Abstract
Anxiety disorders are the most common of the psychiatric disorders affecting as many as 10% of youth, with a peak during adolescence. A core component of these disorders is an unremitting fear in the absence of present threat. One of the most commonly used therapies to treat these disorders is exposure-based cognitive behavioral therapy that identifies the source of the fear and anxiety and then desensitizes the individual to it. This treatment builds on basic principles of fear extinction learning. A number of patients improve with this therapy, but 40–50% do not. This paper provides an overview of recent empirical studies employing both human imaging and cross-species behavioral genetics to examine how fear regulation varies across individuals and across development, especially during adolescence. These studies have important implications for understanding who may be at risk for anxiety disorders and for whom and when during development exposure-based therapies may be most effective.
Keywords: anxiety, fear regulation, development, individual differences
Introduction
Fear learning is an adaptive and evolutionarily conserved process that allows one to respond appropriately to cues associated with danger. However, unremitting fear that persists even when a threat is no longer present is a core component of many anxiety and stress-related disorders. These psychiatric disorders are among the most common in youth today, affecting as many as one in 10.1–4 The only evidence-based behavioral treatment for these disorders are cognitive behavioral therapies (CBT) that identify the cause of the anxiety and then desensitize the individual to that fear. This desensitization process of repeated exposure to the fear-eliciting event is based on the principles of fear extinction learning, in which a fear response is diminished through the association of a once-threatening stimulus with a new state of safety. Despite growing interest in fear extinction learning and retention because of its obvious clinical relevance to the treatment of various anxiety disorders,5 extinction-based therapies have limitations. First, only about 40–50% of individuals with anxiety respond to these treatments.6 Second, fear responses frequently recover spontaneously following the desensitization (i.e., extinction).7 Third, our work8 and that of others9 suggests that extinction learning is diminished during adolescence and therefore extinction-based therapies may have decreased efficacy during specific developmental periods. Finally, genetic factors in mice and humans have been shown to impact extinction learning10 and extinction retention,11,12 suggesting a genetic basis for treatment efficacy.
In this paper, we examine developmental and individual variation in brain circuitry supporting fear learning and regulation, and discuss the implications of this research for the risk and treatment of anxiety disorders. We provide a brief overview of fear neurocircuitry and how this circuitry changes across development, focusing on the period of adolescence, when there is a peak in the diagnosis of anxiety disorders.3 We then present findings from both human neuroimaging and cross-species behavioral studies suggesting that the normal developmental trajectory of the fear circuitry produces a transitory period of inefficient fear regulation during adolescence that may represent a window of vulnerability to anxiety. Finally, we use a translational genetic approach to examine sources of individual variation in fear regulation and their supporting neural substrates in both humans and mouse models. Collectively, these studies inform our understanding of developmental and individual differences in the risk for the impaired regulation of fear that characterizes anxiety. This information may, in turn, guide the identification of the optimal treatment for a given individual and the developmental stage at which an intervention is most likely to be effective.
Neurocircuitry of fear learning and regulation
The ability to rapidly associate aversive outcomes with the stimuli or contexts that predict them is a highly adaptive skill that is conserved across species. Learned fear associations are long lasting; however, when a stimulus ceases to predict threat, fear expression tends to gradually diminish through a process called extinction. Forming this new extinction memory does not overwrite the initial fear association, but inhibits its expression. The persistence of the original fear memory is evidenced by the common return of extinguished fear responses under circumstances such as the return to a fear-associated context (fear renewal), exposure to a stressor (fear reinstatement), or the mere passage of time (spontaneous recovery of fear).7 Following extinction, conflicting threat or safety associations engage in competition to determine which will drive behavior. Thus, effective fear regulation depends critically on the ability to form, maintain, and preferentially retrieve extinction memories when a stimulus previously associated with threat now signals safety. Impairment in this ability may put one at increased risk for anxiety.
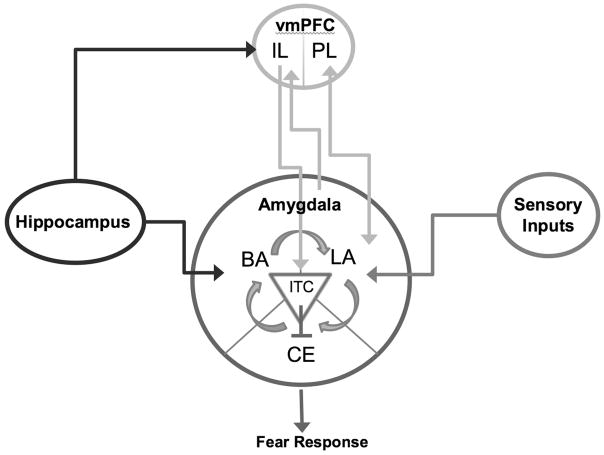
Extensive research in both humans and animal models has produced a detailed model of the neural circuitry supporting fear learning and regulation (Fig. 1). The amygdala, a medial temporal lobe structure composed of multiple subnuclei, critically supports the learning and expression of fear associations.13,14 Specifically, the lateral nucleus (LA) of the amygdala establishes and maintains fear memories and activates the central nucleus (CE) when a threat-associated stimulus is present, triggering the coordinated physiological and behavioral expression of fear via descending projections from the CE to the brainstem and hypothalamus.
Figure 1.
