Abstract
Despite new therapies, breast cancer continues to be the second leading cause of cancer mortality in women a consequence of recurrence and metastasis. In recent years, a population of cancer cells has been identified, called cancer stem cells (CSCs) with self-renewal capacity, proposed to underlie tumor recurrence and metastasis. We previously showed that the adipose tissue cytokine LEPTIN, increased in obesity, promotes the survival of CSCs in vivo. Here, we tested the hypothesis that the Leptin Receptor (LEPR), expressed in mammary cancer cells, is necessary for maintaining CSC-like and metastatic properties. We silenced LEPR via shRNA lentivirus transduction and determined that expression of stem cell self-renewal transcription factors NANOG, SOX2, and OCT4 are inhibited. LEPR-NANOG signaling pathway is conserved between species because we can rescue NANOG expression in human LEPR-silenced cells with the mouse LepR. Using a NANOG promoter GFP reporter, we showed that LEPR is enriched in NANOG promoter active (GFP+) cells. Using lineage tracing, we showed that the GFP+ cells exhibit symmetric and asymmetric division and cell death. LEPR silenced MDA-MB-231 cells exhibit a mesenchymal to epithelial transition morphologically, increased E-CADHERIN and decreased VIMENTIN expression compared to control cells. Finally, LEPR silenced cells exhibit reduced cell proliferation, self-renewal in tumorsphere assays, and tumor outgrowth in xenotransplant studies. Given the emergence of NANOG as a pro-carcinogenic protein in multiple cancers, these studies suggest that inhibition of LEPR may be a promising therapeutic approach to inhibit NANOG and thereby neutralize CSC functions.
Keywords: Obesity, Breast cancer, Cancer Stem cell, Leptin Receptor, NANOG
Introduction
Breast cancer is the most common noncutaneous cancer in US women, and while early detection and therapeutic advances have improved outcome, nearly 25% of diagnosed patients go on to develop secondary tumors at either primary or distant sites (Abdulkarim, et al. 2011; Carey 2011; Hudis and Gianni 2011; Rakha, et al. 2007; Stingl and Caldas 2007; Vargo-Gogola and Rosen 2007). These secondary tumors are the cause of the poor outcomes and reduced survival in these patients (Sorlie, et al. 2001). Obesity is an established breast cancer risk factor and leads to poorer breast cancer outcomes in both pre- and postmenopausal women (Calle, et al. 2003).
The mechanisms underlying the connection between obesity and tumorigenesis remain poorly understood (Park, et al. 2011; Rose and Vona-Davis 2009). Adipose tissue has long been thought to be an inert lipid-storing tissue (Halberg, et al. 2008; Park et al. 2011; Vona-Davis and Rose 2007). However adipose tissue is now recognized as an endocrine organ secreting cytokines, hormones, and inflammatory mediators (Halberg et al. 2008), many of which can influence tumor growth, including leptin (LEP) (Brakenhielm, et al. 2004).
LEP is a metabolic hormone primarily secreted from fat cells and necessary for regulation of body weight (Zhang, et al. 1994). LEP binds to LEP receptor (LEPR) and stimulates the associated Janus Kinase 2 (JAK2) and activates Signal Transducer and Activator of Transcription 3 and 5 (STAT3, 5) extracellular regulated kinase (ERK), and PI3 Kinase/Akt (Friedman 1999; Myers, et al. 2008).
LEPR is also detected in peripheral tissues and is highly expressed in multiple tumors including breast, colon, prostate, and brain (Ando and Catalano 2011; Garofalo and Surmacz 2006; Park and Scherer 2011; Somasundar, et al. 2004). In human breast cancer, LEPR expression is directly correlated with poor overall prognosis (Garofalo, et al. 2006; Ishikawa, et al. 2004; Miyoshi, et al. 2006). Moreover, LEPR is expressed in 92% of triple negative breast cancers (TNBCs)(Otvos, et al. 2011), so called because these tumors do not express the estrogen and progesterone receptors or the HER2 oncogene and are refractory to current clinical strategies (Dent, et al. 2007; Hudis and Gianni 2011).
At the root of these leptin-associated processes is the ability of the LEPR to trigger JAK2/STAT signaling (Myers 2004), known pro-oncogenic pathways. JAK2/STAT3 signaling promotes cancer stem cell (CSC) survival and self-renewal thus LEPR may participate in stem cell signaling pathways. CSCs are recently discovered cancer cells that reside in subsets of breast tumors including TNBCs. CSCs have the ability to self-renew and may be associated with recurrence and metastasis in breast cancer (Al-Hajj, et al. 2003; Liu and Wicha 2010).
CSCs are molecularly characterized based on expression of cell surface receptors including integrin α6 (CD49f), integrin β1 (CD29), hylauronan receptor (CD44), and the stem cell self-renewal transcription factors NANOG, OCT4, and SOX2. NANOG has emerged as a pro-carcinogenic factor in cancer cell lines with CSC behaviors (Jeter, et al. 2009). Compared to control cells, NANOG silencing in cancer cells leads to reduced proliferation, self-renewal based on tumorsphere assays and tumors in xenograft transplant studies (Jeter et al. 2009; Jeter, et al. 2011). Thus, inhibition of NANOG expression may provide a novel therapeutic, though as a transcription factor, NANOG is a difficult drug target.
Research in our lab and others has led to the proposal that LEPR maintains cancers in a stem cell-like state (Feldman, et al. 2011; Zheng, et al. 2011). To interrogate this hypothesis, we generated LEPR silenced mammary cancer cells and assessed self-renewal, cell proliferation, and tumorigenicity in xenograft models. Moreover, because JAK2/STAT3 cytokine signaling is implicated in expression of the stem cell transcription factors, we assessed whether LEPR is necessary for expression of NANOG, OCT4, and SOX2, and in maintenance of cancer cells in an undifferentiated mesenchymal stem cell state. Our studies point to a necessary role of LEPR in maintenance of the stem cell and mesenchymal phenotype in TNBCs and suggest silencing of LEPR may be used to inhibit cancer progression by blocking expression of stem cell transcription factors in cancer stem cells.
Materials & Methods
Cell culture
M-Wnt cells were derived from spontaneous tumors that develop in MMTV-Wnt-1 transgenic mice (Dunlap, et al. 2012). Cells were maintained in RPMI with L-glutamine and 5% fetal bovine serum (FBS). MDA-MB-231 cells were purchased from American Tissue Culture Collection (ATCC, Manassas, VA) and maintained in Leibovitz L-15 medium (Sigma, St. Louis, MO) with 10 % fetal bovine serum (FBS).
Mice
Wild type C57BL/6J mice were purchased from the Jackson Laboratory. All mice were maintained in microisolator units and provided free access to food and water. All mouse procedures were performed under strict adherence to protocols approved by the Institute Animal Care and Use Committee at the Lerner Research Institute, Cleveland Clinic Foundation.
M-Wnt cells were orthotopically transplanted (200,000 cells/mouse) into the right mammary fat pad #4 of female mice at 6 weeks of age (n=3). Mice were monitored twice weekly until tumors were palpable then daily. 4 weeks post-inject, mice were euthanized and the tumors collected for histological analysis. Tumor volume was measured using an electronic caliper, applying the formula [volume = 0.52 × (width) × (height) × (length)] for approximating the volume of a spheroid.
