prevented novelty-induced memory persistence over 24 h. Those characteristics are consistent with our earlier rat data [37]. The next step is to substitute novelty exploration with photo-activation of VTA DA neurons or LC NA neurons using optogenetics (figure 3d). With this procedure, our prediction is that activating these neurons in some appropriate temporal pattern should convert a recently encoded short-term memory into a long-term memory.
Figure 3.
One-shot spatial memory task on the event arena for mice. (a) Event arena for one-shot spatial memory task. The event arena during a daily choice phase. Five sand wells are open but only one contains the reward pellets. All open sand wells contain several pellets that are inaccessible to the mouse in order to control for olfactory artefacts. (b) Daily spatial memory performance (errors). Every day mice have two trials to encode the new sand well location, followed by a choice phase. They quickly reach a stable performance level of less than one error (with two errors being the chance level). (c) Novelty-induced enhancement of memory persistence. Critical sessions involve one sample trial followed by an unrewarded probe test 24 h later. 5 min exploration of a novel environment 30 min after encoding results in enhanced persistence of one-shot spatial memory, as demonstrated by increased dig time in the correct location. (d) Prediction of memory enhancement by optogenetic stimulation of catecholaminergic nuclei. We predict that photoactivation of DA cells of the VTA or DA-releasing NA cells of LC in TH-Cre mice injected with Cre-dependent ChR2 virus (AAV-Flex-ChR2) after weak memory encoding will result in enhancement of memory persistence that mimics the novelty effect. Error bars, ±s.e.m; dotted lines, chance level.
5. Conclusion
Martin et al. [16] laid out a framework for testing rigorously the widely held notion that synaptic potentiation and depression are key players in mediating the creation of memory traces or engrams. That framework has stood the test of time, with exciting new approaches using contemporary techniques exploring the idea further with respect to detectability, anterograde and retrograde alteration. Perhaps most exciting are the first steps being taken towards testing and possibly satisfying the mimicry criterion using optogenetic and other cell-type-specific molecular tools. Critical experiments remain to be done, but the neuroscience community can justifiably feel tantalizingly close to having tested one of the great ideas of modern neuroscience. Forty years on, LTP continues to excite us all as it slowly gives up its mechanistic secrets and reveals its important functional role in learning and memory.
Acknowledgements
We are grateful to Patrick Spooner for technical assistance with the event arena and Jacqueline Friel for assistance with behavioural training of mice.
Funding statement
This work was supported by a European Research Council Advanced Investigator Grant to R.G.M.M. and Guillén Fernández (NEUROSCHEMA, no. 268800). T.T. was supported by the Mitsubishi Tanabe Pharma Corporation and the UK Medical Research Council, to whom we are also grateful for past funding to R.G.M.M. and for a studentship and in vivo skills award currently held by A.J.D.
References
- 1.Jones EG. 1994. Santiago Ramon y Cajal and the Croonian Lecture. Trends Neurosci. 17, 190–192. ( 10.1016/0166-2236(94)90100-7) [DOI] [PubMed] [Google Scholar]
- 2.Hebb DO. 1949. The organization of behaviour. New York, NY: Wiley. [Google Scholar]
- 3.Konorski J. 1948. Conditioned reflexes and neuron organisation. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 4.Kandel ER. 1978. A cell-biological approach to learning. Bethesda, MD: Society for Neuroscience. [Google Scholar]
- 5.Kandel ER. 2001. The molecular biology of memory storage: a dialogue between genes and synapses. Science 294, 1030–1038. ( 10.1126/science.1067020) [DOI] [PubMed] [Google Scholar]