A simplified model of the neurocircuitry supporting fear learning and regulation. Fear conditioning yields an association between a conditioned stimulus (CS) and the unconditioned stimulus (US) that is maintained within the lateral nucleus of the amygdala (LA) and drives fear expression via the central nucleus of the amygdala (CE). Following fear learning, reciprocal connections between the LA and the prelimbic (PL) subregion of the ventromedial prefrontal cortex (vmPFC) are also involved in the sustained expression of fear. During fear extinction, connections are established between the infralimbic (IL) subregion of the vmPFC and the inhibitory intercalated (ITC) cell masses, which inhibit activity in the CE. When extinction memories are retrieved, these connections are activated, decreasing fear expression. The IL and PL also inhibit one another, mediating a competition between fear and extinction memory for behavioral control. Contextual modulation of extinction expression is mediated by projections from the hippocampus to the vmPFC and the amygdala. Adapted, with permission, from Casey et al.39
Bidirectional connections between the amygdala and the ventromedial prefrontal cortex play an important role in modulating fear expression.15 The prelimbic region (PL) of the rodent ventromedial prefrontal cortex (vmPFC) receives information from the amygdala signaling the presence of threat, and appears to transform this phasic signal into sustained firing that drives fear expression via its CE projections.16,17 The human dorsal anterior cingulate cortex has been proposed as a potential human homologue of the PL cortex.18 In contrast, the infralimbic region (IL) of the rodent vmPFC is implicated in the inhibition of fear expression and plays a critical role in the storage and retrieval of extinction memory.19 Cells within the LA and basal nucleus (B) of the amygdala activate the IL via ascending projections in response to a stimulus associated with safety learning.20,21 In turn, the IL has descending projections to a cluster of inhibitory cells, the intercalated cell masses (ITC), which inhibit CE activation and the corresponding fear response. The distinct amygdala–prefrontal IL and PL subnetworks compete for the control of behavior, respectively, driving low-fear and high-fear states.15 The hippocampus also informs this competitive dynamic, supplying contextual information about the degree of threat or safety22–24 that influences whether extinction or the original fear learning is expressed.
Collectively, these findings indicate that the amygdala supports the formation, maintenance, and expression of learned fears, while the regulation of fear expression depends critically on the ventromedial prefrontal cortex. Neuroimaging research examining fear and extinction learning in humans has been largely consistent with these findings from rodent studies, suggesting that the circuitry supporting fear learning and regulation in animal models is highly conserved across species.10,18,25–29 Highlighting the importance of this circuitry for normal emotion regulation, altered vmPFC–amygdala interactions appear to contribute to anxiety. Trait anxiety is associated with heightened amygdala blood oxygen level–dependent (BOLD) activation during fear acquisition30 and impaired extinction learning and retention30–32 that likely stems from impaired prefrontal-amygdala regulation.30,33 Imaging-based connectivity studies reveal that the integrity of the vmPFC–amygdala fiber tract is inversely correlated with trait anxiety,34 suggesting that anatomically compromised inhibitory function contributes to heightened threat reactivity and impaired fear regulation in anxiety. Understanding how prefrontal-amygdala interactions change across development may help to elucidate why adolescence represents a window of heightened vulnerability to anxiety.
Development of fear circuitry and fear regulation
Evidence from developmental neuroanatomical studies suggests that the different trajectories of maturation of components of the fear regulation circuitry might generate a period of inefficient regulation of threat responses during adolescence. Although the basic nuclear structure of the amygdala is present at birth,35,36 the tuning of its connections, especially with later-maturing regions, continues throughout childhood and adolescence. The prefrontal cortex is one of these later-maturing regions, showing a linear pattern of development and not reaching a mature functional architecture until early adulthood.37,38 The early maturation of subcortical regions supporting fear reactivity, coupled with the protracted maturation of the prefrontal cortex may result in an imbalance in the dynamic regulation of fear that yields a heightened sensitivity to threat during adolescence.39
In a recent human neuroimaging study, we40 found that adolescents initially exhibit a heightened amygdala response to threat-related cues (fearful faces) relative to children and adults (Fig. 2), a finding supported by other studies reporting greater amygdala reactivity to emotional pictures in adolescents than adults.41,42 In this task, threat cues generated behavioral inhibition, as measured by slowing in the latency to respond relative to the non-threatening facial expression.40 Degree of threat-related slowing correlated positively with amygdala activation, and negatively with vmPFC activation, consistent with the central role of these regions in the modulation of fear expression. By assessing habituation in the amygdala BOLD response to repeated presentation of fearful faces, we obtained a measure of the ability to diminish fear expression by learning that these expressions posed no real danger (an empty threat) in the current context. Participants reporting higher trait anxiety showed less habituation in the amygdala response as well as reduced amygdala–vmPFC functional connectivity, consistent with evidence that the efficacy of the fear regulation circuitry is diminished in anxious individuals.34,43
Figure 2.
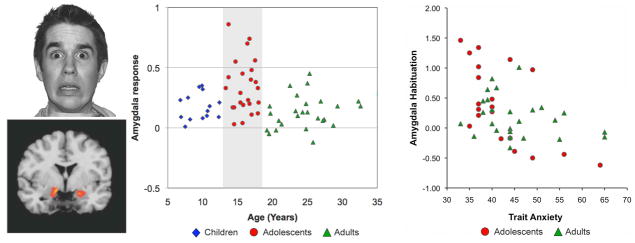
Amygdala activity to fearful faces presented in an emotional go/no-go task (left) was elevated during adolescence compared to children and adults (middle). Habituation (decrease from early to late trials) of this amygdala activity for teens and adults correlated with Spielberger trait anxiety (this scale was not appropriate for participants under 13 years of age). Adapted, with permission, from Hare et al.40 and reprinted from Casey et al.103
Heightened threat reactivity may support adaptive coping with novel dangers during adolescence, when exploratory behaviors increase. In a study of healthy adolescents, the amygdala response showed an initial heightened response to cues signaling potential threat (fearful faces), which gradually decreased with the recognition that these cues posed no actual threat in the current situation. However, persistent fear may confer increased risk of anxiety. In an imaging study using similar paradigms, we have observed elevated amygdala activity in response to repeated presentation of fearful faces in clinically anxious and depressed youth relative to healthy controls.44 Moreover, we observed that females tend to show less habituation of this response over time relative to males,45 which may play a mechanistic role in the higher incidence of anxiety and impaired regulation of mood later in life for females.
The previous studies used the presentation of fearful faces to examine fear regulation. Such cues come to be associated with threat throughout a lifetime of associative learning, although we appear to be biologically prepared to view them as fear-relevant.46 Differential experience with such threat cues poses an experimental confound for interpreting developmental variation in responses to these stimuli. Moreover, such cues are difficult to use with other species, especially rodents.