Immunoblotting
Cells were lysed in buffer containing 20 mM Tris, pH 7.4, 137 mM NaCl, 1% NP-40, 10% glycerol, 20 mM NaF, 1 mM Na orthovanadate, and 1 mM PMSF. Protein concentrations were measured using BCA protein assay (Thermo, Rockford, IL). Membranes were incubated overnight at 4°C with primary antibodies. NANOG, integrin α6, STAT3, P-STAT3, Akt, P-Akt, ERK, and P-ERK were purchased from Cell Signaling (Beverly, MA) and actin from sigma (St. Louis, MO). Anti-rabbit IgG antibodies conjugated to Horseradish Peroxidase (HRP) (Amersham, Piscataway, NJ) were used as secondary antibodies and visualized using the West Pico Chemiluminescent substrate from Pierce (Rockford, IL).
RT-PCR analysis
Total RNA was isolated using TRI reagent (Ambion, Austin, TX) and stored at −80°C until use. RNA concentration was determined using NanoDrop 1000 Spectrophotometer (Thermo, Wilmington, DE). Reverse transcriptase (RT) reactions were prepared using a high capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). 2 μg of total RNA was used as template for first strand cDNA. Amplification of transcripts was performed using Taq DNA polymerase kit (Qiagen, Valencia, CA) with 200 ng of total RNA. Semi-quantitative RT-PCR was quantified by scanning the gels digitally followed by analysis using ImageJ (NIH, Bethesda, MD). Real-time PCR was performed on Steponeplus Real Time PCR system from Applied Biosystems via SYBR-Green Mastermix (Applied Biosystems). The threshold cycle (CT) values for each gene were normalized to expression levels of GAPDH (Applied Biosystems). Primer sequences provided in Supplemental Table 2.
Flow cytometry
Cells were resuspended in PBS containing 2 % FBS and Phycoerythrin (PE)-conjugated rat anti-mouse CD49f (1:100, eBioGoH3, eBioscience), PE-conjugated rat anti-mouse CD24 (1:100, 30-F1, eBioscience) and APC-conjugated rat anti-mouse CD44 (1:100, Pgp-1, eBioscience) at a concentration of 1 million cells/ml for 1 h on ice. After washing twice, cells were subjected to FACS analysis and sorting on BD LSR II cytometer (BD biosciences, CA). Data analysis was performed on the FlowJo version 8.8.6 software (Tree Star, Inc).
MTS cell growth assay
5,000 cells per well were cultured in 96-well plates. Cell proliferation was evaluated using colorimetric MTS assay (Promega) that measures restoration of 3-(4,5-dimethylthiazol-2-yl)-5-(3 carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) to formazan in metabolically active cells. Absorbance of the formazan at 490 nm was determined on a Spectramax 190 plate reader (Molecular Devices, Sunnyvale, CA).
Tumorsphere assays
M-Wnt cells, 1 cell per well was cultured in ultra low 96-well plates (Corning, NY) with 200 μl serum-free DMEM/F12 medium supplemented with 20 ng/ml basic fibroblast growth factor (invitrogen), 10ng/ml epidermal growth factor (Biosource), 2% B27 (invitrogen), 10μg/ml Insulin and 1μg /ml hydrochloride (Sigma). For MDA-MB-231cells, 1,000 cells per well were cultured in ultra low six-well plates. After 5-7 days, tumorspheres were counted under a Leica dissecting scope.
Lentiviral production and transduction
The lentiviral plasmid vector pLKO.1-puro based shRNA clones and control shRNA vector were purchased from Sigma-Aldrich (St Louis, MO, USA)(Supplemental Table 1). The pLKO.1/LEPR shRNA and CONT lentiviruses were purchased from Sigma. Transductions were carried out in serum free medium with either shRNA targeting LepR or control shRNA. After overnight incubation, viral particle containing medium was removed and replaced with fresh complete medium. After 2 days, cells were treated with 2.5 μg/ml puromycin. Cells were harvested 3 days after puromycin selection for RT-PCR, Western blotting and flow cytometry. NANOG promoter GFP lentiviral construct was obtained from System Bioscience (Mountain View, CA). Virus was transduced into MDA-MB-231 as described for shRNA viruses without puromycin selection.
Lineage Tracing Analysis
Lineage tracing was performed as previously described (Lathia, et al. 2011). Briefly, phase-contrast, time-lapse image stacks consisting of 288 frames (5 min intervals) and corresponding fluorescence image stacks (72 frames, 20 min intervals) were imported into Image-Pro Plus (v6.2, Media Cybernetics, Silver Spring, MD, USA). Lineage analysis was performed in a semiautomated manner using customized visual basic Image-Pro Plus macros. Briefly, intensity in each fluorescence stack was normalized across all time-points to account for photobleaching. Synchronized phase-contrast and time-lapse stacks were then displayed side by side and a user marked the center of a daughter cell, tracking the cell until either the final frame was reached, another division event was encountered, or cell death occurred. For each click a circular region of interest 6 pixels in diameter was placed at the click point on the corresponding fluorescence frame and mean intensity was recorded (au tomated process). This procedure was repeated for the corresponding daughter cell and any additional progeny for subsequent divisions of these daughter cells. Once the tracking of two daughter cells for a particular division event was completed, the mean fluorescence intensities, click coordinates, frame numbers, and cell health (live/dead) were exported to Excel for each tracking point for both daughter cells. Finally, the fluorescence image stacks were superimposed upon the corresponding phase-contrast images for validation and illustration.
Results
Targeting of leptin receptor with short hairpin RNA
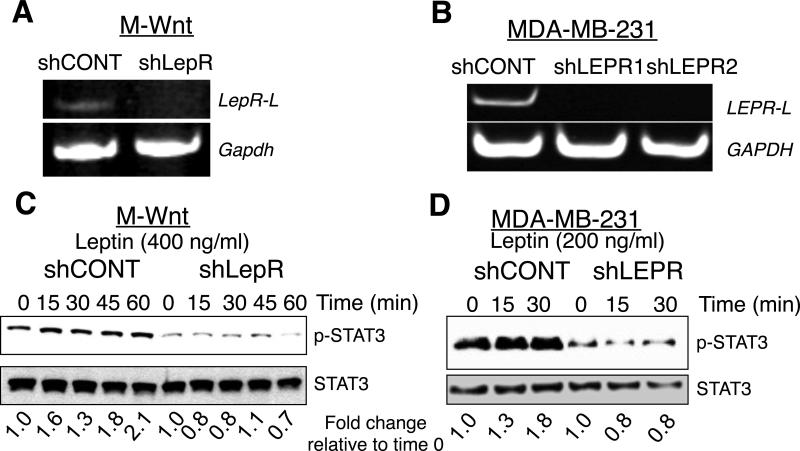
Tumor cells transplanted into LEP-deficient ob/ob mice exhibit reduced tumor initiating activity and reduced CSCs compared to wild type (WT) mice (Zheng et al. 2011). To test the hypothesis that LEPR was necessary for survival, self-renewal, and tumorigenicity of mammary cancer cells, we silenced LEPR using shRNA lentivirus. Since LEPR has not been silenced in mammary cancer cells, we screened shRNA lentiviral constructs for inhibition of mouse and human LEPR expression. Several shRNA constructs were identified, one mouse construct and two human constructs (Supplemental table 1). LEPR was inhibited in both the mouse mammary cancer M-Wnt cells (Dunlap et al. 2012) (Fig. 1A) and the human breast cancer MDA-MB-231 cells (Fig. 1B). LEP activates PI3 Kinase/Akt, extracellular regulated kinase (ERK), and Signal Transducer and Activator of Transcription 3 (STAT3). LEP activation of these pathways is documented for MDA-MB-231 cells but not for M-Wnt cells. Thus, we confirmed that silencing of LEPR in M-Wnt mammary cancer cells led to inhibition in phosphorylation of Akt, ERK, and STAT3 (supplemental Fig. 1).