- 6.Bliss TV, Lomo T. 1973. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 232, 331–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scoville WB, Milner B. 1957. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 20, 11–21. ( 10.1136/jnnp.20.1.11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho VM, Lee JA, Martin KC. 2011. The cell biology of synaptic plasticity. Science 334, 623–628. ( 10.1126/science.1209236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. 2009. Translational control of long-lasting synaptic plasticity and memory. Neuron 61, 10–26. ( 10.1016/j.neuron.2008.10.055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. 2010. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 33, 121–129. ( 10.1016/j.tins.2010.01.001) [DOI] [PubMed] [Google Scholar]
- 11.Murakoshi H, Yasuda R. 2012. Postsynaptic signaling during plasticity of dendritic spines. Trends Neurosci. 35, 135–143. ( 10.1016/j.tins.2011.12.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnes CA, McNaughton BL. 1985. An age comparison of the rates of acquisition and forgetting of spatial information in relation to long-term enhancement of hippocampal synapses. Behav. Neurosci. 99, 1040–1048. ( 10.1037/0735-7044.99.6.1040) [DOI] [PubMed] [Google Scholar]
- 13.Alberini CM. 2009. Transcription factors in long-term memory and synaptic plasticity. Physiol. Rev. 89, 121–145. ( 10.1152/physrev.00017.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansen JP, Cain CK, Ostroff LE, LeDoux JE. 2011. Molecular mechanisms of fear learning and memory. Cell 147, 509–524. ( 10.1016/j.cell.2011.10.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YS, Silva AJ. 2009. The molecular and cellular biology of enhanced cognition. Nat. Rev. Neurosci. 10, 126–140. ( 10.1038/nrn2572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin SJ, Grimwood PD, Morris RG. 2000. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu. Rev. Neurosci. 23, 649–711. ( 10.1146/annurev.neuro.23.1.649) [DOI] [PubMed] [Google Scholar]
- 17.Hubener M, Bonhoeffer T. 2010. Searching for engrams. Neuron 67, 363–371. ( 10.1016/j.neuron.2010.06.033) [DOI] [PubMed] [Google Scholar]
- 18.Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hubener M. 2009. Experience leaves a lasting structural trace in cortical circuits. Nature 4577, 313–317. ( 10.1038/nature07487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawkins RD, Kandel ER. 1984. Is there a cell-biological alphabet for simple forms of learning? Psychol. Rev. 91, 375–391. ( 10.1037/0033-295X.91.3.375) [DOI] [PubMed] [Google Scholar]
- 20.Rumpel S, LeDoux J, Zador A, Malinow R. 2005. Postsynaptic receptor trafficking underlying a form of associative learning. Science 308, 83–88. ( 10.1126/science.1103944) [DOI] [PubMed] [Google Scholar]
- 21.Morris RG, Frey U. 1997. Hippocampal synaptic plasticity: role in spatial learning or the automatic recording of attended experience? Phil. Trans. R. Soc. Lond. B 352, 1489–1503. ( 10.1098/rstb.1997.0136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNaughton BL, Morris RGM. 1987. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends Neurosci. 10, 408–415. ( 10.1016/0166-2236(87)90011-7) [DOI] [Google Scholar]
- 23.Klausberger T, Somogyi P. 2008. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 321, 53–57. ( 10.1126/science.1149381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dayan P, Abbott L. 2005. Theoretical neuroscience. Cambridge, MA: MIT Press. [Google Scholar]
- 25.Turrigiano GG, Nelson SB. 2000. Hebb and homeostasis in neuronal plasticity. Curr. Opin. Neurobiol. 10, 358–364. ( 10.1016/S0959-4388(00)00091-X) [DOI] [PubMed] [Google Scholar]
- 26.Levy WB, Steward O. 1983. Temporal contiguity requirements for long-term associative potentiation/depression in the hippocampus. Neuroscience 8, 791–797. ( 10.1016/0306-4522(83)90010-6) [DOI] [PubMed] [Google Scholar]
- 27.Markram H, Lubke J, Frotscher M, Sakmann B. 1997. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science 275, 213–215. ( 10.1126/science.275.5297.213) [DOI] [PubMed] [Google Scholar]
- 28.Xu S, Jiang W, Poo MM, Dan Y. 2012. Activity recall in a visual cortical ensemble. Nat. Neurosci. 15, 449–455. ( 10.1038/nn.3036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Connor DH, Huber D, Svoboda K. 2009. Reverse engineering the mouse brain. Nature 461, 923–929. ( 10.1038/nature08539) [DOI] [PubMed] [Google Scholar]
- 30.Rogan SC, Roth BL. 2011. Remote control of neuronal signaling. Pharmacol. Rev. 63, 291–315. ( 10.1124/pr.110.003020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. 2011. Optogenetics in neural systems. Neuron 71, 9–34. ( 10.1016/j.neuron.2011.06.004) [DOI] [PubMed] [Google Scholar]
