Abstract
Antiviral therapy using nucleoside reverse transcriptase inhibitors (NRTIs) is neurotoxic and has low efficiency in eradication of HIV-1 harbored in central nervous system (CNS). Previously, we reported that active 5′-triphosphates of NRTIs encapsulated in cationic nanogels (nano-NRTIs) suppress HIV-1 activity more efficiently than NRTIs and exhibit reduced mitochondrial toxicity1,2. Here, we demonstrated low neurotoxicity and excellent antiviral activity of nano-NRTIs decorated with the peptide (AP) binding brain-specific apolipoprotein E receptor. Nano-NRTIs induced lower levels of apoptosis and formation of reactive oxygen species, a major cause of neuron death, than free NRTIs. Optimization of size, surface decoration with AP significantly increased brain accumulation of nano-NRTIs. The efficient CNS delivery of nano-NRTIs resulted in up to 10-fold suppression of retroviral activity and reduced virus-associated inflammation in humanized mouse model of HIV-1 infection in the brain. Our data provide proof of the advanced efficacy of nano-NRTIs as safer alternative of current antiviral drugs.
Keywords: nanogel, nucleoside reverse transcriptase inhibitors, HIV-1 infection, CNS drug delivery, neurotoxicity
Introduction
Highly Active Anti-Retroviral Therapy (HAART), a workhorse of the current AIDS treatment, was successful in reducing the titer of HIV-1 in the blood and decreasing overall mortality. However, the chronic antiviral treatment was increasingly associated with serious side effects, such as muscular dystrophy and peripheral neurotoxicity, and demonstrated a weak efficiency in the CNS due to the poor blood-brain barrier (BBB) permeability of most antiretroviral drugs3. Low drug accumulation in the brain results in neurological complications in chronic AIDS patients4. Strong peripheral control by HAART in some patients with brain HIV compartmentalization requires new better CNS penetrating drugs with low neurotoxicity.
In recent years, various approaches to drug targeting to the CNS have been developed and demonstrated their practical potential in promoting brain-specific transport, reducing free drug concentrations in the blood, and diminishing drug accumulation in peripheral tissues and nervous system associated with non-specific toxicity5. A wide range of drug nanocarriers (such as nanoparticles, liposomes, micelles and polymeric conjugates) was evaluated for CNS delivery by systemic routes, and in many cases they showed preclinical advantages compared to free drugs6. Different vector molecules have been evaluated for brain delivery via intravenous (i.v.) drug administration, from low-molecular weight compounds like DHA to combinatorially selected peptides and BBB receptor-specific antibodies and proteins7. Brain targeting of cationic albumin-conjugated nanocarriers was also demonstrated8, as well as lipophilic particles opsonized with brain-specific blood components, such as apolipoproteins9. Cellular nanodelivery of antiviral drugs using monocytes and macrophages as vehicles for CNS delivery was recently added to the existing nanomedicine approaches10. The nano-ART strategy was applied to deliver various non-nucleoside RT inhibitors encapsulated in biodegradable nanoparticles. Lately, a new sustained release drug delivery system based on wet-milling technology has been proposed that could potentially reduce the frequency of drug administration to weekly IV injections11. Despite the overall progress made in the development of CNS-targeted drug nanoformulations, they are still far from clinical applications, because of the generally low drug accumulation in the brain as a percentage of the injected dose per gram (%ID/g) and yet unsolved problem of peripheral toxicity12. Nanotherapies of HIV infection in the brain require highly efficient antiviral drugs with low neurotoxicity, development of nanocarriers with low rate of non-specific retention, and finding new vector molecules allowing specific accumulation of nanocarriers in the brain in order to apply nanodrugs in clinic. The need for improved targeted and inexpensive drugs continues to be a major impulse in further efforts to completely eradicate the HIV-1 infection and make the antiviral therapy safe.
NRTIs are the first-line drugs in AIDS treatment and important components of the multi-drug cocktails of the HAART. The efficacy of NRTIs is often hampered by low rate of accumulation of these drugs in CNS due to the activity of mostly MRP4, MRP5 and BCRP drug efflux transporters in the BBB13. Generally, at the therapeutic concentrations in blood, the concentration of antiviral drug in the brain is hundred to thousand-fold lower14. Another problem is the drug penetration in mitochondria and their interference with the ATP production and synthesis of mitochondrial DNA, which results in neurotoxicity, including death of peripheral and CNS neurons15. And the third problem is non-specific accumulation of NRTIs in many organs and tissues leading to various toxic side effects such as muscular dystrophy, hepatic or renal failure16,17. NRTIs are the prodrugs which need activation, a conversion into 5′-triphosphorylated derivatives to block the viral RT activity and the synthesis of viral DNA in lymphatic cells and macrophages. In our approach, the triphosphorylated NRTIs are suggested for application as active drugs, because (1) they can avoid the removal from the BBB by drug efflux transporters, (2) they cannot penetrate mitochondria, and (3) they have poor cellular accumulation, mostly, due to negative charge. However, 5′-triphosphates are unstable in vivo to be used for direct targeted delivery, so nanoformulation of these compounds is necessary for therapeutic applications.
Previously, we developed innovative formulations of cationic nanogels with bioactive nucleoside analogs in active triphosphorylated form in order to enhance targeted drug delivery and efficacy18. Such formulations of phosphorylated NRTIs, also called nano-NRTIs, demonstrated fast uptake by macrophages and successful inhibition of HIV-1 activity in these cells without negative effects associated with mitochondrial toxicity of NRTIs at the prolonged treatment1,2. We determined the most efficient core-shell structure of nano-NRTIs vectorized by brain-specific peptides in order to achieve strong virus inhibition without affecting macrophage viability. Here, we report successful applications of targeted antiviral nano-NRTIs in humanized mouse model of HIV-1 infection in the brain. Nano-NRTIs have also been evaluated by their neurotoxicity to conclude on the safety of these new drug nanoformulations.
Methods
All reagents, if not mentioned separately, were purchased from Sigma-Aldrich (St Louis, MO) and used without additional purification. Maleimide-PEG-NHS ester was purchased from GenKem Technology USA (Allen, TX). N-Succinimidyl [2,3-3H] propionate was obtained from Moravek Radiochemicals (Brea, CA). FPLC Sephacryl S-300 (1.5 × 45 cm) and NAP-25 columns for gel filtration were purchased from GE Healthcare Biosciences (Piscataway, NJ). Dialysis tubes were obtained from Thermo Fisher Scientific (Waltham, MA).
