Abstract
Ganciclovir (GCV) is a deoxyguanosine analog that is effective in inhibiting human cytomegalovirus (HCMV) replication. In infected cells GCV is converted to GCV-triphosphate which competes with dGTP for incorporation into the growing DNA strand by the viral DNA polymerase. Incorporated GCV promotes chain termination as it is an inefficient substrate for elongation. Because viral DNA synthesis also relies on cellular ribonucleotide reductase (RR) to synthesize deoxynucleotides, RR inhibitors are predicted to inhibit HCMV replication. Moreover, as dGTP competes with GCV-triphosphate for incorporation, RR inhibitors may also synergize with GCV by reducing intracellular dGTP levels and there by promoting increased GCV-triphosphate utilization by DNA polymerase. To investigate potential of RR inhibitors as anti-HCMV agents both alone and in combination with GCV, HCMV-inhibitory activities of three RR inhibitors, hydroxyurea, didox, and trimidox, were determined. In both spread inhibition and yield reduction assays RR inhibitors had modest anti-HCMV activity with 50% inhibitory concentrations ranging from 36 ± 1.7 to 221 ± 52 µM. However, all three showed significant synergy with GCV at concentrations below their 50% inhibitory and 50% toxic concentrations. These results suggest that combining GCV with relatively low doses of RR inhibitors could significantly potentiate the anti-HCMV activity of GCV in vivo and could improve clinical response to therapy.
Keywords: Cytomegalovirus, Antivirals, Ganciclovir, Hydroxyurea, Didox, Trimidox
1. Introduction
Human cytomegalovirus (HCMV) causes a spectrum of diseases in immune compromised patients, including retinitis in HIV patients, pneumonitis in transplant patients, and serious birth defects characterized by sensorineural hearing loss and severe mental retardation when acquired during pregnancy. Presently there are three drugs licensed for the treatment of systemic HCMV infections: ganciclovir (and its prodrug valganciclovir), foscarnet and cidofovir. Ganciclovir (GCV) is the first drug found to be effective in treating established HCMV infections and continues to be the first-line treatment for HCMV infections in AIDS and organ transplant patients. GCV is a deoxyguanosine analog that is converted to the monophosphate form by the HCMV-encoded protein kinase pUL97, and subsequently, to its di and triphosphate form by host cell kinases. GCV-triphosphate inhibits synthesis of viral DNA by competing with dGTP for incorporation into the growing DNA strand by the viral DNA polymerase. Once inserted GCV provides an inefficient substrate and thereby impairs elongation.
Acyclovir (ACV) is a deoxyguanosine analog whose mechanism of action is similar to that of GCV. It is significantly less toxic and has demonstrated efficacy and safety for treating herpes simplex viruses type 1 and type 2 (HSV-1, HSV-2) infections during pregnancy (Kang et al., 2011). However, ACV has only weak activity against HCMV at clinically useful doses and is therefore not commonly used to treat HCMV infections.
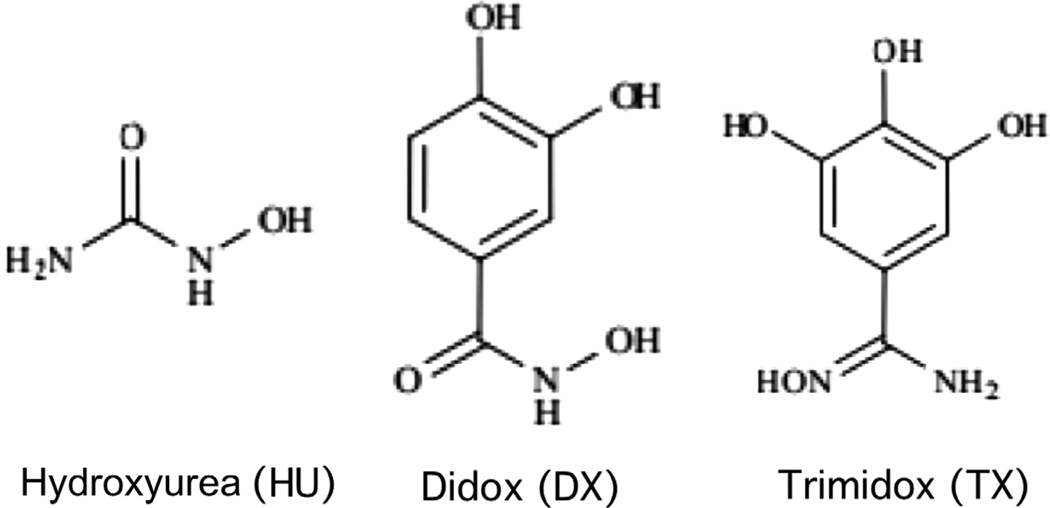
Modest antiviral activity of existing drugs coupled with dose-limiting toxicities limits therapeutic effectiveness and often results in the development of resistance. Development of new antiviral therapies that have improved efficacy as well as reduced toxicity is needed. Here we explored the potential of “combination therapy” to augment the antiviral potency of GCV by co-administration with drugs that reduce intracellular deoxynucleotide pools by inhibiting ribonucleotide reductase (RR), the cellular enzyme that catalyzes the reductive conversion of ribonucleotides into deoxynucleotides. Three RR inhibitors were selected for study: hydroxyurea (HU), didox (DX), and trimidox (TX) (Fig. 1).
Figure 1.
Structures of HU, DX, and TX.
2. Materials and methods
2.1. Virus and cell culture
Human MRC-5 fibroblasts (ATCC CCL-171) were propagated in modified Eagle medium (Gibco-BRL) supplemented with 10% fetal calf serum (HyClone Laboratories), 10,000 IU/L penicillin, and 10 mg/L streptomycin (Gibco-BRL) (MEM). Human ARPE-19 epithelial cells (ATCC CRL-2302) were propagated in high glucose Dulbecco’s modified Eagle medium (Gibco-BRL) supplemented as above (DMEM). Viruses were propagated as described (Cui et al., 2012, 2008; Saccoccio et al., 2011). Virus BADrUL131-Y4 (a gift from Thomas Shenk and Dai Wang) is a variant of HCMV strain AD169 that contains a green fluorescent protein (GFP) expression cassette and in which a mutation in UL131 has been repaired to permit replication in epithelial cells (Wang and Shenk, 2005). Virus RC2626 is a variant of HCMV strain Towne containing a luciferase expression cassette (McVoy and Mocarski, 1999).
