Abstract
Discovering the stress-buffering effects of social relationships has been one of the major findings in psychobiology in the last century. However, an understanding of the underlying neurobiological and psychological mechanisms of this buffering is only beginning to emerge. An important avenue of this research concerns the neurocircuitry that can regulate the activity of the hypothalamic-pituitary-adrenocortical (HPA) axis. The present review is a translational effort aimed at integrating animal models and human studies of the social regulation of the HPA axis from infancy to adulthood, specifically focusing on the process that has been named social buffering. This process has been noted across species and consists of a dampened HPA axis stress response to threat or challenge that occurs with the presence or assistance of a conspecific. We describe aspects of the relevant underlying neurobiology when enough information exists and expose major gaps in our understanding across all domains of the literatures we aimed to integrate. We provide a working conceptual model focused on the role of oxytocinergic systems and prefrontal neural networks as two of the putative biological mediators of this process, and propose that the role of early experiences is critical in shaping later social buffering effects. This synthesis points to both general future directions and specific experiments that need to be conducted to build a more comprehensive model of the HPA social buffering effect across the lifespan that incorporates multiple levels of analysis: neuroendocrine, behavioral, and social.
Keywords: stress, social support, early caregiving, oxytocin, prefrontal cortex
It is an empirical reality that some individuals succumb, while others thrive when confronted with similar stressors. Having access to social support may be an important modulator of these widespread individual differences in responses to potentially stressful events. Indeed, some exciting experiments in humans (e.g., Heinrichs, Baumgartner, Kirschbaum, & Ehlert, 2003; Kirschbaum, Klauer, Filipp, & Hellhammer, 1995; Taylor et al., 2008) and animals (e.g., Hennessy, 1984, 1986; Vogt, Coe, & Levine, 1981) have identified a dampening of the hypothalamic-pituitary-adrenocortical (HPA) axis response to stressors by social factors as one of the possible mechanisms underlying the benefits of social support. Longitudinal studies also reveal relations between social support and basal levels of stress hormones such as salivary cortisol (Rosal, King, Ma, & Reed, 2004). Understanding the social buffering processes affecting this neuroendocrine axis would allow the possibility of interventions that might have cascading positive effects across multiple biological and psychological systems. Despite the important implications of this knowledge, our understanding of the underlying neurobiology and relevant components of social interaction that permit these HPA activity-regulating effects remains vastly incomplete.
General Framework
We adopt a developmental framework to study the social buffering phenomenon due to an extensive body of research indicating that the quality of adult relationships has roots in early development (Sroufe, Egeland, Carlson, & Collins, 2005) and due to growing evidence that stress systems in general and the HPA axis in particular are not only regulated powerfully by social stimuli early in life, but are also subjected to programming effects that shape their future levels of activity (Levine, 2001, 2005a; Meaney & Szyf, 2005). We review studies conducted from infancy to adulthood in humans, as well as animal models that may parallel these findings. We incorporate emerging neuroimaging studies of social buffering in humans, which have primarily been conducted with adults and have not always measured cortisol. In contrast, studies on non-human animals have yielded a well-established and growing literature investigating social buffering in early development. The goal of this review is to integrate animal and human studies to begin to understand the neurobiological structures and processes involved in the stress-relieving properties of social interaction. Furthermore, social support is such a broad construct in the current literature that it is not clear what would need to be manipulated if we were to deploy it as an anti-stress remedy in psychological interventions. Some have even argued that the construct is so broad it is not useful, and we need to abandon it in favor of more specific behaviors (Barrera, 1986). Consequently, we also aim to organize our knowledge of social support behavior. We begin by discussing dimensions and typologies of social support behavior as they have been described in behavioral science, then we briefly review the neurobiology of the HPA axis and what is known about the central neural mechanisms that regulate it (including the role of the prefrontal cortex). Next we address the neurobiology of human social bonding, including the role of the neuropeptides oxytocin and vasopressin and how they may interact with the HPA axis. This will be followed by a review of animal models of HPA activity modulation by social factors, continuing with a review of human studies in children and adults that provide hints about the neurobiology that may be involved in the social buffering effect. We then propose a developmental working model of the social buffering effect focusing on oxytocin and prefrontal cortical systems, emphasizing the role of early experience in shaping neurobehavioral development relevant to this phenomenon (see Fig. 1). The goal throughout will be to lay out the evidence and inferences leading up to the model depicted in Figure 1. We conclude with a discussion of future directions that could lead to a more complete and integrated model of this process in humans.
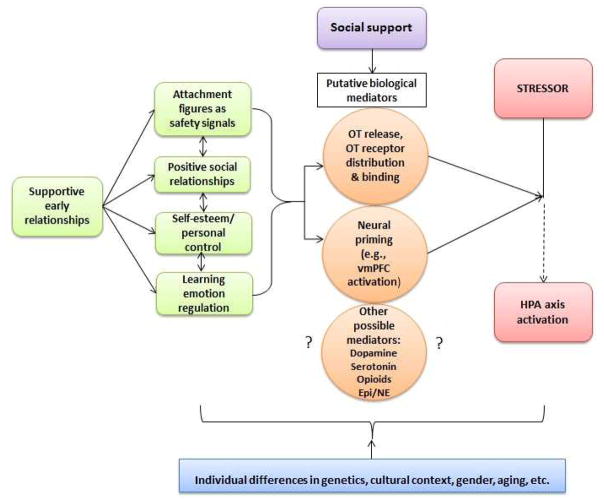
Figure 1. A Developmental Working Model of Social Buffering of the HPA Axis in Humans.
OT = oxytocin, vmPFC = ventro-medial prefrontal cortex, Epi = epinephrine, NE = norepinephrine.
Social Support Dimensions
The importance of social relationships for most aspects of human development has been recognized by psychologists for over a century (Hartup & Laursen, 1999), but our ability to define which aspects of relationships matter in which contexts and to incorporate them in other fields of study has not yet matured (Reis & Collins, 2004). We know that human beings have a fundamental need to belong, experiencing distress when their connections with others are lacking or damaged (Baumeister & Leary, 1995; Miller, 2011). We also know that infants are predisposed to form attachments to their caregivers and possess behavioral systems that allow them to rely on these bonds for survival (Bowlby, 1969) and we will discuss some neurobiological evidence that our species is wired for sociality in the next section. Describing the complexity of different types of relationships and their functions, as well as how they shape individual development is beyond the scope of this review (for a discussion, see Reis, Collins, & Berscheid, 2000). Instead, the main focus here is on social behaviors that have been grouped under the construct of “social support” and that may be relevant for dampening stress reactivity.
Social support has been broadly defined as “information leading the subject to believe that he [she] is cared for and loved, esteemed, and a member of a network of mutual obligations” (Cobb, 1976, p. 300). One definition that allows for an easier operationalization divides social support along three dimensions: emotional, instrumental, and informational (e.g., House & Kahn, 1985). Early studies in this field typically only measured the quantitative or structural aspects of social support networks (House, Landis, & Umberson, 1988), which have been conceptualized as measures of social integration (Cohen, 2004), but recent work has also emphasized the emotional qualities of relationships (their functional aspects) and increasing individuals’ social skills to enhance their ability to use social ties (Reblin & Uchino, 2008). Testifying to the importance of emotional support, studies have shown that perceived or subjective loneliness is an important predictor of psychosocial functioning and morbidity (Caccioppo & Hawkley, 2009). Another distinction that could be drawn is that between minor daily support actions and major stressor-related support (Thoits, 2011), which can be quite different phenomena.
Other studies have focused on the negative effects of some relationships (the “dark side” of relationships as Thoits, 2011, calls it), which in some instances can have stronger and more reliable effects on psychological well-being than the positive effects of social support (Kiecolt-Glaser & Newton, 2001; Pagel, Erdly, & Becker, 1987; Rook, 1984). Even when support is provided, there is some evidence that there are costs associated with it, as some studies suggest that so-called invisible support (reported by the provider but not the recipient) is more effective than support that is acknowledged by the recipient, presumably due to the emotional cost associated with receiving or needing support (Bolger & Amarel, 2007; Bolger, Zuckerman, & Kessler, 2000). There seem to also be cultural differences in the extent to which individuals use social support as a coping mechanism (Taylor et al., 2004), in the type of support that is considered most beneficial and the goals that support providers have in mind when trying to assist (Chen, Kim, Mojaverian, & Morling, 2012). For instance, Chen et al. (2012) found that European Americans reported providing more emotion-focused support, with the goal of closeness and increasing the recipient’s self-esteem, whereas Japanese Americans report providing emotion-focused and problem-focused support equally, and do not report increasing self-esteem as a goal. Cultural differences are biologically relevant, as one study found that European Americans showed lower levels of salivary cortisol in response to the Trier Social Stress Test in the “explicit support” condition, when they imagined asking for support from close others, compared to the “implicit support” condition, when they were asked to write about a group they are close to and about the characteristics of that group. Asians and Asian Americans showed the opposite pattern, with higher levels of cortisol reactivity in the “explicit” compared to the “implicit” support writing task (Taylor, Welch, Kim, & Sherman, 2007). The study suggests different norms about what the primary source of social support for individuals may be.
Furthermore, men and women who report relying primarily on social support as a coping strategy show higher levels of salivary cortisol in response to conflict with their partner compared to participants who rely on other coping strategies (Gunlicks-Stoessel & Powers, 2009). Thus, being able to use this resource may place one at heightened risk of experiencing stress when conflict occurs and, since few relationships are conflict-free, these findings need to inform interventions designed to increase the importance of social support among other coping strategies. Despite negative effects of some aspects of relationships, epidemiological studies seem to suggest a net gain from social support. Perhaps social support interventions need to not only enhance the ability to cope with stress through social support, but should also promote conflict-management skills and other strategies to improve relationship quality.
The methodological challenges of studying social support have been recognized since the beginning of this area of research (Thoits, 1982). The major challenge in many of the initial studies of social support as a stress buffer was their correlational nature. Experimental paradigms have tried to circumvent some of these challenges by randomly assigning individuals to support and no-support conditions in the laboratory, with success in showing a cortisol dampening effect in support conditions (Thorsteinsson & James, 1999). However, these types of studies with adults have also identified individual characteristics associated with how much participants benefit from the provision of social support. These beneficial characteristics include: high self-esteem, extraversion, optimism, a sense of mastery/personal control (Taylor et al., 2008), high self-enhancing cognitions (Taylor, Lerner, Sherman, Sage, & McDowell, 2003), and compassion (Cosley, Mccoy, Saslow, & Epel, 2010). Self-esteem monitors one’s social acceptance and interpersonal effectiveness and can change in response to cues about one’s relational value (Leary et al., 1995; Leary & Baumeister, 2000). Furthermore, there is evidence that self-esteem is not merely a neutral and objective cognition about the self in various domains, but rather that it is linked to a broad range of positive and negative emotions, motives, goals and self-preservation efforts (Leary, 2007). It is no surprise then that laboratory stressors posing self-evaluative threats are the ones which most reliably activate the HPA axis in humans (see the meta-analysis by Dickerson & Kemeny, 2004). Future research needs to not only measure social support, but assess these important self-constructs linked to social resources.
Interestingly, the characteristics making individuals more disposed to benefit from social support are not independent from one’s relationship history. For instance, prior research has linked positive self-esteem with a history of social support across development (Harter, 1999), and we have reasons to believe that a developmental history of positive social relationships directly impacts the individual’s self-concept, emotion regulation abilities, and social competence (Sroufe et al., 2005). The Minnesota Longitudinal Study of Parents and Children –an ongoing 35-year longitudinal study of a high-risk poverty sample- has provided evidence that infants who are securely attached to their mother or primary caregiver are more likely to exhibit high emotion-regulation skills, positive affect, and high self-esteem later in childhood according to observational measures (Sroufe, Schork, Motti, Lawroski, & LaFreniere, 1984; Sroufe, 2005) and they are more likely to be self-reliant in the classroom (Sroufe, Fox, & Pancake, 1983). Furthermore, infants in secure relationships were more likely have competent social interactions with peers according to both teacher and self-ratings across childhood (ages 4–5, 8, 12), which in turn predicted measures of social support and socio-emotional functioning in late adolescence at age 19 (Carlson, Sroufe, & Egeland, 2004). Because infants can have secure attachments to one parent and an insecure attachment to the other, attachment security is a relationship quality, not a trait of the child (Main & Weston, 1981). It reflects the history of the relationship and, specifically, the sensitivity and responsiveness of the care the child has received (Ainsworth, Bell, & Stayton, 1974). Not surprisingly, then, early parenting quality also has been related to positive, secure representations of romantic partners in young adulthood (ages 26–28, Haydon et al., 2012). Conversely, negative early caregiving experiences such as child maltreatment are associated with detrimental social outcomes, beginning with insecure attachment patterns in infancy and preschool (Cicchetti & Barnett, 1991; Cicchetti, Rogosch, & Toth, 2006) and extending to problems with peers which suggest a transfer of social dysfunction from the family context to new relationships that place them at risk of being unable to use social support later (Bolger, Patterson, & Kupersmidt, 1998; Rogosch, Cicchetti, & Aber, 1995). This is because maltreated children show lower social effectiveness and greater aggressive behaviors, often eliciting peer rejection (Rogosch et al., 1995). They also tend to have lower self-esteem with increasing maltreatment severity (Bolger, Patterson, & Kupersmidt, 1998), develop negative expectations about social interactions, and studies show that these expectations mediate the association between maltreatment and low social status among peers (Salzinger, Feldman, Ng-mak, Mojica, & Stockhammer, 2001). Adolescents who were maltreated as children similarly exhibit more relational problems with peers, as well as poor emotional regulation, higher rates of drug use, depression, and self-harm (Scott, Wolfe, & Wekerle, 2003). However, having a close friend is associated with increasing slopes in self-esteem over time for some children experiencing maltreatment (Bolger, Patterson, & Kupersmidt, 1998), suggesting the fact that early experience does not deterministically lead to later social outcomes, but only that it sets certain trajectories in motion which dynamically interact with later experiences and self-representations.
To summarize, it is increasingly recognized that social integration, perceived emotional support, negative social interactions, and self-constructs associated with social resources capture different and important dimensions of what has been termed “social support”, each of which may have distinct effects on stress responses. Psychological studies rarely measure all these social dimensions, at the risk of overlooking important effects on well-being and this shortcoming in the literature will need to be addressed by future research. Furthermore, we have yet to fully understand the psychological mediators of the stress buffering effect and how they operate in different contexts. While some of socially supportive effects are straight-forward and likely do not require a biological explanation (e.g., a friend’s financial assistance can terminate a stressor), others remain to be understood –for instance, understanding how the mere presence of a conspecific reduces the production of stress hormones when confronted with a stressor.
An additional challenge in this area is that social support intervention studies are plagued by differences in methodologies and definitions of social support, such that it is not clear what type of intervention needs to be deployed for which type of problem (Hogan, Linden, & Najarian, 2002). Many clinical studies did not include measures of support -e.g., gathered people into support groups but specifics about the process were not measured (Hogan, Linden, & Najarian, 2002). As a result, there is minimal understanding of the underlying mechanisms operating when positive outcomes do occur. Few of these studies measure physiology. Experimental studies have made some progress in measuring physiological mediators, but they fare no better in deconstructing social support into its components or measuring relationship histories that predict successful reactions to social support in the laboratory. This will be further exemplified in the section reviewing human studies of the social buffering effect. Before we discuss these studies showing dampening of stress physiology by social support, we provide brief overviews of stress neurobiology and of some of the biological substrates of human social interactions.
The Neurobiology of Stress
The Hypothalamic-Pituitary-Adrenal System
Stress can be defined as a “real or interpreted threat to the physiological or psychological integrity of an individual that results in physiological and/or behavioral responses” (McEwen, 2000, p. 508). As Levine (2005b) eloquently discussed, there are three types of constructs usually subsumed by the concept: the inputs/challenges, the systems that process them, and the outputs/responses. To distinguish between these three potential connotations of the term, we will refer to events that trigger stress reactions as stressors (similar to Selye, 1975), and the outputs of this cascade will be called stress responses. We will refer to the biological and psychological systems that process the challenging inputs as stress mediators or simply stress systems. It must be noted that the experience of stress has dissociable emotional, behavioral, and physiological components, with many studies finding dissociations between distress behavior and cortisol production (e.g., Gunnar, Fisch, Korsvick, & Donhowe, 1981).
Stress results when the demands of internal or external events exceed immediately available resources (Lazarus & Folkman, 1984). These demands can be very diverse, ranging from temperature challenges, to infections, to real or perceived psychological threats. Psychological and physical challenges to the organism trigger a neuroendocrine cascade that results in the production of glucocorticoids (GCs; cortisol in humans, corticosterone in rodents) through the activation of the HPA axis (Cone, Low, Elmquist, & Cameron, 2003). The production of stress hormones can occur in reaction to both psychogenic stressors and physiological challenges to homeostasis (e.g., infection, pain). Based on rodent research, which has been corroborated in human and nonhuman primates, the HPA axis and associated brain regions have been identified. Neurons in the medial parvocellular region of the paraventricular nuclei of the hypothalamus (PVN) secrete corticotrophin-releasing hormone (CRH) and arginine vasopressin (AVP), which travel to the anterior pituitary and cause the release of adrenocorticotropic hormone (ACTH) into general circulation (Gunnar & Vazquez, 2006). ACTH subsequently binds to its receptors in the cortex of the adrenal glands, leading to the release of GCs. Circulating hormones bind to receptors distributed throughout the brain and the body, and under normal conditions also exert negative feedback inhibition at multiple levels of the HPA axis, such that the axis can shut down its own release when GC levels are high (Cone et al., 2003). Current evidence also suggests that negative feedback involves GC receptors in other areas outside the HPA axis, including but not limited to, the prefrontal cortex and limbic structures like the hippocampus and amygdala (Oitzl, Champagne, van der Veen, & de Kloet, 2009). The role of these limbic and cortical areas will be discussed in more detail below. The HPA axis is one of the arms of the mammalian stress system, while the autonomic nervous system is the other important branch. A synthesis of findings involving the autonomic nervous system in the social regulation of stress is beyond the scope of this review, but Figure 2 illustrates both systems and their complex interactions.
Figure 2. Neurobiology of Stress.
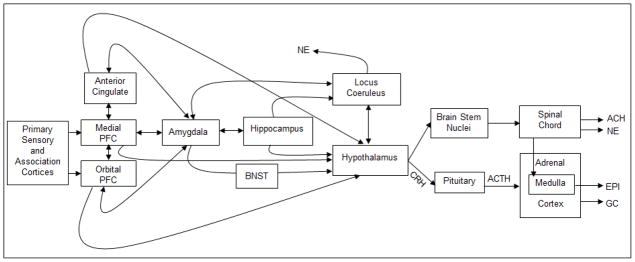
The primary sensory and association cortices relay information to the medial and orbital prefrontal cortex. The anterior cingulate cortex (ACC), medial prefrontal cortex (mPFC) and orbital prefrontal cortex (OFC) then transmit signals to subcortical structures involved in the stress response. The ACC, mPFC and OFC are reciprocally interconnected with each other and with the amygdala. Both the hippocampus and the amygdala also maintain connections to the locus coeruleus (LC) which releases norepinephrine (NE) to brain areas involved in alerting. The ACC, mPFC, OFC, and amygdala, as well as the hippocampus, all provide inputs to the hypothalamus. Nuclei in the lateral hypothalamus activate highly interconnected nuclei in the brainstem, that regulate the sympathetic (norepinephrine, NE and epinephrine, EPI) and parasympathetic (acetylcholine, ACH) nervous system via pathways traveling through the spinal cord to preganglionic nuclei or to target organs (e.g. the adrenal medulla). In the paraventricular region of the hypothalamus, corticotropin releasing hormone is produced, which travels through the hypophysial portal system to the anterior pituitary gland, stimulates the production and release of adrenocorticotropic hormone (ACTH). ACTH stimulates cells in the adrenal cortex to produce glucocorticoids (GC, cortisol in humans). Adapted from Gunnar and Davis (2003, p. 117). This material is reproduced with permission of John Wiley & Sons, Inc. (Copyright © 1999–2012).