Nano-NRTIs
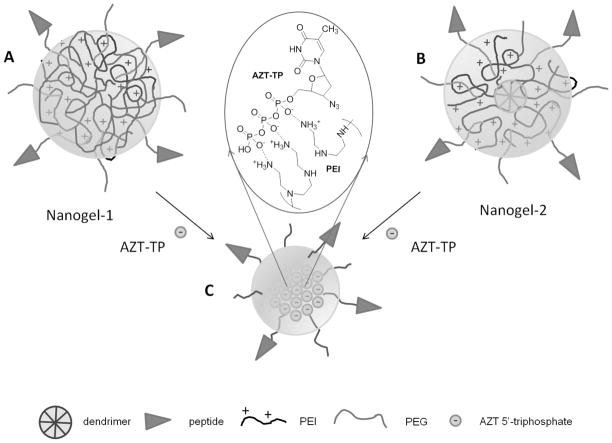
Nanogel NG1 was synthesized starting from a biodegradable PEI (PEIss, M.w. 29,000) consisting of the PEI segments (M.w. 1,800) connected with disulfide bridges. These PEIss molecules have been crosslinked with a 1,1′-carbonyldiimidazole-activated PEG (M.w. 5,000) linker taken in a 50% excess using an ‘emulsification-solvent evaporation’ method as previously described19. In the swollen conjugate, PEG and PEI molecules are evenly distributed forming a macroporous network. The surface of the nanogel was then decorated with MAL-PEG-NHS (M.w. 5,000, 33% wt) linker molecules (Figure 1A). Nanogel NG2 with a core-shell structure design was synthesized stepwise starting from the modification of carbodiimide-activated carboxylated PAMAM dendrimer (Generation 5) with an excess of branched PEI (M.w. 1,200) to obtain a PAMAM-PEI core conjugate. The PAMAM-PEI core was then decorated with MAL-PEG-NHS (M.w. 5,000, 4-fold excess) linker molecules (Figure 1B). The PEG/PEI ratio was determined by elemental analysis of the nitrogen content (Supplemental Materials, Table S1). For purpose of brain targeting, nanogels NG1 and NG2 have been modified with multiple molecules of apolipoprotein E receptor-specific peptide (AP, M.w. 1,550)20. The synthetic peptide contained cysteine at the N-end and was protected by C-end amidation. Non-reacted maleimide moieties have been quenched by reaction with an excess of cysteine. This approach was applied to obtain nanogels without peptide used in the work. The nanogel products were dialyzed in membrane tubes (MWCO 12,000; 2 × 24 h) against water at 4°C to remove non-conjugated linker and peptide molecules. The AP-decorated nanogels have been analyzed and purified if necessary by size-exclusion FPLC on a Sephacryl S-300 (1.5 × 45 cm) column equilibrated in 20% ethanol/0.2M sodium chloride at elution rate 1mL/min (Supplemental Materials, Figure S1). The yield of nanogels in lyophilized form was 60–75%. The peptide content was determined by the amino acid analysis after acidic hydrolysis of AP-nanogels and corresponded to the peptide conjugation rate of 62±6% (Supplemental Materials, Table S1).
Figure 1.
Structures of nanogels (A) AP-NG1, (B) AP-NG2 and (C) preparation of AP-nano-AZT formulation. The insert shows polyionic complex between charged phosphate groups of AZT-TP and amino groups of PEI.
Nano-AZT formulations were prepared from concentrated solutions of AZT 5′-triphosphate21 and nanogels mixed at 1:3–1:6 wt ratios. After incubation for 1 h on ice, nano-AZTs have been purified by gel filtration on NAP-25 column and lyophilized. The drug (AZT) content was calculated from UV absorbance of nano-AZTs using AZT extinction coefficient ε260 9,700 (M−1) (Supplemental Materials, Table S1). The hydrodynamic diameter (dh) and zeta-potential of free and drug-loaded nanogels were measured by dynamic light scattering using Malvern Zetasizer Nano-90 (Supplemental Materials, Table S1). Nanogel morphology was determined by transmission electron microscopy using vanadate contrast staining (Supplemental Materials, Figure S2).
Rat neuron culturing
Perinatal neurons from 18-days old rat pups were purchased from BrainBits (Springfield, IL) in the form of brain tissue. The brain tissue was dispersed by trituration in the supplied Neurobasal medium using a sterile pipette tip (usually, about 50 times). Dispersed neurons were left in the supernatant to settle down by gravity, transferred in a sterile 15-ml tube, and centrifuged at 1,100 rpm for 1 min. Neurons re-suspended in Neurobasal medium (5ml) were distributed into 48–96 wells of a black walled 96-well plate coated with poly-D-lysine (50 μg/mL) and incubated for 4 days at 37°C to allow them to differentiate.
Cytotoxicity
Cytotoxicity of nano-NRTIs to neurons was evaluated following 24h-treatment using a MTT assay22. Initially, cells were treated for 4 h at 37°C in order to allow the internalization of nano-NRTI’s, and then incubated for additional 20 h at 37°C before the MTT assay. The MTT cytotoxicity assay was also used in normalization of the viral RT activity in cultured HIV-1 infected human monocyte-derived macrophages (MDM).
The neuronal death was studied using TUNEL apoptosis assay23. Briefly, nano-NRTI-treated cells (4μg/ml, 24–48 h) were stained with fluorescein (FL)-labeled dUTP/terminal deoxynucleotidyl transferase from TUNEL labeling kit (R&D Systems, Minneapolis, MN). In conjunction with propidium iodide (PI) as a counterstain, the number of apoptotic cells in the treated neuron population was quantified by counting using a confocal microscope. All samples were counted in triplicates.
