Abstract
Background
Acute pulmonary exacerbations are central events in the lives of individuals with cystic fibrosis (CF). Pulmonary Exacerbations lead to impaired lung function, worse quality of life, and shorter survival. We hypothesized that aggressive early treatment of acute pulmonary exacerbation may improve clinical outcomes.
Purpose
Describe the rationale of an ongoing trial designed to determine the efficacy of home monitoring of both lung function measurements and symptoms for early detection and subsequent early treatment of acute CF pulmonary exacerbations.
Study Design
A randomized, non-blinded, multi-center trial in 320 individuals with CF age 14 years and older. The study compares usual care to a twice a week assessment of home spirometry and CF respiratory symptoms using an electronic device with data transmission to the research personnel to identify and trigger early treatment of CF pulmonary exacerbation. Participants will be enrolled in the study for 12 months. The primary endpoint is change in FEV1 (L) from baseline to 12 months determined by a linear mixed effects model incorporating all quarterly FEV1 measurements. Secondary endpoints include time to first acute protocol-defined pulmonary exacerbation, number of acute pulmonary exacerbations, number of hospitalization days for acute pulmonary exacerbation, time from the end of acute pulmonary exacerbation to onset of subsequent pulmonary exacerbation, change in Health related quality of life, change in treatment burden, change in CF respiratory symptoms, and adherence to the study protocol.
Conclusions
This study is a first step in establishing alternative approaches to the care of CF pulmonary exacerbations. We hypothesize that early treatment of pulmonary exacerbations has the potential to slow lung function decline, reduce respiratory symptoms and improve the quality of life for individuals with CF.
Keywords: Cystic Fibrosis, Spirometry, Exacerbation
1. Introduction
Cystic Fibrosis (CF) is the most common life-shortening inherited disease in Caucasians and affects approximately 30,000 individuals in the U.S. It is an autosomal recessive genetic disease caused by mutations a chloride channel, the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR). CF is a systemic disease, which has profound effects on the respiratory and digestive systems. Individuals with CF have abnormally viscous mucus in their airways and develop chronic pulmonary infections. Most with CF suffer from pancreatic insufficiency and do not absorb nutrients normally.[1;2] Despite extensive advances in our understanding of the basic science of CF, the large majority of individuals with CF die from respiratory failure after repeated events termed acute pulmonary exacerbations. Exacerbations are common and present clinically with increased cough, increased sputum production, dyspnea, decreased energy level and appetite, weight loss, and decreases in spirometry[3]. These episodes are likely related to a complex relationship between host defense and airway microorganisms that impact sputum production and airflow obstruction. Pulmonary exacerbations have been associated with decreased survival[4-7], diminished future lung function [8], CF related diabetes[9], sleep disturbances and worse health related quality of life.[10;11]. The mainstay of exacerbation treatment is antibiotic therapy (intravenous, oral, or inhaled), airway clearance, mucolytics, and sometimes corticosteroids as an immune-modulator[12]. Pulmonary function tests, and the forced expiratory volume in 1 second (FEV1) in particular, are the best clinical method for objectively evaluating lung health in CF[13]. Changes in FEV1 can be used, in part, to define exacerbations, and to monitor response to treatment. Currently, unlike patients with asthma, individuals with CF do not routinely monitor their lung function at home, nor do they objectively track respiratory symptoms. Consequently, CF pulmonary exacerbations can be diagnosed weeks after onset when their symptoms progress to a point at which they seek medical care[14]. Use of home monitoring of lung function and symptoms may allow for earlier detection of pulmonary exacerbations, which would allow earlier treatment. This study will test the hypothesis that earlier treatment of CF exacerbations will result in better clinical outcomes.
Investigators reported on their experiences with home monitoring in CF in the late 1980's and early 1990's.[15;16] For a two-year period, 111 CF individuals maintained daily diaries recording vital capacity, weight, respiratory rate, pulse, and symptoms. The daily participation rate was approximately 80%. Subsequently, these investigators carried out a non-concurrent cohort study on 50 individuals with CF.[16] Twenty-five participants were selected randomly from the group that had used home monitoring and were matched to 25 participants that had not performed home monitoring. The groups were matched on age and gender and followed for four years. FEV1 declined from 73.1% predicted to 70.1% predicted in the home monitoring group (N.S.) and declined from 72.3% predicted to 60.8% (p<0.001) in the control group. Later extensions of this early work demonstrated the ability of patients to transmit the results of their home spirometry to the CF clinic via computer modem.[17] Our group has completed several pilot studies demonstrating that home spirometry and symptom measurement with a single electronic device is feasible [18-20]. We also completed a randomized pilot study showing that home monitoring can detect more exacerbations than standard care[21]. The current trial is the first large, randomized trial to assess the efficacy of home symptom and lung function monitoring on change in FEV1. Enrollment began in October 2011 and completion is expected in the Spring of 2014 (Figure 1). The study objectives are listed in Table 1.
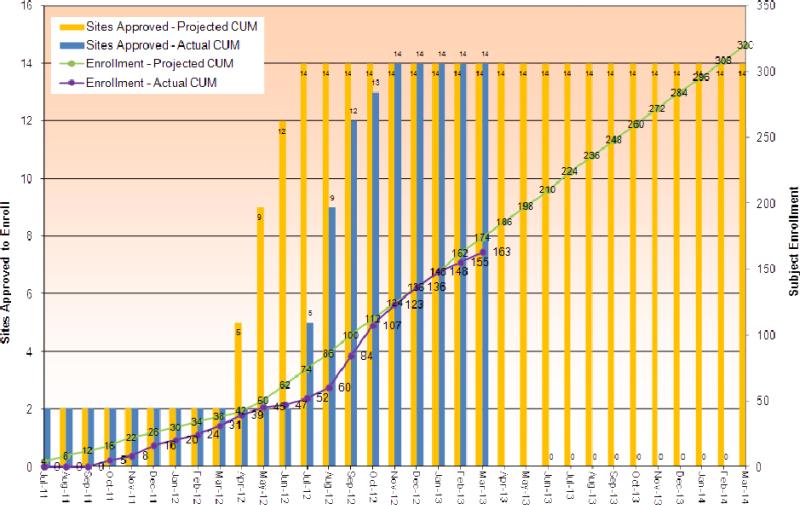
Figure 1.
