Abstract
Maternal exposure to significant prenatal stress can negatively affect infant neurobiological development and increase the risk for developmental and health disturbances. These effects may be pronounced in low SES and ethnic minority families. We explored prenatal partner support as a buffer of the impact of prenatal stress on cortisol reactivity of infants born to low-income Mexican American women. Women (N=220; age 18–42; 84% Spanish-speaking; 89% foreign born; modal family income $10,000–$15,000) reported on economic stress and satisfaction with spousal/partner support during the prenatal period (26–38 weeks gestation), and infant salivary cortisol reactivity to mildly challenging mother-infant interaction tasks was assessed at women’s homes at six weeks postpartum. Multilevel models estimated the interactive effect of prenatal stress and partner support on cortisol reactivity, controlling for covariates and potential confounds. Infants born to mothers who reported high prenatal stress and low partner support exhibited higher cortisol reactivity relative to those whose mothers reported high support or low stress. The effects did not appear to operate through birth outcomes. For low-income Mexican American women, partner support may buffer the impact of prenatal stress on infant cortisol reactivity, potentially promoting more adaptive infant health and development.
Keywords: prenatal stress, infant cortisol, partner support
The foundations for lifespan health begin in the prenatal environment. For low-income and ethnic minority women, the prenatal period can be associated with a number of physical, emotional, social, and financial stressors (Bloom et al., 2013; Bermúdez-Millán et al., 2011; Dominguez et al., 2008). Stressful experiences have negative implications for mothers’ wellbeing, but can also have deleterious consequences for fetal and infant development. Prenatal stress can be conceptualized as a teratogen, a factor that can directly affect the developing fetus and is capable of interfering with normal development (DiPietro, 2012). Effects of prenatal stress have been documented on a wide range of infant outcomes including low birth weight, pre-term birth, dysregulated neurobiological activity, and behavioral, emotional, and neurodevelopmental disturbances (Lazinski, Shea & Steiner, 2008). The impact of prenatal stress can persist into childhood, adolescence, and adulthood (Talge, Neal, & Glover, 2007).
The development of infant physiological stress response systems represents a potential mechanism linking prenatal exposures to infant health and developmental outcomes. The hypothalamic-pituitary-adrenocortical (HPA) axis, a primary mediator of the stress response, regulates the production and release of the glucocorticoid hormone cortisol. Scholars investigating associations between maternal stress and infant birth, health, and developmental outcomes have described a “fetal programming” hypothesis, wherein high stress during pregnancy results in an adverse intrauterine environment that can negatively impact infant growth and contribute to a variety of negative health effects across the lifespan. In part, prenatal stress may affect fetal development via stress-related activation of the maternal HPA axis, resulting in transmission of cortisol across the placenta (Reynolds, 2013). High maternal stress and resulting excess fetal cortisol exposure is believed to disrupt the normal adaptive function of coregulatory maternal stress systems (nervous, endocrine, and immune) that support healthy fetal development (Coussons-Read, 2012). Interference in the normal development of the HPA axis may disrupt subsequent capacities for physiological and behavioral regulation (Weinstock, 2005). O’Connor et al (2013) reported that higher cortisol exposure in utero was associated with dysregulated cortisol reactivity in infancy.
Deleterious effects on birth outcomes represent a potential mechanism through which prenatal stress may be linked to HPA regulation and later infant health and developmental outcomes, although evidence for such a pathway is limited. Reynolds (2013) outlined a theoretical model in which low birth weight mediates the effects of maternal distress on child HPA dysregulation, which in turn increases the risk of obesity and metabolic disorders later in life. A fairly consistent literature identifies prenatal stress, depression, and anxiety as risk factors for preterm birth and low birth weight (Dunkel Schetter, 2011). However, the role of birth outcomes in the relation of prenatal stress to infant HPA axis activity is less clear. Field et al (2004) reported that low birth weight and prematurity did not explain the poorer neonatal outcomes (i.e., less habituation, less orientation, poorer motor skills, and decreased autonomic stability) observed in infants exposed to maternal prenatal depression.
The impact of prenatal stress may be accentuated in low-income Mexican or Mexican American families. Hispanic women commonly face stressors such as discrimination, occupational barriers, language barriers, and deportation fears (Cervantes et al., 1991). Compared to Caucasian families, Hispanic families are more than twice as likely to live below the poverty level ($21,954 for a family of 4; 22.7% of Hispanic families compared to 9.3% of Caucasian respondents; US Census Bureau, 2012), and considerable research documents deleterious effects of poverty on birth outcomes, child stress physiology, and child health and developmental outcomes (Blair et al., 2011; Evans, 2003). However, despite high traditional risk factors, Hispanic immigrant women tend to have more positive birth outcomes compared to African American and other ethnic minority groups in the US (i.e., lower incidence of low birth weight infants, fewer preterm births), and comparable or lower risk for low birth weight compared to non-Hispanic white groups (Flores et al., 2012; McGlade et al., 2004). Better than expected birth outcomes may reflect a Healthy Migrant effect, which posits that health benefits are due to better mental and physical health of those who choose to emigrate compared to those who stay. Alternatively, the “Latina Paradox” hypothesizes that Latinas in the US experience protective cultural factors that promote resilience in the presence of stressful conditions (McGlade et al., 2004), potentially translating into better birth outcomes. Powerful culture-specific values such as strong family and kinship ties and a high value placed on the maternal role may provide protective emotional and practical support for pregnant Latina women.
Support from a romantic partner or spouse is a particularly salient protective factor for pregnant women (Rini et al., 2006). For low-income Mexican American women, strong cultural values relating to the family may enhance the protective effects of support during pregnancy (Bender & Castro, 2000; Diaz et al., 2007; Campos et al., 2008). Further, partner support during pregnancy may promote subsequent infant well-being. More perceived support from a partner has been associated with reduced emotional distress among mothers and distress to novelty among their six to eight week infants (Stapleton et al., 2012). With regards to infant cortisol, existing studies have predominantly focused on postpartum partner support, with relatively consistent findings that partner relationship functioning can significantly affect child cortisol (e.g., Pendry & Adam, 2007).
Many of these studies have been limited by cross-sectional designs that preclude the ability to determine the directionality of influence. DiPietro (2012) notes a number of related methodological issues in the inference of causality in research on the implications of maternal stress for infant outcomes. These issues include a reliance on subjective maternal reports for both predictor (e.g., stress) and outcome (e.g., child health), shared genetic contributions, and potential confounding by other prenatal and postnatal exposures and behaviors. For example, despite being unique psychological constructs, maternal depression and stress are generally significantly correlated, creating a challenge to empirically separate their effects. Further, effects of prenatal stress are difficult to disentangle from effects due to stress in the postpartum environment. In short, while intriguing evidence supports hypothesized causal effects of prenatal stress and support on infant development, longitudinal research beginning in the prenatal period that addresses potential confounding factors is needed to better understand the transmission of risk and protective influences from mother to infant.