ROS assay
Production of the reactive oxygen species (ROS) in drug-treated neurons was evaluated using an OxiSelect ROS assay kit (Cell Biolabs, San Diego, CA). Differentiated neurons were treated with working concentrations (4ug/ml) of nano-NRTIs in growth medium for 24 h, then the medium was removed and cells washed twice with PBS. Hydrogen peroxide was applied for 24 h as a positive control. Non-treated neurons served as a negative control. The 1x dichloro-dihydro-fluorescein diacetate dissolved in growth medium was added to all cells and incubated for 1 h at 37°C, then the medium was removed and cells washed twice with PBS. Ten minutes before reading the plate, supplied standards were prepared and added to empty wells on the plate. The range of serial dilutions was from 102 to 105 nM. The reaction was terminated by adding 100 μL of Cell Lysis Buffer to the standards and cell samples for 5 min at 20°C, and the fluorescence was measured using a FLX-800 plate reader (BioTek US, Vinooski, VT) and analyzed by KC Junior software.
In vivo treatment of infected mice
4–5 week-old C.B-17/scid were obtained from the Charles River Laboratories, Inc., and kept in microisolator cages with free access to food and water under a 12-hour/12-hour light/dark cycle in accordance with ethical guidelines for care of laboratory animals approved by the Institutional Animal Care and Use Committee, University of Nebraska Medical Center.
Mice were stereotactically injected with the HIV-1 infected overnight MDM (MOI = 0.1) as described before24. Animals were split in three groups (n = 5–7): non-treated control, AZT-treated and nano-AZT-treated ones. First intraperitoneal (i.p.) injection of control saline, AZT, and nano-AZT solutions was done once 4 h before HIV-1-infected MDM intracranial inoculation. Next day after the i.c. injection of HIV-1-infected MDM, i.p. injections of AZT or nano-AZT were performed every other day: the control groups were injected with 100uL saline, the AZT-treated groups - with 0.2 mg drug/100 μL, and the nano-AZT-treated groups - with 1 mg nanoformulation/100 μL. On the Day 15, all mice were euthanized, and their brains processed for analysis.
For histological evaluation, brains were removed immediately after euthanasia and fixed with 4% paraformaldehyde overnight followed by the tissue processing using Shandon Citadel 1000 (Thermo Fisher Scientific, Waltham, MA) and embedding in paraffin. 5-Micron-thick sections were cut from the paraffin blocks using a Leica RM2235 microtome (Leica Microsystems, USA) and mounted on Superfrost Plus glass slides. Slides were baked for one hour at 62°C in the presence of an unmasking reagent Trilogy (Cell Marque, Rocklin, CA). Immunohistochemical staining was performed using mouse monoclonal and rabbit polyclonal antibodies for Glial Fibrillary Acidic Protein (GFAP), Vimentin (clone Vim 3B4), and HIV-1 p24 (clone Kal-1, 1:10) from Dako USA (Carpinteria, CA). The polymer-based HRP-conjugated anti-mouse and anti-rabbit Dako EnVision systems were used as secondary detection reagents and then developed with 3, 3′-diaminobenzidine (DAB). All paraffin-embedded sections were counterstained with Mayer’s hematoxylin. Images were obtained by the Optronics digital camera Eclipse E800 (Nikon Instruments, Melville, NY) using MagnaFire 2.0 software.
Real-Time Reverse Transcriptase PCR
Brain samples were homogenized using a ball-mill homogenizer, and total RNA was isolated using the RNA Tissue Kit SII (Autogen, Holliston, MA) with the addition of DNase I (Promega, Madison, WI) using Autogen QuickGene Mini 80 columns. RNA concentration in the samples was measured by spectrophotometry at 260 nm, and RNA purity – by the absorbance 260/280 ratio (pure RNA has the ratio between 1.85 and 2.1).
RNA was stored in the frozen solutions until the reverse transcription. RNA was converted to cDNA using the iScript cDNA synthesis kit (BioRad, Hercules, CA) and subsequently analyzed for expression of various genes. Taqman Gene Expression Assay (Applied Biosystems/Life Technologies, Grand Island, NY) was used to determine the expression of mGAPDH (Mm03302249_g1) and HLA-DQA1 (Hs00824939_gh). In HIV-1 gag analysis, we used the forward primer, 5′-ACATCAAGCCATGCAAAT-3′, the reverse primer, 5′-ATCTGGCCTGGTGCAATAGG-3′, and the TAMRA-labeled probe 5′-CATCAATGAGGAAGCTGCAGAATGGGATAGA-3′. All PCR reactions have been performed using FastStart Universal Probe Master Mix (Roche Applied Sciences, USA) and a StepOnePlus Real-Time PCR detection system (Applied Biosystems, USA). The real time PCR conditions were as follows: 50°C for 2 min, 95°C for 10 min, and then 40 amplification cycles of 95°C for 15s followed by 60°C for 1 min. The data were analyzed using DataAssist Software v2.0 and expressed as a ratio of HIV-1 gag/HLA-DQ, both being normalized by the mGAPDH expression.
Results
Nanogel synthesis and optimization in vivo
We report the preparation of brain-vectorized nano-NRTIs from cationic nanogels NG1 and NG2 loaded with activated antiviral drug, AZT 5′-triphosphate (Figure 1). Nanogels in free and drug-loaded form were analyzed by dynamic light scattering (Table S1). Drug-loaded nanogels had narrow size distribution with a low polydispersity index of 0.22–0.28 and zeta-potential (0–7.5 mV). The median collapse of nanogels after loading with nucleoside 5′-triphosphate was 100 and 50%, respectively, for NG1 and NG2. Nanogels could incorporate AZT 5′-triphosphate up to 25% by mass, forming core-shell particles with near neutral charge (Figure 1C). All nanogels and nano-NRTIs have low viscosity that allowed us to use 10% aqueous solutions for injections.