This is a graph of cumulative study enrollment up to March 2013. The bars represent the number of study sites approved, yellow is projected and blue is actual. The lines represent subject enrollment, green is projected and purple is actual.
Table 1.
Study Objectives
| Primary Objective | To determine the efficacy of home lung function testing and symptom diary use for early intervention in the treatment of adolescent and adult CF acute pulmonary exacerbations. The primary outcome is lung function as measured with forced expiratory volume in the first second (FEV1). We hypothesize that if pulmonary exacerbations are identified and treated earlier, the progression of lung disease will be slowed, indicated by a slower annual decline in FEV1. |
| Secondary Objectives | 1. Assess the efficacy of the multi-faceted intervention compared to usual care using a standardized treatment on health related quality of life as measured by the CFQ-R. |
| 2. Assess the efficacy of the multi-faceted intervention compared to usual care using a standardized treatment on respiratory symptoms as measured by the CFRSD. | |
| 3. Assess whether the intervention will be associated with improved intermediate outcome measures (earlier identification of acute pulmonary exacerbation, change in lung function and respiratory symptoms from onset of acute pulmonary exacerbation to the 14 day follow-up visit, number of hospitalization days for acute pulmonary exacerbation, time from the onset of acute pulmonary exacerbation to onset of subsequent pulmonary exacerbation). | |
| 4. Assess the safety of the multi-faceted intervention compared to usual care. | |
| 5. Assess the tolerability as measured by treatment burden of the multi-faceted intervention compared to usual care. | |
| 6. Assess the adherence to the multi-faceted intervention. |
2. Methods
2.1 General Overview
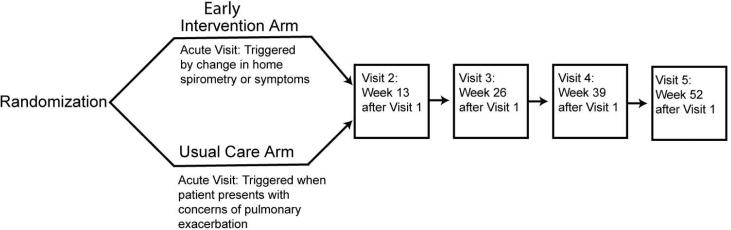
This is a randomized, non-blinded, multi-center trial in individuals with CF. The study compares a usual care arm to an early intervention arm. The intervention arm uses small electronic devices capable of electronic data transmission, to perform home spirometry and assessment of patient reported respiratory symptoms. We are using computerized remote data collection to identify and trigger the treatment of pulmonary exacerbation in adolescents and adults with CF (Figure 2). Additionally, subjects are seen for study visits every three months. Those in the usual care arm attend clinic every 3 months, as is the current standard of care and is combined with a study visit, and they are asked to contact their CF care center for acute visits. Individuals participate in the study for 12 months with five planned study visits and additional acute pulmonary exacerbation visits. For both arms, treatment of a pulmonary exacerbation is determined by the patient's clinician but is encouraged to follow treatment guidelines from the CF Foundation[12]. Recruitment and enrollment is occurring over a 36-month period. Due to the nature of the study intervention, it is not possible for participants to be blinded. Additionally, given that clinical decisions may need to be made based on the results of home monitoring, the investigators and research teams cannot be blinded either.
Figure 2.
Schematic showing an overview of the study design and study visits.
2.2 Study Population
Individuals with CF who are 14 years of age or older are recruited from the thirteen participating centers. Those who meet all of the inclusion criteria and none of the exclusion criteria are eligible for participation in this study. The participating sites are listed in the appendix. Participants need to be in stable condition at enrollment, with no evidence of a pulmonary exacerbation in the preceding two weeks. Individuals who have undergone solid organ transplants are excluded. Burkholderia cenocepacia infection is generally uncommon but is associated with worse outcomes. Given the low frequency of B. cenocepacia, it would be difficult to ensure even distribution of individuals with B. cenocepacia in the two study arms and therefore they are excluded. The study design is meant to be inclusive of the majority of CF patients. However, individuals need to have either a telephone land line or access to a personal computer to participate. If at the time of enrollment, individuals do not have either a computer or a home telephone, they are excluded. It is very unusual for individuals at the participating sites to lack the technology to participate. The inclusion and exclusion criteria are shown in table 2.
Table 2.
Inclusion and Exclusion Criteria
| Inclusion Criteria |
| 1. Documentation of a CF diagnosis as evidenced by one or more clinical features consistent with the CF phenotype and one or more of the following criteria: sweat chloride ≥ 60 mEq/liter, two well-characterized mutations in the cystic fibrosis transmembrane conductive regulator gene, or abnormal nasal potential difference |
| 2. Age ≥ 14 years of age at the Screening Visit |
| 3. Able to perform spirometry |
| 4. Clinically stable without antibiotic treatment for a pulmonary exacerbation in the 2 weeks prior to the Screening Visit |
| 5. At screening, forced expiratory volume in one second (FEV1) greater than 25% of predicted for age |
| Exclusion Criteria |
| 1. History of solid organ transplantation |
| 2. Participation in any interventional trial within the last 30 days |
| 3. Inability to speak and read the English language well enough to complete questionnaires |
| 4. Colonization with Burkholderia cenocepacia within the last 24 months |
| 5. Currently receiving antimicrobial therapy to treat non-tuberculous mycobacterium (e.g., M. abscessus, M. avium complex) |
| 6. Confirmed diagnosis of allergic bronchopulmonary aspergillosis (ABPA), as defined by the CFF guidance document [48], that is being actively treated |
| 7. Inability to access technology required to transmit home spirometry data |
2.2.1 Randomization
Study personnel use the Medidata® Rave and Balance™ systems to randomize participants. An adaptive randomization (dynamic allocation based on minimization[22]) is employed with the goal of ensuring balance between study arms by site, FEV1 and age. Participants are randomly assigned in a 1:1 ratio to one of two arms: 1) Early Intervention or 2) Usual Care. The adaptive randomization algorithm includes the following factors: site, FEV1 (<50%, 50-75%, and >75% predicted) and age (14-18 & 19+). The dynamic allocation algorithm seeks to optimize randomization balance by minimizing a weighted average of the marginal imbalance [23] of treatment allocation for each factor and for the study overall. A random element is added to the otherwise deterministic minimization algorithm to reduce allocation predictability by using a biased coin [24] to include a chance of allocation to a treatment arm other than the arm that optimizes balance. After baseline data collection, participants are randomized to one of the two study groups.