Existing literature points to negative influences of prenatal stress and positive influences of support on infant health and developmental outcomes, but little is known about the extent to which partner support may buffer the impact of stress on infant outcomes, particularly in ethnic minority populations. The present study investigates the interactive impact of prenatal stress and partner support on infant salivary cortisol reactivity in Mexican American women and infants. Due to the extremely low-income of our sample, we focused attention on economic hardship as our measure of prenatal stress, or the stress experienced by mothers when available resources are not adequate for appraised needs (Barrera et al., 2001). Such a measure recognizes that objective indicators of poverty, such as family income, do not adequately capture the psychological sense of disparity at the heart of stress. We hypothesized that higher prenatal support from the spouse/romantic partner would buffer the impact of economic stress on infant cortisol reactivity at six weeks postpartum, such that higher prenatal stress would predict elevated cortisol only for infants whose mothers also reported lower partner support. We also examined potential confounding factors such as maternal postpartum cortisol reactivity, prenatal and postpartum maternal depressive symptoms, and postpartum partner support. Finally, we explored birth outcomes as a potential intermediate pathway linking prenatal stress and support to infant cortisol reactivity.
Method
Participants
Participants included 220 women (mean age = 28.2, SD = 6.4) and their infants. Data were collected at two time points: prenatal and six weeks postpartum. Women were recruited from a hospital-based prenatal clinic that serves low-income women from the surrounding community. Eligibility criteria included: 1) self-identification as Mexican or Mexican American, (2) fluency in English or Spanish, (3) age 18 or older, (4) low-income status (family income below $25,000 or eligibility for Medicaid or Federal Emergency Services coverage for the birth), and (5) no prenatal evidence of a serious infant health or developmental problem. Demographic characteristics are displayed in Table 1.
Table 1.
Sample Demographics
| Prenatal marital status – N (%) | |
| Married or Living w/Partner | 196 (89%) |
| Not married | 24 (11%) |
| Education – N (%) | |
| Did not attend school | 2 (1%) |
| 1 through 8 years of school | 63 (29%) |
| Some high school completed | 73 (33%) |
| High school graduate/GED | 56 (25%) |
| Some college, vocational or technical school | 9 (4%) |
| Associates/Vocational/Technical School | 4 (2%) |
| College degree (BS/BA) or Above | 13 (6%) |
| Maternal Age – Range; M (SD) | 18–42; 28.2 (6.4) |
| Number of biological children – Range; M (SD) | 1–8; 2 (1.6) |
| Country of birth – N (%) | |
| U.S. | 25 (11%) |
| Mexico | 193 (88%) |
| Other | 2 (1%) |
| Age of immigration to U.S. – Range; M (SD) | 0–35; 16.4 (7.5) |
| Family Income N (%) | |
| ≤ $5,000 | 24 (11%) |
| $5,001 – $10,000 | 41 (19%) |
| $10,001 – $15,000 | 61 (28%) |
| $15,001 – $20,000 | 26 (12%) |
| $20,001 – $25,000 | 28 (13%) |
| $25,001 – $30,000 | 13 (6%) |
| $30,001 – $40,000 | 13 (6%) |
| ≥ $40,000 | 10 (5%) |
| Language spoken in the home - N (%) | |
| English | 36 (16%) |
| Spanish | 184 (84%) |
| Birth weight (g) – Range; M (SD) | 1190–4935; 3404 (464) |
| Low birth weight (<2500 g) - N (%) | 2 (1%) |
| Gestational age (wks) – Range; M (SD) | 26–42; 39.3 (1.5) |
| Preterm birth (< 37wks) – N (%) | 8 (3.6%) |
| APGAR (5 min) – Range; M (SD) | 5–10; 8.9 (.49) |
| Infant cortisol (non-transformed) – M (SD) | |
| T0 (pre interaction tasks) | .196 (.19) |
| T1 (immediately post interaction tasks) | .308 (.34) |
| T2 (20-min post interaction tasks) | .332 (.32) |
| T3 (40-min post interaction tasks) | .274 (.32) |
Recruitment and retention
During prenatal care appointments, women were approached by a female, bilingual interviewer who explained the study and assessed eligibility. Of women who were eligible, 56% agreed to a home visit between 26–38 weeks gestation (mean 35.4 weeks, SD = 2.8) during which informed consent was obtained. Of the 263 women who consented to the study at the prenatal visit, 254 (97%) completed a six week visit (two women dropped out of the study after the prenatal visit, one could not be contacted, and six could not be scheduled within one week of the baby’s six week birthday). The current analyses only include those who reported being married or in a romantic relationship at the prenatal visit (n = 220).
Procedure
All interviews were conducted at participants’ homes in their choice of Spanish (85%) or English (15%). Due to variability in literacy, survey questions were read aloud to all participants. Women were given visual aids with written and graphic descriptions of item response formats. The 6-week interview was scheduled within one week of the infant’s chronological age of six weeks, but for infants born at less than 37 weeks gestation (n=8)1, the interview was conducted at six weeks corrected age. At the six week interview, salivary cortisol samples were collected from mothers and infants before and after video-recorded mother-infant interaction tasks that began approximately 30 minutes after arrival in the home. To aid in sample retention, interviews were scheduled at the mother’s convenience; the first cortisol was collected between 8:30 AM and 5 PM (median 12:00 PM). The total duration of the home visits was approximately 2 hours. Women were compensated $75 and small gifts (e.g., bath oils) at the prenatal interview, and $50 and small gifts (e.g., bibs) at the six week interview.
Measures
Partner support
At the prenatal visit, women completed measures assessing two components of partner support: prenatal relationship satisfaction/emotional support, and expectations for tangible and emotional support after the birth. First, a seven item version of the Dyadic Adjustment Scale (Spanier, 1989) measured women’s satisfaction in their relationships and emotional support from their spouse/romantic partner. Previous research supports the validity of the DAS for Hispanic Americans (Youngblut, Brooten, & Menzies, 2006). Sample items include “In general, how often do you think things between you and your partner are going well?” and “How often do you confide in your partner?” One additional question asked: “Overall, how satisfied are you with the support you get from your partner?” Response options ranged from “1” (never/not at all) to “5” (all the time/extremely). A sum score was calculated (Cronbach’s α = .79). Second, the partner support subscale of the Prenatal Expectations Scale for Mexican Americans (PES-MA; Gress-Smith et al., 2013) provided a six item measure of expectations for partner support after the birth (Cronbach’s α = .93). Women also completed the PES-MA at six weeks postpartum, reworded to assess actual experiences of support (α = .93). Developed specifically for low-acculturated Mexican American women, the PES-MA is a culturally-relevant measure of women’s prenatal expectations and postpartum experiences. Sample items include “Your spouse/partner will be there for you when you need him”, and “Your spouse/partner will help take care of the baby”. Items were rated on a five point scale from “1” (Not at all) to “5” (Completely) and a sum score was calculated. Higher scores represent higher expectations for postpartum support (prenatal assessment), or higher experiences of support (postpartum assessment). The DAS and PES-MA scales were highly correlated (R = .66, p < .001), and a composite score for partner support was calculated by summing the z-scores for each scale.
