Abstract
Human cocaine users report that the initial “high” produced by cocaine administration is followed by an anxiogenic “crash”. Given that cocaine has such robust and opposing properties, it is likely that both the positive and negative effects of cocaine contribute to an individual’s motivation to administer the drug. Despite this likelihood, the neurobiology underlying cocaine’s dual processes remains unclear. While much literature supports a role for dopamine (DA) in cocaine reward, it is uncertain if DA also contributes to the drug’s negative effects. Our laboratory has extensively utilized a modified conditioned place test to explore cocaine’s opponent processes. In this paradigm rats develop conditioned place preferences (CPPs) for an environment paired with the immediate/positive effects of cocaine, and conditioned place aversions (CPAs) for an environment paired with the delayed/negative effects present 15-min after i.v. injection. In the current study rats were conditioned to associate an environment with either the immediate or delayed effects of i.v. cocaine (1 mg/kg/0.1 ml) three hours after i.p. pre-treatment with either the DA D1/D2 receptor antagonist cis-flupenthixol (0.5 mg/kg/ml) or saline vehicle. As expected, vehicle-treated control animals developed the normal pattern of CPPs for cocaine’s immediate effects or CPAs for the delayed effects of cocaine. However, while DA receptor antagonism prevented the expression of cocaine CPPs it did not alter the expression of cocaine-induced CPAs. These data confirm a role for DA transmission in cocaine reward but suggest that different neural pathways mediate the drug’s negative/anxiogenic properties.
Keywords: cocaine, dopamine, reward, aversion, conditioned place preference, cis-flupenthixol
1. Introduction
Human drug users report that the intense pleasure produced by cocaine administration is typically followed by feelings of anxiety, dysphoria, irritability, and craving (Anthony et al., 1989; Resnick et al., 1977; Rohsenow et al., 2007; Williamson et al., 1997). Cocaine’s dual positive and negative effects can also be observed in a variety of animal models. For instance, while animals readily self-administer intravenous cocaine (Deneau et al., 1969; Hill & Powell, 1976) and develop preferences for environments paired with the immediate/positive effects of the drug (Mucha et al., 1982; Spyraki et al., 1982), cocaine also increases anxiogenic behaviors in rodents (Simon et al., 1994; Yang et al., 1992; Costall et al., 1989; Rogerio and Takahashi, 1992) and animals develop aversions to environments paired with the drug’s delayed/negative effects (Ettenberg et al., 1999; Ettenberg and Bernardi, 2007; Jhou et al., 2013; Knackstead et al., 2002; Su et al., 2013). These dual effects of cocaine align well with Solomon and Corbit’s (1974) classic Opponent Process Theory, which postulates that motivation stems from the algebraic summation of diametrically opposite and temporally dissociated biological responses to affective stimuli. Given that cocaine administration results in such robust and opposing effects, it seems reasonable to presume that the relative balance between the positive/euphoric and negative/anxiogenic effects of cocaine determine the strength of an individual’s motivation to seek and administer the drug. However, while the neural mechanisms underlying the positive/euphoric effects of cocaine have been well documented, the neurobiology underlying cocaine’s negative/anxiogenic effects remains unclear.
A wide body of research suggests a role for mesolimbic dopamine in reinforcement for natural incentive stimuli such as food or water as well as for drugs of abuse (Berridge, 2009; Ikemoto, 2007; Taber et al., 2012; Wise and Rompre, 1989). Cocaine itself is a potent synaptic dopamine (DA) agonist by way of transporter inhibition (Koe, 1976; Reith et al., 1986), and its administration results in a rise in extracellular DA levels within terminal fields of the mesolimbic system (Church et al., 1987; Hernandez and Hoebel, 1988; Moghaddam and Bunney, 1989). The role of DA in cocaine reward and reinforcement has been suggested by both pharmacological and lesion studies. Disruption of DA-ergic activity of the mesolimbic system via lesion or the administration of DA antagonist drugs, attenuates both conditioned place preferences for cocaine (Isaac et al., 1989; Morency and Beninger, 1986; Spyraki et al., 1987; Veeneman et al., 2011) as well as operant cocaine self-administration (Ettenberg et al., 1982; Pettit et al., 1984; Roberts et al., 1977; Roberts and Koob, 1982). Human studies also support a link between DA systems and reward. Decreased dopaminergic function is thought to contribute to the experience of anhedonia in both clinical and normal populations (Belmaker and Wald, 1977; Fibiger, 1984; Healy, 1989). More specifically in regards to cocaine, humans drug users’ subjective descriptions of euphoria have been shown to be highly correlated with cocaine-induced increases in striatal DA activity (Volkow et al., 1996), and the administration of DA antagonists dose-dependently decreases self-reported ratings of the cocaine “high” (Romach et al., 1999).
Interestingly, aversive and stressful events have also been shown to excite midbrain dopamine neurons. For example, electrophysiological recordings of putative DA neurons within the ventral tegmental area (VTA) show increased activity in response to negative stimuli such as restraint stress, tail pinch, and foot shock (Anstrom and Woodward, 2005; Brischoux et al., 2009; Mantz et al., 1989), and DA levels are elevated in terminal fields of the mesolimbic system following such aversive events (Abercrombie et al., 1989; Bassareo et al., 2002). Research on cocaine-induced anxiety also highlights a role for mesolimbic DA. Simon and colleagues (1993, 1994) utilized an open field test and a light/dark box test to show that cocaine-induced anxiogenic behaviors are exacerbated by injection of the selective DA reuptake inhibitor GBR-12783 or other DA agonists. Furthermore, DA signaling within the mesolimbic system has been shown to be integral for the demonstration of stress-induced reinstatement of cocaine seeking following a period of drug abstinence (Capriles et al., 2003; McFarland et al., 2004; Wang et al., 2005; Xi et al., 2004). It should be noted, however, that previous research has identified other catecholamine systems as potential substrates for the expression of cocaine’s negative/anxiogenic effects (Ettenberg et al., 2011; Ettenberg and Bernardi, 2006; Ettenberg and Bernardi, 2007; Schank et al., 2008; Smith and Aston-Jones, 2008; Wenzel et al., 2012). Thus the precise nature of the neuronal systems underlying these effects of cocaine remains unclear.
