SUMMARY
Presenilins (PS), endoplasmic reticulum (ER) transmembrane proteins, form the catalytic core of γ-secretase, an amyloid precursor protein processing enzyme. Mutations in PS lead to Alzheimer's disease (AD) by altering γ-secretase activity to generate pathologic amyloid beta and amyloid plaques in the brain. Here, we identified a novel mechanism where binding of the soluble, cytosolic N-terminal domain (NTF) of PS to intracellular Ca2+ release channels, ryanodine receptors (RyR), controls Ca2+ release from the ER. While PS1NTF decreased total RyR-mediated Ca2+ release, PS2NTF had no effect at physiological Ca2+ concentrations. This differential function and isotype-specificity is due to four cysteines absent in PS1NTF, present, however, in PS2NTF. Site-directed mutagenesis targeting these cysteines converted PS1NTF to PS2NTF function and vice versa, indicating differential RyR binding. This novel mechanism of intracellular Ca2+ regulation through the PS-RyR interaction represents a novel target for AD drug development and the treatment of other neurodegenerative disorders that critically depend on ryanodine receptor and presenilin signaling.
Keywords: neuroprotection, oxidative stress, endoplasmic reticulum, Alzheimer's disease, intracellular calcium
INTRODUCTION
Alzheimer's disease (AD) is the most common form of dementia (Alzheimer's Association, 2012), and a number of causative mutations have been identified that underlie the familial form of the disease (FAD) (Mattson, 2004, Mattson, 2010). Mutations of presenilin (PS), a protein that forms the enzymatic core of the γ-secretase complex, have profound effects on amyloid precursor protein (APP) processing by increasing amyloid beta (Aβ) production and shifting cleavage towards higher levels of the neurotoxic peptide Aβ42 (Borchelt, et al., 1996, Duff, et al., 1996, Sato, et al., 2001, Scheuner, et al., 1996, Tomita, et al., 1997). Almost 200 PS mutations have been identified, many causative of AD (Mattson, 2010, Schellenberg and Montine, 2012). Most mutations are located in the gene encoding presenilin-1 (PS1) (Alzheimer's Disease Collaborative Group, 1995, Borchelt, et al., 1996, Sherrington, et al., 1995), while presenilin-2 (PS2) mutations typically cause later onset FAD (Levy-Lahad, et al., 1995, Rogaev, et al., 1995, Tomita, et al., 1997).
Every clinically relevant mutation of PS has been found to affect intracellular calcium signaling (Cowburn, et al., 2007, LaFerla, 2002). Previous studies have identified elevated luminal calcium concentrations in the endoplasmic reticulum (ER) during AD, potentiating increased Ca2+ release through the ryanodine receptor (RyR) (Chan, et al., 2000, Meyers, et al., 1995) and the inositol 1,4,5-trisphosphate receptor (IP3R) (Leissring, et al., 1999). RyRs critically contribute to the regulation of cytosolic calcium by the calcium-induced calcium release (CICR) mechanism (Zalk, et al., 2007) and contribute to the pathologic, elevated intracellular Ca2+ concentrations observed in AD (Chan, et al., 2000, Demuro, et al., 2010, Goussakov, et al., 2010, Smith, et al., 2005, Stutzmann, et al., 2006, Stutzmann, et al., 2007). The RyR is also controlled by the cellular redox potential and contributes to abnormal cytosolic Ca2+ homeostasis under oxidative stress as experienced by the cell during aging and disease (Xu, et al., 1998).
A potential function of PS as an ER calcium leak channel prior to its proteolytic cleavage and trafficking to the plasma membrane has been proposed (Tu, et al., 2006). However, a selective knock out of PS resulted in lower ER calcium levels in hippocampal neurons, contradicting this hypothesis (Kasri, et al., 2006, Zhang, et al., 2009). Similarly, no differences in ER calcium leak were identified when comparing wildtype (WT) PS with a loss of function mutant (Shilling, et al., 2012). Pharmacological evidence also suggests a different mechanism of action distinct from PS as an ER calcium leak channel. Dantrolene and ryanodine, both selective antagonists of RyR, ameliorate the disruption of intracellular Ca2+ homeostasis by either PS overexpression (Chan, et al., 2000, Guo, et al., 1999) or by FAD-causing mutant PS (Cedazo-Minguez, et al., 2002, Guo, et al., 1999, Smith, et al., 2005, Stutzmann, et al., 2007), indicating that RyR-mediated Ca2+ release from the ER causes the elevated cytosolic calcium concentrations seen in AD.
Endogenous proteolysis of PS results in two distinct N- and C-terminal cleavage products (Fig. 1), both have been found to bind RyR2 in a calcium dependent manner (PS1, (Chan, et al., 2000); PS2 (Lee, et al., 2006, Takeda, et al., 2005)). PS also interacts with the IP3R resulting in increased channel activity and Ca2+ release from the ER (Cheung, et al., 2010, Cheung, et al., 2008).
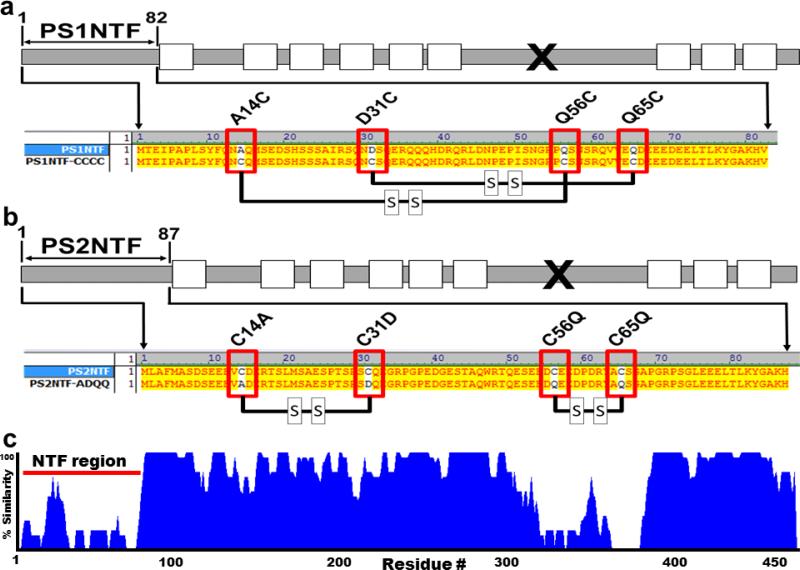
Figure 1. Alignment and predicted disulfide bridges of PS1NTF and PS2NTF and their mutants.
Cysteine sites of PS2NTF were mutated to the complementary residues of PS1NTF and vice versa, resulting in the PS2NTF-ADQQ and PS1NTF-CCCC constructs, respectively. A) Schematic representation of PS1. The soluble N-terminus fragment used in this study is indicated as PS1NTF (white rectangles, TM domains; “x”, endogenous cleavage site). Red boxes indicate mutagenesis sites, where the ADQQ residues of PS1NTF were replaced with complementary cysteine residues of PS2 resulting in the mutant PS1NTF-CCCC. Thick black lines between red boxes represent predicted disulfide bridges (http://scratch.proteomics.ics.uci.edu/; 3/6/2013). B) Schematic representation of PS2 (figure markings the same as 1a). Red boxes indicate mutagenesis sites where cysteine residues of PS2NTF were replaced with complementary residues of PS1 resulting in the mutant PS2NTF-ADQQ (black lines represent predicted disulfide bridges). C) The homology alignment for PS1 and PS2 identifies regions of high variability at the cytosolic N-termini (residues 1-80, blue tracing is proportional to the percent homology between PS1 and PS2) and endogenous cleavage regions (residues #320-370). This contrasts with the high homology of the proteins overall. The different effects of PS1 and PS2 are likely caused by the sequence differences of these two regions between the proteins.