Prenatal Economic Stress
Mothers were administered the Economic Hardship Scale (EHS; Barrera et al., 2001; Cronbach’s α = .86). The 20-item EHS was developed for low-income families as a subjective measure of the psychological sense of hardship. It is advantageous over objective measures such as family income due to difficulties accurately quantifying income in populations with unpredictable income (e.g., day laborers, irregular work hours), as well as problems interpreting the adequacy of a given income level based on unique life circumstances. Instead, the EHS assesses the stress experienced when available resources are not adequate for appraised needs. Evidence for measurement equivalence and validity with Mexican American English and Spanish-speaking adults is detailed in Barrera et al. Four subscales include: Financial Strain, Inability to Make Ends Meet, Not Enough Money, and Economic Adjustments. Subscale scores were converted to z-scores and summed. Higher scores indicate more economic stress.
Depressive symptoms
The 21-item Edinburgh Postnatal Depression Scale (Cox, Holden, & Sagovsky, 1987) was given prenatally and at six weeks postpartum (prenatal α = .86; six week α = .86). The EPDS has been validated in English (Cox et al.) and Spanish (Garcia-Esteve et al., 2003). Higher scores reflect higher depressive symptomatology.
Mother-infant Interaction Task
A series of five structured video-recorded mother-infant observational episodes, selected to elicit mild frustration for the mother and the infant, were conducted. The infant was in an infant seat with the mother seated directly across from him/her. The episodes included 1) Free play (5 mins), 2) Arm Restraint (2 mins; Goldsmith & Rothbart, 1993), 3) Soothing (3 mins), 4) Teaching task (5 mins; mothers are asked to “teach” her child a task from the Bayley Scales of Infant Development II (Bayley, 1993) that reflects a skill 1–2 months beyond the infant’s capabilities, and 5) Peek-a-boo (3 mins). The total duration of the tasks was 25–30 minutes.
Cortisol sampling
Saliva samples were obtained from infants and mothers at the 6-week postpartum home visit. Samples were collected immediately prior to the first task (T0), and at 0 (T1), 20 (T2), and 40 (T3) minutes after all the tasks were complete, using Salivette (mothers) and Sorbette (infants) sampling devices (Sarstedt, Rommelsdorf, Germany; Salimetrics Inc, State College, PA). Oral stimulants were not used, and mothers were asked not to feed the baby for 30 minutes prior to collection. Saliva samples were frozen and mailed to Salimetrics Inc where they were assayed for free cortisol. Missing infant cortisol data were due to the following: significant interference in assays (n=3), insufficient saliva (n=1), infant illness (n=1), and problems in the home (e.g., no electricity) that prevented the interaction task (n=3). Cortisol values were log-transformed (base 10) to correct for deviations from normality. Maternal postpartum cortisol values were used to calculate area under the curve with respect to ground (AUCg) as a measure of total cortisol across the task, and area under the curve with respect to increase (AUCi) as a measure of cortisol reactivity to the task.
Selection of control variables
We considered three types of control variables for evaluation: confounds, covariates, and intermediate variables. Cortisol reactivity can be influenced by a wide range of factors not expected to be related to the independent variables (IV; economic stress, partner support), including time of day, infant waking, or last infant feeding; breastfeeding status; temperature in the home; and mother’s use of medications, caffeine, or tobacco. These variables were evaluated as potential covariates. Covariates do not explain relations between the IVs and the dependent variable (DV) because they are not in a causal pathway between the IVs and the DV (MacKinnon & Luecken, 2008). It is not necessary to control for covariates, but doing so may increase efficiency in estimation (Sauer et al., 2013). We selected covariates by empirical evaluation of their relation to infant cortisol. Although covariates are not expected to change the relation between the IVs and the DV, we elected to take a conservative approach by analyzing models both with and without covariates.
Confounding variables are factors that are associated with both the IV and the DV, introducing bias into estimation of hypothesized causal relations (MacKinnon & Luecken, 2008). Potential confounds were selected by theoretical identification of variables that may be associated with prenatal support, economic stress, and infant cortisol, potentially changing or explaining the relations between them. Potential confounds included: household income, maternal age, education, number of children or country of birth; weeks pregnant at prenatal visit; maternal postpartum cortisol levels and reactivity; and prenatal depression. For example, lower household income or lower education may be associated with both higher prenatal economic stress and dysregulated infant cortisol (Barrera et al., 2001; Blair et al., 2011). Dysregulated maternal cortisol may be associated with a stressful environment and may be a marker of genetic risk to infants. Because prenatal exposures that persist into the postpartum environment complicate efforts to isolate the impact of prenatal factors, we also evaluated maternal depression and partner support at six weeks postpartum2. Potential confounds were evaluated empirically – those related to both the IV and DV were included in final models.
Finally, variables on an intermediate pathway between the IV and DV that may explain the effects of the IV on the DV were considered. These included birth outcomes obtained from medical records at the hospital of birth (birth weight, gestational age, five minute APGAR). For example, a high stress prenatal environment may increase the risk of low birth weight, which may explain six week infant cortisol responses.
Data Analyses
Preliminary analyses
Zero-order correlations between study variables are shown in Table 2. Analyses of outliers identified eight infants with high cortisol values (>4 SD from the sample mean) who were removed from analyses3. We first considered potential covariates. Time of day of sampling was near-significant in predicting the linear slope of cortisol, p = .063. Infants who were breastfed had a higher intercept, p = .001 and increasing linear slope, p = .027. A longer time since the last feeding predicted higher cortisol intercept, p= .002. No other potential covariates were significant predictors of any measure of cortisol activity. Therefore, time of day, breastfeeding status, and time of last feeding were selected as covariates.
Table 2.
