Abstract
The adolescent hippocampus is highly vulnerable to alcohol-induced damage, which could contribute to their increased susceptibility to alcohol use disorders. Altered adult hippocampal neurogenesis represents one potential mechanism by which alcohol (ethanol) affects hippocampal function. Based on the vulnerability of the adolescent hippocampus to alcohol-induced damage, and prior reports of long-term alcohol-induced effects on adult neurogenesis, we predicted adverse effects on adult neurogenesis in the adolescent brain following abstinence from alcohol dependence. Thus, we examined neurogenesis in adolescent male rats during abstinence following a four-day binge model of alcohol dependence. Bromodeoxyuridine and Ki67 immunohistochemistry revealed a 2.2-fold increase in subgranular zone cell proliferation after 7 days of abstinence. Increased proliferation was followed by a 75% increase in doublecortin expression and a 56% increase in surviving bromodeoxyuridine-labeled cells 14 and 35 days post-ethanol exposure, respectively. The majority of newborn cells in ethanol and control groups co-localized with NeuN, indicating a neuronal phenotype and therefore a 1.6-fold increase in hippocampal neurogenesis during abstinence. Although these results mirror the magnitude of reactive neurogenesis described in adult rat studies, ectopic bromodeoxyuridine and doublecortin positive cells were detected in the molecular layer and hilus of adolescent rats displaying severe withdrawal symptoms, an effect that has not been described in adults. The presence of ectopic neuroblasts suggests that a potential defect exists in the functional incorporation of new neurons into the existing hippocampal circuitry for a subset of rats. Age-related differences in functional incorporation could contribute to the increased vulnerability of the adolescent hippocampus to ethanol.
Keywords: Alcoholism, Ethanol, Withdrawal
Introduction
Alcohol consumption during adolescence dramatically increases the probability of developing an alcohol use disorder (AUD; Grant and Dawson, 1997). Remarkably, 6.4% of adolescents drink excessively to the point of meeting diagnostic criteria for an AUD (Merikangas et al., 2010). The adolescent brain reacts distinctly to alcohol compared to adults, especially the adolescent hippocampus which appears particularly vulnerable to excessive alcohol intake (White and Swartzwelder, 2004). Alcohol dependent adolescents have reduced hippocampal volumes and binge-drinking adolescents perform worse on hippocampal-dependent tasks (De Bellis et al., 2000; Parada et al., 2011; Schweinsburg et al., 2010). Studies in animal models support that ethanol affects adolescent hippocampal function differently compared to adults. For example, adolescent rats exhibit greater sensitivity to ethanol-mediated inhibition of trace fear conditioning and spatial memory performance (Yttri et al., 2004; Markwiese et al., 1998). In addition, ethanol inhibits NMDA receptor-mediated long-term potentiation of CA1 pyramidal neurons and enhances GABAA receptor-mediated tonic inhibition of dentate gyrus granule neurons to a greater extent in adolescent rats compared to adults (Pyapali et al., 1999; Fleming et al., 2007). These studies support that developmental differences in plasticity contribute to the adolescent hippocampus' vulnerability to ethanol-induced dysfunction.
Adult neurogenesis represents an important form of hippocampal plasticity that is critical for declarative memory consolidation (Deng et al., 2010). Multi-potential neural progenitor cells (NPCs) located in the subgranular zone (SGZ) of the dentate gyrus underlie the continuous generation of granule cells (reviewed in Zhao et al., 2008). Neurogenesis is comprised of distinct mechanisms: cell proliferation, neuronal differentiation, cell migration, survival, and circuitry integration, many of which are targeted by ethanol (reviewed in Nixon, 2006; Nixon et al., 2010). In rodents, ethanol intoxication appears to dose dependently interfere with SGZ cell proliferation and newborn neuron survival (Contet et al., 2013; Crews et al., 2006b; Ehlers et al., 2013; Nixon and Crews, 2002; Nixon, 2006; Nixon et al., 2010; Richardson et al., 2009). However, ethanol intoxication alters neurogenesis differently in adolescents versus adults (McClain et al., 2011a; Morris et al., 2010a). In adult rats, ethanol decreased S-phase (bromodeoxyuridine, BrdU) and G1-M phase (Ki67) cell cycle markers, suggesting that the number of actively proliferating NPCs is reduced by ethanol (Crews et al., 2006a; Nixon and Crews, 2002). In contrast, in adolescents, a four-day binge model of dependence decreased BrdU, but not Ki67, which indicates that ethanol alters cell cycle kinetics rather than reducing the number of proliferating NPCs (McClain et al., 2011a; Morris et al., 2010a).
Alcohol dependence results in distinct effects on neurogenesis during abstinence and it is not known whether developmental differences contribute. Although long term exposure decreases NPC proliferation and neurogenesis for weeks (Ehlers et al., 2013; Richardson et al., 2009; Taffe et al., 2010), the four-day binge model of dependence results in a transient burst in NPC proliferation at one week of abstinence, which produces a substantial increase in neurogenesis (Nixon and Crews, 2004). Interestingly, this increase in neurogenesis after short-term abstinence closely matches the time line for “reactive neurogenesis” described in models of hippocampal injury (Parent et al., 2006). As ethanol-induced hippocampal damage has been suggested to stimulate this reactive neurogenesis event (Nixon and Crews, 2004) and the adolescent hippocampus appears more susceptible to ethanol-induced damage (White and Swartzwelder, 2004), we hypothesized that indices of reactive neurogenesis would be greater in alcohol dependent adolescent rats.
Materials and Methods
Ethanol exposure
Adolescent male Sprague-Dawley rats (n = 99; Charles River Laboratories, Portage, MI) were housed individually and maintained on a 12 h light/dark cycle. Water was available at all times, though chow was removed during the four-day exposure period. All procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee and adhered to the Guide for the Care and Use of Laboratory Animals. Postnatal day 35 rats (mid-adolescence; Spear, 2000) received ethanol (25% w/v, Pharmco-AAPER, Brookfield, CT) in Vanilla Ensure® Plus (Abbott Laboratories, Abbott Park, IL) or isocaloric control diet (42.75% w/v dextrose) intragastrically every 8 h for 4 days following a procedure modified from Majchrowicz (1975) as reported previously (Morris et al., 2010b). The model was chosen because it produces dependence in four days and blood alcohol levels mimic the truly problematic adolescent AUD population (van Hoof et al., 2011). Briefly, an initial 5 g/kg dose of ethanol was given with successive doses determined from intoxication behavior assessed from a 6-point scale (Table S1). Physical dependence was confirmed by scoring withdrawal behavior (Table S2) in 30 min intervals 10–26 h following the last dose, identical to Morris et al., 2010b. Tail blood samples were collected 90 min following the first dose on the third binge day. Blood was centrifuged (1800 × g for 5 min) and stored at −20°C until analysis. Blood ethanol concentrations were determined in triplicate from plasma using an AM1 Alcohol Analyzer calibrated with a 300 mg/dL external standard (Analox, Lunenberg, MA).
