Abstract
While feverfew has been used for centuries to treat pain and headaches and is recommended for migraine treatment, the mechanism for its protective action remains unknown. Migraine is triggered by calcitonin gene-related peptide (CGRP) release from trigeminal neurons. Peptidergic sensory neurons, express a series of transient receptor potential (TRP) channels, including the ankyrin 1 (TRPA1) channel. Recent findings have identified agents either inhaled from the environment or produced endogenously, which are known to trigger migraine or cluster headache attacks, as TRPA1 simulants. A major constituent of feverfew, parthenolide, may interact with TRPA1 nucleophilic sites, suggesting that feverfew antimigraine effect derives from its ability to target TRPA1. We found that parthenolide stimulates recombinant (transfected cells) or natively expressed (rat/mouse trigeminal neurons) TRPA1, where it, however, behaves as a partial agonist. Furthermore, in rodents, after initial stimulation, parthenolide desensitizes the TRPA1 channel, and renders peptidergic, TRPA1-expressing nerve terminals unresponsive to any stimulus. This effect of parthenolide abrogates nociceptive responses evoked by stimulation of peripheral trigeminal endings. TRPA1 targeting and neuronal desensitization by parthenolide inhibits CGRP release from trigeminal neurons and CGRP-mediated meningeal vasodilatation, evoked by either TRPA1 agonists or other unspecific stimuli. TRPA1 partial agonism, together with desensitization and nociceptor defunctionalization, ultimately resulting in inhibition of CGRP release within the trigeminovascular system, may contribute to the antimigraine effect of parthenolide.
Keywords: TRPA1, Parthenolide, Migraine, CGRP
1. Introduction
Feverfew (Tanacetum parthenium L.) has been used for centuries as a remedy for pain, fever, and headaches [26]. Feverfew, alone or in combination with other compounds, has been long evaluated as a prophylactic agent for migraine [24, 28, 41]. Positive well-powered, randomized clinical trials [14, 37] have led to the recommendation of MIG-99, a relatively stable extract manufactured with supercritical CO2 from feverfew, for migraine prevention with a level B of evidence [22]. More recently, two similar sublingually administered products containing feverfew and ginger have been successfully tested as an acute treatment for migraine attacks [12, 13]. However, the underlying mechanism of the antimigraine action of feverfew remains unknown.
The major constituent of feverfew is the sesquiterpene lactone, parthenolide (Fig. 1A), which has previously been found to exert anti-inflammatory effects [38, 44]. Parthenolide may interact with nucleophilic sites of proteins via its α-methylene-γ-lactone ring and epoxide moiety [36, 42]. This chemical property qualifies a number of reactive molecules as agonists of the transient receptor potential ankyrin 1 (TRPA1), which by covalent modification of cysteine residues cause channel activation [19, 27, 47]. TRPA1 is uniquely stimulated by an unprecedented series of reactive exogenous and endogenous molecules, generated by oxidative stress [1, 6, 7, 29, 47].
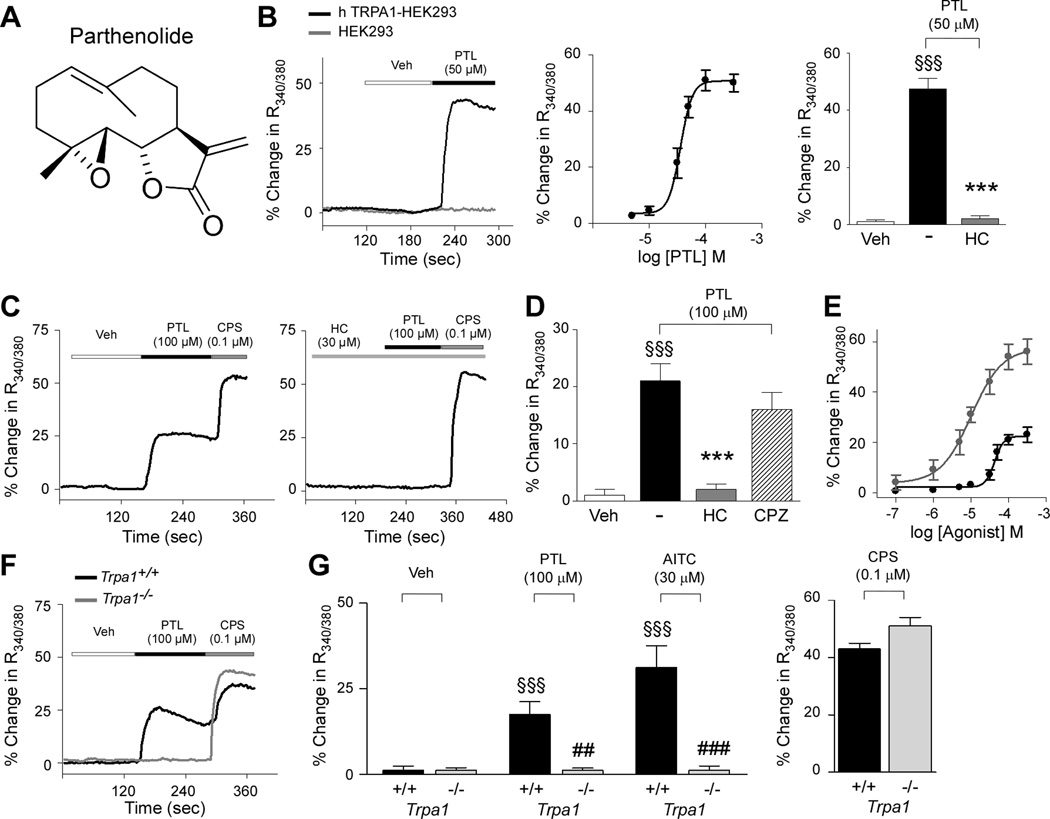
Figure 1.
Parthenolide selectively activates the TRPA1 channel. (A) Chemical structure of parthenolide (PTL). (B) Representative traces and concentration-response curve (CRC) of intracellular calcium mobilization evoked by PTL in HEK293 cells transfected with the cDNA of human TRPA1 (hTRPA1-HEK293; black line) or in untransfected-HEK293 cells (HEK293; grey line). Calcium response evoked by PTL (50 µM) was abolished by the selective TRPA1 antagonist, HC-030031 (HC; 30 µM). (C) Representative traces, (D) pooled data, and (E) CRC of intracellular calcium mobilization evoked by PTL (black dots and line) or allyl isothiocyanate (AITC; grey dots and line) in cultured rat trigeminal ganglion (TG) neurons. Calcium response induced by PTL was abolished by HC (30 µM), and unaffected by the selective TRPV1 antagonist, capsazepine (CPZ; 10 µM) (D). (F,G) Calcium response evoked by PTL or AITC (30 µM) in TG neurons from Trpa1+/+ (black line and columns) was absent in neurons from Trpa1−/− mice (grey line and columns). Responses to capsaicin (CPS; 0.1 µM) were unchanged. Veh is the vehicle of PTL. Each trace represents an average of at least 15 responsive cells/neurons; each point or column represents mean ± SEM of n > 25 cells/neurons. Dash indicates combined vehicles of treatments. §§§P< 0.001 vs. Veh, ***P< 0.001 vs. PTL (ANOVA followed by Bonferroni test). ##P < 0.01 vs. Trpa1+/+treated group (Student’s t-test).
A subset of primary afferents, characterized as ‘capsaicin-sensitive’ because they express the capsaicin receptor, TRP vanilloid 1 (TRPV1), are also enriched with TRPA1 channels, and express and release the sensory neuropeptides, substance P (SP), neurokinin A (NKA), and calcitonin gene-related peptide (CGRP) [10, 23, 43]. CGRP release within the trigeminovascular system is now considered to play a key role in the genesis of migraine headaches [16, 21, 35]. In general, endogenous agents that activate receptors/channels on trigeminal neurons to release CGRP may be considered migraine generators, while exogenous compounds that prevent such activation may be considered potential migraine medicines [31].
