Abstract
Many studies have demonstrated an association between diffuse bilateral testicular microlithiasis (TM) and gonadal and extragonadal germ cell tumors. Nevertheless, it is still uncertain whether ultrasound surveillance is really necessary in patients with TM in the absence of other risk factors such as previous testicular cancer, a history of cryptorchidism or testicular atrophy. We report the cases of a 33- and a 39-year-old man presenting with a retroperitoneal extragonadal tumor. The first patient underwent an MRI examination in order to rule out a lumbosacral hernia: MRI images showed no slipped disks but a voluminous retroperitoneal solid mass. The histological analysis revealed an immature teratoma. The second patient came to the emergency department complaining of abdominal pain, vomiting, weight loss and mild jaundice: ultrasound examination showed a large, ill-defined heterogeneous abdominal mass, confirmed by CT and MRI examination. The histology diagnosed a yolk sac tumor. In both patients, the testicular sonography was performed to rule out a focal lesion, but it displayed bilateral TM without a focal testicular mass. Based on our direct experience, we highlight the importance of annual ultrasonographic surveillance of the testis and the retroperitoneal space in patients with occasionally detected TM.
Key Words: Testicular microlithiasis, Germ cell tumors, Testicular ultrasound
Introduction
Primary retroperitoneal germ cell tumors account for approximately 30% of extragonadal germ cell tumors (EGCTs) and for about 10% of all primary malignant retroperitoneal tumors [1]. Many studies have demonstrated an association between diffuse bilateral testicular microlithiasis (TM) and gonadal germ cell tumors and EGCTs [2, 3]. Nevertheless, it is still uncertain whether ultrasound surveillance is really necessary in patients with TM in the absence of other risk factors such as previous testicular cancer, a history of cryptorchidism or testicular atrophy [4]. We report the cases of a 33- and a 39-year-old man presenting with a retroperitoneal extragonadal tumor and bilateral TM without a focal testicular mass.
Case 1
A 39-year-old man with a 6-month history of lumbar pain came to our hospital to perform an MRI examination in order to rule out a lumbosacral hernia. The MRI images showed no slipped disks, but we unfortunately detected a voluminous retroperitoneal solid mass. Therefore, we decided to perform a total body CT to better characterize the mass and its relationship to adjacent structures. CT images showed a large heterogeneous retroperitoneal mass with curvilinear calcifications and a marked inhomogeneous enhancement after intravenous contrast medium injection due to the presence of necrotic-colliquative areas. This lesion displaced the left renal vein cranially, the abdominal aorta anteriorly and towards the right, and infiltrated the inferior vena cava, the left renal vein, and the left psoas muscle (fig. 1).
Fig. 1.
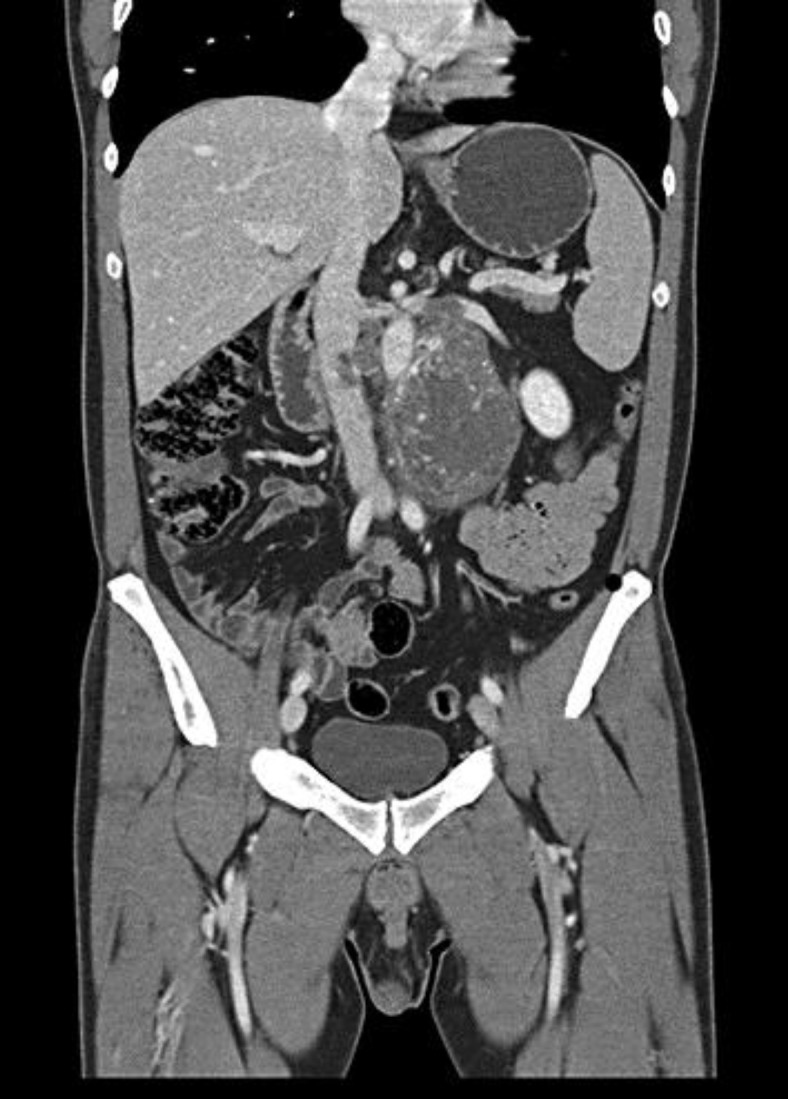
Coronal CT image shows a retroperitoneal heterogeneous mass infiltrating the inferior vena cava and the left renal vein.