It was in this context, that the current investigation was devised to examine the contribution of DA to the dual positive and negative effects of cocaine. The experiment described here involved two phases. First a test of locomotor activity was employed to identify a behaviorally effective dose of cis-flupenthixol. Cis-flupenthixol is a potent and selective DA receptor antagonist (Møller-Nielsen, 1973; Creese et al., 1976), which has been shown to disrupt operant responding for cocaine reinforcement (Ettenberg et al., 1982), conditioned-place preferences for cocaine-paired environments (Veeneman et al., 2011), and cocaine-induced increases in locomotor activity (Delfs et al., 1990). Several investigators have suggested that a drug’s capacity to enhance locomotor activity serves as a predictor of that drug’s rewarding/abuse potential (e.g., Almaric and Koob, 1993; Gold et al., 1989; Uhl et al., 2002; Wise, 2005). Hence our intent in the locomotor activity test was simply to identify a dose of cis-fluenthixol that reversed the locomotor stimulant effects of cocaine (and would therefore be a reasonable challenge dose for effectively antagonizing the positive rewarding effects of cocaine) without abolishing the animal’s capacity for movement. The second phase of the study then utilized this dose of cis-flupenthixol in a modified conditioned place test model. As alluded to above, rats will form conditioned preferences for distinctive environments associated with the immediate rewarding impact of i.v. cocaine and aversions for environments associated with the delayed/anxiogenic effects of the drug present 15-min post-infusion. In the current study, animals were systemically pretreated with cis-flupenthixol during conditioning trials to determine whether compromising DA function would interfere with the development of the learned place preferences (CPP), aversions (CPA), or both. Solomon and Corbit’s (1974) Opponent Process Theory speculated that the immediate “a” and the opponent “b” processes (e.g., the initial “high” and the subsequent “crash” after cocaine administration) were subserved by separate distinct neuronal systems. It was therefore hypothesized that pretreatments with the DA antagonist would attenuate the rewarding properties of cocaine and hence decrease or prevent the establishment of cocaine-induced CPPs (as previously shown by several others; e.g., Morency and Beninger, 1986; Spyraki et al., 1987; Veeneman et al., 2011), but leave the development of CPAs intact.
2.0 Materials and Methods
2.1 Subjects
Subjects were adult male albino Sprague-Dawley rats weighing 275–300g at the time of surgery. Rats were obtained from Charles River Laboratories (Hollister, California, USA) and were pair-housed in hanging plastic cages within a temperature-controlled (23° C) vivarium maintained under a reverse 12-hour light-dark cycle (lights off at 0800h). Animals were provided ad libitum access to food (Purina Rat Chow) and water throughout the duration of the study. All animal handling and procedures adhered to the NIH Guide for the Care and Use of Laboratory Animals and were reviewed and approved by University of California at Santa Barbara’s Institutional Animal Care and Use Committee.
2.2 Surgery
Rats were acclimatized to experimental procedures through daily handling for a minimum of one week prior to surgery. Each rat was then fitted with a chronic indwelling catheter (13 mm of polyethylene tubing, 0.3 mm inner diameter, 0.64 outer diameter; Dow Corning Corporation, Midland, MI, USA) inserted under deep anesthesia induced by an intra-muscular injection of a combined solution of ketamine and xylazine (56.5 and 7.5 mg/kg, respectively; Abbott Laboratories). One end of the catheter was inserted into the right jugular vein and secured in place by silk sutures while the other open end was passed subcutaneously to a stainless steel guide cannula (Item 313G; Plastics One, Roanoke, VA, USA) that exited though a small 2 mm hole on the midline of the animal’s back. Dental cement was used to secure the cannula to a 2 cm square of surgical Mersilene mesh (Bard; Warwick, RI) which allowed the catheter port to lay flat against the subdermal tissue on the animal’s back. During surgery each subject received an injection of the non-opiate analgesic, flunixin meglumine (FluMeglumine; Phoenix Pharmaceuticals, Belmont, California, USA; 2.0 mg/kg, s.c.) to reduce post-surgical pain and 3 ml of 0.9% physiological saline s.c. to prevent dehydration. Additionally, following surgery animals received the antibiotic, ticarcillin disodium/clavulanate potassium (Timentin; 50 mg/kg i.v. in 0.1 ml sterile 0.9 % physiological saline) and 0.1 ml of the anticoagulant heparin (66 IU/0.1 ml i.v. prepared in 0.9% physiological saline) to protect against microbial infection and to promote catheter patency.
All subjects were allowed a minimum of one week to recover from surgery before experimental procedures began. During this time, catheters were flushed once daily with 0.1 ml of Timentin antibiotic (25 mg/kg) followed by 0.1 ml of heparinized 0.9% physiological saline. Prior to the start of the experiment, and every seven days thereafter, catheter patency was confirmed in all animals by observing the behavioral impact of an i.v. injection of the fast-acting barbiturate, methohexital sodium (Brevital; 2.0 mg/kg in 0.1 ml filtered nanopure water). Animals that were unresponsive to the Brevital (i.e. did not exhibit the loss of their righting reflex) when examined prior to testing were re-implanted with a new catheter using the left jugular vein and given additional days for recovery. If catheter patency failed during the course of behavioral testing, that animal was removed from the data analysis.
2.3 Drugs
Cocaine hydrochloride (provided by the National Institute on Drug Abuse) was dissolved in 0.9% physiological saline and sterile filtered. For the locomotor activity assessment, a dose of 15 mg/kg was delivered i.p. – this dose was chosen due to its ability to reliably elevate locomotor activity in rats (e.g., Kosten et al., 1994; Wenzel et al., 2011b). For conditioned place test procedures, cocaine (1.0 mg/kg) was delivered i.v. in a volume of 0.1 ml over 4.3 seconds via a 10 ml syringe nested in a motorized syringe pump (Razel Scientific Instruments, St. Albans, Vermont, USA). The drug dose for this project is the standard dose that has been employed in prior studies in our laboratory and has been shown to consistently produce conditioned place preferences and aversions (Ettenberg et al., 1999; Ettenberg and Bernardi, 2007; Knackstead et al., 2002). Cis-flupenthixol (Sigma Aldrich, St. Louis, Missouri, USA) was dissolved in 0.9% physiological saline vehicle to a dose of 0.125, 0.25, or 0.5 mg/kg and delivered i.p. in a volume of 1 ml/kg.
2.4 Locomotor Activity Apparatus
Locomotor activity was measured in 12 identical Plexiglas chambers each measuring 20 cm L × 40 cm W × 20 cm H (Kinder Scientific, San Diego, California, USA). Each chamber contained a set of 15 infrared photoemitter-detector pairs located 8 cm above the floors and evenly spaced along the long axis; seven more emitter-detector pairs were distributed along the narrow axis of each chamber. A subject’s movement within the chamber was detected via interruption of the infrared-photobeams. A desktop computer running custom software (Kinder Scientific) recorded the animals’ movements in real time during two 1-h test sessions. Activity was measured as distance traveled in cm and summed into 5-min bins.
2.5 Locomotor Activity Procedure
In order to ensure a behaviorally effective dose of DA antagonist, rats (n=21) underwent locomotor activity testing. For this assay, rats were divided into four groups and received a single injection of either 0.0, 0.125, 0.25, or 0.5 mg/kg cis-flupenthixol delivered in a volume of 1 ml/kg. Immediately following cis-flupenthixol administration, rats were then returned to their home cage for two hours. Each rat was then retrieved and individually placed into an assigned locomotor chamber and allowed to habituate to the apparatus for one hour. Following habituation (i.e., 3 h after either cis-flupenthixol pretreatment) each subject was removed from the locomotor chambers, administered an i.p. injection of cocaine (15 mg/kg/1 ml), and returned to the locomotor apparatus for an additional one-hour test session.