More recently, the role of TRPA1 in migraine pathophysiology has been suggested by the observation that both the reactive α,β-unsaturated aldehyde and TRPA1 agonist, acrolein [7], and one major volatile component of the headache tree (U. californica) scent, umbellulone, selectively target TRPA1 to produce a CGRP-dependent meningeal vasodilatation [15, 25, 33], which is considered a predictive migraine model. Thus, we hypothesized that parthenolide exerts its antimigraine effect via TRPA1 targeting.
Here, we found that parthenolide activates both recombinant and neuronal TRPA1. However, parthenolide behaves as a TRPA1 partial agonist, and by targeting TRPA1 causes selective channel desensitization and a non-selective defunctionalization of CGRP-containing sensory neurons. These three peculiar features of parthenolide, ultimately resulting in the inhibition of CGRP release from trigeminal neurons, may contribute to the antimigraine effect of feverfew.
2. Materials and Methods
2.1. Animals
Animal experiments were carried out in conformity to the European Communities Council (ECC) guidelines for animal care procedures and the Italian legislation (DL 116/92) application of the ECC directive 86/609/EEC. Studies were conducted under the University of Florence research permit ✉143/2008-B and ✉204/2012-B. Male C57BL/6 mice (Harlan Laboratories, Milan, Italy), wild type ( Trpa1+/+) or TRPA1- deficient (Trpa1−/−) mice [7], or Sprague-Dawley rats (male, Harlan Laboratories) were used. Animals were sacrificed with intraperitoneal (i.p.) sodium pentobarbital (200 mg/kg).
2.2. Parthenolide powder extraction
Flowered aerial parts of feverfew [Tanacetum parthenium (L.) Schultz Bip., 2 Kg] were extracted with ethanol. Removal of the solvent left a dark gum, which was partitioned between petroleum ether (2 L) and methanol-water (9:1, 2 L). The defatted methanol phase was evaporated, and the residue (37 g) was suspended in petroleum ether-ethyl acetate 1:1, and purified by vacuum chromatography on neutral alumina (300 g), using petroleum ether-ethyl acetate 1:1 as eluent. The fractions containing parthenolide were pooled and evaporated, and the residue was triturated with ether, with a yield of 6.94 g (0.35% on dried plant material) of a white powder, identified by direct comparison with an authentic sample of parthenolide available from a previous study [3].
2.3. Reagents
If not otherwise indicated, all reagents were from Sigma-Aldrich (Milan, Italy). HC-030031 was synthesized as described [2]. Parthenolide extracted powder was dissolved in 100% dimethyl sulfoxide, DMSO, at the final concentration of 100 mM.
2.4. Cell culture and isolation of primary sensory neurons
Human embryonic kidney (HEK293) cells stably transfected with the cDNA of human TRPV1 (hTRPV1-HEK293) or with the cDNA of human TRPA1 (hTRPA1-HEK293) and naive HEK293 cells (American Type Culture Collection, Manassas, VA, USA) were cultured as previously described [33]. A tetracycline-regulated system for inducible expression of TRPA1 in Chinese hamster ovary (CHO) cells transfected with the cDNA of the mouse TRPA1 (mTRPA1-CHO) was used and cultured as previously reported [32]. Naive CHO cells were used as control. For humanTRPA1 (hTRPA1-CHO) wild-type or human mutant 3C/K-Q (C619S, C639S, C663S, K708Q) cDNAs [19] (1 µg/ml) were expressed in naive CHO cells using a polyethylenimine transfection method (PEI, Sigma-Aldrich, Milan, Italy) [4, 45] and cultured as previously reported [19]. All cells were cultured in an atmosphere of 95% air and 5% CO2 at 37 °C.
Trigeminal ganglia (TG) neurons from rats and Trpa1+/+ or Trpa1−/− mice were collected as previously reported [33] (for details see Supplementary Methods).
2.5. Calcium imaging experiments
Calcium fluorescence was measured in rodent in transfected and untransfected HEK293 cells or in trigeminal ganglia neurons, as previously reported [29, 33]. Plated cells were loaded with Fura-2AM-ester (5µM; Alexis Biochemicals; Lausen, Switzerland) added to the buffer solution (37 °C) containing the following (in mM): 2 CaCl2; 5.4 KCl; 0.4 MgSO4; 135 NaCl; 10 D-glucose; 10 HEPES and 0.1% bovine serum albumin at pH 7.4. After 40 min, cells were washed and transferred to a chamber on the stage of a Nikon Eclipse TE2000U microscope for the recording. Cells were excited alternatively at 340 nM and 380 nM to indicate relative intracellular calcium changes by the Ratio340/380 recorded with a dynamic image analysis system (Laboratory Automation 2.0; RCSoftware, Florence, Italy). Cells and neurons were exposed to parthenolide (30–1000 µM), allyl isothiocyanate (AITC, 30 µM), or their vehicles (1%, 0.3 % DMSO). Capsaicin (0.1 µM) was used to identify nociceptive neurons. HC-030031 (30 µM), capsazepine (10 µM) or their respective vehicles (3% and 0.1% DMSO) were used. Results are expressed as the percentage of increase of Ratio340/380 over the baseline normalized to the maximum effect induced by ionomycin (5µM) added at the end of the experiment (% Change in R340/380).
2.6. Electrophysiological recordings
Electrophysiological recordings in the whole-cell mode were performed in CHO transfected cells as previously reported [32] (for details see Supplementary Methods). TRPA1-evoked currents were detected upon cell superfusion with parthenolide (100 µM) and AITC (50 – 100 µM).
2.7. Eye-wiping assay in mice
Conjuctival application of parthenolide (12.5–25–50 nmol, 5 µl) or its vehicle (5 % DMSO) was used to induce an acute nociceptive response in mice [33]. In other experiments, mice received conjunctival AITC (20 nmol) or capsaicin (1 nmol) 40 min after a single (4 mg/kg, i.p.) or repeated (once a day for 5 days, 4 mg/kg, Intragastric, i.g.) parthenolide administration. Data are expressed as number of eye movements following drug application.
2.8. Dural cannulation and behavior testing
Dural cannulation and behavioral studies were performed under protocols approved by the Institutional Animal Care and Use Committee of the University of Arizona and were in accordance with policies and recommendations of the IASP and the NIH guidelines for handling and use of laboratory animals. Rat dura cannulation and cutaneous allodynia measurement were performed as described [49]. AITC (10%, 10 µl), capsaicin (1 µM, 10 µl) or their vehicle (mineral oil) were injected through the cannula 40 min after parthenolide (4 mg/kg, i.p.) or its vehicle (4% DMSO in phosphate-buffered saline). Calibrated von Frey filaments were applied to the midline of the forehead and to the hind paw at 1-hour intervals after injection of the stimuli. Maximum filament strengths were 8 and 15 g for the face and hind paws, respectively. The withdrawal thresholds were determined by the Dixon up-down method.
2.9. CGRP-like immunoreactivity (LI) assay
For CGRP-LI outflow experiments, 0.4 mm slices of rodent tissues (rat spinal cord, trigeminal ganglia and dura mater, and mouse spinal cord) were superfused with an aerated (95% O2 and 5% CO2) Krebs solution with the following composition (in mM): 119 NaCl, 25 NaHCO3, 1.2 KH2PO4, 1.5 MgSO4, 2.5 CaCl2, 4.7 KCl, 11 D-glucose; maintained at 37°C, containing 0.1% bovine serum albumin, and, to minimize peptide degradation, added with the angiotensin converting enzyme inhibitor, captopril (1 µM), and the neutral endopeptidase inhibitor, phosphoramidon (1 µM). Tissues were stimulated with parthenolide (10–30–100 µM) or its vehicle (1% DMSO) dissolved in modified Krebs solution. Some tissues were pre-exposed to capsaicin (10 µM, 20 min) or superfused with a calcium-free buffer containing EDTA (1 mM). Others tissues were pretreated with HC-030031 (30 µM), capsazepine (10 µM) or pre-exposed to parthenolide (300 µM, 30 min) and then, after a prolonged washing, stimulated with parthenolide (100 µM), AITC (100 µM), capsaicin (0.3 µM) or KCl (40 mM). Fractions (4 ml) of superfusate were collected at 10-min intervals before, during, and after administration of stimulus and then freeze-dried, reconstituted with assay buffer, and analyzed for CGRP-LI by using a commercial ELISA kit (Bertin Pharma, France). CGRP-LI was calculated by subtracting the mean prestimulus value from those obtained during or after stimulation. Detection limits of the assays were 5 pg/ml. Results are expressed as femtomoles of peptide per g of tissue. Stimuli did not cross react with CGRP antiserum.
