Abstract
SU-8 negative photoresist is a high tensile strength polymer that has been used for a number of biomedical applications that include cell encapsulation and neuronal probes. Chemically, SU-8 comprises, among other components, an epoxy based monomer and antimony salts, the latter being a potential source of cytotoxicity. We report on the in vitro and in vivo evaluation of SU-8 biocompatibility based on leachates from various solvents, at varying temperature and pH, and upon subcutaneous implantation of SU-8 substrates in mice. MTT cell viability assay did not exhibit any cytotoxic effects from the leachates. The hemolytic activity of SU-8 is comparable to that of FDA approved implant materials such as silicone elastomer, Buna-S and medical steel. In vivo histocompatibility study in mice indicates a muted immune response to subcutaneous SU-8 implants.
Keywords: SU-8, cytotoxicity, biocompatibility, histocompatibility
1. Introduction
The encapsulation and subsequent implantation of biotherapeutic molecules or cells may be valuable in the clinical management of diseases because it can protect the therapeutic cells or molecules from immunorejection [1, 2]. The long term viability of these grafts is determined by the biocompatibility of the encapsulation material. Adverse reaction with host tissue, resulting in fibrous tissue engulfment of the implant, can lead to loss of graft function [3]. Hydrogel microbeads have been widely used for immunoprotective encapsulation [4-6]. Synthetic biocompatible polymers such as polyethylene glycol (PEG), 2-hydroxyethyl methacrylate (HEMA), polylactic acid (PLA), polylactic-co-glycolic acid (PLGA) and polyvinyl alcohol have also been used as encapsulation materials [7]. However, these polymers have limited utility owing to their imprecise porosity that compromises long-term impenetrability to immune molecules [8, 9]. Alternately, encapsulation can also be carried out by engineering microcapsules with precisely defined nanopores or channels on the surfaces. We have reported on the fabrication of such nanoporous microcapsules using SU-8, an epoxy based polymer [10-13]. SU-8 has been used in the fabrication of microelectrodes for measuring electrical impedance in living tissues [14], monitoring neural spikes [15-18] and for intraocular pressure sensing [19]. The biocompatibility of SU-8 as a potential neural probe material was evaluated based on cytotoxicity assays using C6 rat astrocytes [20]. The mechanical strength, biocompatibility and biostability of SU-8 are favorable for the fabrication of long term implants such as neuronal probes. Balciunas et al [21] evaluated the biocompatibility of SU-8 for lab-on-chip applications and established cell viability on SU-8 surfaces using rabbit muscle stem cells. SU-8 based microwells integrated with SH-SY5Y human neuroblastoma cells have been used as biosensors [22]. The effect of SU-8 based implantable neural probes on the viability of three types of cells; human skin fibroblast cells, Schwann cells and explanted neurons from dorsal root ganglia was evaluated by Cho et al [15]. Others have shown that the antimony salts present in SU-8 do not effect the proliferation of mouse embryonic stem cells and human induced pluripotent stem cells on matrigel coated SU-8 surfaces [23].
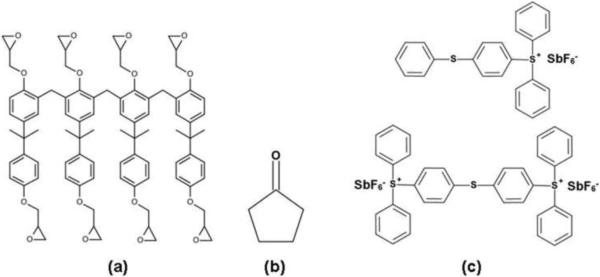
Commercial SU-8 formulations comprise a monomer, containing 8 epoxy moieties (Fig. 1a), a solvent such as cyclopentanone (Fig. 1b) and a photoacid initiator such as triarylsulfonium hexafluoroantimonate (Fig 1c). On exposure to UV radiation, a photoacid is produced that protonates the epoxy moieties, which then react with neutral epoxy groups on heating, resulting in a cross-linked polymer network of high mechanical strength and thermal stability. Process parameters, such as UV dose and baking time, determine the extent of residual epoxy groups and antimony salts [24, 25]. The unreacted photoacid generator is retained within the polymer matrix. Hence, it is essential to evaluate the potential toxicity of leachates from SU-8 under the influence of storage conditions such as temperature, pH and buffer composition. The biocompatibility of SU-8 surfaces has been evaluated using in vitro cell culture models [15, 26, 27]. We have earlier reported on cell viability and function in microcapsules fabricated from SU-8 [11]. However, in addition to the established non-cytotoxic nature of SU-8, a detailed evaluation of SU-8 biocompatibility is essential for potential application as a substrate for long term encapsulation of cellular payload. Here, we present a detailed evaluation of the biocompatibility of SU-8, including cytotoxicity and tissue interaction with implanted substrates, based on the ISO 10993 recommendations for the biological evaluation of medical devices and biomaterials. In vivo studies were performed over a period of 8 weeks following subcutaneous implantation of SU-8 pads in mice.
Fig. 1.
Chemical composition of SU-8 photoresist. SU-8 2075 comprises SU-8 monomer (a), a solvent, cyclopentanone (b) and a photoacid initiator, mixed triarylsulfonium hexafluoroantimonate salts (c).