2.6 Conditioned Place Test Apparatus
Two identical rectangular wooden Conditioned Place Test boxes served as the apparatus. Each box (156 cm long × 34 cm wide × 30 cm high) was subdivided into three distinct compartments separated by removable walls: two equally sized large chambers (61 × 30 cm) separated by a smaller intermediate chamber (34 × 30 cm). One of the larger compartments was painted black with Plexiglas flooring and scented with acetic acid (10% solution, swabbed 5 cm from the top of the compartment). The other large chamber was painted white with soft gray bedding covering the floor (Carefresh; Absorption Corp, Ferndale, Washington, USA) and had no unique odor cues added. The smaller intermediate chamber was painted gray with wood flooring. Each compartment, therefore, had unique visual, tactile, and olfactory properties. Situated above each apparatus was a digital camera that detected and recorded the precise location of the animal in real time via a desktop computer running Any-Maze software (Stoetling Co, Wood Dale, IL, USA).
2.7 Conditioned Place Test Procedure
Place conditioning consisted of three phases: a baseline trial, eight place conditioning trials, and a final preference test. For baseline, subjects were placed into the middle gray section of the apparatus with the interior walls removed and the time spent in each of the three compartments was then recorded over 15-min. Animals that exhibited strong inherent preferences or aversions for one or the other side of the apparatus on baseline (i.e., spent ≥ 500-sec in either the black or white compartment) were removed from data analysis. The following day, the dividing walls were reinserted and place conditioning began. Each rat received an i.p. injection of either 0.5 mg/kg/ml cis-flupenthixol or saline vehicle 3-h prior to each of the eight conditioning trials. This dose was identified in the prior locomotor activity test as reliably reducing cocaine-induced activity without inducing immobility or sedation. Trials consisted of an i.v. injection of either saline-vehicle or cocaine (1.0 mg/kg) and placement into either the white or black compartment for 5-mins. On the following day, each rat received the alternate treatment (cocaine or saline) and was placed in the alternate colored environment. Thus, at the conclusion of conditioning, each subject had experienced four cocaine pairings with one environment and four saline pairings with the alternate environment. Animals were placed into the conditioning chamber either immediately after injection (the “reward” groups) or 15-min post-injection (the delayed “aversion” groups). An “unbiased” experimental design was employed (see Carr et al., 1988) in that the order of i.v. injection (receiving either vehicle or cocaine on the first conditioning trial) and the drug-paired compartment (either black or white) were counterbalanced within each group. 48-h after the 8th conditioning trial, a final 15-min place preference test was conducted exactly as described for baseline. This two-day period between the completion of place conditioning and the final test day allowed for cis-flupenthixol levels to dissipate so that both baseline and test day trials were conducted when animals were in a non-drugged state.
By way of summary, the procedures described above yielded four groups of animals: rats that were pretreated with cis-flupenthixol i.p. prior to each conditioning trial and then placed into a distinctive environment immediately following each i.v. injection of cocaine or vehicle (n=16) or 15-min following each injection (n=13); and rats that were pretreated with saline-vehicle i.p. and underwent place conditioning for either the immediate (n=21) or delayed (n=14) effects of cocaine. CPPs and CPAs were identified as statistically reliable shifts in the time spent on the cocaine-paired side of the apparatus after conditioning relative to the time spent in the same environment prior to conditioning. This was determined by the calculation of “differences scores” in which average time a rat spent in the cocaine-paired compartment on baseline was subtracted from the time spent there on test day (e.g., (Ettenberg et al. 1999; Ettenberg and Bernardi, 2007; Knackstedt et al. 2002). Thus, positive scores indicate that animals spent more time in the drug-paired environment after conditioning (i.e., they developed a CPP), while negative scores indicate an avoidance of the drug-paired side following conditioning trials (i.e., the development of a CPA).
3. Results
3.1 Locomotor Activity
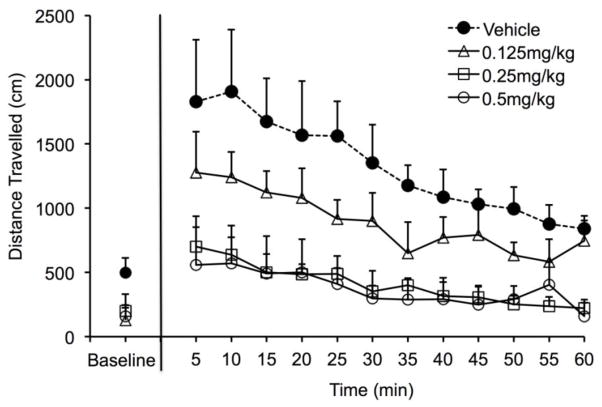
Figure 1 illustrates the mean (+SEM) locomotor activity (expressed as cm traveled during each 5-min interval) of the four cis-flupenthixol pretreatment groups during behavioral testing. The “baseline” data point at the far left of the figure reflects the activity level of each group during last 5-min of the habituation session and the line-graph on the right side of the figure depicts each group’s activity during the 60-min following cocaine administration. The habituation session was intended to reduce exploratory and spontaneous activity of the subjects to low levels so that the psychomotor stimulant effects of cocaine could be readily identified during the second hour of testing. Indeed, by the end of the 60-min of habituation, activity levels were low for all four groups and while the vehicle-treated animals appear to have remained more active than the antagonist-treated animals, the differences in group performance were not statistically reliable (one-way independent group ANOVA on “baseline” scores; F(3,20) = 2.62, p=0.084).
Fig. 1.
Mean (+SEM) distance travelled in cm for each group during the last 5-min of a 1-h habituation session (designated as “baseline” on the far left of the graph) and during the 60-min immediately following i.p. injection of cocaine (15 mg/kg). All animals were pretreated with one of four doses of the DA antagonist cis-flupenthixol (0.0, 0.125, 0.25, or 0.5 mg/kg/ml) 2-h prior to habituation and 3-h hours prior to cocaine administration.
A two-factor (Group x Time) analysis of variance (ANOVA) was computed on the data from the final 60-min of testing. The ANOVA confirmed that all subjects decreased their activity levels over the course of the test session (main effect of “Time”; F(11,187)=12.640, p<.001] and that the patterns of decreased responding were comparable across groups (i.e., there was no statistically significant Group x Time interaction). The ANOVA also revealed that cis-flupenthixol pretreatment dose-dependently reduced cocaine-induced activity (main effect of Group; F(3,17)=6.760, p=.004). Post-hoc Tukey analyses confirmed that those animals pretreated with either 0.25 mg/kg or 0.5 mg/kg cis-flupenthixol were significantly less active than vehicle controls (p < 0.002 in both cases). It should be noted, however, that no dose of antagonist induced immobility in treated animals. Indeed, all four groups demonstrated increases in their activity levels from baseline following cocaine administration with the magnitude of those increases inversely related to the dose of cis-flupenthixol pretreatment. Even animals treated with the high 0.5 mg/kg dose of antagonist increased their locomotor activity following cocaine administration by over 200% (from baseline), traveled over 2000 cm during the first 20-min after cocaine administration, and remained active, albeit at low levels, throughout the test session.