2.10. Meningeal blood flow
Changes in rat middle meningeal artery blood flow were recorded with a Laser Doppler Flowmeter (Perimed Instruments, Milan, Italy) following the procedure reported elsewhere [33]. Briefly, rats were anesthetized (sodium pentobarbital, 50 mg/kg, i.p.), the head fixed in a stereotaxic frame. A cranial window (4 × 6 mm) was realized into the parietal bone to expose the dura mater. The probe (needle type, tip diameter 0.8 mm) was fixed near a branch of the middle meningeal artery (1 mm from the dural outer layer). The window was filled with a modified synthetic interstitial fluid (SIF) containing (in mM): 135 NaCl; 5 KCl; 1 MgCl2; 5 CaCl2; 10 d-glucose and 10 HEPES. Meningeal blood flow was monitored for 30 min after administration of parthenolide (4 mg/kg, i.p.) or AITC (1 µmol/kg, i.p.). Meningeal blood flow was measured also after the administration of acrolein (50 nmol, intranasal, i.n.), ethanol (140 µl/kg, intravenous, i.v.), sodium nitroprusside (SNP, 1 mM/100 µl, topically to the dura surface) or their vehicles 30 min after parthenolide (4 mg/kg, i.p.). Baseline flow was calculated by the mean flow value measured during a 5-min period prior to stimulus. The increase in blood flow was calculated as % change over the baseline.
2.11. Statistical analysis
All values are the mean ± SEM. Statistical analyses were performed by the unpaired Student’s t-test or the two-way or one-way analysis of variance (ANOVA), followed by the post-hoc Bonferroni’s test, or by the Neuman–Keuls test. p<0.05 was considered statistically significant.
3. Results
3.1. Parthenolide activates recombinant and native TRPA1
Parthenolide is known to react with nucleophiles, in particular with cysteine thiol groups, via a Michael addition reaction [42, 48], a mechanism through which many compounds activate TRPA1 [2, 19, 27, 47]. To verify if parthenolide targets TRPA1, we exposed hTRPA1-HEK293 cells to parthenolide, which produced a concentration-dependent increase in intracellular calcium, indicating a stimulatory action. This effect of parthenolide was absent in HEK293 untransfected cells (Fig. 1B). In addition, the calcium response evoked by parthenolide was abolished by the selective TRPA1 antagonist, HC-030031 [30] (Fig. 1B), and absent in hTRPV1-HEK293 cells (data not shown). Next, we examined the effect of parthenolide in cultured rat TG neurons. Parthenolide increased intracellular calcium, and the effect was concentration-dependent (Fig. 1E), and abrogated by HC-030031, but was unaffected by the TRPV1 antagonist, capsazepine (Fig. 1C and D). Parthenolide, similarly to the selective TRPA1 agonist, allyl isothiocyanate (AITC), produced a measurable calcium response in about 40% of TG neurons isolated from Trpa1+/+ mice, an effect that was completely absent in TG neurons isolated from or Trpa1−/− mice (Fig. 1F and G). In contrast, calcium response to capsaicin was unchanged in neurons from both Trpa1+/+ and Trpa1−/− mice (Fig. 1G). All together these findings indicate selectivity of parthenolide for the TRPA1 channel. Furthermore, in rat trigeminal neurons parthenolide showed, in addition to a lower potency, a much-reduced efficacy (maximum response, Emax: 22% ± 2% of ionomycin %, n = 30) than that of AITC (Emax: 58% ± 5% of ionomycin, n = 26, P< 0.001) (Fig. 1E), thus indicating that it behaves as a low potency partial agonist at the TRPA1 channel.
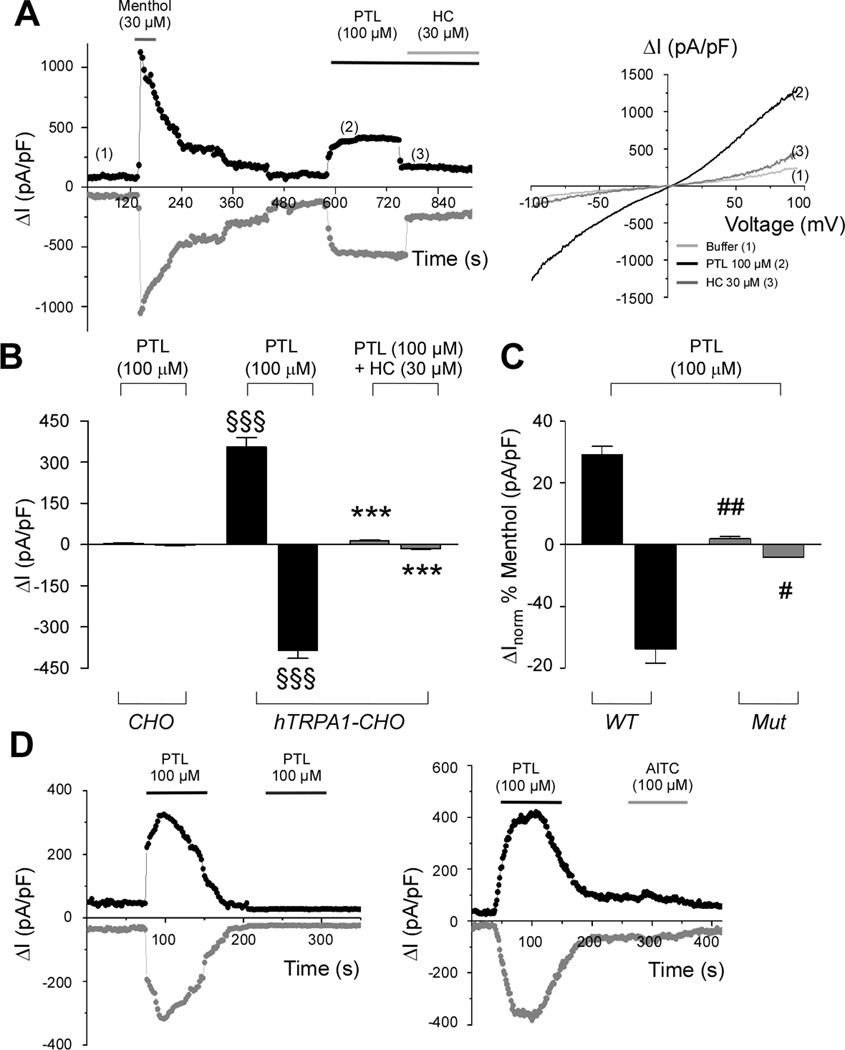
Whole-cell patch-clamp recordings in both hTRPA1-CHO and mTRPA1-CHO transfected cells confirmed the data obtained with calcium experiments, as extracellular application of parthenolide induced a fast activation of both outward and inward currents, which were blocked by the TRPA1-antagonist HC-030031 and were absent in CHO untransfected cells (Fig. 2A and B and Supplementary Fig. 1). To get better insight into the molecular mechanism of the parthenolide and TRPA1 interaction, mutagenesis studies and desensitization studies were undertaken. hTRPA1 activation by electrophilic agonists is dependent on the presence of three key cysteine (C619, C639, C663) and one lysine (K708) residues [19, 27]. Parthenolide activates TRPA1 channel through this mechanism, as it was inactive in CHO transfected with the mutant and human TRPA1 mutant (C619S, C639S, C663S, K708Q) cDNA (3C/K-Q), while the non-electrophilic TRPA1 agonist, menthol [45], activated both mutant and wild-type channel (Fig. 2C). In calcium-containing solutions, application of parthenolide or AITC produced typical inward TRPA1 currents in mTRPA1-CHO cells followed by a rapid calcium-mediated inactivation. Under these conditions, TRPA1 was refractory to stimulation with a second application of parthenolide or AITC (Fig. 2D and Supplementary Fig. 1).