Binding of the PS N-terminus to the RyR was identified as a novel mechanism to alter channel gating kinetics and activity by using soluble fragments of PS1 and PS2, respectively (PS-NTF; PS1NTF residues #1-82, PS2NTF residues #1-87) (Hayrapetyan, et al., 2008, Rybalchenko, et al., 2008). Binding of PS-NTF to RyR2 increased both channel open probability and mean calcium current, but with different inactivation kinetics at higher calcium concentrations. The cytosolic Ca2+ concentration required for modulation of RyR activity by PS1NTF was in the physiological range for neurons (100nM) whereas modulation by PS2NTF required Ca2+ concentrations above 10μM (Hayrapetyan, et al., 2008, Rybalchenko, et al., 2008). PS2NTF blocked the inhibition of RyR2 but only at cytosolic Ca2+ concentrations typically seen during pathophysiological conditions (Hayrapetyan, et al., 2008) while PS1NTF elicited a higher amplitude, shorter duration calcium release from the RyR by increasing channel gating (Rybalchenko, et al., 2008).
The large cytoplasmic face of the RyR is the target of many regulatory proteins of physiologic importance (Wehrens and Marks, 2003) that are lost in the purification procedures prior to electrophysiological experiments (Hwang, et al., 2003, Westhoff, et al., 2003). Therefore, we conducted the following experiments to demonstrate the PS1 and PS2 unique regulation of Ca2+ release through RyR in neuronal cells, thereby producing a Ca2+ dependent control of RyR activity.
PS1 and PS2 have very high sequence homology (>70%) (Fig. 1C) but discrete regions of low homology are present, namely the N-terminal cytosolic domains and the loop regions C-terminal to the endogenous cleavage sites (Fig. 1C). In silico sequence comparison of PS1NTF and PS2NTF indicates potentially significant differences in tertiary protein structure. Disulfide bridges, critical determinants of tertiary structure, were predicted to form between PS2NTF residues Cys14-Cys31 and Cys56-Cys65 (Fig. 1B) (http://scratch.proteomics.ics.uci.edu/; accessed 3/6/2013) and are absent in the cysteine-free PS1NTF.
We hypothesized that the tertiary structures of PS1NTF and PS2NTF were determinants of the PS regulatory effect on RyR-mediated Ca2+ release. We tested this hypothesis with site-directed mutagenesis that eliminated cysteine residues in PS2NTF and introduced cysteines to the PS1NTF sequence at homologous sites (Fig. 1A,B). These complementary mutations were predicted to form disulfide bridges in the mutated PS1NTF and the absence of such structures in the mutated PS2NTF (Fig. 1B) (http://scratch.proteomics.ics.uci.edu/; accessed 3/6/2013).
Our data indicate that the elimination of PS2NTF's cysteines by site-directed mutagenesis renders it functionally identical to PS1NTF with respect to the regulation of RyR-mediated Ca2+ release and, conversely, introduction of cysteines in the homologous sites in the sequence of PS1NTF generates a regulation of RyR similar to PS2NTF. These hypotheses were tested by analysis of intracellular calcium signaling in live cells. Our findings indicate that regulation of RyR-mediated calcium release by PS is physiologically relevant and a potential new target for treatment of diseases characterized by abnormal intracellular calcium homeostasis including but not limited to neurodegeneration in AD.
RESULTS
Expression of critical components of intracellular calcium signaling pathways in the neuronal SH-SY5Y cell line
Expression of intracellular calcium channel isotypes was measured in SH-SY5Y cells using immunocytochemistry and epitope specific antibodies (Methods Tables 2 & 3). SH-SY5Y cells predominantly expressed RyR as the main intracellular calcium channel with all three types of RyR present (Supplementary Fig. 1), while IP3R1 and IP3R3 were not detected, low levels of IP3R2 immunoreactivity was detected in the nucleus (Supplementary Fig. 2). SH-SY5Y cells showed strong endogenous immunoreactivity of both presenilin isoforms, with PS1 distributed ubiquitously and PS2 localized in and around the nucleus (Supplementary Fig. 3). Both PS1 and PS2 colocalized with RyR2 and RyR3 (Supplementary Fig. 4). Over-expression of the PS-NTF proteins was verified by ICC of SH-SY5Y cotransfected with PS-NTF and GFP plasmids (Supplementary Fig. 5). While these endogenous expression levels of PS1 and PS2 in SH-SY5Y cells are generally low, it has been shown that retinoic acid-induced differentiation of SH-SY5Y cells significantly increases PS1 expression, but does not affect levels of PS2 (Flood et al., 2004). Such qualitative Western blots also suggest that endogenous PS levels in undifferentiated SH-SY5Y cells are lower than in neuronal tissue, such as murine brain. Therefore, transfection of PS constructs into undifferentiated SH-SY5Y cells more closely resembles the physiological condition in adult differentiated neuronal tissue, especially in cells undergoing pathological changes such as neurodegeneration, in which both PS1 and PS2 are substantially upregulated (Borghi et al., 2010).
Confirmation of the previously reported binding of recombinant PS-NTF constructs to RyR (Hayrapetyan, et al., 2008, Rybalchenko, et al., 2008) was successfully replicated by Western blotting (Supplementary Fig. 6B) and co-immunoprecipitation (Supplementary Fig. 6B,C).
Differential regulation of RyRs by the N-terminal domains of PS1 and PS2
Changes in intracellular calcium signaling patterns were measured with optical imaging and the Ca2+-specific fluorochrome Fura-2 in live SH-SY5Y cells 24 hours after transfection with PS-NTF constructs. Ca2+ release from the ER was pharmacologically stimulated with the RyR agonist caffeine and parameters of the resulting Ca2+ response were determined. The measured parameters were amplitude (Fmax /F0) (Supplementary Data Table 1), area under the curve (AUC; an integrated correlative measure of the amount of Ca2+ released during the transient) (Supplementary Data Table 2), and time course of the calcium response (Supplementary Data Table 3). RyR-mediated intracellular Ca2+ transients elicited in control transfected cells did not differ from untransfected controls, indicating the use of red fluorescent protein tdTomato (Shaner, et al., 2004) as a transfection marker to identify the cotransfected expression of PS constructs did not interfere with baseline calcium signaling and its pharmacological modulation (Supplementary Fig. 7). Specifically, amplitude (Supplementary Fig. 7A) and AUC (Supplementary Fig. 7B), as a measure of total Ca2+ release, were unchanged after control transfection. The very similar patterns of Ca2+ release are shown in the representative traces (Supplementary Fig. 7C).