3.2 Conditioned Place Testing
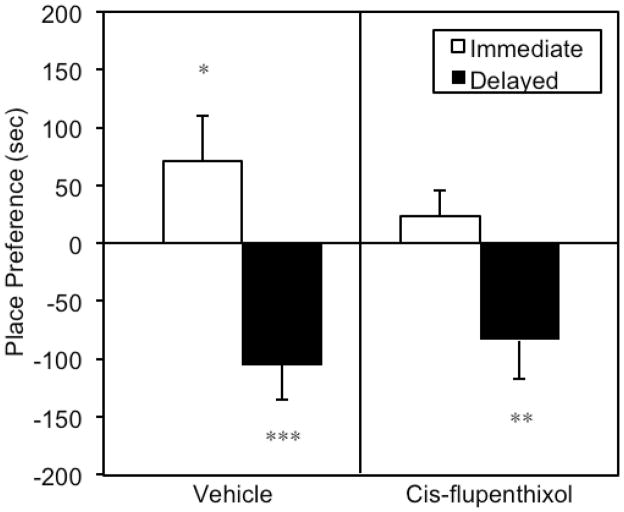
Prior to place conditioning, a repeated-measures t-test confirmed that animals did not have a significant preference for either of the two test environments during baseline (p>.05). Mean (+SEM) Difference Scores (Test-Baseline) are depicted for all four treatment groups in Figure 2. A one-way independent-groups ANOVA computed on those data revealed a significant difference between groups [F(3,63)=6.515, p=.001]. In order to determine if there was a significant mean shift from baseline either toward (preference) or away from (aversion) the cocaine-paired side, directional one-tailed single sample t-tests were used to compare difference scores to zero (a value that would indicate no shift from baseline). As expected, vehicle pre-treated “immediate” group animals exhibited a statistically significant shift toward the cocaine-paired environment on test day relative to baseline [i.e., their difference score was reliably greater than zero; t(20)=1.788, p<.05], while vehicle pre-treated “delayed” group animals spent less time in the cocaine-paired side on test day following conditioning [t(13)=−3.799, p<.002]. Thus, vehicle-pretreated animals developed CPPs for an environment paired with the immediate effects of cocaine, and CPAs for an environment paired with the delayed effects of the drug. Pretreatment with 0.5 mg/kg i.p. cis-flupenthixol prevented the development of CPPs for the immediate effects of i.v. cocaine (p>.05) but had no effect on the negative properties of the drug; i.e., cis-flupenthixol administration did not attenuate the development of CPAs in the delayed-cocaine group [t(12)=−2.514, p<.02].
Fig. 2.
Mean (+SEM) difference scores for animals conditioned to associate a unique environment with either the immediate/positive effects of i.v. cocaine (Immediate) or the delayed/negative effects of the drug present 15-min after injection (Delayed). Difference scores were computed as amount of time spent in the cocaine-paired environment on test day minus the time spent in that same compartment on baseline. Prior to each conditioning trial, each rat was pretreated with either 0.5 mg/kg i.p. cis-flupenthixol (right panel) or saline vehicle (left panel). Asterisks indicate that a group’s difference score was significantly different from zero. *p<.05; ** p<.02; ***p<.002.
4. Discussion
In the current study, animals developed conditioned preferences for environments paired with the immediate effects of i.v. cocaine and aversions for environments paired with the effects of the drug present 15 min after i.v. injection. These findings confirm the results of previous reports (e.g., Ettenberg et al., 1999; Knackstedt et al., 2002; Ettenberg and Bernardi, 2007; Jhou et al., 2013, Su et al., 2013) and suggest that acute cocaine administration produces diametrically opposite and temporally displaced affective actions – i.e., an initial positive/rewarding “high” followed in time by an aversive/anxiogenic “crash” (e.g., Ettenberg, 2004). The notion that a single injection of cocaine can produce both positive and negative reactions corresponds closely to Solomon and Corbit’s Opponent Process Theory of Motivation (1974), which posits that the initial state produced by an affective stimulus (designated as the “a” process) is offset by a subsequent and opposing “b” process that functionally returns the animal to affective homeostasis. Solomon and Corbit further hypothesized that the opponent “a” and “b” processes were each mediated by separate neuronal systems. This position is also supported by the current results in that post-synaptic DA receptor antagonism interfered with the initial positive effects of cocaine, while leaving the negative effects unaltered. Thus, while others have similarly reported that the D1/D2 receptor antagonist, cis-flupenthixol can block cocaine-induced CPPs (e.g., Veeneman et al., 2011), the current results extend these findings by demonstrating that the same antagonist treatment had no effect on the development of the CPAs stemming from the delayed aversive/anxiogenic effects of the drug. Our results are therefore consistent with those of Isaac et al. (1989) who demonstrated that lesions of the dopamine terminals in the prefrontal cortex disrupted cocaine-induced conditioned place preferences but had no effect on the drug’s capacity to produce conditioned taste aversions.
The demonstration of a learned place preference or aversion necessarily requires that the animal is capable of both learning the drug-place association and retrieving that information on test day. Since dopamine antagonism has been shown to impair the acquisition of conditioned behaviors (e.g., El Ghundi et al., 2007; Legault et al., 2006; Wishaw and Dunnett, 1985) one might suggest that the current cis-flupenthixol effects on CPPs stem not from a decrease in cocaine-reward, but rather from an impairment in the animals’ capacity to form the drug-place associations. While one cannot rule out this possibility, such an explanation seems unlikely. The fact that CPPs were attenuated by cis-flupenthixol while CPAs remained unaffected, clearly indicates that the drug did not produce a general disruption in the subjects’ ability to form drug-place associations.
Decreased dopaminergic function achieved through post-synaptic receptor blockade has long been associated not only with deficits in cocaine reward but also with deficits in motoric capacity (Ahlenius et al., 1987; Baldo et al., 1999; Chan and Webster, 1971; Delfs et al., 1990). Indeed, several investigators have suggested that a drug’s capacity to enhance locomotor activity serves as a predictor of, or is associated with, the reinforcing actions of the drug (e.g. Almaric and Koob, 1993; Gold et al., 1989; Uhl et al., 2002; Wise, 2005). In the current study we intentionally selected a DA antagonist (cis-flupenthixol) that had been shown to reverse the locomotor response to cocaine (e.g., Ahlenius et al., 1987), as well as prevent the development of CPPs (Veeneman et al, 2011) and attenuate cocaine reward in an i.v. self-administration paradigm (Negus et al., 1996; Ettenberg et al., 1982). We then identified a dose of cis-flupenthixol that greatly attenuated the psychomotor stimulant effects of cocaine (Fig 1) with the expectation that it would also attenuate cocaine-induced CPPs (as confirmed in Fig 2). The primary question here was whether or not the same dose of antagonist that altered indices of cocaine reward, would similarly affect the aversive/anxiogenic effects of the drug as measured by CPAs (which, of course, it did not). Note that while treatment with the 0.5 mg/kg dose of cis-flupenthixol prevented the psychomotor stimulant response to cocaine, all treated animals continued to exhibit motor activity throughout the one-hour test session. Therefore, all rats maintained their ability to interact with and explore their environment during conditioning trials. Further, during both baseline and test day trials, when the kinetic demands of the task were much higher, the animals were tested undrugged.