Acute release of GCs has numerous effects on the body, including mobilization of energy to muscles, enhanced cardiovascular tone, a stimulation of immune function, inhibition of reproductive physiology, decreased appetite, sharpened cognition, and increased local cerebral glucose utilization (Sapolsky, Romero, & Munck, 2000). GCs are not only produced in response to stressors, but are released in pulses across the day to ensure basal levels of hormones that are necessary for energy, motivation, and optimal functioning overall. The release of basal GCs follows a circadian clock, with higher levels in the morning for humans (approximately 30 minutes after wake-up, which has been named the cortisol awakening response and associated with numerous psychological and physical health outcomes (Fries, Dettenborn & Kirschbaum, 2009) and decreasing production throughout the day, reaching minimum levels at night. Studies show that repeated activation of the acute HPA response can lead to enhanced GC release across the day, followed by down-regulation over time, which has been termed hypocortisolism and has been observed in many instances of prolonged trauma or stress (Fries, Hesse, Hellhammer & Hellhammer, 2005; Gunnar & Vazquez, 2001). Both elevated and chronically low levels of GCs can impair behavioral and physical functions, potentially leading to pathology (Chrousos, 2009).
GCs bind to glucocorticoid receptors (GR) and mineralocorticoid receptors (MR) differentially when they are released in response to a stressor versus their basal circadian release. GCs have 10 times higher affinity to MRs and occupy them first, before acting on the GRs (Oitzl et al., 2009). Thus, MRs are almost entirely occupied when GCs are in the basal ranges, and GRs only become occupied when stressors elevate GC concentration above basal levels. Evidence is accumulating for the hypothesis that the balance of MR:GR is crucial for an effective regulation of stress responses and for resilience to psychiatric disorders (Oitzl et al., 2009). Recent studies in rodents have shown that the expression of MR and GR can be affected by environmental factors such as early maternal care, through epigenetic mechanisms (Meaney & Szyf, 2005). Future research will likely continue to explore the biological mechanisms involved in the transduction of early life experiences into lasting patterns of stress reactivity.
Limbic and Cortical Regulation of the HPA Axis
The HPA system does not operate in isolation. Rather, its activation is the result of a fine-tuned cascade of neural events that involve a large number of brain regions and neurotransmitters, creating a true “neuro-symphony of stress” (Joëls & Baram, 2009). In psychologically challenging circumstances, limbic and cortical regions (e.g., the amygdala, hippocampus, the prefrontal cortex) relay information about threats that can activate or terminate stress responses (see Fig. 2), but there are also many other inputs -e.g., sensory, activating the axis in response to physiological challenges. This section reviews some of the major limbic and cortical structures that modulate HPA responses to psychological stress.
The amygdala is an important limbic structure modulating neuroendocrine functions. Animal models have established its role in emotional learning and conditioning, particularly fear learning (Fanselow & LeDoux, 1999). However, the amygdala is also important for homeostasis and integration of neuroendocrine and autonomic stress systems. Research has highlighted the central nucleus of the amygdala (CEA) as an important orchestrator of emotional and stress integration, also impacting memories of challenging events (Ulrich-Lai & Herman, 2009). In this regard, it is noteworthy that CRH-producing cells do not reside exclusively in the hypothalamus, but they are also present in many brain structures that are involved in associating fear and anxiety with activation of the stress system, including the CEA and the prefrontal cortex (Bale & Vale, 2004). CRH acts on two types of receptors, which have different regional distributions and functional properties, with CRF1 mediating acute stress reactions via the activation of the HPA axis and CRF2 being involved in post-stressor recovery and dampening of the HPA response (Korosi & Baram, 2008). Additional evidence from rodent research suggests that the CEA activates the HPA axis in response to systemic stressors, and integrates autonomic responses to psychogenic stressors, whereas the basolateral and the medial nuclei of the amygdala play a preferential role in activating the HPA axis in response to psychological stressors (Ulrich-Lai & Herman, 2009). The amygdala not only acts on the HPA axis, but is itself influenced by GC release. In rodents, stress-induced GC release permits amygdala activation and facilitates fear learning in infant rats (Moriceau, Roth, & Sullivan, 2010) and modulates fear learning in adults (Luksys & Sandi, 2011; Joëls, Fernandez, & Roozendaal, 2011). Fear learning is central for adaptation, as the activation of stress circuitry is energetically demanding, and thus the ability to distinguish threatening from harmless stimuli is essential for survival.
Equally important is modulation of the HPA axis response by the bed nucleus of the stria terminalis (BNST), though different subdivisions of the BNST seem to have contrary effects: antero-ventral regions seem to have excitatory effects on the axis, whereas posterior regions may be inhibitory (Choi et al., 2007). The hippocampus is also a major limbic structure that has inhibitory control of the HPA axis through projections to the PVN (Ulrich-Lai & Herman, 2009). GR and MR expressed in the hippocampus play a crucial role in negative feedback of the HPA axis, and it is likely due to changes in the activity of these receptors that chronically elevated circulating GCs have been linked to deficits in hippocampally-based abilities, such as declarative memory (Sapolsky, 2003). Illustrating the important role of the hippocampus in HPA axis regulation, studies show that hippocampal damage in humans blocks the cortisol awakening response (Buchanan, Kern, Allen, Tranel, & Kirschbaum, 2004).
Limbic structures are regulated by and communicate with frontal cortical regions, and their bidirectional connections impinge on the activity of the HPA system. For instance, the degree and breadth of interconnectivity between the amygdala and frontal cortex in primates has been well-documented in studies from the last two decades (Emery & Amaral, 2000).
The prefrontal cortex (PFC) plays a crucial role in what has been termed “executive function”, or the top-down control of thought and action which includes working memory, inhibitory control, and cognitive flexibility (Zelazo, Carlson, & Kesek, 2008). These higher-order processes are crucial for the development of emotion regulation, compliance with norms, and intelligent planning of behavior from childhood to adult age (Zelazo, Carlson, & Kesek, 2008). The PFC is organized into distinct topographical regions, which serve divergent and specialized regulatory functions. The orbitofrontal cortex (OFC) and medial PFC have numerous reciprocal connections to the amygdala and other limbic regions (Price, 1999), and play important roles in regulating behavioral, neuroendocrine, and autonomic stress responses. In general, ventral and medial areas of the PFC are thought to primarily regulate emotions, having extensive connections with the amygdala, the nucleus accumbens, and the hypothalamus, whereas regions located more dorsally and laterally are thought to regulate thoughts and actions, and have important projections to sensory and motor areas (Arnsten, 2009). In addition, studies in rodents also show that dorsal regions of the mPFC have inhibitory impacts on the HPA axis, whereas ventral regions may have excitatory roles (Sullivan & Gratton, 1999).
A prefrontal region that has received much attention is the anterior cingulate cortex (ACC). This region is not only involved in regulating the HPA axis (Diorio, Viau, & Meaney, 1993), but it has been implicated in the production of distress calls of 2-year old squirrel monkeys during separation from the mother (MacLean & Newman, 1988) and in responses to social pain and rejection in adult humans (Eisenberger, Lieberman, & Williams, 2003). This region has also been identified as playing a role in studies of the social buffering of pain in humans (see later section on human neuroimaging studies).
The various regions of the prefrontal cortex not only play a role in regulating the activity of stress systems, but they also receive bottom-up inputs from them, and their activity can be impaired by acute or chronic stress exposure (Arnsten, 2009). Even though some experimental studies with squirrel monkeys suggest that stress-inoculating experiences (i.e., stressors that are not overwhelming in either duration of magnitude) can enhance prefrontally-mediated cognitive skills that are instrumental for emotion and stress regulation (Lyons & Parker, 2007), multiple studies in humans show that both acute and chronic exposure to stressors can actually impair these higher-order processes that rely on the PFC (Arnsten, 2009).
Chronic stress induces alterations in both cortical and limbic regions, such as dendritic atrophy and decreased GR expression in the medial PFC and the hippocampus, and increased dendritic branching in the basolateral amygdala and enhanced CRH expression in the CEA (Ulrich-Lai & Herman, 2009). Over time, these alterations are likely to impair the capacity for negative feedback of the HPA axis, as well as to decrease the available PFC-based cognitive resources for coping with stressors. The effects of stress on brain development and the sculpting of cortico-limbic circuits across the lifespan are likely mediated by both circulating GCs and by the extra-hypothalamic CRH system (Korosi & Baram, 2008). Future research will need to better characterize the direct and indirect pathways between limbic and cortical regions and the HPA axis, and how they develop across the lifespan, particularly under the influence of stressful life experiences.
The Neurobiology of Human Social Bonding
To investigate the pathways through which social relationships could buffer individuals against stress reactivity, we need to incorporate our current understanding of the neurobiology of social bonding, as there are theoretical reasons to expect overlapping biological processes. For this reason, we will briefly review a quickly-expanding body of evidence supporting the critical role of the neuropeptides oxytocin (OT) and arginine vasopressin (AVP) in regulating human social behavior (Carter, 1998; Donaldson & Young, 2008; Heinrichs & Domes, 2008; Lee, Macbeth, Pagani, & Young, 2009; Neumann, 2008). These neuropeptides have been strongly conserved throughout evolution and play similar roles in social behavior and reproduction across vertebrates (Donaldson & Young, 2008), but they have also been linked to complex social behaviors that are primarily observed in humans, such as altruism, trust, the ability to infer others’ thoughts or emotions, etc. (Donaldson & Young, 2008).
OT and AVP are neuropeptides produced by magnocellular neurons in the supraoptic nucleus and paraventricular nucleus of the hypothalamus and then released from axon terminals in the posterior pituitary from where they are released into the general circulation (Ludwig, 1998). In addition to their roles as neurohormones once released into the general circulation from the pituitary, the neuropeptides can also be found at other extra-hypothalamic sites (e.g., through release from hypothalamic neurons projecting to several limbic areas, including amygdala, hippocampus, septal nuclei, etc.) and these centrally released peptides act as neurotransmitters and neuromodulators within the brain (Landgraff & Neumann, 2004). Furthermore, OT is also synthesized peripherally –e.g., in the uterus, placenta, amnion, corpus luteum, testis, and heart (Gimpl & Fahrenholz, 2001). OT and AVP exert their physiological effects by binding to their receptors. Based on animals models and converging analyses of the human receptors, OT is currently known to have one receptor, which has equal affinity for OT and AVP, whereas AVP binds to four receptors: V1a, V1b, V2 (Caldwell et al., 2008) and to P2 purinoreceptors, which have been identified in guinea pig hearts (Zenteno-Savin, Sada-Ovalle, Ceballos, & Rubio, 2000). AVP receptors tend to have higher affinity for AVP than OT (e.g., the V1a receptor has 30 times higher affinity for AVP than OT –Holmes, Landry, & Granton, 2003). V1a and V1b are the two centrally expressed subtypes, with V1a being most widely implicated in functions related to social behavior in animal models (Donaldson & Young, 2008). Even though there is significant overlap between the social behaviors that OT and AVP have been implicated in, these differences in receptor affinities are presumed to underlie some specificity in the function of each peptide (Carter, 1998). For instance, there is some evidence that AVP plays particular roles in prosocial behavior in males, and it has also been shown to mediate aggressive behaviors and territoriality in many species (Caldwell et al., 2008; Donaldson & Young, 2008; Young & Wang, 2004). Furthermore, it can also increase stress responses through its actions as a secretagogue for ACTH (DeBold, Sheldon, DeCherney, & Jackson, 1984). OT and AVP may show opposite effects due to acting as each other’s antagonists (Carter & Altemus, 1995; Huber, Veinante, & Stoop, 2005). Future studies will need to clarify the complex interactions between these two neuropeptides and their receptors, however in this review we focus on oxytocin primarily since it has been more consistently shown to be a stress attenuator.
The OT receptor is widely distributed throughout the brain and in humans it is most concentrated in the nucleus basalis, the nucleus of the vertical limb of the diagonal band of Broca, the ventral part of the lateral septal nucleus, the preoptic/anterior hypothalamic area, the posterior hypothalamic area, the substantia nigra pars compacta, as well as in spinal nuclei and the nucleus of the solitary tract (Loup et al., 1991). AVP binding sites are prominent in human brains in the dorsal part of the lateral septal nucleus, in midline nuclei and adjacent intralaminar nuclei of the thalamus, in the hilus of the dentate gyrus, the dorsolateral part of the basal amygdaloid nucleus and the brainstem (Loup et al., 1991). These concentrated distributions in limbic and autonomic areas are consistent with the neuromodulatory role of these neuropeptides.
Studies also show long-lasting effects of developmental experiences on neuropeptide systems (for a comprehensive recent review, see Bales & Perkeybille, 2012). For instance, female mice reared communally exhibited higher OT receptor binding in the dorsal and ventral lateral septum, BNST, agranular insular cortex, and endopiriform cortex, while AVP V1a binding was reduced in the dorsal lateral septum (Curley et al., 2009). The quality of early maternal care also seems to play a role in rodents, for instance high licking/grooming behavior by the dams is associated with increased OT receptor binding in the medial preoptic areas, BNST, lateral septum, PVN, and CEA for females (Champagne and Meaney, 2007; Francis, Champagne, & Meaney, 2000; Francis, Young, Meaney, & Insel, 2002), whereas AVP V1a binding is increased in the CEA in male offspring (Francis et al., 2002).
The effects of OT and AVP on brain and behavior have been shown in many studies to be sexually dimorphic (Carter, 2007). For instance, both OT and its receptor are more highly expressed in females (Carter, 2007) and respond to estrogen signaling (Lee, Macbeth, Pagani, & Young, 2009), whereas androgens stimulate the synthesis of AVP (DeVries & Villalba, 1997). In humans, plasma OT correlated with romantic relationship distress in women but not men, while plasma AVP correlated with relational distress in men but not women (Taylor, Saphire-Bernstein, & Seeman, 2010). The effect of intranasally-administered oxytocin on brain activity evoked by fearful or angry facial expressions also exhibits sex differences, with women showing increases and men decreases of activation in regions associated with face perception and emotion encoding (e.g., temporal poles, amygdala, brainstem; Domes et al., 2007a, 2010; for a review, see Zink & Meyer-Lindberg, 2012). There are some theoretical proposals (Taylor et al., 2000) suggesting that in humans OT and AVP may provide the basis for divergent responses to stress in males and females, with females more likely to exhibit OT-based affiliative (“tend-and-befriend”) responses under threatening circumstances compared to men, though there is also great overlap in the type of coping observed in males and females (Taylor et al., 2000).
Many studies report similar involvement of OT or AVP in the social behaviors of both men and women. For instance, increases in human participants’ plasma or urinary OT have been noted in tasks involving empathy with a stranger’s distress (Barrazza & Zak, 2009), trust and generosity in monetary games (Zak, Kurzban & Matzner, 2005), receiving a massage (Morhenn, Park, Piper, & Zak, 2008), and natural variations in both maternal and paternal behaviors with infants (Gordon, Zagoory-Sharon, Leckman, & Feldman, 2010; Feldman Singer, & Zagoory, 2010). Early experience seems to play a role in shaping some of these effects, since children reared in orphanages showed lower levels of urinary oxytocin after an interaction with their adoptive mothers compared to nonadoptive children and their mothers (Wismer Fries, Ziegler, Kurian, Jacoris, & Pollak, 2005), while adult women who had experienced childhood maltreatment exhibited lower levels of OT in CSF (Heim et al., 2009). OT plasma levels do not only show concurrent associations with behavior, but have also been used to predict behavior. In one study, plasma levels of OT during pregnancy were shown to be predictive of postnatal mother-infant interactions (Feldman, Weller, Zagoory-Sharon, & Levine, 2007; for a review of similar studies, see Galbally et al., 2011). Experimental manipulations involving intranasal administration of OT have also revealed effects consistent with the presumed role of OT in prosocial behavior: increases in trust (Kosfeld, Heinrichs, Zak, Fischbacher, & Fehr, 2005), self-reported attachment security in adulthood (Buchheim et al., 2009), ability to “read the mind” of another person (Domes, Heinrichs, Michel, Berger, & Herpertz, 2007), paternal responsiveness to infants during play (Naber, van Ijzendoorn, Deschamps, van Engeland, & Bakermans-Kranenburg, 2010), and positive communication during couple conflict (Ditzen et al., 2009).
The importance of these neuropeptides for the social buffering of stress will be discussed more in depth in the next section, as there is increasing evidence that oxytocin does not only play critical roles in prosocial behavior, but is capable of dampening HPA axis responses and of acting as a stress attenuator. We review animal models of the neurobiological underpinnings of HPA dampening by social factors next, including studies where the mediators seem to be neuropeptides.
Animal Models of the HPA Dampening Effect by Social Factors
Animal research has built upon the human research exploring the stress-buffering effect of social relationships, but diversified its experimental paradigms to capitalize on the ability of animal research to probe neural mechanisms. As we review this animal literature, it is important to note that social buffering does occur in infancy, but it undergoes dramatic changes as infants mature and become independent. Importantly, animal models have been consistent with findings in humans, strongly suggesting that the neural mechanisms for behavioral and neuroendocrine changes due to social buffering in animal models can be informative when applied to humans.
Social Buffering across the Lifespan in Animal Models
The neuroendocrine control of social buffering in infancy was originally identified in squirrel monkeys when the infant’s typical increase in plasma cortisol during separation from the mother or exposure to a predator quickly returned to normal levels when reunited with the mother or being placed in a familiar social environment (Coe, Franklin, Smith, & Levine, 1982; Coe, Mendoza, Smotherman, & Levine, 1978; Stanton & Levine, 1985). Since then, considerable research on mechanisms of social buffering have expanded on these results and demonstrate that the HPA axis and its buffering by social stimuli show dramatic changes during development. This is due, in part, to maturation of the different levels of the HPA axis (Bouret, Drper, & Simerly, 2004; Rinaman, 2001), but also to the enormous control of the HPA axis by social stimuli from the mother early in life.