Figure 2.
Parthenolide elicits characteristic TRPA1 current and causes desensitization. (A) Representative time courses and (B) pooled-data of whole-cell currents through humanTRPA1-CHO (hTRPA1-CHO) transfected cells measured at membrane potential of +50 mV (black curve) and −50 mV (grey curve), activated by parthenolide (PTL; 100 µM), followed by a fast block by the TRPA1 antagonist, HC-030031 (HC; 30 µM). No response was observed in untransfected CHO cells (CHO). Each column represents mean ± SEM of n > 5 cells. §§§P <0.001 vs. Veh and ***P< 0.001 vs. PTL (ANOVA followed by Bonferroni test). (C) Average values ± SEM (n = 5 cells) for increased currents (at +50 and −50 mV) evoked by PTL (100 µM) through hTRPA1 wild-type (WT) and mutant (3C/K-Q; Mut) CHO transfected cells. Values are normalized to activation induced by menthol (30 µM). PTL doesn’t activate Mut, whereas menthol, which interacts with TRPA1 in a non-covalent manner, does. #P< 0.05, ##P < 0.01 vs. WT (Student’s t-test). (D) Representative time course of whole-cell currents through mouseTRPA1-CHO transfected cells measured at membrane potential of +50 mV (black curve) and −50 mV (grey curve) evoked by PTL (100 µM). A second stimulation of TRPA1 by PTL (100 µM) or allyl isothiocyanate (AITC; 100 µM) failed to evoke any current.
3.2. Parthenolide activates trigeminal nociceptive behavior and allodynia and causes desensitization
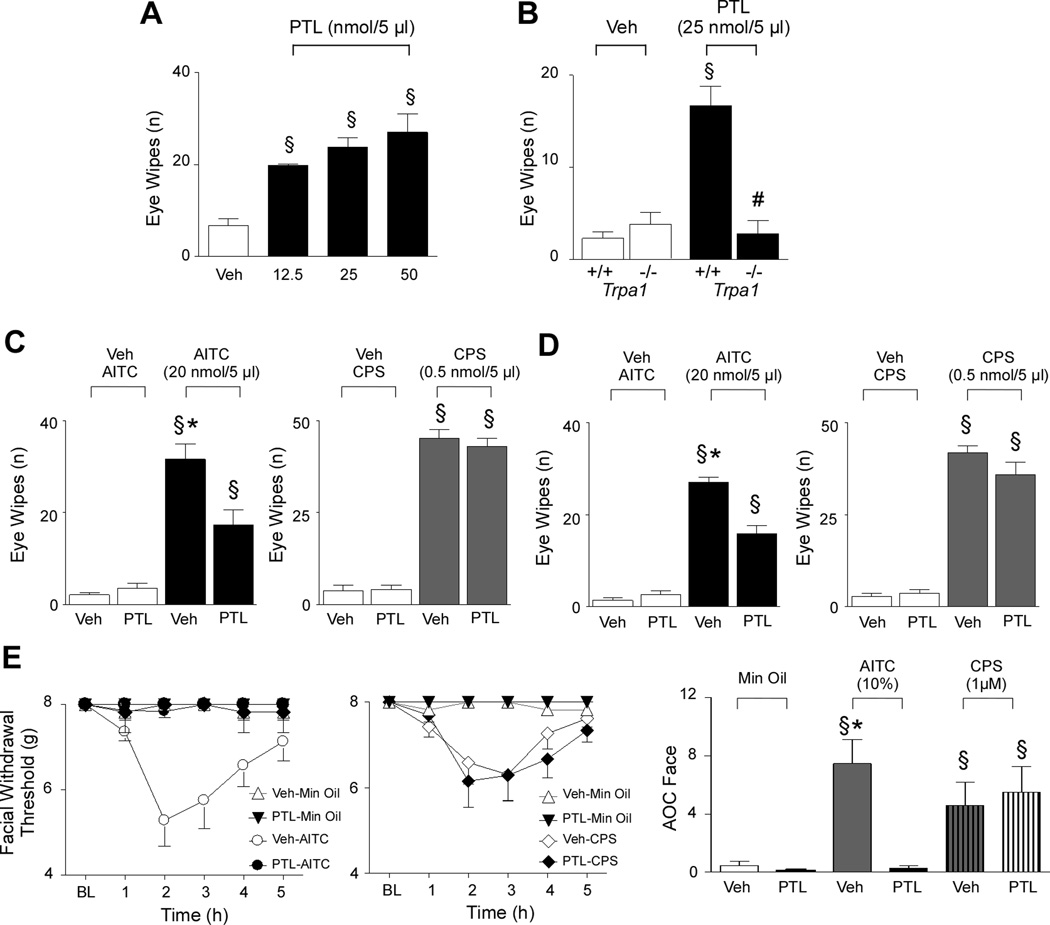
Next, to investigate whether parthenolide, via TRPA1 targeting, affects the functioning of trigeminal nociceptors, we used the eye wiping assay in mice [33]. Conjunctival parthenolide instillation evoked in a concentration-dependent manner an acute nociceptive behavior in C57/BL6 mice and Trpa1+/+ mice, while a negligible effect, indistinguishable from the response produced by parthenolide vehicle, was observed in Trpa1−/− mice (Fig. 3A and B). Of notice, the response evoked by conjunctival instillation of AITC (Fig. 3C) was markedly reduced after systemic administration of parthenolide. AITC was given 40 min after one single dose of parthenolide (4 mg/kg, i.p.), or 40 min after the last of 5 daily treatments with parthenolide (4 mg/kg, i.g.) (Fig. 3D). In contrast, either acute or chronic parthenolide treatment did not affect eye wiping response evoked by instillation of the TRPV1 agonist, capsaicin (Fig. 3C and D). This finding indicates selective desensitization by parthenolide of nociceptive behaviors evoked by TRPA1-specific stimuli.
Figure 3.
Parthenolide activates trigeminal nociceptive behavior in rodents via TRPA1 activation and induces desensitization. (A) Ocular instillation of parthenolide (PTL) in C57/BL6 mice produced a dose-dependent eye wiping nociceptive response. (B) The response to PTL observed in Trpa1+/+ mice was completely absent in Trpa1−/− mice. Values are mean ± s.e.m. of 5 mice. §§§P <0.001 vs. Veh (ANOVA followed by Bonferroni test). ###P<0.001 vs. PTL-Trpa1+/+ (Student’s t-test). (C) The eye wiping response (assessed 40 min after PTL) evoked by ocular instillation of allyl isothiocyanate (AITC; 20 nmol/5 µl), was reduced by intraperitoneal (i.p.) PTL pretreatment (4 mg/kg). PTL pretreatment did not modify the effect induced by ocular instillation of capsaicin (CPS; 0.5 nmol/5 µl). (D) The eye wiping response evoked by ocular instillation of AITC (20 nmol, assessed 40 min after the last PTL administration) was reduced after repeated treatment with Intragastric (i.g.) PTL (4 mg/kg, once day for 5 days). Veh is the vehicle of AITC or CPS, §§§P <0.001 vs. Veh; **P <0.01 and ***P <0.01 vs. PTL pretreated group (ANOVA followed by Bonferroni). (E) Time course and (F) area over the time-effect curve (AOC; baseline to 5 h) of the facial allodynia evoked by the application of AITC (10% in mineral oil, Min Oil) or CPS (10 µl) or their vehicle (Min Oil 100%) on rat dura. The generalized allodynia induced by AITC, but not that evoked by topical application of CPS, was prevented by PTL (4 mg/kg, i.p., given 40 min before dural application). Veh is the vehicle of systemic PTL. Data represent the mean ± SEM of 6 rats per group. §P < 0.05 vs. Min Oil, **P< 0.05 vs. PTL pretreated group. Data were analyzed among groups and across time (baseline to 5 h) by 2-factor ANOVA followed by the Neuman-Keuls test.