The decision to employ an i.p. route of cocaine administration in the locomotor activity test and an i.v. route in the place-conditioning work was based on several factors. While both i.p. and i.v. cocaine administration result in different onsets of action and durations of drug effects (Booze et al., 1997), both routes of administration have been shown to produce comparable effects on locomotor behavior (Porrino,1993). Indeed, Porrino (1993) reported that the similarities in locomotor response to both i.p. and i.v. cocaine stem from the ability of either route of administration to produce similar levels of activation in motor-related brain structures (i.e. the substantia nigra), as measured by 2-[14C] deoxyglucose utilization. In contrast, when reward-related brain regions within the mesocorticolimbic dopamine system were examined, Porrino (1993) found that i.v. cocaine had a significantly greater impact on neuronal activity than i.p. cocaine. Hence while i.p. cocaine was used in the locomotor test, cocaine was delivered i.v. in the place conditioning procedures based both on the data discussed above and to maintain comparability with our previous CPP/CPA findings all of which were conducted with i.v. cocaine.
The current results suggest that while elements of the mesolimbic DA system have been shown to be responsive to the presentation of aversive stimuli and/or stressful events (e.g., Abercrombie et al., 1989; Anstrom and Woodward, 2005; Bassareo et al., 2002; Brischoux et al., 2009; Mantz et al., 1989), the aversive/anxiogenic effects of cocaine remain intact in the absence of normal DA signaling. Such results necessarily imply that other mechanisms are involved in mediating these “negative” actions of the drug. Several possibilities, functioning either alone or in combination, are suggested by the existing literature. For example, cocaine alters benzodiazepine binding in rat brain (Goeders et al., 1997; Keys and Ellison, 1999; Suzuki et al., 2000) and stimulates the release of the stress hormones corticosterone, ACTH, and corticotropin-releasing factor (Borowsky and Kuhn, 1991; Goeders, 2002a, 2002b; Rivier and Vale, 1987; Sarnyai et al., 1995, 2001;Sholar et al., 1998) any one or combination of which could conceivably account for the anxiogenic state produced by the drug. Cocaine is also a potent reuptake inhibitor at the serotonergic (5-hydroxytryptamine; 5-HT) transporter (Filip et al., 2005; Koe, 1976; Ritz et al., 1990) and while manipulations of 5-HT systems have at times produced contradictory and inconclusive results in behavioral tests of anxiety (e.g., Handley et al., 1993) there is a growing literature implicating 5-HT neurons emanating from the dorsal raphé nucleus in the behavioral response to a variety of stressors and anxiogenic drugs (e.g., Abrams et al., 2005; Chaouloff, 2000; Reuter and Jacobs, 1996; Rex et al., 2005; Sena et al., 2003). Indeed, we have previously reported that interfering with 5-HT function via lesion or pharmacological manipulations reduced the aversive response to cocaine while leaving the positive/rewarding effects intact (e.g., Ettenberg and Bernardi, 2006; 2007; Ettenberg et al., 2011). Such results nicely compliment those observed here and suggest a role for 5-HT signaling in the anxiogenic actions of cocaine.
Cocaine also inhibits the norepinephrine (NE) transporter (Raitera et al., 1977; Ritz et al., 1990) and, like 5-HT, enhanced NE neuronal activity has been associated with increases in the behavioral responses to stressful and/or anxiogenic stimuli (Bremner ta al., 1996; Itoi and Sugimoto, 2010). A potential role for NE in the mediation of cocaine’s anxiogenic effects is supported by the finding that NE receptor antagonism within subregions of the extended amygdala reduce the effectiveness of stress to reinstate cocaine-seeking after a period of drug abstinence (e.g., Leri et al., 2002). Additionally, data from our laboratory have shown that NE beta-receptor antagonism within the bed nucleus of the stria terminalis or the central nucleus of the amygdala (both constituent parts of the extended amygdala) dose-dependently attenuates the approach-avoidance conflict behavior observed in rats running an alley for i.v. cocaine (Wenzel et al., 2011a) and prevents the development of cocaine-induced CPAs (Wenzel et al., 2012). Such findings implicate NE signaling, especially within the extended amygdala, as important for the expression of cocaine’s anxiogenic actions. Recent work suggests that structures within the extended amygdala project to a lateral habenula (LHb) – VTA neuronal circuit that Jhou and colleagues have also implicated in the delayed anxiogenic actions of cocaine (Jhou et al., 2009; 2013). Thus, like all other psychoactive drugs, cocaine produces a multitude of actions that influence a wide variety of neuronal systems thought to be involved in stress, anxiety, motivation and reward. Additional research will therefore be needed to more clearly elucidate the contributions of the various endocrine and neurochemical mechanisms in the positive and negative actions of cocaine that ultimately interact to motivate organism’s to seek and ingest the drug.
Highlights.
Cocaine administration produces immediate/positive and delayed/negative effects.
Rats develop preferences for environments paired with cocaine’s immediate effects.
Rats develop aversions for environments paired with the delayed effects of cocaine.