Figure 1. shRNA silencing of leptin receptor inhibits basal and leptin-dependent signaling.
A. and B. Semi-quantitative RT-PCR analysis of leptin receptor long form in M-Wnt (LepR-L) and MDA-MB-231 (LEPR-L) cells transduced with either leptin receptor (LEPR) or control (CONT) shRNA. GAPDH was used as a loading control. C. and D. Analysis of LEP stimulated STAT3 phosphorylation in M-Wnt and MDA-MB-231 cells. M-Wnt cells were treated with 400 ng/ml and MDA-MB-231 cells were treated with 200 ng/ml leptin and at indicated times cells were extracted and analyzed for STAT-3 phosphorylation. Total STAT-3 was used as loading control. Fold induction of p-STAT3 corrected for total STAT3 is shown below each lane. Quantification was performed using ImageJ (NIH). Data are representative of an experiment repeated 3 times.
To determine if reduced LEPR expression is sufficient to cause changes in downstream signaling pathways, we evaluated STAT3 phosphorylation. LEP (400 ng/ml) stimulated endogenous STAT3 phosphorylation (p-STAT3) by 2-fold in control transduced M-Wnt and MDA-MB-231 cells with normal LEPR. In contrast, LEPR-silenced M-Wnt and MDA-MB-231 cells, LEP did not stimulate phosphorylation of STAT3 (Fig. 1C and D). Moreover, basal p-STAT3 was inhibited in LEPR silenced cells (Supplemental Fig. 2). These studies provide evidence that LEPR silencing results in signaling alterations in mouse and human mammary cancer cells.
LEPR regulates NANOG expression
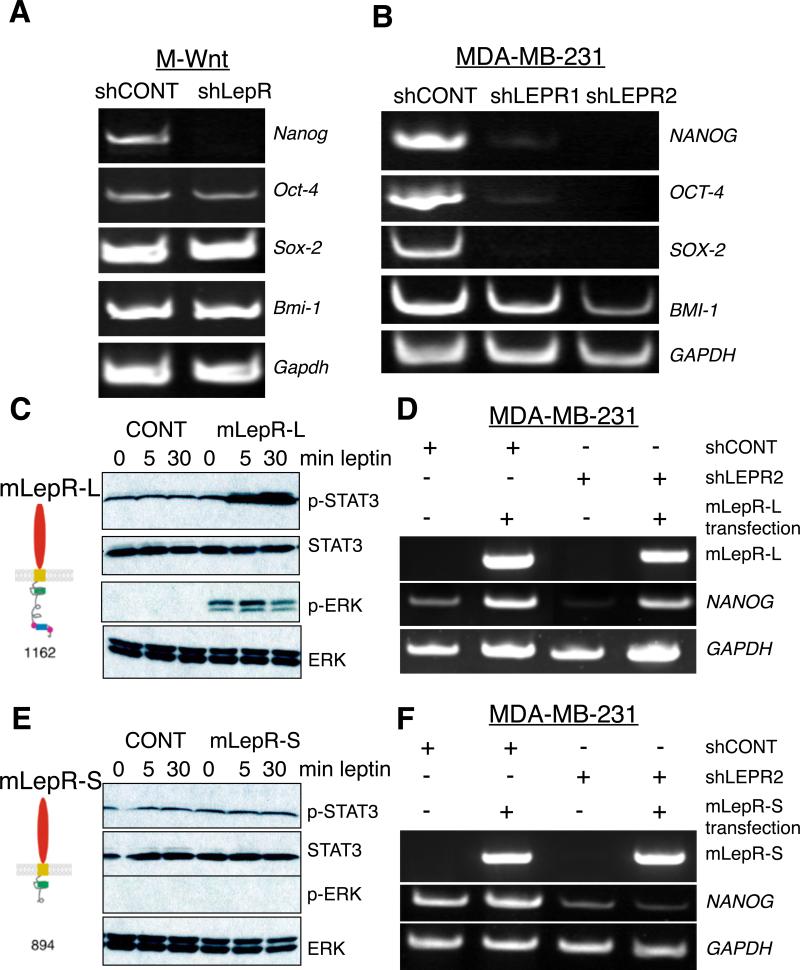
We next assessed whether silencing of LEPR altered the expression of stem cell transcription factors in mammary cancer cells because leptin deficiency leads to reduced CSCs (Zheng et al. 2011). NANOG, SOX2, OCT4, and BMI1 were analyzed by RT-PCR. All of these transcription factors are expressed in M-Wnt and MDA-MB-231 cells (Fig. 2A and B), whereas LEPR silenced cells do not express NANOG in either cell line (Fig. 2 A and B). Further, LEPR silenced MDA-MB-231 cells also do not express SOX2 and OCT4 (Fig. 2B). Relative expression corrected for GAPDH was quantified using NIH ImageJ (Supplemental Fig. 3). Further, we determined that NANOG protein expression is inhibited in LEPR silenced compared to control MDA-MB-231 cells (Supplemental Fig. 4).
Figure 2. Silencing of LEPR in M-Wnt and MDA-MB-231 leads to inhibition of stem cell transcription factors.
Analysis of M-Wnt cells A and MDA-MB-231 B for NANOG, SOX2, OCT4, and BMI1 by RT-PCR in LEPR or CONT shRNA treated cells. GAPDH was used as an RNA loading control. Quantification of data from A and B in is presented in Supplemental Fig. 3. Data are representative of four independent experiments. Average intensity corrected for ACTB or GAPDH for M-Wnt and MDA-MB-231 cells, respectively and presented as mean ± SEM (* p< 0.001). C. Analysis of leptin stimulated STAT3 and ERK in HEK 293 cells transfected with long form of the LepR. HEK293T cells were transfected with CONT or mLepRl cDNA. Cells were incubated with leptin (100 ng/ml) for 0, 5, 30 minutes and pSTAT3 and pERK expression were analyzed by immunoblotting. Total STAT3 and ERK were assayed to control for loading. D. Rescue of NANOG expression in LEPR silenced MDA-MB-231 cells by the long isoform of LepR. LEPR was silenced MDA-MB-231 cells followed by transfection with LepRl cDNA. E. Analysis of LEP stimulated STAT3 and ERK in LepRs cells. HEK293T cells were transfected with empty vector or mLepRs cDNA. Cells were incubated with LEP (100 ng/ml) for 0, 5, 30 minutes and pSTAT3 and pERK expression were analyzed by immunoblotting. Total STAT3 and ERK were assayed to control for loading. F. Lack of rescue of NANOG in LEPR silenced MDA-MB-231 cells by the mouse LepRs. LEPR silenced or CONT MDA-MB-231 cells were transfected with LepRs or empty vector cDNA and expression of NANOG was assayed by RT-PCR.