Prior studies have shown that exposure of the dura to TRPA1 agonists produces time-dependent and reversible cutaneous allodynia of the face and hindpaws, which has been proposed as a preclinical rat behavioral model of migraine [15]. Thus, we evaluated if systemic pretreatment with parthenolide (4 mg/kg i.p., 40 min before dural stimulation) was able to attenuate migraine-related behaviors in rats due to dural TRPA1 activation [15]. The data of the areas-over-time effect curve (AOC) show significant allodynia following AITC stimulation compared to vehicle (mineral oil). Systemic pretreatment with parthenolide (4 mg/kg i.p., 40 min before dural stimulation), but not its vehicle (4% DMSO, in phosphate-buffered saline), prevented the development of the cutaneous allodynia of the rat face (Fig. 3E) and hindpaw (Supplementary Fig. 2) produced by dural application of AITC. However, systemic pretreatment with parthenolide did not alter the cutaneous allodynia evoked by the dural application of capsaicin (Fig. 3E and Supplementary Fig. 2). Again, these findings underline that parthenolide abrogates solely TRPA1 -dependent afferent pathways.
3.3. Parthenolide releases CGRP from primary afferents and causes non-selective desensitization
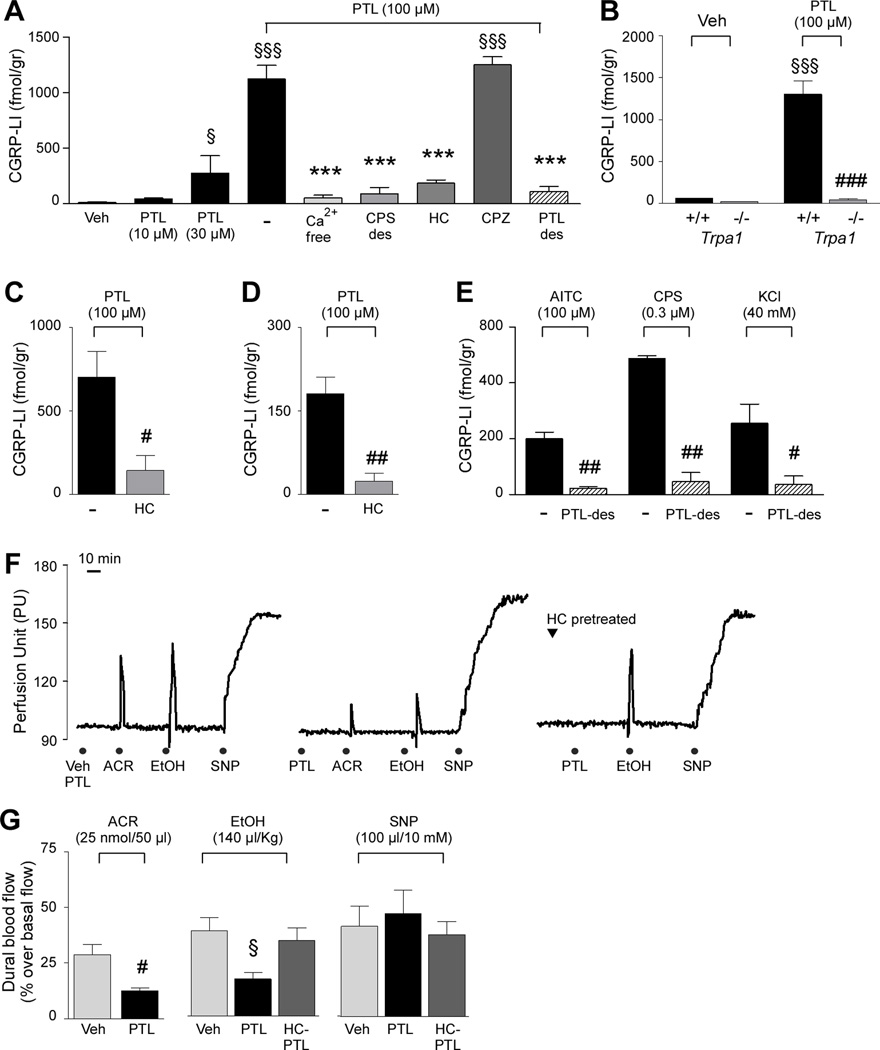
There is evidence that TRPA1 is exclusively localized to peptidergic primary sensory neurons [10, 43]. Experiments described in the previous section support this view. To further examine the ability of parthenolide to affect the release of CGRP from both central and peripheral endings of primary sensory neurons several preparations were used. Parthenolide increased the outflow of CGRP-LI from rat spinal cord slices (Fig. 4A), an effect prevented by pre-exposure to a high concentration of capsaicin [46] and in a calcium-free medium. The effect of parthenolide was abated by HC-030031 but was unaffected by capsazepine (Fig. 4A). In addition, pre-exposure to an elevated concentration of parthenolide prevented parthenolide-evoked CGRP-LI release from rat spinal cord, indicating self-desensitization (Fig. 4A). Similar results were obtained in slices of rat TG (Fig. 4C) or dura mater (Fig. 4D). Exposure to parthenolide increased CGRP-LI outflow from dorsal spinal cord slices obtained from Trpa1+/+ mice, an effect that was completely absent in tissues taken from Trpa1−/− mice (Fig. 4B). Finally, preexposure to an elevated concentration of parthenolide abated the increase in CGRP-LI outflow elicited in rat dura mater by either capsaicin or a high K+ medium (Fig. 4E). Thus, parthenolide by targeting TRPA1, after an initial activation, causes non-selective and complete desensitization of CGRP release from trigeminal neurons.
Figure 4.
Parthenolide via TRPA1 activation releases CGRP from, and causes desensitization of, rat primary sensory neurons. (A) Parthenolide (PTL) increased the calcitonin gene-related peptide-like immunoreactivity (CGRP-LI) outflow from rat dorsal spinal cord slices in a concentration-dependent manner. Effect of PTL (100 µM) was abolished by calcium removal (Ca2+-free), capsaicin desensitization (CPS-des) and the selective TRPA1 antagonist, HC030031 (HC; 30 µM), but not by the selective TRPV1 antagonist, capsazepine (CPZ; 10 µM). Pre-exposure to a high concentration of PTL (300 µM, 30 min; PTL-des) also abolished PTL-evoked CGRP release. (B) PTL increased CGRP-LI outflow from spinal cord slices from Trpa1+/+ mice, but not from Trpa1−/− mice. PTL, elicited a HC-sensitive CGRP-LI release from rat trigeminal ganglia(C) and dura mater (D). (E) Exposure to a high concentration of PTL (300 µM, 30 min) abated CGRP-LI release evoked by allyl isothiocyanate (AITC; 100 µM), capsaicin (CPS; 0.3 µM) or KCl (40 mM). Veh is the vehicle of PTL. Values are mean ± SEM of n = 4 experiments. Dash indicates combined vehicles of the treatments. §P < 0.05 and §§§P< 0.001 vs. Veh; ***P < 0.001 vs. PTL (ANOVA followed by Bonferroni test). #P <0.05,##P < 0.01 and ###P < 0.001 vs. Trpa1 +/+or respective vehicle group (Student’s t-test). (F) Representative traces and (G) pooled data of the increases in dural blood flow evoked by intranasal acrolein (ACR, 50 nmol/50 µl), intravenous ethanol (EtOH, 140 µl/kg, i.v.) or dural application of sodium nitroprusside (SNP; 10 mM/100 µl). PTL (4 mg/kg, i.p.) treatment (middle panel and bar graph) significantly reduced responses to ACR and EtOH, but not to SNP, and HC (300 mg/kg, i.g.) pretreatment (right hand panel and bar graph) prevented the inhibitory effect evoked by systemic PTL (HC-PTL) on EtOH-evoked response. Veh is the vehicle of PTL. Values are mean ± s.e.m. of n > 5 animals. #P<0.05 vs. Veh (Student’s t-test). §P < 0.05 vs. Veh (ANOVA followed by Bonferroni test).