2.2. Drugs
GCV and ACV were purchased from InvivoGen. HU was purchased from Sigma. DX and TX were gifts from Molecules for Health Inc., Richmond, VA. All drugs were solubilized in water and filter sterilized to produce stock solutions of 160 mM (GCV), 45 mM (ACV), 132 mM (HU), 117 mM (DX), or 22.6 mM (TX).
2.3. GFP-based spread inhibition assay
96-well plates containing confluent monolayers of MRC-5 or ARPE-19 cells were infected with virus BADrUL131-Y4 at an MOI of 0.015. One h post infection (hpi) 12 twofold serial dilutions of each drug in MEM (MRC-5s) or DMEM (ARPE-19s) were added. To ensure reproducibility each drug dilution, no-drug controls, and no-virus controls were assayed in triplicate on each plate. After 14 d relative fluorescent units (RFU) of GFP were measured for each well using a Biotek Synergy HT Multi-Mode Microplate Reader. Fifty-percent effective concentration (EC50) values were determined using Prism 5 (GraphPad Software, Inc.) as the inflection points of four-parameter curves fitted to plots of GFP (mean RFUs from triplicate wells converted to % maximum) vs. log[drug] as described previously (Saccoccio et al., 2011).
2.4. Luciferase-based yield reduction assay
96-well plates containing confluent monolayers of MRC-5 fibroblast cells were infected with virus RC2626 at an MOI of 0.03. One hpi 12 twofold serial dilutions of each drug in MEM were added. Each drug dilution, no-drug controls, and no-virus controls were assayed in triplicate on each plate. After incubation for 5 d, 50 µl of supernatant from each well was transferred to corresponding wells in a black-walled, clear/flat-bottomed 96-well plate containing confluent MRC-5 monolayers. After 24 h 100 µl Steady-Gloluciferase assay reagent (Promega) was added and the luciferase activity was measured in relative light units (RLU) using a Biotek Synergy HT Multi-Mode Microplate Reader. EC50 values were determined as described in 2.3.
2.5. Evaluation of RR inhibitors for synergy with GCV
The luciferase-based assay described in 2.4 was modified to evaluate two-drug combinations of GCV-HU, GCV-DX, or GCV-TX. Rows contained twofold dilutions of RR inhibitors while columns contained twofold dilutions of GCV. Each plate included a dilution series of each drug alone, no-drug controls, and no-virus controls. RLU data were analyzed for synergy/antagonism using MacSynergy II software (Prichard and Shipman, 1990). This software uses inhibition data collected for each drug used alone to calculate predicted additive % inhibition values for each drug combination. It then subtracts the predicted additive inhibitions from the observed experimental values and for each drug combination and plots “% inhibition above additive predicted % inhibition” on a three-dimensional graph. Values above zero indicate synergy and negative values indicate antagonism.
2.6. Cytotoxicity
Black-walled, clear/flat-bottomed 96-well plates containing confluent cell monolayers were incubated with duplicate twofold serial dilutions of the drugs for 5 d (MRC-5) or 14 d (MRC-5 and ARPE-19); no-drug controls and no-cell controls were included in triplicate on each plate. After incubation, the drugs were removed by washing with PBS and 100 µL of fresh culture medium was added to each well. CellTiter-Glo assay reagent (100 µL; Promega) was added to each well and luciferase activity (RLU) was measured using a Biotek Synergy HT Multimode Microplate Reader. Fifty-percent cytotoxic dose (TD50) values were determined as described in 2.3. For drug combinations, black-walled clear/flat-bottomed 96-well plates containing confluent MRC-5 cell monolayers were incubated with each drug combination in triplicate and RLU were measured after 5 days as described above. Percent toxicity was calculated as [(RLU (no-drug control) – RLU (drug combination))/RLU (no-drug control)] × 100.
3. Results
3.1. Evaluation of RR inhibitors for inhibition of HCMV spread in fibroblasts and epithelial cells
To first establish whether RR inhibitors HU, DX, and TX have HCMV inhibitory activity when used alone, each drug was evaluated for anti-HCMV activity using a GFP-based spread-inhibition assay. As these drugs target cellular processes that could differ significantly between cell types, inhibition was assessed in two different HCMV-permissive cell types: MRC-5 fibroblasts and ARPE-19 epithelial cells. Known HCMV inhibitors GCV and ACV were assayed for comparison. Confluent cell monolayers were infected at low multiplicity (MOI = 0.015) with virus BADrUL131-Y4, a GFP-tagged derivative of HCMV strain AD169 that replicates efficiently in both cell types (Wang and Shenk, 2005). One h after infection twofold dilutions of drug were added and the cultures were allowed to incubate for 14 d, at which time GFP in each well was quantitated as a measure of viral spread within the monolayer. Antiviral EC50 values were determined from four-parameter curves fitted to plots of GFP vs. log[drug].
In both cell types all three RR inhibitors exhibited HCMV inhibitory activities at relatively high concentrations with EC50s ranging from 43 ± 5.5 to 182 ± 23 µM (Table 1). In contrast, GCV was far more potent, with EC50s of 0.5 ± 0.2 µM (MRC-5) and 1.5 ± 0.2 µM (ARPE-19), while ACV was moderately inhibitory with EC50s of 24 ± 4.2 µM (MRC-5s) and 66 ± 3.8 µM (ARPE-19s). All drugs except HU were 2- to 3-fold more potent in MRC-5 cells compared to ARPE-19s (Table 1).
Table 1.
| Drug | Spread inhibition | Yield inhibition | ||||
|---|---|---|---|---|---|---|
| MRC-5 | ARPE-19 | MRC-5 | ||||
| EC50c | TD50d | EC50c | TD50d | EC50e | TD50f | |
| GCV | 0.5 ± 0.2 | ND | 1.5 ± 0.2 | ND | 0.6 ± 0.06 | ND |
| ACV | 24 ± 4.2 | ND | 66 ± 3.8 | ND | 24 ± 3.1 | ND |
| HU | 171 ± 46 | 4253 ± 701 | 131 ± 18 | 28,032 ± 1008 | 221 ± 52 | 14,500 ± 5400 |
| DX | 82 ± 32 | 146 ± 0.7 | 182 ± 23 | 276 ± 7.8 | 103 ± 19 | 256 ± 5.7 |
| TX | 43 ± 5.5 | 86 ± 4.2 | 125 ± 19 | 222 ± 13 | 36 ± 1.7 | 166 ± 57 |
ND, not determined.