Dopamine antagonism blocks cocaine-induced conditioned place preferences
Dopamine antagonism does not affect cocaine-induced conditioned place aversions
Acknowledgments
This research was funded by NIH grants DA005041 and DA033370 awarded to AE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52(5):1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Abrams JK, Johnson PL, Hay-Schidt A, Mikkelsen JD, Shekkar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neurosci. 2005;133:983–97. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Ahlenius S, Hillegaart V, Thorell G, Magnusson O, Fowler CJ. Suppression of exploratory locomotor activity and increase in dopamine turnover following the local application of cis-flupenthixol into limbic projection areas of the rat striatum. Brain Res. 1987;402(1):131–138. doi: 10.1016/0006-8993(87)91055-9. [DOI] [PubMed] [Google Scholar]
- Amalric M, Koob GF. Functionally selective neurochemical afferents and efferents of the mesocorticolimbic and nigrostriatal dopamine system. Prog Brain Res. 1993;99:209–26. doi: 10.1016/s0079-6123(08)61348-5. [DOI] [PubMed] [Google Scholar]
- Anstrom K, Woodward DJ. Restraint increases dopaminergic burst firing in awake rats. Neuropsychopharmacology. 2005;30(10):1832–1840. doi: 10.1038/sj.npp.1300730. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Tien AY, Petronis KR. Epidemiologic evidence on cocaine use and panic attacks. Am J Epidemiol. 1989;129(3):543–549. doi: 10.1093/oxfordjournals.aje.a115166. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Markou A, Koob GF. Increased sensitivity to the locomotor depressant effect of a dopamine receptor antagonist during cocaine withdrawal in the rat. Psychopharmacology. 1999;141(2):135–144. doi: 10.1007/s002130050817. [DOI] [PubMed] [Google Scholar]
- Bassareo V, De Luca MA, Di Chiara G. Differential expression of motivational stimulus properties by dopamine in nucleus accumbens shell versus core and prefrontal cortex. J Neurosci. 2002;22:4709–4719. doi: 10.1523/JNEUROSCI.22-11-04709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmaker RH, Wald D. Haloperidol in normals. Br J Psychiatry. 1977;131:222–223. doi: 10.1192/bjp.131.2.222b. [DOI] [PubMed] [Google Scholar]
- Berridge KC. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol Behav. 2009;97(5):537–550. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booze RM, Lehner AF, Wallace DR, Welch MA, Mactutus CF. Dose-response cocaine pharmacokinetics and metabolite profile following intravenous administration and arterial sampling in unanesthetized, freely moving male rats. Neurotoxicol Teratol. 1997;19(1):7–15. doi: 10.1016/s0892-0362(96)00180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky B, Kuhn CM. Monoamine mediation of cocaine-induced hypothalamo-pituitary-adrenal activation. J Pharmacol Exp Ther. 1991;256:204–210. [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse. 1996 May;23(1):28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 2009;106(12):4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick PA. Distinguishing effects of cocaine i.v. and SC on mesoaccumbens dopamineand serotonin release with chloral hydrate anesthesia. Pharmacol Biochem Behav. 1992;43(3):929–937. doi: 10.1016/0091-3057(92)90427-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2003;168(1–2):66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Carr GD, Phillips AG, Fibiger HC. Independence of amphetamine reward from locomotor stimulation demonstrated by conditioned place preference. Psychopharmacology. 1988;94(2):221–226. doi: 10.1007/BF00176849. [DOI] [PubMed] [Google Scholar]
- Chaouloff F. Serotonin, stress and corticoids. J Psychopharmacol. 2000;14:139–51. doi: 10.1177/026988110001400203. [DOI] [PubMed] [Google Scholar]
- Chan OL, Webster RA. Effect of tetrabenazine and alpha-methyl-m-tyrosine on exploratory activity and brain catecholamines in rats. Br J Pharmacol. 1971;41(4):691–699. doi: 10.1111/j.1476-5381.1971.tb07077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church WH, Justice JB, Byrd LD. Extracellular dopamine in rat striatum following uptake inhibition by cocaine, nomifensine and benztropine. Eur J Pharmacol. 1987;139(3):345–348. doi: 10.1016/0014-2999(87)90592-9. [DOI] [PubMed] [Google Scholar]
- Costall B, Kelly ME, Naylor RJ, Onaivi ES. The actions of nicotine and cocaine in a mouse model of anxiety. Pharmacol Biochem Behav. 1989;33(1):197–203. doi: 10.1016/0091-3057(89)90450-4. [DOI] [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH. Dopamine receptors and average clinical doses. Science. 1976;194(4264):546–546. doi: 10.1126/science.194.4264.546. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Schreiber L, Kelley AE. Microinjection of cocaine into the nucleus accumbens elicits locomotor activation in the rat. J Neurosci. 1990;10(1):303–310. doi: 10.1523/JNEUROSCI.10-01-00303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneau G, Yanagita T, Seevers MH. Self-administration of psychoactive substances by the monkey. Psychopharmacology. 1969;16(1):30–48. doi: 10.1007/BF00405254. [DOI] [PubMed] [Google Scholar]
- El Ghundi M, O’Dowd BF, George SR. Insights into the role of dopamine receptor systems in learning and memory. Neurosci Rev. 2007;18(1):37–66. doi: 10.1515/revneuro.2007.18.1.37. [DOI] [PubMed] [Google Scholar]
- Ettenberg A. Opponent process properties of self-administered cocaine. Neurosci Biobehav Rev. 2004;27(8):721–728. doi: 10.1016/j.neubiorev.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Bernardi RE. Anxiolytic-like actions of buspirone in a runway model of intravenous cocaine self-administration. Pharmacol Biochem Behav. 2006;85(2):393–9. doi: 10.1016/j.pbb.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Bernardi RE. Effects of buspirone on the immediate positive and delayed negative properties of intravenous cocaine as measured in the conditioned place preference test. Pharmacol Biochem Behav. 2007;87:171–8. doi: 10.1016/j.pbb.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Ofer OA, Mueller CL, Waldroup S, Cohen A, Ben-Shahar O. Inactivation of the dorsal raphé nucleus reduces the anxiogenic response of rats running an alley for intravenous cocaine. Pharmacol Biochem Behav. 2011;97(4):632–9. doi: 10.1016/j.pbb.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology. 1982;78(3):204–209. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Raven MA, Danluck DA, Necessary BD. Evidence for opponent-process actions of intravenous cocaine. Pharmacol Biochem Behav. 1999;64:507–12. doi: 10.1016/s0091-3057(99)00109-4. [DOI] [PubMed] [Google Scholar]
- Fibiger HC. The neurobiological substrates of depression in Parkinson’s disease: a hypothesis. Can J Neurol Sci. 1984;11(1):105–107. doi: 10.1017/s0317167100046230. [DOI] [PubMed] [Google Scholar]
- Filip M, Frankowska M, Zaniewska M, Golda A, Przegalinski E. The serotonergic system and its role in cocaine addiction. Pharmacol Rep. 2005;57:685–700. [PubMed] [Google Scholar]