- 32.Packer AM, Roska B, Hausser M. 2013. Targeting neurons and photons for optogenetics. Nat. Neurosci. 16, 805–815. ( 10.1038/nn.3427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knopfel T. 2012. Genetically encoded optical indicators for the analysis of neuronal circuits. Nat. Rev. Neurosci. 13, 687–700. ( 10.1038/nrm3461) [DOI] [PubMed] [Google Scholar]
- 34.Wilt BA, Burns LD, Ho ETW, Ghosh KK, Mukamel EA, Schnitzer MJ. 2009. Advances in light microscopy for neuroscience. Annu. Rev. Neurosci. 32, 435–506. ( 10.1146/annurev.neuro.051508.135540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RGM. 2007. Schemas and memory consolidation. Science 316, 76–82. ( 10.1126/science.1135935) [DOI] [PubMed] [Google Scholar]
- 36.Harvey CD, Collman F, Dombeck DA, Tank DW. 2009. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature 461, 941–946. ( 10.1038/nature08499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang SH, Redondo RL, Morris RG. 2010. Relevance of synaptic tagging and capture to the persistence of long-term potentiation and everyday spatial memory. Proc. Natl Acad. Sci. USA 107, 19 537–19 542. ( 10.1073/pnas.0913844107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin SJ, Morris RG. 2002. New life in an old idea: the synaptic plasticity and memory hypothesis revisited. Hippocampus 12, 609–636. ( 10.1002/hipo.10107) [DOI] [PubMed] [Google Scholar]
- 39.Green EJ, McNaughton BL, Barnes CA. 1990. Exploration-dependent modulation of evoked responses in fascia dentata: dissociation of motor, EEG, and sensory factors and evidence for a synaptic efficacy change. J. Neurosci. 10, 1455–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moser E, Mathiesen I, Andersen P. 1993. Association between brain temperature and dentate field potentials in exploring and swimming rats. Science 259, 1324–1326. ( 10.1126/science.8446900) [DOI] [PubMed] [Google Scholar]
- 41.Moser EI, Moser MB, Andersen P. 1994. Potentiation of dentate synapses initiated by exploratory learning in rats: dissociation from brain temperature, motor activity, and arousal. Learn. Mem. 1, 55–73. [PubMed] [Google Scholar]
- 42.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. 2006. Learning induces long-term potentiation in the hippocampus. Science 313, 1093–1097. ( 10.1126/science.1128134) [DOI] [PubMed] [Google Scholar]
- 43.Matsuo N, Reijmers L, Mayford M. 2008. Spine-type-specific recruitment of newly synthesized AMPA receptors with learning. Science 319, 1104–1107. ( 10.1126/science.1149967) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morris RGM, Anderson E, Lynch GS, Baudry M. 1986. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-d-aspartate receptor antagonist, AP5. Nature 319, 774–776. ( 10.1038/319774a0) [DOI] [PubMed] [Google Scholar]
- 45.Sakimura K, et al. 1995. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature 373, 151–155. ( 10.1038/373151a0) [DOI] [PubMed] [Google Scholar]
- 46.Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S. 1996. Subregion- and cell type-restricted gene knockout in mouse brain [see comments]. Cell 87, 1317–1326. ( 10.1016/S0092-8674(00)81826-7) [DOI] [PubMed] [Google Scholar]
- 47.Tsien JZ, Huerta PT, Tonegawa S. 1996. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 87, 1327–1338. ( 10.1016/S0092-8674(00)81827-9) [DOI] [PubMed] [Google Scholar]
- 48.Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. 2006. Storage of spatial information by the maintenance mechanism of LTP. Science 313, 1141–1144. ( 10.1126/science.1128657) [DOI] [PubMed] [Google Scholar]
- 49.Gruart A, Munoz MD, Delgado-Garcia JM. 2006. Involvement of the CA3-CA1 synapse in the acquisition of associative learning in behaving mice. J. Neurosci. 26, 1077–1087. ( 10.1523/JNEUROSCI.2834-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clarke JR, Cammarota M, Gruart A, Izquierdo I, Delgado-Garcia JM. 2010. Plastic modifications induced by object recognition memory processing. Proc. Natl Acad. Sci. USA 107, 2652–2657. ( 10.1073/pnas.0915059107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bredt DS, Nicoll RA. 2003. AMPA receptor trafficking at excitatory synapses. Neuron 40, 361–379. ( 10.1016/S0896-6273(03)00640-8) [DOI] [PubMed] [Google Scholar]