It is important to note that the infant HPA axis and its control by social stimuli is not an immature version of the adult social buffering system and is adapted to unique features of the mother-infant dyad. The very early life social buffering system has been most fully described in the rodent and has slowly uncovered an unexpected and dynamic role of the mother in controlling the infants’ stress-induced corticosterone levels. Over the past five decades, our concept of the development of the HPA axis has undergone major revisions. In the 1950’s, rodent research showed initially surprising results that suggested infants do not mount a stress-induced corticosterone response, which was termed the stress nonresponsive period (SNRP). However, the term was changed to the stress hyporesponsive period (SHRP) when it was realized that pups could mount a stress-induced corticosterone response with an injection of endotoxin (Witek-Janusek, 1988), clearly indicating that the neonate has a stress system but its functioning was being suppressed. Insights to the mechanism of this suppression were based on two areas of research, which together highlighted the role of sensory stimuli pups received from the mother. The first clue that the mother was important for pups’ HPA regulation came from maternal deprivation research, a procedure where pups are removed from their mother for many hours. Indeed, prolonged separation resulted in an increase in pups’ blood corticosterone levels, but also produced a lower GR mRNA expression in the hippocampus and frontal cortex, and increased CRH mRNA expression in the paraventricular nucleus (PVN), amygdala central nucleus (CEA), bed nucleus of the stria terminalis (BNST) and the locus coeruleus (LC) neuradrenergic nucleus (Plotsky et al., 2005). This suggested that the separation procedure was a stressor that activated all levels of the HPA axis. Second, the notion of the mother as a “hidden regulator” of pups’ behavior and physiology was emerging (Hofer, 1973), which produced a paradigm-shifting perspective on the role of maternal behavior in normal development. Specifically, research showed that sensory stimuli normally received from the mother maintain pups at homeostasis, with different stimuli and their patterning controlling balance in specific systems. For example, the tactile stimulation pups receive from the mother maintains high levels of pups’ growth hormone, while maternal odor and warmth controls behavioral activity levels (Hofer, 1984; Kuhn, Butler, & Schanberg, 1978).
Decades later, a conceptual leap occurred when it was recognized that maternal deprivation was removing the maternal stimuli that were homeostatic regulators of the infant HPA axis in rodents. Thus, maternal deprivation served to produce a state of chronic HPA hyper-responding which could be conceived of as a state of chronic neuroendocrine stress. Specifically, maternal sensory stimulation of pups was deconstructed and artificially replaced with sensory stimulation of specific sensory systems, such as tactile stimulation from brushing to mimic maternal licking or intubating pups to fill their stomach with milk (van Oers et al., 1998). Indeed, presenting pups with just tactile stimulation during maternal deprivation was sufficient to block the maternal deprivation-induced corticosterone increase suggesting that chronic social buffering by the mother underlies the SHRP. Importantly, these artificial maternal replacement stimuli also restore the HPA response to normal. Specifically, PVN neural activity as measured by c-fos and CRF-R2 expression in the ventromedial hypothalamus both appear to be returned to normal levels if maternally deprived pups receive stroking tactile stimulation (Eghbal-Ahmadi, Avishai-Eliner, Hatalski, & Baram, 1999). These sensory stimuli also alter HPA axis activity, as indicated by CRF mRNA, and plasma ACTH (van Oers et al., 1998). Thus, together this suggests that the SHRP can be viewed as chronic social buffering because sensory stimuli from the mother prevent pups from mounting a stress response. It is still unclear how maternal sensory stimuli maintain the SHRP.
As pups mature, changes in this chronic social buffering system are reflected in the end of the SHRP in 10- to 11-day-old pups, when pups mount a corticosterone response to stressors in an adult-like fashion. That is, when 12-day-old pups are removed from the nest and stressed, they are able to mount a behavioral and corticosterone elevation consistent with the adult response, although corticosterone levels do not approach adult levels until after weaning. Adult-like social buffering emergence coincides with this developmental change in the stress response. The mother is the critical social buffering signal (Shionoya, Moriceau, Bradstock, & Sullivan, 2007; Stanton, Wallstrom, & Levine, 1987; Stanton & Levine, 1990; Suchecki, Rosenfeld, & Levine, 1993; Wiedenmayer, Magarinos, McEwen, & Barr, 2003). Specifically, at this age, rat pups mount a corticosterone response when given a mild stressor, such as electric shock, although this is completely blocked if the mother is present.
With additional maturation, peers also become potent sources of social buffering (Hennessy, Kaiser, & Sachser, 2009). For instance, the presence of a same-sex conspecific dampens HPA response to novelty in periadolescent rats that have spent PND 21-35 cohabitating with their conspecific (Terranova, Cirulli, & Laviola, 1999). Periadolescence is a period when rats are particularly social and motivated to affiliate. Notably, the effect of treatment was statistically significant in males and followed a similar pattern without significance in females. In adults, corticosterone response to a novel box is lower if rats are tested in pairs than if they are tested alone (File & Peet, 1980). Interestingly, adult rats show lower reactivity to stressful stimuli if they are with a nonfamiliar conspecific than if they are confronting it with a familiar conspecific that they were previously housed with (Armario, Luna, & Balasch, 1983; Armario, Ortiz, & Balasch, 1983). This is thought to be due to a higher novelty effect of the environment (i.e., new location) when the animal is placed there with a familiar conspecific that it can ignore. In squirrel monkeys, the presence of familiar and unfamiliar conspecifics as well as group situations dampens corticosterone response to stressful conditions (Hennessy, 1984, 1986; Vogt, Coe, & Levine, 1981).
In summary, rodents show a SHRP in early life that is controlled by social stimuli from the mother to maintain low levels of stress hormones. This SHRP is disrupted when the social stimuli are removed at separation from the caregiver and the stress hormone rises. While it is unclear if humans even have a SHRP, there are some parallels that can be drawn with the role of the mother in human and nonhuman animal models, as will become evident in later sections.
Neural Control of Social Buffering in Animal Research
Despite the importance of social buffering and its widespread phylogenetic representation, our understanding of the neural circuitry is minimal. While imaging techniques in humans have been helpful in identifying structures that correlate with stress and its regulation, problems inherent with this procedure and ethics in human research with young children limit exploring mechanisms and causation in human studies. Thus, animal research is required to better understand this system. However, there are unique challenges to social buffering neurobiology research. First, different stress sensory stimuli, including psychological and physical stressors, activate the HPA axis in distinct ways and at different levels. Research is continuing to identify and characterize these diverse pathways. Second, the specific source of the stimulus initiating the social buffering response is still unknown. For example, when a mother socially buffers her child or pup, is the infant responding to her scent, touch, voice or in children, simply the knowledge that she is there? Third, different social stimuli likely have different impacts on the HPA axis, perhaps at different levels of the axis or indirectly through the myriad brain areas known to modulate HPA activity. For this reason, animal research has attempted to explore these very basic features of the neurobiology of social buffering.
To understand the neural circuitry of social buffering, it is important to understand that psychological and physical stressors activate the HPA axis differently. For example, physical stressors such as shock activate the HPA axis at the level of the PVN via norepinephrine, most likely originating from A1-A2 brainstem noradrenergic nuclei (Cunningham & Sawchenko, 1988; Figueiredo et al., 2003; Herman et al., 2003). Psychological stressors, on the other hand, appear to have a more complex control of the HPA axis, with clear implication of the amygdala, specifically two subareas implicated in controlling the HPA axis: central (Beaulieu, Paolo, & Barden, 1986; Roozendaal, Koolhaas, & Bohus, 1991) and medial (Prewitt & Herman, 1997). The medial amygdala appears to control release of CRF in the median eminence to increase ACTH, although this is likely indirectly through the BNST (Canteras et al., 1995; Herman, Ostrander, Mueller, & Figueiredo, 2005; Prewitt & Herman, 1997, 1998). This indirect route appears to ultimately increase CRF in the median eminence (Dong, Petrovich & Swanson, 2001; Prewitt & Herman, 1998).
How social stimuli interface with this complex stress circuit has yet to be determined, although clues are emerging. Importantly, social buffering directly impacts the HPA axis to reduce stress hormone release. For example, rats exposed to a shock box accompanied by a partner show decreases in c-fos immunoreactivity in the PVN compared to rats that face the fear stimulus alone (Kiyokawa, Kikusui, Takeuchi, & Mori, 2004). Indirect effects of social buffering on the HPA axis have also been documented for the prefrontal cortex (PFC) in both human and nonhuman research. This brain area has strong reciprocal connections with the amygdala and can modulate the PVN (Figueiredo et al., 2003; Stefanacci & Amaral, 2002; Vertes, 2004). Research on juvenile rhesus monkeys that were exposed to a novel environment but were socially buffered by their mother showed increased activity in the PFC (Rilling et al., 2001). Specifically, this PET study found greater right dorsolateral PFC activation and higher plasma cortisol levels when the mother was absent compared to mother being present. Importantly, lesion studies in rodents support a causal link between the PFC (prelimbic or anterior cingulate subareas) and this buffering process. Specifically, lesioning these PFC subareas blocked the social buffering effects of an adult partner (Herman et al., 2005). The underlying neural circuitry of the social buffering effect is hypothesized to involve stressor appraisal by the PFC (modified by sensory cues of a social partner), which then sends information to limbic regions such as the amygdala and BNST, which are in turn heavily connected to the PVN (Hennessy et al., 2009). This model is biologically plausible, and is currently being tested. As noted in the section on human social buffering, human neuroimaging studies support the role of the PFC in social buffering (Eisenberger et al., 2007).
Oxytocin has also been a major focus in the social buffering literature and has been shown to reduce the stress response in humans and nonhuman animal models, although its association with social buffering is less well established and not always predictable. Social buffering is only one of many behaviors that implicate oxytocin. Specifically, the stimuli that initiate the release of oxytocin and its role in controlling behavior are diverse, although there are some similarities across species, such as lactation’s milk ejection reflex, affiliation, pain reduction and stress reduction (Carter, 2003; Nelson & Panksepp, 1998; Neumann, 2008; Onaka, 2004; Young, 2009). As described above, oxytocin is not only released into general circulation from the pituitary, but is also centrally released by hypothalamic neurons that have been found to project to extra-hypothalamic sites such as some limbic areas (Landgraf & Neumann, 2004).
The role of oxytocin in generalized stress reduction on the behavioral and neural level is well established. For example, oxytocin knockout mice have higher corticosterone levels and attenuated levels of medial amygdala activity, as indicated by fos immunohistochemistry (Mantella, Vollmer, Rinaman, Li, & Amico, 2004). The psychological stressor (shaker) produced similar increases in c-fos expression in the PVN, BNST, and central amygdala for wild-type and knockout mice, thus the OT mutation only had evident effects in the medial amygdala. Furthermore, it is known that social activities enhance oxytocin release (Carter, 1998) and that central administration of oxytocin inhibits the HPA axis response in prairie voles and rats (DeVries, Cho, Cardillo, & Carter, 1997; Neumann, Kromer, Toschi, & Ebner, 2000; Windle, Shanks, Lightman, & Ingram, 1997). In one study (Detillion, Craft, Glasper, Prendergast, & DeVries, 2004), Siberian hamsters exhibited increased cortisol concentrations in response to a skin wound and subsequent immobilization stress, but only in socially isolated animals. Socially housed ones thus showed a buffering of the HPA axis. Importantly, treating the isolated hamsters with OT eliminated the stress-induced increases in cortisol and facilitated wound healing, implicating OT in HPA axis suppression. These findings are consistent with those from the human literature. For instance, intranasal administration of oxytocin paired with the provision of social support dampens HPA axis responses in human males (Heinrichs et al., 2003). Social support from the romantic partner did not have this effect by itself in women, but when it was paired with a massage from the romantic partner it dampened salivary cortisol responses (Ditzen et al., 2007). Lactating women who breastfed before a laboratory stress task showed blunted ACTH, plasma total cortisol and salivary free cortisol in response to the task (Heinrichs et al., 2001). It is known that suckling leads to a release of OT and prolactin in lactating women.
As we examine possible neural mechanisms for oxytocin’s effect on stress, its action can be direct or indirect. A direct role would be at the level of the HPA axis and via suppression of ACTH (Gibbs, 1986). Indirect effects of oxytocin on social buffering appear more common. When released centrally, oxytocin can be distributed widely in the brain and oxytocin receptors are found throughout the brain. In rats, this includes the ventromedial nucleus of the hypothalamus (VMH), amygdala, pituitary, the lateral septum, BNST, the anterior olfactory nucleus, the preoptic (POA), ventral tegmental areas (VTA), and the hippocampus (Adan et al., 1995; Gimpl & Fahrenholz, 2001; Ostrowski, 1998; Veinante & Freund-Mercier, 1997; Windle et al., 2004). It should also be noted that the receptors either increase or decrease firing of cells that project to many additional brain areas including additional subareas of the PFC such as the anterior cingulate, orbitofrontal and prelimbic areas (Febo, Shields, Ferris, & King, 2009). As a result, rodent models indicate that oxytocin impacts several limbic (amygdala, hippocampus, BNST) and hypothalamic regions and suggest a basic understanding of oxytocin’s direct and indirect effects on attenuating the stress response.
In summary, there are likely many central nervous system (CNS) pathways supporting multiple social buffering pathways, each with distinct characteristics and effects. Both animal models and parallel studies in humans are beginning to uncover these non-mutually exclusive mechanisms. Human studies are limited in the methodologies that they can deploy to investigate CNS processes, however advances in neuroimaging and neuroendocrine assays have allowed for some evidence that the social buffering effects in humans may share some of the same underlying biological mechanisms. While there is some inconsistency in the oxytocin social buffering literature, there is also strong evidence for its ubiquitous effect across species. It seems that the complexity of interactions between the social buffering and stress circuits combines with other myriad factors that influence the likelihood of its occurrence -e.g., social organization of the species, prior social experiences, integration into a social unit, the sex, and the developmental stage of the animal are likely contributing factors (Hennessy, Kaiser, & Sachser, 2009; Kikusui, Winslow, & Mori, 2006).
As we consider animal models of the neural mechanisms of social buffering in early life, it is important to note that many of the brain structures considered important for mediating the attenuation of the stress response in adults are not yet developed in early life. For example, the PFC is a late developing structure in most mammals and is unlikely capable of being involved in social buffering until rat pups are over two or three weeks old. However, in rodents, adult-like social buffering as indicated by attenuation of the stress-induced corticosterone release has been demonstrated in 10-12-day-old rat pups (Moriceau & Sullivan, 2006). Specifically, maternal presence blocks the initiation of the HPA response to a mild shock as indicated by elevated PVN neural activity (2-DG autoradiography) that is blocked if the mother is present. PVN microdialysis, which measured neurotransmitter release during shock showed a robust release of PVN norepinephrine (NE) in 12-day old rat pups, with NE release previously shown to be a potent activation of the HPA axis to produce an increase in corticosterone. However, if the mother was present, these shocked pups showed no increase in PVN NE and no corticosterone increase. Indeed, the mother’s ability to socially buffer pups’ stress response can be overridden by infusion of intra-PVN NE in pups while being shocked, and the social buffering can be mimicked without the maternal presence by blocking PVN NE receptors (Shionoya et al., 2007).
It should be noted that the relationship between the mother and pup is reciprocal and the pups can also influence the mother’s corticosterone level, although in the opposite direction (Deschamps, Woodside, & Walker, 2003; Walker et al., 2004). That is, the mother has a robust stress response with the pups and a SHRP while away from her pups. The ability of social stimuli to control corticosterone is similar in both the mother and infant via modulation of NE in the PVN (Toufexis & Walker, 1996). The robust stress response mounted by the mother presumably enables the mother to protect her pups, for instance through maternal aggression.
The Role of Early Life Experience in Social Buffering
Experience is important for social buffering. The strength of the relationship and previous experience with the social figure greatly impacts the strength of social buffering. For example, while the mother is generally the most potent social buffer, the role of primary caregiver appears to be a critically important variable. For instance, in titi monkeys the father is the infant’s preferred caregiver and the father is the potent social buffer stimulus, not the mother (Hoffman, Mendoza, Hennessy, & Mason, 1995). Furthermore, as social relationships change across development and the role of the caregiver diminishes, the social buffering stimulus can expand to other familiar social figures, such as cage mates (DeVries, DeVries, Taymans, & Carter, 1995; Hennessy, Hornschuh, Kaiser, & Sachser, 2006; Hennessy, Young, O’Leary, & Maken, 2003; Maken & Hennessy, 2009; Mason, 1970). For example, with respect to buffering effects of long-term cohabitation, we know that rats living isolated during adolescence show higher adrenal responses to acute stress in adulthood if they are female and lower reactivity if they are male (Weintraub, Singaravelu, & Bhatnagar, 2010). Thus, there is a clear interaction between experience-dependent changes in the HPA axis and social buffering.
The level of maternal care received by infant animals can also influence oxytocin levels, which in turn, can greatly impact the stress response and social buffering (Bales & Perkeybile, 2012; Carter, 2003). The mother’s level of oxytocin can also influence her maternal care, and variations in maternal care have long been shown to influence brain and behavioral development (Levine, 2005a). In neonatal prairie voles with reduced oxytocin levels, social behavior, adult pair bonding and parenting are reduced (Young, 2009). Research on nonhuman primates suggests that early life experience is important for social buffering. Specifically, adverse rearing conditions (peer rearing) greatly attenuate the ability of social stimuli to socially buffer, although oxytocin was not implicated as the mechanism for this effect (Winslow, Noble, Lyons, Sterk, & Insel, 2003). Importantly, the relationship between effects of early life experience on the development of the HPA axis and the effectiveness of later social buffering highlighted by animal work parallels our understanding of the dynamics of human social buffering.
Some argue that the social buffering effects of oxytocin might be due to experience. Hennessy et al. (2009) speculate that oxytocin release during stressors may be a result of classical conditioning, as pairings of stressors with tactile stimulation or social comforting occur throughout life and start early in development. This hypothesis may also explain the failure of social support to buffer stress responses in some animals deprived of maternal care (Winslow et al., 2003). Specifically, release of oxytocin as a part of the stress response may be a way to restore homeostasis, given its role in HPA-response dampening (Taylor, 2006). The argument is that oxytocin may motivate social behaviors that serve this function, particularly in women, given theoretical proposals that they might lean towards a “tend-and-befriend” coping style (Taylor et al., 2000).
Human Studies of Stress Buffering by Social Support across Development
There is now a vast literature documenting the social regulation of the HPA axis in humans during early development (Gunnar & Donzella, 2002; Gunnar, 2006), but a surprising dearth of studies examining this question in middle childhood and adolescence. Starting in infancy, characteristics of caregiving begin to be associated with aspects of cortisol reactivity. Maternal sensitivity and cooperation while taking a 3-month-old out of the bath influence the infant’s post-stressor cortisol recovery but not the magnitude of cortisol response to the drop in temperature itself, in a study using salivary samples (Albers, Riksen-Walraven, Sweep, & de Weerth, 2008). Some animal studies suggest that cold stressors may have independent access to the HPA axis (Walker, Scribner, Cascio, & Dallman, 1991). It is not only primary caregivers’ behavior that can buffer infants’ stress reactivity. A 30-minute separation from the mother elevates salivary cortisol in 9-month olds, but being with a babysitter who is sensitive and responsive to the infant during this time dampens reactivity compared to being with a babysitter that only responds to the infant when he/she is visibly upset (Gunnar, Larson, Hertsgaard, Harris, & Brodersen, 1992).