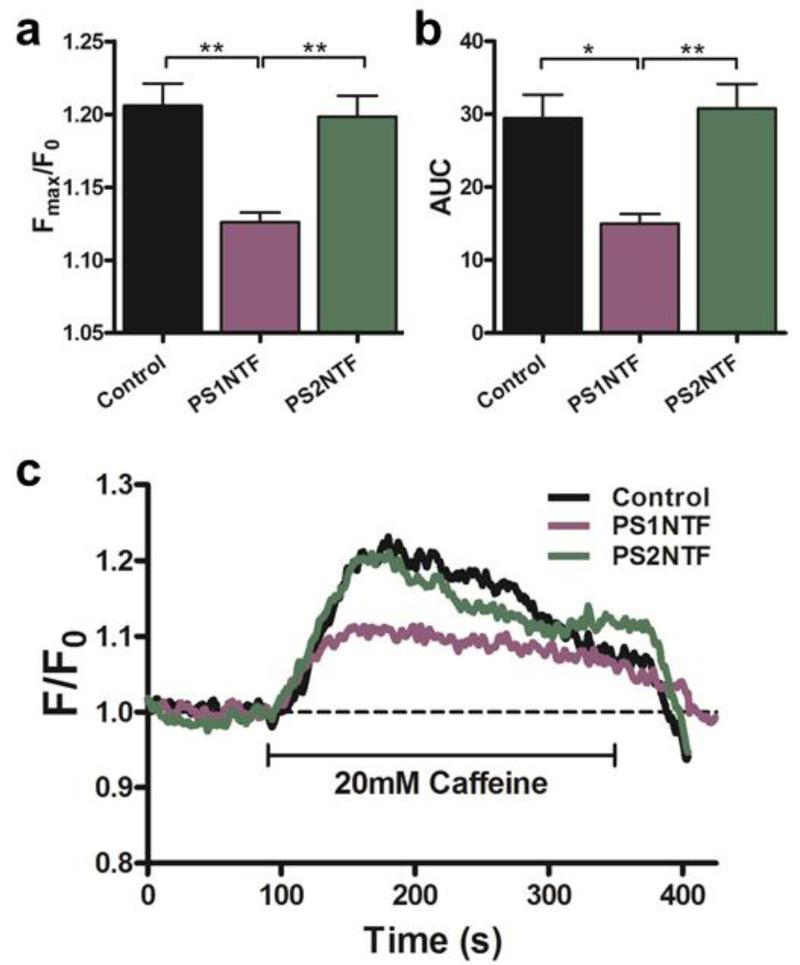
Expression of the N-terminal domain of PS1 (PS1NTF) in the cytosol of SH-SY5Y attenuated the amplitude of RyR-mediated Ca2+ transients by 38.8% ± 3.4 % (Fig. 2A; Supplementary Data Table 1). However, cytosolic expression of the homologous region of PS2 had no effect on the amplitude of the Ca2+ transients (Fig. 2A). Similarly, overall Ca2+ release from intracellular stores measured as AUC was unchanged after expression of PS2NTF but significantly decreased in cells overexpressing PS1NTF (49.01 ± 4.42 %; Fig. 2B; Supplementary Data Table 2). Representative traces show that Ca2+ transients remain unchanged in cells expressing PS2NTF while expression of PS1NTF significantly reduced the release of Ca2+ from intracellular stores when compared to untransfected controls (Fig. 2C).
Figure 2. Pharmacologically elicited intracellular calcium release from RyR is significantly attenuated by PS1NTF but not PS2NTF.
A) Cytoplasmic expression of PS1NTF reduced the maximum amplitude (Fmax/F0) of the RyR mediated calcium transient after pharmacological stimulation of SH-SY5Y cells with 20mM caffeine (n=8), however, cytoplasmic expression of PS2NTF (n=11) has no effect when compared to untransfected control (n=8). B) AUC of calcium transients measured after pharmacological stimulation as a measure of total intracellular calcium release was significantly reduced in cells expressing PS1NTF but remained unaltered in PS2NTF expressing cells (n= 8 and 11, respectively). Data is shown as mean ± SEM with significance determined by one-way ANOVA and Bonferroni's post-hoc test. [* P <0.05; ** P <0.01]. C) Representative traces of calcium transients after pharmacological stimulation of SH-SY5Y cells expressing cytoplasmic PS1NTF or PS2NTF.
Specificity of the caffeine sensitive Ca2+ release by RyR was confirmed by inhibition with the RyR specific antagonist dantrolene (Koulen and Thrower, 2001). Pretreatment with 20 μM dantrolene resulted in significant reduction of caffeine sensitive Ca2+ release (Supplementary Fig. 8).
Domain structure and related redox sensitivity determine how the N-terminal domain of PS controls RyR activity
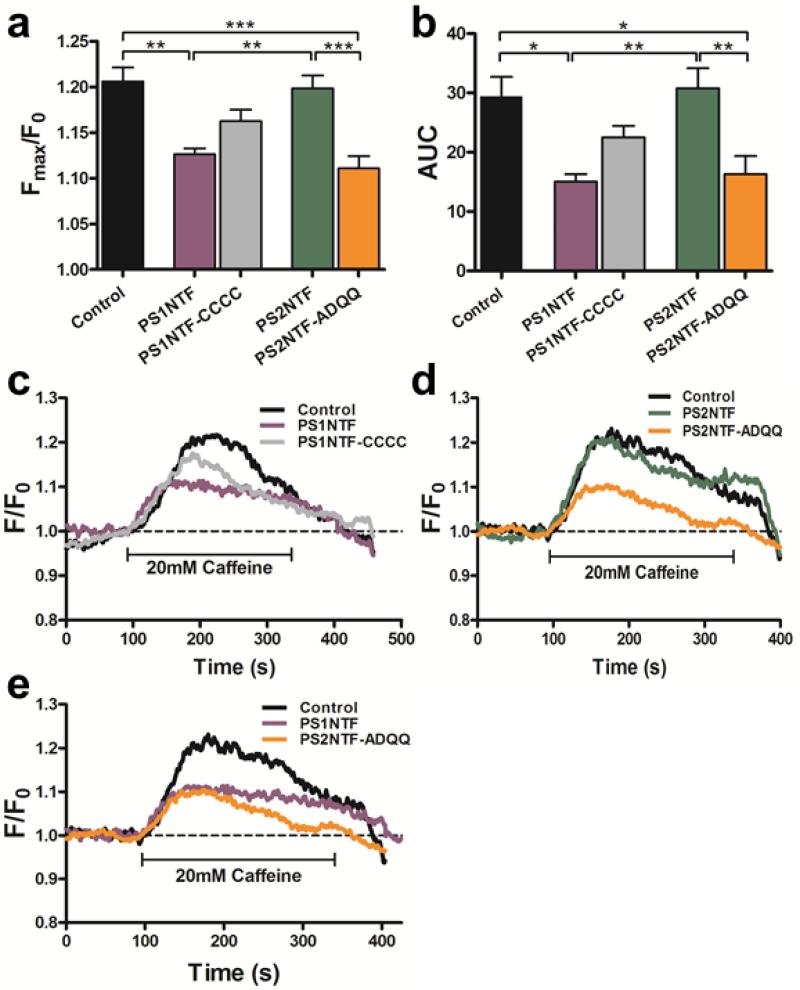
Mutation of the four cysteine residues of the N-terminal cytosolic domain of PS2 to the redox insensitive construct PS2NTF-ADQQ (C14A, C31D, C56Q, C65Q) exchanged the amino acids of the equivalent domain from PS1NTF. PS2NTF-ADQQ's activity was similar to that observed for PS1NTF and significantly decreased from PS2NTF and controls with respect to amplitude (46.1 ± 6.8 % vs. control; Fig. 3A; Supplementary Data Table 1) and total Ca2+ release (44.7 ± 10.4 % vs. control AUC; Fig. 3B; Supplementary Data Table 2). The reverse experiment, mutation of the same residues of the N-terminal cytosolic domain of PS1 (A14C, D31C, Q56C, Q65C) to the cysteine residues of PS2NTF, made the resulting PS1NTF-CCCC construct more PS2NTF-like in its effects on RyR Ca2+ release. This was determined by the absence of a statistically significant difference of PS1NTF-CCCC regulated Ca2+ transients from either PS1NTF or PS2NTF regulated Ca2+ transients for both the amplitude (21.4 ± 6.3 % decrease vs. control; Fig. 3A; Supplementary Data Table 1) and AUC (23.6 ± 6.6 % decrease vs. control; Fig. 3B; Supplementary Data Table 2). The PS1NTF-CCCC was unique as an intermediate value distinct from all other constructs that caused either a PS1NTF regulatory effect on RyR or, like PS2NTF, were indistinguishable from the untransfected control. This intermediate effect of PS1NTF-CCCC may have biological significance since the PS1NTF-CCCC mutant is statistically indistinguishable from two decidedly different treatment groups (PS1NTF vs. PS2NTF or control). Representative traces exemplifying the activity of PS constructs with altered cysteine residues are shown in Figure 3C-E. The cysteine mutations are sufficient to convert the PS2NTF mediated control of Ca2+ transients into PS1NTF-like activity and vice versa. Figure 3E distinguishes PS1NTF and PS2NTF-ADQQ effects from those mediated by control. Supplementary Data Tables 1 and 2 list parameters that characterize Ca2+ transients’ amplitude and total Ca2+ release, respectively, while time course parameters are listed in Supplementary Data Table 3.