Zero-order correlations
| Partner Support |
Economic Stress |
Infant Cortisola | ||||
|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | |||
| Prenatal Partner Support | 1.0 | −.220** | .130 | −.080 | .155* | −.109 |
| Prenatal Economic Stress | −.220** | 1.0 | −.093 | .093 | .102 | −.001 |
| Birth outcomes | ||||||
| Gestational age (wks) | −.116 | .013 | −.033 | .064 | .015 | .014 |
| Birth weight (g) | −.175* | .068 | −.006 | −.032 | −.040 | −.051 |
| 5-min APGAR | −.008 | .149* | −.032 | −.020 | −.057 | −.022 |
| Mother demographics | ||||||
| Age | .041 | .225** | .056 | −.096 | .003 | .028 |
| Education (yrs) | .042 | −.293** | .187** | −.044 | −.035 | .127 |
| Number of children | −.019 | .319** | −.009 | .076 | .021 | −.066 |
| Country of birthb | .007 | .132† | .031 | .137 | .082 | .091 |
| Context | ||||||
| Prenatal depression | −.429** | .307** | −.058 | .049 | .116 | .151† |
| Postpartum depression | −.280** | .156* | −.086 | .055 | .093 | .129 |
| Postpartum support | .680** | −.101 | .119† | −.058 | −.107 | −.092 |
| Breastfeeding statusc | −.097 | .019 | .026 | .140† | .126 | .088 |
| Time of dayd | −.040 | .044 | −.072 | .030 | .147† | .014 |
| Time of last feedinge | −.036 | −.074 | .189** | .212** | .119 | .120 |
Raw (non-logtransformed) cortisol values
coded 0 = US; 1 = Mexico
coded 0 = bottle-feeding only; 1 = partial or exclusive breast-feeding
calculated in minutes since midnight
coded 1 = less than an hour ago; 2 = 1–2 hours ago; 3 = 2–3 hours ago; 4 = 3–4 hour ago; 5 = more than 4 hours ago
p < .05;
p < .01;
p < .10
Next, we evaluated potential confounds: older maternal age, lower household income, and a greater number of biological children were associated with higher economic stress, but these variables were not associated with partner support, the interaction of partner support and economic stress, or any measure of infant cortisol (see Table 2). Mother’s country of birth and number of weeks pregnant at the prenatal visit were not associated with economic stress, partner support, the interaction term, or any measure of infant cortisol. Maternal education was negatively correlated with economic stress (r = −.29, p < .01), was predictive of infant cortisol intercept (estimate = .017; p = .024), and was near significant for the linear slope (estimate = −.020; p = .063) and quadratic response (estimate = .006; p = .059), such that infants whose mothers had less education had a lower baseline cortisol and greater reactivity than those with higher educated mothers. Higher prenatal depressive symptoms were correlated with higher economic stress and lower partner support, but did not significantly predict any measure of cortisol activity (intercept p = .38; linear slope p = .09; quadratic p = .21)).
Potential confounds in the postpartum environment were also considered. Maternal postpartum AUCi and AUCg were not correlated with economic stress, partner support, or the interaction term (p’s > .13). Maternal AUCg and AUCi were also not associated with infant cortisol reactivity or total cortisol (p’s > .22). Postpartum maternal depressive symptoms were correlated with prenatal support and economic stress (see Table 2), and predicted infant cortisol intercept (estimate = −.027, p = .016) and linear slope (estimate = .021, p = .036). Postpartum support was positively correlated with prenatal support and predicted infant cortisol intercept (estimate = .026, p = .009), linear slope (estimate = −.022, p = .009) and quadratic response (estimate = .004, p = .035). Therefore, we controlled for maternal education, postpartum depressive symptoms, and partner support in final models.
Primary analyses
We used multilevel linear modeling (MLM) to evaluate the influence of prenatal stress and partner support on infant cortisol response to the interaction tasks. MLM is valuable for these data because it handles repeated cortisol measures nested within infant, and because it allows for the simultaneous modeling of possible differences in change in cortisol over time across persons and does not limit data analysis to only complete cases. The data were modeled using SPSS MIXED, with the repeated cortisol measures forming the within-person dimension (ICC = .43). A variable “time” was created to reflect within-person cortisol sampling order, coded so that “0” = baseline/intercept, “1” = immediately after mother-infant tasks, “2” = 20 minutes post tasks, and “3” = 40 minutes post tasks. The relation of this Level 1 variable with the DV was included as a random linear and random quadratic (time × time) effect. Partner support, economic stress, and the interaction of partner support and economic stress served as Level 2 (between person) IVs. Continuous variables were centered at the sample mean. Pseudo R2 was calculated as a measure of effect size for the quadratic term (stress × support × time × time), using the covariance parameter estimates for the model without the interaction terms relative to models with the interaction terms: the pseudo R2 represents the change in variance explained by the addition of the interaction of prenatal stress and support to the model.
Models were first estimated with only the IVs. Next, models were estimated with covariates (time of day, breastfeeding status, and time of last feeding). Maternal education, postpartum maternal depressive symptoms, and postpartum support were then included as control variables. Finally, we evaluated birth outcomes as potential intermediate variables.
Results
Infant cortisol response to the interactions
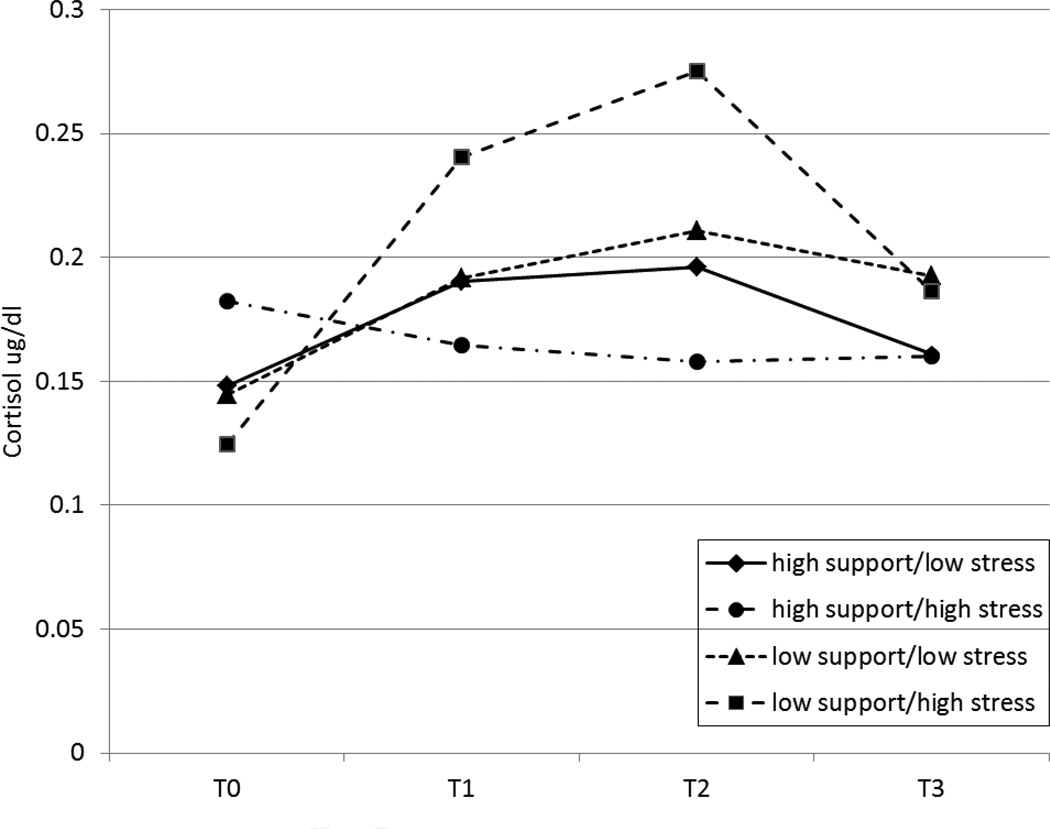
Multilevel models were estimated predicting the repeated measures of infant cortisol from prenatal partner support, economic stress, and the interaction of partner support and economic stress. Results are shown in Table 3. There was a significant quadratic pattern in the cortisol response, F(1,184)= 27.0, p < .001, β = −.053, 95% CI [−0.073, −0.033], indicating significant infant cortisol reactivity to the tasks across the sample. The interaction term (stress by support) was significantly associated with the linear slope, p = .003, and the quadratic pattern of response, p = .003, pseudo R2 = .17 (see Figure 1). There was also a significant effect of partner support for the linear slope, p <.001, and quadratic pattern of response, p = .001, indicating higher reactivity among infants whose mothers reported less prenatal support.