50% effective concentrations (EC50).
50% cytotoxic concentrations (TD50).
GFP-based HCMV spread inhibition assay 14 d after infection.
CellTiter-Glo cytotoxicity assay after 14 d exposure to drugs.
Luciferase-based HCMV yield inhibition assay 5 d after infection.
CellTiter-Glo cytotoxicity assay after 5 d exposure to drugs.
As RR inhibitors target cellular deoxynucleotide biosynthesis and impair cellular DNA replication, they are anticipated to exhibit some cytotoxicity. To compare antiviral potency with cytotoxicity, uninfected MRC-5 and ARPE-19 cultures were incubated with RR inhibitors for 14 days and cell viability was assayed using CellTiter-Glo, a luciferase-based method that quantitates intracellular ATP as a measure of viable cells. Fifty-percent cytotoxic doses (TD50s) listed in Table 1 were determined from four-parameter curves fitted to plots of luciferase RLUs vs. log[drug] (not shown). HU was clearly the least toxic, with TD50s of 4253 ± 701 µM (MRC-5) and 28,032 ± 1008 µM (ARPE-19). DX and TX were significantly more toxic, with cytotoxicity TD50s only 1.5- to 2-fold higher than their antiviral EC50s (Table 1).
3.2. Evaluation of RR inhibitors for HCMV inhibition using a luciferase-based yield reduction assay
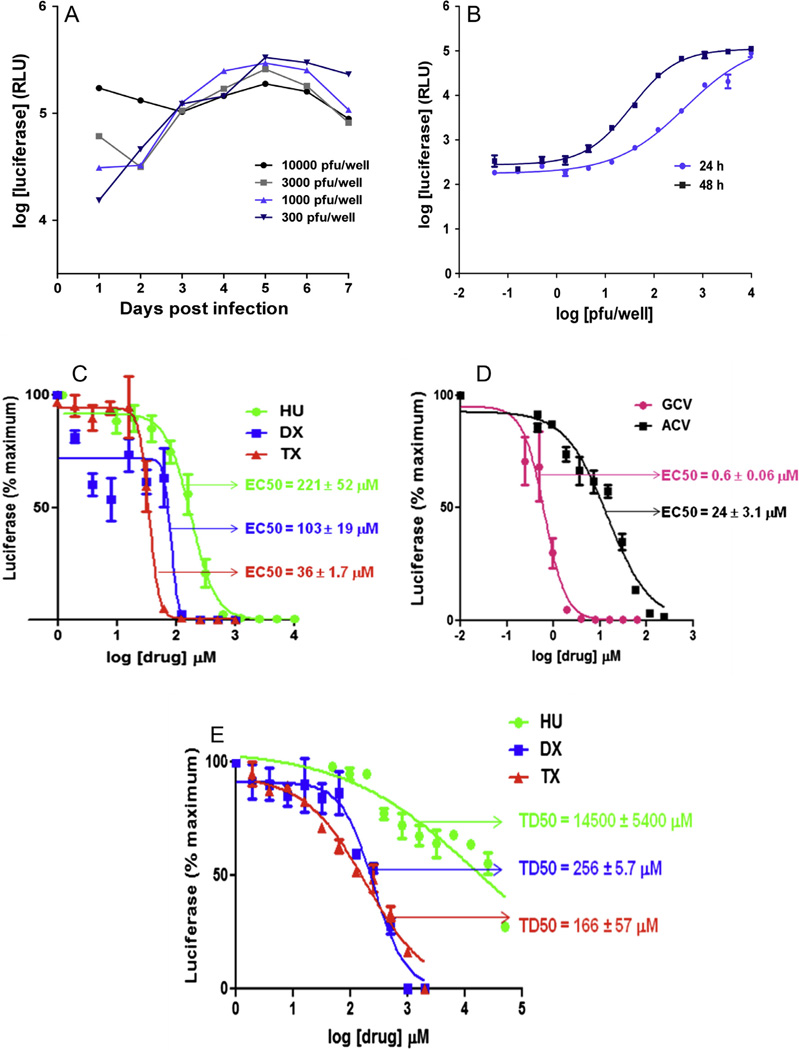
To determine the impact of RR inhibitors on production and release of infectious virus from infected cells, we utilized a virus, RC2626, that expresses luciferase (McVoy and Mocarski, 1999). To first establish the relationship between RC2626 replication and luciferase expression, we inoculated MRC-5 cultures with increasing infectious doses of RC2626 and measured luciferase activity in the cultures at different times after infection. The results revealed that from day 3 post infection and thereafter intracellular luciferase levels did not accurately reflect input levels of infectious virus (Fig. 2A), whereas at 24 and 48 hpi log(luciferase activity) exhibited a sigmoidal relationship with log(virus inoculum) (Fig. 2B). As the 24 h data had a more linear relationship over a broader dynamic range (101–104 pfu/well), subsequent assays used luciferase activity at 24 hpi as a surrogate for viral infectious units in the inoculum. Moreover, since the linear range peaked at 104 pfu/105 RLU and the 48-hpi luciferase activities plateaued at 105 RLU (Fig. 2B), yield assays were empirically optimized to attain maximal (i.e., no drug-treated) luciferase signals slightly under 105 RLU to ensure that RLU values accurately reflect virus infectious units. The final assay design used MRC-5 cells infected with RC2626 at an MOI of 0.03, incubation in the presence of inhibitors for five days, transfer of 50 µl culture supernatants to fresh MRC-5 cultures, and assay for luciferase activity in secondary cultures 24 hpi.
Figure 2.