Figure 3. Mutation of PS2NTF cysteines to the complementary PS1NTF residues exchanges ability to regulate RyR calcium release.
A) The amplitude of RyR-mediated, pharmacologically elicited intracellular calcium release in SH-SY5Y cells was measured as the maximum emission fluorescence value over baseline (Fmax/F0). Cells expressing PS2NTF-ADQQ (amino acids 1-87 of PS2 with the mutations C14A, C31D, C56Q, C65Q; n=8) showed similar attenuation of maximal calcium release (Fmax/F0) as cells expressing PS1NTF (n=8). Mutation of PS1NTF to PS1NTF-CCCC (amino acids 1-82 of PS1 with the mutations A14C, D31C, Q56C, Q65C; n=8) attenuated the PS1NTF effect on RyR calcium release but did not fully restore Fmax/F0 values measured in PS2NTF expressing (n=11) or control cells (n=8) resulting in a distinct intermediate response that was not statistically different from either the PS1NTF or PS2NTF expressing cells. B) AUC of calcium transients measured after pharmacological stimulation of SH-SY5Y cells expressing the recombinant PS-NTF domains mirror the results seen for the Fmax/F0 values. Data is shown as mean ± SEM; asterisks indicate significance by one-way ANOVA with Bonferroni's post-hoc test [* P <0.05; ** P <0.01; *** P <0.001]. Representative traces of calcium transients produced by pharmacological stimulation of SH-SY5Y cells expressing PS1NTF or PS1NTF-CCCC (C) and PS2NTF or PS2NTF-ADQQ (D). Representative traces show the similar lowering effect of PS1NTF and the mutant PS2NTF-ADQQ on RyR calcium release (E).
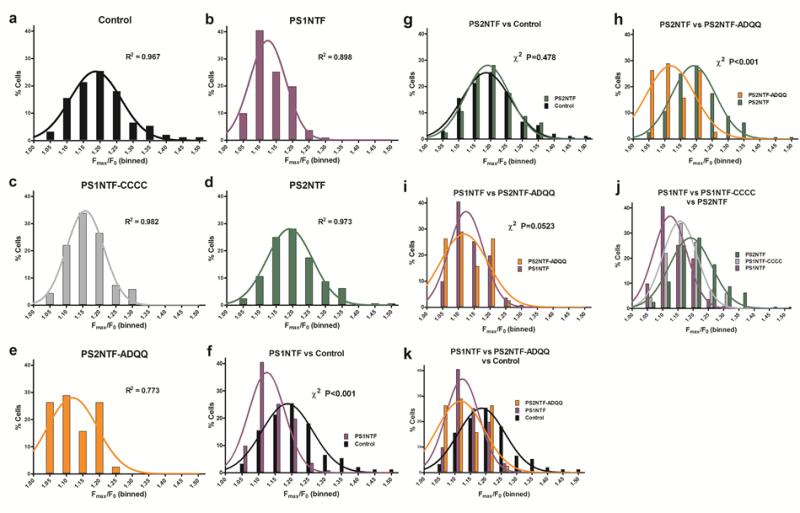
PS fragment transfection shifted the distribution of amplitudes of intracellular calcium release transients in SH-SY5Y cells leftward (Fig. 4). Amplitude data were binned at 5% width with non-linear regression and goodness of fit measures applied to determine the Gaussian distribution (Fig. 4A-E). R2 values indicated normality for all groups (Fig. 4A-E). The Pearson's chi square (χ2) test, a stringent test for direct comparisons, was used to determine the similarity of the amplitude distributions for all experimental groups (Fig. 4F-K, Supplementary Data Table 4). The distribution of amplitudes of RyR-mediated Ca2+ release by PS1NTF (Fig. 4 F-K) was distinctly different compared to control but the distribution of PS2NTF transfected cells (Fig. 4G) did not differ from control. Mutation of PS1NTF to PS1NTF-CCCC ablated the amplitude reducing effect of PS1NTF (Fig. 4J), while mutation of PS2NTF to PS2NTF-ADQQ rendered it functionally PS1NTF-like (Fig. 4H,I,K).
Figure 4. Amplitudes of the RyR mediated calcium transients binned at 5% intervals are Gaussian normally distributed. PS2NTF-ADQQ and PS1NTF shift the distribution curve left, indicating attenuation of RyR-mediated intracellular calcium release.
Fmax/F0 values per cell were binned at 5% width (abscissae) as a percentage of the total number of Fmax/F0 values (ordinates) collected for each experimental condition. Columns indicate the percentage of Fmax/F0 values that fell between the bin indicated on the abscissa (α) but below the next higher value on the abscissa such that α < × < (α+0.05). Lines are the non-linear regression to determine the Gaussian distribution (1,000 iterations) with the corresponding R2 value indicated. All treatment groups were found to be normally distributed. Data and analyses for control SH-SY5Y cells (A), PS1NTF (B), PS1NTF-CCCC (C), PS2NTF (D), and PS2NTF-ADQQ (E). Binned histograms were compared by the stringent Pearson's chi-squared (χ2) test and key comparisons are shown (F-K). Both PS1NTF (F, I, J) and PS2NTF-ADQQ (H, I, K) significantly shift the Fmax/F0 distribution leftward, indicating attenuation of RyR calcium release vs. control. PS2NTF (G) was not different from controls while PS1NTF-CCCC (J) resulted in an intermediate effect. Pearson's χ2 test results not indicated on the graphs above: J) PS1NTF / PS2NTF, χ2 P<0.001; PS1NTF / PS1NTF-CCCC, χ2 P<0.05; PS2NTF / PS1NTF-CCCC, χ2 P<0.05. K) Control / PS1NTF, χ2 P<0.001; Control / PS2NTF-ADQQ, χ2 P<0.001; PS1NTF / PS2NTF-ADQQ, χ2 n.s. Pearson's χ2 tests for all treatment groups are given in Supplementary Data Table 4.