- 52.Kessels HW, Malinow R. 2009. Synaptic AMPA receptor plasticity and behavior. Neuron 61, 340–350. ( 10.1016/j.neuron.2009.01.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bourne J, Harris KM. 2007. Do thin spines learn to be mushroom spines that remember? Curr. Opin. Neurobiol. 17, 381–386. ( 10.1016/j.conb.2007.04.009) [DOI] [PubMed] [Google Scholar]
- 54.Mitsushima D, Ishihara K, Sano A, Kessels HW, Takahashi T. 2011. Contextual learning requires synaptic AMPA receptor delivery in the hippocampus. Proc. Natl Acad. Sci. USA 108, 12 503–12 508. ( 10.1073/pnas.1104558108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. 2000. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287, 2262–2267. ( 10.1126/science.287.5461.2262) [DOI] [PubMed] [Google Scholar]
- 56.Davis S, Butcher SP, Morris RGM. 1992. The NMDA receptor antagonist D-2-amino-5-phosphonopentanoate (D-AP5) impairs spatial learning and LTP in vivo at intracerebral concentrations comparable to those that block LTP in vitro. J. Neurosci. 12, 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Danysz W, Zajaczkowski W, Parsons CG. 1995. Modulation of learning processes by ionotropic glutamate receptor ligands. Behav. Pharmacol. 6, 455–474. ( 10.1097/00008877-199508000-00007) [DOI] [PubMed] [Google Scholar]
- 58.Staubli U, Thibault O, DiLorenzo M, Lynch G. 1989. Antagonism of NMDA receptors impairs acquisition but not retention of olfactory memory. Behav. Neurosci. 103, 54–60. ( 10.1037/0735-7044.103.1.54) [DOI] [PubMed] [Google Scholar]
- 59.Steele RJ, Morris RGM. 1999. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus 9, 118–136. () [DOI] [PubMed] [Google Scholar]
- 60.Day M, Langston R, Morris RG. 2003. Glutamate-receptor-mediated encoding and retrieval of paired-associate learning. Nature 424, 205–209. ( 10.1038/nature01769) [DOI] [PubMed] [Google Scholar]
- 61.Bast T, da Silva BM, Morris RG. 2005. Distinct contributions of hippocampal NMDA and AMPA receptors to encoding and retrieval of one-trial place memory. J. Neurosci. 25, 5845–5856. ( 10.1523/JNEUROSCI.0698-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Habib D, Tsui CK, Rosen LG, Dringenberg HC. In press. Occlusion of low-frequency-induced, heterosynaptic long-term potentiation in the rat hippocampus in vivo following spatial training. Cereb Cortex. ( 10.1093/cercor/bht174) [DOI] [PubMed] [Google Scholar]
- 63.Moser EI, Krobert KA, Moser MB, Morris RG. 1998. Impaired spatial learning after saturation of long-term potentiation. Science 281, 2038–2042. ( 10.1126/science.281.5385.2038) [DOI] [PubMed] [Google Scholar]
- 64.Saucier D, Cain DP. 1995. Spatial learning without NMDA receptor-dependent long-term potentiation. Nature 378, 186–189. ( 10.1038/378186a0) [DOI] [PubMed] [Google Scholar]
- 65.Bannerman DM, Good MA, Butcher SP, Ramsay M, Morris RG. 1995. Distinct components of spatial learning revealed by prior training and NMDA receptor blockade. Nature 378, 182–186. ( 10.1038/378182a0) [DOI] [PubMed] [Google Scholar]
- 66.Wang SH, Finnie PS, Hardt O, Nader K. 2012. Dorsal hippocampus is necessary for novel learning but sufficient for subsequent similar learning. Hippocampus 22, 2157–2170. ( 10.1002/hipo.22036) [DOI] [PubMed] [Google Scholar]
- 67.Inglis J, Martin SJ, Morris RGM. 2013. Upstairs-downstairs revisited: spatial pretraining-induced rescue of normal spatial learning during selective blockade of hippocampal N-methyl-d-aspartate receptors. Eur. J. Neurosci. 37, 718–727. ( 10.1111/ejn.12087) [DOI] [PubMed] [Google Scholar]
- 68.Li Y, Erzurumlu RS, Chen C, Jhaveri S, Tonegawa S. 1994. Whisker-related neuronal patterns fail to develop in the trigeminal brainstem nuclei of NMDAR1 knockout mice. Cell 76, 427–437. ( 10.1016/0092-8674(94)90108-2) [DOI] [PubMed] [Google Scholar]
- 69.Bannerman DM, et al. 2008. NMDA receptor subunit NR2A is required for rapidly acquired spatial working memory but not incremental spatial reference memory. J. Neurosci. 28, 3623–3630. ( 10.1523/JNEUROSCI.3639-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McHugh TJ, Blum KI, Tsien JZ, Tonegawa S, Wilson MA. 1996. Impaired hippocampal representation of space in CA1-specific NMDAR1 knockout mice. Cell 87, 1339–1349. ( 10.1016/S0092-8674(00)81828-0) [DOI] [PubMed] [Google Scholar]