3.4. Parthenolide modulates changes in meningeal blood flow by TRP stimulants
A large body of evidence has shown that stimulants of peptidergic trigeminal neurons in rodents cause, among various effects, either extravasation of plasma proteins in the dura mater (an effect mediated by the tachykinins, SP and NKA) or vasodilatation of meningeal arterial vessels (a response mediated by CGRP) [31]. However, while tachykinin-dependent neurogenic edema does not seem to play a relevant role in migraine, CGRP release and the resulting neurogenic vasodilatation seems to represent a major underlying migraine mechanism [20, 21, 35]. Thus, we wondered whether parthenolide affects meningeal blood flow in rats. Whereas administration of AITC (i.p., 1 µmol/kg) produced a TRPA1-dependent and CGRP-mediated increase in meningeal blood flow (data not shown), maximum achievable dose of parthenolide (4 mg/kg, i.p.) was ineffective (Fig. 4F). Parthenolide (4 mg/kg, i.p., 30 min before the stimulus) inhibited the meningeal vasodilatation evoked by both intranasal acrolein instillation, a response that has been previously identified as produced by TRPA1 activation [25] or by intranasal capsaicin or intravenous ethanol, responses that are both mediated by TRPV1 stimulation [34]. Parthenolide did not affect meningeal vasodilatation evoked by the direct vasodilator agent, sodium nitroprusside (Fig. 4F and G). Finally, pretreatment with HC-030031 prevented the inhibitory effect of parthenolide on ethanol-evoked vasodilatation (Fig. 4F and G). Results suggest that parthenolide produces desensitization of perivascular meningeal sensory fibers, which mediate CGRP-dependent vasodilatation. As the desensitizing effect of parthenolide is not exclusively confined to TRPA1-agonists, but also to stimuli, such as acrolein, capsaicin or ethanol, which act via TRPA1-independent pathways [25, 34], it is possible that parthenolide, via TRPA1 targeting, causes non-selective defunctionalization of peptidergic sensory nerve terminals.
4. Discussion
The first novelty of the present study, supported by unequivocal pharmacological and genetic findings, is that parthenolide targets TRPA1. In particular, deletion of TRPA1 makes mouse neurons unresponsive to parthenolide and both genetic and pharmacological evidence indicate that parthenolide activates the human TRPA1 variant, suggesting that responses produced by parthenolide in rodents could also be reproduced in humans. Mutagenesis studies of the human TRPA1 indicate that the loss of function of the mutant channel enlists parthenolide into the larger series of electrophilic and reactive molecules, which activate TRPA1 via covalent binding to key aminoacidic residues of the protein [19, 47].
TRPA1 agonists, including acrolein [25] or umbellulone [8, 33], have been proposed to trigger migraine headache attacks via their ability to activate TRPA1, and the ensuing release of CGRP from perivascular trigeminal nerve endings. Therefore, the clinical use of feverfew in the treatment of migraine headache appears somehow contradictory to the present observation that parthenolide stimulates TRPA1 and releases CGRP. However, the second important novelty of our study consists in the observation that parthenolide acts as a TRPA1 partial agonist, produces selective channel desensitization, and causes defunctionalization of TRPA1-expressing, peptidergic neurons, e.g. renders nerve terminals unresponsive to a number of different stimuli. A reduced Emax of parthenolide as compared to that of AITC was observed in cultured TG neurons. This finding suggests that parthenolide behaves as a partial agonist at the TRPA1 channel. In the presence of endogenously produced agonists, such as reactive oxygen species [9, 40], or their by-products, including 4-hydroxynonenal [47], oxononenal [45], or acrolein [7], the partial agonist activity of parthenolide may result in TRPA1 inhibition.
Another way, by which parthenolide may cause TRPA1 inhibition, derives from its ability to produce desensitization of both afferent (nociceptive) and ‘efferent’ (neuropeptide-mediated and local) responses of primary sensory neurons. In vitro electrophysiological and organ bath studies and in vivo functional experiments showed that parthenolide pretreatment attenuates TRPA1-mediated responses. In particular, after initial activation, parthenolide caused channel desensitization in TRPA1-CHO cells, which quickly became unresponsive to a second application of parthenolide or AITC.
Differences between parthenolide-induced desensitization of afferent and efferent responses seem to depend on the localization of TRPA1 in specific subsets of primary sensory neurons. Studies with TRPA1-deficient mice and pharmacological treatments identified the wiping response to parthenolide and AITC [33] as entirely mediated by activation of TRPA1 expressed in TRPV1-positive neurons. In the case of this purely afferent response, parthenolide pretreatment selectively reduced the wiping response by TRPA1-agonists (AITC or parthenolide), but not that by a TRPV1 agonist (capsaicin) (Fig. 4). Similar results were obtained in experiments of facial allodynia produced by AITC application to the rat dura, an effect that was completely abated by parthenolide, which, in contrast, did not affect capsaicin-evoked response. Thus, desensitization to parthenolide seems to be confined to nociceptive responses solely initiated by TRPA1 activation.
A different scenario emerges from neuropeptide release studies in which the ‘efferent’ function [23] of sensory nerves was investigated. Pre-exposure to parthenolide prevented not only the ability of TRPA1 agonists (AITC or parthenolide) to release CGRP from dural sensory nerve terminals, but also that of a TRPV1 agonist (capsaicin) as well as that induced by high K+, a non-specific depolarizing agent. The most parsimonious explanation for this finding is that parthenolide causes complete defunctionalization of peptidergic trigeminal nerve endings, which are no longer able to release CGRP and SP/NKA in response to any stimulus. Capsaicin has long been known to produce sensory neuron defunctionalization [23], and this property represents the mechanistic basis for the clinical use of topical capsaicin treatments. Present findings are consistent with the notion that TRPA1 agonists, likewise capsaicin, cause homologous and heterologous desensitization [39].
How can the ability of parthenolide, intimately linked to TRPA1 targeting, to produce selective desensitization of TRPA1-mediated afferent responses and non-selective inhibition of neurogenic (neuropeptide-mediated) responses, be clarified? One possible explanation derives from the coexistence of TRPA1 in peptidergic neurons. Although the observation has not been always confirmed [5], TRPA1 seems to colocalize predominantly with neuropeptides (SP/NKA and CGRP) in a specific subset of nociceptive neurons, whereas a substantial portion of TRPV1 positive neurons, which responds to capsaicin, is not peptidergic, and does not express TRPA1 [10, 43]. Due to its selective action at TRPA1, parthenolide desensitizes TRPA1 and defunctionalizes exclusively the subset of TRPA1-expressing neurons, which, however, encompasses the entire peptidergic neuronal subpopulation. Thus, after exposure to parthenolide, peptidergic neurons are no longer able to release CGRP upon exposure to any stimulus, whereas non-peptidergic, TRPA1-negative and TRPV1-positive neurons may still produce nociceptive responses when exposed to a variety of stimuli, including capsaicin.