DISCUSSION
Control of RyR-mediated calcium mobilization from intracellular stores is differentially regulated by presenilin 1 and 2 at the molecular level to maintain cellular calcium homeostasis
The N-terminal cytosolic domain of PS1 controls intracellular calcium release in live cells independently of PS's transmembrane domains through direct interaction with the RyR. This finding provides direct functional and additional mechanistic support for the hypothesis that N-terminal cytosolic domains of presenilins bind to and regulate RyR ion channel activity (Hayrapetyan, et al., 2008, Rybalchenko, et al., 2008). Specifically, single channel electrophysiology experiments showed that PS1NTF increased RyR mean current and channel open probability over the physiological RyR activity range (10 nM – 1 μM free Ca2+) but not at RyR deactivating, high calcium concentrations (> 10 μM free Ca2+) (Fig. 5B) (Rybalchenko, et al., 2008). Consistent with the biophysical evidence obtained for PS2NTF (Hayrapetyan, et al., 2008), our live cell imaging experiments did not show an effect of PS2NTF on RyR at physiological Ca2+ concentrations. Single channel electrophysiology indicated that PS2NTF affected RyR activity at calcium concentrations greater than physiologically normal or healthy cytosolic calcium concentrations (Hayrapetyan, et al., 2008).
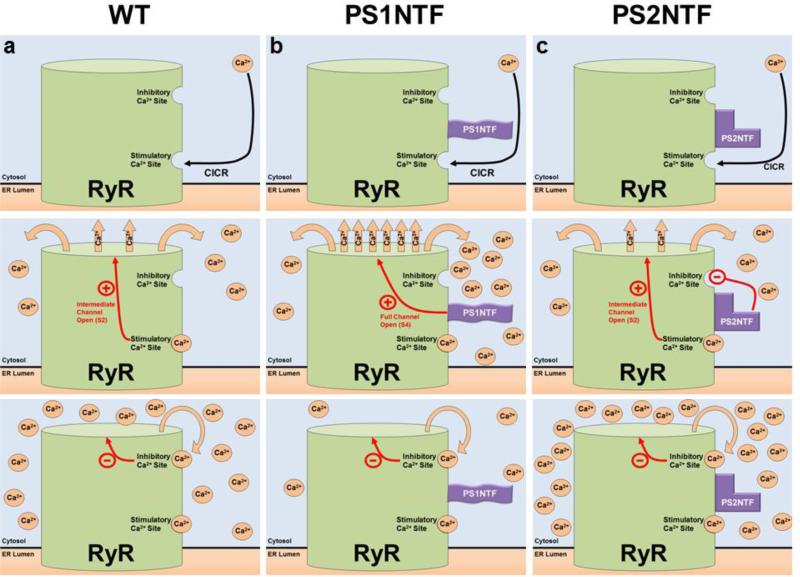
Figure 5. Proposed mechanism of RyR calcium release regulated by the N-termini of PS1 and PS2.
PS1NTF and PS2NTF have distinct effects on RyR calcium induced calcium release (CICR) from ER stores. A) The mechanism of RyR regulation by Ca2+ binding at the high affinity stimulatory site results in moderate channel opening. Ca2+ is released until the local Ca2+ concentration rises to the point at which the low affinity, inhibitory Ca2+ binding site is occupied resulting in closure of the channel. B) The N-terminus of PS1 (PS1NTF) binds RyR and increases calcium release from the ER. Occupation of the RyR high affinity stimulatory site by Ca2+ causes channel gating and Ca2+ release. Bound PS1NTF increases channel opening, causing rapid RyR Ca2+ release until the low affinity inhibitory Ca2+ site is bound, closing the channel and terminating ER Ca2+ release. The increased rate of Ca2+ release due to PS1NTF binding results in overall reduced Ca2+ release because inhibitory concentrations are reached in less time. C) The N-terminus of PS2 (PS2NTF) binds to RyR. Ca2+ ion binding at the high affinity stimulatory site of RyR causes channel gating and Ca2+ release. PS2NTF has no effect on channel gating but blocks Ca2+ inhibition of the RyR channel at high cytosolic calcium concentrations. Significantly elevated cytosolic Ca2+ concentrations result in binding of Ca2+ at the low affinity inhibitory site eventually closing the channel and ending calcium release, however, resulting in an overall higher cytosolic Ca2+ concentration.
The live cell imaging and previously published single channel electrophysiology data combine to describe a novel mechanism of calcium regulation in neurons in general with specific relevance to AD. The interaction between PS1NTF and RyR is especially critical as PS1 is well established as the more pathogenic of the two PS genes (LaFerla, 2002). Figure 5 schematically describes the proposed functional interactions of RyR with PS1NTF or PS2NTF (Fig. 5).
As such, the modulation of calcium signaling resulting from the interaction of PS2NTF with the RyR is not likely to be observed in a live cell at normal or even elevated cytosolic calcium concentrations, suggesting a more predominant role of PS1NTF under physiological conditions, while the PS2NTF/RyR interaction is likely to critically contribute to Ca2+ homeostasis during pathological states.
Advances in bioinformatics have yielded sub-nanometer resolution for the cytoplasmic region of the skeletal RyR1 (Flood et al., 2004) and pseudo-atomic structures of the murine RyR3 (Zhu et al., 2013). However, the sizes of the RyRs pose a significant obstacle in performing mutagenesis experiments required to experimentally validate the exact location of the binding site for presenilins and a complete bioinformatics study modeling the interaction based on these datasets (Flood et al., 2004; Zhu et al., 2013) is beyond the scope of the present study. Therefore, our mechanistic explanation of the model for the PS-NTF/RyR interaction has to rely on published electrophysiological single-channel data (Hayrapetyan, et al., 2008, Rybalchenko, et al., 2008) as well as the functional results presented in the present study.
The organization and related redox sensitivity of the N-terminal domain structure of PS controls RyR-mediated intracellular calcium release
In order to understand underlying structural determinants and cellular mechanisms, our experiments targeting the cysteine residues of PS2NTF (Fig. 1) and mutating them to the complementary residues of PS1NTF successfully exchanged the PS regulatory effect on RyR. Specifically, the PS2NTF-ADQQ mutant lacking all of PS2's N-terminal cysteines controlled RyR activity in a PS1NTF-like fashion (Figs. 3, 4H,K). Mutation of PS1NTF with the analogous cysteines from PS2NTF, viz. the mutant PS1NTF-CCCC, resulted in a loss of function with respect to the PS1NTF-like regulation of RyR mediated Ca2+ release and made the fragment more PS2NTF-like (Figs. 3, 4J). Biologically, the mutant PS1NTF-CCCC exhibited the PS2NTF effect on intracellular calcium release, showing no statistical difference to control or PS2NTF groups. On the other hand, removal of the PS2NTF redox sensitive cysteines and disulfide bridges with the mutant PS2NTF-ADQQ caused a distinct effect on RyR signaling that was statistically indistinguishable from the PS1NTF-mediated effect on RyR calcium release for all measured parameters (Figs. 3, 4K). PS1NTF and PS2NTF-ADQQ were identical in their ability to significantly attenuate total intracellular Ca2+ release (Figs. 3, 4K). The change from the redox sensitive PS2NTF to the redox insensitive PS2NTF-ADQQ represented a gain of function mutation that increased burst firing of RyR and attenuated total intracellular Ca2+ release similar to PS1NTF. Interestingly, the mutation of PS1NTF to PS1NTF-CCCC was not a complete conversion to fully match PS2NTF regulated RyR calcium release. This is likely due to sequence differences between the PS1NTF and PS2NTF (Fig. 1) outside of the mutated cysteines preventing full conversion of the PS1NTF-CCCC interaction with RyR to a PS2NTF-RyR mode of calcium regulation. This obvious trend of PS1NTF-CCCC towards a PS2NTF-like action on RyR becomes evident in that the parameters measured for PS1NTF-CCCC are not significantly different from either PS1NTF or PS2NTF regulation of RyR. This may indicate both a biological relevance and an intermediate effect of PS1NTF-CCCC.