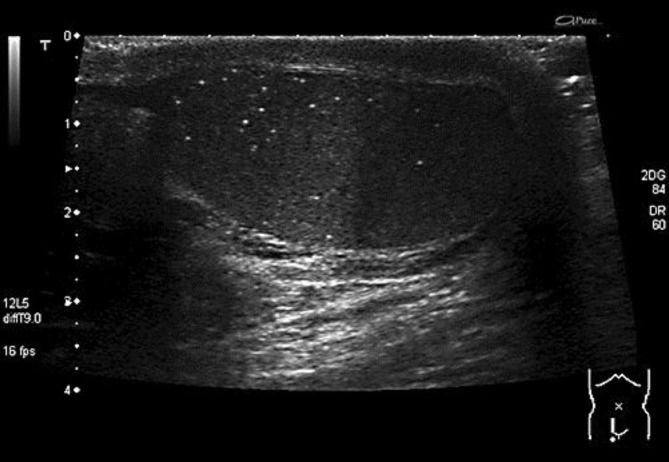
The patient's α-fetoprotein, lactate dehydrogenase, and beta subunit of human chorionic gonadotropin levels were 2.680 IU/l (normal, 90–180 IU/l), 279 ng/ml (normal, 0–7.5 ng/ml), and 4 mIU/ml (normal, <5 mIU/ml). We performed a scrotal ultrasonography (US) to rule out that this mass was a retroperitoneal metastasis of a primary testicular tumor: US showed bilateral classic TM (defined as more than 5 calcifications scattered throughout the testicle), without a focal lesion (fig. 2). Comparing the current ultrasound images with previous US testicular images (the patient underwent a scrotal US when he was 25 years old because of a testicular trauma), we noticed that microcalcification patterns were very similar.
Fig. 2.
Ultrasound longitudinal image shows classic TM of the right testis without focal lesions.
The patient underwent a CT-guided biopsy and at histology, an immature teratoma was diagnosed.
Case 2
A 33-year-old man came to our emergency department complaining of abdominal pain, vomiting, weight loss and mild jaundice. Ultrasound examination detected a large, ill-defined heterogeneous abdominal mass.
The patient's serum α-fetoprotein, lactate dehydrogenase, and beta subunit of human chorionic gonadotropin levels were 2.470 IU/l (normal, 90–180 IU/l), 232 ng/ml (normal, 0–7.5 ng/ml) and 3 mIU/ml (normal, <5 mIU/ml). Besides, the serum markers of cholestasis were high: conjugated bilirubin was 2 mg/100 ml (normal, <0.2 mg/100 ml), γ-glutamyl transpeptidase 70 IU/l (normal, 1–30 IU/l) and alkaline phosphates 300 IU/l (normal, <170 IU/l).
CT and MRI examinations showed a giant retroperitoneal mass made up by multiple necrotic-colliquative fluid areas with a multilocular aspect, which dislocated the inferior vena cava anteriorly and with possible infiltrating signs; it also compressed the portal vein and the common bile duct with moderate dilatation of the intrahepatic ducts (fig. 3).
Fig. 3.