There are two major LEPR isoforms, short (LEPR-S) and long (LEPR-L), expressed in cancer cells. LEPR-L is best studied, as it activates JAK2/STAT signaling pathways. LEPR-S contains a short cytoplasmic domain and does not activate JAK2-dependent signaling pathways and its function remains unknown (Bjorbaek, et al. 2001). To exclude off-target effects of the LEPR shRNA and determine whether LEPR-L or LEPR-S isoforms are sufficient to rescue expression of the NANOG, OCT-4, and SOX-2, we transfected LEPR silenced MDA-MB-231 cells with either the mouse LepR-L or LepR-S cDNA and assayed for NANOG expression. As a negative control, we used an empty pcDNA3.1 plasmid. Human Embryonic Kidney (HEK) 293 cells were used to assess LepR-L and LepR-S signaling, since they are validated cells to assay leptin-stimulated LEPR activity (Banks, et al. 2000). Mouse LepR-L and LepR-S were transfected into HEK 293 cells and STAT3 and ERK phosphorylation was assayed. As expected, LEP stimulates rapid phosphorylation of STAT3 and ERK in HEK 293 cells overexpressing LEPR-L (Fig. 2C), whereas LEP could not stimulate either STAT3 or ERK phosphorylation in cells overexpressing LEPR-S (Fig. 2E). Next, we transfected mouse LepR-L or LepR-S in LEPR silenced MDA-MB-231 and assayed NANOG expression (Fig. 2D and 2F). Only mouse LepR-L was sufficient to rescue NANOG. Collectively, the data indicate the LEPR-L expression is necessary to maintain the cells undifferentiated in a stem cell state.
Time-lapse lineage tracing of NANOG expressing MDA-MB-231 cells identifies symmetric and asymmetric cell division and death
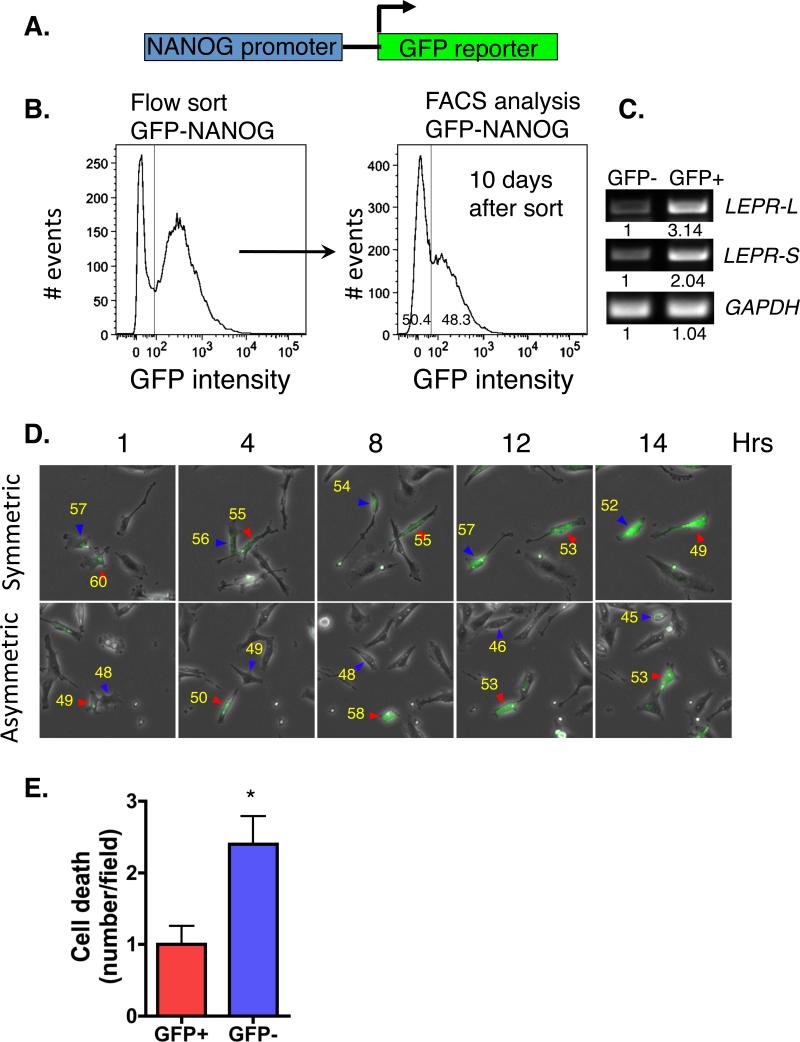
Because NANOG is a stem cell transcription factor, we asked whether it is expressed uniformly in MDA-MB-231 cells, and we assessed the fate of the NANOG expressing cells. We transduced MDA-MB-231 cells with a reporter in which the NANOG promoter drives GFP expression (Fig. 3A). Transduced cells were sorted to enrich for the GFP expressing (NANOG promoter active) population and cultured. Within 2 passages, the GFP enriched cells showed mixed expression of GFP positive (GFP+) and negative (GFP−) cells (Fig. 3B). Since the MDA-MB-231 cells were first sorted for > 95% GFP+ this suggests that these cells are executing multiple modes of division (symmetric, asymmetric). We next assessed expression of LEPR-L and LEPR-S in the GFP+ and GFP− cells and determined that GFP+ express 2-3 fold higher levels of both isoforms of LEPR compared with GFP− cells (Fig. 3C). We independently confirmed that GFP+ cells exhibit increased expression of NANOG compared with GFP− cells.
Figure 3. NANOG expressing MDA-MB-231 cells co-express LEPR and proliferate asymmetrically.
A. Reporter construct used to generate NANOG promoter active cells. MDA-MB-231 cells were transduced with lentivirus and sorted for GFP-expression. B. MDA-MB-231 cells expressing GFP (NANOG-Promoter active, GFP+) divide asymmetrically. GFP-NANOG transduced cells were flow sorted and GFP high, > 103 intensity (left panel) were isolated and cultured for an additional 10 day. GFP+ and GFP− MDA-MB-231 cells quantification by FACS (right panel). C. GFP expressing MDA-MB-231 cells coexpress LEPR. GFP− and GFP+ expressing MDA-MB-231 cells were sorted, RNA extracted and expression of LEPRl, LEPRs, and GAPDH analyzed by RT-PCR. Data are representative of an analysis repeated three times. D. MDA-MB-231 GFP cells exhibit symmetric and asymmetric cell division based on live imaging digital fluorescent microscopy analysis. Differential interference contrast images were obtained every 5 minutes and fluorescent images collected every 20 minutes. Still images at indicated times are presented from time-lapse movies. Data are representative of observations from over 400 cell divisions. Based on fluorescence intensity of the daughter cells (indicated in yellow) collected over 12-15 hours, we calculated symmetric and asymmetric cell division frequency. Red and blue arrowheads denote the individual tracked cells. E. In parallel, cell death was analyzed microscopically. We quantified the number of cell death events in the GFP+ and GFP− MDA-MB-231 in 10 fields with at least 50 cells/field. Data are presented as mean ± SEM (* p < 0.01).
