Abstract
Cytochrome P450 mono-oxygenases (P450s) are the principal enzymes involved in the oxidative metabolism of drugs and other xenobiotics. In this protocol, we describe a fluorescence-based, high-throughput assay for measuring the activity of P450 3A4, one of the key enzymes involved in drug metabolism. The assay involves the oxidative debenzylation of a substituted coumarin, yielding an increase in fluorescence on reaction. The entire procedure can be accomplished in 1 h or less.
INTRODUCTION
Cytochrome P450 (P450) mono-oxygenases are heme-thiolate proteins that are found in almost all living organisms. In addition to the biosynthesis of essential endogenous molecules, such as steroids and eicosanoids, they are also responsible for the oxidative metabolism of a wide variety of xenobiotics1,2. In all, 57 genes are found in humans, but five of the P450 enzymes—1A2, 2C9, 2C19, 2D6 and 3A4—are responsible for >90% of metabolism of drugs currently in clinical use2–4. Considerable emphasis is placed on the role of these P450s in the pharmaceutical industry, in terms of assays for contributions to metabolism of new chemical entities and enzyme inhibition.
Various in vitro assays for measuring the activity and inhibition of P450s have been developed over the years5. The most commonly applied method today is to use recombinant P450s with probe substrates, with the resulting products resolved by high-performance liquid chromatography (HPLC) and detected by a mass spectrometer (MS) coupled to the HPLC system6–8. These HPLC–MS based assays are highly sensitive and have been used as an industry standard method for P450 inhibition assessments. However, this method cannot be fit into a high-throughput format and may be problematic when large numbers of samples need to be tested (e.g., profiling the inhibitory potential of a chemical library against a specific P450 oxygenase). In this situation, fluorescence-based P450 assays are much faster and more cost effective than HPLC–MS assays9,10. In these assays, P450 oxidizes a pro-fluorescent molecule to a fluorescent product. This product can be directly measured using a fluorescence microplate reader. However, all fluorescence measurements must be cautiously monitored for fluorescence interference or fluorescence quenching from tested compounds. In principle such assays can be carried out whenever the fluorescence of a reaction product is sufficient to be detected accurately. Examples include our work with P450 1A2 and resorufins11 and P450 2A6 and coumarin 7-hydroxylation12.
In this protocol, we present a microtiter plate-based fluorescence assay for the activity of P450 3A4. A number of coumarins and other fluorophores have been used in P450 fluorescence assays13–15. 7-Benzoyloxy-4-trifluoromethyl coumarin (BFC) is used in this assay as the probe substrate and undergoes O-dealkylation to give the fluorescent product 7-hydroxy-4-trifluoromethyl coumarin (HFC, λexcitation 405 nm/λemission 510–545 nm) (Fig. 1)15. In this protocol, we demonstrate how this assay can be applied in the determination of the IC50 value of ketoconazole, a potent inhibitor of P450 3A4.
Figure 1.
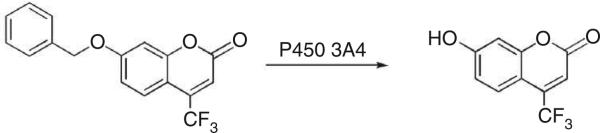
O-Dealkylation of 7-benzoyloxy-trifluoromethyl coumarin by P450 3A4.
The literature contains fluorescent reactions that can be used with other P450s11–15. Another alternative for high-throughput P450 activity assays is luminescence methods. Analogous to the fluorescence assays, a pro-luminescent substrate is used and yields a product that emits luminescence upon the addition of a developing agent16. The advantage of luminescence assays is the improved signal-to-noise ratio compared with fluorescence. The major drawback is that an additional development step is needed and the signal cannot be monitored in a continuous mode.