- Goeders NE. Stress and cocaine addiction. J Pharmacol Exp Ther. 2002a;301:785–9. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002;27:13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Irby BD, Shuster CC, Guerin GF. Tolerance and sensitization to the behavioral effects of cocaine in rats: relationship to benzodiazepine receptors. Pharmacol Biochem Behav. 1997;57:43–56. doi: 10.1016/s0091-3057(96)00122-0. [DOI] [PubMed] [Google Scholar]
- Gold LH, Geyer MA, Koob GF. Neurochemical mechanisms involved in behavioral effects of amphetamines and related designer drugs. NIDA Res Monogr. 1989;94:101–26. [PubMed] [Google Scholar]
- Handley SL, McBlane JW, Critchley MA, Njung’e K. Multiple serotonin mechanisms in animal models of anxiety: environmental, emotional and cognitive factors. Behav Brain Res. 1993;58(1–2):203–10. doi: 10.1016/0166-4328(93)90104-x. [DOI] [PubMed] [Google Scholar]
- Healy D. Neuroleptics and psychic indifference: a review. J R Soc Med. 1989;82(10):615–619. doi: 10.1177/014107688908201018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci. 1988;42(18):1705–1712. doi: 10.1016/0024-3205(88)90036-7. [DOI] [PubMed] [Google Scholar]
- Hill SY, Powell BJ. Cocaine and morphine self-administration: effects of differential rearing. Pharmacol Biochem Behav. 1976;5(6):701–704. doi: 10.1016/0091-3057(76)90315-4. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56(1):27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac WL, Nonneman AJ, Neisewander J, Landers T, Bardo MT. Prefrontal cortex lesions differentially disrupt cocaine-reinforced conditioned place preference but not conditioned taste aversion. Behav Neurosci. 1989;103(2):345–355. doi: 10.1037//0735-7044.103.2.345. [DOI] [PubMed] [Google Scholar]
- Itoi K, Sugimoto N. The brainstem noradrenergic systems in stress, anxiety and depression. J Neuroendocrinol. 2010;22:355–61. doi: 10.1111/j.1365-2826.2010.01988.x. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009;513(6):566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Good CH, Rowley CS, Xu SP, Wang H, Burnham NW, Hoffman AF, Lupica CR, Ikemoto S. Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J Neurosci. 2013;33(17):7501–7512. doi: 10.1523/JNEUROSCI.3634-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keys AS, Ellison GD. Long-term alterations in benzodiazepine, muscarinic and alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor density following continuous cocaine administration. Pharmacol Toxicol. 1999;85:144–50. doi: 10.1111/j.1600-0773.1999.tb00082.x. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Samimi MM, Ettenberg A. Evidence for opponent-process actions of intravenous cocaine and cocaethylene. Pharmacol Biochem Behav. 2002;72:931–6. doi: 10.1016/s0091-3057(02)00764-5. [DOI] [PubMed] [Google Scholar]
- Koe BK. Molecular geometry of inhibitors of the uptake of catecholamines and serotonin in synaptosomal preparations of rat brain. J Pharmacol Exp Ther. 1976;199:649–661. [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Chi S, Nestler EJ. Fischer and Lewis rat strains show differential cocaine effects in conditioned place preference and behavioral sensitization but not in locomotor activity or conditioned taste aversion. J Pharmacol Exp Ther. 1994;269(1):137–144. [PubMed] [Google Scholar]
- Legault G, Smith CT, Beninger RJ. Post-training intra-striatal scopolamine or flupenthixol impairs radial maze learning in rats. Behav Brain Res. 2006;170(1):148–155. doi: 10.1016/j.bbr.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002 Jul 1;22(13):5713–8. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantz J, Thierry AM, Glowinski J. Effect of noxious tail pinch on the discharge rate of mesocortical and mesolimbic dopamine neurons: selective activation of the mesocortical system. Brain Res. 1989;476(2):377–381. doi: 10.1016/0006-8993(89)91263-8. [DOI] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish C, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24(7):1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Bunney BS. Differential effect of cocaine on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens: comparison to amphetamine. Synapse. 1989;4(2):156–161. doi: 10.1002/syn.890040209. [DOI] [PubMed] [Google Scholar]
- Møller Nielsen I. Does a true qualitative difference exist in the mode of action of neuroleptics and thymanaleptics on catecholamine neuron systems. Mod Probl Pharmacopsychiatry. 1970;5:68–70. [PubMed] [Google Scholar]
- Morency MA, Beninger RJ. Dopaminergic substrates of cocaine-induced place conditioning. Brain Res. 1986;399(1):33–41. doi: 10.1016/0006-8993(86)90598-6. [DOI] [PubMed] [Google Scholar]
- Mucha RF, van der Kooy D, O’Shaughnessy M, Bucenieks P. Drug reinforcement studied by the use of place conditioning in rat. Brain Res. 1982;243:91–105. doi: 10.1016/0006-8993(82)91123-4. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Lamas X, Mendelson JH. Acute and chronic effects of flupenthixol on the discriminative stimulus and reinforcing effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 1996;278:879–90. [PubMed] [Google Scholar]
- Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology. 1984;84(2):167–173. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- Porrino LJ. Functional consequences of acute cocaine treatment depend on route of administration. Psychopharmacology. 1993;112(2–3):343–351. doi: 10.1007/BF02244931. [DOI] [PubMed] [Google Scholar]
- Raiteri M, Del Carmine R, Bertollini A, Levi G. Effects of sympathomimetic amines on the svnaptosomal transport of noradrenaline, dopamine and 5-hydroxytryptamine. Eur J Pharmacol. 1977;41:133–143. doi: 10.1016/0014-2999(77)90202-3. [DOI] [PubMed] [Google Scholar]
- Reith ME, Meisler BE, Sershen H, Lajtha A. Structural requirements for cocaine congeners to interact with dopamine and serotonin uptake sites in mouse brain and to induce stereotyped behavior. Biochem Pharmacol. 1986;35(7):1123–1129. doi: 10.1016/0006-2952(86)90148-6. [DOI] [PubMed] [Google Scholar]
- Resnick RB, Kestenbaum RS, Schwartz LK. Acute systemic effects of cocaine in man: a controlled study by intranasal and intravenous routes. Science. 1977;195(4279):696–698. doi: 10.1126/science.841307. [DOI] [PubMed] [Google Scholar]
- Reuter LE, Jacobs BL. A microdialysis examination of serotonin release in the rat forebrain induced by behavioral/environmental manipulations. Brain Res. 1996;739:57–69. doi: 10.1016/s0006-8993(96)00809-8. [DOI] [PubMed] [Google Scholar]
- Rex A, Voigt JP, Fink H. Anxiety but not arousal increases 5-hydroxytryptamine release in the rat ventral hippocampus in vivo. Eur J Neurosci. 2005;22:1185–9. doi: 10.1111/j.1460-9568.2005.04251.x. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Cone E, Kuhar MJ. Cocaine inhibition of ligand binding at dopamine, norepinephrine and serotonin transporters: a structure activity study. Life Sci. 1990;46:635–645. doi: 10.1016/0024-3205(90)90132-b. [DOI] [PubMed] [Google Scholar]
- Rivier C, Vale W. Cocaine stimulates adrenocorticotropin (ACTH) secretion through a corticotropin-releasing factor (CRF)-mediated mechanism. Brain Res. 1987;422:403–6. doi: 10.1016/0006-8993(87)90953-x. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav. 1977;6(6):615–620. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Koob GF. Disruption of cocaine self-administration following 6-hydroxydopamine lesions of the ventral tegmental area in rats. Pharmacol Biochem Behav. 1982;17(5):901–904. doi: 10.1016/0091-3057(82)90469-5. [DOI] [PubMed] [Google Scholar]