- 71.Fukaya M, Kato A, Lovett C, Tonegawa S, Watanabe M. 2003. Retention of NMDA receptor NR2 subunits in the lumen of endoplasmic reticulum in targeted NR1 knockout mice. Proc. Natl Acad. Sci. USA 100, 4855–4860. ( 10.1073/pnas.0830996100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakazawa K, et al. 2002. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science 297, 211–218. ( 10.1126/science.1071795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakazawa K, Sun LD, Quirk MC, Rondi-Reig L, Wilson MA, Tonegawa S. 2003. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron 38, 305–315. ( 10.1016/S0896-6273(03)00165-X) [DOI] [PubMed] [Google Scholar]
- 74.McHugh TJ, et al. 2007. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science 317, 94–99. ( 10.1126/science.1140263) [DOI] [PubMed] [Google Scholar]
- 75.Reisel D, Bannerman DM, Schmitt WB, Deacon RM, Flint J, Borchardt T, Seeburg PH, Rawlins JN. 2002. Spatial memory dissociations in mice lacking GluR1. Nat. Neurosci. 5, 868–873. ( 10.1038/nn910) [DOI] [PubMed] [Google Scholar]
- 76.Schmitt WB, Deacon RM, Seeburg PH, Rawlins JN, Bannerman DM. 2003. A within-subjects, within-task demonstration of intact spatial reference memory and impaired spatial working memory in glutamate receptor-A-deficient mice. J. Neurosci. 23, 3953–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zamanillo D, et al. 1999. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science 284, 1805–1811. ( 10.1126/science.284.5421.1805) [DOI] [PubMed] [Google Scholar]
- 78.Schmitt WB, Sprengel R, Mack V, Draft RW, Seeburg PH, Deacon RM,, Rawlins JN, Bannerman DM. 2005. Restoration of spatial working memory by genetic rescue of GluR-A-deficient mice. Nat. Neurosci. 8, 270–272. ( 10.1038/nn1412) [DOI] [PubMed] [Google Scholar]
- 79.Sanderson DJ, Good MA, Skelton K, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM. 2009. Enhanced long-term and impaired short-term spatial memory in GluA1 AMPA receptor subunit knockout mice: evidence for a dual-process memory model. Learn. Mem. 16, 379–386. ( 10.1101/lm.1339109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sanderson DJ, Bannerman DM. 2012. The role of habituation in hippocampus-dependent spatial working memory tasks: ecvidence from GluA1 AMPA receptor subunit knockout mice. Hippocampus 22, 981–994. ( 10.1002/hipo.20896) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Niewoehner B, Single FN, Hvalby O, Jensen V, Meyer zum Alten Borgloh S, Seeburg PH, Rawlins JN, Sprengel R, Bannerman DM. 2007. Impaired spatial working memory but spared spatial reference memory following functional loss of NMDA receptors in the dentate gyrus. Eur. J. Neurosci. 25, 837–846. ( 10.1111/j.1460-9568.2007.05312.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bannerman DM, et al. 2012. Dissecting spatial knowledge from spatial choice by hippocampal NMDA receptor deletion. Nat. Neurosci. 15, 1153–1159. ( 10.1038/nn.3166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu W. 2011. PSD-95-like membrane associated guanylate kinases (PSD-MAGUKs) and synaptic plasticity. Curr. Opin. Neurobiol. 21, 306–312. ( 10.1016/j.conb.2011.03.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mansuy IM, Shenolikar S. 2006. Protein serine/threonine phosphatases in neuronal plasticity and disorders of learning and memory. Trends Neurosci. 29, 679–686. ( 10.1016/j.tins.2006.10.004) [DOI] [PubMed] [Google Scholar]
- 85.Peng S, Zhang Y, Zhang J, Wang H, Ren B. 2010. ERK in learning and memory: a review of recent research. Int. J. Mol. Sci. 11, 222–232. ( 10.3390/ijms11010222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lisman J, Yasuda R, Raghavachari S. 2012. Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 13, 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yin X, Takei Y, Kido MA, Hirokawa N. 2011. Molecular motor KIF17 is fundamental for memory and learning via differential support of synaptic NR2A/2B levels. Neuron 70, 310–325. ( 10.1016/j.neuron.2011.02.049) [DOI] [PubMed] [Google Scholar]
