Abstract
Stress that is experienced after items have been encoded into memory can protect memories from the effects of forgetting. However, very little is known about how stress impacts recognition memory. The current study investigated how an aversive laboratory stressor (i.e., the cold-pressor test) that occurs after information has been encoded into memory affects subsequent recognition memory in an immediate and a delayed test (i.e., 2-hour and 3-month retention interval). Recognition was assessed for negative and neutral photographs using a hybrid remember/know confidence procedure in order to characterize overall performance and to separate recollection- and familiarity-based responses. The results indicated that relative to a non-stress control condition, post-encoding stress significantly improved familiarity but not recollection-based recognition memory or free recall. The beneficial effects of stress were observed in males for negative and neutral materials at both immediate and long-term delays, but were not significant in females. The results indicate that aversive stress can have long-lasting beneficial effects on the memory strength of information encountered prior to the stressful event.
Keywords: Recognition, Memory, Stress
1. Introduction
Stress can impact memory in various ways (Joëls, Pu, Wiegert, Oitzl, & Krugers, 2006; Lupien & McEwen, 1997; McGaugh & Roozendaal, 2002; Schwabe, Joëls, Roozendaal, Wolf, & Oitzl, 2012). For example, chronic stress can lead to permanent impairments in long term memory (McEwen & Sapolsky, 1995), and acute stress encountered during or just prior to retrieval can lead to decrements in memory performance (e.g., Smeets, Otgaar, Candel, & Wolf, 2008; Schwabe, Wolf, & Oitzl, 2010). However, there is growing evidence that acute stress encountered shortly after encoding can have beneficial effects on free recall, in the sense that post-encoding stress can protect memories from the effects of forgetting (Andreano & Cahill, 2006; Cahill, Gorski, & Le, 2003; Smeets et al., 2008). The beneficial effects of stress during the retention interval are theoretically important because they provide evidence for a consolidation process whereby stress acts to protect or strengthen recently encoded memories (e.g., McGaugh, 2000, Schwabe et al., 2012).
However, whether stress has a direct impact on memory strength is unclear because in almost all of the previous studies of post-encoding stress only free recall was measured rather than recognition memory. Stress may increase recall performance by facilitating the retrieval or search processes that are critical for recall without directly impacting the strength of the underlying memory trace. In the only study that has examined the effects of stress on recognition (Yonelinas, Parks, Koen, Jorgenson, & Mendoza, 2011) subjects encoded a mixture of negative and neutral photographs, then either went skydiving or waited on the ground. After a 2-hour delay period, free recall and recognition memory were tested. Recognition was tested using a remember/know and confidence rating procedure in order to separate recollection and familiarity-based recognition. The results indicated that stress led to an increase in familiarity-based recognition memory, and did not impact recollection-based recognition or free recall. In addition, the beneficial effects of stress were observed in males but not in females, a finding that is consistent with prior studies in humans and animals (e.g., Andreano & Cahill, 2006; Conrad et al., 2004; Cazakoff, Johnson, & Howland, 2010), and that has been attributed to variations in hormonal levels across the estrus cycle (for a review, see Andreano & Cahill, 2009).
The results of Yonelinas et al. (2011) provide support for the idea that stress facilitates the consolidation of recently encoded memories, in the sense that stress influenced familiarity strength, but they raise a number of important questions. First, do the effects of skydiving on recognition generalize to other forms of stress? Skydiving is unusual in that subjects are willing to pay for the experience, and it is a very extreme stress manipulation. That is, the cortisol elevations reported in the skydiving study were about two times the magnitude of those reported in most laboratory studies of stress and memory which have used either the cold-pressor test, in which an arm is held in ice water, or various types of socially-induced stress. Whether recognition memory performance benefits from a stressor such as the cold-pressor test is unknown.
Second, an unexpected finding in the skydiving study was that while stress improved memory for neutral materials, it did not significantly affect memory for negative materials. This contrasts with several previous studies of recall which suggested that stress has larger effects on negative than neutral materials (e.g., Cahill et al., 2003; Smeets et al., 2008). Importantly, memory performance for negative materials was quite high in the study by Yonelinas et al. (2011), and so high levels of performance may have concealed effects of stress on memory for negative materials.