Binning the amplitude values for the measured calcium transients further illustrated the distinct physiological effect of PS1NTF on RyR channel activity (Fig. 4). PS1NTF expression shifted the distribution of calcium transient amplitudes significantly to the left compared to control (Fig. 4F). Conversely, amplitudes for the PS2NTF and control conditions were highly similar (Fig. 4G) indicating that there is no interaction of PS2NTF with RyR at physiological calcium concentrations. Comparison of PS1NTF-CCCC with the other experimental groups supported our finding that the PS1NTF-CCCC group is biologically similar to the PS2NTF group (Fig. 4J). A compelling finding is the distributions of PS1NTF, PS1NTF-CCCC, and PS2NTF with a consistent leftward shift in the direction of the PS1NTF modality (Figure 4J). The leftward shift of the binned histograms for PS2NTF-ADQQ (Fig. 4H) indicated that the redox insensitive mutations to PS2NTF rendered the mutant PS2NTF-ADQQ significantly different from PS2NTF. Direct comparison of amplitudes between PS1NTF and PS2NTF-ADQQ did not yield statistical significance, indicating that the redox insensitive mutation of PS2NTF rendered the protein PS1NTF-like.
These results indicate that the effects of the PS1NTF-RyR interaction can be replicated by mutating PS2NTF to the redox insensitive PS2NTF-ADQQ. These results are suggestive of a redox sensitive mechanism of PS regulation of RyR calcium release.
Our findings further suggest that the differences in the binding of the cytosolic N-terminal domains of PS to RyR are functionally linked to the redox state of the cell. A reducing state, as typically observed for the cytoplasm under physiological conditions, would balance PS-RyR interactions towards the PS1 mode of regulation, while oxidizing conditions in the cytoplasm as encountered during cellular oxidative stress would foster the formation of protein disulfide bonds (Cumming, et al., 2004) thus promoting the PS2 mode of regulation of RyR activity. Combined with a dysregulation of intracellular calcium homeostasis, oxidative environments are often seen during neuronal degeneration (Burroughs, et al., 2012, Mattson, 2004) and could include binding of PS2NTF, which is inactive in a physiological milieu, or inactivation of PS1NTF due to altered folding and domain structures as exemplified by our PS1NTF-CCCC mutation. Both pathways are potentially synergistic in their negative effects on intracellular calcium homeostasis and provide a novel paradigm for disease involvement of PS and represent a potential novel target for therapeutic intervention.
Our data provide critical support for the hypothesis that the soluble N-termini of presenilins interact with RyRs to differentially and isotype-specifically regulate calcium release from the ER, indicating an additional avenue of disease involvement of both presenilins and RyR in AD and other forms of neuronal degeneration. Our data corroborates previous preliminary evidence (Hayrapetyan, et al., 2008, Rybalchenko, et al., 2008) to propose a novel mechanism in which PS1NTF and likely also PS2NTF-ADQQ interact with RyRs to facilitate channel gating to higher conductance states (Fig. 5B). Instead of a rapid flicker between open and closed (S2) RyR channels are gated to full conductance (S4) when facilitated by the binding of the N-terminus of PS1. The increased current caused by higher conductance states raised the cytosolic calcium concentration in the proximal volume of the RyR to an auto-inhibitory level causing channel closure. When the whole cell calcium concentration is monitored, this resulted in a net decrease in total calcium release from intracellular stores (Fig. 5B).
PS1 appears to cause more AD pathologies than PS2 whether assessed by knockout models or number of FAD mutations (Elder, et al., 2010, Schaeffer, et al., 2011). Mouse models show little to no symptoms when PS2 is deleted (Donoviel, et al., 1999) but knockouts of PS1 are not viable past the early stages of development (Shen, et al., 1997). This indicates that the PS1-RyR interaction is of greater physiological importance than the PS2-RyR interaction and that PS2 cannot fully compensate for PS1 effects. This last point is especially meaningful in the case of FAD where about 80% of clinical mutations to PS are localized to PS1 (Elder, et al., 2010). To date 185 clinical mutations have been found on the PS1 gene while only 13 have been found on PS2 (http://www.molgen.vib-ua.be/ADMutations/; accessed 3/6/2013). Such a large number of mutations to PS1 suggest that FAD mutations represent a loss of function of PS1, with gain of function being improbable due to different clinical FAD mutations resulting in similar phenotypes. Our data suggest a novel mechanism for PS1 as an endogenous regulator of the cytosolic Ca2+ concentration. Loss of PS1 regulation of RyR calcium release cannot be compensated by a PS2 effect, thus such a loss of PS1 regulation could lead to elevated cytosolic Ca2+ and AD pathology. The opposite is true as well, mutations to PS2 are less pathologic because PS1 would still properly regulate RyR calcium release.
The different calcium concentrations required for PS1NTF and PS2NTF to bind RyR, namely the non-physiological, higher calcium concentration required for PS2NTF to bind RyR are potentially indicative of a feed-forward mechanism of calcium dyshomeostasis (Fig. 5). This is supported by our findings that indicate that the redox insensitive PS2NTF-ADQQ binds RyR with a PS1NTF-like effect and at a physiologically similar calcium concentration (Figs. 3, 4). As normal aging occurs, or the AD pathology begins to emerge, the cytosolic calcium concentration of an otherwise healthy neuron increases in step with disease progression. Oxidation of the many reactive cysteines of the RyR increases channel opening and calcium flux into the cytosol (Xu, et al., 1998). The failure of PS1 to regulate oxidized RyR increases along with the cytosolic calcium concentration. As the cytosolic calcium increases, the binding of PS2 to RyR may become relevant but, as knockout models (Elder, et al., 2010, Schaeffer, et al., 2011), electrophysiology experiments (Hayrapetyan, et al., 2008, Rybalchenko, et al., 2008), and the data above have shown, PS2 cannot replace PS1 function. To the contrary, electrophysiological experiments have shown that PS2NTF increases the dynamic range of RyR-mediated calcium release into the outright toxic levels by preventing calcium auto-inhibition of the channel (Hayrapetyan, et al., 2008). Loss of PS1 regulation of RyR at high cytosolic calcium concentrations would make the PS2-RyR interaction functionally relevant amongst the other AD symptoms contributing to the calcium hypothesis of AD (LaFerla, 2002, Mattson, 2010). The high cytosolic calcium concentration is permissive of PS2NTF-RyR binding, therefore calcium release from the ER is further potentiated. This mechanism can feed forward to cause irreversible cellular damage or neurodegeneration (LaFerla, 2002, Mattson, 2010). In summary, based on the novel mechanism described herein, it is tentative to speculate that PS1 mutations (independent of their location), will result in the potentiation of RyR-mediated intracellular calcium release. This increase would then contribute to an overall calcium toxicity resulting from oxidative stress-mediated mobilization of intracellular calcium from RyRs, with a significant role of the PS2NTF/RyR interaction augmenting this effect as a result of the overall increased calcium concentration.