2. Materials and Methods
2.1 Materials
SU-8, SU-8 developer, Omnicoat and PG remover were procured from Microchem Inc. (www.microchem.com). Dulbecco's modified eagle medium (DMEM) cell culture medium, trypan blue and dimethylthiazol diphenyltetrazolium (MTT) were procured from HiMedia (us.vwr.com). Hemolysis assay kit was procured from Haemoscan Inc. (www.haemoscan.com).
2.2 Cell culture
9L glioma cells isolated from rat brain tumors (www.atcc.org) were used for cytotoxicity studies. The cells were cultured in DMEM enriched with 10% fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 μg/mL). Cells were incubated at 37 °C in 5% CO2 atmosphere. Cell numbers were determined using a hemocytometer following trypan blue staining.
2.3 Animals
BALB/c mice, aged 8 weeks, were used for in vivo implantation studies and were procured from Charles River (www.criver.com). All animal procedures were carried out in accordance with Institutional Animal Care and Use Committee (IACUC) policies. Biocompatibility studies were designed based on the ISO 10993 recommendations for the evaluation of medical implants.
2.4 Fabrication of SU-8 substrates
SU-8 pads (2.5 mm × 2.5 mm × 200 μm) were fabricated on a silicon wafer as reported earlier [28]. Omnicoat was spun on a silicon wafer at 3000 rpm and baked at 200 °C for 2 min. On this layer, SU-8 2075 was spun at 900 rpm and pre-baked at 65 °C for 10 min followed by 95 °C for 40 min. 2.5 mm × 2.5 mm squares were patterned on the SU-8 layer using a photomask, followed by UV exposure (400 mJ/cm2). The patterned pads were then baked at 65 °C for 10 min and 95 °C for 20 min. The wafers were then cooled to room temperature and developed using SU-8 developer solution. The pads were then hard baked at 150 °C for 15 min and released from the wafer using PG remover to dissolve the Omnicoat layer and release the SU-8 pads. Released SU-8 pads were sequentially washed in isopropanol, water and dried under a stream of nitrogen. The pads were steam sterilized before use.
2.5 Evaluation of residual antimony in leachates
SU-8 formulations contain antimony (Sb) salts as photocatalysts, whose possible leaching from SU-8 pads in various solutions was evaluated. SU-8 pads were incubated in phosphate buffered saline (PBS), isopropanol, vegetable oil and phosphate buffers at different pH. Sterile SU-8 pads (60 mg, i.e. approx. 60 pads) were transferred to clean glass vials. Vials were filled with 2 ml of PBS (4 temperatures: 4° C, 37° C, 43° C and room temperature), isopropanol (room temperature), vegetable oil (room temperature) and incubated for 72 h. The effect of pH on Sb leaching was similarly evaluated following incubation in PBS at room temperature (pH 5.5 and pH 6.51). Residual Sb in the extracts was analyzed by inductively coupled plasma mass spectrometry (ICP-MS) using an Agilent 7500cx instrument (www.chem.agilent.com).
2.6 Agar overlay assay
The effect of soluble leachates on cell viability was evaluated using an agar diffusion assay [29]. 9L rat glioma cells were seeded in 100 cc culture dishes (105 cells/dish) and allowed to adhere overnight. The medium was removed and the adherent cells coated with a layer of 2% agarose-DMEM medium (1:1). The agar layer was allowed to gel and SU-8 substrates placed on the surface, followed by 72 h incubation. Then, the cells were stained with crystal violet (1% solution) and the area under the pads was observed under an optical microscope.
2.7 Cytotoxicity of SU-8 leachates
The cytotoxic effect of leachates from SU-8 was evaluated by MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay [30]. Extracts from SU-8 samples, incubated in PBS for 72 h, were used for the study. 9L rat glioma cells were seeded in a 96 well plate at a density of 2500 cells/well. Cells were incubated overnight in DMEM medium to facilitate adhesion to the plates. The culture medium was replaced with fresh medium supplemented with various concentrations of the SU-8 extract (0, 2.5, 5 and 10 % by volume; each n = 6). The total volume of the additive was maintained constant by the addition of PBS. Cells were incubated at 37 °C in 5 % CO2, 95 % relative humidity for 72 h. Post-incubation, the medium was drained and MTT solution (5 mg/ml, 20 μl) was added to each well and cells were further incubated at 37 °C for 4 h. Subsequently, the MTT solution was drained from the plate and 200 μl of DMSO was added to each well to dissolve the formazan crystals. The density of viable cells was then determined by measuring the optical density at 570 nm. Background correction was performed by measuring the optical density at 670 nm.
2.8 Hemolysis assay
The hemolytic activity of SU-8 substrates was evaluated using a commercial hemolysis assay kit, following the manufacturer's protocol. Briefly, the red blood cells (RBC) supplied with the kit were resuspended in 5ml of the requisite dilution buffer. Four SU-8 pads were placed in a sterile tube. Similarly, the three polymer reference substrates (silicone elastomer, Buna-S and medical steel) provided with the kit were also placed in separate tubes. Then, 0.5 ml of the RBC suspension was added to each tube. Tubes containing the RBC suspension alone served as negative control. Total hemoglobin was determined by the addition of lysis buffer. The tubes were then incubated overnight at 37 °C with end-to-end rotation. Released hemoglobin concentration was measured spectrophotometrically using the equation
where AHb is the absorbance of hemoglobin, A450, A415 and A380 are the absorbances at 450, 410 and 380 nm. Hemoglobin content was quantified against a standard curve generated using the hemoglobin standard provided.