Bromodeoxyuridine (BrdU) administration
To label proliferating cells, rats were administered BrdU (300 mg/kg, i.p.; Fluka, Buchs, Switzerland or Roche, Mannheim, Germany) 2, 7, or 14 days following the last dose and were euthanized 2 h later (Fig. 1A). To examine differentiation and neurogenesis, a second group of rats was injected with BrdU at 7 days post-binge and was euthanized 28 days later (Fig. 1B).
Figure 1.

Two BrdU injection paradigms were used. (A) For analysis of SGZ cell proliferation, BrdU (300 mg/kg, i.p.) was injected 2, 7, or 14 days following four-day binge ethanol exposure and rats were euthanized (Sac) 2 hours later. (B) For assessment of neurogenesis, BrdU (300 mg/kg, i.p.) was injected 7 days post-ethanol and rats were euthanized 28 days later. Combining detection of BrdU with cell-type specific markers (GFAP and NeuN) allowed assessment of neuronal differentiation with confocal microscopy.
Immunohistochemistry
Immunohistochemistry followed standard, published procedures (McClain et al., 2011a; Nixon and Crews, 2004). Primary antibody information can be found in Table 1. Briefly, rats were euthanized via anesthetic overdose (Nembutal®; MWI Veterinary Supplies, Nampa, ID) and transcardially perfused with 0.1 M phosphate buffered saline (pH 7.4) followed by 4% paraformaldehyde (pH 7.4). Brains were extracted, post-fixed in 4% paraformaldehyde for 24 h at 4°C, and stored in 0.1 M phosphate buffered saline at 4°C until sectioning. Twelve series of coronal sections were obtained at 40 μm on a vibrating microtome (Leica Microsystems, Wetzlar, Germany) and stored in cryoprotectant at −20°C until use. Single label immunohistochemistry was conducted on free-floating tissue sections to detect BrdU (proliferation), Ki67 (proliferation), doublecortin (immature neurons), and prospero homeobox 1 (granule cells, Prox1). Tissue was washed 3 × 5 min in tris-buffered saline (TBS; 20 mM Tris, 500 mM NaCl, pH 7.5; BioRad Laboratories, Inc., Hercules, CA) and then incubated for 30 min in 0.6% H2O2 to quench endogenous peroxidase activity. Antigen retrieval (standard sodium citrate for 1 h at 65°C; McClain et al., 2011a) was used for Ki67 and Prox1; whereas BrdU required DNA denaturing as described by Nixon and Crews (2002). Nonspecific binding was blocked in buffer (3% normal serum/0.1–0.2% Triton X-100/TBS) then sections were incubated in primary antibody for 16 (BrdU) or 42 h at 4°C. Secondary antibodies were biotinylated horse anti-mouse or goat anti-rabbit IgG (Vector Laboratories) detected with avidin-biotin-peroxidase complex (ABC elite kit, Vector Laboratories) and nickel-enhanced 3,3-diaminobenzidine tetrahydrochloride (Polysciences, Warrington, PA). Ki67 and BrdU stained sections were counterstained in Neutral Red (Fisher Scientific, Pittsburgh, PA) or cresyl violet (Fisher Scientific), respectively. All were coverslipped with Cytoseal® (Richard Allen Scientific, Kalamazoo, MI).
Table 1.
Primary Antibody Information
| Antibody | Host | Conc. | Source, Catalog no. | Used at Post-Binge Time Point (Days) |
|---|---|---|---|---|
| BrdU | Mouse | 0.22 μg/mL | Millipore, Billerica, MA, MAB3424 | 2, 7, 14 |
| BrdU | Rat | 1:400 | Accurate Chemical, Westbury, NY, OBT0030 | 35 |
| Ki67 | Mouse | 1:200 | Vector Laboratories, Burlingame, CA, VP-K452 | 7, 35 |
| DCX | Goat | 0.2 μg/mL | Santa Cruz Biotechnology, Santa Cruz, CA, sc-8066 | 7, 14 |
| NeuN | Mouse | 1:500 | Millipore, MAB377 | 35 |
| GFAP | Rabbit | 1:2,000 | DakoCytomation, Carpinteria, CA, Z 0334 | 35 |
| Prox1 | Goat | 1 μg/mL | R & D Systems, Minneapolis, MN, AF2727 | 14 |
BrdU = bromodeoxyuridine, DCX = doublecortin, NeuN = neuronal nuclei, GFAP = glial fibrillary acidic protein, Prox-1 = prospero homeobox 1
Fluorescent, triple-label immunohistochemistry for BrdU (rat anti-BrdU), neurons (mouse anti-NeuN), and astrocytes (rabbit anti-glial fibrillary acidic protein (GFAP)) was conducted with goat anti-mouse IgG Alexa Fluor 633, goat anti-rabbit IgG Alexa Fluor 546, and goat anti-rat IgG Alexa Fluor 488 (1:200; Invitrogen, San Diego, CA) secondary antibodies with Pro-long® Gold anti-fade reagent (Invitrogen) identical to that previously described (Nixon and Crews, 2004).
Quantification
All slides were coded to ensure that experimenters were blind to treatment conditions. For proliferation studies, BrdU and Ki67 labeling were analyzed on an Olympus BX-41 microscope equipped with an UPlanSApo 100x oil immersion lens (1.4 numerical aperture; Olympus, Center Valley, PA). Only cells within the SGZ (~50 μm ribbon of tissue between the hilus and granule cell layer) of the dorsal dentate gyrus (Bregma −1.8 to −5.52; Paxinos and Watson, 2009) were counted. Data were expressed as cells/section. A profile counting methodology was chosen due to the low number of cells to be counted, the non-homogeneous distribution of these cells in the SGZ, the lack of change in the volume in the dentate gyrus in this model (Leasure and Nixon, 2010), and previous work demonstrating that this method obtains results similar in relative difference to stereology (Crews et al., 2004; Noori and Fornal, 2011). For differentiation studies, profile counts of the number of BrdU+ cells within the granule cell layer were obtained on an Olympus BX-51 microscope with ProScan II motorized stage, microcator, DP70 digital camera, 488λ epifluorescence cube, and PlanApo 40x lens (0.9 numerical aperture, Olympus). Data were reported as cells/section.