2.3 Study Interventions
2.3.1 Home Monitoring Intervention
Participants assigned to the early intervention arm use the AM2+® Lung Function Monitor (ERT, Inc.).[25] This device determines flow and volume via an infrared rotary flow sensor. It measures FEV1, FEF25-75, FVC, and peak expiratory flow. The accuracy, reproducibility and interdevice variability have been independently tested and meet the American Thoracic Society standards.[26] The AM2 meter is accurate to within ±0.05L. Participants who have a computer at home are supplied an AM2 meter and cable to transmit measurements to the study database. Participants with land phone lines are supplied an AM2 meter and a modem that connects to and transmits via a standard phone line. Participants receive replacement batteries and rotary flow sensors at visit 3. The AM2 monitors are programmed with a modified version of the symptom questionnaire (the Cystic Fibrosis Respiratory Symptom Diary (CFRSD))[27] enabling the participants to answer customized questionnaires by entering responses into the meter.
At the time of randomization, participants receive a demonstration on use of the home monitoring device and written instructions. Baseline spirometry measurements are recorded on the home spirometer. They are shown how to connect their meters to the modem or the computer. Participants measure spirometry twice weekly based on preliminary data from Goss, Edwards, Ramsey, et al.[27] Subjects are instructed to measure their FEV1 after taking inhaled medications and performing airway clearance techniques. They record three trials at each session in a seated position. Each home spirometry session takes less than 5 minutes. Participants are instructed to transmit their lung function results twice a week. The lung function data and the CFRSD responses are transmitted at the same time to a secured database on a centralized server. A study coordinator contacts them if they do not transmit their data within 5 days of the previous transmission. A computer algorithm is used to flag any FEV1 values in liters that are greater than 10% below baseline (absolute change) (ie. for a baseline FEV1 of 2.00 liters, a value below 1.80 liters would trigger a flag). The computer algorithm also generates a trigger based upon symptoms for evaluation for acute pulmonary exacerbation.
Sites are automatically notified by email of the triggers and need for acute pulmonary exacerbation evaluation if either of the following occurs: (1) the FEV1 (L) value falls by greater than 10% from baseline (defined as the spirometry performed at study entry on the AM2 device) or (2) respiratory symptoms worsen in 2 or more of the 8 respiratory symptoms captured in the CFRSD. The site schedules participants for the acute pulmonary exacerbation evaluation within five business days of the alarm. Subjects in the intervention arm are seen for a scheduled study visit every three months.
2.3.2 Usual Care Control Group
The usual care arm of the study relies on the participant contacting the CF clinical care team if he or she perceives a change in their clinical status. Usual care is defined as quarterly CF visits and acute visits triggered by calls from the participant to the clinic triage telephone line. This approach represents the current standard of care for patients with CF.
2.4 Measures
Demographic information (e.g., date of birth, sex, race, employment status, highest educational level attained, insurance status, tobacco use, and marital status) and medical historyare collected at screening. At screening and at the final visit, a complete physical examination is performed by either the site investigator or site sub-investigator. Qualified staff (physician, nurse practitioner, registered nurse, physician's assistant) will complete the abbreviated physical exam at all other visits including acute visits. Pulse oximetry is measured on room air with the participant at rest at all study visits. Spirometry is performed at each study visit and at acute visits, as well as at post-pulmonary exacerbation visits (time period between assessments – 2 weeks). Spirometry is performed in accordance with the most recent American Thoracic Society/European Respiratory Society guidelines.[28]
At every regularly scheduled study visit, the following dimensions are measured:
Assessment of Respiratory Symptoms. CFRSD [27] is the first patient reported outcome developed specifically to measure respiratory symptoms in CF employing standard qualitative methods. The CFRSD includes 16 questions (8 symptom items, 4 emotional impact items, and 4 activity impact items) for patient reporting. For both treatment arms, this instrument will be employed at all study visits and at acute visits for possible pulmonary exacerbation and post-acute pulmonary exacerbation.
Assessment of Health Related Quality of Life. The study instrument includes the Cystic Fibrosis Questionnaire-R (CFQ-R) [29-32]. This is a CF-specific instrument to assess health-related quality of life that has been validated in the CF population in the United States. The CFQ-R contains five generic and four CF specific domains. In addition, there are 3 symptom scales and an overall health perception scale. This instrument takes about 10 minutes to administer and is interviewer or self-administered. The minimally clinically significant difference noted in clinical trials with participants with CF has been established as a change of 4 points in each of the domains.
Assessment of Medication Adherence. The Treatment Adherence Questionnaire-Cystic Fibrosis (TAQ-CF) [33] is the only self-report adherence measure designed for CF patients with data on its psychometric properties. The original version of the TAQ-CF had 10-items and assessed aerosolized medications, chest physiotherapy, and enzyme use. This scale exhibited moderate to high agreement between mothers and teenagers and good 1-year test-retest reliabilities. The TAQ-CF was expanded to 57 items to include new drugs and methods of airway clearance as well as assessing the name and dose of each medication, the frequency of each treatment on a 7-point Likert scale (ranging from “not at all” to “3 or more times per day”) and its duration. The questionnaire takes less than 10 minutes to complete. This measure is used at all study visits to assess adherence to pulmonary medications, airway clearance and nutritional recommendations.