2.9 Evaluation of in vivo biocompatibility in mice
Eight-week-old male BALB/c mice (n=24) were used for the study. The animals were randomly divided into 4 groups (n = 6) corresponding to time points of 1, 2, 4 and 8 weeks at which the animals were euthanized for analysis. Animals were monitored for distress during the course of the study. Implantation procedures were carried out under aseptic conditions. National Institute of Health (NIH) guidelines for the care and use of laboratory animals were observed. Prior to implantation, mice were anesthetized using an IP injection of ketamine (80 mg/kg)-xylazine (10 mg/kg). An incision of approximately 5 mm was made on both the right and left flanks and a subcutaneous pocket formed. Two SU-8 pads were placed in the right flank and the incision closed with sutures. The left flank, without any implants served as control. At the end of 1, 2, 4 and 8 weeks, mice were euthanized by carbon dioxide inhalation and tissue surrounding the implant site was excised. The excised tissue was fixed in buffered formalin for 24 h, dehydrated and embedded in paraffin wax. Tissue sections were mounted on glass slides and stained with hematoxylin and eosin (H&E) for histopathological evaluation.
A 1.0 mm linear segment of the best oriented interface between SU-8 and surrounding tissue was identified and the following parameters were evaluated; a) number of eosinophils and neutrophils, b) fibrotic capsule thickness, c) number of mast cells and d) percent of SU-8 interface occupied by multinucleated giant cells (MNGC).
2.10 Statistics
Exact Wilcoxon Rank Sum Tests were used to determine statistical differences, with p < =0.05 considered statistically significant (Origin Lab, Version 8.6, http://www.originlab.com).
3.0 Results and Discussion
3.1 Antimony leaching from SU-8
The effect of various solvents and buffers on Sb leaching from the SU-8 substrates was evaluated by ICP-MS. Table 1 shows that, at room temperature, maximum leaching of Sb occurs at pH 5.5 and in isopropanol. Reduced leaching was observed in vegetable oil (hydrophobic) and PBS (hydrophilic). Sb leaching in PBS was the highest at 43 °C and was comparable to the values observed in isopropanol. Enhanced Sb leaching was observed at acidic pH (pH 5.5) as compared to near neutral pH. It has been reported that exposure to an acidic environment results in the etching of SU-8 surfaces [31]. The presence of trace levels of residual antimony, on the surface of processed SU-8 polymers has been reported [32]. Surface treatments including oxygen plasma treatment, which etch the surface of the polymer lead to the migration of antimony from the inside to the surface of the polymer.
Table 1.
Inductively coupled plasma mass spectrometry (ICP-MS) analysis of antimony in SU-8 leachates. SU-8 substrates (60 mg) were incubated in 2 ml of each of the solvents and incubated for 72 h prior to analysis.
| S. No. | Sample | Sb (ppb) |
|---|---|---|
| 1 | PBS 4 °C | 7.5 |
| 2 | PBS (room temp) | 1.60 |
| 3 | PBS 37 °C | 3.5 |
| 4 | PBS 43 °C | 23.39 |
| 5. | Isopropanol | 21.2 |
| 6. | Vegetable oil* | 1.0 |
| 7. | Phosphate buffer; pH 5.50 | 23.4 |
| 8. | Phosphate buffer; pH 6.51 | 2.6 |
Leachate from vegetable oil was diluted in isopropanol (1:10).
The cytotoxic and genotoxic effects of Sb compounds have been widely studied. It has been reported, that Sb(V) compounds are less toxic than Sb(III) [33]. Soluble forms of Sb(V) are used in the treatment of leishmaniasis [34]. Sb(V) can be reduced to the toxic ionic form Sb(III) under acidic conditions [35]. However, under normal physiological conditions, Sb(V) is reported to be non-toxic in humans [36]. The abundance of Sb in natural waters is < 5 ppb [36]. The United States Environmental Protection Agency (USEPA) recommends a daily limit of 6 ppb in drinking water [37]. Our results indicate that under normal physiological conditions Sb leaching from SU-8 is minimal and is below the recommendations of the USEPA.
3.2 Agar overlay assay
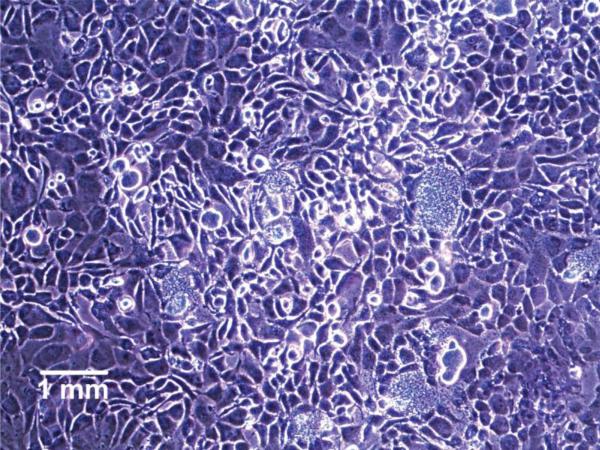
Cytotoxic leachates from SU-8 can diffuse through the agar barrier and potentially inhibit cell proliferation at localized regions, resulting in bare patches when cell surfaces are stained with crystal violet. We did not observe any inhibition of cell proliferation resulting from the presence of SU-8 pads on the agar layer (Fig. 2). Crystal violet staining was uniform under the region where the SU-8 substrates were placed on the agar layer, indicating that under these conditions, SU-8 was not toxic to cells. This is in agreement with reports demonstrating viable cell growth on SU-8 surfaces without any observable cytotoxic effects [15, 38].