Inhibition of HCMV yield in fibroblasts by RR inhibitors. (A) Confluent MRC-5 cultures in 96-well plates were infected with increasing infectious units (pfu/well) of RC2626 and luciferase activities in cell lysates were determined at the indicated times after infection (for simplicity, data for only four inocula are shown). (B) Luciferase data collected 24 and 48 hpi were plotted vs. pfu/well. (C and D) Confluent MRC-5 cultures in 96-well plates were infected with RC2626 (MOI = 0.03) and incubated in the presence of different concentrations of the indicated drugs for five days. 50 µl of day-five culture supernatants were transferred to fresh confluent MRC-5 cultures. After 24 h luciferase activities in cell lysates were determined. Data from three independent experiments were normalized by converting RLU to “ percent of maximum RLU” for each experiment and then averaged. Best-fit four-parameter curves were fitted to the data and used to calculate EC50 values for each drug. (E) Toxicity of RR inhibitors. MRC-5 cultures in 96-well plates were incubated in the presence of different concentrations of the indicated drugs for 5 days and cell viability was measured using CellTiter-Glo. Best-fit four-parameter curves were fitted to the data and used to calculate TD50 values for each drug. Each data point represents the mean of two replicate wells.
This assay was used to determine the ability of RR inhibitors to inhibit production of infectious virus; GCV and ACV were again assayed as comparators. Dose–response curves are shown in Fig. 2C and D and EC50s are in Table 1. The EC50s were quite similar to those obtained using the GFP-based spread inhibition assay in MRC-5s, with RR inhibitors exhibiting EC50s in the 36 ± 1.7 to 221 ± 52 µM range while GCV was more potent at 0.6 ± 0.06 µM and ACV was moderately active at 24 ± 3.1 µM. Cytotoxicity was evaluated using CellTiter-Glo with uninfected MRC-5 cells after five days incubation of cells with RR inhibitors. The results were quite similar to those obtained above for 14 d incubation with RR inhibitors (Fig. 2E and Table 1).
3.3. Evaluation of RR inhibitors for synergy with GCV
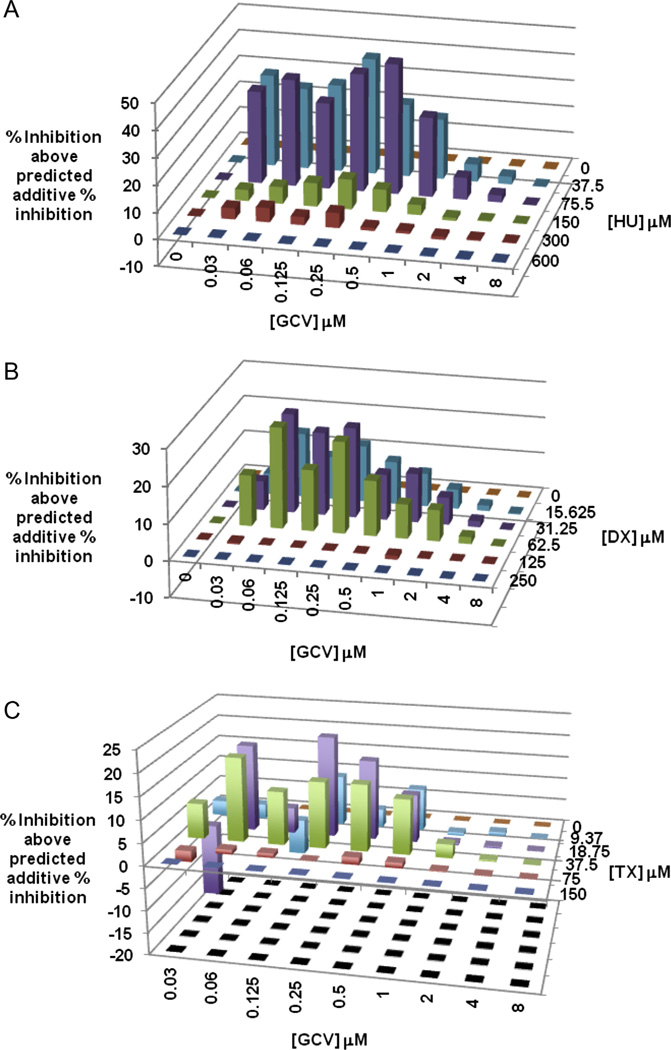
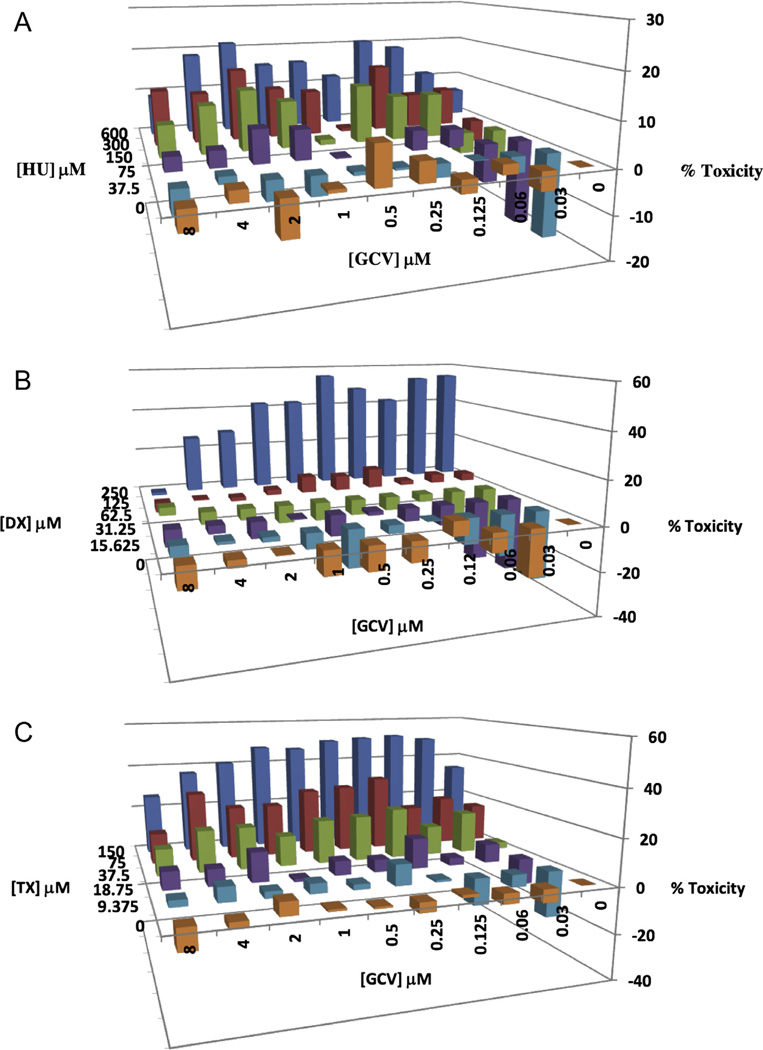
To determine if RR inhibitors, when used in combination with GCV, can potentiate the anti-HCMV activity of GCV, the luciferase-based yield reduction assay was utilized to test a checkerboard of different GCV concentrations in combination with different RR inhibitor concentrations. The resulting luciferase values were analyzed using MacSynergy II (Prichard and Shipman, 1990). MacSynergy II plots for HCMV inhibition by GCV-HU or GCV-DX combinations revealed considerable synergy at RR inhibitor concentrations below their EC50s for HCMV inhibition. For example, the combination of 75 µM HU with 0.5 µM GCV exhibited maximum synergy wherein the observed inhibitory effect of the combination was nearly 50 percentage units greater than the predicted additive inhibition for this drug combination (Fig. 3A). Similar although somewhat lower synergies (5–30 percentage units) were observed for GCV combined with DX (Fig. 3B) and for GCV combined with TX (Fig. 3C). MacSynergy II also determines an overall synergy volume based on observed inhibitions that lie outside the 95% confidence limits of the predicted additive inhibitions. Volumes greater than 100 µM2% indicate strong synergy that may be relevant in vivo (Prichard and Shipman, 1990). For GCV-HU, -DX, and -TX combinations the synergy scores were 501, 314, and 197 µM2%, respectively. Importantly, combination of GCV with HU, DX, or TX did not result in enhanced cytotoxic effects greater than those of the RR inhibitors when used alone (Fig. 4).
Figure 3.