Third, to what degree is the stress-related enhancement of memory maintained across time? Yonelinas and colleagues (2011) tested memory two hours after the stressor (a time that was sufficient to allow cortisol levels to return to normal levels), and found that stress affected familiarity-based recognition, but not recollection or recall. However, prior studies reporting effects of stress on recall have used longer delays, such as one day (Smeets et al., 2008), 48 hours (Beckner, Tucker, Delville, & Mohr, 2006), or one week (Cahill et al., 2003). Whether the beneficial effects of stress on familiarity-based recognition are maintained across a longer delay is unknown. Moreover, it is possible that the beneficial effects of stress on recollection and recall simply require more time to emerge. Thus, it is critical to determine the effects of stress across short and long delays.
The current study examined the effect of post-encoding stress on recognition, using the cold-pressor test. The primary questions were whether recollection or familiarity would be enhanced by post-encoding stress induced by the cold-pressor test, whether stress would benefit memory for both negative and neutral materials, and whether any such effects would be maintained across a long retention interval. In addition, because prior studies have indicated that the stress effects are more robust for males than for females (e.g., Andreano & Cahill, 2006) we included both males and females to determine if the stress effects were modulated by sex. Participants first encoded negative and neutral pictures, then completed either a stress-induction procedure (i.e., cold-pressor with ice water) or a non-stress control procedure (i.e., warm water). After a two hour delay, recall and recognition for the pictures was tested. To reduce possible ceiling effects, the presentation rate during the study phase was shorter than that used by Yonelinas et al. (2011; i.e., 800 vs. 2000ms/image). A remember/know confidence procedure was used to separate the effects on recollection and familiarity-based recognition. Finally, subjects were brought back to the lab several months later and received a second set of recall and recognition tests for the originally-encoded target materials.
2. Methods
2.1. Participants
A total of 40 undergraduates (20 female) were recruited from an online participant pool, and received Psychology course credit for participating. All testing was conducted between 11:00 and 15:00h. We chose the 11:00–15:00 testing period to avoid the rapid decline of cortisol associated with waking up (Lommer et al., 1976), and to facilitate comparisons to other related studies that had tested at this time (e.g., Yonelinas et al., 2011). Twenty subjects (10 female) were assigned to the stress group (Mean age = 19.2 years, Mean years education = 13) and twenty (10 female) were assigned to the control group (Mean age = 19.7 years, Mean years education = 13.5). Three participants reported use of oral contraceptives (1 stress, 2 controls), but excluding these subjects did not influence the pattern of results. All 40 subjects were contacted for a follow-up session. Twenty-one subjects (11 female) returned for the long-term memory assessment, for which they were paid $30. Ten had been originally assigned to the control condition (5 female), and eleven had been assigned to the stress condition (6 female). None of our subjects reported use of tobacco or medications. The study was approved by the Internal Review Board at the University of California, Davis.
2.2. Stimuli
The current study used a set of 368 pictures, half neutral and half negative, that was used in previous research (Yonelinas et al., 2011). The pictures were selected primarily from the International Affective Photo Series (IAPS) based on their standard scores of emotional arousal and emotional valence (Lang, Bradley, & Cuthbert, 2008), as well as from our own set (to balance the two sets for factors such as visual complexity, color, and the presence of humans). Images were approximately 315 pixels square, with minor variation in size and shape. Eight of the images were used as example trials: two prior to encoding and six prior to the recognition task. In the encoding phase, 60 neutral and 60 negative pictures were presented to each participant in a random order. In the initial recognition test, each participant was presented with 120 studied images and 120 new images (60 neutral) in a random order. Participants who returned for the long-term assessment were presented with the 120 studied images and the remaining 120 new images (60 neutral).