Experimental design
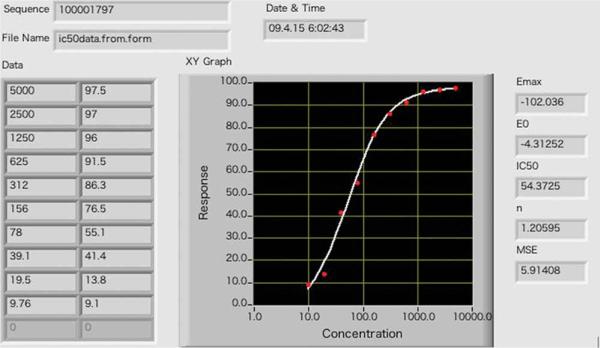
The protocol can be applied to directly determine IC50 values for compounds that are potential P450 3A4 inhibitors. Recombinant P450 3A4 protein without other P450s is recommended in this assay, because other P450s, e.g., 1A2, can also transform BFC to HFC. The compounds to be studied should be dissolved in water if they are soluble. Otherwise, prepare the compounds in acetonitrile or methanol. Adjust the concentration of the inhibitor so that the organic solvent is < 1% (vol/vol) in the final reaction to avoid interference with P450 catalytic activities. The plate setup used in this protocol is described in Table 1. The reaction is carried out in duplicate in rows A and B. Varying amounts of ketoconazole are added to the wells (columns 1 through 10). Well 11 serves as the blank control, because the stopping buffer is added before the addition of the NADPH-regenerating system. The fraction of activity remaining with inactivated P450 3A4 (obtained in the presence of ketoconazole) compared with the activity in the absence of any added compound to the reaction mixture (column 12, control, absence of P450 3A4 inhibition) is used in the calculations. The ratio of inactivated P450 is plotted as a function of ketoconazole concentration using a 4-parameter logistic equation: y = a + (b – a)/(1 + 10(x–c)d)) using the software available at http://www.changbioscience.com/stat/ec50.html.
TABLE 1.
Plate setup: The concentration of ketoconazole (nM) in each well is listed. The row designations are from A to B, and the column designations are from 1 to 12.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 5000 | 2500 | 1250 | 625 | 312 | 156 | 78 | 39.1 | 19.5 | 9.76 | Blank | No inhibitor |
| B | 5000 | 2500 | 1250 | 625 | 312 | 156 | 78 | 39.1 | 19.5 | 9.76 | Blank | No inhibitor |
MATERIALS
REAGENTS
Human P450 3A4 ‘bicistronic’ membranes17 containing both P450 and NADPH-P450 reductase (concentration of P450 is 1 μM). Similar preparations are available from BD Bioscience, cat. no. 456202.
Potassium phosphate (Sigma-Aldrich, cat. no. P9791)
NADP+ (Sigma-Aldrich, cat. no. N5755)
Glucose-6-phosphate (Sigma-Aldrich, cat. no. G7879)
Yeast glucose-6-phosphate dehydrogenase (Sigma-Aldrich, cat. no. G6378)
Tris base (Sigma-Aldrich, cat. no. T1503)
Methanol (Fisher, cat. no. A452-4) ! CAUTION Flammable and toxic. Use goggles and work in a fume hood.
Acetonitrile (Fisher, cat. no. A998-4) ! CAUTION Flammable and toxic. Use goggles and work in a fume hood.
BFC (BD Biosicences, cat. no. 451731)
Ketoconazole (Sigma-Aldrich, cat. no. K1003)
EQUIPMENT
96-well black assay plate (Corning Costar, cat. no. 3915)
Polarstar microplate reader (BMG Labtech)
Microchannel pipetter (Gilson)
Software to calculate IC50 (http://www.changbioscience.com/stat/ec50.html)
REAGENT SETUP
Water for all buffers and incubations should be purified using a Milli-Q (Millipore, Billerica, MA, USA) system.
NADPH-generating system: combine 50 parts 10 mM NADP+, 50 parts 0.1 M glucose-6-phosphate and 1 part 1 mg ml–1 yeast glucose-6-phosphate dehydrogenase; prepare fresh daily; store on ice when not in use.