DCX immunoreactivity was measured by Visiomorph image analysis software on the BX-51 system (version 3.6.4.0, Visiopharm, Hoersholm, Denmark). Multi-panel images containing the dorsal dentate gyrus were collected at 100x. The region of interest was drawn around the SGZ and granule cell layer, image segmentation was performed, and DCX immunoreactivity was calculated as pixels/mm2. Ectopic DCX and Prox1-positive profile counts were obtained from the hilus and molecular layer using the BX-51 microscope and reported as cells/section.
Triple label immunohistochemistry was quantified similar to previous reports (Morris et al., 2010a) using a laser scanning confocal microscope (Leica TCS SP5; Wetzlar, Germany) equipped with argon, helium-neon 543, and helium-neon 633 lasers. The phenotype of new cells was determined by sampling 50 BrdU+ cells in the granule cell layer of each subject using a 63x oil immersion objective. Z-plane image stacks (1,024 × 1,024 pixels) were taken at 0.84 μm thickness then reconstructed and analyzed for colabeling in the X, Y, and Z plane. BrdU+ cells that co-labeled with NeuN, GFAP, or neither were classified as neurons, astrocytes, or other, respectively. The number of new neurons, astrocytes, or other cells was calculated by multiplying the number of BrdU+ cells/section by the cell type proportion.
Brain-derived neurotrophic factor (BDNF) ELISA
Rats were rapidly decapitated at 2, 7, or 14 days following ethanol exposure. Brains were immediately extracted and the hippocampus was dissected out, snap-frozen on dry ice and stored at −80°C. Identical to previous reports, tissue was homogenized and growth factor content was determined via ELISA kit (Millipore CYT306) following the manufacturer's instructions (McClain et al., 2011b). Duplicates of all samples, standards, and positive controls were run. Absorbance was measured at 450 nm on a DXT880 Multimode Detector plate reader (Beckman Coulter, Brea, CA). BDNF protein concentration was divided by the total protein concentration obtained in a BCA assay and expressed as pg of BDNF/mg of total protein.
Statistics
Statistical analyses were conducted using GraphPad Prism® (version 5.04; Graphpad Software, La Jolla, CA). Immunohistochemical data, blood ethanol concentrations, and ELISA measurements were compared by two-tailed t-test or ANOVA with post-hoc tests as appropriate. Non-continuous variables (e.g. behavior scores) were analyzed using the non-parametric Kruskal-Wallis test.
Bartlett's test was used to determine equality of variance between groups. When variances were proportional to the square of the group mean (ectopic DCX and Prox1), cell counts were log transformed prior to ANOVA and post-hoc Tukey's tests (Bland and Altman, 1996). Correlations between non-continuous variables (intoxication and withdrawal scores) and continuous neurogenesis measures were examined with Spearman rank correlation coefficients whereas BECs and neurogenesis were correlated via Pearson's. Data is reported as mean ± standard error of the mean and differences considered significant at P < 0.05.
Results
NPC proliferation increased in adolescent rats during early abstinence
In the BrdU proliferation time course study (Fig. 2), two-way ANOVA indicated a significant diet × time interaction on the number of BrdU+ cells located along the dentate gyrus SGZ [F(2,34) = 5.2, P = 0.01]. Specifically, SGZ BrdU increased 2.2-fold after 7 days of abstinence (P < 0.01), while no difference between control and ethanol groups were observed at the 2 or 14 day time points. At the 2-day time point, BrdU was observed throughout the hippocampus (Fig. 2B). Our previous work demonstrated that these cells are proliferating microglia, an effect which is remarkably similar between adults (Nixon et al., 2008) and adolescents (McClain et al., 2011b). The current study focused solely on the SGZ as it is the neurogenic region of the hippocampus. Changes in BrdU immunoreactivity were confirmed by measuring Ki67, a protein expressed by all proliferating cells regardless of cell cycle stage (Kee et al., 2002). Similar to BrdU, Ki67 increased 2.3-fold [t(8) = 3.4, P = 0.009] in ethanol dependent rats. Changes in BrdU and Ki67 were not due to differences in intoxication or withdrawal severity since blood ethanol concentrations, peak withdrawal, and mean withdrawal behavior were similar across groups (Table 2). In addition, BECs and withdrawal scores did not correlate with BrdU immunoreactivity at any time point (Tables S3–5).
Figure 2.
SGZ cell proliferation increases in alcohol dependent adolescent rats after 1 week of abstinence. Representative images show BrdU immunoreactivity present within multi-cell clusters scattered along the length of the SGZ in control (A) and ethanol dependent rats after 2 (B), 7 (D), and 14 (E) days of abstinence. Ethanol exposure caused easily discernible changes to BrdU immunoreactivity that varied by recovery time point. (G) Profile counts revealed that the number of BrdU+ cells located within the SGZ increased significantly 7 days after binge ethanol exposure. BrdU+ cells detected outside of the neurogenic SGZ at the 2-day time point were previously shown to be proliferating microglia (McClain et al., 2011b). Representative images show SGZ Ki67 immunoreactivity in control (C) and ethanol dependent rats after 7 days of abstinence (F). (H) Similar to BrdU, the number of Ki67+ cells increased significantly after 7 days of abstinence in ethanol dependent rats. Arrows point to areas represented in insets. Scale bars = 100 μm (10 μm for insets). *P < 0.05. Samples sizes are as follows: n = 10/group for 2-day time point, n = 4–6/group for 7-day time point, and n = 5/group for 14-day time point. BrdU = bromodeoxyuridine, Con = control, EtOH = ethanol, GCL = granule cell layer, SGZ = subgranular zone.
Table 2.