2.3. Procedure
The procedure is illustrated in Figure 1. After providing informed consent, participants provided a baseline saliva sample. The participant was offered a piece of gum and produced approximately 3 mL of saliva into a Salivette tube. Then the participant completed an incidental encoding procedure, in which 120 IAPS pictures (60 neutral, 60 negative) were presented via computer (using e-Prime 2.0) and the participant rated each picture for visual complexity. Each picture was presented for 800 ms, after which the participant had up to 2000 ms to respond. After an inter-trial interval of 500 ms, the next trial was initiated. These ratings were not analyzed. The participant completed questionnaires for approximately 10 min, providing demographic, medical, and sleep-related information.
Figure 1.
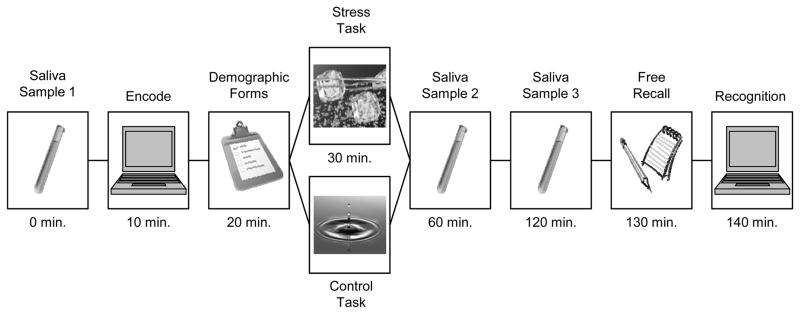
Methodological timeline for the first experimental session. The time values represent cumulative time from the onset of the experiment. In addition, subjects returned after 3 months for a second recall and recognition test.
Each participant then completed the cold-pressor test or a control task. The participant submerged their non-dominant arm in either an ice-water bath (M = 0.6° C) or tepid water (M = 23.4° C). The participant was instructed to keep their arm submerged for 3 min, or as long as possible, and to refrain from talking during the task. After a twenty minute delay, a second saliva sample was taken. This was followed by a one hour delay before a third saliva sample was taken. During the delay period, the participant was permitted to drink water (but not to eat), and was permitted to read or complete course work so long as it did not involve viewing pictures. Finally, the participant completed a recall and a recognition test.
The participant was given 10 min to recall as many pictures as possible by writing short descriptions of studied pictures. The recall test was followed immediately by a recognition test in which a mix of 120 studied and 120 new pictures were presented for 1500 ms each. Participants rated each picture as either being Recollected, or on a scale of 1–5, in which 1 = Sure new and 5 = Sure old. After the participant responded, a 500 ms interval preceded the subsequent trial.
For the long-term assessment, the testing procedure was identical to the initial test phase. That is, upon arriving to the lab and giving informed consent, participants were given 10 min to recall pictures from the original encoding phase. Then participants completed a recognition test in which the 120 studied images were mixed with 120 new images that had not previously been used in the experiment.
2.4. Analysis of Saliva Samples
Salivary cortisol concentrations were estimated in duplicate using a commercially-available radioimmunoassay kit (Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA). A detailed description of the assay procedure can be found in Yonelinas et al. (2011). The lower sensitivity for cortisol detection was 1.39nmol/L. One sample from one participant fell below this threshold, we did not obtain cortisol data from one participant, and one participant’s saliva samples were taken at incorrect intervals. Data from those three participants were removed from all analyses. Salivary cortisol data was subjected to an ANOVA with stress group (control/stress) and sex (male/female) as between-subjects factors and time of sample (sample 1/sample 2/sample 3) as a within-subject factor.