Synergistic inhibition of HCMV replication by combinations of GCV with HU, DX, or TX. Checkerboard arrays of GCV-HU (A), GCV-DX (B), GCV-TX (C) combinations were evaluated using the luciferase-based yield reduction assay described in figure 2. MacSynergy II software was used to calculate % inhibition above predicted additive % inhibitions for each drug combination. Positive values in the Z-axis indicate synergy for a given drug combination. Data shown represent means of data from three independent experiments.
Figure 4.
Toxicity of GCV-RR inhibitor combinations. MRC-5 cultures in 96-well plates were incubated with checkerboard arrays of GCV combinations with HU, DX, or TX for 5 days, then cell viability was measured using CellTiter-Glo. Toxicity (Z-axis) for all drug combinations was calculated as described in materials and methods. Data shown represent means of data from three independent experiments.
Together, these results suggest that RR inhibitors, when present below their effective concentrations for HCMV inhibition and well below their toxic concentrations, can substantially increase the effectiveness of GCV against HCMV.
4. Discussion
RR activity is important for efficient replication of herpesvirus DNA. Viruses in the alpha and gamma subfamilies encode functional RRs (Boehmer and Lehman, 1997), whereas betaherpesviruses, including human and animal CMVs, encode RR homologs that lack RR function but have acquired unrelated functions (Lembo and Brune, 2009). Consequently, CMVs presumably rely upon host RR to provide deoxynucleotides for viral DNA synthesis. Consistent with this, HCMV and murine CMV (MCMV) upregulate expression of cellular RR (Lembo et al., 2000; Patrone et al., 2003).
Antiherpesviral activities of RR inhibitors have been explored primarily using HSV-1 and HSV-2, with limited studies on varicella zoster virus (VZV) and HCMV. In vitro studies have shown that inhibitors of cellular RR or the HSV-1 or VZV RRs (including HU, FMdC, A723U, A1110U, BW348U87, and the “BILD” series of peptidomimetics) exhibit antiviral activity when used alone and either potentiate or result in synergy when used in combination with ACV against wild type or drug-resistant strains of VZV, HSV-1, or HSV-2 (Bridges et al., 1995; Duan et al., 1998; Ellis et al., 1989; Lawetz and Liuzzi, 1998; Liuzzi et al., 1994; Moss et al., 1996, 1995; Neyts and De Clercq, 1999; Prichard and Shipman, 1995; Sergerie and Boivin, 2008; Spector et al., 1985, 1987, 1989). HU has also been shown to potentiate the activity of cidofovir and to synergize with GCV to inhibit replication of wild type or drug-resistant strains of HSV-1 or HSV-2 (Neyts and De Clercq, 1999; Sergerie and Boivin, 2008). One HSV-1 RR inhibitor, A1110U, has been shown to inhibit HCMV replication in vitro and to potentiate the anti-HCMV activity of GCV, presumably through affects on cellular RR (Hamzeh et al., 1993).
The present study extends these findings by examining inhibition of HCMV by the RR inhibitors HU, DX, and TX using spread inhibition and yield reduction assays. The EC50s that were determined for HU (131 ± 18 to 221 ± 52 µM) are consistent with a prior report in which titer reduction data suggest an EC50 of less than 500 µM (Anders et al., 1986). In contrast, with a reported EC50 of 3 µM (Lembo et al., 2000), MCMV appears to be significantly more sensitive to HU than HCMV. This extends also to DX, as EC50s of 10–25 µM have been reported for MCMV (Go et al., 2011) while for HCMV our EC50s ranged from 82 ± 32 to 182 ± 23 µM. Inhibition of HCMV or animal CMVs by TX has not been previously reported. That EC50s differed by only 2- to 3-fold between fibroblasts (MRC-5) and epithelial (ARPE-19) cells suggests that anti-HCMV activity of RR inhibitors is not significantly cell-type dependent, at least with respect to the two cell types represented here. DX and TX were more potent than HU but were also notably more toxic, with TC50s only 2-fold higher than their anti-HCMV EC50s. Consistent with studies showing synergistic effects of RR inhibitors with drugs targeting HSV-1, HSV-2, VZV, or HCMV (discussed above), the three RR inhibitors examined here acted synergistically with GCV to inhibit HCMV replication.