To evaluate the modes of cell division, cells were cultured, monitored, and tracked over a 48-hour time period. We determined that the GFP+ MDA-MB-231 cells divide through symmetric division, leading to the generation of two daughter cells that both express GFP (Fig. 3D). Cells could also undergo asymmetric division leading to the production of one daughter cell that is GFP+ and second daughter cell, which is GFP− (Fig. 3D). Thus, GFP+ MDA-MB-231 cells generate both NANOG promoter active and inactive cells. In parallel, cell death was quantified. We determined that GFP− cells exhibited significantly (1.5 times) greater cell death compared to cells that are GFP + (Fig. 3E). The data suggest that inhibiting NANOG expression may lead to differentiation, cell death, and reduced CSC behaviors.
LEPR is necessary for maintenance of MDA-MB-231 in a mesenchymal state
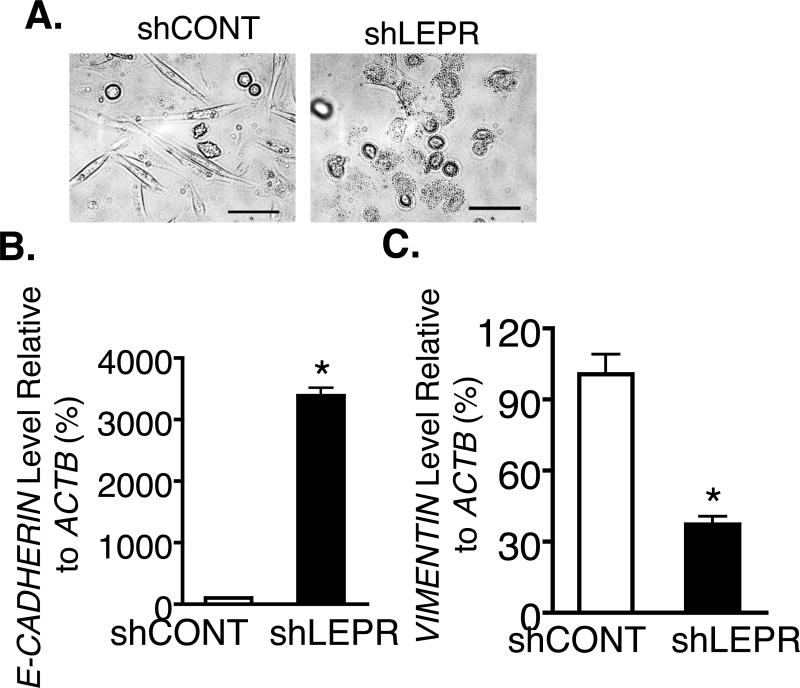
MDA-MB-231 cells are TNBCs with mesenchymal stem cell-like character (Lehmann, et al. 2011). In contrast, LEPR silenced cells exhibit a non-mesenchymal rounded epithelial-like morphology (Fig. 4A). Further, real Time PCR indicated that E-cadherin, a terminal differentiation epithelial marker, is significantly induced 300-fold in LEPR silenced MDA-MB-231 cells compared to controls (Fig. 4B). In parallel, vimentin, a mesenchymal marker, was significantly inhibited by 60% in LEPR silenced cells (Fig. 4C). This suggests that LEPR silenced cells undergo a mesenchymal to epithelial transition.
Figure 4. Inhibition of LEPR leads to mesenchymal to epithelial transformation.
A. LEPR silenced and control MDA-MB-231 were imaged phase contrast microscopy. Representative images shown (bar = 100 μm). B and C. Real Time PCR analysis of E-CADHERIN and VIMENTIN in CONT and LEPR shRNA transduced MDA-MB-231 cells. Results are representative of an analysis repeated three times.
LEPR silenced mammary cancer cells exhibit reduced CSC frequency
These findings indicate that LEPR silenced breast cancer cells were differentiated and indicated that LEP promotes stem cell behaviors. We performed cell proliferation assays using an MTS assay and determined that LEPR silenced cells exhibit reduced viability (Supplemental Fig. 5). Thus, we assessed cell death using the DeadEnd Fluorometric TUNEL system (Promega) but we did not detect a significant increase in apoptosis in shLEPR compared to shCONT cells. We speculate that the inhibition in LEPR expression leads to a reduction in cell proliferation with limited increase in cell death.
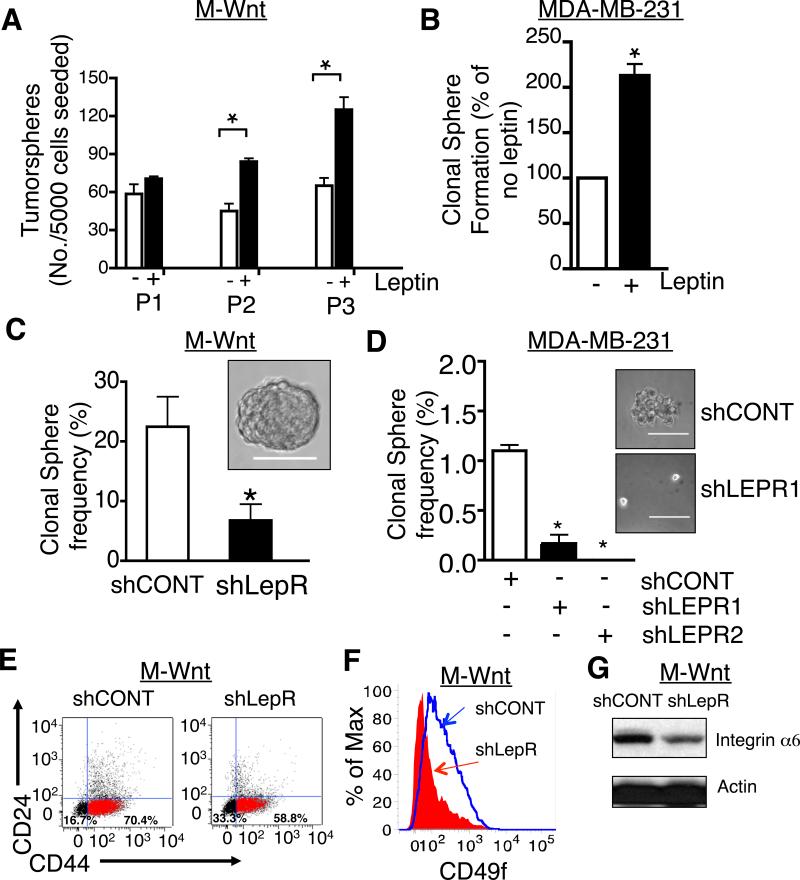
Next, we evaluated the effect of LEP on CSC self-renewal in tumorsphere cultures. M-Wnt cells were plated in tumorsphere cultures in the absence or presence of LEP (400 ng/ml). Once tumorspheres formed spheroids were counted, collected and recultured as tumorspheres and the procedure was repeated. The analysis revealed that LEP led to an increase in number of tumorspheres compared to cells cultured in the absence of leptin (Fig. 5A). In parallel, we assayed the effect of LEP on tumorsphere formation in MDA-MB-231 cells. LEP (200 ng/ml) increased tumorsphere formation by 2-fold compared to its absence (Fig. 5B). This indicates that LEP via LEPR is sufficient to increase the viability of CSCs in culture.
Figure 5. Inhibition of LEPR expression in mammary cancer cells results in reduced tumorsphere formation.