An alternative approach that can be used more objectively across development and species is that of Pavlovian conditioning, which involves the repeated pairing of a neutral cue (e.g., an image or a tone) with an intrinsically aversive outcome (e.g., electric shock) until it acquires negative significance. The same cue can subsequently undergo extinction by presenting the cue repeatedly without the aversive stimulus. Extinction retention can be assessed by presenting the cue at subsequent time points to see whether the safety learning is retained or the fear memory recovers. These paradigms are not only applicable across development, but also across species, enabling the use of techniques for probing underlying mechanisms that cannot be employed in humans. Moreover, the associative learning processes and underlying neural mechanisms that support Pavlovian fear learning and extinction have been well characterized13,14 and are altered in anxiety.31 Thus, developmental variation in these processes may play a role in the vulnerability to anxiety during adolescence.
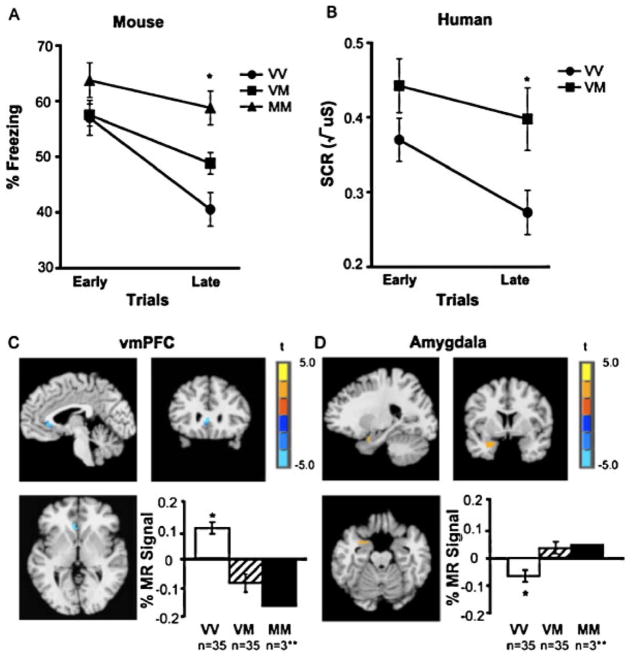
In a recent study, we examined whether immature prefrontal-amygdala connectivity might influence the extinction of learned fear during adolescence.8 In this study, children (aged 5–11), adolescents (aged 12–17), and adults (aged 18–28) took part in a fear conditioning paradigm in which neutral cues (colored squares) were paired with an aversive white noise during the acquisition phase, and then were presented alone during a subsequent extinction phase. A skin conductance response to stimulus presentations was used as an index of fear expression. While there were no differences between age groups in fear acquisition, adolescents showed a reduction in fear extinction relative to both children and adults (Fig. 3A), who did not differ in extinction learning. A parallel experiment in mice examined fear learning and extinction in mice at preadolescent (postnatal day 23, P23), adolescent (P29), and adult (P70) time points. In this study, a tone served as the conditioned stimulus and electric shock as the unconditioned stimulus, and freezing in response to the tone was measured as an index of conditioned fear. Consistent with the findings in humans, adolescent mice showed a selective attenuation in fear extinction relative to both pre- and post-adolescent age groups (Fig. 3B). These findings suggest that diminished extinction learning may render learned fear associations particularly persistent during adolescence, highlighting the importance of identifying approaches for treating anxiety in this developmental period that do not rely upon extinction learning.
Figure 3.
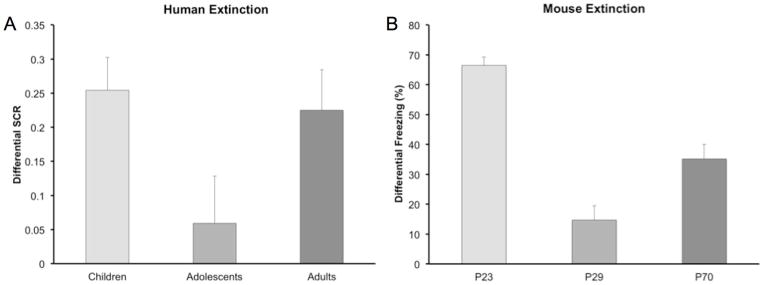
Both (A) humans and (B) mice exhibit attenuated fear extinction learning during adolescence compared to pre-adolescent juveniles and adults. Adapted, with permission, from Pattwell et al.8
During adolescence, the relative immaturity of the vmPFC may give rise to inefficient regulation of threat responses driven by enhanced amygdala activity. Numerous studies in animals,47 humans,48–52 and in childhood and adolescent mood and anxiety disorders,41,53 indicate that an inverse relationship between the amygdala and vmPFC governs the normal regulation of negative affect. This inverse coupling supports adaptive learning and cognition, promoting both fear extinction29 and positive interpretations of emotionally ambiguous information,54 both of which are impaired in anxiety.55 A developmental imbalance in this dynamic relationship may contribute to the heightened emotional reactivity characteristic of adolescence, and might increase vulnerability to anxiety disorders in certain individuals. One important but little-studied question is, what factors place an individual at heightened risk for developing the excessive fear that characterizes anxiety?
Individual differences in fear regulation
Fear reactivity and regulation vary not only across development, but also among individuals at each developmental stage. Recent research suggests that such individual differences in fear expression are relatively stable trait-like qualities;56,57; however, the mechanisms governing such variation are not well understood. As with all complex behavior, individual variation in fear expression stems from two fundamental factors: the inherited genetic profile intrinsic to each individual and one’s unique extrinsic life experiences. These factors interactively shape the neurobiological processes that ultimately drive behavior. In the following discussion, we present evidence that individual variation in both our experiences and our genes contributes to differences in fear reactivity and regulation, and may inform both the understanding of who might be at heightened risk for anxiety disorders, as well as what treatment approaches may be best suited for a particular individual.