Assessment of Anxiety and Depression. The Hospital Anxiety and Depression Scale (HADS) [34;35] is a 14-item measure that is widely used and accepted to assess symptoms of depression and anxiety in adults. Scores for both anxiety and depression can range from 0-21, with higher scores indicating greater symptomatology. Scores of 11 or higher are considered indicative of anxiety or depression.
Assessment of Social Support. The Medical Outcome Study Social Support Survey (MOS-SSS) [36] is a 19-item measure of four separate social support subscales (Emotional/Informational, Tangible, Affectionate, and Positive Social Interaction) and an overall functional social support index. Items are on a 5-point Likert scale ranging from “none of the time” to “all of the time.” A higher score for an individual scale or the overall support index indicates more support. Research indicates good discriminant validity and reliability (all alphas > 0.90).
At participants’ final study visit a global assessment of protocol burden is assessed. Treatment burden is assessed by employing a domain from the CFQ-R that specifically addresses the impact of treatments for CF on health related quality of life. At the end of a participant's participation in the study, the participant will be asked to respond to a scale of 0 (very easy, no impact on my daily activities) to 10 (very inconvenient, took a lot of time and so inconvenient I would not do this routinely) to the question: “Overall, how much of a burden on you was participating in this study?”
Additionally, at screening and at the final study visit, the microbiology results from the most recent routine respiratory culture (e.g., sputum and pharyngeal cultures) done for clinical purposes are recorded. Blood is also being drawn at baseline and at the time of the first pulmonary exacerbation to be stored for future research on markers of inflammation, antibodies against microorganisms, viral DNA load and so on.
All subjects are seen at regularly scheduled study visits and at the time of any acute change in respiratory status. Subjects in the home monitoring arm are also seen when there is a trigger from the home monitoring device. At each study visit, a standardized review of pulmonary signs and symptoms is conducted to assess for presence of acute pulmonary exacerbations. Criteria for a protocol defined acute pulmonary exacerbation is based on modified definition used in the Early Pseudomonas Infection Control (EPIC001) clinical trial (U01HL080310)[37] with one modification: the duration of symptoms for a minor criteria will be ≥ 3 days (Table 3). This modification was used in a recent CF clinical trial of oral azithromycin [38]. This definition will be used to compare the rates of protocol defined acute pulmonary exacerbations between the study arms.
Table 3.
Protocol Definition of a Pulmonary Exacerbation
| The presence of a protocol-defined pulmonary exacerbation is defined by the following: One of the major criteria alone or Two of the minor signs/symptoms and fulfillment of symptom duration |
| Major Criteria: (One finding alone establishes the presence of a pulmonary exacerbation) |
| (1) Absolute decrease in FEV1 (L) of ≥ 10% from best baseline at study initiation using office spirometry, unresponsive to albuterol (in participants able to reproducibly perform spirometry) |
| (2)Oxygen saturation <90% on room air or absolute decrease of ≥ 5% from previous baseline |
| (3) New lobar infiltrate(s) or atelectasi(e)s on chest radiograph |
| (4) Hemoptysis (more than streaks on more than one occasion in past week) |
| Minor Signs/Symptoms: (Two minor signs/symptoms are required with duration criteria in the absence of major criteria) |
| (1) Increased work of breathing or respiratory rate |
| (2) New or increased adventitial sounds on lung exam |
| (3) Weight loss ≥5% of body weight or decrease across 1 major percentile in weight percentile for age in past 6 months |
| (4) Increased cough |
| (5) Decreased exercise tolerance or level of activity |
| (6) Increased chest congestion or change in sputum |
| Sign/Symptom Duration: (Required with two minor signs/symptoms in absence of major criteria): Initial sign or symptom present for ≥ 3 days |
| Duration of sign/symptoms ≥3 days |
2.5 Outcomes
2.5.1 Primary Outcome
The primary endpoint will be the difference between study arms in the change in FEV1 (L) from baseline to 12 months (end of study).
2.5.2 Secondary Outcomes
The secondary endpoints are time to first acute protocol-defined pulmonary exacerbation, number of acute pulmonary exacerbations, number of hospitalization days for acute pulmonary exacerbation, time from the end of acute pulmonary exacerbation to onset of subsequent pulmonary exacerbation, change in Health related quality of life as measured by the CFQ-R, change in treatment burden as measured by the treatment burden domain of the CFQ-R, change in CF respiratory symptoms as measured by the CFQ-R respiratory domain and the CFRSD, and adherence to the study protocol.
There are also several important safety outcomes in this study. These are incidence of adverse events, incidence of serious adverse events, and change in prevalence of resistant species of bacteria (Methicillin Resistant S. aureus, Multiply Resistant P. aeruginosa, B. cepacia, S. maltophilia, A. xylosoxidans) in sputum between baseline and final visit (Visit 5 or early withdrawal).
2.5 Quality Assurance and monitoring
2.5.1 Quality Assurance
After data have been entered into the study database, a system of computerized data validation checks are implemented and applied to the database on a regular basis. Queries are entered, tracked, and resolved through the electronic data capture system directly. The study database is updated in accordance with the resolved queries. All changes to the study database will be documented. The principal investigators (PI's) and study managers meet bimonthly by conference call to discuss study progress and issues. Research coordinators also have periodic conference calls at times determined by the PIs and the study managers. There is also an annual in person meeting of all study investigators and research coordinators.
2.5.2 Monitoring
Online monitoring is conducted by the data coordinating center according to the U.S. CFR Title 21 Parts 50, 56, and 312 and ICH Guidelines for good clinical practice (E6). An independent medical monitor reviews significant adverse events (SAEs) to adjudicate relatedness to the study intervention.
