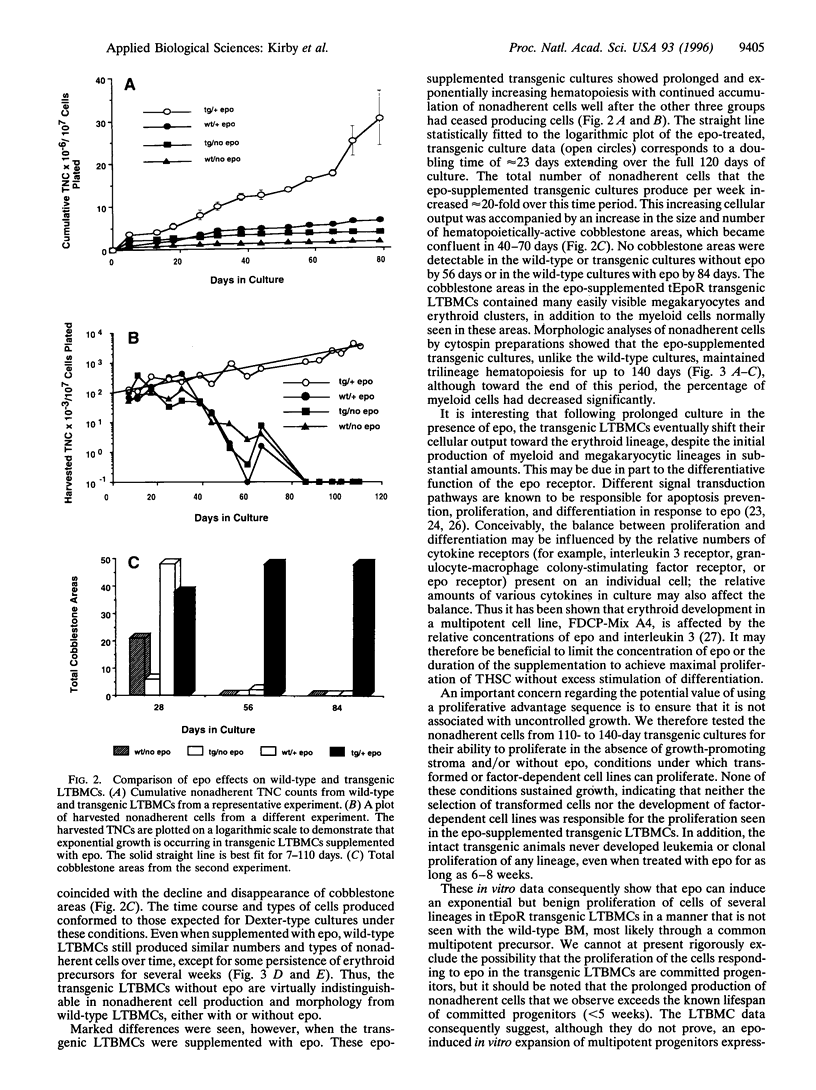
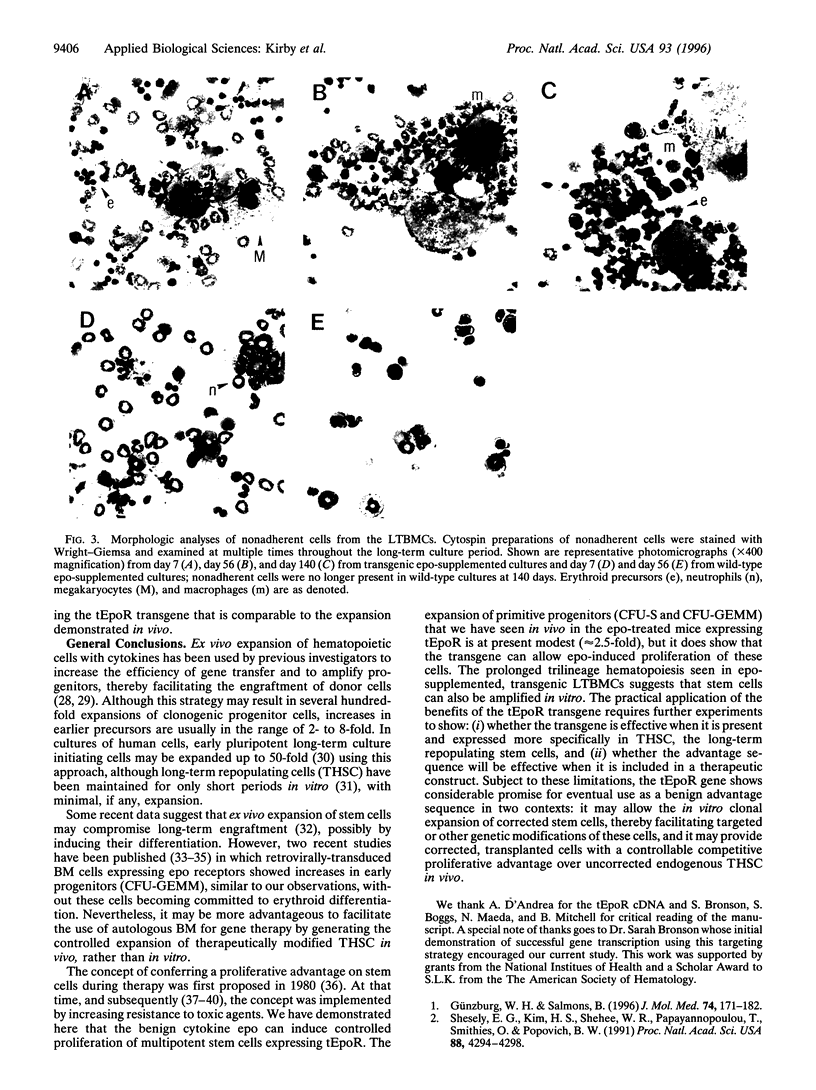
Abstract
The long-term efficacy of gene therapy using bone marrow transplantation requires the engraftment of genetically altered totipotent hematopoietic stem cells (THSCs). Ex vivo expansion of corrected THSCs is one way to increase the efficiency of the procedure. Similarly, selective in vivo expansion of the therapeutic THSCs rather than the endogenous THSCs could favor the transplant. To test whether a conferred proliferative advantage gene can facilitate the in vitro and in vivo expansion of hematopoietic stem cells, we have generated transgenic mice expressing a truncated receptor for the growth factor erythropoietin. These mice are phenotypically normal, but when treated in vivo with exogenous erythropoietin they exhibit a marked increase in multipotent, clonogenic hematopoietic cells [colony-forming units in the spleen (CFU-S) and CFUs that give rise to granulocytes, erythroid cells, macrophages, and megakaryocytes within the same colony (CFU-GEMM)] in comparison with the wild-type mice. In addition, long-term in vitro culture of tEpoR transgenic bone marrow in the presence of erythropoietin induces exponential expansion of trilineage hematopoietic stem cells not seen with wild-type bone marrow. Thus, the truncated erythropoietin receptor gene shows promise as a means for obtaining cytokine-inducible hematopoietic stem cell proliferation to facilitate the direct targeting of THSCs and to provide a competitive repopulation advantage for transplanted therapeutic stem cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bronson S. K., Smithies O. Altering mice by homologous recombination using embryonic stem cells. J Biol Chem. 1994 Nov 4;269(44):27155–27158. [PubMed] [Google Scholar]
- Carr F., Medina W. D., Dube S., Bertino J. R. Genetic transformation of murine bone marrow cells to methotrexate resistance. Blood. 1983 Jul;62(1):180–185. [PubMed] [Google Scholar]
- Cline M. J., Stang H., Mercola K., Morse L., Ruprecht R., Brown J., Salser W. Gene transfer in intact animals. Nature. 1980 Apr 3;284(5755):422–425. doi: 10.1038/284422a0. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Yoshimura A., Youssoufian H., Zon L. I., Koo J. W., Lodish H. F. The cytoplasmic region of the erythropoietin receptor contains nonoverlapping positive and negative growth-regulatory domains. Mol Cell Biol. 1991 Apr;11(4):1980–1987. doi: 10.1128/mcb.11.4.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMartino J., Carroll M., Mathey-Prevot B., D'Andrea A. D. Erythropoietin receptor contains both growth-promoting activity and differentiation-promoting activity. Ann N Y Acad Sci. 1994 Apr 15;718:213–222. doi: 10.1111/j.1749-6632.1994.tb55720.x. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Allen T. D., Lajtha L. G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977 Jun;91(3):335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- Dubart A., Feger F., Lacout C., Goncalves F., Vainchenker W., Dumenil D. Murine pluripotent hematopoietic progenitors constitutively expressing a normal erythropoietin receptor proliferate in response to erythropoietin without preferential erythroid cell differentiation. Mol Cell Biol. 1994 Jul;14(7):4834–4842. doi: 10.1128/mcb.14.7.4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einerhand M. P., Bakx T. A., Kukler A., Valerio D. Factors affecting the transduction of pluripotent hematopoietic stem cells: long-term expression of a human adenosine deaminase gene in mice. Blood. 1993 Jan 1;81(1):254–263. [PubMed] [Google Scholar]
- Fauser A. A., Messner H. A. Identification of megakaryocytes, macrophages, and eosinophils in colonies of human bone marrow containing neurtophilic granulocytes and erythroblasts. Blood. 1979 May;53(5):1023–1027. [PubMed] [Google Scholar]
- Günzburg W. H., Salmons B. Development of retroviral vectors as safe, targeted gene delivery systems. J Mol Med (Berl) 1996 Apr;74(4):171–182. doi: 10.1007/BF00204747. [DOI] [PubMed] [Google Scholar]
- He T. C., Jiang N., Zhuang H., Quelle D. E., Wojchowski D. M. The extended box 2 subdomain of erythropoietin receptor is nonessential for Jak2 activation yet critical for efficient mitogenesis in FDC-ER cells. J Biol Chem. 1994 Jul 15;269(28):18291–18294. [PubMed] [Google Scholar]
- Heath D. S., Axelrad A. A., McLeod D. L., Shreeve M. M. Separation of the erythropoietin-responsive progenitors BFU-E and CFU-E in mouse bone marrow by unit gravity sedimentation. Blood. 1976 May;47(5):777–792. [PubMed] [Google Scholar]
- Heberlein C., Fischer K. D., Stoffel M., Nowock J., Ford A., Tessmer U., Stocking C. The gene for erythropoietin receptor is expressed in multipotential hematopoietic and embryonal stem cells: evidence for differentiation stage-specific regulation. Mol Cell Biol. 1992 Apr;12(4):1815–1826. doi: 10.1128/mcb.12.4.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth C. M., Alauldin M., Cross M. A., Fairbairn L. J., Dexter T. M., Whetton A. D. Erythroid development of the FDCP-Mix A4 multipotent cell line is governed by the relative concentrations of erythropoietin and interleukin 3. Br J Haematol. 1995 Sep;91(1):15–22. doi: 10.1111/j.1365-2141.1995.tb05238.x. [DOI] [PubMed] [Google Scholar]
- Hooper M., Hardy K., Handyside A., Hunter S., Monk M. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature. 1987 Mar 19;326(6110):292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- Ihle J. N. Interleukin-3 and hematopoiesis. Chem Immunol. 1992;51:65–106. doi: 10.1159/000420755. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Witthuhn B. A., Quelle F. W., Yamamoto K., Silvennoinen O. Signaling through the hematopoietic cytokine receptors. Annu Rev Immunol. 1995;13:369–398. doi: 10.1146/annurev.iy.13.040195.002101. [DOI] [PubMed] [Google Scholar]