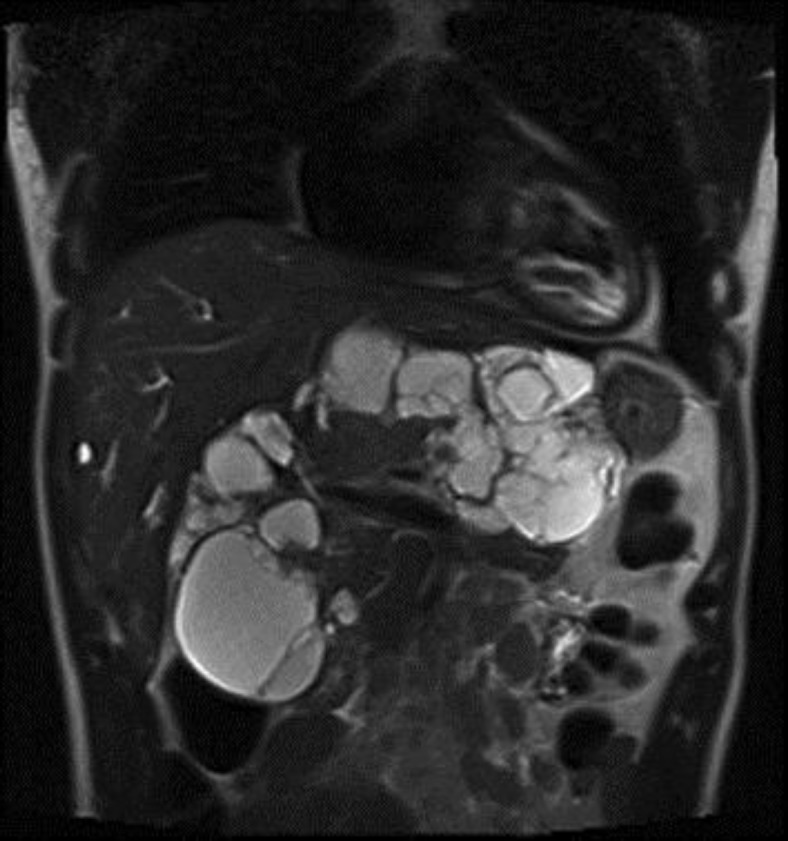
Coronal T2-weighted MR image shows a large retroperitoneal mass composed by multiple necrotic-colliquative fluid areas with a multilocular appearance.
The patient was sent to do an US to rule out the presence of a primary testicular tumor, which revealed bilateral TM without a focal hypoechoic lesion; the microcalcification pattern was quite similar to that of a past ultrasound exam that was performed when the patient was 22 years old because of a suspected varicocele.
The patient underwent a CT-guided biopsy and at histology, a yolk sac tumor was diagnosed.
Discussion
EGCTs account for 1–5% of all germ cell tumors [1]. An EGCT is by definition a germ cell neoplasm, displaying one of the histologic types associated with gonadal origin, but located outside the gonads [5]. The most widely accepted theory suggests that EGCTs arise from primordial germ cells misplaced during their migration to the gonads [1]. It remains uncertain, however, whether such tumors develop primarily at extragonadal sites or represent metastases of a primary testicular tumor [2].
Regarding the latter case, EGCT may have developed from ‘burned out testicular tumors’ or they may just be metastatic lesions from primary testicular tumors that were not detected at the time of the diagnosis [6]. A burned out gonadal primary tumor is a regressed tumor which is seen as an echogenic scar or a hypoechoic tissue on testicular ultrasound and which clinically presents with metastasis.
Histologically, EGCTs comprise seminomas (30–40%) and nonseminomatous tumors (60–70%) in men and dysgerminomas and nondysgerminomas in women. Nonseminomatous germ cell tumors (NSGCTs) include teratoma, embryonal carcinoma, endodermal sinus tumor (yolk sac tumor), choriocarcinoma, and tumors with mixed histology. NSGCTs usually have a more aggressive course than seminomatous tumors [1]. Extragonadal nonseminomatous tumors present with an elevated α-fetoprotein or human chorionic gonadotropin level [5]. α-Fetoprotein is produced by endodermal sinus tumors, either alone or in association with other types of germ cell tumors. Human chorionic gonadotropin is only produced by syncytiotrophoblasts occurring as a component of choriocarcinoma. These are useful serum markers in the diagnosis, prognosis, and follow-up of patients with germ cell tumors [5].
The majority of the EGCTs occur in men, except benign mature teratoma, which occurs with equal frequency in men and women. EGCTs are usually seen in children or young adults, and typically arise in midline locations. In adults, the most common sites of primary EGCTs are, in descending order, the mediastinum, the retroperitoneum and the cranium. In children, the cranium and sacrococcygeal region are the common sites [1].
Primary retroperitoneal germ cell tumors account for about 10% of all primary malignant retroperitoneal tumors and about 30–40% of EGCTs[1]. EGCTs are often seen in or near the midline, especially between the T6 and S2 vertebrae. A midline mass is more suggestive of a primary EGCT than of metastasis [7]. These tumors are usually large at presentation. Encasement, displacement, and compression of the abdominal vessels are common. Patients may present with metastases: brain, liver, lungs and bones are the common sites of metastases [1].