Fig. 2.
Crystal violet staining of 9L glioma cells cultured under an agar diffusion layer, directly below a SU-8 substrate pad.
3.3 MTT cell viability assay
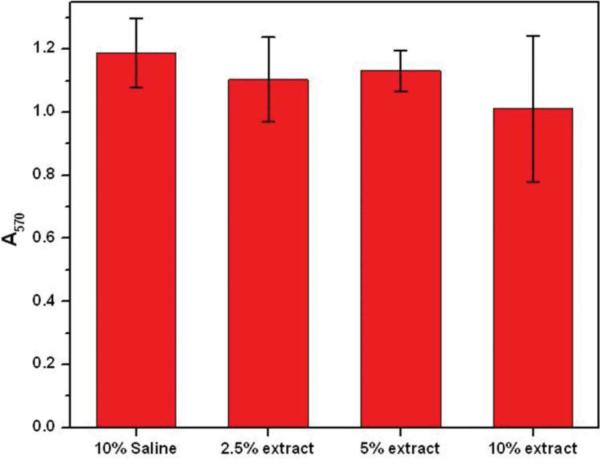
Cytotoxicity of SU-8 leachates was evaluated by a quantitative MTT cell viability assay on 9L glioma cells (Fig. 3). MTT assay provides a quantitative assessment of cellular response to toxic agents which can impair mitochondrial function and cell viability. Bisphenol A, the primary constituent of SU-8, is cytotoxic and is known to induce apoptosis [39]. SU-8 extracts from PBS did not cause a significant inhibition of viable cell growth for 2.5% and 5% extracts while exhibiting significant inhibition for the 10% extract. The negative control containing PBS alone had no effect on cell viability.
Fig. 3.
MTT cell viability assay of 9L glioma cells cultured with DMEM medium supplemented 0, 2.5, 5 and 10% of SU-8 leachate in PBS. Culture medium supplemented with 10% PBS was used as a control. Cells were incubated for 72 h before the MTT assay and the viable cell population was quantified by the absorbance at 570 nm.
3.4 Hemolysis assay
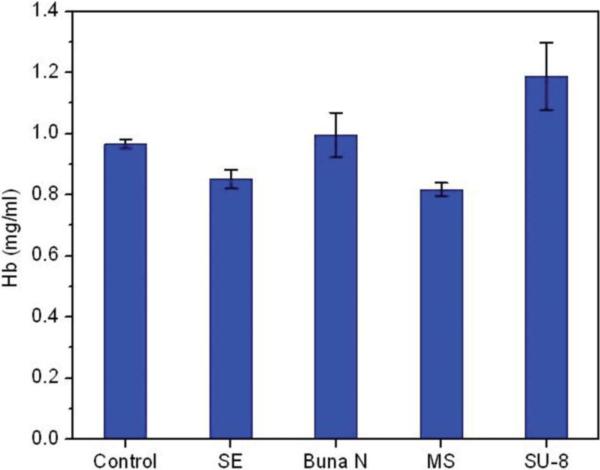
SU-8 displayed higher hemolytic activity in comparison to the biocompatible reference standards provided with the kit (Fig. 4). Hemoglobin release from the reference standards and SU-8 was corrected for spontaneous lysis observed in the control. The quantity of hemoglobin released from RBCs after 24 h incubation at 37 °C was 1.1% of the total hemoglobin, which is significantly higher than the reference standards (p<0.05), but is below the recommended limit of 2%. There were no statistical differences between the controls and any of the other polymer reference substrates. It should be noted that the control samples showed a higher concentration of Hb than recommended by the manufacturer. This may be due to the degradation of the RBCs during storage and transport.
Fig. 4.
Hemolysis assay of SU-8 substrate pads. The hemolytic activity of SU-8 substrates was compared to the activity of three FDA approved biocompatible reference materials, i) Silicon elastomer (SE), ii) Buna N and iii) Medical steel (MS) supplied with the assay kit and a control without any polymer additive.
The interaction of implantable polymers with blood can result in the release of hemoglobin from RBC. Hemolysis is influenced by a variety of factors including geometry and surface profile of the polymer (which influences shear stress) and chemical interaction of RBC with leachates. including metal ions [40]. Our study demonstrates the favorable hemolytic characteristic of SU-8 as an implant material.
3.5 In vivo biocompatibility
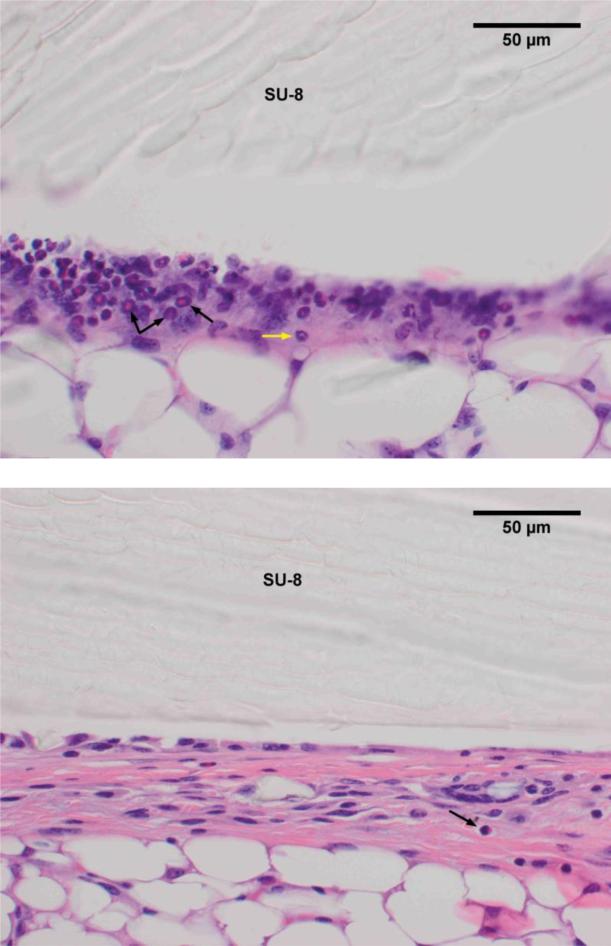
Macroscopic inspection of the implantation site reveals an immediate mild inflammatory reaction to SU-8 implantation, evidenced by the presence of eosinophils and neutrophils. The intensity and number of the eosinophils and neutrophils decreases after week 1 suggesting that their formation is primarily due to the stress of implantation rather than interaction with SU-8 (Fig. 5).