Environmental factors
Studies of the development of the visual system have clearly demonstrated the existence of early periods during which atypical sensory input results in aberrant wiring of the brain, yielding alterations in visual perception that typically last a lifetime.58 Early life experiences of abuse, adversity, or deprivation can yield persistent alterations in emotional processing,59 suggesting that similar critical periods exist in affective development. Recently, we examined the effect of early-life institutional rearing on emotion regulation ability and its neural substrates.60 In this study, children adopted from foreign orphanages either early (15 months or younger) or late in life (older than 15 months) were compared to children reared from birth in a family environment. Later-adopted children exhibited heightened behavioral interference in the presence of threat-related stimuli, suggesting that the ability to regulate threat responses was impaired in these individuals. This behavioral pattern was paralleled by heightened amygdala activity to these potential cues of threat. These findings suggest that exposure to adverse early life experience generates an amygdala-dependent propensity toward threat reactivity, with length of exposure determining the magnitude of the effect.
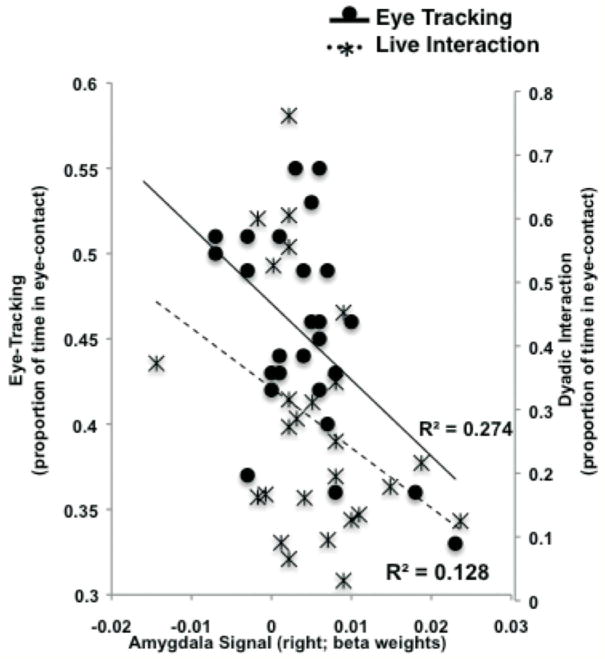
The most striking result from this study was the association between heightened amygdala activity to threat cues and live dyadic interaction between the child and parent. Specifically, we used video recordings of the child’s gaze with his/her parent both after a brief separation, as well as to simple face stimuli in a laboratory task. We found that greater activity within the amygdala was associated with decreased eye contact both in the dyadic interaction with their parents and to static face stimuli during the laboratory task (Fig. 4), an effect that was most pronounced in those children adopted later in life. These findings are consistent with evidence from animal studies showing that chronic stress exposure yields heightened amygdala reactivity, with relatively slow recovery.61,62 Moreover, these results underscore the importance of top-down modulation of amygdala-driven threat reactivity for healthy emotional development.
Figure 4.
Amygdala activity in individuals who faced early life adversity is associated with behavioral avoidance of potential threat. Amygdala signal change to fearful faces in institutionally reared children was inversely correlated with the amount of eye contact children made during eye tracking (proportion of frames spent looking at the eye region). Adapted, with permission, from Tottenham et al.60
Genetic factors
While our experiences may tune our response to threat, fear learning and expression is also substantially heritable.63 Given the complexity of the learning processes and circuitry governing fear expression, this heritability almost certainly reflects the contribution of multiple genes that influence different aspects of fear reactivity and regulation. Our laboratory has examined how normal variation in brain-derived neurotrophic factor (BDNF) and the serotonin transporter genes influence fear extinction learning and extinction retention, processes that critically regulate the expression of fear and anxiety.
BDNF is a neurotrophin that mediates the plastic synaptic changes supporting various forms of learning and memory, including fear learning and extinction.64,65 The human BDNF gene contains a common single nucleotide polymorphism that leads to a valine (Val) to methionine (Met) substitution at codon 66 (Val66Met). This substitution produces decreased trafficking of BDNF into the regulated secretory pathway, which in turn leads to reduced activity-dependent release of BDNF.66 In a genetically modified knock-in mouse, identical to the wild type except that it contains methionine at codon 66 (BDNFMet), the Met allele has been associated with heightened anxiety-like behavior.67 In a recent study, we conducted parallel studies in both adult mice and humans to examine whether BDNF might modulate anxiety through an influence on the fear regulation circuitry.10 Across species, Met allele carriers showed less extinction than Val allele carriers (Fig. 5A and B). Human functional magnetic resonance imaging (fMRI) data revealed corresponding alterations in frontoamygdala circuitry, with Met allele carriers exhibiting less vmPFC activity (Fig. 5C) and greater amygdala activity (Fig. 5D) than noncarriers. Subsequent studies suggest that this extinction deficit stems from impaired synaptic plasticity in the IL medial prefrontal cortex68 and that infusion of BDNF into this area is sufficient to produce the reduction in fear typically observed in fear extinction.69 These findings suggest that inherited genetic variation in BDNF function may modulate the capacity to regulate fear through safety learning, representing a potential source of individual vulnerability toward anxiety.
Figure 5.
The BDNF Val66Met Met allele is associated with decreased extinction learning in both (A) mice and (B) humans. Met allele carriers showed (C) reduced ventromedial prefrontal cortex (vmPFC) activation and (D) elevated amygdala activation in response to the conditioned stimulus during extinction. Adapted, with permission, from Soliman et al.10
Serotonin is theorized to play an important role in the processing of aversive stimuli70 and is proposed to contribute to anxiety through its effects on fear conditioning.71 Mice lacking the serotonin transporter gene show marked deficits in extinction retention72,73 as well as heightened anxiety and depression-like behavior.74 These findings suggest that serotonin may contribute to these clinical disorders through the modulation of extinction retention. In a recent study,11 we examined whether a common functional serotonin transporter polyadenylation polymorphism (STPP/rs3813034)75 might yield similar effects in adult humans. We genotyped participants who completed a two-day conditioning paradigm for the STPP. Participants underwent fear conditioning followed immediately by extinction. Twenty four hours later, a second extinction phase enabled evaluation of whether participants retained initial extinction learning. We found a dose-dependent effect of the STPP on extinction retention, with individuals carrying an increasing number of the lower serotonin transporter–expressing STPP G allele and showing greater spontaneous recovery of fear (Fig. 6A), as well as higher self-reported trait anxiety and depressive symptoms (Fig. 6B and C). These findings mirror the behavioral effects of genetic knockout of the serotonin transporter in the mouse, and suggest that through genetic modulation of extinction retention, the STPP may confer a genetic predisposition toward recurrent fear, a characteristic feature of anxiety.