It is not clear how early the social buffering effect emerges. In one study, newborns undergoing circumcision 3–5 days after birth showed robust increases in cortisol, but while providing them with a pacifier decreased behavioral distress in half of the babies (i.e., reduced crying), it did not significantly dampen the spike in serum cortisol compared to the no-pacifier group (Gunnar et al., 1981). However, behavioral distress and cortisol responses are not always correlated, such that it is possible for infants to experience a “buffered” cortisol response but still exhibit distress, and vice versa, for infants to show reduced expressions of distress but elevated levels of cortisol reactivity. Little is understood about these dissociations between negative affect, fear, and hormonal stress responses, but further investigations would likely yield important clues about the neurobiological pathways that are involved in the social buffering of the HPA axis, as well as some of the pathways that are not.
Turning from newborns to other studies of the first year of life, one longitudinal study (Gunnar, Mangelsdorf, Larson, & Hertsgaard, 1989) found that maternal separation did not elevate levels of salivary cortisol in 9-month-olds but did so when they were 13 months old. Maternal presence also does not prevent salivary cortisol elevations to inoculations in 2-, 4-, and 6-month-olds (Gunnar, Brodersen, Krueger, & Rigatuso, 1996), but by the time these same infants are 18 months old having a secure attachment figure present moderates the link between fearful child temperament and cortisol reactivity; children who are both fearful and insecurely attached to the parent who is present have higher salivary cortisol levels in response to both inoculations and the Strange Situations procedure, which involves brief separations from and then reunions with the attachment figure (Gunnar, Brodersen, Nachmias, Buss, & Rigatuso, 1996). The interaction between secure attachment relationships and child temperament in predicting HPA axis responses have been replicated by other studies as well (Nachmias, Gunnar, Mangelsdorf, Parritz, & Buss, 1996; Spangler & Schieche, 1998). Importantly, these studies show that maternal presence dampens cortisol reactivity to threats even when the children exhibit fear behaviorally (Nachmias et al., 1996). Similar results are observed in naturalistic situations, with a recent study showing that securely attached toddlers who are accompanied by their mothers during the first few days of child care showed lower levels of salivary cortisol compared to children who are in insecure relationships but still accompanied by the mothers (Ahnert, Gunnar, Lamb, & Bartel, 2004). Secure attachments are considered to reflect a history of sensitive, responsive, and consistent caregiving (Ainsworth, Bell, & Stayton, 1974), whereby the child learns that the parent is available and will respond to his/her needs.
Touch is another factor that can dampen cortisol reactivity in infants. For instance, 6-month-olds whose mother provides tactile stimulation during a still-face paradigm (a distressing event for the infant during which the mother suddenly displays a still face) have lower cortisol reactivity and decreased salivary cortisol levels after this episode compared to infants who are not touched during the procedure (Feldman, Singer & Zagoory, 2010). Premature infants, who are frequently separated from their mothers for long periods of time after birth, greatly benefit from daily massage therapy, which lowers their salivary cortisol levels and promotes their growth (Field, 1995). Finally, tactile stimulation has a multitude of beneficial effects for all infants, including the facilitation of calm states as indicated by reductions in serum levels of norepinephrine and epinephrine, as well as in urinary cortisol levels, and the regulation of sleeping patterns through increases in levels of melatonin (for a review, see Underdown, Barlow, & Stewart-Brown, 2010).
In conclusion, there is little evidence of social buffering of adrenocortical responses early in the first year of life, but reliable effects have been repeatedly documented by the end of this first year. Social buffering may require a certain level of cognitive development for infants to experience social referencing and a more solid organization of the attachment system. This may suggest that the PFC is critical for the type of social buffering observed in human development. It is also possible that these developmental processes –attachment and social referencing– could facilitate different types of social buffering effects. For instance, we know that 12-month-olds are capable of using affective cues from the mother (positive, neutral, but especially negative) to decide whether to play with or avoid toys later in a free-play situation (Hornik, Risenhoover, & Gunnar, 1987). This may explain some instances in which children are not afraid of stimuli in the presence of the mother. However, for instances such as inoculations described in the studies reviewed above, the mother’s affective cues do not seem to make the event appear less threatening and indeed most children still show fear despite the fact that older children no longer show cortisol responses in the presence of the mother. Thus, most social buffering effects cannot be explained by social referencing. Furthermore, Nachmias et al. (1996) have suggested that securely attached infants have more coping resources, because they know they can rely on their caregivers to protect them from threat, and we know from both the older infant and adult human studies that availability of coping resources predicts lower adrenocortical responses. However, it must be noted that extended separations (e.g., first few days in child care) produce salivary cortisol elevations 60 minutes from mothers’ departure in all children (Ahnert et al., 2004), thus the social buffering effects of attachment may be limited to briefer separations.
Despite the richness of data on the early social regulation of the HPA axis (for reviews of additional studies, see Gunnar & Donzella, 2002; Gunnar, 2006), there are no human prospective longitudinal studies that objectively assess early caregiving and then HPA axis function from infancy to adulthood, to explore the long-term impact of early caregiving on stress reactivity. There are also no studies tracking social buffering effects from infancy to childhood or adolescence.
However, there are some longitudinal studies of children who experience early social deprivation in orphanages worldwide and later experience enriched social environments through adoption. These children initially show alterations in HPA axis function and subsequent show blunted cortisol levels in saliva suggestive of downregulation due to chronic stress (Carlson & Earls, 1997; Gunnar, 2001) which nevertheless seem to somewhat normalize after being adopted into benevolent families (Dozier et al., 2006; Fisher, Stoolmiller, Gunnar, & Burraston, 2007). However, by 6–7 years after being adopted by families, children from this population tend to exhibit elevated basal cortisol levels (Gunnar, Morison, Chisholm, & Schuder, 2001), particularly if they were growth stunted at adoption (Johnson, Bruce, Tarullo, & Gunnar, 2011). Furthermore, a study of 4–5 year olds adopted internationally after social deprivation in orphanage care revealed higher levels of urinary cortisol after a laboratory interpersonal interaction with their mother compared to that with a stranger, which was the opposite of the pattern observed in non-adopted children (Wismer Fries, Shirtcliff, & Pollak, 2008). Even though this study had a small sample size (18 adopted, 21 non-adopted children), the findings are noteworthy as they show not only the cortisol-buffering effect of the parent for non-adopted children, but also the failure of this protective mechanism to occur in children who did not experience a normative attachment relationship early in life.
The existence of a social buffering of the HPA axis in humans is also documented by a small number of experimental studies in older children. One study of 7–12 year olds girls and their mothers showed that a phone conversation with the mother after the Trier Social Stress Test (TSST; performing a speech in front of an audience and a mental arithmetic task) is sufficient to produce lower salivary cortisol levels post-stressor, and also results in higher levels of urinary oxytocin (Seltzer, Ziegler & Pollak, 2010). Girls who had their mother present showed even stronger cortisol dampening and higher oxytocin release. In another study with 10–11-year-olds, events reported in daily diaries were associated with lower levels of cortisol if children were with a best friend at the time of the event (Adams, Santo, & Bukowski, 2011).
In adults, one study found that men but not women display a reduced salivary cortisol response to the TSST when preparing a speech with their romantic partner (Kirschbaum et al., 1995). The finding in men has been replicated and a more recent study shows an enhanced buffering effect of social support with intranasal administration of oxytocin (Heinrichs et al., 2003). As noted earlier, in women partner support coupled with a brief neck and shoulder massage had the same cortisol-dampening effect (Ditzen et al., 2007). These sex differences are inconsistent with the study of 7–12-year-old girls who show social buffering of cortisol reactivity through telephone conversations with their mothers (Seltzer et al., 2010). Do adult females not exhibit buffering of cortisol reactivity with mere provision of social support, or is it simply the case that their adult male partners did not have comparable supportive actions to those provided by mothers to girls? Studies are needed to examine this question empirically, for instance by recruiting a best female friend to provide the support. However, the studies by Seltzer et al. and Ditzen et al. are consistent in showing that adding a physical comfort component seems to magnify the effect in females in both age groups.
With respect to the psychological factors that could be moderating the effect of social support on cortisol reactivity, one experimental study investigated adult attachment security as a potential moderator and it failed to show any effects. This runs contrary to predictions based on social developmental literature (see the section titled Social Support Dimensions above), but it might be explained by the fact that adult attachment was only assessed with a self-report measure (Ditzen et al., 2008). Observational measures of the quality of a major adult relationship might be able to lend support to this prediction, but again a direct empirical examination is needed. More research is also needed to examine stress buffering effects across development, especially throughout childhood and adolescence, and to understand these sex differences in the social modulation of the HPA axis.
Despite the evidence that the HPA-buffering effect occurs in humans, little is understood about the neural processes that underlie this effect. Neuroimaging studies exploring these processes have been primarily conducted with adults. These studies provide hints about the circuitry relevant for regulating the stress response in general and they have implicated the mPFC (Buchanan et al., 2010), hippocampus (Pruessner et al., 2008), and amygdala (Taylor et al., 2008), though other neural regions have been correlated with stress response regulation. No studies have yet directly assessed neural activation correlated with social support provision in the laboratory, but we review studies that may indirectly measure related processes. In the next section, we divide the relevant literature in three parts: 1) neuroimaging studies of the regulation of HPA responses to laboratory stressors; 2) neuroimaging studies of general negative affect regulation tasks; and 3) neuroimaging studies of pain and social pain regulation tasks. We include the last two categories despite their failure to measure HPA axis responses because they provide evidence of buffering against negative affect or pain through social support and because they involve some of the same circuitry linked to the regulation of HPA responses. We adopt a definition of negative affect as referring to “unpleasant feeling states that are accompanied by both behavioral and physiological changes” (Stone & Gorin, 2000, p. 8). One such negative affect is pain. Physical pain is defined as “the unpleasant experience that is associated with actual or potential tissue damage” (IASP, 1994, p. 209), whereas social pain has been defined as “the unpleasant experience that is associated with actual or potential damage to one’s sense of social connection or social value” (Eisenberger, 2012, p. 421). In this review we focus on pain as an affective state supported by cortical and cortical-subcortical circuits, not the nociceptive responses triggered by noxious stimuli, as research has shown these are dissociable components of pain (Eisenberger, 2012). Both pain and other types of negative affect (sadness, anger, fear, etc.) have the potential to constitute stressors and to trigger the activity of stress-mediating systems such as the HPA axis, but they do not necessarily always do so. This depends on whether they are perceived as a “threat to the physiological or psychological integrity of an individual” (McEwen, 2000, p. 508) and whether the intensity of the stimulus overwhelms the individual’s capacity to cope, according to most definitions of stress (Lazarus & Folkman, 1984). Thus these studies may be informative for the goals of this review.
Neuroimaging Studies of the Regulation of HPA Responses to Laboratory Stressors
The number of neuroimaging studies examining the neural correlates of exposure to a laboratory stress task has quickly burgeoned in the past decade (for a review, see Dedovic, D’Aguiar, & Pruessner, 2009). However, only some of the studies have measured cortisol to ascertain an HPA response (e.g., Dedovic et al., 2005; Pruessner et al., 2008; Pruessner, Champagne, Meaney, & Dagher, 2004; Soliman et al., 2008; Wang et al., 2005, 2007). These studies attempt to infer regulatory roles for various neural regions by correlating changes in neural activation with changes in cortisol levels. Whether these inferences are always correct remains to be determined, but these studies are laying the groundwork for more complex hypotheses. The most consistent findings across these studies were the association between the cortisol response and changes in activation in orbitofrontal cortex, ventral right PFC, the anterior cingulate cortex, and deactivation of limbic regions, especially the hippocampus (Pruessner et al., 2008). Interestingly, one of these studies measured early life care using self-report and showed that participants reporting low parental care had higher dopamine release in the ventral striatum compared to high parental care subjects, and dopamine release was correlated with the magnitude of the salivary cortisol response to a laboratory stressor (Pruessner et al., 2004). Some of these studies also report sex differences in the circuitry activated or deactivated by various stressor paradigms (e.g., Wang et al., 2007), with one study demonstrating that the pattern of sex differences depends on female hormonal cycle, suggesting that there are powerful hormonal effects on the neural circuitry that impinges on the HPA axis (Goldstein et al., 2010).
To our knowledge, only two studies in humans have examined the neural structures associated with the social regulation of the HPA axis response to a stressor, and these have only measured social support indirectly. The first relevant study was conducted by Taylor et al. (2008) and used fMRI to reveal greater right ventrolateral PFC activation and lower amygdala activation during a threat regulation task for participants with high psychosocial resources, who were also more likely to have diminished salivary cortisol reactivity to the Trier Social Stress Test conducted outside the scanner. Psychosocial resources were defined as a composite measure of self-esteem, optimism, and a sense of psychological control/mastery. Interestingly, this study also showed that individuals with high psychosocial resources were not different in their sensitivity to detect threat or their amygdala activation to it, but only in the regulation of threat condition. Given the previously mentioned links between self-esteem and one’s social history, this study may have measured a proxy for the lifelong availability of social support in the form of psychosocial resources. One study with adults has shown that early parental care predicts both hippocampal size and self-esteem (Engert et al., 2010). However, this study measured early parental care using self-report, making it impossible to rule out the possibility that individuals with lower self-esteem may be biased in their report of early life experiences and more prone to experience stressful life events, thus leading to hippocampal atrophy. Both younger and elderly adults with low self-esteem have smaller hippocampi compared to adults with high self-esteem (Pruessner et al., 2005), but again causality is not clear in any of these studies.
The second relevant study found that individuals having frequent interactions with supportive others across a 10-day period showed both reduced salivary cortisol reactivity to the Trier Social Stress Test and diminished dorsal ACC (dACC) and Brodmann’s Area 8 activation (frontal cortex) in response to a social rejection paradigm (Eisenberger et al., 2007). The social rejection paradigm used in this study is called Cyberball and it is frequently used in a neuroimaging environment (Williams, Cheung, & Choi, 2000; Williams & Jarvis, 2006). It involves playing a computer game of ball tossing with two virtual (pre-programmed) others, and suddenly being excluded from receiving the ball by the two other presumed players. Even though the individual differences in neural activation found in this study mediated the relationship between self-reported social support and the cortisol response to the TSST, these measures were not collected at the same time and are correlational in nature. Thus we ultimately cannot conclude that social support orchestrates the HPA response by activating these regions, but this study is an important first step. While animal studies discussed above also suggest a role for the PFC subareas, these studies were also based on correlations.
Neuroimaging Studies of General Affect Regulation Tasks
An increasing number of studies are attempting to link specific neural regions with various emotion regulation tasks. For instance, studies show an inverse coupling of ventromedial PFC (vmPFC) and amygdala activation during successful regulation of negative affect during laboratory tasks in both younger (Ochsner & Gross, 2008) and older adults (Urry et al., 2006). Many of these tasks are very simple –for instance, participants are asked to engage in reappraisal when exposed to an image with a negative valence. In one study, adults reared in environments marked by harsh parenting and conflict showed elevated right ventrolateral PFC (vlPFC) and amygdala activation in response to angry/fearful faces, whereas participants from non-risk homes showed increased right vlPFC activity coupled with decreased amygdala activation to these faces, a pattern associated in many studies with regulation of negative affect (Taylor et al., 2006). These studies did not measure cortisol, but they involve the same type of inverse coupling of prefrontal and limbic circuitry involved in the regulation of the HPA axis described previously. Stress researchers have long acknowledged the fact that cognitive appraisal defines the stressful nature of stimuli (Lazarus, 1993; Lazarus & Folkman, 1984), thus it is not surprising that effortful regulation of negative affect engages similar regions as self-regulation during social stress tasks. Interestingly, early caregiving experiences seem to be associated with alterations in the later functioning of the PFC, the amygdala, and their connections. For instance, some studies of orphanage-reared children show increased amygdala volume compared to normative samples (Tottenham et al., 2010), reduced white matter between the amygdala and the PFC as shown by diffusion tensor imaging (Govindan et al., 2010), as well as reduced glucose metabolism in the infralimbic PFC and orbito-frontal gyrus as shown in a PET study (Chugani et al., 2001).
Few studies have investigated the social regulation of affect in a neuroimaging environment. In one study of threat regulation, adult women were subjected to the threat of electric shock and had their hand held by their husband, a stranger or did not have their hand held by anyone (Coan, Schaefer, & Davidson, 2006). Higher marital quality reported by the woman was related to lower threat-related activation in the right anterior insula, superior frontal gyrus, and hypothalamus during the husband but not the stranger hand-holding condition. These findings suggest some of the circuitry involved in dampening responses to threat, but unfortunately cortisol measures were not taken thus we can only infer that this is one possible pathway that may be involved in reducing HPA axis output, particularly with the finding regarding the hypothalamus.
Neuroimaging Studies of Pain and Social Pain Regulation Tasks
Another rapidly expanding area of research is assessment of the social buffering of pain. These studies (particularly those of social pain or exclusion) could shed some light on the neural circuitry activated by social support. They can also show that social buffering applies to non-social stressors as well, for instance thermal pain. One study found that images of the participant’s romantic partner are sufficient to dampen thermal pain experiences, concurrent with the activation of reward and limbic circuitry including the caudate head, nucleus accumbens, lateral orbitofrontal cortex, amygdala, and dorsolateral PFC (Younger, Aron, Parke, Chatterjee, & Mackey, 2010). Importantly, this experimental manipulation had a more powerful analgesic effect than a simple distraction task. While this study seemed to emphasize the reward-like qualities of images of romantic partners, another study proposed that attachment figures serve as safety signals. Specifically, Eisenberger et al. (2011b) showed that women exposed to images of their long-term romantic partners reported less pain in response to heat stimuli and exhibited lower levels of pain-related neural activation (dACC and anterior insula) as well as increased vmPFC activation, which correlated with relationship duration and perceived partner support. Given previous studies linking vmPFC activation to cues which signal safety through conditioned learning and also to the extinction of conditioned fear (Phelps, Delgado, Nearing, & LeDoux, 2004; Quirk, Garcia, & Gonzalez-Lima, 2006; Schiller, Levy, Niv, LeDoux, & Phelps, 2008), this finding can be interpreted as providing evidence that attachment figures serve as safety signals or stimuli that neutrally prime subsequent responses with safety cues.
Turning to social pain experiments, individuals undergoing the Cyberball social exclusion paradigm who received emotional support by text message showed reduced ventral ACC activity (associated with social pain in previous experiments with adults –e.g., Eisenberger et al., 2003) and increased left lateral and medial PFC activation that seems to be associated with decreased negative affect (Onoda et al., 2009). Developmental differences must be noted here, as studies using similar exclusion paradigms with adolescents involve not only the same regions described above but also a positive relationship between ventral striatum activation and decreased social pain (Masten et al., 2009).
Self-esteem seems to be an important factor in determining the magnitude of the social pain response. Eisenberger et al. (2011a) showed that individuals with low self-esteem exhibit higher dACC and anterior insula activation in response to negative feedback, and individuals whose self-esteem changes from pre- to post-scan also show activity in mPFC, arguably reflecting changes in self-referential thoughts. Onoda et al. (2010) also found that low trait self-esteem correlates with higher dACC activation during a Cyberball task. One fMRI study found that individuals with low self-esteem show a positive linear relationship between ventral ACC and medial PFC activation and the level of positive versus negative feedback received from others, whereas individuals with high self-esteem did not show any relationship between activation in these areas and feedback type (Sommerville, Kelley, & Heatherton, 2010).