Conclusion
The mechanism proposed here represents a novel pharmacological target for the treatment of AD and other neurodegenerative processes. The next step in translating these findings to the clinics is identification of a pharmacological agent that can replicate, facilitate, or antagonize the PS-RyR interaction. This could include the design of peptide mimetics of PS1NTF that would be able to bind RyR, though intracellular delivery of peptides in patients is no simple task. Alternatively, a high throughput strategy could be adopted to screen compound libraries and to identify small molecule targets that modify the binding of PS1 to RyR. Further study is required before translation to the clinic can begin in earnest but the data above describe a novel PS-RyR interaction in neuronal cells capable of leading to AD pathology. PS1 and PS2 regulation of RyR is a novel mechanism of cytosolic calcium control that further supports and advances the calcium hypothesis of AD.
Supplementary Material
HIGHLIGHTS.
Novel mechanism: presenilin N-termini control intracellular calcium release.
Isotype-specific effect due to 4 cysteines absent in presenilin 1 and present in 2.
Mutagenesis converted function of presenilin 1 to 2 and vice versa.
Novel target for Alzheimer's disease drug development and treatment of neurodegeneration.
ACKNOWLEDGEMENTS
Research reported in this publication was supported in part by grants from the National Eye Institute (EY014227 and EY022774), the Institute on Aging (AG010485, AG022550 and AG027956), the National Center for Research Resources and National Institute of General Medical Sciences (RR022570 and RR027093) of the National Institutes of Health (PK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support by a Fight for Sight Post-Doctoral Award (SLG), the Felix and Carmen Sabates Missouri Endowed Chair in Vision Research, the Vision Research Foundation of Kansas City, and a Challenge Grant from Research to Prevent Blindness (PK) is gratefully acknowledged. The authors thank Dr. Roger Y. Tsien, University of California San Diego, for the generous gift of the red fluorescent protein tdTomato construct (41).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Alzheimer's Association 2012 Alzheimer's disease facts and figures. Alzheimers Dement. 2012;8:131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Alzheimer's Disease Collaborative Group The structure of the presenilin 1 (S182) gene and identification of six novel mutations in early onset AD families. Alzheimer's Disease Collaborative Group. Nat Genet. 1995;11:219–222. doi: 10.1038/ng1095-219. [DOI] [PubMed] [Google Scholar]
- 3.Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS. Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 4.Burroughs SL, Duncan RS, Rayudu P, Kandula P, Payne AJ, Clark JL, Koulen P, Kaja S. Plate reader-based assays for measuring cell viability, neuroprotection and calcium in primary neuronal cultures. J Neurosci Methods. 2012;203:141–145. doi: 10.1016/j.jneumeth.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borghi R, Piccini A, Barini E, Cirmena G, Guglielmotto M, Tamagno E, Fornaro M, Perry G, Smith MA, Garuti A, Tabaton M. Upregulation of presenilin 1 in brains of sporadic, late-onset Alzheimer's disease. J Alzheimers Dis. 2010;22(3):771–775. doi: 10.3233/JAD-2010-100729. [DOI] [PubMed] [Google Scholar]
- 6.Cedazo-Minguez A, Popescu BO, Ankarcrona M, Nishimura T, Cowburn RF. The presenilin 1 deltaE9 mutation gives enhanced basal phospholipase C activity and a resultant increase in intracellular calcium concentrations. J Biol Chem. 2002;277:36646–36655. doi: 10.1074/jbc.M112117200. [DOI] [PubMed] [Google Scholar]
- 7.Chan SL, Mayne M, Holden CP, Geiger JD, Mattson MP. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J Biol Chem. 2000;275:18195–18200. doi: 10.1074/jbc.M000040200. [DOI] [PubMed] [Google Scholar]
- 8.Cheung KH, Mei L, Mak DO, Hayashi I, Iwatsubo T, Kang DE, Foskett JK. Gain-of-function enhancement of IP3 receptor modal gating by familial Alzheimer's disease-linked presenilin mutants in human cells and mouse neurons. Sci Signal. 2010;3:ra22. doi: 10.1126/scisignal.2000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung KH, Shineman D, Muller M, Cardenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VM, Foskett JK. Mechanism of Ca2+ disruption in Alzheimer's disease by presenilin regulation of InsP3 receptor channel gating. Neuron. 2008;58:871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowburn RF, Popescu BO, Ankarcrona M, Dehvari N, Cedazo-Minguez A. Presenilin-mediated signal transduction. Physiol Behav. 2007;92:93–97. doi: 10.1016/j.physbeh.2007.05.053. [DOI] [PubMed] [Google Scholar]
- 11.Cumming RC, Andon NL, Haynes PA, Park M, Fischer WH, Schubert D. Protein disulfide bond formation in the cytoplasm during oxidative stress. J Biol Chem. 2004;279:21749–21758. doi: 10.1074/jbc.M312267200. [DOI] [PubMed] [Google Scholar]
- 12.Demuro A, Parker I, Stutzmann GE. Calcium signaling and amyloid toxicity in Alzheimer disease. J Biol Chem. 2010;285:12463–12468. doi: 10.1074/jbc.R109.080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donoviel DB, Hadjantonakis AK, Ikeda M, Zheng H, Hyslop PS, Bernstein A. Mice lacking both presenilin genes exhibit early embryonic patterning defects. Genes Dev. 1999;13:2801–2810. doi: 10.1101/gad.13.21.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- 15.Elder GA, Gama Sosa MA, De Gasperi R, Dickstein DL, Hof PR. Presenilin transgenic mice as models of Alzheimer's disease. Brain Struct Funct. 2010;214:127–143. doi: 10.1007/s00429-009-0227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flood F, et al. Presenilin expression during induced differentiation of the human neuroblastoma SH-SY5Y cell line. Neurochem Int. 2004;44(7):487–96. doi: 10.1016/j.neuint.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Goussakov I, Miller MB, Stutzmann GE. NMDA-mediated Ca(2+) influx drives aberrant ryanodine receptor activation in dendrites of young Alzheimer's disease mice. J Neurosci. 2010;30:12128–12137. doi: 10.1523/JNEUROSCI.2474-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo Q, Fu W, Sopher BL, Miller MW, Ware CB, Martin GM, Mattson MP. Increased vulnerability of hippocampal neurons to excitotoxic necrosis in presenilin-1 mutant knock-in mice. Nat Med. 1999;5:101–106. doi: 10.1038/4789. [DOI] [PubMed] [Google Scholar]
- 19.Hayrapetyan V, Rybalchenko V, Rybalchenko N, Koulen P. The N-terminus of presenilin-2 increases single channel activity of brain ryanodine receptors through direct protein-protein interaction. Cell Calcium. 2008;44:507–518. doi: 10.1016/j.ceca.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Hwang SY, Wei J, Westhoff JH, Duncan RS, Ozawa F, Volpe P, Inokuchi K, Koulen P. Differential functional interaction of two Vesl/Homer protein isoforms with ryanodine receptor type 1: a novel mechanism for control of intracellular calcium signaling. Cell Calcium. 2003;34:177–184. doi: 10.1016/s0143-4160(03)00082-4. [DOI] [PubMed] [Google Scholar]