A. Effect of LEP on tumorsphere formation in M-Wnt cells. 5000 cells were plated in 6 well ultra-low plates in the absence or presence of 400 ng/ml leptin. After 10 days, spheres were counted from each well (P1). Spheres were dissociated and protocol repeated 2 times, P2 and P3. Data are presented as mean ± SEM. B. Effect of LEP on tumorsphere formation in MDA-MB-231 cells. 5000 cells were plated in 6 well ultra-low plates in the absence or presence of 100 ng/ml leptin. After 10 days, spheres were counted from each well. Data are presented as % mean ± SEM corrected for no leptin. Analysis was repeated at least 3 times. C. Tumorsphere formation in LepR and CONT M-Wnt cells. Single M-Wnt cells from LepR or CONT transduced lentiviruses were cultured in ultra low 96-well plates. After 5 days, the tumorsphere containing wells were counted under a Leica dissecting scope. Representative tumorsphere shown in panel (bar = 100 μm). Data are average results from four independent experiments and presented as mean ± SEM (* p <0.05). D. Tumorsphere formation in LEPR and CONT shRNA transduced MDA-MB-231 cells. LEPR1, LEPR2, and CONT shRNA transduced cells were cultured at a density of 1000 cells/well in 6 well ultra-low plates. After 5-7 days, tumorspheres were counted under a Leica dissecting scope. Each bar represents the mean ± SEM, * p <0.05. Analysis is representative of data from 3 different experiments. Representative tumorsphere shown in panel. E. CD44+CD24− population was quantified in LepR and CONT transduced M-Wnt cells. Cell were stained with APC-CD44 and PE-CD 24 and analyzed by fluorescence activated cell sorting (FACS). F. CD49f/integrin α6 was quantified in LepR and CONT shRNA transduced M-Wnt cells. Data shown is a representative analysis of FACS experiment that was repeated three times. G. Analysis of expression of Integrin α6 levels by immunoblotting LepR and CONT shRNA transduced M-Wnt cells.
We next assessed whether LEPR silencing would inhibit tumorsphere formation in M-Wnt and MDA-MB-231 cells. To determine the stem cell frequency, we cultured single viable cells per well in a 96-well ultra-low binding plate. After 7-10 days, wells containing single tumorspheres were counted (Fig. 5C). The clonal sphere formation frequency in shRNA control transduced M-Wnt cells was 22.5 ± 10 and in LepR shRNA transduced cells was 6.8 ± 5.4 (p <0.05). This indicates that LepR silencing leads to decreased CSCs. Likewise, we performed tumorsphere formation assays in LEPR shRNA and control transduced MDA-MB-231 cells. LEPR silencing led to a >80% reduction in sphere formation frequency (shCONT 1.1 ± 0.1, shLEPR1 0.17 ± 0.15, and shLEPR2 no sphere formation frequency; Fig. 5D).
As further evidence that silencing LEPR reduces the percentage of CSCs, cells were analyzed by fluorescence activated cell sorting (FACS) for the breast CSC markers CD44+CD24− and CD49f. We determined that the proportion of CD44+CD24− cells was reduced by greater than 10% in LEPR-silenced relative to control M-Wnt cells (Fig. 5E). In parallel, we observed a 4-fold reduction in CD49f/integrin α6 immunoreactive cells in LepR silenced compared to control cells (Fig. 5F). Further, integrin α6 expression, based on immunoblotting, was significantly inhibited in LepR silenced compared to control M-Wnt cells (Fig. 5G). Collectively, the data indicate that silencing of LEPR expression in mouse and human mammary cancer cells leads to fewer CSCs.
LEPR silenced mammary cancer cells exhibit inhibition in tumor outgrowth
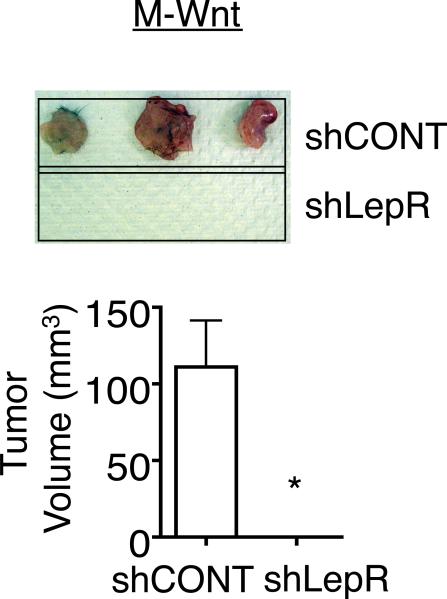
To assess the affect of LEPR silencing on tumor formation, control and LepR shRNA transduced M-Wnt cells were orthotopically transplanted (200,000 cells/mouse) into syngeneic C57Bl/6J female mice. 100% of control transduced cells formed tumors that were > 100 mm3 within 4 weeks (Fig. 6). In contrast, no tumors formed in mice transplanted with the LEPR silenced cells (Fig. 6). This provides evidence that LEPR is necessary for maintenance of a tumorigenic phenotype.
Figure 6. shRNA inhibition of LEPR leads to tumor outgrowth in vivo.
Tumor outgrowth in mice injected with LEPR and CONT shRNA transduced M-Wnt cells. Wild type (WT) mice were injected s.c. with 200,000 cells. After 4 weeks, mice were euthanized, tumors excised, and the tumor volume was measured. Data are presented as mean ± SEM of 3 mice (* p< 0.001).
Discussion
Our findings indicate that in both murine and human mammary cancer cells, LEPR is necessary for maintenance and self-renewal of undifferentiated cancer cells possessing CSC hallmarks. Inhibition of LEPR expression leads to inhibition of NANOG, a master regulator of self-renewal in normal stem cells, viability of CSCs, proliferation, and tumor-initiating activity. Importantly, LEPR-NANOG signaling is highly conserved because we showed that mouse LepR is able to rescue NANOG expression in LEPR silenced human breast cancer cells. Further, mouse LepR rescues the inhibited cell proliferation in LEPR silenced cells. Moreover, LEPR is necessary to maintain a mesenchymal and invasive state as evidenced by the transcriptional profiling of LEPR silenced and control breast cancer cells.
LEPR is a cytokine receptor and LEP binding leads to activation of PI3K/Akt, ERK, and STAT3 signaling pathways. Previous studies have linked PI3K/Akt, ERK, and/or STAT3 signaling to cellular behaviors including cell proliferation, migration, and angiogenesis (Gonzalez and Leavis 2003; Hu, et al. 2002; Mauro, et al. 2007; Sharma, et al. 2006). By silencing LEPR, the current studies reveal a necessary role for LEPR in CSC phenotypes including proliferation, invasion, self-renewal, and tumorigenicity.
The LEPR silenced cancer cells provide critical evidence that LEPR is necessary for expression of the core stem cell transcription factor NANOG in both mouse and humans. In humans, LEPR is also necessary for expression of SOX2 and OCT4. The differential regulation of SOX2 and OCT4 may indicate that the upstream regulation of these transcription factors is independent of JAK2/STAT3 signaling pathways in mouse cancer stem cells. Indeed, in embryonic stem cells, SOX2 is regulated by STAT3 whereas NANOG is regulated by AKT signaling. Thus, we speculate that in mouse cancer stem cells LepR via Akt signaling regulates Nanog expression, whereas in human cancer stem cells LEPR via STAT3 and AKT signaling pathways regulates NANOG, SOX2, and OCT4 expression.