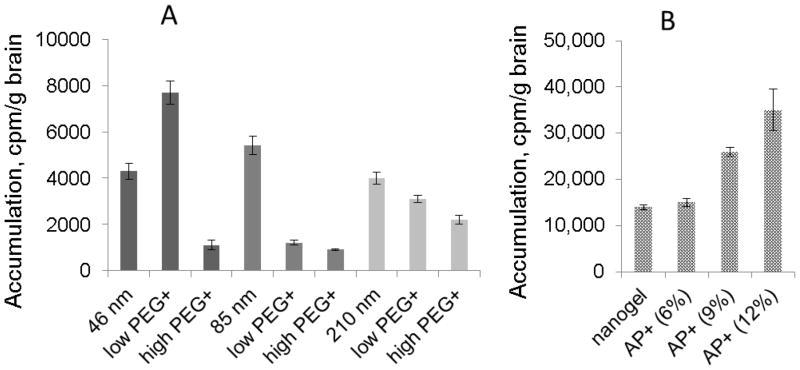
To determine the optimal parameters of brain accumulation, we investigated effects of particle size, surface PEGylation, and modification of drug-loaded NG1 with a brain specific peptide, apolipoprotein E receptor-binding peptide (AP). Usually, the ‘emulsification-solvent evaporation’ method of nanogel synthesis produces a rather wide range of polymer networks of different size, which can be fractionated by size-exclusion chromatography18. Nanogel fractions with an average dh of 46, 85 and 210 nm have been isolated and then tritium-labeled using N-succinimidyl [2,3-3H] propionate25. In the second experiment, nanogel surface was modified with PEG molecules at low and high modification rates. In the third experiment, a selected NG1 fraction (dh 46 nm) was modified with PEG linker and AP to obtain different modification rates. Average efficacy of nanogel modification by peptide was high as determined by quantitative amino acid analysis.
Brain accumulation data measured after injection of tritium-labeled nanogel samples at equal doses are summarized in Figure 2. It is evident that nanogels NG1 and NG2 have similar levels of accumulation (Figure 2A). The smallest studied nanogel (dh 46 nm) with additional PEGylation demonstrated a more than 2 times higher brain accumulation compared to larger nanogels (Figure 2B). Higher PEGylation caused strong screening effect that, evidently, prevented effective interaction of nanogels with the BBB endothelium. Modified nanogels also showed low retention in bypassing organs: liver, spleen and lungs (data not shown). The fraction of nanogels with dh 46 nm was modified with AP peptide at three conjugation rates (6, 9 and 12% wt). No increase of brain accumulation was detected at low peptide content (6%) in comparison with the only PEGylated nanogel (Figure 2C). At higher peptide content (9–12%), nanogel accumulation in the brain was 3–4 times more efficient, evidently, because of the cooperative binding with multiple ApoE receptor sites on the BBB endothelium. In animal experiments, the peptide content of 9% in AP-nano-AZT was sufficient to obtain high HIV-1 inhibition in the brain.
Figure 2.
Optimization of the accumulation of nanogel (3H-labeled NG1) in the brain following i.v. injection in the mouse tail vein. (A) Nanogel size (dh 46, 85 and 210 nm) and rate of PEGylation (low PEG: 25% wt; high PEG: 50% wt) dependence. (B) Peptide decoration rate dependence: AP content in nanogel is given in wt% (rounded). Data shown are means ± SEM (n = 3).
Neurotoxicity of nano-NRTIs
Comparative cytotoxicity of nano-NRTIs and free NRTIs was tested in cultured rat neurons. NG2 showed lower toxicity to neurons than NG1; IC50 values for nano-AZT were 20 times higher compared to free AZT (Figure 3A). These data correlated well with the results of TUNEL apoptosis assay. Short 4h-treatment with high doses of AZT demonstrated a striking 7–14 fold protective effect of nano-AZT compared to free drug (Figure 3B). The observed level of apoptosis in nano-AZT-treated neurons was also 6–8 times lower than in AZT-treated neurons. At longer treatment (24h) these differences becomes less expressed, e.g. the neuron death was 30% lower for nano-AZT compared to AZT at the same drug concentrations (data not shown). The neurotoxicity of NRTIs is closely related to the mitochondrial damage in neurons26. Earlier we discovered that mitochondrial DNA was less depleted after the treatment with nano-NRTIs as compared to free NRTIs, evidently, due to the low penetration ability of 5′-phosphorylated NRTIs through the mitochondrial membrane1. The lower mitochondrial toxicity of nano-AZT may explain the observed protection of neurons by nanoformulations.
Figure 3.
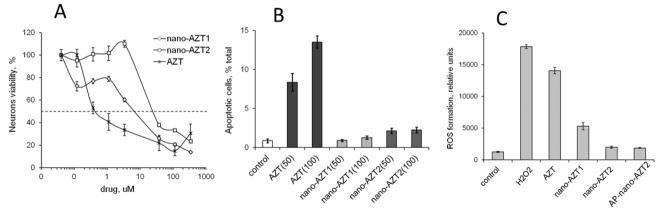
Neurotoxicity of nano-AZT formulations in cultured rat neurons. (A) Neurons viability measured by colorimetric MTT assay following the 24h-treatment with increasing concentrations of AZT or nano-AZT formulations. Data shown are means ± SEM (n = 8). 50% Viability values (IC50): 0.3 μM (AZT), 6 μM (nano-AZT1) and 21 μM (nano-AZT2). (B) Apoptosis measured by TUNEL staining following the 2h-treatment with 50–100 μM concentrations of AZT or nano-AZT formulations. Data shown are means ± SEM (n = 3). (C) ROS formation following the 24h-treatment with 15 μM concentrations of AZT or nano-AZT formulations. Hydrogen peroxide (H2O2) was used as a positive control. Data shown are means ± SEM (n = 3).
Another important parameter characterizing toxic effect of NRTIs on mitochondria is the production of reactive oxygen species (ROS)27. Here, we measured the ROS concentration in neurons after 24h- incubation with AZT or nano-AZT formulations (Figure 3C). The protective effect of nano-AZT versus AZT in neurons was generally preserved.
Antiviral activity of nano-NRTIs
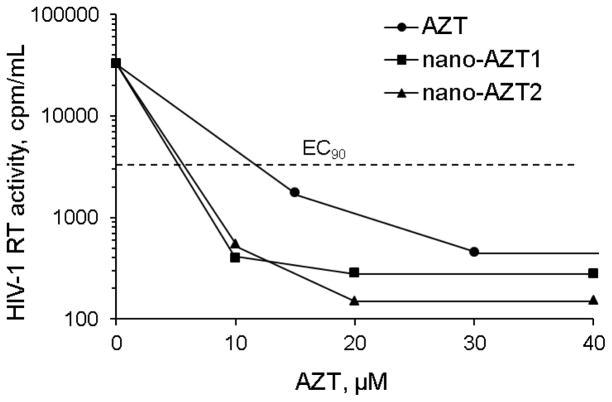
Antiviral efficacy was measured in human MDMs pre-treated with AZT, nano-AZT and AP-nano-AZT formulations for 4 h and, then, infected with HIV-1. Inhibition of RT activity of HIV-1 was observed on Day 5 after treatment. Nano-AZTs demonstrated similar antiviral activity expressed as EC90, an effective drug concentration reducing the virus activity 10-fold, which was more than 2 times lower than EC90 of AZT (13 μM). Thus, the HIV-1 activity could be reduced 100-fold by application of 6 μM nano-AZTs. Based on cytotoxicity of nano-NRTIs, their therapeutic index (IC50/EC50) could be as high as 1000.