A Data Safety Monitoring Board (DSMB) has been created to function as an independent group of experts who advise the study investigators. The members of the DSMB serve in an individual capacity and provide their expertise and recommendations. The DSMB is composed of four members, including a statistician. The members are not co-investigators of the study and have no undisclosed conflict of interest. The DSMB reviews enrollment data and periodic safety monitoring reports, submitted by the data management group, as long as participants are being enrolled or evaluated. The DSMB holds meeting at least every six months. The DSMB also reviews the following activities associated with the intervention's evaluation: 1) participants’ responses to the burden item; and 2) any complaints that may be received from participants, family members, and clinicians about any aspect of the study including recruitment procedures and study implementation. All potential participants are provided contact information to register complaints, if they experience coercion or other problems. The DSMB will recommend modifying or stopping the study if any such complaints represent a legitimate concern about the study procedures or methods.
2.6 Data Management and Statistics
2.6.1 Data Management
Study personnel at each site enter data from source documents corresponding to a participant's visit into the protocol-specific electronic case report form (eCRF) when the information corresponding to that visit is available. Participants are not identified by name in the study database or on any study documents collected but will be identified by a site number, subject number and initials. If a correction is required for an eCRF, the time and date stamps track the person entering or updating eCRF data and creates an electronic audit trail. A copy of the eCRF will remain at the Investigator's site at the completion of the study.
The data is entered into a validated database. The data management group will be responsible for data processing, in accordance with procedural documentation. Database lock will occur once quality assurance procedures have been completed. All procedures for the handling and analysis of data will be conducted using good computing and clinical data management practices.
At all times, appropriate backup copies of the database and related software files will be maintained and the information will be appropriately protected from illegitimate access. Databases are backed up by the database administrator in conjunction with any updates or changes to the database. At critical junctures of the protocol (e.g., production of interim reports and final reports), a permanent archive of the database will be made. Archived versions of the database will be saved for at least ten years after the study publication.
2.6.2 Sample Size Justification
To simplify calculations, sample size and power estimates are based on a two-sample t-test rather than the longitudinal modeling approach that will be used in analysis, and therefore estimated sample size should be conservative.
The primary outcome is the difference in slope of FEV1 change over 52 weeks between early intervention and usual care groups. Given a sample size of 148 per group, the study will have 80% power to detect a 72 ml change in FEV1 from the time of enrollment to 52 weeks follow-up in the early intervention group compared to the change in the usual care arm assuming a standard deviation (SD) of 220 ml from a prior study of inhaled tobramycin in CF [39] and a two-tailed significance level of 0.05. If we assume a lower SD of 190 found in a more recent clinical trial [40], there would be 80% power to detect a mean difference between the two groups of 62 ml. This treatment effect is comparable to treatment effect sizes seen in 6-month treatment trials of therapies that have now been integrated into clinical care and represents <4% difference in slope of change. Due to improvements in general clinical care, changes in FEV1 have become harder to detect, but targeting a smaller change would not be clinically relevant.[39;41;42]
Based on preliminary data, we anticipate that approximately 7% of participants will drop out of the study prematurely. Therefore we plan to enroll 160 participants per arm for a total enrollment of 320.
2.6.3 General Analysis Plan
A detailed Statistical Analysis Plan (SAP) has been written describing all analyses that will be performed.
All eligible participants who comprise the intent to treat population and who are randomized into the study will be included in the efficacy and safety analysis.
The following demographic variables at screening will be summarized by study arm: race, gender, age, height, weight, spirometry, genotype, CF related complications, microbiology, tobacco use, and rates of standard chronic therapies. In addition, the following socioeconomic factors will be categorized: employment status, highest educational level attained, insurance status and marital status.
2.6.4 Primary Analysis
The primary outcome variable is FEV1 (as measured via office spirometry, not home spirometry), which will be obtained at quarterly study visits. The primary analysis will use a linear mixed effects model incorporating all quarterly FEV1 measurements to estimate the 52-week change in FEV1 and test for the differences between the two study arms (Early Intervention and Usual Care).[43] In the primary model, the outcome variable will be FEV1 (in liters), and the predictor variables will be baseline randomization factors such as FEV1 (<50%, 50-75%, and >75% predicted) and age (14-18 & >19 years), study arm assignment, time (in weeks) and the interaction between study arm and time. The primary test will be the test for the interaction between treatment and time. The slope of the mean regression line will be used to estimate change from baseline to 52 weeks in each arm and the difference between them.
2.6.5 Analysis of Secondary Outcomes
The secondary endpoints of change in health related quality of life and treatment burden as measured by the CFQ-R, and change in respiratory symptoms as measured by the CFQ-R respiratory domain and the CFRSD will be analyzed using a linear mixed effects model incorporating baseline randomization factors FEV1 (<50%, 50-75%, and >75% predicted) and age (14-18 & 19+), study arm, time (in weeks) and the interaction between study arm and time. Time to first acute protocol-defined pulmonary exacerbation and time from the start of acute pulmonary exacerbation to the onset of a subsequent pulmonary exacerbation will be analyzed using Cox proportional hazards regression. An Anderson-Gill model for recurrent events will be used to handle multiple subsequent pulmonary exacerbations.[44] Number of acute pulmonary exacerbations and number of hospitalization days for acute pulmonary exacerbation will be analyzed using Poisson regression. Because the time subjects will be in the study may vary, the number of days on study will be taken into account. For all these regression models, adjustment for baseline demographics that show imbalance will be explored.
Safety and tolerability data will be summarized by study arm. Adverse event rates will be coded by body system and MedDRA classification term. Adverse events will be tabulated by study arm and will include the number of participants for whom the event occurred, the rate of occurrence, and the severity and relationship to study participation or study procedures.
Change in prevalence of resistant species of bacteria (Methicillin Resistant S. aureus, Multiply Resistant P. aeruginosa, B. cepacia, S. maltophilia, A xylosoxidans) in sputum between baseline and final visit will be summarized by treatment group.