LEPR-stem cell signaling is highly conserved between species because the mouse LepR can rescue the human silenced breast cancer cells. Further, we reveal that LEPR is necessary for maintaining breast cancer cells in an invasive mesenchymal state. Our approach is unprecedented in studying LEPR in cancer cells and we will use the same approach to tease apart the LEPR signaling pathways necessary for proliferation, migration/invasion, and angiogenesis.
Recent studies by Machida and colleagues indicate that LEPR is regulated by the self-renewal transcription factors SOX2 and OCT4 (Feldman et al. 2011). Further, their data suggest that LEP, in a STAT3-dependent manner, regulates SOX2 and OCT4. In their studies, Machida and colleagues present a LEPR-SOX/OCT4 self-reinforcing loop. Our studies complement Machida, though there are several notable differences. Here we show that LEPR is necessary for regulation of NANOG expression in both mice and human cells and for cell proliferation and tumorigenesis. Moreover, LEPR silenced cancer cells are not capable of initiating tumor outgrowth pointing to a necessary role for LEPR in tumorigenesis. Collectively, these studies are the first to show that LEPR has a necessary role, not just an accessory one, in breast cancer and specifically self-renewal of CSCs and tumorigenesis.
The MDA-MB-231 cells tagged with the GFP-NANOG reporter provide a unique model to track NANOG expressing breast cancer cells and their contribution to tumor progression and metastasis. Using these cells we can determine whether NANOG promoter active (GFP+) cells (putative CSCs) home to specific niches in the tumor as well as target to other organs/tissues such as bone, lung, and brain, known sites of breast cancer metastasis (Aslakson and Miller 1992; Charafe-Jauffret, et al. 2009; Lin, et al. 2008). Because these cells coexpress the LEPR we will determine whether inhibiting LEP or LEPR is sufficient to block survival, migration/invasion, and tumorigenicity in NANOG expressing CSCs. Finally, these reporter cells may prove to be a unique model to study the role of obesity in breast cancer progression, recurrence, and metastasis.
Obesity is an established risk factor for multiple cancers including those of the breast (Calle et al. 2003; Roberts, et al. 2010). Leptin was an early candidate thought to link obesity and cancer, though leptin receptor antagonists have yet to progress to the clinic (Ando and Catalano 2011; Garofalo and Surmacz 2006; Hu et al. 2002; Lautenbach, et al. 2009; Surmacz 2007). The studies of Goodwin and colleagues further suggest that leptin is a promoter of breast cancer recurrence and distant metastasis leading to overall poor patient survival, particularly in late stage breast cancer (Goodwin, et al. 2012). This clinical observation is consistent with a role for leptin and leptin receptor in cancer stem cells. Because CSCs have been implicated in recurrence and metastasis, our studies suggest a mechanistic link between obesity, leptin, and cancer recurrence and metastasis. This leads us to speculate that obesity and its associated increase level in leptin would exhibit an increase in maintenance of CSCs and thus increased recurrence, distant metastasis, and overall poor patient survival.
Our studies, as well as several recent reports in the literature in breast and other tumor cell models (Jeter et al. 2009; Jeter et al. 2011) suggest that NANOG is a key regulator of CSC self-renewal, clonogenic growth and tumorigenicity. However, NANOG is a particularly challenging therapeutic target given its role as a master transcription factor important in several cellular functions in CSC and non-cancer stem cells, including adipose-derived and mammary gland stem cells (Dentelli, et al. 2012; Kaimala, et al. 2012). Our novel findings of the inhibitory effects of LEPR silencing on NANOG expression, CSC self-renewal, cell proliferation, and tumor outgrowth in murine and human breast cancer cell lines suggest LEPR or components of its downstream signaling pathway may be promising as a druggable targets to inhibit the pro-cancer effects associated with NANOG. This may be particularly important for treating breast and other cancers in the ever-increasing population of obese, hyperleptinemic women, who relative to normoweight women typically have a poorer prognosis.
Supplementary Material
Acknowledgements
We would like to thank members of the Lerner Research Institute Flow Cytometry Core (Cathy Shemo and Sage O'Bryant) for assistance with the FACS and cell sorting.
Funding:
Research was supported by funds to OR from the Cleveland Clinic Foundation, NCI TREC development grant (U54CA116867, Berger (PI)), Case Comprehensive Cancer Center Pilot grant and Special Funds in Aging Cancer Energy Balance Research (P30 CA043703), the American Cancer Society (Grant #IRG-91-022-15), and the Cleveland Clinical Translational Sciences Collaborative (UL1RR024989). This work was also supported by a K99/R00 Pathway to Independence Award CA157948 (JDL) from the NIH, V Foundation for Cancer Research (JDL), Voices Against Brain Cancer (JDL), American Cancer Society (JDL), and the Ohio Cancer Research Associates (JDL). SD was funded by USMRMC FY08 Breast Cancer Research Program Postdoctoral Fellowship (W81XWH-09-1-0720).
Footnotes
The authors declare no potential conflicts of interest with findings presented in this manuscript. AV is an employee of Image IQ.
Declaration of interest:
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Abdulkarim BS, Cuartero J, Hanson J, Deschenes J, Lesniak D, Sabri S. Increased risk of locoregional recurrence for women with T1-2N0 triple-negative breast cancer treated with modified radical mastectomy without adjuvant radiation therapy compared with breast-conserving therapy. J Clin Oncol. 2011;29:2852–2858. doi: 10.1200/JCO.2010.33.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando S, Catalano S. The multifactorial role of leptin in driving the breast cancer microenvironment. Nat Rev Endocrinol. 2011 doi: 10.1038/nrendo.2011.184. [DOI] [PubMed] [Google Scholar]
- Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52:1399–1405. [PubMed] [Google Scholar]
- Banks AS, Davis SM, Bates SH, Myers MG., Jr. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Buchholz RM, Davis SM, Bates SH, Pierroz DD, Gu H, Neel BG, Myers MG, Jr., Flier JS. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem. 2001;276:4747–4755. doi: 10.1074/jbc.M007439200. [DOI] [PubMed] [Google Scholar]
- Brakenhielm E, Cao R, Gao B, Angelin B, Cannon B, Parini P, Cao Y. Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ Res. 2004;94:1579–1588. doi: 10.1161/01.RES.0000132745.76882.70. [DOI] [PubMed] [Google Scholar]
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- Carey LA. Directed therapy of subtypes of triple-negative breast cancer. Oncologist. 2011;16(Suppl 1):71–78. doi: 10.1634/theoncologist.2011-S1-71. [DOI] [PubMed] [Google Scholar]
- Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, Hur MH, Diebel ME, Monville F, Dutcher J, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–1313. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- Dentelli P, Barale C, Togliatto G, Trombetta A, Olgasi C, Gili M, Riganti C, Toppino M, Brizzi MF. A diabetic milieu promotes OCT4 and NANOG production in human visceral-derived adipose stem cells. Diabetologia. 2012 doi: 10.1007/s00125-012-2734-7. [DOI] [PubMed] [Google Scholar]