Antiviral activity of nano-NRTIs in vivo
AP-nano-AZT formulations have been evaluated for antiviral treatment in vivo using a humanized mouse model of HIV-1 infection in CNS28. In this model, cultured MDMs infected with the strain HIV-1ADA are stereotactically injected in the brain of immunodeficient C.B-17/scid mice. For antiviral treatment, mice received i.p. injections of of AZT or AP-nano-AZT formulations over a two weeks period. In the end of experiment, the brain samples were separated in order to obtain sections for immunostaining by HIV-1 anti-p24 and macrophage-specific anti-vimentin antibodies, or analyzed by gene expression using isolated RNA samples and a real-time quantitative RT-PCR to measure expression of HIV-1gag and human cell-specific HLA-DQ genes. Figure 5 shows data analysis after the treatment with AP-nano-AZT1. We observed nearly a 6-fold reduction of HIV-1-infected cells in nano-AZT-treated animals compared to AZT alone. Very low residual virus staining could be seen in the nano-AZT-treated brain tissue (Figure 5B). Infected animals similarly treated with AP-nano-AZT2 showed as much as 10-fold reduction in HIV-1 load in MDM (Figure 6A). The MDM amount was reduced non-significantly during the treatment period. The group treated with AZT demonstrated very low curative effect, evidently because of the poor drug penetration through the BBB29. Thus, both AP-nano-AZT formulations demonstrated high efficacy in the suppression of HIV-1 activity in vivo.
Figure 5.
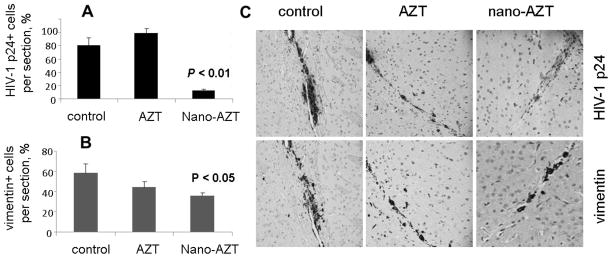
Antiviral effects of nano-AZT in humanized mouse model of HIV-1 infection in the brain. NOD-SCID mice received intracranial injections of HIV-1 infected macrophages. Starting the next day, animals (three groups, n = 5–6) were treated by i.p. injections of saline (control), AZT (dose: 8 mg/kg) and AP-nano-AZT1 (dose: 40 mg/kg) every other day over the two weeks period. Brain sections were immunostained with monoclonal antibodies to HIV-1 p24 protein (A, C: upper row) or macrophage-specific protein vimentin (B, C: lower row). The number of positive cells per section was counted, and the corresponding data for non-treated, AZT-treated and AP-nano-AZT treated animals groups are shown as a percentage of total number of cells. Data shown are means ± SEM (n = 3). The difference between groups was considered statistically significant at P < 0.05 (Student’s t-test). (C) Examples of the stained brain sections are shown. Brown color of DAB staining shows the presence of corresponding mAb in the sites of intracranial injections of HIV-1 infected macrophages.
Figure 6.
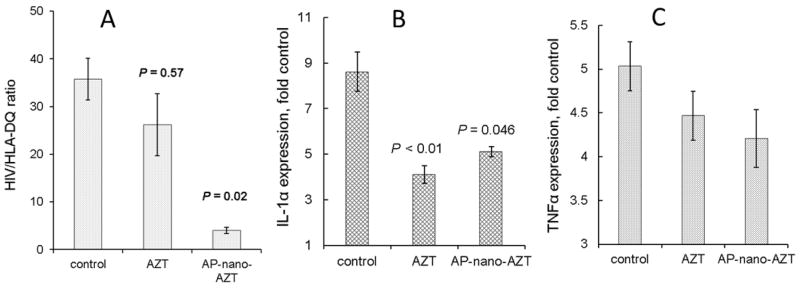
Antiviral and anti-inflammatory effects of nano-AZT in humanized mouse model of HIV-1 infection in the brain. Experimental conditions were the same as in Figure 5. RNA was isolated from brain left (ipsilateral) and right (control) hemispheres. Samples were analyzed by real-time quantitative RT-PCR for HIV-1 gag, human cell-related HLA-DQ, IL-1β and TNFα, and normalized to GAPDH. (A) This graph shows the content of HIV-1 in MDM calculated as a ratio of HIVgag/HLA-DQ expression levels in the ipsilateral hemisphere. Data shown are means ± SEM (n = 5–6). (B and C) Expression of inflammatory factors IL-1β and TNFα. The graph shows data as ratio of ipsilateral to control hemisphere. All data shown in B and C are means ± SEM (n = 3). The difference between groups was considered statistically significant at P < 0.05 (Student’s t-test).
Macrophage activation and release of inflammatory cytokines as a result of HIV-1 infection in the brain have been identified as critical factors in neuron death and the development of HIV-associated encephalopathy30. We evaluated the effect of AP-nano-AZTs on the expression of inflammatory cytokines TNFα and IL-1β in the brain of treated miceby real-time quantitative RT-PCR31. We observed a significant two-fold reduction of IL-1β expression (Figure 6B). The effect on TNFα between non-treated and treated brains was smaller but statistically significant (P < 0.05)(Figure 6C). In conclusion, AP-nano-AZTs reduced inflammatory response on HIV-1 infection in the brain in correlation with their high antiviral activity.