10 mM NADP+: 382 mg in 50 ml of (Milli-Q) water, remains stable for several months if it is stored at 4 °C.
0.1M glucose-6-phosphate: 3.4 g in 100 ml of (Milli-Q) water, remains stable for several months if it is stored frozen at –20 °C.
103 IU ml–1 yeast glucose-6-phosphate dehydrogenase: prepare at 1 mg ml–1 in 10 mM Tris-acetate buffer (pH 7.4), containing 1.0 mM EDTA and 20% glycerol (vol/vol); it is to be stored at 4 °C. This remains stable for several months.
0.1M potassium phosphate buffer (pH7.4): 13.96 g dibasic potassium phosphate (Sigma-Aldrich cat. no. P288) and 2.69 g monobasic potassium phosphate (Sigma-Aldrich cat. no. P9791) per 1 liter (Milli-Q) water (add phosphate salts to water, not vice-versa); remains stable for several months at 4 °C.
Stop buffer: 80% acetonitrile and 20% 0.5 M Tris-base (vol/vol), stored at an ambient temperature (23 °C); it remains stable for several months. ! CAUTION Acetonitrile is flammable and toxic. Use goggles and work in a fume hood.
4 mM BFC: 2.56 mg in 2ml methanol, stored in a Teflon-sealed amber glass vial at 4 °C. This is stable for several months. ! CAUTION Methanol is flammable and toxic. Use goggles and work in a fume hood.
1 mM ketoconazole: 5.31 mg in 10 ml methanol, stored in a Teflon-sealed glass vial at 4 °C. This is stable for several months. ! CAUTION Methanol is flammable and toxic. Use goggles and work in a fume hood.
2× enzyme-substrate mix: mix P450 3A4 and BFC in 0.1 M potassium phosphate buffer so the final concentration of P450 is 20 nM and BFC is 40 μM. Prepare the mix fresh daily.
EQUIPMENT SETUP
Configure the Polarstar reader to ‘Standard and Time-Resolved Fluorescence’. Install the appropriate excitation filters (405 nm in this study) and emission filter (445 nm in this study) in the filter wheels. Define the test method in the ‘Well’ mode.
PROCEDURE
Plate setup ● TIMING 30 min
Dispense 0.06 ml of 0.1 M potassium phosphate buffer into each of the wells (columns 2–12) of a 96-well plate using a multichannel pipette.
Dispense 0.118 ml of 0.1 M potassium phosphate buffer and 0.002 ml of 1 mM ketoconazole into the wells in column 1.
Serially dilute 0.06 ml from the well (in column 1) to the other wells (columns 2–10). Remove the extra 0.06 ml in the well in column 10.
Dispense 0.1 ml of 2× enzyme–substrate mix in all the wells. Add 0.075 ml of stop buffer to the wells in column 11.
Incubate the plate in a 37 °C incubator for 5 min.
Remove the plate from the incubator and dispense 0.04 ml of NADPH-generating system into each well to initiate the reaction.
Plate incubation ● TIMING 20 min
-
7
Incubate the plate for 15 min in a 37 °C incubator, and then add 0.075 ml of stop buffer to each well (except wells in column 11).
IC50 calculation ● TIMING 20 min
-
8
Scan the plate using the Polarstar plate reader, average the replicates of data for each column.
-
9
Subtract the blank from the mean value of all other columns and calculate the percent of inactivated enzyme for each dilution of ketoconazole (designated as I%): I = (1 – (mean of individual column – mean of column 11)/(mean of column 12 – mean of 11)) × 100
● TROUBLESHOOTING
-
10Fit the data with the 4-parameter logistic fit
to determine the IC50 using the free web-based software at TMDU Chemical Biology Database (Tokyo, Japan): http://www.changbioscience.com/stat/ec50.html.
● TIMING
Steps 1–6, Plate setup: 30 min
Step 7, Plate incubation: 20 min
Steps 8–10, IC50 calculation: 20 min
● TROUBLESHOOTING
Troubleshooting advice can be found in Table 2.
TABLE 2.