In murine models of HSV-1- or HSV-2-induced cutaneous or ocular lesions, RR inhibitors administered topically showed therapeutic efficacy and exhibited potentiation or synergy when used in combination with ACV (Brandt et al., 1996; Bridges et al., 1995; Duan et al., 1998; Ellis et al., 1989; Liuzzi et al., 1994; Lobe et al., 1991; Moss et al., 1995, 1996; Spector et al., 1992). Combination therapies were also effective for treating lesions caused by ACV-resistant strains (Duan et al., 1998; Ellis et al., 1989; Lobe et al., 1991; Spector et al., 1992). Despite encouraging results in mice, clinical studies found that topical formulations of ACV combined with the HSV RR inhibitor 348U87 were ineffective at preventing or treating UV-induced herpes labialis (Bernstein and Rheins, 1994) or for treating ACV-resistant anogenital herpes in HIV-infected subjects (Safrin et al., 1993). However, both reports cite the general inadequacy of topical delivery as the probable cause of treatment failure and suggest that topical formulations with improved penetration or systemic delivery might be more effective.
In vivo the efficacy of RR inhibitors as monotherapy against CMV infections has been little studied. In one study, DX treatment of sub-lethal MCMV infection in mice failed to decrease viral load in livers and spleen; paradoxically, DX prophylaxis was detrimental, resulting in elevated hepatic inflammatory cytokines and suppressed CD8cell responses (Go et al., 2011). However, the in vitro findings presented here suggest that in combination with GCV RR inhibitors such as HU, DX, or TX can provide significant augmentation of HCMV inhibition even when used at concentrations well below their TD50s (and indeed, below their EC50s for HCMV inhibition). Thus, combination therapy using relatively low doses of RR inhibitors could significantly potentiate the anti-HCMV activity of GCV in vivo and improve clinical response to therapy that might be particularly helpful in cases of GCV-resistance.
HU is approved by the FDA for the treatment of sickle cell disease and some cancers. HU also has anti-retroviral activity, can synergize with deoxynucleoside analog anti-retrovirals (Lori and Lisziewicz, 2000), and has been used to treat HIV infections (Lori et al., 2004). DX and TX are experimental drugs that have been evaluated as possible cancer therapeutics (Carmichael et al., 1990; Rubens et al., 1991; Veale et al., 1988) and as anti-retrovirals both alone and in combination with reverse transcriptase inhibitors in murine models of retrovirus-induced lymphoproliferative disease and immune deficiency (Mayhew et al., 1997, 2002, 2005; Sumpter et al., 2004). DX has also been investigated as an alternative to HU for treatment of sickle cell disease (Kaul et al., 2006; Pace et al., 1994). That sickle cell patients undergoing HU therapy experience peak plasma HU concentrations of 264–660 µM (NTP-CERHR, 2008) suggests that HU levels sufficient to synergize with GCV in vivo are achievable. TX has not been evaluated in humans; however, DX has completed phase I and II cancer trials (Carmichael et al., 1990; Rubens et al., 1991; Veale et al., 1988). Doses associated with only minor toxicities resulted in peak DX plasma levels of approximately 300 µM (Veale et al., 1988), again indicating that DX levels sufficient to synergize with GCV may be attainable in vivo without significant toxicity.
5. Conclusions
The RR inhibitors HU, DX, and TX used alone have anti-HCMV activity. While inherently toxic at higher concentrations, RR inhibitors exhibit significant synergy with GCV at concentrations that are non-toxic in vitro and, at least for HU and DX, feasible in vivo. Based on these findings further studies are warranted, both in vitro and in vivo using animal models of CMV infection.
Acknowledgments
This work was supported by grants R01AI088750 and R21AI073615 (to M.A.M) from the National Institutes of Health. We thank Dai Wang and Thomas Shenk for BAC clone BADrUL131-Y4 and Mark Prichard for MacSynergy II.
References
- Anders DG, Irmiere A, Gibson W. Identification and characterization of a major early cytomegalovirus DNA-binding protein. J. Virol. 1986;58:253–262. doi: 10.1128/jvi.58.2.253-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DI, Rheins LA. Solar simulator-induced herpes simplex labialis: use in evaluating treatment with acyclovir plus 348U87. Antiviral Res. 1994;23:225–233. doi: 10.1016/0166-3542(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Boehmer PE, Lehman IR. Herpes simplex virus DNA replication. Annu. Rev. Biochem. 1997;66:347–384. doi: 10.1146/annurev.biochem.66.1.347. [DOI] [PubMed] [Google Scholar]
- Brandt CR, Spencer B, Imesch P, Garneau M, Deziel R. Evaluation of a peptidomimetic ribonucleotide reductase inhibitor with a murine model of herpes simplex virus type 1 ocular disease. Antimicrob. Agents Chemother. 1996;40:1078–1084. doi: 10.1128/aac.40.5.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CG, Ahmed SP, Sunkara PS, McCarthy JR, Tyms AS. The ribonucleotide reductase inhibitor (E)-2′-fluoromethylene-2′-deoxycytidine (MDL 101,731): a potential topical therapy for herpes simplex virus infection. Antiviral Res. 1995;27:325–334. doi: 10.1016/0166-3542(95)00015-e. [DOI] [PubMed] [Google Scholar]