Binge Data for Proliferation Study
| Time Point (Days) | N | BEC (mg/dL) | Peak Withdrawal | Mean Withdrawal |
|---|---|---|---|---|
| 2 | 10 | 362 ± 20.9 | 3.2 ± 0.14 | 1.5 ± 0.20 |
| 7 | 6 | 314 ± 19.6 | 3.3 ± 0.16 | 1.7 ± 0.26 |
| 14 | 5 | 398 ± 24.5 | 3.1 ± 0.20 | 1.6 ± 0.43 |
BEC = Blood ethanol concentration
An increase in immature neurons follows increased NPC proliferation
DCX is expressed in developing neuroblasts up to two weeks after making the commitment to differentiate into a hippocampal granule cell (Brown et al., 2003). To determine if increased proliferation at the 7-day time point enhances granule cell neurogenesis, DCX immunoreactivity was quantified in the dentate gyrus SGZ and granule cell layer after 14 days of abstinence (Fig. 3). A 1.6-fold increase in the ethanol treated group was observed [t(8) = 5.9, P < 0.001].
Figure 3.

Increased proliferation along the SGZ is followed by enhanced DCX expression 14 days post-ethanol exposure. Representative images show DCX+ cells densely packed along the inner side of the granule cell layer with developing dendrites extending towards the molecular layer 14 days after control (A) or ethanol (B) treatment. Arrows point to areas represented in insets. Scale bars = 100 μm (10 μm for insets). (C) Analysis of immunoreactivity revealed a significant increase in DCX expression in ethanol dependent rats compared to controls. n = 5/group. *P < 0.05. GCL = granule cell layer, DCX = doublecortin.
Neuronal differentiation is not altered during abstinence
Newly born neuroblasts require 3–4 weeks to express mature neuronal markers such as NeuN (Zhao et al., 2008) and incorporate into the hippocampal circuitry (Espósito et al., 2005). To determine the fate of cells born during the proliferative burst, rats were injected with BrdU at the 7-day time point and euthanized 28 days later. Triple-label immunofluorescence (BrdU/NeuN/GFAP) demonstrated that most BrdU+ cells co-labeled with NeuN (Fig. 4A–I). No BrdU/GFAP co-labeling was observed, similar to previous reports (Morris et al., 2010a), while ~10% of BrdU+ cells did not co-label with NeuN or GFAP (“other”). No difference between groups was observed in the proportion of BrdU+ cells that co-labeled with NeuN or neither cell-type marker (“other”).
Figure 4.
Adult hippocampal neurogenesis increases during abstinence, but neuronal differentiation is unaltered. (A) BrdU, (B) NeuN, (C) GFAP, and (D) orthogonal view of a representative confocal Z-stack image from a control rat 35 days post-treatment (28 days after BrdU injection to label cells during the proliferative burst). (E) BrdU, (F) NeuN, (G) GFAP, and (H) orthogonal view of a representative confocal Z-stack image from an ethanol rat 35 days post-treatment. Scale bars = 20 μm. (I) Graph shows the proportion of sampled BrdU+ cells that were co-labeled with NeuN (neuron), GFAP (astrocyte), or neither (other). No BrdU/GFAP+ cells were observed and there was no effect on the number of BrdU+ cells that were not co-labeled with NeuN or GFAP. (J) Graph shows calculated neurogenesis based on the number of BrdU+ cells present in the granule cell layer multiplied by the cell phenotype proportions. The number of surviving BrdU+ cells was significantly higher in ethanol-treated rats compared to controls. The increase in BrdU combined with cell phenotype data showed that more neurons were generated during abstinence in ethanol dependent rats than in controls. There was no significant difference in the number of “other” cells generated. n = 11 for the control group and n = 9 for the ethanol group. *P < 0.05. BrdU = bromodeoxyuridine, Con = control, EtOH = ethanol, GFAP = glial fibrillary acidic protein, NeuN = neuronal nuclei.
Enhanced NPC proliferation during early abstinence leads to an increase in new granule cells
To determine if effects on NPC proliferation augment the number of new cells produced during abstinence, surviving BrdU+ cells labeled during the proliferative burst were quantified 28 days later (i.e. 35 days post-ethanol exposure). Profile counts revealed that 1.6-fold more BrdU+ cells survived in the ethanol group compared to the control group [t(18) = 3.3, P = 0.004] (Fig. 4J). The number of new neurons was estimated by multiplying BrdU counts by the proportion of BrdU+ cells that co-localized with NeuN (Fig. 4J). Similarly, a 1.6-fold increase in the number of new granule cells was observed [t(18) = 2.7, P = 0.015]. No statistically significant effect was detected among cells that differentiated into cells other than neurons or astrocytes [t(18) = 2.1, P = 0.054].
Ectopic neurogenesis in adolescent rats that experience severe withdrawal
While quantifying NPC proliferation, a striking number of BrdU+ cells were detected in the dentate gyrus molecular layer of some rats (Fig. 5A,C). In addition, ectopic DCX staining was evident in the hilus and molecular layer of a subset of rats at the 7 and 14-day time points, suggesting that immature neuronal cells were generated in non-neurogenic areas (Fig. 5B,D). Knowing the relationship between seizures and ectopic neurogenesis (Parent et al., 2006) and that some rats in this model experience seizures, we examined the relationship between ethanol withdrawal severity and ectopic BrdU and DCX. Therefore, a second study was done to generate additional rats for the 14-day time point (4 control, 11 ethanol). Intoxication and SGZ DCX measurements closely matched those described for rats used in the 14-day BrdU proliferation experiment (Table 3 and Fig. S1); therefore, rats from both studies were combined (9 control, 16 ethanol).
Figure 5.
Evidence for ectopic neurogenesis in a subset of rats that experienced severe ethanol withdrawal. Images show a single case study from the 7 and 14 day time points to demonstrate ectopic appearance of proliferation (BrdU) and neurogenesis (DCX) markers. The SGZ was outlined to show where BrdU and DCX immunoreactive cells are normally found. Arrows point to ectopic cells in the molecular layer and hilus where BrdU and DCX are not normally expressed. Ectopic BrdU (A) and DCX staining (B) was detected in the molecular layer at the 7-day time point in an ethanol dependent adolescent rat that experienced severe symptoms of ethanol withdrawal. Clusters of BrdU+ cells (C) and DCX+ cells (D) persist in the molecular layer after 14 days of abstinence. DCX+ cells are also evident in the hilus at this time point. Compare images of ectopic neurogenesis in this figure to images showing a lack of ectopic neurogenesis in other ethanol dependent rats in figures 2 and 3. * denotes areas represented in insets. Scale bars = 100 μm (20 μm for insets). BrdU = bromodeoxyuridine, GCL = granule cell layer, ML = molecular layer.