Interestingly, one of the most recent studies using this paradigm (DeWall et al., 2011) reported that adult attachment style modulates the neural response to social rejection, such that individuals with anxious attachment styles show increased activation in the dACC and insula, whereas individuals with avoidant attachment show reduced activation in these regions. Unfortunately, the Cyberball social exclusion paradigm does not elevate cortisol in women, who have been deemed to be more vulnerable to social stressors than men in the first place (Zöller, Maroof, Weik, & Deinzer, 2010). Thus, this paradigm cannot be used to test the social buffering of the HPA axis, but it does highlight the importance of the ACC in signaling social pain and its relationship to self-esteem, which has been associated with social support measures. Given the previously mentioned links between the ACC and the HPA axis, it will be important to find paradigms that can both activate the ACC and elevate cortisol.
To summarize, adult neuroimaging studies implicate increased PFC activation (with specific regions differing by task –mPFC, dlPFC, vlPFC, OFC) and decreased limbic activity (amygdala, hippocampus) in negative affect regulation tasks (including pain, fear) that in some cases correlated with HPA axis reactivity. Even though no study to date has used neuroimaging to examine neural activity during the stressor and measure cortisol output to the same stressor, these are biologically plausible pathways given what is known about HPA axis physiology (see Fig. 2).
A Developmental Working Model of the Stress-Dampening Effects of Social Support
Almost two decades have passed since the discovery that support from a romantic partner before a laboratory stress task can dampen cortisol reactivity in adult males (Kirschbaum et al., 1995) and we are still lacking an integrated psychobiological model that explains the social buffering of stress in humans. In this review we highlight many empirical gaps that prevent a definitive description of the mechanisms involved in this complex process and suggest future experiments and directions to address these gaps. Additionally, we propose a working model to guide future research in this area drawing upon the studies of human and non-human animals described thus far. The model (Fig. 1, Table 1) postulates that early social experiences shape the psychobiological efficacy of social support in containing HPA axis responses, and as a consequence the tendency to seek support as a strategy for coping with stress. We propose that two of the biological factors that underpin this efficacy are parameters of oxytocinergic function (levels of OT release, changes in receptor expression and binding across the brain throughout development) and neural priming by attachment figures which involves activation of PFC areas frequently associated with successful emotion regulation and extinction of conditioned fear in adult neuroimaging studies. The neural wirings which facilitate biological interactions between neural systems subserving social behavior and those implementing stress responses (e.g., synaptic contacts between OT- and CRH-expressing neurons in the PVN, as well as the presence of OT receptors on CRH-producing neurons in the PVN and BNST, Dabrowska et al., 2011) are likely to be evolutionarily selected characteristics of humans and other species, given the importance of conspecifics for survival (Carter, 1998; 2003). Thus, the fact that attachment figures can serve as stress buffers is likely a product of both experience-expectant and experience-dependent neurobehavioral development. Evidence that this is an experience-expectant process consists of the fact that attachment to caregivers and retreating to them for safety is a nearly universal phenomenon, with human infants having strong propensities to form attachments even when caregivers are abusive (Bowlby, 1969). However, forming an attachment to a specific caregiver is experience-dependent and requires learning (likely including classical conditioning, but also more complex forms of learning) about the availability of a specific adult in times of distress, with studies showing that the quality of infant attachment is predicted by observations of consistent and sensitive responding to the infants’ needs and especially distress signals (Ainsworth, Bell, & Stayton, 1974). Furthermore, neuroimaging studies show that images of significant others activate the vmPFC (Eisenberger et al., 2011b), which has been implicated in learning safety cues and the extinction of fear conditioning, suggesting a neural basis for the experience-dependent process of associating specific individuals with anti-threat and anti-stress cues. As shown in our model (Table 1), early caregiving experiences lay the foundation for later social competence with peers and romantic partners who can become attachment figures in their own right, which in turn promotes positive emotional outcomes and higher levels of self-esteem and personal control. Models of abnormal rearing (peer rearing in primates, child maltreatment or orphanage-rearing in humans) support the experience-dependent characteristics of the social buffering of stress, given some evidence for an absent social buffering in these instances as well as evidence of co-occurring alterations in OT levels and in the coupling between the PFC and the amygdala (inferences 6, 9 in Table 1). A final point is that there are likely developmental changes in the types of cues triggering the social buffering of stress and by inference also in the neurobiology involved. During early development, sensory cues and homeostatic regulators (e.g., feeding, warmth, tactile stimulation) seem to play an important role in buffering the HPA axis in both human infants and rodents. With brain development and the maturation of the PFC, the simple presence of caregivers or peers seems to be enough in many instances for both human and nonhuman animals. In humans, there is increased complexity with cognitive development, as social partners can provide coping assistance, coach the cognitive reappraisal of stressors, and support the development of self-esteem. Furthermore, experiencing the stress-relieving effects of caregivers early on may teach the organism to seek social partners and may entrain certain neural activation patterns that promote affiliative behaviors across development, thus serving as positive feedback signals in the process of becoming disposed to exhibit a buffering of stress by social stimuli. The core inferences for our model and the corresponding evidence for each is presented below in Table 1 (selected studies from those described in previous sections).
Table 1.
Core inferences for the proposed developmental working model of social buffering.
| Inference | Evidence |
|---|---|
| 1. Sensitive early caregiving predicts later positive self-esteem, emotion regulation, and competent peer/romantic relationships. | |
| a) Children who are securely attached to their parents in infancy exhibit higher emotion regulation, positive affect, self-esteem, and self-reliance in the classroom. | ○ Sroufe, Fox, and Pancake (1983); Sroufe et al. (1984); Sroufe, 2005 |
| b) Securely attached infants are more likely to have competent peer relations across childhood and report higher levels of social support in late adolescence. | ○ Carlson et al. (2004); |
| c) Quality of caregiving received in the first years of life is related to positive, secure representations of one’s romantic partner in young adulthood (ages 26–28). | ○ Haydon et al. (2012); |
|
| |
| 2. Conversely, abnormal rearing (e.g., child maltreatment) is related to impaired social development. | |
| a) Child maltreatment is associated with insecure attachment patterns in infancy and preschool. | ○ Cicchetti & Barnett (1991); Cicchetti et al. (2006); |
| b) Maltreated children exhibit lower social effectiveness, greater aggression, often eliciting higher peer rejection. | ○ Bolger et al. (1998); Rogosch et al. (1995); |
| c) Adolescents with maltreatment histories show poor emotional regulation, higher rates of drug use, depression, and self-harm. | ○ Scott et al. (2003); |
| d) However, maltreated children who have a close friend show increasing slopes in self-esteem over time. | ○ Bolger et al. (1998); |
|
| |
| 3. Animal models and human studies show that social input can dampen HPA axis reactivity. | |
| a) Rat pups’ corticosterone responses to mild stressors (e.g., electric shock) are blocked if the mother is present. | ○ Shionoya et al. (2007); Stanton & Levine (1990); Suchecki et al. (1993); Wiedenmayer et al. (2003); |
| b) With maturation, other conspecifics (cohabitating companions) begin to show buffering effects in rodents. | ○ Terranova et al. (1999); |
| c) In squirrel monkeys, the presence of familiar and unfamiliar conspecifics dampens corticosterone responses to stressful conditions. | ○ Hennessy (1984, 1986); Vogt, Coe, & Levine (1981); |
| d) Behaviorally inhibited infants who are securely attached to their parents show lower cortisol reactivity to stressors. | ○ Gunnar et al. (1996); Nachmias et al. (1996); |
| e) Men display a reduced salivary cortisol response to a public speaking stressor with support from their romantic partner. Women show the same reduction with the addition of massage. | ○ Ditzen et al. (2007); Kirschbaum et al. (1995); Heinrichs et al. (2003); |
| f) Individuals high in psychosocial resources (including high self- esteem) show lower HPA-axis reactivity to stressors. | ○ Taylor et al. (2008); |
|
| |
| 4. Oxytocin release is stimulated by social contact. | |
| a) OT increases are associated with mating and pair-boding in diverse species (e.g., prairie voles). | ○ Carter (1998, 2003); |
| b) Increases in human participants’ plasma or urinary OT have been associated with maternal/paternal play behaviors with infants, mother-daughter communication by telephone post- stressor in older children. | ○ Gordon et al. (2010); Feldman et al. (2010); Seltzer et al. (2010); |
| c) Expression of trust and generosity towards strangers is associated with increases in plasma OT in laboratory economic games. | ○ Zak et al. (2005); |
| d) Massage increases OT release in adult humans. | ○ Morhenn et al. (2008); |
|
| |
| 5. Oxytocin has stress-reducing properties based on animal models and human studies. | |
| a) Central administration of OT inhibits the HPA axis response in prairie voles and rats. | ○ DeVries et al. (1997); Neumann et al. (2000); Windle et al. (1997); |
| b) Treating socially isolated hamsters with OT eliminates stress- induced increases in glucocorticoids (post-injury) and facilitates wound healing. | ○ Detillion et al. (2004); |
| c) Intranasal administration of oxytocin paired with the provision of social support dampens HPA axis responses in human males. | ○ Heinrichs et al. (2003); |
| d) Lactating women who breastfed before a laboratory stress task showed blunted ACTH, plasma total cortisol, and salivary free cortisol in response to the task. | ○ Heinrichs et al. (2001); |
|
| |
| 6. Early social experience is associated with profiles of later OT release and OT, AVP receptor expression and binding. | |
| a) Communal rearing in female mice results in higher OT receptor binding in the dorsal and ventral lateral septum, BNST, agranular insular cortex, and endopiriform cortex, while AVP V1a binding is reduced in the dorsal lateral septum. | ○ Curley et al. (2009); |
| b) High maternal licking/grooming behavior in rodents is associated with increased OT receptor binding in the medial preoptic areas, BNST, lateral septum, PVN, and CEA for females, and increased AVP V1a binding in the CEA in male offspring. | ○ Champagne & Meaney (2007); Francis et al. (2000, 2002); |
| c) Adult women who have experienced childhood maltreatment exhibit lower levels of OT in cerebrospinal fluid. | ○ Heim et al. (2009); |
|
| |
| 7. Some instances of abnormal rearing in primates and humans are associated with an absent social buffering of the HPA axis. | |
| a) Peer-reared monkeys did not show reductions in cortisol responses to a novel cage in the presence of a social companion. | ○ Winslow et al. (2003); |
| b) Children reared in orphanages did not show the same increases in levels of urinary OT and decreases in cortisol that non-adopted children showed after an interaction with their mothers. | ○ Wismer Fries et al. (2005); |
|
| |
| 8. Prefrontal cortex activation is implicated in stress-buffering studies in animals and in human adult neuroimaging studies of negative affect regulation. | |
| a) Juvenile rhesus monkeys that are socially buffered by their mother while being exposed to a novel environment show increased activity in the PFC. | ○ Rilling et al. (2001); |
| b) Participants high in psychosocial resources show greater right ventrolateral PFC activation and lower amygdala activation during a threat regulation task and diminished salivary cortisol reactivity to the Trier Social Stress Test. | ○ Taylor et al. (2008); |
| c) Individuals reporting frequent interactions with supportive others (across 10 days) show both reduced salivary cortisol reactivity to the TSST and diminished dACC and Brodmann’s Area 8 activation (frontal cortex) in response to social rejection. | ○ Eisenberger et al. (2007); |
| d) Women presented with images of their long-term romantic partner show decreased pain-related neural activation (dACC, anterior insula) and increased vmPFC activation, which correlates with relationship duration and perceived partner support. | ○ Eisenberger et al. (2011b); |
|
| |
| 9. Early caregiving experiences may shape connections between PFC and limbic areas that have excitatory inputs to the HPA axis (e.g., amygdala). | |
| a) Previously institutionalized children show reduced white matter between the PFC and amygdala, enlarged amygdalas, and reduced glucose metabolism in the orbito-frontal gyrus and infralimbic PFC. | ○ Chugani et al. (2001); Govindan et al. (2010); Tottenham et al. (2010); |
| b) Adults who have grown up with harsh parenting and conflict show both elevated right vlPFC and increased amygdala activation in response to angry/fearful faces, whereas participants from non- risk homes showed increased right vlPFC activity coupled with decreased amygdala activation to these faces. | ○ Taylor et al. (2006); |
| c) Animal models show that chronic stress induces dendritic atrophy and decreased GR expression in the medial PFC and the hippocampus (which typically have inhibitory input on the HPA axis), as well as increased dendritic branching in the basolateral amygdala and enhanced CRH expression in the central amygdala. | ○ Ulrich-Lai & Herman (2009); |
Conclusions and Future Directions
The working model we propose is incomplete due to gaps in empirical research highlighted throughout this review, but we suggest several directions for future research that could help illuminate the contingencies associated with the social buffering phenomenon.
Behavioral Assays of Social Support
Behavioral studies in humans need to expand their measurement of different dimensions of social support in order to better understand which ones are beneficial in which context. Most prior studies have instructed significant others to provide support, without objectively assessing the content or nature of the social support provided in the laboratory (for a recent exception, see Meuwly et al., 2012). Dyadic coding of affect, communication patterns, and the nature of supportive interactions would benefit this area of research. Observational measures of both conflict and support elicited in the laboratory need to be combined with existing self-report measures to better understand the “active ingredients” of relational support as it relates to stress reactivity. We predict that encouragement, validation, cognitive restructuring, and instrumental help all likely facilitate reduced stress reactivity, but based on our model we also predict that once we control for the quality and history of the relationship these effects would be reduced, as it is likely that the stress-buffering conferred by the presence of a consistently supportive partner prevails even when their momentary support behavior in the laboratory might not exhibit the same qualities. Conversely, we predict that displays of supportive behavior in the laboratory will be ineffective against the backdrop of an inconsistently supportive or conflicted relationship history. However, studying the specific behaviors of supportive partners that are associated with both a positive relationship history and stress buffering in the laboratory would be useful for future interventions promoting relationship quality.
Cultural Context
There is insufficient information at this time for incorporating cultural context in our model, despite some emerging evidence for its role in predicting the effectiveness of supportive actions and individual differences in types of support provided or desired (see section on Social Support Dimensions above). Measurement of these aspects needs to be more consistently embedded in future research designs, as it is likely that culture plays a major role in the socialization and transmission of culturally-accepted stress-coping strategies.
Other Possible Biological Mediators
There are additional neurobiological systems that could be involved in the social buffering of stress, but their role is currently poorly understood. For instance, dopamine may play a role in human pair bonding, given animal models showing concurrent activation of OT and dopamine in the nucleus accumbens being associated with pair bonding in prairie voles (Young & Wang, 2004). In humans, one study found that images of the participant’s romantic partner activated regions considered critical for reward learning, including the caudate head, nucleus accumbens, lateral orbitofrontal cortex, amygdala, and dorsolateral PFC (Younger et al., 2010). However, to our knowledge no studies have investigated links with the social regulation of HPA axis responses in humans. Furthermore, it seems that social-affiliative behaviors share neurochemical control with other behavioral systems, being influenced by endogenous opioids, ascending serotonin and norepinephrine systems, as well as fear neural systems that can be observed during separation distress in older infancy as one example (Panksepp, Nelson, & Bekkedal, 1997). There is a rich animal literature on the role of opioids in distress associated with social isolation (Eisenberger, 2012), but it has been difficult to study parallel processes in humans.
Beyond the HPA Axis
Studies have also shown that social partners can decrease stress-responses mediated by the autonomic nervous system, with much of the early work in this area showing significant effects of experimentally-provided social support on heart rate, systolic blood pressure, diastolic blood pressure, and skin conductance measures collected during a stressor paradigm (Lepore, 1998; Thorsteinsson & James, 1999). These studies are informative for our current review on the HPA axis, as they expose some pitfalls in this area of research. For instance, some of these experimental studies have caused increased cardiovascular reactivity by having the friend or stranger present during the stress task, thus potentially increasing the social-evaluative threat to the participant. Furthermore, even though social support provided before the stressor by a stranger or best friend can diminish cardiovascular reactivity in women (Fontana, Diegnan, Villeneuve, & Lepore, 1999), the quality of the relationship with the friend moderates the effect (Uno, Uchino, & Smith, 2002). Studies of the HPA axis need to incorporate this insight and measure relationship quality in these experimental set-ups.
Developmental Questions
Future studies would also benefit from applying a developmental framework to the study of social support and its stress buffering effect. We need to begin to developmentally track the quality of participants’ relationships over time, and how they impact later HPA axis reactivity, analyzing their bidirectional connections at different time points. It is likely that there will be significant associations between social relationships and HPA responsiveness across development, with moderate continuity from early caregiving experiences to later stress reactivity given relations between early and later social development (see Table 1). There is a striking dearth of information on the social HPA-buffering effect in middle childhood and adolescence, and no information on the developmental precursors of the effect observed in adults. For instance, we do not understand how social experiences across the lifespan may lead to the noted associations between positive self-esteem and lower cortisol responses to social stressors in adults, but we expect that these associations are mediated by a sense of personal control/predictability that should be positively correlated with consistent parenting early in life. These studies are crucial if we are to understand how individual differences in receptivity to the benefits of social support emerge.
Sex Differences
Despite sexually dimorphic effects in neuropeptide actions, HPA axis functioning, as well patterns of support-seeking and coping with stress based on differential socialization of the two genders, this area of research remains perhaps the most poorly understood of all reviewed here. We reported several studies in humans which examined buffering effects only in males or only in females, making it difficult to draw direct comparisons. One of the challenges hindering this area of research is that we need sample sizes large enough to study these processes in both males and females. The second challenge is that it has been more convenient to exclude one of the genders than to include physiological measures of sex steroids or to control for stage of menstrual cycle. However, we propose that a developmental approach may help further this area. By investigating when these divergences among the sexes emerge, we expect that we may be more prepared to understand sex differences in the social buffering of stress. For instance, we predict that differences in social support behavior due to socialization should already be notable in early childhood, while some of the effects of sex steroids would become more prominent starting with puberty.
Neuroimaging Studies
In order to better understand the neurobiology of the social buffering of the HPA axis, neuroimaging studies in adults need to experimentally expose participants to a laboratory stressor with social support provided by a stranger, significant other, or no social support provided. This has yet to be done in a neuroimaging context with measurement of glucocorticoids, even though we now have stress tasks appropriate for this environment (e.g., the Montreal Neuroimaging Stress Test –Dedovic et al., 2005) and there is the possibility of text messaging, vocal or physical support provided by a partner while in the scanner. Additionally, we need better mapping of the timing of the activation of different neural regions, as some of the studies reviewed measure activation over very short periods (Wang et al., 2005) and may tap into anticipatory cognitive processes pre-stressor, while others measure activation over longer timeframes (Kern et al., 2008), capturing negative-feedback processes after the production of GCs and their binding to PFC or limbic receptors, thus specific regions activated sometimes differ across studies. We also need to expand our arsenal of stressor paradigms that activate the HPA axis in the scanner, in order to investigate whether social support has a general buffering effect of both social and non-social stressors.