Fig. 5.
H&E staining of excised tissue surrounding SU-8 implant after 1 wk (a) and 8 wk (b) shows a reduction in the number of eosinophils (black arrows) and neutrophils (yellow arrow) over time.
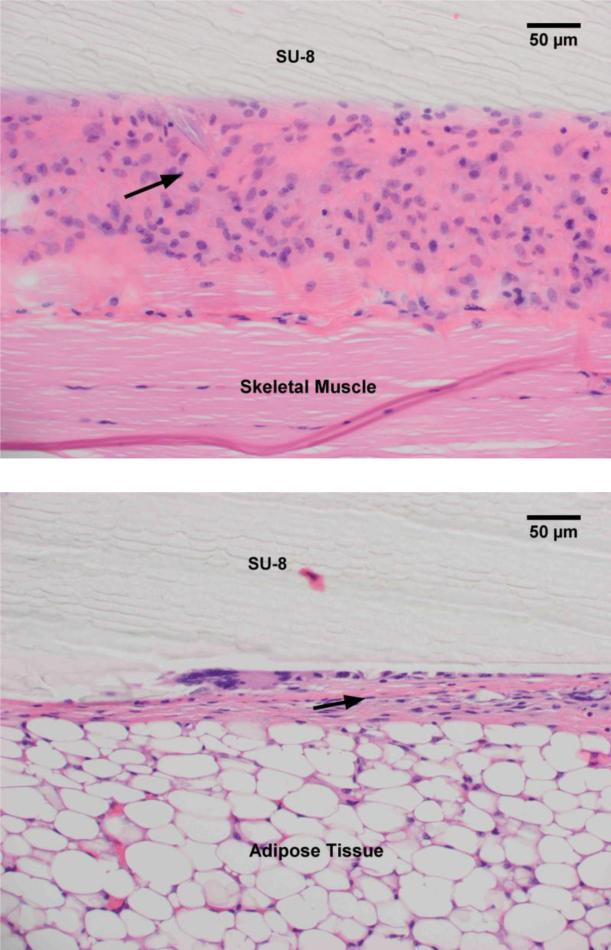
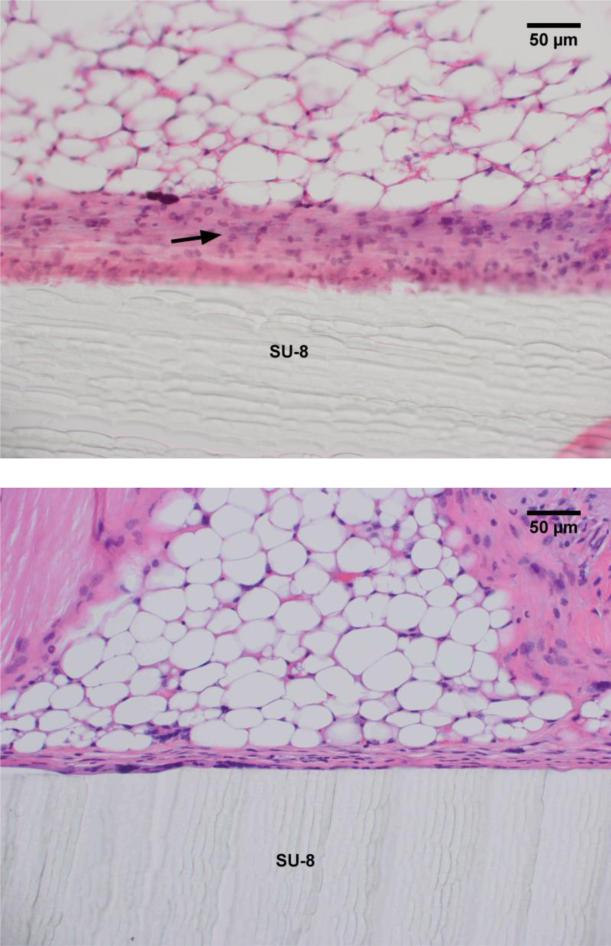
SU-8 interfaces with either adipose tissue or skeletal muscle in the mice evaluated. The fibrotic capsule thickness is larger when SU-8 contacts skeletal muscle, probably due to the friction/irritation with muscle contraction. The capsule is approximately twice as thick at the interface with skeletal muscle as compared with adipose tissue (Fig. 6). SU-8 exhibits minimal reactivity with the adipose tissue and elicits the formation of a thin capsule at the interface involving a few strands of collagen. As observed from a lower magnification image of Fig. 5, this begins with a thicker band of loose, reactive connective tissue at week 1, and matures to a thin fibrous capsule at week 8 (Fig. 7).