- 21.Kasri NN, Kocks SL, Verbert L, Hebert SS, Callewaert G, Parys JB, Missiaen L, De Smedt H. Up-regulation of inositol 1,4,5-trisphosphate receptor type 1 is responsible for a decreased endoplasmic-reticulum Ca2+ content in presenilin double knock-out cells. Cell Calcium. 2006;40:41–51. doi: 10.1016/j.ceca.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Koulen P, Thrower EC. Pharmacological modulation of intracellular Ca(2+) channels at the single-channel level. Mol Neurobiol. 2001;24:65–86. doi: 10.1385/MN:24:1-3:065. [DOI] [PubMed] [Google Scholar]
- 23.LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer's disease. Nat Rev Neurosci. 2002;3:862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- 24.Lee SY, Hwang DY, Kim YK, Lee JW, Shin IC, Oh KW, Lee MK, Lim JS, Yoon DY, Hwang SJ, Hong JT. PS2 mutation increases neuronal cell vulnerability to neurotoxicants through activation of caspase-3 by enhancing of ryanodine receptor-mediated calcium release. FASEB J. 2006;20:151–153. doi: 10.1096/fj.05-4017fje;1. [DOI] [PubMed] [Google Scholar]
- 25.Leissring MA, Parker I, LaFerla FM. Presenilin-2 mutations modulate amplitude and kinetics of inositol 1, 4,5-trisphosphate-mediated calcium signals. J Biol Chem. 1999;274:32535–32538. doi: 10.1074/jbc.274.46.32535. [DOI] [PubMed] [Google Scholar]
- 26.Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu CE, Jondro PD, Schmidt SD, Wang K, et al. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science. 1995;269:973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 27.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattson MP. ER calcium and Alzheimer's disease: in a state of flux. Sci Signal. 2010;3:e10. doi: 10.1126/scisignal.3114pe10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyers MB, Pickel VM, Sheu SS, Sharma VK, Scotto KW, Fishman GI. Association of sorcin with the cardiac ryanodine receptor. J Biol Chem. 1995;270:26411–26418. doi: 10.1074/jbc.270.44.26411. [DOI] [PubMed] [Google Scholar]
- 30.Rogaev EI, Sherrington R, Rogaeva EA, Levesque G, Ikeda M, Liang Y, Chi H, Lin C, Holman K, Tsuda T, et al. Familial Alzheimer's disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer's disease type 3 gene. Nature. 1995;376:775–778. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- 31.Rybalchenko V, Hwang SY, Rybalchenko N, Koulen P. The cytosolic N-terminus of presenilin-1 potentiates mouse ryanodine receptor single channel activity. Int J Biochem Cell Biol. 2008;40:84–97. doi: 10.1016/j.biocel.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 32.Sato N, Imaizumi K, Manabe T, Taniguchi M, Hitomi J, Katayama T, Yoneda T, Morihara T, Yasuda Y, Takagi T, Kudo T, Tsuda T, Itoyama Y, Makifuchi T, Fraser PE, St George-Hyslop P, Tohyama M. Increased production of beta-amyloid and vulnerability to endoplasmic reticulum stress by an aberrant spliced form of presenilin 2. J Biol Chem. 2001;276:2108–2114. doi: 10.1074/jbc.M006886200. [DOI] [PubMed] [Google Scholar]
- 33.Schaeffer EL, Figueiro M, Gattaz WF. Insights into Alzheimer disease pathogenesis from studies in transgenic animal models. Clinics. 2011;66(Suppl 1):45–54. doi: 10.1590/S1807-59322011001300006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schellenberg GD, Montine TJ. The genetics and neuropathology of Alzheimer's disease. Acta Neuropathol. 2012;124:305–323. doi: 10.1007/s00401-012-0996-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 36.Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 37.Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 38.Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, Tsuda T, Mar L, Foncin JF, Bruni AC, Montesi MP, Sorbi S, Rainero I, Pinessi L, Nee L, Chumakov I, Pollen D, Brookes A, Sanseau P, Polinsky RJ, Wasco W, Da Silva HA, Haines JL, Perkicak-Vance MA, Tanzi RE, Roses AD, Fraser PE, Rommens JM, St George-Hyslop PH. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 39.Shilling D, Mak DO, Kang DE, Foskett JK. Lack of evidence for presenilins as endoplasmic reticulum Ca2+ leak channels. J Biol Chem. 2012;287:10933–10944. doi: 10.1074/jbc.M111.300491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith IF, Hitt B, Green KN, Oddo S, LaFerla FM. Enhanced caffeine-induced Ca2+ release in the 3xTg-AD mouse model of Alzheimer's disease. Journal of neurochemistry. 2005;94:1711–1718. doi: 10.1111/j.1471-4159.2005.03332.x. [DOI] [PubMed] [Google Scholar]
- 41.Stutzmann GE, Smith I, Caccamo A, Oddo S, Laferla FM, Parker I. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer's disease mice. J Neurosci. 2006;26:5180–5189. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stutzmann GE, Smith I, Caccamo A, Oddo S, Parker I, Laferla F. Enhanced ryanodine-mediated calcium release in mutant PS1-expressing Alzheimer's mouse models. Ann N Y Acad Sci. 2007;1097:265–277. doi: 10.1196/annals.1379.025. [DOI] [PubMed] [Google Scholar]
- 43.Takeda T, Asahi M, Yamaguchi O, Hikoso S, Nakayama H, Kusakari Y, Kawai M, Hongo K, Higuchi Y, Kashiwase K, Watanabe T, Taniike M, Nakai A, Nishida K, Kurihara S, Donoviel DB, Bernstein A, Tomita T, Iwatsubo T, Hori M, Otsu K. Presenilin 2 regulates the systolic function of heart by modulating Ca2+ signaling. FASEB J. 2005;19:2069–2071. doi: 10.1096/fj.05-3744fje. [DOI] [PubMed] [Google Scholar]
- 44.Tomita T, Maruyama K, Saido TC, Kume H, Shinozaki K, Tokuhiro S, Capell A, Walter J, Grunberg J, Haass C, Iwatsubo T, Obata K. The presenilin 2 mutation (N141I) linked to familial Alzheimer disease (Volga German families) increases the secretion of amyloid beta protein ending at the 42nd (or 43rd) residue. Proc Natl Acad Sci U S A. 1997;94:2025–2030. doi: 10.1073/pnas.94.5.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wehrens XH, Marks AR. Altered function and regulation of cardiac ryanodine receptors in cardiac disease. Trends Biochem Sci. 2003;28:671–678. doi: 10.1016/j.tibs.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Westhoff JH, Hwang SY, Duncan RS, Ozawa F, Volpe P, Inokuchi K, Koulen P. Vesl/Homer proteins regulate ryanodine receptor type 2 function and intracellular calcium signaling. Cell Calcium. 2003;34:261–269. doi: 10.1016/s0143-4160(03)00112-x. [DOI] [PubMed] [Google Scholar]
- 48.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 49.Zalk R, Lehnart SE, Marks AR. Modulation of the ryanodine receptor and intracellular calcium. Annu Rev Biochem. 2007;76:367–385. doi: 10.1146/annurev.biochem.76.053105.094237. [DOI] [PubMed] [Google Scholar]
- 50.Zhang C, Wu B, Beglopoulos V, Wines-Samuelson M, Zhang D, Dragatsis I, Sudhof TC, Shen J. Presenilins are essential for regulating neurotransmitter release. Nature. 2009;460:632–636. doi: 10.1038/nature08177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu L, et al. Modeling a ryanodine receptor N-terminal domain connecting the central vestibule and the corner clamp region. J Biol Chem. 2013;288(2):903–14. doi: 10.1074/jbc.M112.429670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.