Animal models will remain essential in testing cellular and molecular mediators, as well as more conclusive probes into underlying neural function. The largest gap in this literature concerns the effect of early rearing on later predisposition to social buffering effects, as most studies have focused on the effects of rearing on later stress reactivity but not on its social regulation (for an exception, see Winslow et al., 2003).
The Role of Genetics
In humans, there is increasing evidence that genetic variations in the oxytocin receptor gene may underlie some individual differences in human social behavior and receptivity to social support under stress. For instance, Chen et al. (2011) have shown that an oxytocin receptor gene (OXTR) polymorphism moderates the magnitude of the social buffering effect in adult human males, such that only individuals with one or two G alleles on the rs53576 polymorphism of the OXTR gene showed lower salivary cortisol levels in response to a social evaluative stressor task when receiving support, compared to individuals receiving no support. Furthermore, parents with two copies of the G allele on the rs53576 polymorphism of the OXTR gene or at least one copy of the long allele of the serotonin transporter gene (5-HTT) displayed more sensitive behavior to their 2-year-olds (Bakermans-Kranenburg & van Ijzendoorn, 2009). The exact functionality of these genes is not fully known, and more studies are needed to link genetic variations to neural function and to receptivity to this effect.
The studies described above are essential for developing a more complete and integrated model of the stress buffering effect of social support that incorporates neuroendocrine, genetic, behavioral, and social levels of analysis. Given widely recognized benefits of social support for broader health outcomes (Cassel, 1976; Cobb, 1976; Cohen, 1988, 2004; Cohen & Wills, 1985; Kiecolt-Glaser, Gouin & Hantsoo, 2010; Reblin & Uchino, 2008; Taylor, 2007; Thoits, 1995, 2011; Uchino, Caccioppo, & Kiecolt-Glaser, 1996) and an increasingly rich knowledge base concerning the deleterious effects of stress on health (McEwen, 2008; McEwen & Gianaros, 2011; Miller, Chen, & Parker, 2011), examining the regulation of the HPA axis by social relationships across the lifespan may prove informative. With an understanding of both behavior and the underlying biology, interventions that deploy social support to relieve stress would be able to select the most effective behaviors and use biomarkers to evaluate their effectiveness in relation to the goal of reducing the burden of stress and mental or physical illness.
Acknowledgments
This work was supported by the Eva O. Miller Fellowship to the first author and, in part, by grants NIH-DC009910, NIH-MH091451 to RMS, and grants NIH-MH080905, NIH-MH078105 to MRG.
Contributor Information
Camelia E. Hostinar, Institute of Child Development, University of Minnesota, Minneapolis, MN
Regina M. Sullivan, Emotional Brain Institute, Nathan S. Kline Institute, Department of Child and Adolescent Psychiatry, NYU Langone Medical Center, New York NY
Megan R. Gunnar, Institute of Child Development, University of Minnesota, Minneapolis, MN
References
- Adams RE, Santos JB, Bukowski WM. The presence of a best friend buffers the effects of negative experiences. Developmental Psychology. 2011;47(6):1786–1791. doi: 10.1037/a0025401. [DOI] [PubMed] [Google Scholar]
- Adan R, Van Leeuwen F, Sonnemans M, Brouns M, Hoffman G, Verbalis JG, Burbach JP. Rat oxytocin receptor in brain, pituitary, mammary gland, and uterus: Partial sequence and immunocytochemical localization. Endocrinology. 1995;136:4022–4028. doi: 10.1210/en.136.9.4022. [DOI] [PubMed] [Google Scholar]
- Ahnert L, Gunnar M, Lamb M, Barthel M. Transition to child care: Associations with infant-mother attachment, infant negative emotion and cortisol elevations. Child Development. 2004;75:639–650. doi: 10.1111/j.1467-8624.2004.00698.x. [DOI] [PubMed] [Google Scholar]
- Ainsworth MDS, Bell SM, Stayton DJ. Infant–mother attachment and social development: Socialisation as a product of reciprocal responsiveness to signals. In: Richards MPM, editor. The integration of a child into a social world. New York: Cambridge University Press; 1974. pp. 99–137. [Google Scholar]
- Albers EM, Riksen-Walraven JM, Sweep FC, de Weerth C. Maternal behavior predicts infant cortisol recovery from a mild everyday stressor. Journal of Child Psychology & Psychiatry. 2008;49:97–103. doi: 10.1111/j.1469-7610.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- Armario A, Luna G, Balasch J. The effect of conspecifics on corticoadrenal response of rats to a novel environment. Behavioral and Neural Biology. 1983;37:332–337. doi: 10.1016/s0163-1047(83)91425-5. [DOI] [PubMed] [Google Scholar]
- Armario A, Ortiz R, Balasch J. Corticoadrenal and behavioral response to open field in pairs of male rats either familiar or non-familiar to each other. Experientia. 1983;39:1316–1317. doi: 10.1007/BF01990391. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience. 2009;10(6):410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Social Cognitive and Affective Neuroscience. 2008;3:128–134. doi: 10.1093/scan/nsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: Role in stress responsivity and other behaviors. Annual Review of Pharmacology and Toxicology. 2004;44:525–527. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bales KL, Perkeybile AM. Developmental experiences and the oxytocin receptor system. Hormones and Behavior. 2012;61(3):313–9. doi: 10.1016/j.yhbeh.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Barraza JA, Zak PJ. Empathy toward strangers triggers oxytocin release and subsequent generosity. Annals of the New York Academy of Sciences. 2009;1167:182–9. doi: 10.1111/j.1749-6632.2009.04504.x. [DOI] [PubMed] [Google Scholar]
- Barrera M. Distinctions between social support concepts, measures, and models. American Journal of Community Psychology. 1986;14:413–445. Retrieved from http://link.springer.com/article/10.1007%2FBF00922627. [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117(3):497–529. doi: 10.1037/0033-2909.117.3.497. [DOI] [PubMed] [Google Scholar]
- Beaulieu S, Di Paolo T, Barden N. Control of ACTH secretion by the central nucleus of the amygdala: Implication of the serotoninergic system and its relevance to the glucocorticoid delayed negative feedback mechanism. Neuroendocrinology. 1986;44(2):247–254. doi: 10.1159/000124652. [DOI] [PubMed] [Google Scholar]
- Berkman LF, Syme SL. Social networks, host resistance, and mortality: A nine- year followup study of Alameda County residents. American Journal of Epidemiology. 1979;109:186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- Bolger N, Amarel D. Effects of social support visibility on adjustment to stress: Experimental evidence. Journal of Personality and Social Psychology. 2007;92:458–475. doi: 10.1037/0022-3514.92.3.458. [DOI] [PubMed] [Google Scholar]
- Bolger KE, Patterson CJ, Kupersmidt JB. Peer relationships and self-esteem among children who have been maltreated. Child Development. 1998;69(4):1171–97. Retrieved from http://www.jstor.org/stable/1132368. [PubMed] [Google Scholar]
- Bolger N, Zuckerman A, Kessler RC. Invisible support and adjustment to stress. Journal of Personality and Social Psychology. 2000;79:953–961. doi: 10.1037/0022-3514.79.6.953. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Drper SJ, Simerly RB. Foration of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. Journal of Neuroscience. 2004;24:2789–2797. doi: 10.1523/JNEUROSCI.5369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby J. Attachment and loss: Vol. 1: Attachment. New York: Basic Books; 1969. [Google Scholar]
- Buchanan TW, Kern S, Allen JS, Tranel D, Kirschbaum C. Circadian regulation of cortisol after hippocampal damage in humans. Biological Psychiatry. 2004;56:651–656. doi: 10.1016/j.biopsych.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Driscoll D, Mowrer SM, Sollers JJ, Thayer JF, Kirschbaum C, Tranel D. Medial prefrontal cortex damage affects physiological and psychological stress responses differently in men and women. Psychoneuroendocrinology. 2010;35(1):56–66. doi: 10.1016/j.psyneuen.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchheim A, Heinrichs M, George C, Pokorny D, Koops E, Henningsen P, Gündel H. Oxytocin enhances the experience of attachment security. Psychoneuroendocrinology. 2010;34(9):1417–22. doi: 10.1016/j.psyneuen.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC. Perceived social isolation and cognition. Trends in Cognitive Sciences. 2009;13(10):447–454. doi: 10.1016/j.tics.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, Lee HJ, Macbeth AH, Young WS., III Vasopressin: Behavioral roles of an “original” neuropeptide. Progress in Neurobiology. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: A PHAL study in the rat. Journal of Comparative Neurology. 1995;360:213–245. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- Cassel J. The contribution of the social environment to host resistance. American Journal of Epidemiology. 1976;104(2):107–123. doi: 10.1093/oxfordjournals.aje.a112281. [DOI] [PubMed] [Google Scholar]
- Carlson M, Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Annals of the New York Academy of Sciences. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- Carlson EA, Sroufe LA, Egeland B. The construction of experience: A longitudinal study of representation and behavior. Child Development. 2004;75(1):66–83. doi: 10.1111/j.1467-8624.2004.00654.x. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23(8):779–818. doi: 10.1016/S0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter CS. Developmental consequences of oxytocin. Physiology and Behavior. 2003;79(3):383–397. doi: 10.1016/S0031-9384(03)00151-3. [DOI] [PubMed] [Google Scholar]
- Carter CS. Sex differences in oxytocin and vasopressin: Implications for autism spectrum disorders? Behavior and Brain Research. 2007;176:170–186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Carter CS, Altemus M. Integrative functions of lactational hormones in social behavior and stress management. Annals of the New York Academy of Sciences. 1997;807:164–174. doi: 10.1111/j.1749-6632.1997.tb51918.x. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behavioral Neuroscience. 2007;121:1353–1363. doi: 10.1037/0735-7044.121.6.1353. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Barnett D. Attachment organization in maltreated preschoolers. Development and Psychopathology. 1991;3:397–411. doi: 10.1017/S0954579400007598. [DOI] [Google Scholar]
- Cicchetti D, Rogosch FA, Toth SL. Fostering secure attachment in infants in maltreating families through preventive interventions. Development and Psychopathology. 2006;18(3):623–49. doi: 10.1017/S0954579406060317. [DOI] [PubMed] [Google Scholar]
- Chen FS, Kumsta R, von Dawans B, Monakhov M, Ebstein RP, Heinrichs M. Common oxytocin receptor gene (OXTR) polymorphism and social support interact to reduce stress in humans. Proceedings of the National Academy of Sciences. 2011;108(50):19937–19942. doi: 10.1073/pnas.1113079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JM, Kim HS, Mojaverian T, Morling B. Culture and social support provision: Who gives it and why? Personality and Social Psychology Bulletin. 2012;38(3):3–13. doi: 10.1177/0146167211427309. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhasz C, Nagy F, Chugani DC. Local brain functional activity following early deprivation: A study of postinstitutionalized Romanian orphans. NeuroImage. 2001;14:1290–1301. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: Implications for the integration of limbic inputs. Journal of Neuroscience. 2007;27(8):2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nature Reviews Endocrinology. 2009;5:374–381. doi: 10.1038/nrendo.2009. [DOI] [PubMed] [Google Scholar]
- Coan JA, Schaefer HS, Davidson RJ. Lending a hand: Social regulation of the neural response to threat. Psychological Science. 2006;17(12):1032–1039. doi: 10.1111/j.1467-9280.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- Cobb S. Social support as moderator of life stress. Psychosomatic Medicine. 1976;38:300–314. doi: 10.1097/00006842-197609000-00003. Retrieved from http://www.psychosomaticmedicine.org/content/38/5/300.full.pdf. [DOI] [PubMed] [Google Scholar]
- Coe CL, Franklin D, Smith ER, Levine S. Hormonal responses accompanying fear and agitation in the squirrel monkey. Physiology and Behavior. 1982;29(6):1051–1057. doi: 10.1016/0031-9384(82)90297-9. [DOI] [PubMed] [Google Scholar]
- Coe CL, Mendoza SP, Smotherman WP, Levine S. Mother–infant attachment in the squirrel monkey: Adrenal response to separation. Behavioral Biology. 1978;22:256–263. doi: 10.1016/s0091-6773(78)92305-2. [DOI] [PubMed] [Google Scholar]
- Cohen S. Psychosocial models of the role of social support in the etiology of physical disease. Health Psychology. 1988;7(3):269–297. doi: 10.1037/0278-6133.7.3.269. [DOI] [PubMed] [Google Scholar]
- Cohen S. Social relationships and health. American Psychologist. 2004;59:676–684. doi: 10.1037/0003-066X.59.8.676. Retrieved from http://psycnet.apa.org.ezp1.lib.umn.edu/journals/amp/59/8/676.pdf. [DOI] [PubMed] [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychological Bulletin. 1985;98:310–357. doi: 10.1037/0033-2909.98.2.310. [DOI] [PubMed] [Google Scholar]
- Cone RD, Low MJ, Elmquist JK, Cameron JL. Williams Textbook of Endocrinology. Philadelphia, PA: Saunders; 2003. Neuroendocrinology; pp. 81–176. [Google Scholar]
- Cosley BJ, McCoy SK, Saslow LR, Epel ES. Is compassion for others stress buffering? Consequences of compassion and social support for physiological reactivity to stress. Journal of Experimental Social Psychology. 2010;46:816–823. doi: 10.1016/j.jesp.2010.04.008. [DOI] [Google Scholar]
- Cunningham ET, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. Journal of Comparative Neurology. 1988;274:60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- Curley JP, Davidson S, Bateson P, Champagne FA. Social enrichment during postnatal development induces transgenerational effects on emotional and reproductive behavior in mice. Frontiers in Behavioral Neuroscience. 2009;3:1–14. doi: 10.3389/neuro.08.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Ahern TH, Guo J-D, McDonald AJ, Mascagni F, Rainnie DG. Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: Implications for balancing stress and affect. Psychoneuroendocrinology. 2011;36(9):1312–26. doi: 10.1016/j.psyneuen.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBold CR, Sheldon WR, DeCherney GS, Jackson RV, Alexander AN, Vale W, Orth DN. Arginine vasopressin potentiates adrenocorticotropin release induced by ovine corticotropin-releasing factor. Journal of Clinical Investigation. 1984;73:533–8. doi: 10.1172/JCI111240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, D’Aguiar C, Pruessner JC. What stress does to your brain: A review of neuroimaging studies. The Canadian Journal of Psychiatry. 2009;54(1):6–15. doi: 10.1177/070674370905400104. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19175975. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, Pruessner JC. The Montreal Imaging Stress Task: Using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. Journal of Psychiatry and Neuroscience. 2005;30:319–325. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16151536. [PMC free article] [PubMed] [Google Scholar]
- Deschamps S, Woodside B, Walker CD. Pups presence eliminates the stress hyporesponsiveness of early lactating females to a psychological stress representing a threat to the pups. Journal of Neuroendocrinology. 2003;15:486–497. doi: 10.1046/j.1365-2826.2003.01022.x. [DOI] [PubMed] [Google Scholar]
- Detillion CE, Craft TK, Glasper ER, Prendergast BJ, DeVries C. Social facilitation of wound healing. Psychoneuroendocrinology. 2004;29:1004–1011. doi: 10.1016/j.psyneuen.2003.10.003. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Cho MM, Cardillo S, Carter CS. Oxytocin can suppress the HPA axis in prairie voles. Society for Neuroscience Abstract. 1997;22:1851. [Google Scholar]
- DeVries AC, DeVries MB, Taymans ST, Carter CS. Modulation of pair bonding in female prairie voles (Microtus ochrogaster) by corticosterone. Proceedings of the National Academy of Sciences. 1995;92:7744–7748. doi: 10.1073/pnas.92.17.7744. Retrieved from http://www.pnas.org/content/92/17/7744.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries GF, Villalba C. Brain sexual dimorphism and sex differences in parental and other social behaviors. Annals of the New York Academy of Sciences. 1997;807:273–286. doi: 10.1111/j.1749-6632.1997.tb51926.x. [DOI] [PubMed] [Google Scholar]
- DeWall CN, Masten CL, Powell C, Combs D, Schurtz DR, Eisenberger NI. Do neural responses to rejection depend on attachment style? An fMRI study. Social Cognitive and Affective Neuroscience. 2011:1–9. doi: 10.1093/scan/nsq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. Journal of Neuroscience. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzen B, Schmidt S, Strauss B, Nater UM, Ehlert U, Heinrichs M. Adult attachment and social support interact to reduce psychological but not cortisol responses to stress. Journal of Psychosomatic Research. 2008;64:479–486. doi: 10.1016/j.jpsychores.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Neumann ID, Bodenmann G, von Dawans B, Turner RA, Ehlert U, Heinrichs M. Effects of different kinds of couple interaction on cortisol and heart rate responses to stress in women. Psychoneuroendocrinology. 2007;32:565–574. doi: 10.1016/j.psyneuen.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biological Psychiatry. 2009;65(9):728–31. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biological Psychiatry. 2007a;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biological Psychiatry. 2007b;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Research. Brain Research Reviews. 2001;38:192–246. doi: 10.1016/S0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dozier M, Peloso E, Lindhiem O, Gordon MK, Manni M, Sepulveda S, Levine S. Developing evidence-based interventions for foster children: An example of a randomized clinical trial with infants and toddlers. Journal of Social Issues. 2006;62:767–785. doi: 10.1111/j.1540-4560.2006.00486.x. [DOI] [Google Scholar]
- Eghbal-Ahmadi M, Avishai-Eliner S, Hatalski CG, Baram TZ. Differential regulation of the expression of corticotropin-releasing factor receptor type 2 (CRF2) in hypothalamus and amygdala of the immature rat by sensory input and food intake. Journal of Neuroscience. 1999;19:3982–3991. doi: 10.1523/JNEUROSCI.19-10-03982.1999. Retrieved from http://www.jneurosci.org/content/19/10/3982.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Muscatell KA, Byrne Haltom KE, Leary MR. The neural sociometer: Brain mechanisms underlying state self-esteem. Journal of Cognitive Neuroscience. 2011a;23(11):3448–55. doi: 10.1162/jocn_a_00027. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Master SL, Inagaki TK, Taylor SE, Shirinyan D, Lieberman MD, Naliboff BD. Attachment figures activate a safety signal-related neural region and reduce pain experience. Proceedings of the National Academy of Sciences. 2011b;108(28):11721–6. doi: 10.1073/pnas.1108239108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. NeuroImage. 2007;35:1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery NJ, Amaral DG. The role of the amygdala in primate social cognition. In: Lane RD, Nadel L, editors. Cognitive neuroscience of emotion. New York: Oxford University Press; 2000. pp. 156–191. [Google Scholar]
- Engert V, Buss C, Khalili-Mahani N, Wadiwalla M, Dedovic K, Pruessner JC. Investigating the association between early life parental care and stress responsivity in adulthood. Developmental Neuropsychology. 2010;35(5):570–581. doi: 10.1080/87565641.2010.494752. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/S0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Febo M, Shields J, Ferris C, King J. Oxytocin modulates unconditioned fear response in lactating dams: An fMRI study. Brain Research. 2009;1302:183–193. doi: 10.1016/j.brainres.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Singer M, Zagoory O. Touch attenuates infants’ physiological reactivity to stress. Developmental Science. 2010;13(2):271–278. doi: 10.1111/j.1467-7687.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: Plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychological Science. 2007;18(11):965–70. doi: 10.1111/j.1467-9280.2007.02010.x. Retrieved from http://www.jstor.org/stable/40064854. [DOI] [PubMed] [Google Scholar]