Figure 6.
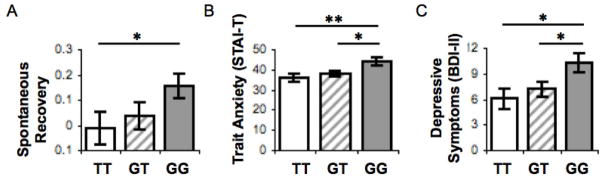
The lower-expressing variant (G allele) of a serotonin transporter polyadenylation polymorphism (STPP/rs38130304) is associated with (A) decreased retention of fear extinction learning, as well as higher self-reported (B) Spielberger trait anxiety (STAI-T) and (C) Beck Depression Inventory (BDI-II) scores. Adapted, with permission, from Hartley et al.11
Beyond the roles of BDNF and serotonin demonstrated in our work, genetic variation in both the dopamine76,77 and endocannabinoid systems78 also appear to modulate fear regulation. Polymorphisms in the dopamine transporter gene (DAT1) and the gene coding for catechol-O-methyltransferase (COMT), a dopamine-degrading enzyme, have been associated with variation in extinction learning.76,77 Individuals carrying a lower-expressing variant of the gene that codes for fatty acid amid hydrolase (FAAH), an enzyme that degrades the endogenous cannabinoid anandamide, show quicker habituation of the amygdala response to threat-related stimuli.78 These genetic findings in humans are consistent with evidence from pharmacological manipulations across species demonstrating that these signaling systems modulate fear extinction learning and retention.79–81 In addition to the influence of interindividual genomic variation of fear extinction, recent research indicates that fear extinction processes are also under epigenetic regulation,82,83 suggesting a mechanism by which environmental influence may interact with genetic processes to modulate fear regulation.
Collectively these findings indicate that genetic and experiential variation among individuals may confer associative learning tendencies that modulate the regulation of fear expression. Such learning biases may, in turn, influence both individual vulnerability for anxiety and resistance to standard behavioral treatment approaches. The studies discussed above examined genetic differences in fear regulation in adults. One important question that has received little attention to date is how these genetic factors might influence the maturation of fear regulation. Future research examining genotype–development interactions will be necessary in order to understand both when and how genetic individual differences give rise to the behavioral influences that have been observed in adulthood.
Implications for the treatment of anxiety
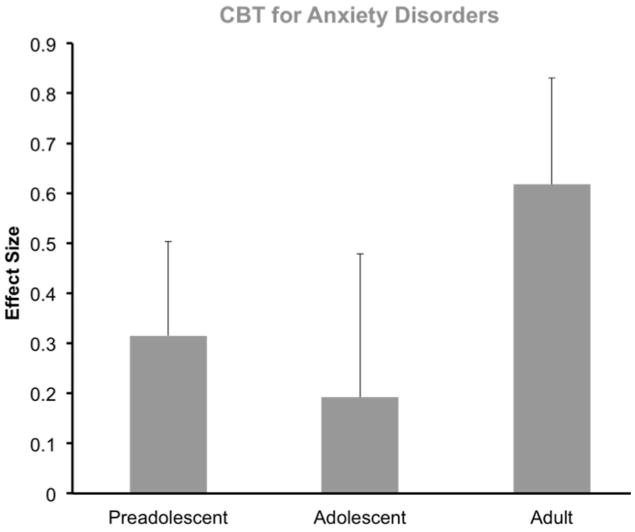
Individual and developmental variation in the efficiency of the fear regulatory circuitry has meaningful implications for the treatments that are likely to be effective for a given individual at a particular stage in life. A common approach to the treatment of anxiety involves exposure therapy in which cues that elicit fear responses are identified and then recalled in a safe context in an attempt to diminish the associated fear response.84 Although exposure therapies are based on extinction learning principles, the actual application of the therapy within the clinic differs substantially from laboratory fear extinction procedures. While further research is needed to establish the validity of laboratory extinction measures as predictors of therapeutic success, theoretically, the efficacy of exposure therapy should depend at least in part on the ability to both acquire and retain extinction learning.85 The studies described here detailing developmental variation in fear reactivity and regulation suggest that exposure-based approaches may be less effective for treating fear during adolescence, when extinction learning is attenuated, than at other timepoints. To date, this hypothesis has not yet been tested directly, however a recent developmental re-analysis of studies assessing the efficacy of CBT suggests that this proposal might have merit (Fig. 7).86 A future clinical study examining exposure therapy efficacy across development will be required to conclusively address this question.
Figure 7.
An age based re-analysis of data from studies examining the efficacy of cognitive behavioral therapy (CBT) in juveniles104 and adults105 reveals a non-significant trend suggesting the possibility of reduced effectiveness of CBT during adolescence. Adapted, with permission, from Dale et al.86
Similarly, genotype may modulate exposure therapy efficacy in individuals carrying alleles that compromise the efficacy of extinction learning and retention. Although the prescription of selective serotonin-reuptake inhibitors (SSRIs) is a common pharmacological approach to the treatment of anxiety, their efficacy is unclear.87 Recent reports in mice showing that chronic SSRI administration only prevented the return of conditioned fear when coupled with extinction training suggest that coupling SSRI prescription with concurrent extinction-based therapies may improve outcomes.88,89 In these studies, serotonin may foster increased plasticity that permits the restructuring of the fear regulation circuitry through new learning.90 A recent large-scale clinical study suggests that this combined therapeutic approach improves treatment response in children and adolescence.6
A number of pharmacological agents can enhance extinction learning.19 The most studied of these compounds is D-cycloserine (DCS), which has been found to both speed extinction learning and increase extinction retention,91,92 Two clinical studies have reported improved responses to therapy when incorporating DCS administration into the regimen93,94 and future clinical trials may establish more clearly the efficacy of DCS and other potential agents for reducing anxiety. These findings suggest that using extinction-enhancing substances as an adjunct to exposure-based therapy may be one promising avenue for ameliorating a reduced capacity to diminish fear.