- Katayama N., Clark S. C., Ogawa M. Growth factor requirement for survival in cell-cycle dormancy of primitive murine lymphohematopoietic progenitors. Blood. 1993 Feb 1;81(3):610–616. [PubMed] [Google Scholar]
- Koller B. H., Smithies O. Inactivating the beta 2-microglobulin locus in mouse embryonic stem cells by homologous recombination. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8932–8935. doi: 10.1073/pnas.86.22.8932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacout C., Dubart A., Vainchenker W., Duménil D. Pluripotent stem cells constitutively expressing a normal erythropoietin receptor give rise to normal hematopoiesis in lethally irradiated recipient mice. Exp Hematol. 1996 Jan;24(1):18–25. [PubMed] [Google Scholar]
- Leavitt J., Gunning P., Porreca P., Ng S. Y., Lin C. S., Kedes L. Molecular cloning and characterization of mutant and wild-type human beta-actin genes. Mol Cell Biol. 1984 Oct;4(10):1961–1969. doi: 10.1128/mcb.4.10.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux M. E., Rebel V. I., Lansdorp P. M., Eaves C. J. Characterization and purification of a primitive hematopoietic cell type in adult mouse marrow capable of lymphomyeloid differentiation in long-term marrow "switch" cultures. Blood. 1995 Aug 15;86(4):1339–1347. [PubMed] [Google Scholar]
- Longmore G. D., Lodish H. F. An activating mutation in the murine erythropoietin receptor induces erythroleukemia in mice: a cytokine receptor superfamily oncogene. Cell. 1991 Dec 20;67(6):1089–1102. doi: 10.1016/0092-8674(91)90286-8. [DOI] [PubMed] [Google Scholar]
- Lu L., Ge Y., Li Z. H., Keeble W., Kabat D., Bagby G. C., Broxmeyer H. E., Hoatlin M. E. Retroviral transfer of the recombinant human erythropoietin receptor gene into single hematopoietic stem/progenitor cells from human cord blood increases the number of erythropoietin-dependent erythroid colonies. Blood. 1996 Jan 15;87(2):525–534. [PubMed] [Google Scholar]
- McLachlin J. R., Eglitis M. A., Ueda K., Kantoff P. W., Pastan I. H., Anderson W. F., Gottesman M. M. Expression of a human complementary DNA for the multidrug resistance gene in murine hematopoietic precursor cells with the use of retroviral gene transfer. J Natl Cancer Inst. 1990 Aug 1;82(15):1260–1263. doi: 10.1093/jnci/82.15.1260. [DOI] [PubMed] [Google Scholar]
- Miura O., D'Andrea A., Kabat D., Ihle J. N. Induction of tyrosine phosphorylation by the erythropoietin receptor correlates with mitogenesis. Mol Cell Biol. 1991 Oct;11(10):4895–4902. doi: 10.1128/mcb.11.10.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y., Miura O., Ihle J. N., Aoki N. Activation of the mitogen-activated protein kinase pathway by the erythropoietin receptor. J Biol Chem. 1994 Nov 25;269(47):29962–29969. [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S. O., Kittler E. L., Ramshaw H. S., Quesenberry P. J. Ex vivo expansion of murine marrow cells with interleukin-3 (IL-3), IL-6, IL-11, and stem cell factor leads to impaired engraftment in irradiated hosts. Blood. 1996 Jan 1;87(1):30–37. [PubMed] [Google Scholar]