- Rogerio R, Takahashi RN. Anxiogenic properties of cocaine in the rat evaluated with the elevated plus maze. Pharmacol Biochem Behav. 1992;43:631–3. doi: 10.1016/0091-3057(92)90203-r. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Martin RA, Eaton CA, Monti PM. Cocaine craving as a predictor of treatment attrition and outcomes after residential treatment for cocaine dependence. J Stud Alcohol Drugs. 2007;68(5):641–648. doi: 10.15288/jsad.2007.68.641. [DOI] [PubMed] [Google Scholar]
- Romach MK, Glue P, Kampman K, Kaplan HL, Somer GR, Poole S, Clarke L, Coffin V, Cornish J, O’Brien CP, Sellers EM. Attenuation of the euphoric effects of cocaine by the dopamine D1/D5 antagonist ecopipam (SCH 39166) Arch Gen Psychiatry. 1999;56(12):1101–1106. doi: 10.1001/archpsyc.56.12.1101. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G. Brain corticotropin-releasing factor mediates ‘anxiety-like’ behavior induced by cocaine withdrawal in rats. Brain Res. 1995;675:89–97. doi: 10.1016/0006-8993(95)00043-p. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev. 2001;53:209–244. [PubMed] [Google Scholar]
- Schank JR, Liles LC, Weinshenker D. Norepinephrine signaling through beta-adrenergic receptors is critical for expression of cocaine-induced anxiety. Biol Psychiatry. 2008;63(11):1007–1012. doi: 10.1016/j.biopsych.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena LM, Bueno C, Pobbe RL, Andrade TG, Zangrossi H, Jr, Viana MB. The dorsal raphé nucleus exerts opposed control on generalized anxiety and panic-related defensive responses in rats. Behav Brain Res. 2003;142:125–33. doi: 10.1016/s0166-4328(02)00399-6. Erratum in: Behav Brain Res, 2003, 145, 233. [DOI] [PubMed] [Google Scholar]
- Sholar MB, Mendelson JH, Mello NK, Siegel AJ, Kaufman MJ, Levin JM, Renshaw PF, Cohen BM. Concurrent pharmacokinetic analysis of plasma cocaine and adrenocorticotropic hormone in men. J Clin Endocrinol Metab. 1998;83:966–968. doi: 10.1210/jcem.83.3.4654. [DOI] [PubMed] [Google Scholar]
- Simon P, Dupuis R, Constentin J. Thigmotaxis as an index of anxiety in mice: influence of dopaminergic transmissions. Behav Brain Res. 1994;61:59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Simon P, Panissaud C, Costentin J. Anxiogenic-like effects induced by stimulation of dopamine receptors. Pharmacol Biochem Behav. 1993;45(3):685–690. doi: 10.1016/0091-3057(93)90525-x. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Aston Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008;213(1–2):43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–45. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Spyraki C, Fibiger HC, Phillips AG. Cocaine-induced place preference conditioning: lack of effects of neuroleptics and 6-hydroxydopamine lesions. Brain Res. 1982;253(1–2):195–203. doi: 10.1016/0006-8993(82)90686-2. [DOI] [PubMed] [Google Scholar]
- Spyraki C, Nomikos GG, Varonos DD. Intravenous cocaine-induced place preference: attenuation by haloperidol. Behav Brain Res. 1987;26(1):57–62. doi: 10.1016/0166-4328(87)90016-7. [DOI] [PubMed] [Google Scholar]
- Su Z, Santoostaroam A, Wenzel J, Ettenberg A. On the persistence of cocaine-induced place preferences and aversions in rats. Psychopharmacology. 2013 doi: 10.1007/s00213-013-3086-9. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Abe S, Yamaguchi M, Baba A, Hori T, Shiraishi H, Ito T. Effects of cocaine administration on receptor binding and subunits mRNA of GABA(A)-benzodiazepine receptor complexes. Synapse. 2000;38:198–215. doi: 10.1002/1098-2396(200011)38:2<198::AID-SYN11>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Taber KH, Black DN, Porrino LJ, Hurley RA. Neuroanatomy of dopamine: reward and addiction. J Neuropsychiatry Clin Neurosci. 2012;24(1):1–4. doi: 10.1176/appi.neuropsych.24.1.1. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Hall FS, Sora I. Cocaine, reward, movement and monoamine transporters. Mol Psychiatry. 2002;7:21–6. doi: 10.1038/sj.mp.4000964. [DOI] [PubMed] [Google Scholar]
- Veeneman MM, Boleij H, Broekhoven MH, Snoeren EM, Guitart Masip M, Cousijn J, Spooren W, Vanderschuren LJ. Dissociable roles of mGlu5 and dopamine receptors in the rewarding and sensitizing properties of morphine and cocaine. Psychopharmacology. 2011;214(4):863–876. doi: 10.1007/s00213-010-2095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Ding YS, Logan J, Dewey SL, Hitzemann R, Lieberman J. Relationship between psychostimulant-induced “high” and dopamine transporter occupancy. Proc Natl Acad Sci U S A. 1996;93(19):10388–10392. doi: 10.1073/pnas.93.19.10388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25(22):5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel JM, Lane JE, Su Z-I, Ettenberg A. Program No. 779.11. Neuroscience Abstracts. New Orleans, LA: Society for Neuroscience; 2012. Norepinephrine antagonism in the central nucleus of the amygdala blocks the development of conditioned place aversions stemming from the delayed negative effects of i.v. cocaine in rats. [Google Scholar]
- Wenzel JM, Su Z-I, Haber ZM, Ettenberg A. Program No. 264.20. Neuroscience Abstracts. Washington, DC: Society for Neuroscience; 2011a. Noradrenergic antagonism within the extended amygdala attenuates the anxiogenic effects of cocaine in a self-administration runway model. [Google Scholar]
- Wenzel JM, Waldroup SA, Haber ZM, Su Z-I, Ben-Shahar O, Ettenberg A. Effects of lidocaine-induced inactivation of the bed nucleus of the stria terminalis, the central or the basolateral nucleus of the amygdala on the opponent-process actions of self-administered cocaine in rats. Psychopharmacology. 2011b;217(2):221–30. doi: 10.1007/s00213-011-2267-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson S, Gossop M, Powis B, Griffiths P, Fountain J, Strang J. Adverse effects of stimulant drugs in a community sample of drug users. Drug Alcohol Depend. 1997;44:87–94. doi: 10.1016/s0376-8716(96)01324-5. [DOI] [PubMed] [Google Scholar]
- Wise RA. Forebrain substrates of reward and motivation. J Comp Neurol. 2005;493(1):115–21. doi: 10.1002/cne.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Dunnett SB. Dopamine depletion, stimulation or blockade in the rat disrupts spatial navigation and locomotion dependent upon beacon or distal cues. Behav Brain Res. 1985;18(1):11–29. doi: 10.1016/0166-4328(85)90165-2. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gilbert J, Campos AC, Kline N, Ashby CR, Jr, Hagan JJ, Heidbreder CA, Gardner EL. Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacology. 2004;176(1):57–65. doi: 10.1007/s00213-004-1858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X-M, Gorman AL, Dunn AJ, Goeders NE. Anxiogenic effects of acute and chronic cocaine administration: neurochemical and behavioral studies. Pharmacol Biochem Behav. 1992;41:643–50. doi: 10.1016/0091-3057(92)90386-t. [DOI] [PubMed] [Google Scholar]