2.6.6 Interim Analyses
Review of interim data analyses for safety will occur semi-annually with the DSMB. A more formal interim data analysis for safety will occur once, when approximately half of the total patient population has been enrolled. An approximate Haybittle-Peto boundary will be used as a guideline, based on two-sided p-value less than 0.0027 for difference in the rate of unexpected SAEs related to procedure[45]. The DSMB can recommend stopping the study if based on the board's judgment an unanticipated and unacceptable number of SAEs have occurred.
3. Discussion
The early intervention in CF exacerbation (eICE) trial is a multicenter randomized trial of home symptom and lung function monitoring, with the goal of identifying acute pulmonary exacerbations earlier than with routine care, thus allowing for earlier treatment. The study objective is to determine if the home monitoring intervention will result in better clinical outcomes, with lung function as the primary outcome measure. Accredited CF care centers nationwide are very familiar with clinical drug trials and the CF Foundation has been instrumental in developing an infrastructure to ensure efficient, well-conducted trials. The eICE study utilizes the expertise and resources of the CF Foundation Therapeutics Development Network [46]. The eICE trial is unique in this research landscape for a number of reasons. Home electronic monitoring places new responsibility on participants to use the device and transmit the data to the study coordinators. Also, the device and data transmission poses challenges unique to this study. This study will provide a unique opportunity to characterize physiology and symptoms leading up to, during and following acute pulmonary exacerbations. Related to this, there are several ancillary studies planned and ongoing that will utilize the participants and data from the current study. These ancillary studies will examine the CF microbiome and biomarkers associated with exacerbations. The study will also provide a platform for assessing factors that influence treatment adherence in CF. Enrollment is expected to be complete in March 2014 and we hope to learn whether early identification of pulmonary exacerbations through home monitoring will lead to better health for the CF population.
While use of an electronic device that allows users to complete a symptom questionnaire and perform spirometry does not pose risks inherent in taking an experimental drug, the intervention places different responsibilities on study participants including performing the measurements and transmitting the data via computer or telephone modem. The technology for home spirometry and symptom monitoring is rapidly evolving. In an initial feasibility study[47], one of the PIs (NL) used the PIKO-1 meter, but the device had limited storage capacity and did not allow for electronic transmission of data. This led to a subsequent pilot study using the AM2 devices and data transmission via telephone modems. Increasingly the U.S. population uses cell phones rather than land lines but also has access to home computers. Additionally, in order to perform a multisite study in which exacerbations were to be detected prospectively, better systems for data capture and analysis was needed. In an additional pilot randomized trial using the Piko-6 device with the Philips Telehealth system, it became clear that “real time” submission of data was critical along with a computer based upload given the lack of standard land based telephone lines in use by many prospective participants. The lessons learned from these earlier studies led to the decision to use the AM2 devices for the present study since they were accurate, small, easy to use, and could capture both spirometry and symptom questions. To avoid the limitation of requiring a land phone line, we provided participants the option of connecting the AM2 to a computer via a USB port for data transfer or using a telephone modem. In order to allow for real time identification of exacerbations from multiple study sites, we implemented a centralized web-based server. Data from the participants’ devices is stored on the server, where FEV1 and symptom score triggers are computed and individual research coordinators are notified.
An important potential limitation of the study design is the lack of blinding, which is unfortunately not possible given the nature of the intervention. To address the potential bias that could arise from lack of blinding we have carefully developed protocols and strict definitions to limit systematic differences in study conduct on the part of participants or research team members. Additionally, our primary outcome is an objective measure that should not be biased.
Generalizability is frequently a concern in clinical trials. By enrolling Individuals with CF at 13 sites across the US, the study population should be representative of the general US CF population. Subjects enrolled in the standard care arm may still be more vigilant about their symptoms than usual, which could diminish our ability to detect differences between the two study groups.
The eICE study is currently enrolling patient at the goal rate (Figure 1) and should complete enrollment in March 2014. First and foremost this study will demonstrate whether Individuals with CF can and will perform home monitoring and whether home monitoring can detect CF exacerbations at a time earlier than with usual care. Furthermore, it will provide valuable insights into the physiology and symptoms in the periods around and during exacerbations. This study will be able to assess the impact of treatment adherence on exacerbations and also the role of self monitoring on treatment adherence. The eICE study will also provide a platform for important ancillary studies about the complex CF lung microbiome and how this influences pulmonary exacerbations.
Acknowledgements
This research was supported by grants from the NIH/NHBI (R01 HL103965) and the CF Foundation.
Funding Support: NHLBI R01HL103965, NIDDK P30DK089507, Cystic Fibrosis Foundation
List of Abbreviations
- ABPA
allergic bronchopulmonary aspergillosis
- AE
adverse event
- BCDM
Biostatistics and Clinical Data Management
- CF
cystic fibrosis
- CFRSD
Cystic Fibrosis Respiratory Symptom Diary
- CFQ-R
Cystic Fibrosis Questionnaire - Revised
- DSMB
Data Safety Monitoring Board
- FEV1
forced expiratory volume in one second
- FVC
forced vital capacity
- HADS
Hospital Anxiety and Depression Scale
- HRQOL
Health related quality of life
- MOS-SSS
Medical Outcomes Study Social Support Survey
- PI
Principal Investigator
- SAE
serious adverse event
- TAQ-CF
Treatment Adherence Questionnaire-CF
- TDNCC
Therapeutics Development Network Coordinating Center
Appendix
Appendix.