Table 3.
Binge Data for Ectopic Study
| Time Point (Days) | N | BEC (mg/dL) | Peak Withdrawal | Mean Withdrawal |
|---|---|---|---|---|
| 2 (ELISA) | 7 | 346 ± 31.1 | 2.6 ± 0.20 | 0.52 ± 0.20 |
| 7 (ELISA) | 6 | 382 ± 14.3 | 3.4 ± 0.14 | 1.2 ± 0.30 |
| 14 (ELISA) | 5 | 428 ± 20.6 | 3.4 ± 0.18 | 1.8 ± 0.31 |
| 14 (IHC) | 11 | 382 ± 16.6 | 3.3 ± 0.17 | 1.5 ± 0.33 |
BEC = Blood ethanol concentration
Because of the positive correlation between the number of ectopic DCX+ cells in the hilus and mean (P = 0.04) and peak (P = 0.02) withdrawal scores (Fig. 6), ethanol dependent rats were divided into 2 groups: rats that experienced convulsions or had mean withdrawal scores greater than 2.2 were classified as high withdrawal (HW), while rats with mean withdrawal scores less than 2.2 were classified as low withdrawal (LW). Though arbitrary, 2.2 was chosen because it is the starting point for the more severe, rostral symptoms and was approximately the mean withdrawal score (2.13) for all rats that had an observed seizure in this study. Of the 16 ethanol dependent rats, 7 met HW criteria. Because variance was obviously non-homogenous [P < 0.0001 for hilus and molecular layer], data were log transformed then analyzed via one-way ANOVA. Ethanol withdrawal had a significant effect on the presence of ectopic DCX+ cells in both the molecular layer [F(2,22) = 23, P < 0.001] and hilus [F(2,22) = 11, P < 0.001]. Post-hoc Tukey's tests confirmed that more DCX+ cells were observed in the hilus of the HW group compared to the control and LW groups (Fig. 7) and that the LW group had greater hilar DCX than the control group. In the molecular layer, the HW group had more DCX+ cells than the other two groups; however, the number of DCX+ cells in the LW group was not different from controls (Fig. 7).
Figure 6.
Correlation between measures of ectopic neurogenesis and withdrawal severity. (A) Correlations with mean withdrawal. Spearman correlation measurements show positive relationships between 14-day DCX (SGZ+GCL) densitometry measurements and mean withdrawal (B) and between 14-day hilus DCX cell counts and mean withdrawal (C). (D) Correlations with peak withdrawal. Spearman correlation measurements show positive relationships between 14-day DCX (SGZ+GCL) densitometry measurements and peak withdrawal (E) and between 14-day hilus DCX cell counts and peak withdrawal (F). BDNF = brain-derived neurotrophic factor, DCX = doublecortin, GCL = granule cell layer, Prox1 = prospero homeobox 1, SGZ = subgranular zone.
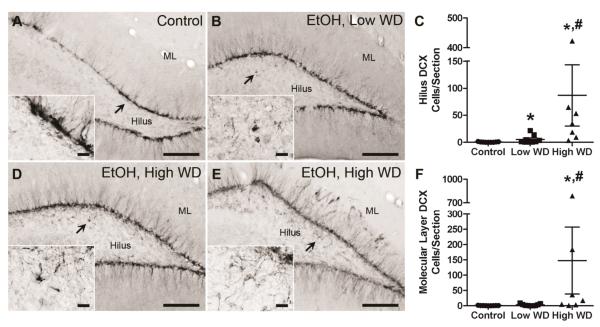
Figure 7.
Ectopic neurogenesis was quantified at the 14-day time point by counting DCX+ cells in both the molecular layer and hilus. Representative images show DCX immunoreactivity in control (A, n = 9) and low withdrawal (B, n = 9) groups. Due to the variability within the high withdrawal group (n=7), two examples are shown. In some, ectopic DCX was restricted to the hilus (D), while in others, ectopic DCX was detected in both the hilus and molecular layer (E). Scale bars = 200 μm (20 μm for insets). Arrows point to areas represented in insets. Ethanol withdrawal resulted in a significant increase in the number of ectopic DCX+ cells in the dentate gyrus hilus, regardless of withdrawal severity; however, high withdrawal severity was associated with the greatest degree of ectopic neurogenesis in this region (C). Increased ectopic neurogenesis was only detected in the molecular layer of rats that experienced severe ethanol withdrawal (F). Based on results from post-hoc Tukey's tests, * and # denote significance from control and low WD group, respectively. DCX = doublecortin, EtOH = ethanol, ML = molecular layer, WD = withdrawal.
Prox1, a transcription factor expressed by late-stage subgranular NPCs (Karalay et al., 2011; Iwano et al., 2012), was measured to determine if ectopic neuroblasts expressed markers consistent with granule cell commitment (Fig. 8). Variance between groups was statistically different [P < 0.001 for hilus and molecular layer] therefore, data were log transformed and analyzed by one-way ANOVA. Ethanol withdrawal had a significant effect on the number of Prox1+ cells located in the hilus [F(2,22) = 9.4, P = 0.001]. Post-hoc Tukey's tests revealed that the number of hilar Prox1+ cells was greater in the HW group compared to the LW or control groups (Fig. 8C). Surprisingly, no withdrawal effect was observed on molecular layer Prox1 expression [F(2,22) = 2.4, P = 0.12].
Figure 8.
Ectopic cells in the hilus and molecular layer express granule cell-specific Prox1. Representative images show Prox1 expression in control (A, n = 9) and low withdrawal groups (B, n = 9). Due to the variability within the high withdrawal group (n=7), two examples are shown. In some, ectopic Prox1 was restricted to the hilus (D), while in others, ectopic Prox1 was detected in both the hilus and molecular layer (E). Scale bars = 200 μm (20 μm for insets). Arrows point to areas represented in insets. Severe ethanol withdrawal was associated with a significant increase in the number of Prox1+ cells present in the hilus (C). No significant effect of withdrawal severity was found in the molecular layer despite clear evidence of numerous Prox1+ cells observed in a select few rats of the high ethanol withdrawal group (F). Based on results from post-hoc Tukey's tests, * denotes significance compared to the control and low WD groups. EtOH = ethanol, ML = molecular layer, Prox1 = prospero homeobox 1, WD = withdrawal.