Table 3.
Multilevel model prediction of infant cortisol response to mother-infant task by prenatal economic stress and prenatal partner support
| Intercept β (CI) | Linear β (CI) | Quadratic β (CI) | |
|---|---|---|---|
| Model 1: no covariates | |||
| Economic Stress | −.001 [−.019, .016] | .011 [−.012, .035] | −.004 [−.011, .003] |
| Partner Support | .029 [−.002, .060] | −.079 [−.120, −.039]** | .021 [.008, .033]** |
| Partner Support × Economic Stress | .008 [−.003, .019] | −.024 [−.040, −.008]** | .007 [.003, .012]** |
| Model 2: controlling for covariatesa | |||
| Economic Stress | −.001 [−.016, .018] | .009[−.015, .032] | −.003 [−.010, .004] |
| Partner Support | .032 [.001, .062]* | −.075 [−.116, −.035]** | .020 [.008, .032]** |
| Partner Support × Economic Stress | .011 [−.001, .023] | −.024 [−.039, −.008]** | .007 [.002, .012]** |
| Model 3: controlling for potential confoundsb | |||
| Maternal education | .019 [.003, .035]* | −.019 [−.040, .002] | .006 [−.001, .012] |
| Postpartum depression | −.008 [−.018, .002] | .009 [−.004, .023] | −.002 [−.006, .003] |
| Postpartum support | .0001 [−.011, .011] | .005 [−.010, .020] | −.002 [−.007, .002] |
| Economic Stress | .007 [−.011, .026] | .002 [−.022, .027] | −.001 [−.009, .006] |
| Partner Support | .024 [−.018, .065] | −.085 [−.140, −.030]** | .025 [.008, .042]** |
| Partner Support × Economic Stress | .008 [−.003, .019] | −.024[−.040, −.009]** | .007 [.002, .012]** |
covariates included time of day, time of last feeding, and breastfeeding status.
confounds included maternal education, postpartum depressive symptoms, and postpartum partner support
p < .05
p < .01
Figure 1.
Prenatal stress and partner support interact to predict infant cortisol reactivity1
1 For display purposes, “low” support and stress were set at 1 SD below the sample means; “high” support and stress were set at 1 SD above the sample means. T0= pre-task; T1 = immediately post-tasks, T2 = 20 mins post-tasks; T3 = 40 mins post-tasks. Predicted log-transformed cortisol values were subjected to anti-log-transformation in order to display cortisol values in an interpretable metric.
Exploratory analyses were conducted to further understand the nature of the interaction by dividing the sample into high and low economic stress groups using a median split. Within the low stress group, prenatal partner support had no effects on infant cortisol intercept, slope, or quadratic pattern of response (all p’s > .58). However, within the high stress group, partner support was highly significant in predicting cortisol intercept (p = .02), linear slope (p < .001), and quadratic response (p < .001).
Additional exploratory analyses addressed the graphical pattern of results which suggested that infants whose mothers reported high stress and high support may have had elevated baseline cortisol and attenuated reactivity. However, prenatal support, economic stress, and their interaction did not predict baseline cortisol (p’s > .14). When the sample was divided into high and low support groups using a median split, economic stress only predicted reactivity for infants in the low support (p = .015) group (high support p = .35).
Controlling for covariates
The second model controlled for covariates including time of day, time of last feeding, and breastfeeding status. The quadratic pattern of response remained significant for partner support p = .002, and the interaction of partner support × economic stress, p = .005.
Controlling for potential confounds
Finally, analyses were repeated controlling for potential confounds (Model 3). The quadratic pattern of results remained significant after controlling for maternal education, postpartum depressive symptoms, and postpartum partner support (economic stress × prenatal partner support: p = .003; prenatal partner support: p = .004). Postpartum partner support did not predict any measure of cortisol response (all p’s > .34). Postpartum depressive symptoms also did not predict any measure of cortisol response (p’s > .11). Maternal education remained a significant predictor of the intercept (p = .018), and was near significant for the linear slope (p = .075) and quadratic response (p = .071).
Evaluation of birth outcomes as intermediate variables
The interaction of prenatal economic stress and partner support did not predict any of the birth outcomes (birth weight: p = .36; APGAR: p = .48; gestational age: p = .33). Contrary to predictions, higher economic stress predicted a higher APGAR score (B = .028, p = .029), and higher prenatal support predicted lower birth weight (B = −40.8, p = .048).
Finally, we entered birth outcomes as predictors in a model with economic stress, partner support, and the interaction of economic stress and partner support, covarying for time of day, breastfeeding status, and time of last feeding. None of the birth outcomes were significantly related to any measure of infant cortisol (all p’s > .19), and did not significantly affect any of the relations of economic stress and partner support to infant cortisol.