- Field T. Massage therapy for infants and children. Journal of Behavioral and Developmental Pediatrics. 1995;16:105–111. [PubMed] [Google Scholar]
- Figueiredo HF, Bodie BL, Tauchi M, Dolgas CM, Herman JP. Stress integration after acute and chronic predator stress: Differential activation of central stress circuitry and sensitization of the hypothalamo-pituitary-adrenocortical axis. Endocrinology. 2003;144:5249–5258. doi: 10.1210/en.2003-0713. [DOI] [PubMed] [Google Scholar]
- File SE, Peet LA. The sensitivity of the rat corticosterone response to environmental manipulations and to chronic chlordiazepoxide treatment. Physiology and Behavior. 1980;25:753–758. doi: 10.1016/0031-9384(80)90379-0. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on daytime cortisol activity. Psychoneuroendocrinology. 2007;32:892–905. doi: 10.1016/j.psyneuen.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana AM, Diegnan T, Villeneuve A, Lepore SJ. Nonevaluative social support reduces cardiovascular reactivity in young women during acutely stressful performance situations. Journal of Behavioral Medicine. 1999;22(1):75–91. doi: 10.1023/A:1018751702934. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. Journal of Neuroendocrinology. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Francis DD, Young LJ, Meaney MJ, Insel TR. Naturally occurring Differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: Gender differences. Journal of Neuroendocrinology. 2002;14:349–353. doi: 10.1046/j.0007-1331.2002.00776.x. [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): Facts and future directions. International Journal of Psychophysiology. 2009;72(1):67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer D. A new view on hypocortisolism. Psychoneuoendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Galbally M, Lewis AJ, IJzendoorn M, Permezel M. The role of oxytocin in mother-infant relations: A systematic review of human studies. Harvard Review of Psychiatry. 2011;19:1–14. doi: 10.3109/10673229.2011.549771. [DOI] [PubMed] [Google Scholar]
- Gibbs DM. Stress-specific modulation of ACTH secretion by oxytocin. Neuroendocrinology. 1986;42(6):456–458. doi: 10.1159/000124487. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiological Reviews. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. Retrieved from http://physrev.physiology.org/content/81/2/629.long. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. Journal of Neuroscience. 2010;30(2):431–438. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin and the development of parenting in humans. Biological Psychiatry. 2010;68(4):377–82. doi: 10.1016/j.biopsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan RM, Behen ME, Helder E, Makki MI, Chugani HT. Altered water diffusivity in cortical association tracts in children with early deprivation identified with tract-based spatial statistics (TBSS) Cerebral Cortex. 2010;20(3):561–9. doi: 10.1093/cercor/bhp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunlicks-Stoessel ML, Powers SI. Romantic partners’ coping strategies and patterns of cortisol reactivity and recovery in response to relationship conflict. Journal of Social and Clinical Psychology. 2009;28(5):630–649. doi: 10.1521/jscp.2009.28.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR. Effects of early deprivation: Findings from orphanage-reared infants and children. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. Cambridge, MA: MIT Press; 2001. pp. 617–629. [Google Scholar]
- Gunnar MR. Social regulation of stress in early child development. In: McCartney K, Phillips D, editors. The handbook of early child development. Oxford, UK: Blackwell Publishing; 2006. pp. 106–125. [Google Scholar]
- Gunnar MR, Brodersen L, Krueger K, Rigatuso J. Dampening of adrenocortical responses during infancy: Normative changes and individual differences. Child Development. 1996;67(3):877–889. doi: 10.1111/1467-8624.ep9704150171. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Brodersen L, Nachmias M, Buss K, Rigatuso R. Stress reactivity and attachment security. Developmental Psychobiology. 1996;29:191–204. doi: 10.1002/(SICI)1098-2302(199604)29:3<191::AID-DEV1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Davis EP. Stress and emotion in early childhood. In: Weiner IB, Freedheim DK, Lerner RM, editors. Handbook of Psychology. Hoboken, NJ: Wiley; 2003. pp. 113–134. [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/S0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Fisch R, Korsvik S, Donhowe J. The effect of circumcision on serum cortisol and behavior. Psychoneuroendocrinology. 1981;6(3):269–276. doi: 10.1016/0306-4530(81)90037-8. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Larson M, Hertsgaard L, Harris M, Brodersen L. The stressfulness of separation among 9-month-old infants: Effects of social context variables and infant temperament. Child Development. 1992;63:290–303. doi: 10.1111/1467-8624.ep9207061011. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Mangelsdorf S, Larson M, Hertsgaard L. Attachment, temperament and adrenocortical activity in infancy: A study of psychoendocrine regulation. Developmental Psychology. 1989;25:355–363. doi: 10.1037/0012-1649.25.3.355. [DOI] [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Development and Psychopathology. 2001;13:611–628. doi: 10.1017/S095457940100311X. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez D. Low cortisol and a flattening of expected daytime rhythm: Potential índices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1017/S0954579401003066ER. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez D. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen D, editors. Developmental Psychopathology: Developmental Neuroscience. 2. Vol. 2. New York: Wiley; 2006. pp. 533–577. [Google Scholar]
- Haydon KC, Collins WA, Salvatore JE, Simpson JA, Roisman GI. Shared and distinctive origins and correlates of adult attachment representations: The developmental organization of romantic functioning. Child Development. 2012;83(5):1689–702. doi: 10.1111/j.1467-8624.2012.01801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter S. The construction of the self: A developmental perspective. New York: Guilford; 1999. [Google Scholar]
- Hartup WW, Laursen B. Relationships as developmental contexts: Retrospective themes and contemporary issues. In: Collins WA, Laursen B, editors. Relationships and the developmental significance: The Minnesota Symposia on Child Psychology. Vol. 29. 1999. pp. 13–35. [Google Scholar]
- Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Molecular psychiatry. 2009;14(10):954–8. doi: 10.1038/mp.2008. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry. 2003;54(12):1389–1398. doi: 10.1016/S0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Domes G. Neuropeptides and social behaviour: Effects of oxytocin and vasopressin in humans. Progress in Brain Research. 2008;170:337–350. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Meinlschmidt G, Neumann I, Wagner S, Kirschbaum C, Ehlert U, Hellhammer DH. Effects of suckling on hypothalamic-pituitary-adrenal axis responses to psychosocial stress in postpartum lactating women. Journal of Clinical Endocrinology and Metabolism. 2001;86(10):4798–804. doi: 10.1210/jc.86.10.4798. [DOI] [PubMed] [Google Scholar]
- Hennessy MB. Presence of companion moderates arousal of monkeys with restricted social experience. Physiology and Behavior. 1984;33:693–698. doi: 10.1016/0031-9384(84)90033-7. [DOI] [PubMed] [Google Scholar]
- Hennessy MB. Effects of social partners on pituitary-adrenal activity during novelty exposure in adult female squirrel monkeys. Physiology and Behavior. 1986;38:803–807. doi: 10.1016/0031-9384(86)90046-6. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Hornschuh G, Kaiser S, Sachser N. Cortisol responses and social buffering: A study across the life span. Hormones and Behavior. 2006;49:383–390. doi: 10.1016/j.yhbeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Young TL, O’Leary SK, Maken DS. Social preferences of developing guinea pigs (Cavia porcellus) from the preweaning to the periadolescent periods. Journal of Comparative Psychology. 2003;117:406–413. doi: 10.1037/0735-7036.117.4.406. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Kaiser S, Sachser N. Social buffering of the stress response: Diversity, mechanisms, and functions. Frontiers in Neuroendocrinology. 2009;30:470–482. doi: 10.1016/j.yfrne.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Frontiers in Neuroendocrinology. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical axis. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29(8):1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hofer MA. The effects of brief maternal separations on behavior and heart rate of two- week old rat pups. Physiology and Behavior. 1973;10:423–427. doi: 10.1016/0031-9384(73)90200-X. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Relationships as regulators: A psychobiologic perspective on bereavement. Psychosomatic Medicine. 1984;46:183–197. doi: 10.1097/00006842-198405000-00001. Retrieved from http://www.psychosomaticmedicine.org/content/46/3/183.long. [DOI] [PubMed] [Google Scholar]
- Hoffman KA, Mendoza SP, Hennessy MB, Mason WA. Responses of infant titi monkeys to removal of one or both parents: Evidence for paternal attachment. Developmental Psychobiology. 1995;28:399–407. doi: 10.1002/dev.420280705. [DOI] [PubMed] [Google Scholar]
- Hogan BE, Linden W, Najarian B. Social support interventions: Do they work? Clinical Psychology Review. 2002;22:381–440. doi: 10.1016/S0272-7358(01)00102-7. [DOI] [PubMed] [Google Scholar]
- Holmes CL, Landry DW, Granton JT. Science review: Vasopressin and the cardiovascular system part 1 –Receptor physiology. Critical care (London, England) 2003;7(6):427–34. doi: 10.1186/cc2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornik R, Risenhoover N, Gunnar MR. The effects of maternal positive, neutral, and negative affective communications on infant responses to new toys. Child Development. 1987;58:937–944. Retrieved from http://www.jstor.org/stable/1130534. [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- House JS, Kahn RL. Measures and concepts of social support. In: Cohen S, Syme SL, editors. Social support and health. New York: Academic Press; 1985. pp. 83–108. [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–8. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Merskey H, Bogduk N, editors. International Association for the Study of Pain (IASP. Task Force on Taxonomy in Classification of Chronic Pain, 2nd edition. IASP Press; Washington, DC: 1994. pp. 209–214. [Google Scholar]
- Joëls M, Baram TZ. The neuro-symphony of stress. Nature Reviews Neuroscience. 2009;10(6):459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, Fernandez G, Roozendaal B. Stress and emotional memory: A matter of timing. Trends in Cognitive Sciences. 2011;15(6):280–288. doi: 10.1016/j.tics.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Johnson AE, Bruce J, Tarullo AR, Gunnar MR. Growth delay as an index of allostatic load in young children: Predictions to disinhibited social approach and diurnal cortisol activity. Development and Psychopathology. 2011;23:859–871. doi: 10.1017/S0954579411000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33:517–529. doi: 10.1016/j.psyneuen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton TL. Marriage and health: His and hers. Psychological Bulletin. 2001;127(4):472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Hantsoo LV. Close relationships, inflammation, and health. Neuroscience and Biobehavioral Reviews. 2010;35:33–38. doi: 10.1016/j.neubiorev.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, Mori Y. Social buffering: Relief from stress and anxiety. Philosophical Transactions of the Royal Society. Biological Sciences. 2008;361:2215–2228. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosomatic Medicine. 1995;57:23–31. doi: 10.1097/00006842-199501000-00004. Retrieved from http://www.psychosomaticmedicine.org/content/57/1/23.long. [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Partner’s stress status influences social buffering effects in rats. Behavioral Neuroscience. 2004;118:798–804. doi: 10.1037/0735-7044.118.4.798. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–6. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Korosi A, Baram TZ. The central corticotropin releasing factor system during development and adulthood. European Journal of Pharmacology. 2008;583:204–214. doi: 10.1016/j.ejphar.2007.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn CM, Butler SR, Schanberg SM. Selective depression of growth hormone during maternal deprivation in rat pups. Science. 1978;201:1034–1036. doi: 10.1126/science.684424. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: A dynamic concept of multiple and variable modes of neuropeptide communication. Frontiers in Neuroendocrinology. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lazarus RS. From psychological stress to the emotions: A history of changing outlooks. Annual Reviews of Psychology. 1993;44:1–21. doi: 10.1146/annurev.ps.44.020193.000245. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal, and coping. New York: Springer; 1984. [Google Scholar]
- Leary M. Motivational and emotional aspects of the self. Annual Review of Psychology. 2007;58:317–344. doi: 10.1146/annurev.psych.58.110405.085658. [DOI] [PubMed] [Google Scholar]
- Leary MR, Baumeister RF. The nature and function of self-esteem: Sociometer theory. Advances in Experimental Social Psychology. 2000;32:1–62. doi: 10.1016/S0065-2601(00)80003-9. [DOI] [Google Scholar]
- Leary MR, Tambor ES, Terdal SK, Downs DL. Self-esteem as an interpersonal monitor: The sociometer hypothesis. Journal of Personality and Social Psychology. 1995;68(3):518–530. doi: 10.1037//0022-3514.68.3.518. [DOI] [Google Scholar]
- Lee H-J, Macbeth A, Pagani J, Young S. Oxytocin: The great facilitator of life. Progress in Neurobiology. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore SJ. Problems and prospects for the social support-reactivity hypothesis. Annals of Behavioral Medicine. 1998;20:257–269. doi: 10.1007/BF02886375. [DOI] [PubMed] [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic- pituitary-adrenal axis in the rat. Physiology and Behavior. 2001;73:255–260. doi: 10.1016/S0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005a;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Levine S. Stress: An historical perspective. In: Steckler T, Kalin N, Reul JMHM, editors. Handbook on Stress, Immunology and Behavior. Amsterdam: Elsevier Science; 2005b. pp. 3–23. [Google Scholar]
- Light KC, Smith TE, Johns JM, Brownley KA, Hofheimer JA, Amico JA. Oxytocin responsivity in mothers of infants: A preliminary study of relationships with blood pressure during laboratory stress and normal ambulatory activity. Health Psychology. 2000;19:560–567. doi: 10.1037/0278-6133.19.6.560. [DOI] [PubMed] [Google Scholar]
- Link H, Dayanithi G, Föhr K, Gratzl M. Oxytocin at physiological concentrations evokes adrenocorticotropin (ACTH) release from corticotrophs by increasing intracellular free calcium mobilized mainly from intracellular stores. Oxytocin displays synergistic or additive effects on ACTH-releasing factor or arginine vasopressin-induced ACTH secretion, respectively. Endocrinology. 1992;130:2183–2191. doi: 10.1210/en.130.4.2183. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high- affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Research. 1991;555:220–232. doi: 10.1016/0006-8993(91)90345-V. [DOI] [PubMed] [Google Scholar]
- Ludwig M. Dendritic release of vasopressin and oxytocin. Journal of Neuroendocrinology. 1998;10:881–895. doi: 10.1046/j.1365-2826.1998.00279.x. [DOI] [PubMed] [Google Scholar]
- Luksys G, Sandi C. Neural mechanisms and computations underlying stress effects on learning and memory. Current Opinion in Neurobiology. 2011;21(3):502–508. doi: 10.1016/j.conb.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ. Stress inoculation-induced indications of resilience in monkeys. Journal of Traumatic Stress. 2007;20:423–433. doi: 10.1002/jts.20265. [DOI] [PubMed] [Google Scholar]
- Mantella RC, Vollmer RR, Rinaman L, Li X, Amico JA. Enhanced corticosterone concentrations and attenuated Fos expression in the medial amygdala of female oxytocin knockout mice exposed to psychogenic stress. American Journal of Physiology. Regulatory, integrative and comparative physiology. 2004;287(6):R1494–504. doi: 10.1152/ajpregu.00387.2004. [DOI] [PubMed] [Google Scholar]
- Mason WA. Motivational factors in psychosocial development. In: Arnold WJ, Page MM, editors. Nebraska Symposium on Motivation. University of Nebraska Press; Lincoln: 1970. pp. 35–67. [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, Dapretto M. Neural correlates of social exclusion during adolescence: Understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience. 2009;4(2):143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean PD, Newman JD. Role of midline frontolimbic cortex in production of the isolation call of squirrel monkeys. Brain Research. 1988;450:111–123. doi: 10.1016/00068993(88)91550-8. [DOI] [PubMed] [Google Scholar]
- Maken DS, Hennessy MB. Development of selective social buffering of the plasma cortisol response in laboratory-reared male guinea pigs (Cavia porcellus) Behavioral Neuroscience. 2009;123:347–355. doi: 10.1037/a0015034. [DOI] [PubMed] [Google Scholar]
- Main M, Weston DR. The quality of the toddler’s relationship to mother and to father: Related to conflict behavior and the readiness to establish new relationships. Child Development. 1981;52(3):932–940. Retrieved from http://www.jstor.org/stable/1129097. [Google Scholar]
- McEwen B. Stress, Definition and concepts of. In: Fink G, editor. Encyclopedia of Stress. Vol. 3. San Diego: Academic Press; 2000. pp. 508–509. [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;583:174–185. doi: 10.1016/j.ejphar.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annual Reviews of Medicine. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: Life at the interface between a dynamic environment and a fixed genome. Dialogues in Clinical Neuroscience. 2005;7(2):103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16262207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuwly N, Bodenmann G, Germann J, Bradbury TN, Ditzen B, Heinrichs MJ. Dyadic coping, insecure attachment, and cortisol stress recovery following experimentally induced stress. Journal of Family Psychology. 2012;26(6):937–47. doi: 10.1037/a0030356. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving towards a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morhenn V, Park J, Piper E, Zak P. Monetary sacrifice among strangers is mediated by endogenous oxytocin release after physical contact. Evolution and Human Behavior. 2008;29:375–383. doi: 10.1016/j.evolhumbehav.2008.04.004. [DOI] [Google Scholar]
- Moriceau S, Roth TL, Sullivan RM. Rodent model of infant attachment learning and stress. Developmental Psychobiology. 2010;52(7):651–660. doi: 10.1002/dev.20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neuroscience. 2006;9:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naber F, van Ijzendoorn MH, Deschamps P, van Engeland H, Bakermans-Kranenburg MJ. Intranasal oxytocin increases fathers’ observed responsiveness during play with their children: A double-blind within-subject experiment. Psychoneuroendocrinology. 2010;35(10):1583–6. doi: 10.1016/j.psyneuen.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Nachmias M, Gunnar MR, Mangelsdorf S, Parritz R, Buss KA. Behavioral inhibition and stress reactivity: Moderating role of attachment security. Child Development. 1996;67(2):508–522. doi: 10.1111/1467-8624.ep9605280324. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Panksepp J. Brain substrates of infant-mother attachment: Contributions of opioids, oxytocin, and norepinephrine. Neuroscience and Biobehavioral Reviews. 1998;22(3):437–452. doi: 10.1016/S0149-7634(97)00052-3,. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Brain oxytocin mediates beneficial consequences of close social interactions: From maternal love and sex. In: Pfaff D, Kordon C, Chanson P, Christen Y, editors. Hormones and Social behaviour. Heidelberg: Springer Verlag; 2008. pp. 81–101. [Google Scholar]