An increase in BDNF can trigger aberrant hippocampal NPC migration (Scharfman et al., 2005). To examine if hippocampal BDNF expression changes in parallel with the appearance of ectopic neuroblasts, rats were subjected to the 4-day ethanol exposure paradigm and then sacrificed 2, 7, or 14 days later (Table 3). BDNF was detected in all samples; however, the amount of hippocampal BDNF was unchanged after 2 (control = 249 ± 8.7 μg BDNF/mg protein; ethanol = 211 ± 25.8 μg BDNF/mg protein), 7 (control = 231 ± 9.7 μg BDNF/mg protein; ethanol = 238 ± 17.8 μg BDNF/mg protein) and 14 (control = 257 ± 10.1 μg BDNF/mg protein; ethanol = 248 ± 18.4 μg BDNF/mg protein) days of abstinence. In addition, BDNF levels did not correlate with withdrawal severity at any time point measured (Fig. 6).
Discussion
These studies demonstrate that adolescent rats exhibit reactive hippocampal neurogenesis after ethanol dependence similar to adult rats. However, striking ectopic neurogenesis was observed in severely withdrawing rats, an effect that may be specific to adolescents in this AUD model. An increase in hippocampal cell proliferation was detected seven days following four-day binge ethanol exposure, followed by two indices of increased neurogenesis: enhanced expression of the immature neuronal marker, DCX, and increased BrdU/NeuN co-localization two and four weeks post-intoxication, respectively. The extent of reactive hippocampal neurogenesis in adolescent rats is similar to that first reported in adult rats subjected to the same four-day exposure paradigm (Nixon and Crews, 2004) suggesting that the underlying mechanisms that regulate NPC proliferation and neuronal differentiation during abstinence are similar in the adolescent and adult brain. This was unexpected given the evidence showing that the adolescent brain, especially the hippocampus, appears more vulnerable to the damaging effects of alcohol compared to adults (Crews et al., 2000; De Bellis et al., 2000; White and Swartzwelder, 2004) and that alcohol effects on adult neurogenesis differ between adolescent and adult rats (McClain et al., 2011a).
Ectopic neurogenesis and severe ethanol withdrawal
Most distinct in these data was a clear relationship between severe ethanol withdrawal and aberrant or ectopic neurogenesis, an effect that may be adolescent-specific. Evidence for ectopic neurogenesis included detection of BrdU-labeled and DCX immunoreactive cells within the non-neurogenic molecular layer and hilus of the dentate gyrus in rats that experienced withdrawal-induced seizures or had mean withdrawal scores greater than 2.2. The phenotype of ectopic cells was characterized by examining the expression of Prox1, a member of the homeobox transcription factor family that is required for differentiation of NPCs into granule cells (Karalay et al., 2011) and is essential for the maintenance of the granule cell phenotype (Iwano et al., 2012). Comparable to DCX, severe ethanol withdrawal was associated with an increase in the number of Prox1+ cells in the hilus, indicating that aberrantly migrating NPCs maintain a granule cell phenotype. Similar to the effects of severe ethanol withdrawal in adolescent rats, pilocarpine-induced status epilepticus induces the appearance of Prox1+ cells in the hilus 14 days following seizure induction (Parent et al., 2006). This similarity suggests that it is the seizure component of withdrawal that underlies this phenomenon. However, the withdrawal monitoring protocol used has limitations as the rats are observed only 30 min/hour and we cannot rule out that seizures and other critical withdrawal symptoms were missed. Intriguingly, ectopic neurogenesis has not been observed in adult rats exposed to this same alcohol paradigm (Nixon and Crews, 2004). Given that withdrawal severity is similar between adolescent and adult rats in this model (Morris et al., 2010b), severe ethanol withdrawal-induced ectopic neurogenesis may be adolescent-specific. When considered along with the abnormal functional characteristics described for seizure-induced ectopic granule neurons, the association between adolescence and ethanol-induced ectopic neurogenesis may explain, in part, the high sensitivity of the adolescent hippocampus to alcohol-induced dysfunction (Parada et al., 2011; Schweinsburg et al., 2010; White and Swartzwelder, 2004).
The presence of ectopic neurogenesis indicates that severe withdrawal following adolescent alcohol dependence interferes with mechanisms that control hippocampal NPC migration. In the dentate gyrus, a role for BDNF in NPC migration has been suggested as direct infusion of BDNF into the hilus results in the appearance of ectopic granule cells (Scharfman et al., 2005). Coupled with evidence from adult rodent alcohol models and human alcoholics supporting a potential role for BDNF in AUDs (Costa et al., 2011; Miller, 2004), we hypothesized that severe ethanol withdrawal and ectopic neurogenesis would be linked with an increase in hippocampal BDNF expression. Surprisingly, analysis of BDNF protein at 2, 7 and 14 days post-ethanol exposure revealed no change in BDNF expression and no correlation between BDNF and measures of withdrawal severity. Although we cannot completely rule out a role for BDNF based on limited ELISA measurements, the data suggests that other factors are involved in aberrant NPC migration. For example, reduced reelin expression and altered radial glial positioning and morphology have been associated with ectopic neurogenesis (Gong et al., 2007; Haas et al., 2002; Mooney et al., 2004). Future studies will examine the contribution of reelin and radial glia to ectopic neurogenesis observed following alcohol dependence in adolescent rats.