- Carmichael J, Cantwell BM, Mannix KA, Veale D, Elford HL, Blackie R, Kerr DJ, Kaye SB, Harris AL. A phase I and pharmacokinetic study of didox administered by 36 hour infusion. The cancer research campaign phase I/II clinical trials committee. Br. J. Cancer. 1990;61:447–450. doi: 10.1038/bjc.1990.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Meza BP, Adler SP, McVoy MA. Cytomegalovirus vaccines fail to induce epithelial entry neutralizing antibodies comparable to natural infection. Vaccine. 2008;26:5760–5766. doi: 10.1016/j.vaccine.2008.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Adler SP, Davison AJ, Smith L, Habib el SE, McVoy MA. Bacterial artificial chromosome clones of viruses comprising the Towne cytomegalovirus vaccine. J. Biomed. Biotechnol. 2012;2012:428498. doi: 10.1155/2012/428498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J, Liuzzi M, Paris W, Lambert M, Lawetz C, Moss N, Jaramillo J, Gauthier J, Deziel R, Cordingley MG. Antiviral activity of a selective ribonucleotide reductase inhibitor against acyclovir-resistant herpes simplex virus type 1 in vivo. Antimicrob. Agents Chemother. 1998;42:1629–1635. doi: 10.1128/aac.42.7.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis MN, Lobe DC, Spector T. Synergistic therapy by acyclovir and A1110U for mice orofacially infected with herpes simplex viruses. Antimicrob. Agents Chemother. 1989;33:1691–1696. doi: 10.1128/aac.33.10.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go V, Tang-Feldman YJ, Lochhead SR, Lochhead GR, Yu CQ, Elford HL, Inayat MS, Oakley OR, Pomeroy C. Paradoxical response to prophylactic didox (N-3, 4 trihydroxybenzamide) treatment in murine cytomegalovirus-infected mice. Antiviral Ther. 2011;16:1277–1286. doi: 10.3851/IMP1893. [DOI] [PubMed] [Google Scholar]
- Hamzeh FM, Spector T, Lietman PS. 2-Acetylpyridine 5-[(dimethylamino)thiocarbonyl]-thiocarbonohydrazone (1110U81) potently inhibits human cytomegalovirus replication and potentiates the antiviral effects of ganciclovir. Antimicrob. Agents Chemother. 1993;37:602–604. doi: 10.1128/aac.37.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SH, Chua-Gocheco A, Bozzo P, Einarson A. Safety of antiviral medication for the treatment of herpes during pregnancy. Can. Fam. Physician. 2011;57:427–428. [PMC free article] [PubMed] [Google Scholar]
- Kaul DK, Kollander R, Mahaseth H, Liu XD, Solovey A, Belcher J, Kelm RJ, Jr, Vercellotti GM, Hebbel RP. Robust vascular protective effect of hydroxamic acid derivatives in a sickle mouse model of inflammation. Microcirculation. 2006;13:489–497. doi: 10.1080/10739680600778456. [DOI] [PubMed] [Google Scholar]
- Lawetz C, Liuzzi M. The antiviral activity of the ribonucleotide reductase inhibitor BILD 1351 SE in combination with acyclovir against HSV type-1 in cell culture. Antiviral Res. 1998;39:35–46. doi: 10.1016/s0166-3542(98)00028-x. [DOI] [PubMed] [Google Scholar]
- Lembo D, Brune W. Tinkering with a viral ribonucleotide reductase. Trends Biochem. Sci. 2009;34:25–32. doi: 10.1016/j.tibs.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Lembo D, Gribaudo G, Hofer A, Riera L, Cornaglia M, Mondo A, Angeretti A, Gariglio M, Thelander L, Landolfo S. Expression of an altered ribonucleotide reductase activity associated with the replication of murine cytomegalovirus in quiescent fibroblasts. J. Virol. 2000;74:11557–11565. doi: 10.1128/jvi.74.24.11557-11565.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi M, Deziel R, Moss N, Beaulieu P, Bonneau AM, Bousquet C, Chafouleas JG, Garneau M, Jaramillo J, Krogsrud RL, et al. A potent peptidomimetic inhibitor of HSV ribonucleotide reductase with antiviral activity in vivo. Nature. 1994;372:695–698. doi: 10.1038/372695a0. [DOI] [PubMed] [Google Scholar]
- Lobe DC, Spector T, Ellis MN. Synergistic topical therapy by acyclovir and A1110U for herpes simplex virus induced zosteriform rash in mice. Antiviral Res. 1991;15:87–100. doi: 10.1016/0166-3542(91)90027-o. [DOI] [PubMed] [Google Scholar]
- Lori F, Lisziewicz J. Rationale for the use of hydroxyurea as an anti-human immunodeficiency virus drug. Clin. Infect. Dis. 2000;30(Suppl. 2):S193–S197. doi: 10.1086/313851. [DOI] [PubMed] [Google Scholar]
- Lori F, Kelly LM, Foli A, Lisziewicz J. Safety of hydroxyurea in the treatment of HIV infection. Expert Opin. Drug Saf. 2004;3:279–288. doi: 10.1517/14740338.3.4.279. [DOI] [PubMed] [Google Scholar]
- Mayhew C, Oakley O, Piper J, Hughes NK, Phillips J, Birch NJ, Elford HL, Gallicchio VS. Effective use of ribonucleotide reductase inhibitors (Didox and Trimidox) alone or in combination with didanosine (ddI) to suppress disease progression and increase survival in murine acquired immunodeficiency syndrome (MAIDS) Cell Mol. Biol. (Noisy-le-grand) 1997;43:1019–1029. [PubMed] [Google Scholar]
- Mayhew CN, Mampuru LJ, Chendil D, Ahmed MM, Phillips JD, Greenberg RN, Elford HL, Gallicchio VS. Suppression of retrovirus-induced immunodeficiency disease (murine AIDS) by trimidox and didox: novel ribonucleotide reductase inhibitors with less bone marrow toxicity than hydroxyurea. Antiviral Res. 2002;56:167–181. doi: 10.1016/s0166-3542(02)00108-0. [DOI] [PubMed] [Google Scholar]
- Mayhew CN, Sumpter R, Inayat M, Cibull M, Phillips JD, Elford HL, Gallicchio VS. Combination of inhibitors of lymphocyte activation (hydroxyurea, trimidox, and didox) and reverse transcriptase (didanosine) suppresses development of murine retrovirus-induced lymphoproliferative disease. Antiviral Res. 2005;65:13–22. doi: 10.1016/j.antiviral.2004.09.003. [DOI] [PubMed] [Google Scholar]
- McVoy MA, Mocarski ES. Tetracycline-mediated regulation of gene expression within the human cytomegalovirus genome. Virology. 1999;258:295–303. doi: 10.1006/viro.1999.9724. [DOI] [PubMed] [Google Scholar]