Troubleshooting guide for P450 3A4 fluorescence assay.
| Step number | Problem | Possible reason | Solution |
|---|---|---|---|
| 9 | Low signal/noise ratio | Inactive NADPH-generating system | Verify that the NADPH-generating system is working |
| Decreased P450 activity | Increase the concentration of P450 in the reaction | ||
| Inconsistent amounts of product formation between replicates | Failure to equilibrate the temperature of plates before initiating the reaction | Pre-incubate plate for 5 min at 37°C |
ANTICIPATED RESULTS
Fluorescence readings from dealkylation of BFC by P450 3A4 and the data processing are shown in Table 3. Background fluorescence reading is shown in well 11, where the NADPH-generating system is added after the addition of stop buffer. The 100% enzyme activity value is obtained in well 12, where no inhibitor was added. Sometimes low signal/noise ratios (well 12/well 11< 2) can be problematic. This is often caused by decreased P450 activities and can normally be corrected by increasing the enzyme concentration. The IC50 determined by the 4-parameter logistic fitting was 54 nM, using the web-based program (Fig. 2).
TABLE 3.
Fluorescence readings and IC50 calculation of the P450 3A4 inhibitor ketaconazole.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 7845 | 8072 | 8229 | 8729 | 9536 | 11753 | 15018 | 18213 | 22589 | 23408 | 7489 | 24886 |
| B | 7571 | 7647 | 7850 | 8861 | 9827 | 10940 | 14914 | 16373 | 20459 | 21789 | 7223 | 23760 |
| Mean | 7708 | 7859 | 8039 | 8795 | 9681 | 11346 | 14966 | 17293 | 21524 | 22598 | 7356 | 24323 |
| Mean–Blank | 424 | 503 | 683 | 1439 | 2325 | 3990 | 7610 | 9937 | 14168 | 15242 | 0 | 16967 |
| I% | 97.5 | 97 | 96 | 91.5 | 86.3 | 76.5 | 55.1 | 41.4 | 13.8 | 9.1 | ||
| Inhibitor concentration (nM) | 5000 | 2500 | 1250 | 625 | 312 | 156 | 78 | 39.1 | 19.5 | 9.76 |
These fluorescence readings were obtained using ketoconozale as an inhibitor of P450 3A4. Concentrations of ketaconozale (nM) 5000, 2500, 1250, 625, 312, 156, 78, 39.1, 19.5 and 9.76 in columns 1–10, respectively. Column 11 blank and column 12 no inhibitor.
Figure 2.
ACKNOWLEDGMENTS
Cytochrome P450 research in this laboratory is supported by United States Public Health Service grant R37 CA090426.
Footnotes
AUTHOR CONTRIBUTIONS Q.C. performed and optimized the assays. Q.C. wrote most of the paper, with the assistance of F.P.G. and C.D.S. C.D.S. checked the protocols and helped Q.C. with data processing.