Functional consequences of reactive hippocampal neurogenesis
Adult born granule cells become fully integrated into the existing hippocampal circuitry 4–8 weeks following birth (Espósito et al., 2005; Zhao et al., 2008). Although the precise function of adult hippocampal neurogenesis remains heavily debated, evidence from behavioral studies links neurogenesis to hippocampal-dependent learning and memory processes (Deng et al., 2010). The emerging data implicating adult born granule cells in learning and memory suggest that restoration of hippocampal neurogenesis may be involved in cognitive improvement exhibited by abstinent alcoholics (Bartels et al., 2007; Nixon and Crews, 2004; Sullivan et al., 2000). The beneficial effects of neurogenesis combined with increased neurogenesis detected in early abstinence supports this intriguing idea. Although ethanol-exposed adolescent rats develop deficits in hippocampal-dependent learning and memory tasks (reviewed in White and Swartzwelder, 2004; Yttri et al., 2004), it is not known if alterations in neurogenesis are involved, nor is it clear if reactive neurogenesis contributes to recovery of these deficits. Most tests of hippocampal function occur within several days to 3 weeks of ethanol exposure (Coleman et al., 2011; Yttri et al., 2004), while functional effects of reactive neurogenesis may not be observed until 6 weeks after cell birth (Kee et al., 2007). Additional work is needed to determine if new neurons generated during abstinence properly integrate into the existing hippocampal circuitry to provide beneficial effects on hippocampal function. These studies will be particularly important given the evidence for ectopic neurogenesis in adolescent rats that experienced severe ethanol withdrawal.
Conclusions
Human and animal studies consistently demonstrate that excessive ethanol intake typical of AUDs causes impairments in hippocampal structure and function. These detrimental effects have been hypothesized to underlie the role of the hippocampus in the development of AUDs (Nixon et al., 2010). Effects of adolescent ethanol intoxication on neurogenesis, which include disruption of NPC proliferation and reduced survival of adult born granule neurons (McClain et al., 2011a; Morris et al., 2010a; Taffe et al., 2010), could have important implications for how impairments in hippocampal integrity contribute to the development of addiction (Mandyam and Koob, 2012). Reactive neurogenesis may be an attempt to compensate for lost neurogenesis and could represent a key intrinsic mechanism to repair structural damage and improve cognitive function during recovery (Mandyam and Koob, 2012; Nixon, 2006). Conversely, ectopic neurogenesis potentially disrupts normal dentate gyrus circuitry and may hinder functional recovery (Parent, 2007), underscoring the importance for investigating whether neurons born during reactive neurogenesis integrate correctly into the existing hippocampal circuitry. A more complete understanding of the causes and role of alcohol-induced reactive neurogenesis is critical for developing therapies aimed at promoting recovery of hippocampal function in adolescents with AUDs.
Supplementary Material
Acknowledgements
The authors would like to thank M. Ayumi Deeny for her expert technical assistance with immunohistochemistry and densitometry measurements. This work was supported by NIAAA grants R21 AA016307 and R01 AA016959 and NIDA grant T32 DA016176.
Footnotes
Author Contributions JM, SA Morris, and KN were responsible for study concept and design. JM performed cell proliferation and ectopic neurogenesis experiments. SA Morris completed experiments examining NPC differentiation and survival. SA Marshall performed ELISA experiments. JM, SA Morris, SA Marshall, and KN analyzed data and interpreted the findings. JM drafted the manuscript. JM and KN critically evaluated and revised the manuscript for intellectual content. All authors critically reviewed the manuscript and approved the final version for publication.
References
- Bartels C, Kunert HJ, Stawicki S, Kröner-Herwig B, Ehrenreich H, Krampe H. Recovery of hippocampus-related functions in chronic alcoholics during monitored long-term abstinence. Alcohol Alcohol. 2007;42:92–102. doi: 10.1093/alcalc/agl104. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistics notes: Transforming data. BMJ. 1996;312:770. doi: 10.1136/bmj.312.7033.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Coleman LG, Jr, He J, Lee J, Styner M, Crews FT. Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcohol Clin Exp Res. 2011;35:671–688. doi: 10.1111/j.1530-0277.2010.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contet C, Kim A, Le D, Iyengar SK, Kotzebue RW, Yuan CJ, Kieffer BL, Mandyam CD. μ-Opioid receptors mediate the effects of chronic ethanol binge drinking on the hippocampal niche. Addict Biol. 2013 doi: 10.1111/adb.12040. doi:10.1111/adb.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M-A, Girard M, Dalmay F, Malauzat D. Brain-derived neurotrophic factor serum levels in alcohol-dependent subjects 6 months after alcohol withdrawal. Alcohol Clin Exp Res. 2011;35:1966–1973. doi: 10.1111/j.1530-0277.2011.01548.x. [DOI] [PubMed] [Google Scholar]
- Crews F, Nixon K, Kim D, Joseph J, Shukitt-Hale B, Qin L, Zou J. BHT blocks NF-kappaB activation and ethanol-induced brain damage. Alcohol Clin Exp Res. 2006a;30:1938–1949. doi: 10.1111/j.1530-0277.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006b;137:437–445. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K, Wilkie ME. Exercise reverses ethanol inhibition of neural stem cell proliferation. Alcohol. 2004;33:63–71. doi: 10.1016/j.alcohol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Liu W, Wills DN, Crews FT. Periadolescent ethanol vapor exposure persistently reduces measures of hippocampal neurogenesis that are associated with behavioral outcomes in adulthood. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.03.058. doi: 10.1016/j.neuroscience.2013.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espósito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming RL, Wilson WA, Swartzwelder HS. Magnitude and ethanol sensitivity of tonic GABAA receptor-mediated inhibition in dentate gyrus changes from adolescence to adulthood. J Neurophysiol. 2007;97:3806–3811. doi: 10.1152/jn.00101.2007. [DOI] [PubMed] [Google Scholar]
- Gong C, Wang T-W, Huang HS, Parent JM. Reelin regulates neuronal progenitor migration in intact and epileptic hippocampus. J Neurosci. 2007;27:1803–1811. doi: 10.1523/JNEUROSCI.3111-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSMIV alcohol abuse and dependence: results from the national longitudinal alcohol epidemiologic survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Haas CA, Dudeck O, Kirsch M, Huszka C, Kann G, Pollak S, Zentner J, Frotscher M. Role for reelin in the development of granule cell dispersion in temporal lobe epilepsy. J Neurosci. 2002;22:5797–5802. doi: 10.1523/JNEUROSCI.22-14-05797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwano T, Masuda A, Kiyonari H, Enomoto H, Matsuzaki F. Prox1 postmitotically defines dentate gyrus cells by specifying granule cell identity over CA3 pyramidal cell fate in the hippocampus. Development. 2012;139:3051–3062. doi: 10.1242/dev.080002. [DOI] [PubMed] [Google Scholar]