Fig. 6.
H&E staining of excised tissue surrounding SU-8 substrates implanted adjacent to skeletal muscle (a) and adipose tissue (b). The fibrous capsule (black arrow) thickness formed due to the interaction with the skeletal muscle is approximately twice that formed due to interaction with the adipose tissue.
Fig. 7.
H&E staining of excised tissue surrounding SU-8 substrates indicates the formation of loose connective tissue (black arrow) after 1 wk (a) which matures into a thin capsular layer at wk 8 (b).
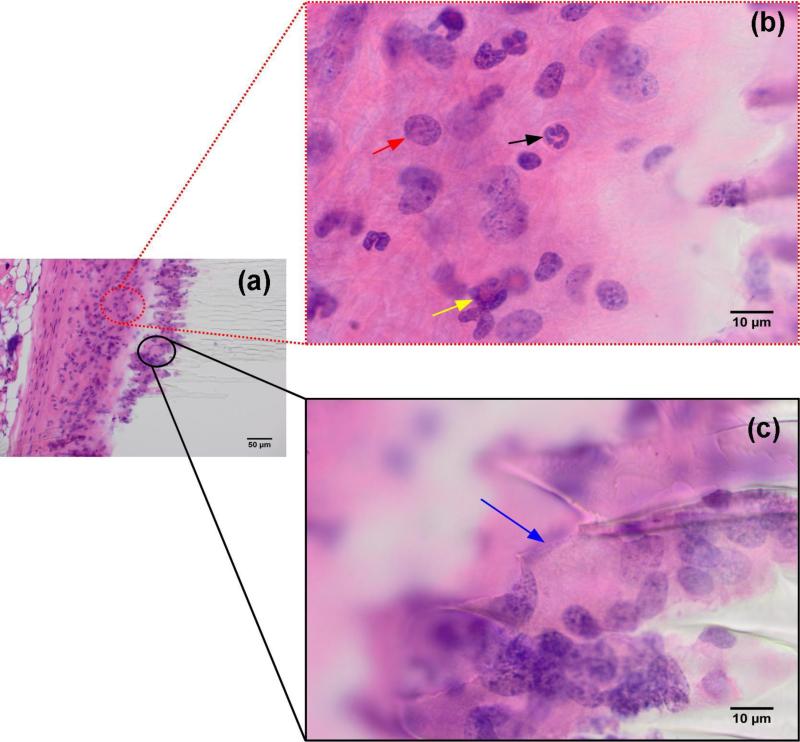
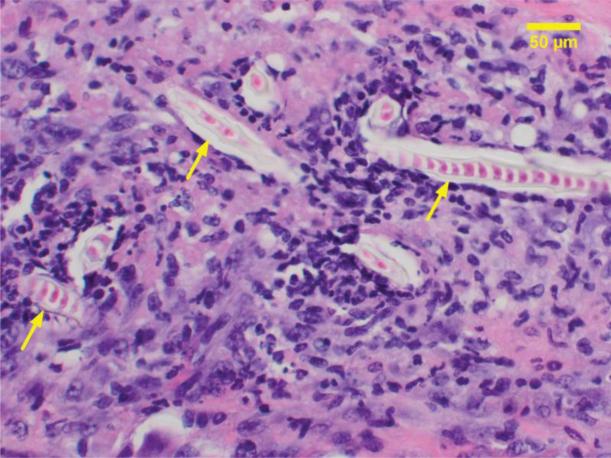
When SU-8 substrate had serrated edges resulting from breakage during implantation, macrophages and multinuclear giant cells were observed to intercalate between surface irregularities (Fig 8). The periodicity of these surface defects indicates a mechanical cause rather than a biological effect. No such effect was observed in a majority of the samples where the SU-8 surface had smooth edges. During the course of the study over 8 weeks, no degradation of the SU-8 by macrophages was observed. There is no tissue necrosis associated with the SU-8 substrates. No increase in the number of mast cells was observed. A single cell layer of mast cells was recruited early and covers most of the SU-8 substrate. Multinucleated giant cells cover between 30 and 60% of the SU-8 surface over a period of 8 weeks with an average of 30% involvement as early as the first week.
Fig. 8.
Immune response to the serrated edges of SU-8 substrate broken during implantation (a). Inset (b) macrophages (red arrow), neutrophils (black arrow) and eosinophils (yellow arrow); (c) multinucleated giant cells (blue arrow).
The reaction between mice varies somewhat in intensity at each time interval, as might be expected from factors such as 1) slightly different anatomic location of implantation within the animal and 2) introduction of other material, such as hair strands, at the time of implantation. It was observed that naked hairs adjacent to SU-8 elicited a brisk inflammatory response, which dwarfs the response to SU-8 alone (Fig. 9).
Fig. 9.
Acute inflammatory response elicited by a hair shaft (yellow arrows) embedded into the tissue during implantation.