References
- 1.Isin EM, Guengerich FP. Complex reactions catalyzed by cytochrome P450 enzymes. Biochim. Biophys. Acta. 2007;1770:314–329. doi: 10.1016/j.bbagen.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Guengerich FP. Human cytochrome P450 enzymes. in Cytochrome P450: Structure, Mechanism, and Biochemistry. In: Ortiz de Montellano PR, editor. 3rd edn. Kluwer Academic/Plenum Press; NY, USA: 2005. pp. 377–530. [Google Scholar]
- 3.Williams JA, et al. Drug-drug interactions for UDP-glucuronosyltransferase substrates: a pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug Metab. Dispos. 2004;32:1201–1208. doi: 10.1124/dmd.104.000794. [DOI] [PubMed] [Google Scholar]
- 4.Wienkers LC, Heath TG. Predicting in vivo drug interactions from in vitro drug discovery data. Nat. Rev. Drug Discov. 2005;4:825–833. doi: 10.1038/nrd1851. [DOI] [PubMed] [Google Scholar]
- 5.Fowler S, Zhang H. In vitro evaluation of reversible and irreversible cytochrome P450 inhibition: current status on methodologies and their utility for predicting drug-drug interactions. AAPS J. 2008;10:410–424. doi: 10.1208/s12248-008-9042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turpeinen M, Uusitalo J, Jalonen J, Pelkonen O. Multiple P450 substrates in a single run: rapid and comprehensive in vitro interaction assay. Eur. J. Pharm. Sci. 2005;24:123–132. doi: 10.1016/j.ejps.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Lin T, Pan K, Mordenti J, Pan L. In vitro assessment of cytochrome P450 inhibition: strategies for increasing LC/MS-based assay throughput using a one-point IC50 method and multiplexing high-performance liquid chromatography. J. Pharm. Sci. 2007;96:2485–2493. doi: 10.1002/jps.20884. [DOI] [PubMed] [Google Scholar]
- 8.Youdim KA, Lyons R, Payne L, Jones BC, Saunders K. An automated, high-throughput, 384 well cytochrome P450 cocktail IC50 assay using a rapid resolution LC-MS/MS end-point. J. Pharm. Biomed. Anal. 2008;48:92–99. doi: 10.1016/j.jpba.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto T, Suzuki A, Kohno Y. Application of microtiter plate assay to evaluate inhibitory effects of various compounds on nine cytochrome P450 isoforms and to estimate their inhibition patterns. Drug Metab. Pharmacokinet. 2002;17:437–448. doi: 10.2133/dmpk.17.437. [DOI] [PubMed] [Google Scholar]
- 10.Schaeffner I, Petters J, Aurich H, Frohberg P, Christ B. A microtiter plate-based screening assay to assess diverse effects on cytochrome P450 enzyme activities in primary rat hepatocytes by various compounds. Assay Drug Dev. Technol. 2005;3:27–38. doi: 10.1089/adt.2005.3.27. [DOI] [PubMed] [Google Scholar]
- 11.Kim D, Guengerich FP. Enhancement of 7-methoxyresorufin O-demethylation of human cytochrome P450 1A2 by molecular breeding. Arch. Biochem. Biophys. 2004;432:102–108. doi: 10.1016/j.abb.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Kim D, Wu Z-L, Guengerich FP. Analysis of coumarin 7-hydroxylation activity of cytochrome P450 2A6 using random mutagenesis. J. Biol. Chem. 2005;280:40319–40327. doi: 10.1074/jbc.M508171200. [DOI] [PubMed] [Google Scholar]
- 13.Crespi CL, Miller VP, Penman BW. Microtiter plate assays for inhibition of human, drug-metabolizing cytochromes P450. Anal. Biochem. 1997;248:188–190. doi: 10.1006/abio.1997.2145. [DOI] [PubMed] [Google Scholar]
- 14.Onderwater RCA, Venhorst J, Commandeur JNM, Vermeulen NPE. Design, synthesis, and characterization of 7-methoxy-4-(aminomethyl) coumarin as a novel and selective cytochrome P450 2D6 substrate suitable for high-throughput screening. Chem. Res. Toxicol. 1999;12:555–559. doi: 10.1021/tx980248q. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura K, Hanna IH, Cai H, Nishimura Y, Williams KM, Guengerich FP. Coumarin substrates for cytochrome P450 2D6 fluorescence assays. Anal. Biochem. 2001;292:280–286. doi: 10.1006/abio.2001.5098. [DOI] [PubMed] [Google Scholar]
- 16.Cali JJ, et al. Luminogenic cytochrome P450 assays. Expert Opin. Drug Metab. Toxicol. 2006;2:629–645. doi: 10.1517/17425255.2.4.629. [DOI] [PubMed] [Google Scholar]
- 17.Parikh A, Gillam EMJ, Guengerich FP. Drug metabolism by Escherichia coli expressing human cytochromes P450. Nat. Biotechnol. 1997;15:784–788. doi: 10.1038/nbt0897-784. [DOI] [PubMed] [Google Scholar]