Discussion
The present study examined the interactive influence of prenatal stress and partner support on infant salivary cortisol reactivity in very low-income Mexican American families. Previous literature, conducted predominantly with Caucasian samples, has focused primarily on main effects of prenatal stress on infant neuroendocrine and developmental outcomes. We hypothesized that partner support during pregnancy would buffer against the negative impact of economic stress on infants’ cortisol reactivity at six weeks of age. Our results suggest that support from a romantic partner during pregnancy may play an important protective role in the development of infants’ stress reactivity systems for pregnant Mexican American women experiencing considerable stress. Specifically, we found that infants evidenced higher cortisol reactivity when mothers reported high economic stress during pregnancy coupled with low partner support relative to women reporting higher partner support or lower economic stress.
Consistent with our results, strong emphases on family ties and higher social support are theorized to be mechanisms through which the Latina paradox is activated. Because our sample only included Mexican American women, we cannot generalize our results to majority groups or other ethnic minority groups. Given the unique cultural characteristics of our sample, the powerful buffering effects of partner support may be unique among the Latina community. Future research that attempts to replicate these results with other ethnic groups can provide valuable insight into the extent to which these processes are consistent across other cultures. Studies suggest that infant health benefits associated with the Latina paradox may dissipate for more acculturated Latinas (D’Anna-Hernandez et al., 2012). Given the number of first generation immigrants and low acculturative status of our sample, it will also be important to evaluate whether the results hold in later generation or more acculturated families.
Prenatal partner support appeared not merely to act as buffer against the negative influence of prenatal stress on infants’ cortisol reactivity, but also to exert a unique protective effect over and above the impact of prenatal stress. This effect held even after controlling for postpartum partner support, suggesting that observed differences in cortisol reactivity may be traced to the unique influences of partner support during pregnancy. Although most studies investigating the role of partner support on infant outcomes have focused on postpartum support, some have found similar evidence that prenatal partner support may exert unique influences. For example, Feldman and colleagues (2000) found that support during pregnancy predicted as much as 31% of the variance in fetal growth. Our study is unique in evaluating the impact of prenatal support while controlling for support received in the postpartum period.
Unlike previous studies, we did not find main effects of prenatal stress on infant cortisol reactivity. Rather, our results support a risk and protective model in which higher economic stress during pregnancy was associated with heightened infant cortisol reactivity only when combined with low partner support. The low incomes of all participants in the sample may have limited the power to detect main effects of stress if it can be assumed that all participants were experiencing elevated stress relative to higher SES populations. Alternatively economic stress may not exert the same impact on infant cortisol activity as other forms of prenatal stress. However, our results suggest that in the face of considerable economic stressors, supportive resources in the prenatal period are an important buffer of the impact on infant cortisol. These results may be useful for the development of interventions aimed at the promotion of partner support during pregnancy for women with few economic resources.
The graphical pattern of results shown in Figure 1 was such that infants whose mothers reported high economic stress coupled with high partner support appeared to show attenuated cortisol reactivity. This pattern of results was not statistically significant: exploratory analyses did not support significant differences in baseline cortisol or reactivity among infants whose mothers reported high support coupled with either high or low stress. However, similar to theories of stress inoculation (e.g., Lyons et al., 2009), it is interesting to speculate about the potential for strong support in the face of high stress to promote adaptive maternal self-regulation, which may influence infant cortisol reactivity via biological or behavioral coregulatory mechanisms. Alternatively, attenuated reactivity may be an early marker of HPA axis dysregulation associated with early life stress (e.g., Gunnar & Quevedo, 2007). Assessment of cortisol reactivity, health, and development at later ages will help determine whether attenuated reactivity is present, and if so, whether it is adaptive or maladaptive.
An additional aim of our study was to evaluate whether prenatal influences on infant cortisol reactivity operated through effects on birth outcomes. However, partner support, prenatal stress, and their interaction did not predict any of the birth outcomes, and none of the birth outcomes were associated with cortisol reactivity. Our results contrast with previous findings that prenatal social support predicts better birth outcomes (Collins et al., 1993; Feldman, et al., 2000) and prenatal stress predicts worse birth outcomes (Entringer, Buss, & Wadhwa, 2010). Differences in sample characteristics may help explain this discrepancy. Our sample is unique because it only included low-acculturated Hispanic families, a population shown to have healthier birth outcomes than would be expected given elevated socioeconomic risk (e.g., D’Anna-Hernandez et al., 2012). Our finding that birth outcomes did not mediate the relation of prenatal stress and support to infant cortisol reactivity may not generalize to other ethnic groups, more acculturated Mexican Americans, or higher SES populations. It is also possible that more highly stressed women, for whom effects on birth outcomes may have been stronger, declined to participate in the study. Finally, our sample size may not have provided enough power to detect small effect sizes in the relation of prenatal stress to birth outcomes.
The observed effects of prenatal stress and partner support on infant cortisol reactivity may have acted through a third variable not considered here. For example, parenting and the parent-infant bond are potential intermediary pathways. Harsh or neglectful parenting styles have been associated with insecure infant attachment, and insecure attachment has been linked to elevated cortisol reactivity (e.g., Bernard & Dozier, 2010). Women who experience high stress and low support during the prenatal period may be less effective at coregulating their infant’s reactivity during the mildly stressful interaction task. Aspects of the postnatal environment, such as maternal depression, may also induce epigenetic variation leading to altered biological responses (Monk, Spicer, & Champagne, 2012). Although we found no evidence that postpartum depression or support explained the effects of prenatal experiences on infant cortisol reactivity, it will be important for future studies to closely examine additional mechanistic pathways.
There are several limitations to the analyses. First, the study only included low-income participants who self-identified as Mexican or Mexican American, the majority of whom were foreign-born, Spanish-speaking, and low in acculturation. Although this sampling strategy provides a critical contribution to understanding the health of a high risk and understudied population, our results cannot be generalized to other racial/ethnic groups, or more acculturated Mexican Americans. Similarly, our analyses only included women who reported a current romantic partner at the prenatal interview; results should not be generalized to women without romantic partners. Second, this was not an experimental study; despite the prospective design, there may be other unmeasured confounders that could explain results. Third, infant HPA axis reactivity reflects the influence of genetic variation and gene-environment interactions (deKloet et al., 2005; Ellis et al., 2006). Infants of mothers who experience considerable prenatal stress and/or low support may have inherited genetic vulnerabilities that influence biological sensitivity to the postpartum environment. Although we do not have genetic measures, we address this concern by controlling for mother-rated measures of prenatal and postpartum depressive symptoms and postpartum partner support, which would presumably be similarly affected by personality or genetic factors. We also evaluated maternal postpartum cortisol a potential marker of genetic influence; it did not predict infant reactivity or explain the relation of prenatal factors to infant cortisol. However, even if an unmeasured genetic factor is the underlying mechanism, our results suggest that prenatal stress and partner support are easily measured markers of prenatal influences on infant neurobiological outcomes. Further, stress and support are potentially modifiable targets for intervention in the prenatal period. For example, Urizar and Muñoz (2011) reported that infants born to low-income Spanish-speaking women who received a prenatal stress management intervention had lower salivary cortisol levels than those in the control group. Finally, because we did not measure maternal HPA activity during pregnancy, we cannot evaluate in utero hormone exposure as a mechanism.