Surface chemistry influences the biocompatibility of implant materials with the host tissue. Though most implant materials are designed to be non-toxic, non-immunogenic and chemically inert, they induce an acute inflammatory response at the time of implantation. This culminates in the formation of a fibrous capsule around the implant which acts as a diffusion barrier and impedes the exchange of nutrients, metabolites and therapeutic drugs exchange with the host environment. Multinucleated giant cells penetrate the implants and degrade them [41, 42]. Our results indicate that SU-8 implants are surrounded by a thin fibrous capsule which can facilitate efficient diffusion and can hence support long term viability of cellular payload. We didn't observe excessive formation of MNGC's, except on serrated SU-8 edges generated due to the breaking of the substrates during implantation. Our study indicates the biocompatibility and biostability of SU-8 and its suitability as an implant material.
4.0 Conclusions
The biocompatible nature of polymerized SU-8 is evidenced by cell viability and minimal hemolytic activity. The effect of leachates from SU-8 is comparable to the effect of normal saline. Residual antimony analysis by ICP-MS indicates minimal leaching in PBS under physiological conditions but an enhanced leaching in isopropanol and acidic buffer. Residual antimony levels can be further reduced by additional UV photoexposure and increased baking times during fabrication. In vivo histocompatibility studies indicate minimal interaction with host tissue, with the number of eosinophils and neutrophils reducing over time, indicating an initial transplant related stress. SU-8 elicits a very thin fibrous capsule formation, facilitating efficient exchange of metabolites and nutrients with the host environment. Our study indicates the suitability of SU-8 as an implant material, supporting cell viability and hence its potential application as a biocompatible matrix for biomolecular encapsulation. Biocompatibility may be further enhanced by surface treatments including oxygen plasma treatment [32], chemical treatment with acid and base [43], ethanolamine and grafting of the surface with polyethylene glycol [44].
Highlights.
In vitro and in vivo biocompatibility of SU-8 was evaluated.
Antimony leaching from SU-8 substrates in various solvents and conditions evaluated by ICP-AES.
Cytotoxicity, cell viability and hemocompatibility assays were performed.
Subcutaneous implantation of SU-8 substrates in Balb/C mice elicited muted immune response.
Acknowledgments
We acknowledge funding from NIH R01 EB007456
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krishnamurthy NV, Gimi B. Crit Rev Biomed Eng. 2011;39:473–491. doi: 10.1615/critrevbiomedeng.v39.i6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanza RP, Cooper DK. Mol Med Today. 1998;4:39–45. doi: 10.1016/S1357-4310(97)80544-8. [DOI] [PubMed] [Google Scholar]
- 3.Rihova B. Adv Drug Deliv Rev. 2000;42:65–80. doi: 10.1016/s0169-409x(00)00054-5. [DOI] [PubMed] [Google Scholar]
- 4.Nafea EH, Marson A, Poole-Warren LA, Martens PJ. J Control Release. 2011;154:110–122. doi: 10.1016/j.jconrel.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Hunt NC, Grover LM. Biotechnol Lett. 2010;32:733–742. doi: 10.1007/s10529-010-0221-0. [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann H, Shirley SG, Zimmermann U. Curr Diab Rep. 2007;7:314–320. doi: 10.1007/s11892-007-0051-1. [DOI] [PubMed] [Google Scholar]
- 7.Santos E, Zarate J, Orive G, Hernandez RM, Pedraz JL. Adv Exp Med Biol. 2010;670:5–21. doi: 10.1007/978-1-4419-5786-3_2. [DOI] [PubMed] [Google Scholar]
- 8.Iwata H, Kobayashi K, Takagi T, Oka T, Yang H, Amemiya H, Tsuji T, Ito F. J Biomed Mater Res. 1994;28:1003–1011. doi: 10.1002/jbm.820280905. [DOI] [PubMed] [Google Scholar]
- 9.Shoichet MS, Li RH, White ML, Winn SR. Biotechnol Bioeng. 1996;50:374–381. doi: 10.1002/(SICI)1097-0290(19960520)50:4<374::AID-BIT4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 10.Gimi B, Kwon J, Kuznetsov A, Vachha B, Magin RL, Philipson LH, Lee JB. J Diabetes Sci Technol. 2009;3:297–303. doi: 10.1901/jaba.2009.3-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gimi B, Kwon J, Liu L, Su Y, Nemani K, Trivedi K, Cui Y, Vachha B, Mason R, Hu W, Lee JB. Biomed Microdevices. 2009;11:1205–1212. doi: 10.1007/s10544-009-9338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gimi B, Kwon J, Trivedi K, Krishnamurthy N, Hu W, Lee J. XVIIth International Conference on Bioencapsulation; Groningen, Netherlands. 2009. [Google Scholar]