Discussion
Current HAART provides highly efficient treatment of AIDS, allowing patients to live long lives. However, there are now evident two major factors affecting the patient quality of life. The first factor is the toxicity of many antiviral drugs, leading to peripheral neuropathy that is especially notable during the chronic course of the therapy. The second factor is the inefficient drug accumulation in the brain and neurodegeneration resulting from HIV-associated inflammation of the CNS. Recently, we issued a hypothesis that active 5′-triphosphates of NRTIs can be more efficient and less toxic drug form in the anti-HIV therapy1. Nanoencapsulated 5′-triphosphates of NRTIs (nano-NRTIs) demonstrated fast accumulation and efficient suppression of HIV-1 activity at much lower concentrations than free drugs in the infected MDMs in vitro. In the previous work, we observed that the architecture of cationic nanogels used in nano-NRTI preparation could result in lower mitochondrial toxicity of nano-NRTIs, one of major causes of therapy-related neuron death. Cationic nanogels also demonstrated an efficient accumulation in the brain following the i.v. injection that depended on the nanogel charge and surface decoration with brain-specific polypeptide ligands25. Thus, application of nano-NRTIs could potentially be at the same time effective against general HIV-1 infection, without inducing a peripheral toxicity, and provide cure of the HIV-1 infection in the CNS. Here, we investigated neurotoxicity of selected nano-NRTIs in vitro, optimized these nanoformulations for efficient brain delivery in vivo and, finally, evaluated the therapeutic antiviral and antiinflammatory effects of targeted nano-NRTIs in humanized mouse model of HIV-1 infection in the CNS.
Recent findings indicated that mitochondrial toxicity of antiviral drugs plays a decisive role in the peripheral toxicity and neuronal death32–34. They demonstrated that strong inhibition of DNA polymerase γ, a significant drop in mitochondrial ATP production, and the release of reactive oxygen species (ROS), which damage nuclear DNA, induce apoptosis, especially in sensitive neurons. Peripheral HIV-associated neuropathies and pain can be caused by activation of peripheral macrophages, which serve as an important depot of the virus besides T-lymphocytes35. In the CNS infection that is notoriously difficult for chemotherapy due to the limited drug penetration through the BBB, brain-associated macrophages play a similar role36. Increased level of ROS was observed also in various neurodegenerative diseases, confirming the important effect of ROS on neuronal death37. We found that a 4h-treatment with AZT results in significant ROS production in the cultured rat neurons that was comparable with the effect of hydrogen peroxide. By contrast, the ROS production was 2 times lower after the treatment with nano-AZT formulations. Even long incubation with nano-AZT (24 h) resulted in a 50% reduced ROS formation as compared to the treatment with AZT (data not shown). The lower ROS level seems to be directly correlated with the lower neuronal toxicity of nano-AZT formulations. Our neuron cytotoxicity study is based on the MTT colorimetric assay, which measures the formation of blue-colored formazan by active cellular mitochondria. In dead cells, an absence of mitochondrial activity results in the absence of cell coloration. Our data clearly show the correlation of ROS production with higher cytotoxicities of free AZT compared to nano-AZTs. This protective effect is due to low accumulation of phosphorylated nucleoside analogs in mitochondria. Nanogels can fast release the AZT 5′-triphosphate in the cells after interactions with cellular membrane, and the phosphorylated AZT should have a lower binding to the negatively charged mitochondrial membrane as compared to AZT21.
Successful chemotherapy of CNS diseases relies on the efficacy of drug delivery into the brain. Lately, significant efforts have been focused on the development of nanocarriers that could enhance targeted drug delivery and accumulation in the brain38. Nanoencapsulated drugs can bypass drug efflux transporters, showing an enhanced accumulation in the brain, although the overall rate of the accumulation is usually low (< 1%ID/g). Nanocarrier-based drug delivery to the brain is significantly affected by the surface modification with the serum proteins (opsonization), by phagocytic cells of the reticuloendothelial system (RES), and by the ability to bind with the receptors responsible for a high mass transport of nutrients to the brain on the surface of the BBB endothelium. Recent experiments with metal nanoparticles of different sizes for diagnostic purposes allowed to determine the optimal size range for the efficient brain accumulation39,40. Particles with the diameters between 10 and 70 nm, mostly in the range of 10–40 nm, accumulated the most easily in the brain. Kidney can remove very fast particles smaller than 10 nm from circulation. A longer nanocarrier circulation can be achieved by PEGylation. In nanogels, the cationic core with loaded drug molecules is shielded by the PEG brush on the surface. Our experiments showed comparable results: small nanogels (46 nm) with low PEG density demonstrated the highest brain accumulation. When the PEG decoration was enhanced by the attachment of brain-specific peptides, e.g. binding with apolipoprotein E receptors, we observed a 3- to 5-fold increase of brain accumulation of modified nanogels. The BBB retention of the peptide-modified nanogels can be effective only if multiple peptide molecules participate in the process (‘velcro’ effect). Another factor that may work in favor of nanogels in the hydrodynamic circulation is the softness of nanogel network41. Flattening of nanogels which are bound to the surface of the BBB endothelium significantly reduce sharing forces that would easily remove the larger particles. And the peptide decoration can change biodistribution of nanogels in vivo. It is extremely difficult to correctly balance all factors affecting the fate of nanogels and other nanocarriers in vivo42. However, the strong therapeutic effect observed in the treatment of otherwise non-treatable HIV-1 infection in the brain by nano-NRTIs can overweight some potential side effects. Besides drug delivery to the BBB endothelium, injected nanogel particles are accumulated in bypassing organs, mostly captured by peripheral macrophages of RES, phagocytes of liver (Kupfer cells) and spleen. Our results show that particle size may be one of the principal factors in the process. Macrophage play important part in the body defense against infections. They can capture viral particles recognized by antibodies and activate a protective inflammation. We studied an in vitro antiviral activity of nano-NRTIs in HIV-infected human macrophages and also used a humanized mouse model of HIV-1 infection in the brain. In recent years, this model was extensively evaluated in the development of new treatments against HIVE43. One of new very promising treatment modalities, nanoART, was developed for non-NRTI drugs11. The antiviral efficacy of nano-NRTIs in vivo resulted in up to 10-fold suppression of HIV-1 activity in the brain after the treatment with AP-nano-AZT formulations. In conclusion, our results demonstrate the significant advantage of nano-NRTIs over free drugs in the antiviral therapy of HIV-1 infection in the central nervous system. Nano-NRTIs may complement the nanoART strategy by extending the nanomedicine approach to the full range of current antiviral drugs against AIDS.