This study has a number of strengths that contribute to a growing literature on prenatal programming effects on infant neurobiological development. Latinos are the largest ethnic minority group in the U.S., increasing by nearly 45% since 2000 (U.S. Census Bureau, 2011). Latinos of Mexican origin represent the largest proportion of this growth. The risks for infant health associated with prenatal stress are particularly salient for low-income Hispanic women who, compared to non-Hispanic White women, are more likely to live in poverty, less likely to have graduated from high school, and less likely to have health insurance (US Census Bureau, 2003; Pew Hispanic Center, 2011). However, strong cultural values of family and social support may provide important protection for Mexican American infants. Longitudinal studies such as this, beginning in the prenatal period and controlling for potential confounds, offer an ideal opportunity for a comprehensive understanding of the short- and long-term risk and protective factors contributing to the health of Mexican American children. Our study expands literature on the effect of prenatal stress by evaluating partner support as a buffer of the negative consequences for cortisol reactivity in infants born to low-income Mexican American women. The results suggest that partner support may dampen the impact of prenatal stress on infant cortisol regulation, potentially promoting more adaptive infant health and development.
ACKNOWLEDGMENTS
This research was funded by the National Institute of Mental Health (R01 MH083173-01). We thank the mothers and infants for their participation; Kirsten Letham, Anne Mauricio, Danielle Roubinov, Rika Tanaka, Monica Gutierrez, and Craig Enders, for their assistance with data collection and management; Dr. Dean Coonrod and the Maricopa Integrated Health Systems for their assistance with recruitment; and the interviewers for their commitment and dedication to this project.
ROLE OF THE FUNDING SOURCE
Funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
One infant was born at 26 weeks, 7 infants were born at 36 weeks. All remaining infants were full-term.
Economic stress was not assessed at six weeks postpartum. Postpartum partner support did not interact with prenatal economic stress to predict infant cortisol reactivity (p = .10).
Primary study results remained significant with these infants included, however they were removed from analyses because the values were not considered reliable and could distort interpretation of results.
CONFLICT OF INTEREST
The authors have no conflicts of interest financial or otherwise.
CONTRIBUTORS
Luecken made substantial contributions to the conception and design, acquisition of data, analysis and interpretation of data, drafting and critical revision of the manuscript, statistical analyses, and obtaining funding. Lin made substantial contributions to the collection of data, interpretation of data, and to the drafting and critical revision of the manuscript. Coburn made substantial contributions to the collection of data, interpretation of data, and to the drafting and critical revision of the manuscript. MacKinnon made substantial contributions to analysis and interpretation of data, drafting and critical revision of the manuscript, statistical analyses, and obtaining funding. Gonzales made substantial contributions to the conception and design, acquisition of data, critical revision of the manuscript, and obtaining funding. Crnic made substantial contributions to the conception and design, acquisition of data, critical revision of the manuscript, and obtaining funding.
References
- Barrera M, Caples H, Tein J-Y. The psychological sense of economic hardship: Measurement models, validity, and cross-ethnic equivalence for urban families. Am J Community Psychol. 2001;29:493–517. doi: 10.1023/a:1010328115110. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley Scales of Infant Development - Second Edition (BSID-II) 1993 [Google Scholar]
- Bender DE, Castro D. Explaining the birth weight paradox: Latina immigrants' perceptions of resilience and risk. J. Immigrant Health. 2000;2:155–173. doi: 10.1023/A:1009513020506. [DOI] [PubMed] [Google Scholar]
- Bermúdez-Millán A, Damio G, Cruz J, D'Angelo K, Segura-Pérez S, Hromi-Fiedler A, Pérez-Escamilla R. Stress and the social determinants of maternal health among Puerto Rican women: a CBPR approach. J. Health Care Poor Underserved. 2011;22:1315–1330. doi: 10.1353/hpu.2011.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K, Dozier M. Examining infants' cortisol response to laboratory tasks among children varying in attachment disorganization: stress reactivity or return to baseline? Dev. Psychol. 2010;46:1771–1778. doi: 10.1037/a0020660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Raver CC, Granger D, Mills-Koonce R, Hibel L. Allostasis and allostatic load in the context of poverty in early childhood. Dev. Psychopathol. 2011;23:845–857. doi: 10.1017/S0954579411000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom T, Glass N, Curry MA, Hernandez R, Houck G. Maternal stress exposures, reactions, and priorities for stress reduction among low-income, urban women. J. Midwifery Womens Health. 2013;58:167–174. doi: 10.1111/j.1542-2011.2012.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos B, Dunkel Schetter C, Abdou CM, Hobel CJ, Glynn LM, Sandman CA. Familialism, social support, and stress: Positive implications for pregnant Latinas. Cultur. Divers. Ethnic Minor. Psychol. 2008;14(2):155–162. doi: 10.1037/1099-9809.14.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes RC, Padilla AM, Salgado de Snyder N. The Hispanic Stress Inventory: A culturally relevant approach to psychosocial assessment. Psychol. Assess.: J. Consult. Clin. Psychol. 1991;3(3):438. [Google Scholar]
- Collins NL, Dunkel-Schetter C, Lobel M, Scrimshaw SC. Social support in pregnancy: Psychosocial correlates of birth outcomes and postpartum depression. J. Pers. Soc. Psychol. 1993;65(6):1243–1258. doi: 10.1037//0022-3514.65.6.1243. [DOI] [PubMed] [Google Scholar]
- Coussons-Read ME. The psychoneuroimmunology of stress in pregnancy. Curr. Dir. Psychol. Sci. 2012;21:323–328. [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- D'Anna-Hernandez KL, Hoffman MC, Zerbe GO, Coussons-Read M, Ross RG, Laudenslager ML. Acculturation, maternal cortisol, and birth outcomes in women of Mexican descent. Psychosom. Med. 2012;74:296–304. doi: 10.1097/PSY.0b013e318244fbde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deKloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosc. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Diaz MA, Le HN, Cooper BA, Muñoz RF. Interpersonal factors and perinatal depressive symptomatology in a low-income Latina sample. Cultur. Divers. Ethnic Minor. Psychol. 2007;13(4):328–336. doi: 10.1037/1099-9809.13.4.328. [DOI] [PubMed] [Google Scholar]