Non-extinction-based behavioral methods for reducing fear may provide alternative therapies for treatment-resistant forms of anxiety. A recent study suggested that if the retrieval of a fear memory is followed immediately by extinction, this leads to a disruption of memory reconsolidation that effectively overwrites the fear memory through new safety learning.95,96 Furthermore, this effect appears to be amygdala dependent,97 circumventing the inhibitory vmPFC–amygdala circuitry that might be compromised in individuals in whom fear regulation is attenuated. Future studies examining whether this reconsolidation effect is present across development might clarify whether this technique might be an effective approach for treatment-resistant anxiety in adolescence.
Another route to treatment might lie in strengthening the vmPFC–amygdala circuit through behavioral intervention. Recent studies in animal models suggest that coping actively with a stressor diminishes fear, activating an alternative amygdala-striatal circuit that biases the organism toward active instead of passive responses to threat stimuli.98 Active coping also activates the vmPFC, recruiting the fear regulation circuitry in a non-extinction-dependent manner.99 This vmPFC activation appears to foster prolonged resilience to subsequent stressors,100,101 possibly by strengthening this inhibitory circuitry. Therapeutic approaches that teach behavioral strategies to actively cope with fear may function in a similar manner102 and might be particularly effective during childhood and adolescence, when prefrontal plasticity is higher.
Conclusions
In summary, research across species has identified both developmental as well as individual differences in fear regulation ability that may confer heightened risk toward anxiety through modulation of the circuitry that supports the downregulation of fear. This research yields important insights that may inform the development of novel evidence-based therapies. An increased understanding of how to augment or circumvent inefficiencies in fear regulation will move the field of psychiatry toward a more personalized therapeutic approach, targeting anxiety in a manner that is most likely to be effective for a particular person at a given developmental stage.
Acknowledgments
This paper was presented as the 2012 Salmon Lecture and supported in part by the National Institute of Mental Health P50 MH62196, R01 DA018879, the Mortimer D. Sackler M.D. family, Dewitt Wallace Reader’s Digest Fund and the Weill Cornell Medical College Department of Psychiatry, Citigroup Biomedical Imaging Center and Imaging Core.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49:980–9. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman DL, Moffitt TE, Caspi A, Magdol L, Silva PA, Stanton WR. Psychiatric disorder in a birth cohort of young adults: prevalence, comorbidity, clinical significance, and new case incidence from ages 11 to 21. J Consult Clin Psychol. 1996;64:552–62. [PubMed] [Google Scholar]
- 4.Pollack MH, Otto MW, Sabatino S, Majcher D, Worthington JJ, McArdle ET, et al. Relationship of childhood anxiety to adult panic disorder: correlates and influence on course. Am J Psychiatry. 1996;153:376–81. doi: 10.1176/ajp.153.3.376. [DOI] [PubMed] [Google Scholar]
- 5.Anderson KC, Insel TR. The promise of extinction research for the prevention and treatment of anxiety disorders. Biol Psychiatry. 2006;60:319–21. doi: 10.1016/j.biopsych.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359:2753–66. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouton M. Context and Behavioral Processes in Extinction. Learning & Memory. 2004;11:485–94. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- 8.Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, et al. Altered fear learning across development in both mouse and human. Proc Natl Acad Sci U S A. 2012;109:16318–23. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCallum J, Kim JH, Richardson R. Impaired Extinction Retention in Adolescent Rats: Effects of D-Cycloserine. Neuropsychopharmacology. 2010;35:2134–42. doi: 10.1038/npp.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, et al. A Genetic Variant BDNF Polymorphism Alters Extinction Learning in Both Mouse and Human. Science. 2010;327:863–6. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartley CA, McKenna MC, Salman R, Holmes A, Casey B, Phelps EA, et al. Serotonin transporter polyadenylation polymorphism modulates the retention of fear extinction memory. Proceedings of the National Academy of Sciences. 2012;109:5493–8. doi: 10.1073/pnas.1202044109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson R-M, Saksida LM, et al. Impaired Fear Extinction Learning and Cortico-Amygdala Circuit Abnormalities in a Common Genetic Mouse Strain. Journal of Neuroscience. 2008:8074–85. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeDoux J. The amygdala. Current Biology. 2007;17:868–74. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- 15.Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Current Opinion in Neurobiology. 2010;20:231–5. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained Conditioned Responses in Prelimbic Prefrontal Neurons Are Correlated with Fear Expression and Extinction Failure. Journal of Neuroscience. 2009;29:8474–82. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch S. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learning & Memory. 2006;13:728–33. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milad M, Quirk G, Pitman R, Orr S, Fischl B. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007;62:1191–4. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 19.Quirk GJ, Mueller D. Neural Mechanisms of Extinction Learning and Retrieval. Neuropsychopharmacology. 2008:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–6. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- 21.Repa JC, Muller J, Apergis J, Desrochers TM, Zhou Y, LeDoux JE. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nature Neuroscience. 2001;4:724–31. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- 22.Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behavioural Brain Research. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- 23.Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. 2007;17:749–58. doi: 10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- 24.Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron. 2012;76:804–12. doi: 10.1016/j.neuron.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottfried J, Dolan R. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nature neuroscience. 2004;7:1145–53. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- 26.Kalisch R, Korenfeld E, Klaas S, Weiskopf N, Seymour B, Dolan R. Context-Dependent Human Extinction Memory Is Mediated by a Ventromedial Prefrontal and Hippocampal Network. Journal of Neuroscience. 2006;26:9503–11. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knight DC, Smith CN, Cheng DT, Stein EA, Helmstetter FJ. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cogn Affect Behav Neurosci. 2004;4:317–25. doi: 10.3758/cabn.4.3.317. [DOI] [PubMed] [Google Scholar]