The radiologic findings for primary EGCTs are nonspecific. The imaging appearances are similar to those of gonadal germ cell tumors. Seminoma is rare in the retroperitoneum and is seen as a large, lobulated, well-defined homogeneous solid mass with fibrous septa and ring-like or speckled calcifications. NSGCTs are depicted as heterogeneous tumors with areas of hemorrhage, necrosis, and heterogeneous enhancement. Flow voids as well as invasion of adjacent structures that are due to hypervascularity may be seen[7].
Primary testicular malignancy and EGCTs are often associated with TM [2, 8]. This is an uncommon pathologic condition that is detected by scrotal US and is defined as the presence within the substance of the testis of 5 or more speckled bright foci, 1–2 mm in diameter, with little or no acoustic shadowing; the microcalcifications usually affect both testes, but may be unilateral and can be focal or diffuse [3, 4].
Conclusion
J. Richenberg and N. Brejt affirm that ultrasound surveillance is unlikely to benefit patients with TM in the absence of other risk factors; on the contrary, in the presence of additional risk factors (previous testicular cancer, a history of maldescent or testicular atrophy), patients are likely to be under clinical and ultrasound surveillance [4].
Nevertheless, it is still controversial whether performing sonographic surveillance is better than regular testicular self-examination in adult patients with classic TM and the absence of any known testicular tumor.
In this setting, on the basis of our direct experience, we highlight the importance of annual ultrasonographic surveillance of the testis and retroperitoneal space in patients with occasionally detected TM. Besides, taking into account the increased risk of metachronous testicular malignancy in patients with previous EGCT [6, 9], we recommend yearly testicular ultrasound follow-up after surgical removal of retroperitoneal gonadic tumor.
Disclosure Statement
The authors declare that there are no conflicts of interest.
References
- 1.Shinagare AB, Jagannathan JP, Ramaiya NH, Hall MN, Van den Abbeele AD. Adult extragonadal germ cell tumors. AJR Am J Roentgenol. 2010;195:W274–W280. doi: 10.2214/AJR.09.4103. [DOI] [PubMed] [Google Scholar]
- 2.Scholz M, Zehender M, Thalmann GN, Borner M, Thöni H, Studer UE. Extragonadal retroperitoneal germ cell tumor: evidence of origin in the testis. Ann Oncol. 2002;13:121–124. doi: 10.1093/annonc/mdf003. [DOI] [PubMed] [Google Scholar]
- 3.Yee WS, Kim YS, Kim SJ, Choi JB, Kim SI, Ahn HS. Testicular microlithiasis: prevalence and clinical significance in a population referred for scrotal ultrasonography. Korean J Urol. 2011;52:172–177. doi: 10.4111/kju.2011.52.3.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richenberg J, Brejt N. Testicular microlithiasis: is there a need for surveillance in the absence of other risk factors? Eur Radiol. 2012;22:2540–2546. doi: 10.1007/s00330-012-2520-4. [DOI] [PubMed] [Google Scholar]
- 5.Meyer MA, Gilbertson-Dahdal DL. Retroperitoneal extragonadal endodermal sinus tumor with bilateral diffuse classic testicular microlithiasis. J Ultrasound Med. 2010;29:1843–1847. doi: 10.7863/jum.2010.29.12.1843. [DOI] [PubMed] [Google Scholar]
- 6.Yamada Y, Tomita K, Fujimura T, Nishimatsu H, Takeuchi T, Kitamura T. Metachronous testicular tumor developing eight years after retroperitoneal extragonadal germ cell tumor. Int J Urol. 2008;15:267–269. doi: 10.1111/j.1442-2042.2007.01971.x. [DOI] [PubMed] [Google Scholar]
- 7.Rajiah P, Sinha R, Cuevas C, Dubinsky TJ, Bush WH, Jr, Kolokythas O. Imaging of uncommon retroperitoneal masses. Radiographics. 2011;31:949–976. doi: 10.1148/rg.314095132. [DOI] [PubMed] [Google Scholar]
- 8.Comiter CV, Renshaw AA, Benson CB, Loughlin KR. Burned-out primary testicular cancer: sonographic and pathological characteristics. J Urol. 1996;156:85–88. [PubMed] [Google Scholar]
- 9.Ando R, Yasui T, Tozawa K, Sasaki S, Hayashi Y, Kohri K. Testicular seminoma occurring 8 years after treatment of a metastatic extragonadal germ cell tumor. Int J Urol. 2007;14:85–86. doi: 10.1111/j.1442-2042.2006.01544.x. [DOI] [PubMed] [Google Scholar]