- Dunlap SM, Chiao LJ, Nogueira L, Usary J, Perou CM, Varticovski L, Hursting SD. Dietary Energy Balance Modulates Epithelial-To-Mesenchymal Transition and Tumor Progression in Murine Claudin-Low and Basal-Like Mammary Tumor Models. Cancer Prev Res (Phila) 2012 doi: 10.1158/1940-6207.CAPR-12-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE, Chen C, Punj V, Tsukamoto H, Machida K. Pluripotency factor- mediated expression of the leptin receptor (OB-R) links obesity to oncogenesis through tumor-initiating stem cells. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1114438109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JM. Leptin and the regulation of body weight. Harvey Lect. 1999;95:107–136. [PubMed] [Google Scholar]
- Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, Russo A, Sulkowski S, Surmacz E. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res. 2006;12:1447–1453. doi: 10.1158/1078-0432.CCR-05-1913. [DOI] [PubMed] [Google Scholar]
- Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol. 2006;207:12–22. doi: 10.1002/jcp.20472. [DOI] [PubMed] [Google Scholar]
- Gonzalez RR, Leavis PC. A peptide derived from the human leptin molecule is a potent inhibitor of the leptin receptor function in rabbit endometrial cells. Endocrine. 2003;21:185–195. doi: 10.1385/ENDO:21:2:185. [DOI] [PubMed] [Google Scholar]
- Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Taylor SK, Hood N. Insulin- and obesity-related variables in early-stage breast cancer: correlations and time course of prognostic associations. J Clin Oncol. 2012;30:164–171. doi: 10.1200/JCO.2011.36.2723. [DOI] [PubMed] [Google Scholar]
- Halberg N, Wernstedt-Asterholm I, Scherer PE. The adipocyte as an endocrine cell. Endocrinol Metab Clin North Am. 2008;37:753–768. x–xi. doi: 10.1016/j.ecl.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin--a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst. 2002;94:1704–1711. doi: 10.1093/jnci/94.22.1704. [DOI] [PubMed] [Google Scholar]
- Hudis CA, Gianni L. Triple-negative breast cancer: an unmet medical need. Oncologist. 2011;16(Suppl 1):1–11. doi: 10.1634/theoncologist.2011-S1-01. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Kitayama J, Nagawa H. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin Cancer Res. 2004;10:4325–4331. doi: 10.1158/1078-0432.CCR-03-0749. [DOI] [PubMed] [Google Scholar]
- Jeter CR, Badeaux M, Choy G, Chandra D, Patrawala L, Liu C, Calhoun-Davis T, Zaehres H, Daley GQ, Tang DG. Functional evidence that the self-renewal gene NANOG regulates human tumor development. Stem Cells. 2009;27:993–1005. doi: 10.1002/stem.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeter CR, Liu B, Liu X, Chen X, Liu C, Calhoun-Davis T, Repass J, Zaehres H, Shen JJ, Tang DG. NANOG promotes cancer stem cell characteristics and prostate cancer resistance to androgen deprivation. Oncogene. 2011 doi: 10.1038/onc.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaimala S, Bisana S, Kumar S. Mammary gland stem cells: more puzzles than explanations. J Biosci. 2012;37:349–358. doi: 10.1007/s12038-012-9200-z. [DOI] [PubMed] [Google Scholar]
- Lathia JD, Hitomi M, Gallagher J, Gadani SP, Adkins J, Vasanji A, Liu L, Eyler CE, Heddleston JM, Wu Q, et al. Distribution of CD133 reveals glioma stem cells self- renew through symmetric and asymmetric cell divisions. Cell Death Dis. 2011;2:e200. doi: 10.1038/cddis.2011.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenbach A, Budde A, Wrann CD, Teichmann B, Vieten G, Karl T, Nave H. Obesity and the associated mediators leptin, estrogen and IGF-I enhance the cell proliferation and early tumorigenesis of breast cancer cells. Nutr Cancer. 2009;61:484–491. doi: 10.1080/01635580802610115. [DOI] [PubMed] [Google Scholar]
- Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;113:2638–2645. doi: 10.1002/cncr.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Wicha MS. Targeting Breast Cancer Stem Cells. J Clin Oncol. 2010 doi: 10.1200/JCO.2009.27.5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro L, Catalano S, Bossi G, Pellegrino M, Barone I, Morales S, Giordano C, Bartella V, Casaburi I, Ando S. Evidences that leptin up-regulates E-cadherin expression in breast cancer: effects on tumor growth and progression. Cancer Res. 2007;67:3412–3421. doi: 10.1158/0008-5472.CAN-06-2890. [DOI] [PubMed] [Google Scholar]
- Miyoshi Y, Funahashi T, Tanaka S, Taguchi T, Tamaki Y, Shimomura I, Noguchi S. High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. Int J Cancer. 2006;118:1414–1419. doi: 10.1002/ijc.21543. [DOI] [PubMed] [Google Scholar]
- Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- Myers MG., Jr. Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res. 2004;59:287–304. doi: 10.1210/rp.59.1.287. [DOI] [PubMed] [Google Scholar]
- Otvos L, Jr., Kovalszky I, Riolfi M, Ferla R, Olah J, Sztodola A, Nama K, Molino A, Piubello Q, Wade JD, et al. Efficacy of a leptin receptor antagonist peptide in a mouse model of triple-negative breast cancer. Eur J Cancer. 2011 doi: 10.1016/j.ejca.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Park J, Euhus DM, Scherer PE. Paracrine and Endocrine Effects of Adipose Tissue on Cancer Development and Progression. Endocr Rev. 2011 doi: 10.1210/er.2010-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Scherer PE. Leptin and cancer: from cancer stem cells to metastasis. Endocr Relat Cancer. 2011;18:C25–29. doi: 10.1530/ERC-11-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, Ellis IO. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109:25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61:301–316. doi: 10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- Rose DP, Vona-Davis L. Influence of obesity on breast cancer receptor status and prognosis. Expert Rev Anticancer Ther. 2009;9:1091–1101. doi: 10.1586/era.09.71. [DOI] [PubMed] [Google Scholar]
- Sharma D, Saxena NK, Vertino PM, Anania FA. Leptin promotes the proliferative response and invasiveness in human endometrial cancer cells by activating multiple signal-transduction pathways. Endocr Relat Cancer. 2006;13:629–640. doi: 10.1677/erc.1.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasundar P, McFadden DW, Hileman SM, Vona-Davis L. Leptin is a growth factor in cancer. J Surg Res. 2004;116:337–349. doi: 10.1016/j.jss.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl J, Caldas C. Molecular heterogeneity of breast carcinomas and the cancer stem cell hypothesis. Nat Rev Cancer. 2007;7:791–799. doi: 10.1038/nrc2212. [DOI] [PubMed] [Google Scholar]
- Surmacz E. Obesity hormone leptin: a new target in breast cancer? Breast Cancer Res. 2007;9:301. doi: 10.1186/bcr1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargo-Gogola T, Rosen JM. Modelling breast cancer: one size does not fit all. Nat Rev Cancer. 2007;7:659–672. doi: 10.1038/nrc2193. [DOI] [PubMed] [Google Scholar]
- Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer. 2007;14:189–206. doi: 10.1677/ERC-06-0068. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Dunlap SM, Zhu J, Downs-Kelly E, Rich J, Hursting SD, Berger NA, Reizes O. Leptin deficiency suppresses MMTV-Wnt-1 mammary tumor growth in obese mice and abrogates tumor initiating cell survival. Endocr Relat Cancer. 2011;18:491–503. doi: 10.1530/ERC-11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.