- 28.LaBar K, Gatenby J, Gore J, LeDoux J. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–45. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 29.Phelps E, Delgado M, Nearing K, LeDoux J. Extinction Learning in Humans Role of the Amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 30.Indovina I, Robbins TW, Núñez-Elizalde AO, Dunn BD, Bishop SJ. Fear-Conditioning Mechanisms Associated with Trait Vulnerability to Anxiety in Humans. Neuron. 2011:563–71. doi: 10.1016/j.neuron.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, et al. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behaviour research and therapy. 2005:1391–424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Rauch SL, Milad MR, Orr SP, Quinn BT, Fischl B, Pitman RK. Orbitofrontal thickness, retention of fear extinction, and extraversion. Neuroreport. 2005;16:1909. doi: 10.1097/01.wnr.0000186599.66243.50. [DOI] [PubMed] [Google Scholar]
- 33.Sehlmeyer C, Schöning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, et al. Human Fear Conditioning and Extinction in Neuroimaging: A Systematic Review. PLoS ONE. 2009:e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim MJ, Whalen PJ. The Structural Integrity of an Amygdala-Prefrontal Pathway Predicts Trait Anxiety. Journal of Neuroscience. 2009;29:11614–8. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humphrey T. The development of the human amygdala during early embryonic life. Journal of Comparative Neurology. 1968;132:135–65. doi: 10.1002/cne.901320108. [DOI] [PubMed] [Google Scholar]
- 36.ULFIG N, SETZER M, BOHL J. Ontogeny of the human amygdala Annals of the New York. Academy of Sciences. 2003;985:22–33. doi: 10.1111/j.1749-6632.2003.tb07068.x. [DOI] [PubMed] [Google Scholar]
- 37.Huttenlocher PR. Synaptic density in human frontal cortex: Developmental changes and effects of aging. Brain Research. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 38.Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- 39.Casey BJ, Pattwell SS, Glatt CE, Lee FS. Treating the Developing Brain: Implications from Human Imaging and Mouse Genetics. Annu Rev Med. 2012 doi: 10.1146/annurev-med-052611-130408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008:927–34. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, et al. A developmental examination of amygdala response to facial expressions. J Cogn Neurosci. 2008;20:1565–82. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–8. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- 43.Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci. 2012;15:1736–41. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, et al. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58:1057–63. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- 45.Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, et al. Amygdala response to facial expressions in children and adults. Biol Psychiatry. 2001;49:309–16. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- 46.Öhman A, Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- 47.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–4. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 48.Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–82. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 49.Haas BW, Omura K, Constable RT, Canli T. Emotional conflict and neuroticism: personality-dependent activation in the amygdala and subgenual anterior cingulate. Behav Neurosci. 2007;121:249–56. doi: 10.1037/0735-7044.121.2.249. [DOI] [PubMed] [Google Scholar]
- 50.Holmes AJ, Lee PH, Hollinshead MO, Bakst L, Roffman JL, Smoller JW, et al. Individual Differences in Amygdala-Medial Prefrontal Anatomy Link Negative Affect, Impaired Social Functioning, and Polygenic Depression Risk. J Neurosci. 2012;32:18087–100. doi: 10.1523/JNEUROSCI.2531-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–84. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Urry HLvRC, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:568–76. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003:2317. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- 55.Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci (Regul Ed) 2007:307–16. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 56.Bush D, Sotres-Bayon F, LeDoux J. Individual differences in fear: Isolating fear reactivity and fear recovery phenotypes. J Trauma Stress. 2007 doi: 10.1002/jts.20261. [DOI] [PubMed] [Google Scholar]
- 57.Zeidan MA, Lebron-Milad K, Thompson-Hollands J, Im JJY, Dougherty DD, Holt DJ, et al. Test-Retest Reliability during Fear Acquisition and Fear Extinction in Humans. CNS Neuroscience & Therapeutics. 2011:no–no. doi: 10.1111/j.1755-5949.2011.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–79. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- 59.Fox SELP, Nelson CA., 3rd How the timing and quality of early experiences influence the development of brain architecture. Child Development. 2010;81:28–40. doi: 10.1111/j.1467-8624.2009.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Dev Sci. 2011;14:190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adamec RE, Blundell J, Burton P. Neural circuit changes mediating lasting brain and behavioral response to predator stress. Neurosci Biobehav Rev. 2005;29:1225–41. doi: 10.1016/j.neubiorev.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 62.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hettema JM, Annas P, Neale MC, Kendler KS, Fredrikson M. A twin study of the genetics of fear conditioning. Archives of General Psychiatry. 2003:702. doi: 10.1001/archpsyc.60.7.702. [DOI] [PubMed] [Google Scholar]
- 64.Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 65.Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2012;35:24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003:257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 67.Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–3. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pattwell SS, Bath KG, Perez-Castro R, Lee FS, Chao MV, Ninan I. The BDNF Val66Met Polymorphism Impairs Synaptic Transmission and Plasticity in the Infralimbic Medial Prefrontal Cortex. Journal of Neuroscience. 2012;32:2410–21. doi: 10.1523/JNEUROSCI.5205-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of Fear Extinction with Hippocampal-Infralimbic BDNF. Science. 2010:1288–90. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dayan P, Huys Q. Serotonin in Affective Control. Annual Review of Neuroscience. 2009 doi: 10.1146/annurev.neuro.051508.135607. [DOI] [PubMed] [Google Scholar]
- 71.Deakin J, Graeff F. 5-HT and mechanisms of defence. Journal of Psychopharmacology. 1991:305. doi: 10.1177/026988119100500414. [DOI] [PubMed] [Google Scholar]