In agreement with this hypothesis, we found that parthenolide pretreatment reduces CGRP-mediated meningeal vasodilatation evoked by either intranasal acrolein, a phenomenon entirely TRPA1-dependent [25], or by i.v. ethanol, an entirely TRPV1-dependent response [34]. In addition, reversal by HC-030031 of parthenolide-evoked inhibition of vasodilatation induced by ethanol robustly supports the role of TRPA1 in this phenomenon. Defunctionalization by parthenolide of neuropeptide-mediated and TRPA1-dependent responses in in vitro experiments was complete. However, in vivo meningeal vasodilatation experiments, most likely because of poor drug pharmacokinetics, defunctionalization seems to be partial. Poor ability of gaining adequate concentrations for channel activation at perivascular trigeminal endings and/or weak agonism may also be the reasons why intraperitoneal injection of parthenolide failed to produce any measurable meningeal vasodilatation.
Parthenolide and related sesquiterpene lactones have been shown, among other effects, to inhibit the activation of the proinflammatory transcription factor, nuclear factor-κB (NF-κB), by different stimuli, such as phorbol esters, tumor necrosis factor-a, and hydrogen peroxide [11, 17]. These findings are of relevance in migraine as the nitric oxide (NO) donor, glyceryl trinitrate (GTN), known to provoke delayed migraine attacks when infused in migraineurs, increased the expression of inducible NO synthase (iNOS) by a NF-κB-dependent mechanism [38]. The observation that both NF-κB activation, and iNOS expression were attenuated by parthenolide [38] suggested that the antimigraine action of parthenolide may depend upon the inhibition of this key proinflammatory pathway. The underlying pharmacokinetic and/or pharmacodynamic mechanism responsible for the neuronal defunctionalization of TRPA1-expressing neurons by parthenolide remains to be elucidated. Nevertheless, this novel property of parthenolide may add up to previously described anti-inflammatory actions of the compound [18, 36, 38] to account for the ability of chronically or acutely administered feverfew to prevent migraine attacks [12–14].
Supplementary Material
Parthenolide, a major constituent of feverfew, acts as a partial agonist of TRPA1.
Parthenolide ability to target TRPA1 could explain its therapeutic effects on migraine.
Acknowledgments
This study was supported in part by the Regione Toscana (FABER – POR CREO, FESR 2007–2013 1.1.C.) to P.G., in part by Ente Cassa di Risparmio di Firenze to R.N. and M.R.M., as well as the NIH/NINDS (NS 072204) to G.D.. We are grateful to prof. David Julius (UCSF, CA USA) for the kind gift of the TRPA1-deficient mice and humanTRPA1 wild-type and humanTRPA1 mutant (C619S, C639S, C663S, K708Q) cDNAs; to prof. Bernd Nilius (Katholieke Universiteit, Leuven, Belgium) for providing mouseTRPA1-CHO transfected cells and to prof. Alyn H. Morice (Academic Department of Medicine, Castle Hill Hospital, UK) for providing humanTRPA1-HEK293 stably transfected cells. We thank Dr. Delia Preti and Prof. Pier Giovanni Baraldi (University of Ferrara, Italy) for providing HC-030031.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
P.G. is a member of the editorial boards of Physiological Reviews, Pain and Molecular Pain, and receives research support from Chiesi Farmaceutici, Merck Sharp & Dohme, Italian Institute of Technology, Regione Toscana, Italian Ministry of University and Research, and Ente Cassa di Risparmio di Firenze. M.R.M. receives research support from MIUR and Ente Cassa di Risparmio di Firenze. All other authors reported no bio-medical financial interests or potential conflicts of interest. R.P. is full-time employee at Chiesi Farmaceutici SpA. The other authors declare no competing interests.
References
- 1.Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M, Baraldi PG, Poole DP, Bunnett NW, Geppetti P, Patacchini R. Cigarette smoke-induced neurogenic inflammation is mediated by alpha, beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest. 2008;118:2574–2582. doi: 10.1172/JCI34886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appendino GG, Nano P, Crispolide GM. An Unusual Hydroperoxysesquiterpene Lactone from Tanacetum vulgare. Phytochemistry. 1982;21:1099–1102. [Google Scholar]
- 4.Aricescu AR, Lu W, Jones EY. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr D Biol Crystallogr. 2006;62:1243–1250. doi: 10.1107/S0907444906029799. [DOI] [PubMed] [Google Scholar]
- 5.Barabas ME, Kossyreva EA, Stucky CL. TRPA1 is functionally expressed primarily by IB4-binding, non-peptidergic mouse and rat sensory neurons. PLoS One. 2012;7:e47988. doi: 10.1371/journal.pone.0047988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baraldi PG, Preti D, Materazzi S, Geppetti P. Transient receptor potential ankyrin 1 (TRPA1) channel as emerging target for novel analgesics and anti-inflammatory agents. J Med Chem. 2010;53:5085–5107. doi: 10.1021/jm100062h. [DOI] [PubMed] [Google Scholar]
- 7.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 8.Benemei S, Appendino G, Geppetti P. Pleasant natural scent with unpleasant effects: cluster headache-like attacks triggered by Umbellularia californica. Cephalalgia. 2009;30:744–746. doi: 10.1111/j.1468-2982.2009.01988.x. [DOI] [PubMed] [Google Scholar]
- 9.Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. 2008;118:1899–1910. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhattacharya MR, Bautista DM, Wu K, Haeberle H, Lumpkin EA, Julius D. Radial stretch reveals distinct populations of mechanosensitive mammalian somatosensory neurons. Proc Natl Acad Sci U S A. 2008;105:20015–20020. doi: 10.1073/pnas.0810801105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bork PM, Schmitz ML, Kuhnt M, Escher C, Heinrich M. Sesquiterpene lactone containing Mexican Indian medicinal plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-kappaB. FEBS Lett. 1997;402:85–90. doi: 10.1016/s0014-5793(96)01502-5. [DOI] [PubMed] [Google Scholar]
- 12.Cady RK, Goldstein J, Nett R, Mitchell R, Beach ME, Browning R. A double-blind placebo-controlled pilot study of sublingual feverfew and ginger (LipiGesic M) in the treatment of migraine. Headache. 2011;51:1078–1086. doi: 10.1111/j.1526-4610.2011.01910.x. [DOI] [PubMed] [Google Scholar]
- 13.Cady RK, Schreiber CP, Beach ME, Hart CC. Gelstat Migraine (sublingually administered feverfew and ginger compound) for acute treatment of migraine when administered during the mild pain phase. Med Sci Monit. 2005;11:PI65–PI69. [PubMed] [Google Scholar]
- 14.Diener HC, Pfaffenrath V, Schnitker J, Friede M, Henneicke-von Zepelin HH. Efficacy and safety of 6.25 mg t.i.d. feverfew CO2-extract (MIG-99) in migraine prevention--a randomized, double-blind, multicentre, placebo-controlled study. Cephalalgia. 2005;25:1031–1041. doi: 10.1111/j.1468-2982.2005.00950.x. [DOI] [PubMed] [Google Scholar]
- 15.Edelmayer RM, Le LN, Yan J, Wei X, Nassini R, Materazzi S, Preti D, Appendino G, Geppetti P, Dodick DW, Vanderah TW, Porreca F, Dussor G. Activation of TRPA1 on dural afferents: a potential mechanism of headache pain. Pain. 2012;153:1949–1958. doi: 10.1016/j.pain.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goadsby PJ, Edvinsson L. Human in vivo evidence for trigeminovascular activation in cluster headache. Neuropeptide changes and effects of acute attacks therapies. Brain. 1994;117:427–434. doi: 10.1093/brain/117.3.427. [DOI] [PubMed] [Google Scholar]