Participating Sites
| Organization Name | Site PI(s) |
|---|---|
| Johns Hopkins University School of Medicine (Baltimore, MD) | Lechtzin, Noah |
| University of Alabama (Birmingham, AL) | Antony, Veena |
| University of North Carolina at Chapel Hill (Chapel Hill, NC) | Donaldson, Scott |
| Northwestern University (Chicago, ILL) | McColley, Susanna |
| Cincinnati Children's Hospital (Cincinnati, OH) | Clancy, John |
| Case Western Reserve University (Cleveland, OH) | Dasenbrook, Elliott |
| Nationwide Children's Hospital (Columbus, OH) | McCoy, Karen |
| University of Colorado (Denver, CO) | Nick, Jerry |
| University of Colorado (Denver, CO) | Accurso, Frank |
| University of Iowa (Iowa City, IA) | Ahrens, Richard |
| University of Minnesota (Minneapolis, MN) | Billings, Joanne |
| Stanford University (Palo Alto, CA) | Milla, Carlos |
| University of Pittsburgh (Pittsburgh, PA) | Orenstein, David |
| Seattle Children's Hospital (Seattle, WA) | Gibson, Ronald |
| University of Washington School of Medicine (Seattle, WA) | Goss, Christopher |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Ratjen F, Doring G. Cystic fibrosis. Lancet. 2003;361(9358):681–9. doi: 10.1016/S0140-6736(03)12567-6. [DOI] [PubMed] [Google Scholar]
- 2.Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med. 2006;173(5):475–82. doi: 10.1164/rccm.200505-840OE. [DOI] [PubMed] [Google Scholar]
- 3.Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: Epidemiology and pathogenesis. Thorax. 2007;62(4):360–7. doi: 10.1136/thx.2006.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001;153(4):345–52. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liou TG, Adler FR, Cahill BC, Fitzsimmons SC, Huang D, Hibbs JR, et al. Survival effect of lung transplantation among patients with cystic fibrosis. JAMA. 2001;286(21):2683–9. doi: 10.1001/jama.286.21.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liou TG, Adler FR, Huang D. Use of lung transplantation survival models to refine patient selection in cystic fibrosis. Am J Respir Crit Care Med. 2005;171(9):1053–9. doi: 10.1164/rccm.200407-900OC. [DOI] [PubMed] [Google Scholar]
- 7.Mayer-Hamblett N, Rosenfeld M, Emerson J, Goss CH, Aitken ML. Developing Cystic Fibrosis Lung Transplant Referral Criteria Using Predictors of Two Year Mortality. Am J Respir Crit Care Med. 2002 doi: 10.1164/rccm.200202-087OC. [DOI] [PubMed] [Google Scholar]
- 8.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34(2):91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 9.Marshall BC, Butler SM, Stoddard M, Moran AM, Liou TG, Morgan WJ. Epidemiology of cystic fibrosis-related diabetes. J Pediatr. 2005;146(5):681–7. doi: 10.1016/j.jpeds.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 10.Britto MT, Kotagal UR, Hornung RW, Atherton HD, Tsevat J, Wilmott RW. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest. 2002;121(1):64–72. doi: 10.1378/chest.121.1.64. [DOI] [PubMed] [Google Scholar]
- 11.Dobbin CJ, Bartlett D, Melehan K, Grunstein RR, Bye PT. The effect of infective exacerbations on sleep and neurobehavioral function in cystic fibrosis. Am J Respir Crit Care Med. 2005;172(1):99–104. doi: 10.1164/rccm.200409-1244OC. [DOI] [PubMed] [Google Scholar]
- 12.Flume PA, Mogayzel PJ, Robinson KA, Goss CH, Rosenblatt RL, Kuhn RJ, et al. Cystic Fibrosis Pulmonary Guidelines: Treatment of Pulmonary Exacerbations. Am J Respir Crit Care Med. 2009 doi: 10.1164/rccm.200812-1845PP. [DOI] [PubMed] [Google Scholar]
- 13.Ramsey BW, Boat TF. Outcome measures for clinical trials in cystic fibrosis. Summary of a Cystic Fibrosis Foundation consensus conference. J Pediatr. 1994;124(2):177–92. doi: 10.1016/s0022-3476(94)70301-9. [DOI] [PubMed] [Google Scholar]
- 14.Sanders DB, Hoffman LR, Emerson J, Gibson RL, Rosenfeld M, Redding GJ, et al. Return of FEV1 after pulmonary exacerbation in children with cystic fibrosis. Pediatr Pulmonol. 2010;45(2):127–34. doi: 10.1002/ppul.21117. [DOI] [PubMed] [Google Scholar]
- 15.Finkelstein SM, Budd JR, Warwick WJ, Kujawa SJ, Wielinski CL, Ewing LB. Feasibility and compliance studies of a home measurement monitoring program for cystic fibrosis. J Chronic Dis. 1986;39(3):195–205. doi: 10.1016/0021-9681(86)90024-x. [DOI] [PubMed] [Google Scholar]
- 16.Finkelstein SM, Wielinski CL, Kujawa SJ, Loewenson R, Warwick WJ. The impact of home monitoring and daily diary recording on patient status in cystic fibrosis. Pediatr Pulmonol. 1992;12(1):3–10. doi: 10.1002/ppul.1950120104. [DOI] [PubMed] [Google Scholar]
- 17.Shultz EK, Finkelstein SM, Budd JR, Moore A, Warwick WJ. A home-based pulmonary function monitor for cystic fibrosis. Med Instrum. 1988;22(5):234–9. [PubMed] [Google Scholar]
- 18.West NE, Boyle MP, Mogayzel PJ, Riekert KA, Lechtzin N. The Ability of Home Spirometry and Symptom Monitoring to Predict Exacerbations in Cystic Fibrosis. <[11] Journal>. 2009;44(S10):A376. [Google Scholar]
- 19.Goss CH, McKone EF, Mathews D, Kerr D, Wanger JS, Millard SP. Experience using centralized spirometry in the phase 2 randomized, placebo-controlled, double-blind trial of denufosol in patients with mild to moderate cystic fibrosis. J Cyst Fibros. 2008;7(2):147–53. doi: 10.1016/j.jcf.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Lechtzin N, Merlo C, meade j, podliska m, Watts S, Boyle MP. Home FEV1 Monitoring to Assess Response to Therapy in Adults with Cystic Fibrosis. Pediatr Pulmonol. 2007;42(S30):379. [Google Scholar]
- 21.Aitken ML, Caldwell E, Wilhelm E, Goss CH. Early Intervention in Pulmonary Exacerbation. Pediatr Pulmonol. 2011;46(S34):A331. [Google Scholar]
- 22.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–15. [PubMed] [Google Scholar]