- 72.Narayanan V, Heiming RS, Jansen F, Lesting J, Sachser N, Pape H-C, et al. Social Defeat: Impact on Fear Extinction and Amygdala-Prefrontal Cortical Theta Synchrony in 5-HTT Deficient Mice. PLoS ONE. 2011:e22600. doi: 10.1371/journal.pone.0022600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, et al. Impaired Stress-Coping and Fear Extinction and Abnormal Corticolimbic Morphology in Serotonin Transporter Knock-Out Mice. Journal of Neuroscience. 2007;27:684–91. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murphy DL, Fox MA, Timpano KR, Moya PR, Ren-Patterson R, Andrews AM, et al. How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology. 2008;55:932–60. doi: 10.1016/j.neuropharm.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gyawali S, Subaran R, Weissman MM, Hershkowitz D, McKenna MC, Talati A, et al. Association of a Polyadenylation Polymorphism in the Serotonin Transporter and Panic Disorder. Biol Psychiatry. 2010;67:331–8. doi: 10.1016/j.biopsych.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lonsdorf TB, Weike AI, Nikamo P, Schalling M, Hamm AO, Öhman A. Genetic Gating of Human Fear Learning and Extinction Possible Implications for Gene-Environment Interaction in Anxiety Disorder. Psychological science. 2009;20(2):198–206. doi: 10.1111/j.1467-9280.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- 77.Raczka KA, Mechias ML, Gartmann N, Reif A, Deckert J, Pessiglione M, Kalisch R. Empirical support for an involvement of the mesostriatal dopamine system in human fear extinction. Translational psychiatry. 2011;1(6):e12. doi: 10.1038/tp.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gunduz-Cinar O, Macpherson KP, Cinar R, Gamble-George J, Sugden K, Williams B, Holmes A. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Molecular psychiatry. 2012;18(7):813–823. doi: 10.1038/mp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lutz B. The endocannabinoid system and extinction learning. Molecular neurobiology. 2007;36(1):92–101. doi: 10.1007/s12035-007-8004-x. [DOI] [PubMed] [Google Scholar]
- 80.Rabinak CA, Angstadt M, Sripada CS, Abelson JL, Liberzon I, Milad MR, Phan KL. Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haaker J, Gaburro S, Sah A, Gartmann N, Lonsdorf TB, Meier K, Kalisch R. Single dose of L-dopa makes extinction memories context-independent and prevents the return of fear. Proceedings of the National Academy of Sciences. 2013 doi: 10.1073/pnas.1303061110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learning & memory. 2007;14(4):268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stafford JM, Raybuck JD, Ryabinin AE, Lattal KM. Increasing histone acetylation in the hippocampus-infralimbic network enhances fear extinction. Biological psychiatry. 2012;72(1):25–33. doi: 10.1016/j.biopsych.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Foa EB. Psychosocial therapy for posttraumatic stress disorder. J Clin Psychiatry. 2006;67:40–5. [PubMed] [Google Scholar]
- 85.Berry AC, Rosenfield D, Smits JAJ. Extinction retention predicts improvement in social anxiety symptoms following exposure therapy. Depress Anxiety. 2009;26:22–7. doi: 10.1002/da.20511. [DOI] [PubMed] [Google Scholar]
- 86.Dale AT, Hartley CA, Pattwell SS, Ruberry EJ, Somerville LH, Compton SN, Lee FS, Casey BJ, Walkup JT. (in press) Fear and Anxiety from Principle to Practice: Implications for when to treat youth with anxiety disorders. Biological Psychiatry. doi: 10.1016/j.biopsych.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scott Allan, Davidson A, Palmer K. Antidepressant drugs in the treatment of anxiety disorders. Advances in Psychiatric Treatment. 2001:275–82. [Google Scholar]
- 88.Deschaux O, Spennato G, Moreau J-L, Garcia R. Chronic treatment with fluoxetine prevents the return of extinguished auditory-cued conditioned fear. Psychopharmacology. 2011:231–7. doi: 10.1007/s00213-010-2134-y. [DOI] [PubMed] [Google Scholar]
- 89.Karpova NN, Pickenhagen A, Lindholm J, Tiraboschi E, Kulesskaya N, Agustsdottir A, et al. Fear Erasure in Mice Requires Synergy Between Antidepressant Drugs and Extinction Training. Science. 2011:1731–4. doi: 10.1126/science.1214592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci. 2010;30:14964–71. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mao SC, Hsiao YH, Gean PW. Extinction training in conjunction with a partial agonist of the glycine site on the NMDA receptor erases memory trace. J Neurosci. 2006;26:8892–9. doi: 10.1523/JNEUROSCI.0365-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Walker DLRK, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. Journal of Neuroscience. 2002;22:6. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, et al. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- 94.Ressler KJRB, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Archives of General Psychiatry. 2004;61:1136–44. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 95.Monfils M-H, Cowansage KK, Klann E, Ledoux JE. Extinction-Reconsolidation Boundaries: Key to Persistent Attenuation of Fear Memories. Science. 2009;324:951–5. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schiller D, Monfils M-H, Raio CM, Johnson DC, Ledoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2009;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Agren T, Engman J, Frick A, Bjorkstrand J, Larsson EM, Furmark T, et al. Disruption of reconsolidation erases a fear memory trace in the human amygdala. Science. 2012;337:1550–2. doi: 10.1126/science.1223006. [DOI] [PubMed] [Google Scholar]
- 98.Amorapanth P, LeDoux J, Nader K. Different lateral amygdala outputs mediate reactions and actions elicited by a fear-arousing stimulus. Nature Neuroscience. 2000;3:74–9. doi: 10.1038/71145. [DOI] [PubMed] [Google Scholar]
- 99.Amat J, Baratta M, Paul E, Bland S, Watkins L. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe …. Nature Neuroscience. 2005 doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- 100.Amat J, Paul E, Zarza C, Watkins L, Maier S. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. Journal of Neuroscience. 2006;26:13264–72. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maier SF, Watkins LR. Role of the medial prefrontal cortex in coping and resilience. Brain Research. 2010;1355:52–60. doi: 10.1016/j.brainres.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.LeDoux JE, Gorman JM. A call to action: overcoming anxiety through active coping. Am J Psychiatry. 2001;158:1953–5. doi: 10.1176/appi.ajp.158.12.1953. [DOI] [PubMed] [Google Scholar]
- 103.Casey BJ, Ruberry EJ, Libby V, Glatt CE, Hare T, Soliman F, Duhoux S, Frieligsdorf H, Tottenham N. Transitional and translational studies of risk for anxiety. Depression and Anxiety. 2011;28(1):18–28. doi: 10.1002/da.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753–2766. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davidson JRT, Foa EB, Huppert JD, Keefe FJ, Franklin ME, Compton JS, et al. Fluoxetine, comprehensive cognitive behavioral therapy, and placebo in generalized social phobia. Arch Gen Psychiatry. 2004;61:1005–1013. doi: 10.1001/archpsyc.61.10.1005. [DOI] [PubMed] [Google Scholar]