- 88.Yin X, Feng X, Takei Y, Hirokawa N. 2012. Regulation of NMDA receptor transport: a KIF17-cargo binding/releasing underlies synaptic plasticity and memory in vivo. J. Neurosci. 32, 5486–5499. ( 10.1523/JNEUROSCI.0718-12.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Day JJ, Sweatt JD. 2011. Epigenetic mechanisms in cognition. Neuron 70, 813–829. ( 10.1016/j.neuron.2011.05.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang W, Kwon EJ, Tsai LH. 2012. MicroRNAs in learning, memory, and neurological diseases. Learn. Mem. 19, 359–368. ( 10.1101/lm.026492.112) [DOI] [PubMed] [Google Scholar]
- 91.Loebrich S, Nedivi E. 2009. The function of activity-regulated genes in the nervous system. Physiol. Rev. 89, 1079–1103. ( 10.1152/physrev.00013.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brun VH, Ytterbo K, Morris RG, Moser MB, Moser EI. 2001. Retrograde amnesia for spatial memory induced by NMDA receptor-mediated long-term potentiation. J. Neurosci. 2, 356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Staubli U, Chun D. 1996. Factors regulating the reversibility of long-term potentiation. J. Neurosci. 16, 853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sacktor TC. 2011. How does PKM zeta maintain long-term memory? Nat. Rev. Neurosci. 12, 9–15. ( 10.1038/nrn2949) [DOI] [PubMed] [Google Scholar]
- 95.Volk LJ, Bachman JL, Johnson R, Yu Y, Huganir RL. 2013. PKM-zeta is not required for hippocampal synaptic plasticity, learning and memory. Nature 493, 420–423. ( 10.1038/nature11802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee AM, et al. 2013. Prkcz null mice show normal learning and memory. Nature 493, 416–419. ( 10.1038/nature11803) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hernández AI, Oxberry WC, Crary JF, Mirra SS, Sacktor TC. 2014. Cellular and subcellular localization of PKMζ. Phil. Trans. R. Soc. B 369, 20130140 ( 10.1098/rstb.2013.0140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hardt O, Migues PV, Hastings M, Wong J, Nader K. 2010. PKMzeta maintains 1-day- and 6-day-old long-term object location but not object identity memory in dorsal hippocampus. Hippocampus 20, 691–695. [DOI] [PubMed] [Google Scholar]
- 99.Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, Nader K. 2010. PKMzeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat. Neurosci. 13, 630–634. ( 10.1038/nn.2531) [DOI] [PubMed] [Google Scholar]
- 100.Redondo RL, Morris RG. 2011. Making memories last: the synaptic tagging and capture hypothesis. Nat. Rev. Neurosci. 12, 17–30. ( 10.1038/nrn2963) [DOI] [PubMed] [Google Scholar]
- 101.Sanhueza M, Fernandez-Villalobos G, Stein IS, Kasumova G, Zhang P, Bayer KU,, Otmakhov N, Hell JW, LIsman J. 2011. Role of the CaMKII/NMDA receptor complex in the maintenance of synaptic strength. J. Neurosci. 31, 9170–9178. ( 10.1523/JNEUROSCI.1250-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Halt AR, et al. 2012. CaMKII binding to GluN2B is critical during memory consolidation. EMBO J. 31, 1203–1216. ( 10.1038/emboj.2011.482) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Johansen JP, Hamanaka H, Monfils MH, Behnia R, Deisseroth K, Blair HT, LeDoux JE. 2010. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proc. Natl Acad. Sci. USA 107, 12 692–12 697. ( 10.1073/pnas.1002418107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S. 2012. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, U381–U415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Garner AR, Rowland DC, Hwang SY, Baumgaertel K, Roth BL, Kentros C, Mayford M. 2012. Generation of a synthetic memory trace. Science 335, 1513–1516. ( 10.1126/science.1214985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Neves G, Cooke SF, Bliss TV. 2008. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat. Rev. Neurosci. 9, 65–75. ( 10.1038/nrn2303) [DOI] [PubMed] [Google Scholar]
- 107.Yang SN, Tang YG, Zucker RS. 1999. Selective induction of LTP and LTD by postsynaptic [Ca2+]i elevation. J. Neurophysiol. 81, 781–787. [DOI] [PubMed] [Google Scholar]
- 108.Schneider F, Gradmann D, Hegemann P. 2013. Ion selectivity and competition in channelrhodopsins. Biophys. J. 105, 91–100. ( 10.1016/j.bpj.2013.05.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Frey U, Morris RG. 1997. Synaptic tagging and long-term potentiation. Nature 385, 533–536. ( 10.1038/385533a0) [DOI] [PubMed] [Google Scholar]