Supplementary Material
Figure 4.
Antiviral activity of nano-AZT formulations in cultured monocyte-derived macrophages (MDM) infected by HIV-1ADA at MOI 0.05. MDM were pre-incubated for 2 h with different concentrations of AZT or nano-AZT before infection. HIV-1 reverse transcriptase (RT) activity was measured by the inclusion of 3H-thymidine on Day 5 post-infection. 90% Effective concentration (EC90) for both Nano-AZT: 6 μM. At 13 μM concentration of Nano-AZT, HIV-1 RT activity was reduced 100-fold. The data are averages of two independent measurements.
Acknowledgments
This work was supported by the NIH R01 grant NS076386 to Serguei V. Vinogradov.
Footnotes
Authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vinogradov SV, Poluektova LY, Makarov E, Gerson T, Senanayake MT. Nano-NRTIs: efficient inhibitors of HIV type-1 in macrophages with a reduced mitochondrial toxicity. Antivir Chem Chemother. 2010;21:1–14. doi: 10.3851/IMP1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makarov E, Gerson T, Senanayake T, Poluektova LY, Vinogradov Efficient suppression of Human Immunodeficiency Virus in Macrophages by Nano-NRTIs. Antiviral Res. 2010;86(1):A38–9. [Google Scholar]
- 3.Varatharajan L, Thomas SA. The transport of anti-HIV drugs across blood-CNS interfaces: summary of current knowledge and recommendations for further research. Antiviral Res. 2009;82(2):A99–109. doi: 10.1016/j.antiviral.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taiwo B, Hicks C, Eron J. Unmet therapeutic needs in the new era of combination antiretroviral therapy for HIV-1. J Antimicrob Chemother. 2010;65(6):1100–7. doi: 10.1093/jac/dkq096. [DOI] [PubMed] [Google Scholar]
- 5.Rao KS, Ghorpade A, Labhasetwar V. Targeting anti-HIV drugs to the CNS. Expert Opin Drug Deliv. 2009;6(8):771–84. doi: 10.1517/17425240903081705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mallipeddi R, Rohan LC. Progress in antiretroviral drug delivery using nanotechnology. Int J Nanomedicine. 2010;5:533–47. [PMC free article] [PubMed] [Google Scholar]
- 7.Malakoutikhah M, Teixidó M, Giralt E. Shuttle-mediated drug delivery to the brain. Angew Chem Int Ed Engl. 2011;50(35):7998–8014. doi: 10.1002/anie.201006565. [DOI] [PubMed] [Google Scholar]
- 8.Lu W, Tan YZ, Hu KL, Jiang XG. Cationic albumin conjugated pegylated nanoparticle with its transcytosis ability and little toxicity against blood-brain barrier. Int J Pharm. 2005;295(1–2):247–60. doi: 10.1016/j.ijpharm.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 9.Kreuter J, Shamenkov D, Petrov V, Ramge P, Cychutek K, Koch-Brandt C, Alyautdin R. Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J Drug Target. 2002;10(4):317–25. doi: 10.1080/10611860290031877. [DOI] [PubMed] [Google Scholar]
- 10.Nowacek AS, Miller RL, McMillan J, Kanmogne G, Kanmogne M, Mosley RL, et al. NanoART synthesis, characterization, uptake, release and toxicology for human monocyte-macrophage drug delivery. Nanomedicine (Lond) 2009;4(8):903–17. doi: 10.2217/nnm.09.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dash PK, Gendelman HE, Roy U, Balkundi S, Alnouti Y, Mosley RL, et al. Long-acting nanoformulated antiretroviral therapy elicits potent antiretroviral and neuroprotective responses in HIV-1-infected humanized mice. AIDS. 2012;26(17):2135–44. doi: 10.1097/QAD.0b013e328357f5ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tiwari SB, Amiji MM. A review of nanocarrier-based CNS delivery systems. Curr Drug Deliv. 2006;3(2):219–32. doi: 10.2174/156720106776359230. [DOI] [PubMed] [Google Scholar]
- 13.Dallas S, Miller DS, Bendayan R. Multidrug resistance-associated proteins: expression and function in the central nervous system. Pharmacol Rev. 2006;58(2):140–61. doi: 10.1124/pr.58.2.3. [DOI] [PubMed] [Google Scholar]
- 14.Banks WA, Ercal N, Price TO. The blood-brain barrier in neuroAIDS. Curr HIV Res. 2006;4(3):259–66. doi: 10.2174/157016206777709447. [DOI] [PubMed] [Google Scholar]
- 15.Clark US, Cohen RA. Brain dysfunction in the era of combination antiretroviral therapy: implications for the treatment of the aging population of HIV-infected individuals. Curr Opin Investig Drugs. 2010;11(8):884–900. [PMC free article] [PubMed] [Google Scholar]
- 16.Jones M, Núñez M. Liver toxicity of antiretroviral drugs. Semin Liver Dis. 2012;2(2):167–76. doi: 10.1055/s-0032-1316472. [DOI] [PubMed] [Google Scholar]
- 17.Calza L. Renal toxicity associated with antiretroviral therapy. HIV Clin Trials. 2012;13(4):189–211. doi: 10.1310/hct1304-189. [DOI] [PubMed] [Google Scholar]
- 18.Vinogradov SV, Zeman AD, Batrakova EV, Kabanov AV. Polyplex Nanogel formulations for drug delivery of cytotoxic nucleoside analogs. J Contr Release. 2005;107:143–157. doi: 10.1016/j.jconrel.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohli E, Han H-Y, Zeman AD, Vinogradov SV. Formulations of biodegradable Nanogel carriers with 5′-triphosphates of nucleoside analogs that display a reduced cytotoxicity and enhanced drug activity. J Contr Release. 2007;121:19–27. doi: 10.1016/j.jconrel.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sauer I, Dunay IR, Weisgraber K, Bienert M, Dathe M. An apolipoprotein E-derived peptide mediates uptake of sterically stabilized liposomes into brain capillary endothelial cells. Biochemistry. 2005;44(6):2021–9. doi: 10.1021/bi048080x. [DOI] [PubMed] [Google Scholar]