- 13.Kwon J, Trivedi K, Krishnamurthy NV, Hu W, Lee J-B, Gimi B. J Vac Sci Technol B. 2009;27:2795–2800. doi: 10.1116/1.3258146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tijero M, Gabriel G, Caro J, Altuna A, Hernandez R, Villa R, Berganzo J, Blanco FJ, Salido R, Fernandez LJ. Biosens Bioelectron. 2009;24:2410–2416. doi: 10.1016/j.bios.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Cho SH, Lu HM, Cauller L, Romero-Ortega MI, Lee JB, Hughes GA. IEEE Sens J. 2008;8:1830–1836. [Google Scholar]
- 16.Altuna A, Gabriel G, de la Prida LM, Tijero M, Guimera A, Berganzo J, Salido R, Villa R, Fernandez LJ. J Micromech Microeng. 2010;20 [Google Scholar]
- 17.Ismailova E, Doublet T, Khodagholy D, Quilichini P, Ghestem A, Yang SY, Bernard C, Malliaras GG. Int J Nanotechnol. 2012;9:517–528. [Google Scholar]
- 18.Altuna A, de la Prida LM, Bellistri E, Gabriel G, Guimera A, Berganzo J, Villa R, Fernandez LJ. Biosens Bioelectron. 2012;37:1–5. doi: 10.1016/j.bios.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 19.Xue N, Chang SP, Lee JB. Conf Proc IEEE Eng Med Biol Soc. 2011:2854–2857. doi: 10.1109/IEMBS.2011.6090788. [DOI] [PubMed] [Google Scholar]
- 20.Ereifej ES, Khan S, Newaz G, Zhang JS, Auner GW, VandeVord PJ. J Biomed Mater Res A. 2011;99A:141–150. doi: 10.1002/jbm.a.33170. [DOI] [PubMed] [Google Scholar]
- 21.Balciunas E, Jonusauskas L, Valuckas V, Baltriukiene D, Bukelskiene V, Gadonas R, Malinauskas M. Proc. SPIE 8427, Biophotonics: Photonic Solutions for Better Health Care III. 2012;8427:84271X–84271X. [Google Scholar]
- 22.Wang L, Wu ZZ, Xu BQ, Zhao YP, Kisaalita WS. Sens Actuators B Chem. 2009;140:349–355. [Google Scholar]
- 23.Hirai Y, Kamei K.-i., Makino Y, Liu L, Yuan Q, Chen Y, Tabata O. Procedia Engineering. 2011;25:1233–1236. [Google Scholar]
- 24.Abgrall P, Conedera V, Camon H, Gue AM, Nguyen NT. Electrophoresis. 2007;28:4539–4551. doi: 10.1002/elps.200700333. [DOI] [PubMed] [Google Scholar]
- 25.del Campo A, Greiner C. J Micromech Microeng. 2007;17:R81–R95. [Google Scholar]
- 26.Kotzar G, Freas M, Abel P, Fleischman A, Roy S, Zorman C, Moran JM, Melzak J. Biomaterials. 2002;23:2737–2750. doi: 10.1016/s0142-9612(02)00007-8. [DOI] [PubMed] [Google Scholar]
- 27.Voskerician G, Shive MS, Shawgo RS, von Recum H, Anderson JM, Cima MJ, Langer R. Biomaterials. 2003;24:1959–1967. doi: 10.1016/s0142-9612(02)00565-3. [DOI] [PubMed] [Google Scholar]
- 28.Nemani K, Kwon J, Trivedi K, Hu W, Lee JB, Gimi B. J Microencapsul. 2011;28:771–782. doi: 10.3109/02652048.2011.621552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guess WL, Rosenbluth SA, Schmidt B, Autian J. J Pharm Sci. 1965;54:1545–1547. doi: 10.1002/jps.2600541036. [DOI] [PubMed] [Google Scholar]
- 30.Mosmann T. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 31.Wang YL, Pai JH, Lai HH, Sims CE, Bachman M, Li GP, Allbritton NL. J Micromech Microeng. 2007;17:1371–1380. [Google Scholar]
- 32.Walther F, Davydovskaya P, Zurcher S, Kaiser M, Herberg H, Gigler AM, Stark RW. J Micromech Microeng. 2007;17:524. [Google Scholar]
- 33.Patterson TJ, Ngo M, Aronov PA, Reznikova TV, Green PG, Rice RH. Chem Res Toxicol. 2003;16:1624–1631. doi: 10.1021/tx034146y. [DOI] [PubMed] [Google Scholar]
- 34.Frezard F, Demicheli C. Expert Opin Drug Deliv. 2010;7:1343–1358. doi: 10.1517/17425247.2010.529897. [DOI] [PubMed] [Google Scholar]
- 35.Frezard F, Demicheli C, Ferreira CS, Costa MA. Antimicrob Agents Chemother. 2001;45:913–916. doi: 10.1128/AAC.45.3.913-916.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sundar S, Chakravarty J. Int J Environ Res Public Health. 2010;7:4267–4277. doi: 10.3390/ijerph7124267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.EPA 811-F-95-002j-T, National Primary Drinking Water Regulations. Antimony, Office of Water, U.E.P.A. 1995 [Google Scholar]
- 38.Hennemeyer M, Walther F, Kerstan S, Schurzinger K, Gigler AM, Stark RW. Microelectron Eng. 2008;85:1298–1301. [Google Scholar]
- 39.LaPensee EW, LaPensee CR, Fox S, Schwemberger S, Afton S, Ben-Jonathan N. Cancer Lett. 2010;290:167–173. doi: 10.1016/j.canlet.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henkelman S, Rakhorst G, Blanton J, van Oeveren W. Mater Sci Eng C Mater Biol Appl. 2009;29:1650–1654. [Google Scholar]
- 41.Kamath S, Bhattacharyya D, Padukudru C, Timmons RB, Tang L. J Biomed Mater Res A. 2008;86:617–626. doi: 10.1002/jbm.a.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thevenot P, Hu W, Tang L. Curr Top Med Chem. 2008;8:270–280. doi: 10.2174/156802608783790901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deepu A, Sai VV, Mukherji S. J Mater Sci. 2009;20(Suppl 1):S25–28. doi: 10.1007/s10856-008-3471-9. [DOI] [PubMed] [Google Scholar]
- 44.Tao SL, Popat KC, Norman JJ, Desai TA. Langmuir. 2008;24:2631–2636. doi: 10.1021/la703066z. [DOI] [PubMed] [Google Scholar]