- Karalay Ö , Doberauer K, Vadodaria KC, Knobloch M, Berti L, Miquelajauregui A, Schwark M, Jagasia R, Taketo MM, Tarabykin V, Lie DC, Jessberger S. Prospero-related homeobox 1 gene (Prox1) is regulated by canonical Wnt signaling and has a stage-specific role in adult hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2011;108:5807–5812. doi: 10.1073/pnas.1013456108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee N, Sivalingam S, Boonstra R, Wojtowicz JM. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods. 2002;115:97–105. doi: 10.1016/s0165-0270(02)00007-9. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Nixon K. Exercise neuroprotection in a rat model of binge alcohol consumption. Alcohol Clin Exp Res. 2010;34:404–414. doi: 10.1111/j.1530-0277.2009.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacology. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Koob GF. The addicted brain craves new neurons: putative role for adult-born progenitors in promoting recovery. Trends Neurosci. 2012;35:250–260. doi: 10.1016/j.tins.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- McClain JA, Hayes DM, Morris SA, Nixon K. Adolescent binge alcohol exposure alters hippocampal progenitor cell proliferation in rats: Effects on cell cycle kinetics. J Comp Neurol. 2011a;519:2697–2710. doi: 10.1002/cne.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain JA, Morris SA, Deeny MA, Marshall SA, Hayes DM, Kiser ZM, Nixon K. Adolescent binge alcohol exposure induces long-lasting partial activation of microglia. Brain Behav Immun. 2011b;25:S120–S128. doi: 10.1016/j.bbi.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW. Repeated episodic exposure to ethanol affects neurotrophin content in the forebrain of the mature rat. Exp Neurol. 2004;189:173–181. doi: 10.1016/j.expneurol.2004.05.026. [DOI] [PubMed] [Google Scholar]
- Mooney SM, Siegenthaler JA, Miller MW. Ethanol induces heterotopias in organotypic cultures of rat cerebral cortex. Cereb Cortex. 2004;14:1071–1080. doi: 10.1093/cercor/bhh066. [DOI] [PubMed] [Google Scholar]
- Morris SA, Eaves DW, Smith AR, Nixon K. Alcohol inhibition of neurogenesis: A mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus. 2010a;20:596–607. doi: 10.1002/hipo.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SA, Kelso ML, Liput DJ, Marshall SA, Nixon K. Similar withdrawal severity in adolescents and adults in a rat model of alcohol dependence. Alcohol. 2010b;44:89–98. doi: 10.1016/j.alcohol.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K. Alcohol and adult neurogenesis: roles in neurodegeneration and recovery in chronic alcoholism. Hippocampus. 2006;16:287–295. doi: 10.1002/hipo.20162. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. J Neurosci. 2004;24:9714–9722. doi: 10.1523/JNEUROSCI.3063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Kim DH, Potts EN, He J, Crews FT. Distinct cell proliferation events during abstinence after alcohol dependence: microglia proliferation precedes neurogenesis. Neurobiol Dis. 2008;31:218–229. doi: 10.1016/j.nbd.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Morris SA, Liput DJ, Kelso ML. Roles of neural stem cells and adult neurogenesis in adolescent alcohol use disorders. Alcohol. 2010;44:39–56. doi: 10.1016/j.alcohol.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noori HR, Fornal CA. The appropriateness of unbiased optical fractionators to assess cell proliferation in the adult hippocampus. Front Neurosci. 2011;5 doi: 10.3389/fnins.2011.00140. 10.3389/fnins.2011.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada M, Corral M, Caamaño-Isorna F, Mota N, Crego A, Holguín SR, Cadaveira F. Binge drinking and declarative memory in university students. Alcohol Clin Exp Res. 2011;35:1475–1484. doi: 10.1111/j.1530-0277.2011.01484.x. [DOI] [PubMed] [Google Scholar]
- Parent JM. Adult neurogenesis in the intact and epileptic dentate gyrus. In: Scharfman HE, editor. Progress in Brain Research. Elsevier; 2007. pp. 529–540. [DOI] [PubMed] [Google Scholar]
- Parent JM, Elliott RC, Pleasure SJ, Barbaro NM, Lowenstein DH. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann Neurol. 2006;59:81–91. doi: 10.1002/ana.20699. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Compact 6th ed Academic Press; New York: 2009. [Google Scholar]
- Pyapali GK, Turner DA, Wilson WA, Swartzwelder HS. Age and dose-dependent effects of ethanol on the induction of hippocampal long-term potentiation. Alcohol. 1999;19:107–111. doi: 10.1016/s0741-8329(99)00021-x. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Chan SH, Crawford EF, Lee YK, Funk CK, Koob GF, Mandyam CD. Permanent impairment of birth and survival of cortical and hippocampal proliferating cells following excessive drinking during alcohol dependence. Neurobiol Dis. 2009;36:1–10. doi: 10.1016/j.nbd.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, McQueeny T, Nagel BJ, Eyler LT, Tapert SF. A preliminary study of functional magnetic resonance imaging response during verbal encoding among adolescent binge drinkers. Alcohol. 2010;44:111–117. doi: 10.1016/j.alcohol.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Lim KO, Pfefferbaum A. Longitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: relationships to changes in brain structure. Neuropsychology. 2000;14:178–188. [PubMed] [Google Scholar]
- Taffe MA, Kotzebue RW, Crean RD, Crawford EF, Edwards S, Mandyam CD. Long-lasting reduction in hippocampal neurogenesis by alcohol consumption in adolescent nonhuman primates. Proc Natl Acad Sci U S A. 2010;107:11104–11109. doi: 10.1073/pnas.0912810107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoof JJ, Van Der Lely N, Bouthoorn SH, Van Dalen WE, Pereira RR. Adolescent alcohol intoxication in the Dutch hospital departments of pediatrics: a 2-year comparison study. J Adolesc Health. 2011;48:212–214. doi: 10.1016/j.jadohealth.2010.06.001. [DOI] [PubMed] [Google Scholar]
- White AM, Swartzwelder HS. Hippocampal function during adolescence: a unique target of ethanol effects. Ann N Y Acad Sci. 2004;1021:206–220. doi: 10.1196/annals.1308.026. [DOI] [PubMed] [Google Scholar]
- Yttri EA, Burk JA, Hunt PS. Intermittent ethanol exposure in adolescent rats: dose-dependent impairments in trace conditioning. Alcohol Clin Exp Res. 2004;28:1433–1436. doi: 10.1097/01.alc.0000147657.51745.a7. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.