- Petzer A. L., Hogge D. E., Landsdorp P. M., Reid D. S., Eaves C. J. Self-renewal of primitive human hematopoietic cells (long-term-culture-initiating cells) in vitro and their expansion in defined medium. Proc Natl Acad Sci U S A. 1996 Feb 20;93(4):1470–1474. doi: 10.1073/pnas.93.4.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebel V. I., Dragowska W., Eaves C. J., Humphries R. K., Lansdorp P. M. Amplification of Sca-1+ Lin- WGA+ cells in serum-free cultures containing steel factor, interleukin-6, and erythropoietin with maintenance of cells with long-term in vivo reconstituting potential. Blood. 1994 Jan 1;83(1):128–136. [PubMed] [Google Scholar]
- Reid L. H., Shesely E. G., Kim H. S., Smithies O. Cotransformation and gene targeting in mouse embryonic stem cells. Mol Cell Biol. 1991 May;11(5):2769–2777. doi: 10.1128/mcb.11.5.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shesely E. G., Kim H. S., Shehee W. R., Papayannopoulou T., Smithies O., Popovich B. W. Correction of a human beta S-globin gene by gene targeting. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4294–4298. doi: 10.1073/pnas.88.10.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol L., Luhovy M., Guan Y., Prchal J. F., Semenza G. L., Prchal J. T. Primary familial polycythemia: a frameshift mutation in the erythropoietin receptor gene and increased sensitivity of erythroid progenitors to erythropoietin. Blood. 1995 Jul 1;86(1):15–22. [PubMed] [Google Scholar]
- Sorrentino B. P., Brandt S. J., Bodine D., Gottesman M., Pastan I., Cline A., Nienhuis A. W. Selection of drug-resistant bone marrow cells in vivo after retroviral transfer of human MDR1. Science. 1992 Jul 3;257(5066):99–103. doi: 10.1126/science.1352414. [DOI] [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961 Feb;14:213–222. [PubMed] [Google Scholar]
- Traycoff C. M., Cornetta K., Yoder M. C., Davidson A., Srour E. F. Ex vivo expansion of murine hematopoietic progenitor cells generates classes of expanded cells possessing different levels of bone marrow repopulating potential. Exp Hematol. 1996 Feb;24(2):299–306. [PubMed] [Google Scholar]
- Williams D. A., Hsieh K., DeSilva A., Mulligan R. C. Protection of bone marrow transplant recipients from lethal doses of methotrexate by the generation of methotrexate-resistant bone marrow. J Exp Med. 1987 Jul 1;166(1):210–218. doi: 10.1084/jem.166.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witthuhn B. A., Quelle F. W., Silvennoinen O., Yi T., Tang B., Miura O., Ihle J. N. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993 Jul 30;74(2):227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- de la Chapelle A., Träskelin A. L., Juvonen E. Truncated erythropoietin receptor causes dominantly inherited benign human erythrocytosis. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4495–4499. doi: 10.1073/pnas.90.10.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]