- DiPietro JA. Maternal stress in pregnancy: Considerations for fetal development. J. Adol. Health. 2012;51(2) Suppl:S3–S8. doi: 10.1016/j.jadohealth.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez TP, Dunkel Schetter C, Glynn LM, Hobel C, Sandman CA. Racial differences in birth outcomes: the role of general, pregnancy, and racism stress. Health Psychol. 2008;27:194–203. doi: 10.1037/0278-6133.27.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkel Schetter C. Psychological science on pregnancy: Stress processes, biopsychosocial models, and emerging research issues. Ann. Rev. Psychol. 2011;52:531–558. doi: 10.1146/annurev.psych.031809.130727. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Jackson JJ, Boyce WT. The stress response systems: Universality and adaptive individual differences. Dev. Rev. 2006;26(2):175–212. [Google Scholar]
- Entringer S, Buss C, Wadhwa PD. Prenatal stress and developmental programming of human health and disease risk: concepts and integration of empirical findings. Curr. Opin. Endocrinol. Diabetes Obes. 2010;17(6):507–516. doi: 10.1097/MED.0b013e3283405921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Dev. Psychol. 2003;39(5):924–933. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- Feldman P, Dunkel-Schetter C, Sandman CA, Wadhwa PD. Maternal social support predicts birth weight and fetal growth in human pregnancy. Psychosom. Med. 2000;62(5):715–725. doi: 10.1097/00006842-200009000-00016. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, Dieter J, Hernandez-Reif M, Schanberg S, Kuhn C, Bendell D. Prenatal depression effects on the fetus and the newborn. Infant Beh. Dev. 2004;27(2):216–229. 216. [Google Scholar]
- Flores MES, Simonsen SE, Manuck TA, Dyer JM, Turok DK. The "Latina Epidemiologic Paradox": Contrasting patterns of adverse birth outcomes in US-born and foreign-born Latinas. Womens Health Issues. 2012;22:e501–e507. doi: 10.1016/j.whi.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Garcia-Esteve L, Ascaso C, Ojuel J, Navarro P. Validation of the Edinburgh Postnatal Depression Scale (EPDS) in Spanish mothers. J. Affect. Disord. 2003;75(1):71–76. doi: 10.1016/s0165-0327(02)00020-4. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Rothbart MK. The laboratory temperament assessment battery (LAB-TAB) University of Wisconsin; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gress-Smith JL, Roubinov DS, Tanaka R, Crnic K, Gonzales N, Enders C, Luecken LJ. Prenatal expectations in mexican american women: Development of a culturally sensitive measure. Arch. Womens Ment. Health. 2013;16:303–314. doi: 10.1007/s00737-013-0350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The Neurobiology of Stress and Development. Ann. Rev. Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Lazinski MJ, Shea AK, Steiner M. Effects of maternal prenatal stress on offspring development: A commentary. Arch. Womens Ment. Health. 2008;11(5–6):363–375. doi: 10.1007/s00737-008-0035-4. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ, Katz M, Schatzberg AF. Developmental cascades linking stress inoculation, arousal regulation, and resilience. Front. Beh. Neurosci. 2009;3 doi: 10.3389/neuro.08.032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Luecken LJ. How and for whom? Mediation and moderation in health psychology. Health Psychol. 2008;27:99–100. doi: 10.1037/0278-6133.27.2(Suppl.).S99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlade MS, Saha S, Dahlstrom ME. The Latina paradox: an opportunity for restructuring prenatal care delivery. Am. J. Public Health. 2004;94:2062–2065. doi: 10.2105/ajph.94.12.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C, Spicer J, Champagne FA. Linking prenatal maternal adversity to developmental outcomes in infants: the role of epigenetic pathways. Dev. Psychopathol. 2012;24(4):1361–1376. doi: 10.1017/S0954579412000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor TG, Bergman K, Sarkar P, Glover V. Prenatal cortisol exposure predicts infant cortisol responses to acute stress. Dev. Psychobiol. 2013;55:145–155. doi: 10.1002/dev.21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendry P, Adam EK. Associations between parents' marital functioning, maternal parenting quality, maternal emotion and child cortisol levels. Int. J. Beh. Dev. 2007;31(3):218–231. [Google Scholar]
- Pew Hispanic Center. Statistical portrait of Hispanics in the United States, 2011. Washington, DC: Pew Research Center; 2011. [Google Scholar]
- Reynolds RM. Glucocorticoid excess and the developmental origins of disease: Two decades of testing the hypothesis—2012 Curt Richter Award Winner. Psychoneuroendocrinol. 2013;38(1):1–11. doi: 10.1016/j.psyneuen.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Rini C, Dunkel Schetter C, Hobel CJ, Glynn LM, Sandman CA. Effective social support: Antecedents and consequences of partner support during pregnancy. Pers. Relationsh. 2006;13(2):207–229. [Google Scholar]
- Sauer B, Brookhart MA, Roy JA, Vanderweele TJ. Covariate selection. In: Venentgas P, Dreyer NA, Nourjah P, Smith SR, Torchia MM, editors. Developing a Protocol for Observational Comparitive Effectiveness Research: A User's Guide. Rockville, MD: Agency for Healthcare Research and Quality; 2013. [PubMed] [Google Scholar]
- Spanier GB. Manual for the dyadic adjustment scale. New York: Multi-Health System; 1989. [Google Scholar]
- Stapleton LRT, Dunkel Schetter CD, Westling E, Rini C, Glynn LM, Hobel CJ, Sandman CA. Perceived partner support in pregnancy predicts lower maternal and infant distress. J. Fam. Psychol. 2012;26(3):453. doi: 10.1037/a0028332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talge NM, Neal C, Glover V Early Stress, Translational Research and Prevention Science Network. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? J. Child Psychol. Psychiatry. 2007;48(3–4):245–261. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urizar GG, Muñoz RF. Impact of a prenatal cognitive-behavioral stress management intervention on salivary cortisol levels in low-income mothers and their infants. Psychoneuroendocrinol. 2011;36:1480–1494. doi: 10.1016/j.psyneuen.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Census Bureau. The Hispanic population in the United States: March, 2002. Washington, DC: U.S. Department of Commerce Economics and Statistics Administration; 2003. [Google Scholar]
- US Census Bureau. The Hispanic population: 2010. Washington, DC: U.S. Department of Commerce Economics and Statistics Administration; 2011. [Google Scholar]
- US Census Bureau. Income, expenditures, poverty, and wealth. Washington, DC: Statistical Abstracts of the United States; 2012. [Google Scholar]
- Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Beh. Immun. 2005;19:296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Youngblut JM, Brooten D, Menzies V. Psychometric properties of Spanish versions of the FACES II and Dyadic Adjustment Scale. J. Nurs. Meas. 2006;14:181–189. doi: 10.1891/jnm-v14i3a003. [DOI] [PubMed] [Google Scholar]