- 23.Han B, Enas NH, McEntegart D. Randomization by minimization for unbalanced treatment allocation. Stat Med. 2009;28(27):3329–46. doi: 10.1002/sim.3710. [DOI] [PubMed] [Google Scholar]
- 24.Brown S, Thorpe H, Hawkins K, Brown J. Minimization--reducing predictability for multi-centre trials whilst retaining balance within centre. Stat Med. 2005;24(24):3715–27. doi: 10.1002/sim.2391. [DOI] [PubMed] [Google Scholar]
- 25.Wagner FM, Weber A, Park JW, Schiemanck S, Tugtekin SM, Gulielmos V, et al. New telemetric system for daily pulmonary function surveillance of lung transplant recipients. Ann Thorac Surg. 1999;68(6):2033–8. doi: 10.1016/s0003-4975(99)01140-6. [DOI] [PubMed] [Google Scholar]
- 26.Richter K, Kanniess F, Mark B, Jorres RA, Magnussen H. Assessment of accuracy and applicability of a new electronic peak flow meter and asthma monitor. Eur Respir J. 1998;12(2):457–62. doi: 10.1183/09031936.98.12020457. [DOI] [PubMed] [Google Scholar]
- 27.Goss CH, Edwards TC, Ramsey BW, Aitken ML, Patrick DL. Patient-reported respiratory symptoms in cystic fibrosis. J Cyst Fibros. 2009;8(4):245–52. doi: 10.1016/j.jcf.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 29.Quittner AL. Measurement of quality of life in cystic fibrosis. Curr Opin Pulm Med. 1998;4(6):326–31. doi: 10.1097/00063198-199811000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Quittner AL, Sweeny S, Watrous M, Munzenberger P, Bearss K, Gibson NA, et al. Translation and linguistic validation of a disease-specific quality of life measure for cystic fibrosis. J Pediatr Psychol. 2000;25(6):403–14. doi: 10.1093/jpepsy/25.6.403. [DOI] [PubMed] [Google Scholar]
- 31.Quittner AL, Buu A, Messer MA, Modi AC, Watrous M. Development and validation of The Cystic Fibrosis Questionnaire in the United States: a health-related quality-of-life measure for cystic fibrosis. Chest. 2005;128(4):2347–54. doi: 10.1378/chest.128.4.2347. [DOI] [PubMed] [Google Scholar]
- 32.Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire-Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest. 2009;135(6):1610–8. doi: 10.1378/chest.08-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quittner A, Drotar D, Ievers-Landis C, Slocum N. Adherence to Medical Treatments in Adolescents With Cystic Fibrosis: The Development and Evaluation of Family-Based Interventions. In: Drotar D, editor. Promoting Adherence to Medical Treatment in Chronic Childhood Illness; Concepts, Methods and Intervention. Lawrence Erlbaum Associates; New Jersey: 2000. pp. 383–407. [Google Scholar]
- 34.Smith AB, Selby PJ, Velikova G, Stark D, Wright EP, Gould A, et al. Factor analysis of the Hospital Anxiety and Depression Scale from a large cancer population. Psychol Psychother. 2002;75(Pt 2):165–76. doi: 10.1348/147608302169625. [DOI] [PubMed] [Google Scholar]
- 35.White D, Leach C, Sims R, Atkinson M, Cottrell D. Validation of the Hospital Anxiety and Depression Scale for use with adolescents. Br J Psychiatry. 1999;175:452–4. doi: 10.1192/bjp.175.5.452. [DOI] [PubMed] [Google Scholar]
- 36.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–14. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 37.Rabin HR, Butler SM, Wohl ME, Geller DE, Colin AA, Schidlow DV, et al. Pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol. 2004;37(5):400–6. doi: 10.1002/ppul.20023. [DOI] [PubMed] [Google Scholar]
- 38.Saiman L, Anstead M, Mayer-Hamblett N, Lands LC, Kloster M, Hocevar-Trnka J, et al. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2010;303(17):1707–15. doi: 10.1001/jama.2010.563. [DOI] [PubMed] [Google Scholar]
- 39.Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med. 1999;340(1):23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 40.Goss CH, McKone EF, Mathews D, Kerr D, Wanger JS, Millard SP. Experience using centralized spirometry in the phase 2 randomized, placebo-controlled, double-blind trial of denufosol in patients with mild to moderate cystic fibrosis. J Cyst Fibros. 2008;7(2):147–53. doi: 10.1016/j.jcf.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354(3):229–40. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- 42.Saiman L, Marshall BC, Mayer-Hamblett N, Burns JL, Quittner AL, Cibene DA, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290(13):1749–56. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 43.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–74. [PubMed] [Google Scholar]
- 44.Therneau TM, Hamilton SA. rhDNase as an example of recurrent event analysis. Stat Med. 1997;16(18):2029–47. doi: 10.1002/(sici)1097-0258(19970930)16:18<2029::aid-sim637>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 45.Piantadosi S. Data Dependent Stopping. In: Piantadosi S, editor. Clinical Trials A Methodologic Perspective. 1 ed. Wiley-Interscience; New York: 1997. pp. 230–269. [Google Scholar]
- 46.Rowe SM, Borowitz DS, Burns JL, Clancy JP, Donaldson SH, Retsch-Bogart G, et al. Progress in cystic fibrosis and the CF Therapeutics Development Network. Thorax. 2012;67(10):882–90. doi: 10.1136/thoraxjnl-2012-202550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lechtzin N, Merlo C, meade j, podliska m, Watts SL, Boyle MP. Home FEV1 Monitoring to Assess Response to Therapy in Adults with Cystic Fibrosis. Pediatric Pulmonology. 2007;42(S30):379. [Google Scholar]
- 48.Stevens DA, Moss RB, Kurup VP, Knutsen AP, Greenberger P, Judson MA, et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis--state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin Infect Dis. 2003;37(Suppl 3):S225–S264. doi: 10.1086/376525. [DOI] [PubMed] [Google Scholar]