- Neumann ID, Kromer SA, Toschi N, Ebner K. Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitaryadrenal axis in male rats: Involvement of hypothalamic and limbic brain regions. Regulatory Peptides. 2000;96:31–38. doi: 10.1016/S0167-0115(00)00197-X. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oitzl S, Champagne DL, van der Veen R, de Kloet ER. Brain development under stress: Hypotheses of glucocorticoid actions revisited. Neuroscience and Biobehavioral Reviews. 2009;34:853–866. doi: 10.1016/j.neubiorev.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Onaka T. Neural pathways controlling central and peripheral oxytocin release during stress. Journal of Neuroendocrinology. 2004;16:308–312. doi: 10.1111/j.0953-8194.2004.01186.x. [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Nakashima K, Nittono H, Ura M, Yamawaki S. Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Social Neuroscience. 2009;4(5):443–454. doi: 10.1080/17470910902955884. [DOI] [PubMed] [Google Scholar]
- Ostrowski N. Oxytocin receptor mRNA expression in rat brain: Implications for behavioral integration and reproductive success. Psychoneuroendocrinology. 1998;23:989–1004. doi: 10.1016/S0306-4530(98)00070-5. [DOI] [PubMed] [Google Scholar]
- Pagel MD, Erdly WW, Becker J. Social networks: We get by with (and in spite of) a little help from our friends. Journal of Personality and Social Psychology. 1987;53:793–804. doi: 10.1037/0022-3514.53.4.793. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Nelson E, Bekkedal M. Brain systems for the mediation of social separation-distress and social-reward: Evolutionary antecedents and neuropeptide intermediaries. In: Carter S, Lederhendler II, Kirkpatrick B, editors. The Integrative Neurobiology of Affiliation. NY: The New York Academy of Sciences; 1997. pp. 78–100. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Prewitt CM, Herman JP. Hypothalamo-pituitary-adrenocortical regulation following lesions of the central nucleus of the amygdala. Stress. 1997;1:263–280. doi: 10.3109/10253899709013746. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9787250. [DOI] [PubMed] [Google Scholar]
- Prewitt CM, Herman JP. Anatomical interactions between the central amygdaloid nucleus and the hypothalamic paraventricular nucleus of the rat: A dual tract-tracing analysis. Journal of Chemical Neuroanatomy. 1998;15:173–185. doi: 10.1016/S0891-0618(98)00045-3. [DOI] [PubMed] [Google Scholar]
- Price JL. Prefrontal cortical networks related to visceral function and mood. Annals of the New York Academy of Sciences. 1999;877:383–396. doi: 10.1111/j.1749-6632.1999.tb09278.x. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: A positron emission tomography study using [11C]raclopride. Journal of Neuroscience. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani Engert V, Pruessner M, Buss C, Lupien S. Deactivation of the limbic system during acute psychosocial stress: Evidence from positron emission tomography and functional magnetic resonance imaging studies. Biological Psychiatry. 2008;63(2):234–240. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Baldwin MW, Dedovic K, Renwick R, Mahani NK, Lord C, Meaney M, Lupien S. Self-esteem, locus of control, hippocampal volume, and cortisol regulation in young and old adulthood. NeuroImage. 2005;28:815–826. doi: 10.1016/j.neuroimage.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, González-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biological Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Reblin M, Uchino BN. Social and emotional support and its implication for health. Current Opinions in Psychiatry. 2008;21(2):201–205. doi: 10.1097/YCO.0b013e3282f3ad89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis HT, Collins WA. Relationships, human behavior, and psychological science. Current Directions in Psychological Science. 2004;13(6):233–237. Retrieved from http://www.jstor.org/stable/20182964. [Google Scholar]
- Reis HT, Collins WA, Berscheid E. The relationship context of human behavior and development. Psychological Bulletin. 2000;126:844–872. doi: 10.1037/0033-2909.126.6.844. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Winslow JT, O’Brien D, Gutman DA, Hoffman JM, Kilts CD. Neural correlates of maternal separation in rhesus monkeys. Biological Psychiatry. 2001;49:146–157. doi: 10.1016/s0006-3223(00)00977-x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11164761. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Postnatal development of the catecholamine inputs to the paraventricular nucleus of the hypothalamus in rats. Journal of Comparative Neurology. 2001;438:411–422. doi: 10.1002/cne.1324. [DOI] [PubMed] [Google Scholar]
- Rogosch FA, Cicchetti D, Aber JL. The role of child maltreatment in early deviations in cognitive and affective processing abilities and later peer relationship problems. Development and Psychopathology. 1995;7(04):591. doi: 10.1017/S0954579400006738. [DOI] [Google Scholar]
- Rook KS. The negative side of social interaction: Impact on psychological well-being. Journal of Personality and Social Psychology. 1984;46:1097–1108. doi: 10.1037/0022-3514.46.5.1097. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Koolhaas JM, Bohus B. Attenuated cardiovascular, neuroendocrine, and behavioral responses after a single footshock in central amygdaloid lesioned male rats. Physiology and Behavior. 1991;50(4):771–775. doi: 10.1016/0031-9384(91)90016-H. [DOI] [PubMed] [Google Scholar]
- Rosal MC, King J, Ma Y, Reed GW. Stress, social support, and cortisol: Inverse associations? Behavioral Medicine. 2004;30:11–21. doi: 10.3200/BMED.30.1.11-22. [DOI] [PubMed] [Google Scholar]
- Salzinger S, Feldman RS, Ng-mak DS, Mojica E, Stockhammer TF. The effect of physical abuse on children’s social and affective status: A model of cognitive and behavioral processes explaining the association. Development and Psychopathology. 2001;13:805–825. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11771909. [PubMed] [Google Scholar]
- Sapolsky RM. Stress and plasticity in the limbic system. Neurochemical Research. 2003;28(11):1735–1742. doi: 10.1023/A:1026021307833. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/er.21.1.55. [DOI] [PubMed] [Google Scholar]
- Seltzer LJ, Ziegler TE, Pollak SD. Social vocalizations can release oxytocin in humans. Proceedings of the Royal Society. Biological Sciences. 2010;277:2661–2666. doi: 10.1098/rspb.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye H. Confusion and controversy in the stress field. Journal of Human Stress. 1975;1(2):37–44. doi: 10.1080/0097840X.1975.9940406. [DOI] [PubMed] [Google Scholar]
- Shionoya K, Moriceau S, Bradstock P, Sullivan RM. Maternal attenuation of hypothalamic paraventricular nucleus norepinephrine switches avoidance learning to preference learning in preweanling rat pups. Hormones and Behavior. 2007;52:391–400. doi: 10.1016/j.yhbeh.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott KL, Wolfe DA, Wekerle C. Maltreatment and trauma: Tracking the connections in adolescence. Child and Adolescent Psychiatric Clinics of North America. 2003;12(2):211–230. doi: 10.1016/S1056-4993(02)00101-3. [DOI] [PubMed] [Google Scholar]
- Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA. From fear to safety and back: Reversal of fear in the human brain. Journal of Neuroscience. 2008;28:11517–11525. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman A, O’Driscoll GA, Pruessner J, Holahan AV, Boileau I, Gagnon D, Dagher A. Stress-induced dopamine release in humans at risk of psychosis: A [11C]raclopride PET study. Neuropsychopharmacology. 2008;33:2033–2041. doi: 10.1038/sj.npp.1301597. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nature Neuroscience. 2006;9(8):1007–1008. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Spangler G, Schieche M. Emotional and adrenocortical responses of infants to the strange situation: The differential function of emotional expression. International Journal of Behavioral Development. 1998;22(4):681–706. doi: 10.1080/016502598384126. [DOI] [Google Scholar]
- Sroufe LA. Attachment and development: A prospective, longitudinal study from birth to adulthood. Attachment and Human Development. 2005;7(4):349–67. doi: 10.1080/14616730500365928. [DOI] [PubMed] [Google Scholar]
- Sroufe LA, Egeland B, Carlson E, Collins WA. The development of the person: The Minnesota study of risk and adaptation from birth to adulthood. New York: Guilford; 2005. [Google Scholar]
- Sroufe LA, Fox N, Pancake V. Attachment and dependency in developmental perspective. Child Development. 1983;54:1615–1627. Retrieved from http://www.jstor.org/stable/1129825. [Google Scholar]
- Sroufe LA, Schork E, Motti F, Lawroski N, LaFreniere P. The role of affect in social competence. In: Izard CE, Kagan J, Zajonc R, editors. Emotions, cognition, and behavior. New York: Plenum; 1984. pp. 289–319. [Google Scholar]
- Stanton ME, Levine S. Brief separation elevates cortisol in mother and infant squirrel monkeys. Physiology and Behavior. 1985;34(6):1007–1008. doi: 10.1016/0031-9384(85)90029-0. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Levine S. Inhibition of infant glucocorticoid stress response: Specific role of maternal cues. Developmental Psychobiology. 1990;23:411–426. doi: 10.1002/dev.420230504. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Wallstrom J, Levine S. Maternal contact inhibits pituitary-adrenal stress responses in preweanling rats. Developmental Psychobiology. 1987;20:131–145. doi: 10.1002/dev.420200204. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Amaral DG. Some observations on cortical inputs to the macaque monkey amygdala: An anterograde tracing study. Journal of Comparative Neurology. 2002;451(4):301–323. doi: 10.1002/cne.10339. [DOI] [PubMed] [Google Scholar]
- Stone AA, Gorin AA. Negative affect. In: Fink G, editor. Definition and concepts ofEncyclopedia of Stress. Vol. 3. San Diego: Academic Press; 2000. pp. 8–11. [Google Scholar]
- Suchecki D, Rosenfeld P, Levine S. Maternal regulation of the hypothalamic- pituitary-adrenal axis in the infant rat: The roles of feeding and stroking. Brain Research. Developmental Brain Research. 1993;75:185–192. doi: 10.1016/0165-3806(93)90022-3. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. Journal of Neuroscience. 1999;19:2834–2840. doi: 10.1523/JNEUROSCI.19-07-02834.1999. Retrieved from http://www.jneurosci.org/content/19/7/2834.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE. Tend and befriend. Biobehavioral bases of affiliation under stress. Current Directions in Psychological Science. 2006;15(6):273–277. doi: 10.1111/j.1467-8721.2006.00451.x. [DOI] [Google Scholar]
- Taylor SE. Social support. In: Friedman HS, Cohen Silver R, editors. Foundations of health psychology. New York: Oxford University Press; 2007. pp. 145–171. [Google Scholar]
- Taylor SE. Mechanisms linking early life stress to adult health outcomes. Proceedings of the National Academy of Sciences. 2010;107(19):8507–8512. doi: 10.1073/pnas.1003890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Burklund LJ, Eisenberger NI, Lehman BJ, Hilmert CJ, Lieberman MD. Neural bases of moderation of cortisol stress responses by psychosocial resources. Journal of Personality and Social Psychology. 2008;95(1):197–211. doi: 10.1037/0022-3514.95.1.197. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural responses to emotional stimuli are associated with childhood family stress. Biological Psychiatry. 2006;60:296–301. doi: 10.1016/j.biopsych.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lerner JS, Sherman DK, Sage RM, McDowell NK. Are self- enhancing cognitions associated with healthy or unhealthy biological profiles? Journal of Personality and Social Psychology. 2003;85:605–615. doi: 10.1037/0022-3514.85.4.605. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Saphire-Bernstein S, Seeman TE. Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychological Science. 2010;21(1):3–7. doi: 10.1177/0956797609356507. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Sherman DK, Kim HS, Jarcho J, Takagi K, Dunagan MS. Culture and social support: Who seeks it and why? Journal of Personality and Social Psychology. 2004;87:354–362. doi: 10.1037/0022-3514.87.3.354. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Welch W, Kim HS, Sherman DK. Cultural differences in the impact of social support on psychological and biological stress responses. Psychological Science. 2007;18:831–837. doi: 10.1111/j.1467-9280.2007.01987.x. Retrieved from http://www.jstor.org/stable/40064822. [DOI] [PubMed] [Google Scholar]
- Terranova ML, Cirulli F, Laviola G. Behavioral and hormonal effects of partner familiarity in periadolescent rat pairs upon novelty exposure. sychoneuroendocrinology. 1999;24:639–656. doi: 10.1016/S0306-4530(99)00019-0. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry T, Nurse M, Gilhooly T, Casey BJ. Prolonged institutional rearing is associated with atypically large amygdala volume and emotion regulation difficulties. Developmental Science. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoits PA. Conceptual, methodological, and theoretical problems in studying social support as a buffer against life stress. Journal of Health and Social Behavior. 1982;23(2):145–159. Retrieved from http://www.jstor.org/stable/2136511. [PubMed] [Google Scholar]
- Thoits PA. Stress, coping, and social support processes: Where are we? What next? Journal of Health and Social Behavior, Extra Issue. 1995:53–79. Retrieved from http://www.jstor.org/stable/2626957. [PubMed]
- Thoits PA. Mechanisms linking social ties and support to physical and mental health. Journal of Health and Social Behavior. 2011;52:145–161. doi: 10.1177/0022146510395592. [DOI] [PubMed] [Google Scholar]
- Thorsteinsson E, James J. A meta-analysis of the effects of experimental manipulations of social support during laboratory stress. Psychological Health. 1999;14:869–886. doi: 10.1080/08870449908407353. [DOI] [Google Scholar]
- Toufexis DJ, Walker CD. Noradrenergic facilitation of the adrenocorticotropin response to stress is absent during lactation in the rat. Brain Research. 1996;737:71–77. doi: 10.1016/0006-8993(96)00627-0. [DOI] [PubMed] [Google Scholar]
- Uchino BN. Social support and health: A review of physiological processes potentially underlying links to disease outcomes. Journal of Behavioral Medicine. 2006;29:377–387. doi: 10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: A review with emphasis on underlying mechanisms and implications for health. Psychological Bulletin. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underdown A, Barlow J, Stewart-Brown S. Tactile stimulation in physically healthy infants: Results of a systematic review. Journal of Reproductive and Infant Psychology. 2010;28(1):11–29. doi: 10.1080/02646830903247209. [DOI] [Google Scholar]
- Uno D, Uchino BN, Smith TW. Relationship quality moderates the effect of social support given by close friends on cardiovascular reactivity in women. International Journal of Behavioral Medicine. 2002;9(3):243–262. doi: 10.1207/S15327558IJBM0903_06. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers HJ, de Kloet ER, Whelan T, Levine S. Maternal deprivation effect on the infant’s neural stress markers is reversed by tactile stimulation and feeding but not by suppressing corticosterone. Journal of Neuroscience. 1998;18(23):10171–10179. doi: 10.1523/JNEUROSCI.18-23-10171.1998. Retrieved from http://www.jneurosci.org/content/18/23/10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier M. Distribution of oxytocin- and vasopressin-binding sites in the rat extended amygdala: A histoautoradiographic study. Journal of Comparative Neurology. 1997;383:305–325. doi: 10.1002/(SICI)1096-9861(19970707)383:3<305::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vogt JL, Coe CL, Levine S. Behavioral and adrenocorticoid responsiveness of squirrel monkeys to a live snake: Is flight necessarily stressful? Behavioral and Neural Biology. 1981;32:391–405. doi: 10.1016/s0163-1047(81)90826-8. [DOI] [PubMed] [Google Scholar]
- Walker CD, Scribner KA, Cascio CS, Dallman MF. The pituitary- adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology. 1991;128(3):1385–1395. doi: 10.1210/endo-128-3-1385. [DOI] [PubMed] [Google Scholar]
- Walker CD, Deschamps S, Proulx K, Tu M, Salzman C, Woodside B, Richard D. Mother to infant or infant to mother? Reciprocal regulation of responsiveness to stress in rodents and the implications for humans. Journal of Psychiatry and Neuroscience. 2004;29:364–382. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15486606. [PMC free article] [PubMed] [Google Scholar]
- Walker CD, Scribner KA, Cascio CS, Dallman MF. The pituitary- adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology. 1991;128(3):1385–1395. doi: 10.1210/endo-128-3-1385. [DOI] [PubMed] [Google Scholar]
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, Detre JA. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proceedings of the National Academy of Sciences. 2005;102:17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Korczykowski M, Rao H, Fan Y, Pluta J, Gur RC, Detre JA. Gender difference in neural response to psychological stress. Social Cognitive and Affective Neuroscience. 2007;2:227–239. doi: 10.1093/scan/nsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub A, Singaravelu J, Bhatnagar S. Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Research. 2010;1343:83–92. doi: 10.1016/j.brainres.2010.04.068. [DOI] [PubMed] [Google Scholar]
- Wiedenmayer CP, Magarinos AM, McEwen BS, Barr GA. Mother lowers glucocorticoid levels of preweaning rats after acute threat. Annals of the New York Academy of Sciences. 2003;1008:304–307. doi: 10.1196/annals.1301.038. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. Journal of Neuroscience. 2004;24:2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/en.138.7.2829. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28:910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- Williams KD, Cheung CKK, Choi W. Cyberostracism: Effects of being ignored over the internet. Journal of Personality and Social Psychology. 2000;79:748–762. doi: 10.1037/0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Williams KD, Jarvis B. Cyberball: A program for use in research on interpersonal ostracism and acceptance. Behavioral Research Methods. 2006;38:174–180. doi: 10.3758/BF03192765. [DOI] [PubMed] [Google Scholar]
- Wismer Fries AB, Shirtcliff EA, Pollak SD. Neuroendocrine dysregulation following early social deprivation in children. Developmental Psychobiology. 2008;50(6):588–599. doi: 10.1002/dev.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wismer Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behaviour. Proceedings of the National Academy of Sciences. 2005;102(47):17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witek-Janusek L. Pituitary-adrenal response to bacterial endotoxin in developing rats. American Journal of Physiology. 1988;255:E525–E530. doi: 10.1152/ajpendo.1988.255.4.E525. Retrieved from http://ajpendo.physiology.org/content/255/4/E525.reprint. [DOI] [PubMed] [Google Scholar]
- Young L. Oxytocin. In: Edgen AM, Pfaff DW, editors. Molecular mechanisms of hormones actions on behavior. San Diego, CA: Academic Press; 2009. pp. 783–802. [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nature Neuroscience. 2004;7(10):1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Younger J, Aron A, Parke S, Chatterjee N, Mackey S. Viewing pictures of a romantic partner reduces experimental pain: Involvement of neural reward systems. PLoS ONE. 2010;5:e13309. doi: 10.1371/journal.pone.0013309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak PJ, Kurzban R, Matzner WT. Oxytocin is associated with human trustworthiness. Hormones and Behavior. 2005;48(5):522–7. doi: 10.1016/j.yhbeh.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Carlson SM, Kesek A. Development of executive function in childhood. In: Nelson C, Luciana M, editors. Handbook of developmental cognitive neuroscience. 2. Cambridge, MA: MIT Press; 2008. [Google Scholar]
- Zenteno-Savin T, Sada-Ovalle I, Ceballos G, Rubio R. Effects of arginine vasopressin in the heart are mediated by specific intravascular endothelial receptors. European Journal of Pharmacology. 2000;410:15–23. doi: 10.1016/S0014-2999(00)00853-0. [DOI] [PubMed] [Google Scholar]
- Zink CF, Meyer-Lindenberg A. Human neuroimaging of oxytocin and vasopressin in social cognition. Hormones and behavior. 2012;61(3):400–9. doi: 10.1016/j.yhbeh.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zöller C, Maroof P, Weik U, Deinzer R. No effect of social exclusion on salivary cortisol secretion in women in a randomized controlled study. Psychoneuroendocrinology. 2010;35:1294–1298. doi: 10.1016/j.psyneuen.2010.02.019. [DOI] [PubMed] [Google Scholar]