- Moss N, Beaulieu P, Duceppe JS, Ferland JM, Gauthier J, Ghiro E, Goulet S, Grenier L, Llinas-Brunet M, Plante R, et al. Peptidomimetic inhibitors of herpes simplex virus ribonucleotide reductase: a new class of antiviral agents. J. Med. Chem. 1995;38:3617–3623. doi: 10.1021/jm00018a022. [DOI] [PubMed] [Google Scholar]
- Moss N, Beaulieu P, Duceppe JS, Ferland JM, Garneau M, Gauthier J, Ghiro E, Goulet S, Guse I, Jaramillo J, Llinas-Brunet M, Malenfant E, Plante R, Poirier M, Soucy F, Wernic D, Yoakim C, Deziel R. Peptidomimetic inhibitors of herpes simplex virus ribonucleotide reductase with improved in vivo antiviral activity. J. Med. Chem. 1996;39:4173–4180. doi: 10.1021/jm960324r. [DOI] [PubMed] [Google Scholar]
- Neyts J, De Clercq E. Hydroxyurea potentiates the antiherpesvirus activities of purine and pyrimidine nucleoside and nucleoside phosphonate analogs. Antimicrob. Agents Chemother. 1999;43:2885–2892. doi: 10.1128/aac.43.12.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTP-CERHR. Monograph on the potential human reproductive and developmental effects of hydroxyurea. NTP Cerhr Mon. 2008:vii–viii. v, ix-III1. [PubMed] [Google Scholar]
- Pace BS, Elford HL, Stamatoyannopoulos G. Transgenic mouse model of pharmacologic induction of fetal hemoglobin: studies using a new ribonucleotide reductase inhibitor, Didox. Am. J. Hematol. 1994;45:136–141. doi: 10.1002/ajh.2830450208. [DOI] [PubMed] [Google Scholar]
- Patrone M, Percivalle E, Secchi M, Fiorina L, Pedrali-Noy G, Zoppe M, Baldanti F, Hahn G, Koszinowski UH, Milanesi G, Gallina A. The human cytomegalovirus UL45 gene product is a late, virion-associated protein and influences virus growth at low multiplicities of infection. J. Gen. Virol. 2003;84:3359–3370. doi: 10.1099/vir.0.19452-0. [DOI] [PubMed] [Google Scholar]
- Prichard MN, Shipman C., Jr A three-dimensional model to analyze drug-drug interactions. Antiviral Res. 1990;14:181–205. doi: 10.1016/0166-3542(90)90001-n. [DOI] [PubMed] [Google Scholar]
- Prichard MN, Shipman C., Jr Ribonucleotide reductase: an important enzyme in the replication of herpes simplex virus type 1 and a target for antiviral chemotherapy. Chemotherapy. 1995;41:384–395. doi: 10.1159/000239371. [DOI] [PubMed] [Google Scholar]
- Rubens RD, Kaye SB, Soukop M, Williams CJ, Brampton MH, Harris AL. Phase II trial of didox in advanced breast cancer. Cancer research campaign phase I/II clinical trials committee. Br. J. Cancer. 1991;64:1187–1188. doi: 10.1038/bjc.1991.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccoccio FM, Sauer AL, Cui X, Armstrong AE, Habib ES, Johnson DC, Ryckman BJ, Klingelhutz AJ, Adler SP, McVoy MA. Peptides from cytomegalovirus UL130 and UL131 proteins induce high titer antibodies that block viral entry into mucosal epithelial cells. Vaccine. 2011;29:2705–2711. doi: 10.1016/j.vaccine.2011.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safrin S, Schacker T, Delehanty J, Hill E, Corey L. Topical treatment of infection with acyclovir-resistant mucocutaneous herpes simplex virus with the ribonucleotide reductase inhibitor 348U87 in combination with acyclovir. Antimicrob. Agents Chemother. 1993;37:975–979. doi: 10.1128/aac.37.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergerie Y, Boivin G. Hydroxyurea enhances the activity of acyclovir and cidofovir against herpes simplex virus type 1 resistant strains harboring mutations in the thymidine kinase and/or the DNA polymerase genes. Antiviral Res. 2008;77:77–80. doi: 10.1016/j.antiviral.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Spector T, Averett DR, Nelson DJ, Lambe CU, Morrison RW, Jr, St Clair MH, Furman PA. Poentiation of antiherpetic activity of acyclovir by ribonucleotide reductase inhibition. Proc. Natl. Acad. Sci. USA. 1985;82:4254–4257. doi: 10.1073/pnas.82.12.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T, Stonehuerner JG, Biron KK, Averett DR. Ribonucleotide reductase induced by varicella zoster virus. Characterization, and potentiation of acyclovir by its inhibition. Biochem. Pharmacol. 1987;36:4341–4346. doi: 10.1016/0006-2952(87)90682-4. [DOI] [PubMed] [Google Scholar]
- Spector T, Harrington JA, Morrison RW, Jr, Lambe CU, Nelson DJ, Averett DR, Biron K, Furman PA. 2-Acetylpyridine 5-[(dimethylamino)thiocarbonyl]-thiocarbonohydrazone (A1110U), a potent inactivator of ribonucleotide reductases of herpes simplex and varicellazoster viruses and a potentiator of acyclovir. Proc. Natl. Acad. Sci. USA. 1989;86:1051–1055. doi: 10.1073/pnas.86.3.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T, Lobe DC, Ellis MN, Blumenkopf TA, Szczech GM. Inactivators of herpes simplex virus ribonucleotide reductase: hematological profiles and in vivo potentiation of the antiviral activity of acyclovir. Antimicrob. Agents Chemother. 1992;36:934–937. doi: 10.1128/aac.36.5.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpter LR, Inayat MS, Yost EE, Duvall W, Hagan E, Mayhew CN, Elford HL, Gallicchio VS. In vivo examination of hydroxyurea and the novel ribonucleotide reductase inhibitors trimidox and didox in combination with the reverse transcriptase inhibitor abacavir: suppression of retrovirus-induced immunodeficiency disease. Antiviral Res. 2004;62:111–120. doi: 10.1016/j.antiviral.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Veale D, Carmichael J, Cantwell BM, Elford HL, Blackie R, Kerr DJ, Kaye SB, Harris AL. A phase 1 and pharmacokinetic study of didox: a ribonucleotide reductase inhibitor. Br. J. Cancer. 1988;58:70–72. doi: 10.1038/bjc.1988.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Shenk T. Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J. Virol. 2005;79:10330–10338. doi: 10.1128/JVI.79.16.10330-10338.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]