- 21.Vinogradov SV, Kohli E, Zeman AD. Cross-linked polymeric Nanogel formulations of 5′-triphosphates of nucleoside analogs: role of the cellular membrane in drug release. Mol Pharm. 2005;2005(2):449–461. doi: 10.1021/mp0500364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe W, Konno K, Ijichi K, Inoue H, Yokota T, Shigeta S. MTT colorimetric assay system for the screening of anti-orthomyxo- and anti-paramyxoviral agents. J Virol Methods. 1994;48(2–3):257–65. doi: 10.1016/0166-0934(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 23.Nunnari G, Xu Y, Acheampong EA, Fang J, Daniel R, Zhang C, et al. Exogenous IL-7 induces Fas-mediated human neuronal apoptosis: potential effects during human immunodeficiency virus type 1 infection. J Neurovirol. 2005;11(4):319–28. doi: 10.1080/13550280500187005. [DOI] [PubMed] [Google Scholar]
- 24.Limoges J, Persidsky Y, Poluektova L, Rasmussen J, Ratanasuwan W, Zelivyanskaya M, et al. Evaluation of antiretroviral drug efficacy for HIV-1 encephalitis in SCID mice. Neurology. 2000;54(2):379–89. doi: 10.1212/wnl.54.2.379. [DOI] [PubMed] [Google Scholar]
- 25.Vinogradov SV, Batrakova EV, Kabanov AV. Nanogels for oligonucleotide delivery to the brain. Bioconjug Chem. 2004;15:50–60. doi: 10.1021/bc034164r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kakuda TN. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin Ther. 2000;22(6):685–708. doi: 10.1016/S0149-2918(00)90004-3. [DOI] [PubMed] [Google Scholar]
- 27.Wang CC, Fang KM, Yang CS, Tzeng SF. Reactive oxygen species-induced cell death of rat primary astrocytes through mitochondria-mediated mechanism. J Cell Biochem. 2009;107(5):933–43. doi: 10.1002/jcb.22196. [DOI] [PubMed] [Google Scholar]
- 28.Persidsky Y, Gendelman HE. Murine models for human immunodeficiency virus type 1-associated dementia: the development of new treatment testing paradigms. J Neurovirol. 2002;8 (Suppl 2):49–52. doi: 10.1080/13550280290167993. [DOI] [PubMed] [Google Scholar]
- 29.Wu D, Clement JG, Pardridge WM. Low blood-brain barrier permeability to azidothymidine (AZT), 3TC, and thymidine in the rat. Brain Res. 1998;791(1–2):313–6. doi: 10.1016/s0006-8993(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 30.Persidsky Y, Zheng J, Miller D, Gendelman HE. Mononuclear phagocytes mediate blood-brain barrier compromise and neuronal injury during HIV-1-associated dementia. J Leukoc Biol. 2000;68(3):413–22. [PubMed] [Google Scholar]
- 31.Gorantla S, Liu J, Sneller H, Dou H, Holguin A, Smith L, Ikezu T, Volsky DJ, Poluektova L, Gendelman HE. Copolymer-1 induces adaptive immune anti-inflammatory glial and neuroprotective responses in a murine model of HIV-1 encephalitis. J Immunol. 2007;179(7):4345–56. doi: 10.4049/jimmunol.179.7.4345. [DOI] [PubMed] [Google Scholar]
- 32.Leung GP. Iatrogenic mitochondriopathies: a recent lesson from nucleoside/nucleotide reverse transcriptase inhibitors. Adv Exp Med Biol. 2012;942:347–69. doi: 10.1007/978-94-007-2869-1_16. [DOI] [PubMed] [Google Scholar]
- 33.Evliyaoglu F, Karadag R, Burakgazi AZ. Ocular neuropathy in peripheral neuropathies. Muscle Nerve. 2012;46(5):681–6. doi: 10.1002/mus.23414. [DOI] [PubMed] [Google Scholar]
- 34.Harrison TB, Smith B. Neuromuscular manifestations of HIV/AIDS. J Clin Neuromuscul Dis. 2011;13(2):68–84. doi: 10.1097/CND.0b013e318221256f. [DOI] [PubMed] [Google Scholar]
- 35.Wiebe LA, Phillips TJ, Li JM, Allen JA, Shetty K. Pain in HIV: an evolving epidemic. J Pain. 2011;12(6):619–24. doi: 10.1016/j.jpain.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Kaul M, Lipton SA. Mechanisms of neuronal injury and death in HIV-1 associated dementia. Curr HIV Res. 2006;4(3):307–18. doi: 10.2174/157016206777709384. [DOI] [PubMed] [Google Scholar]
- 37.Federico A, Cardaioli E, Da Pozzo P, Formichi P, Gallus GN, Radi E. Mitochondria, oxidative stress and neurodegeneration. J Neurol Sci. 2012;322(1–2):254–62. doi: 10.1016/j.jns.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 38.Kabanov AV, Gendelman HE. Nanomedicine in the diagnosis and therapy of neurodegenerative disorders. Prog Polym Sci. 2007;32(8–9):1054–1082. doi: 10.1016/j.progpolymsci.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sonavane G, Tomoda K, Makino K. Biodistribution of colloidal gold nanoparticles after intravenous administration: effect of particle size. Colloids Surf B Biointerfaces. 2008;66(2):274–80. doi: 10.1016/j.colsurfb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 40.De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJ, Geertsma RE. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29(12):1912–9. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 41.Charoenphol P, Huang RB, Eniola-Adefeso O. Potential role of size and hemodynamics in the efficacy of vascular-targeted spherical drug carriers. Biomaterials. 2010;31(6):1392–402. doi: 10.1016/j.biomaterials.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Vinogradov SV. Nanogels in the race for drug delivery. Nanomedicine (Lond) 2010;5(2):165–8. doi: 10.2217/nnm.09.103. [DOI] [PubMed] [Google Scholar]
- 43.Gorantla S, Poluektova L, Gendelman HE. Rodent models for HIV-associated neurocognitive disorders. Trends Neurosci. 2012;35(3):197–208. doi: 10.1016/j.tins.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.