- 110.Frey U, Morris RG. 1998. Synaptic tagging: implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci. 21, 181–188. ( 10.1016/S0166-2236(97)01189-2) [DOI] [PubMed] [Google Scholar]
- 111.Okada D, Ozawa F, Inokuchi K. 2009. Input-specific spine entry of soma-derived Vesl-1S protein conforms to synaptic tagging. Science 324, 904–909. ( 10.1126/science.1171498) [DOI] [PubMed] [Google Scholar]
- 112.Okuno H, et al. 2012. Inverse synaptic tagging of inactive synapses via dynamic interaction of Arc/Arg3.1 with CaMKIIbeta. Cell 149, 886–898. ( 10.1016/j.cell.2012.02.062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.MacGurn JA, Hsu PC, Emr SD. 2012. Ubiquitin and membrane protein turnover: from cradle to grave. Annu. Rev. Biochem. 81, 231–259. ( 10.1146/annurev-biochem-060210-093619) [DOI] [PubMed] [Google Scholar]
- 114.Abraham WC, Logan B, Greenwood JM, Dragunow M. 2002. Induction and experience-dependent consolidation of stable long-term potentiation lasting months in the hippocampus. J. Neurosci. 22, 9626–9634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Barnes CA. 1983. The physiology of the senescent hippocampus. In Neurobiology of the hippocampus (ed. Siefert W.), pp. 87–108. London, UK: Academic Press. [Google Scholar]
- 116.Cooke SF, Bear MF. 2010. Visual experience induces long-term potentiation in the primary visual cortex. J. Neurosci. 30, 16 304–16 313. ( 10.1523/JNEUROSCI.4333-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Canals S, Beyerlein M, Merkle H, Logothetis NK. 2009. Functional MRI evidence for LTP-induced neural network reorganization. Curr. Biol. 19, 398–403. ( 10.1016/j.cub.2009.01.037) [DOI] [PubMed] [Google Scholar]
- 118.Álvarez-Salvado E, Pallarés V, Moreno A, Canals S. 2014. Functional MRI of long-term potentiation: imaging network plasticity. Phil. Trans. R. Soc. B 369, 20130152 ( 10.1098/rstb.2013.0152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Frey U, Matthies H, Reymann KG. 1991. The effect of dopaminergic D1 receptor blockade during tetanization on the expression of long-term potentiation in the rat CA1 region in vitro. Neurosci. Lett. 129, 111–114. ( 10.1016/0304-3940(91)90732-9) [DOI] [PubMed] [Google Scholar]
- 120.Swanson-Park JL, Coussens CM, Mason-Parker SE, Raymond CR, Hargreaves EL, Dragunow M, Cohen AS, Abraham WC. 1999. A double dissociation within the hippocampus of dopamine D1/D5 receptor and beta-adrenergic receptor contributions to the persistence of long-term potentiation. Neuroscience 92, 485–497. ( 10.1016/S0306-4522(99)00010-X) [DOI] [PubMed] [Google Scholar]
- 121.Lemon N, Manahan-Vaughan D. 2006. Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. J. Neurosci. 26, 7723–7729. ( 10.1523/JNEUROSCI.1454-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.O'Carroll CM, Morris RG. 2004. Heterosynaptic co-activation of glutamatergic and dopaminergic afferents is required to induce persistent long-term potentiation. Neuropharmacology 47, 324–332. ( 10.1016/j.neuropharm.2004.04.005) [DOI] [PubMed] [Google Scholar]
- 123.O'Carroll CM, Martin SJ, Sandin J, Frenguelli B, Morris RG. 2006. Dopaminergic modulation of the persistence of one-trial hippocampus-dependent memory. Learn. Mem. 13, 760–769. ( 10.1101/lm.321006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rossato JI, Bevilaqua LR, Izquierdo I, Medina JH, Cammarota M. 2009. Dopamine controls persistence of long-term memory storage. Science 325, 1017–1020. ( 10.1126/science.1172545) [DOI] [PubMed] [Google Scholar]
- 125.Bethus I, Tse D, Morris RG. 2010. Dopamine and memory: modulation of the persistence of memory for novel hippocampal NMDA receptor-dependent paired associates. J. Neurosci. 30, 1610–1618. ( 10.1523/JNEUROSCI.2721-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smith WB, Starck SR, Roberts RW, Schuman EM. 2005. Dopaminergic stimulation of local protein synthesis enhances surface expression of GluR1 and synaptic transmission in hippocampal neurons. Neuron 45, 765–779. ( 10.1016/j.neuron.2005.01.015) [DOI] [PubMed] [Google Scholar]
- 127.Granado N, Ortiz O, Suarez LM, Martin ED, Cena V, Solis JM, Moratalla R. 2008. D1 but not D5 dopamine receptors are critical for LTP, spatial learning, and LTP-Induced arc and zif268 expression in the hippocampus. Cereb. Cortex 18, 1–12. ( 10.1093/cercor/bhm026) [DOI] [PubMed] [Google Scholar]