- 17.Hehner SP, Heinrich M, Bork PM, Vogt M, Ratter F, Lehmann V, Schulze-Osthoff K, Droge W, Schmitz ML. Sesquiterpene lactones specifically inhibit activation of NF-kappa B by preventing the degradation of I kappa B-alpha and I kappa B-beta. J Biol Chem. 1998;273:1288–1297. doi: 10.1074/jbc.273.3.1288. [DOI] [PubMed] [Google Scholar]
- 18.Heptinstall S, White A, Williamson L, Mitchell JR. Extracts of feverfew inhibit granule secretion in blood platelets and polymorphonuclear leucocytes. Lancet. 1985;1:1071–1074. doi: 10.1016/s0140-6736(85)92371-2. [DOI] [PubMed] [Google Scholar]
- 19.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol. 2010;6:573–582. doi: 10.1038/nrneurol.2010.127. [DOI] [PubMed] [Google Scholar]
- 21.Ho TW, Ferrari MD, Dodick DW, Galet V, Kost J, Fan X, Leibensperger H, Froman S, Assaid C, Lines C, Koppen H, Winner PK. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet. 2008;372:2115–2123. doi: 10.1016/S0140-6736(08)61626-8. [DOI] [PubMed] [Google Scholar]
- 22.Holland S, Silberstein SD, Freitag F, Dodick DW, Argoff C, Ashman E. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346–1353. doi: 10.1212/WNL.0b013e3182535d0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- 24.Johnson ES, Kadam NP, Hylands DM, Hylands PJ. Efficacy of feverfew as prophylactic treatment of migraine. Br Med J (Clin Res Ed) 1985;291:569–573. doi: 10.1136/bmj.291.6495.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunkler PE, Ballard CJ, Oxford GS, Hurley JH. TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain. 2011;152:38–44. doi: 10.1016/j.pain.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levin M. Herbal treatment of headache. Headache. 2012;52(Suppl 2):76–80. doi: 10.1111/j.1526-4610.2012.02234.x. [DOI] [PubMed] [Google Scholar]
- 27.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 28.Maizels M, Blumenfeld A, Burchette R. A combination of riboflavin, magnesium, and feverfew for migraine prophylaxis: a randomized trial. Headache. 2004;44:885–890. doi: 10.1111/j.1526-4610.2004.04170.x. [DOI] [PubMed] [Google Scholar]
- 29.Materazzi S, Nassini R, Andre E, Campi B, Amadesi S, Trevisani M, Bunnett NW, Patacchini R, Geppetti P. Cox-dependent fatty acid metabolites cause pain through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A. 2008;105:12045–12050. doi: 10.1073/pnas.0802354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moskowitz MA. The neurobiology of vascular head pain. Ann Neurol. 1984;16:157–168. doi: 10.1002/ana.410160202. [DOI] [PubMed] [Google Scholar]
- 32.Nassini R, Gees M, Harrison S, De Siena G, Materazzi S, Moretto N, Failli P, Preti D, Marchetti N, Cavazzini A, Mancini F, Pedretti P, Nilius B, Patacchini R, Geppetti P. Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain. 2010;152:1621–1631. doi: 10.1016/j.pain.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 33.Nassini R, Materazzi S, Vriens J, Prenen J, Benemei S, De Siena G, la Marca G, Andre E, Preti D, Avonto C, Sadofsky L, Di Marzo V, De Petrocellis L, Dussor G, Porreca F, Taglialatela-Scafati O, Appendino G, Nilius B, Geppetti P. The 'headache tree' via umbellulone and TRPA1 activates the trigeminovascular system. Brain. 2012;135:376–390. doi: 10.1093/brain/awr272. [DOI] [PubMed] [Google Scholar]
- 34.Nicoletti P, Trevisani M, Manconi M, Gatti R, De Siena G, Zagli G, Benemei S, Capone JA, Geppetti P, Pini LA. Ethanol causes neurogenic vasodilation by TRPV1 activation and CGRP release in the trigeminovascular system of the guinea pig. Cephalalgia. 2008;28:9–17. doi: 10.1111/j.1468-2982.2007.01448.x. [DOI] [PubMed] [Google Scholar]
- 35.Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, Pollentier S, Lesko LM. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–1110. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- 36.Pareek A, Suthar M, Rathore GS, Bansal V. Feverfew (Tanacetum parthenium L.): A systematic review. Pharmacogn Rev. 2011;5:103–110. doi: 10.4103/0973-7847.79105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfaffenrath V, Diener HC, Fischer M, Friede M, Henneicke-von Zepelin HH. The efficacy and safety of Tanacetum parthenium (feverfew) in migraine prophylaxis--a double-blind, multicentre, randomized placebo-controlled dose-response study. Cephalalgia. 2002;22:523–532. doi: 10.1046/j.1468-2982.2002.00396.x. [DOI] [PubMed] [Google Scholar]
- 38.Reuter U, Chiarugi A, Bolay H, Moskowitz MA. Nuclear factor-kappaB as a molecular target for migraine therapy. Ann Neurol. 2002;51:507–516. doi: 10.1002/ana.10159. [DOI] [PubMed] [Google Scholar]
- 39.Ruparel NB, Patwardhan AM, Akopian AN, Hargreaves KM. Homologous and heterologous desensitization of capsaicin and mustard oil responses utilize different cellular pathways in nociceptors. Pain. 2008;135:271–279. doi: 10.1016/j.pain.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawada Y, Hosokawa H, Matsumura K, Kobayashi S. Activation of transient receptor potential ankyrin 1 by hydrogen peroxide. Eur J Neurosci. 2008;27:1131–1142. doi: 10.1111/j.1460-9568.2008.06093.x. [DOI] [PubMed] [Google Scholar]
- 41.Shrivastava R, Pechadre JC, John GW. Tanacetum parthenium and Salix alba (Mig- RL) combination in migraine prophylaxis: a prospective, open-label study. Clin Drug Investig. 2006;26:287–296. doi: 10.2165/00044011-200626050-00006. [DOI] [PubMed] [Google Scholar]
- 42.Skalska J, Brookes PS, Nadtochiy SM, Hilchey SP, Jordan CT, Guzman ML, Maggirwar SB, Briehl MM, Bernstein SH. Modulation of cell surface protein free thiols: a potential novel mechanism of action of the sesquiterpene lactone parthenolide. PLoS One. 2009;4:e8115. doi: 10.1371/journal.pone.0008115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 44.Tassorelli C, Greco R, Morazzoni P, Riva A, Sandrini G, Nappi G. Parthenolide is the component of tanacetum parthenium that inhibits nitroglycerin-induced Fos activation: studies in an animal model of migraine. Cephalalgia. 2005;25:612–621. doi: 10.1111/j.1468-2982.2005.00915.x. [DOI] [PubMed] [Google Scholar]
- 45.Taylor-Clark TE, Ghatta S, Bettner W, Undem BJ. Nitrooleic acid, an endogenous product of nitrative stress, activates nociceptive sensory nerves via the direct activation of TRPA1. Mol Pharmacol. 2009;75:820–829. doi: 10.1124/mol.108.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trevisani M, Geppetti P, Davis JB, Bianchi A, Harrison S, Randall AD, Smith GD, Owen D, Brough SJ, Jerman JC, Gray J, Amadesi S, Campi B, Barbieri M, Tognetto M, Gunthorpe MJ, Smart D. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat Neurosci. 2002;5:546–551. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- 47.Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andre E, Patacchini R, Cottrell GS, Gatti R, Basbaum AI, Bunnett NW, Julius D, Geppetti P. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner S, Kratz F, Merfort I. In vitro behaviour of sesquiterpene lactones and sesquiterpene lactone-containing plant preparations in human blood, plasma and human serum albumin solutions. Planta Med. 2004;70:227–233. doi: 10.1055/s-2004-815539. [DOI] [PubMed] [Google Scholar]
- 49.Yan J, Edelmayer RM, Wei X, De Felice M, Porreca F, Dussor G. Dural afferents express acid-sensing ion channels: a role for decreased meningeal pH in migraine headache. Pain. 2011;152:106–113. doi: 10.1016/j.pain.2010.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.