- 128.Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. 1997. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 88, 615–626. ( 10.1016/S0092-8674(00)81904-2) [DOI] [PubMed] [Google Scholar]
- 129.Barco A, Alarcon JM, Kandel ER. 2002. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell 108, 689–703. ( 10.1016/S0092-8674(02)00657-8) [DOI] [PubMed] [Google Scholar]
- 130.Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. 2004. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell 116, 467–479. ( 10.1016/S0092-8674(04)00115-1) [DOI] [PubMed] [Google Scholar]
- 131.Redondo RL, Okuno H, Spooner PA, Frenguelli BG, Bito H, Morris RG. 2010. Synaptic tagging and capture: differential role of distinct calcium/calmodulin kinases in protein synthesis-dependent long-term potentiation. J. Neurosci. 30, 4981–4989. ( 10.1523/JNEUROSCI.3140-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gasbarri A, Packard MG, Campana E, Pacitti C. 1994. Anterograde and retrograde tracing of projections from the ventral tegmental area to the hippocampal formation in the rat. Brain Res. Bull. 33, 445–452. ( 10.1016/0361-9230(94)90288-7) [DOI] [PubMed] [Google Scholar]
- 133.Gasbarri A, Sulli A, Packard MG. 1997. The dopaminergic mesencephalic projections to the hippocampal formation in the rat. Prog. Neuropsychopharmacol. Biol. Psychiatry 21, 1–22. ( 10.1016/S0278-5846(96)00157-1) [DOI] [PubMed] [Google Scholar]
- 134.Lisman JE, Grace AA. 2005. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron 46, 703–713. ( 10.1016/j.neuron.2005.05.002) [DOI] [PubMed] [Google Scholar]
- 135.Lisman J, Grace AA, Duzel E. 2011. A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends Neurosci. 34, 536–547. ( 10.1016/j.tins.2011.07.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Smith CC, Greene RW. 2012. CNS dopamine transmission mediated by noradrenergic innervation. J. Neurosci. 32, 6072–6080. ( 10.1523/JNEUROSCI.6486-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. 1999. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat. Neurosci. 2, 1120–1124. ( 10.1038/16046) [DOI] [PubMed] [Google Scholar]
- 138.Vazdarjanova A, Guzowski JF. 2004. Differences in hippocampal neuronal population responses to modifications of an environmental context: evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. J. Neurosci. 24, 6489–6496. ( 10.1523/JNEUROSCI.0350-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Morris RG. 2006. Elements of a neurobiological theory of hippocampal function: the role of synaptic plasticity, synaptic tagging and schemas. Eur. J. Neurosci. 23, 2829–2846. ( 10.1111/j.1460-9568.2006.04888.x) [DOI] [PubMed] [Google Scholar]
- 140.Moncada D, Viola H. 2007. Induction of long-term memory by exposure to novelty requires protein synthesis: evidence for a behavioral tagging. J. Neurosci. 27, 7476–7481. ( 10.1523/JNEUROSCI.1083-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Moncada D, Ballarini F, Martinez MC, Frey JU, Viola H. 2011. Identification of transmitter systems and learning tag molecules involved in behavioral tagging during memory formation. Proc. Natl Acad. Sci. USA 108, 12 931–12 936. ( 10.1073/pnas.1104495108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dong Z, Gong B, Li H, Bai Y, Wu X, Huang Y, He W, Li T, Wang YT. 2012. Mechanisms of hippocampal long-term depression are required for memory enhancement by novelty exploration. J. Neurosci. 32, 11 980–11 990. ( 10.1523/JNEUROSCI.0984-12.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Merhav M, Rosenblum K. 2008. Facilitation of taste memory acquisition by experiencing previous novel taste is protein-synthesis dependent. Learn. Mem. 15, 501–507. ( 10.1101/lm.986008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ballarini F, Moncada D, Martinez MC, Alen N, Viola H. 2009. Behavioral tagging is a general mechanism of long-term memory formation. Proc. Natl Acad. Sci. USA 106, 14 599–14 604. ( 10.1073/pnas.0907078106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lindeberg J, Usoskin D, Bengtsson H, Gustafsson A, Kylberg A, Soderstrom S, Ebendal T. 2004. Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis 40, 67–73. ( 10.1002/gene.20065) [DOI] [PubMed] [Google Scholar]