2.5. Analysis of Memory
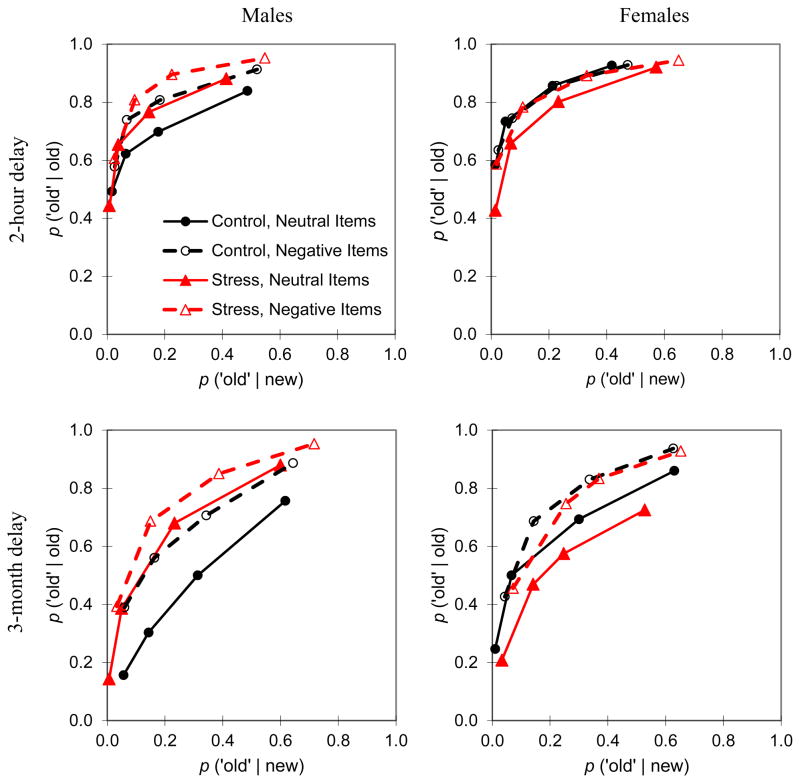
Recognition data were submitted to several analyses. Overall performance was assessed by computing d′ (Macmillan & Creelman, 2005). In this analysis studied items receiving an R, 5, 4, or 3 response were treated as hits and unstudied items receiving an R, 5, 4, or 3 response were treated as false alarms. Each participant’s recognition confidence data were also used to plot cumulative ROCs (MacMillan & Creelman, 2005), whereby the proportions of studied items rated at a given level of confidence were plotted against the proportion of unstudied items rated at the same level of confidence (Figure 2). That is, the left-most point on each curve represents the proportion of R and 5 responses to studied items plotted against the proportion of R and 5 responses to unstudied items. Note that R and 5 responses were combined because prior work has indicated that some familiarity-based responses are associated with high recognition confidence (Yonelinas, 2001). The next point represents the proportion of R, 5, and 4 responses to studied items plotted against the proportion of R, 5, and 4 responses to unstudied items, and so on, through confidence ratings of 2 (note that cumulative ratings at confidence level 1 result in proportions of 1.00). A dual-process signal detection model was fit to each subject’s data by minimizing the sum of squared errors, and a confidence-based ROC analysis was used to compute estimates of recollection and familiarity (Yonelinas, 1994). We also estimated recollection and familiarity using the remember/know method (Yonelinas, 2001), whereby recollection was estimated as the proportion of remember responses and familiarity was estimated as the proportion of recognized items that were familiar but not remembered [P (5 or 4 or 3) / ( 1 - P (remember)].
Figure 2.
Average recognition memory receiver operating characteristics.
Recall, scored by two independent raters, was initially measured as the number of pictures correctly described in the free recall test. Descriptions that lacked enough detail to specify a single target picture were not scored as correct. In a secondary analysis, the average number of details recalled for each type of picture was scored by counting the number of descriptive words (i.e., nouns, verbs, adjectives, adverbs) in each answer. Each measure of memory performance was subjected to a 3-factor ANOVA with stress group and sex as between-subject variables, and stimulus valence (neutral/negative) as a within-subject variable. Given the earlier reports of sex differences in studies of stress (see Andreano & Cahill, 2006) performance was also analyzed separately for males and females, using 2-factor ANOVAs with group as a between-subject variable and valence a within-subject variable. All analyses were conducted with PAS v.18, with an alpha level of 0.05.
3. Results
3.1. Salivary Cortisol
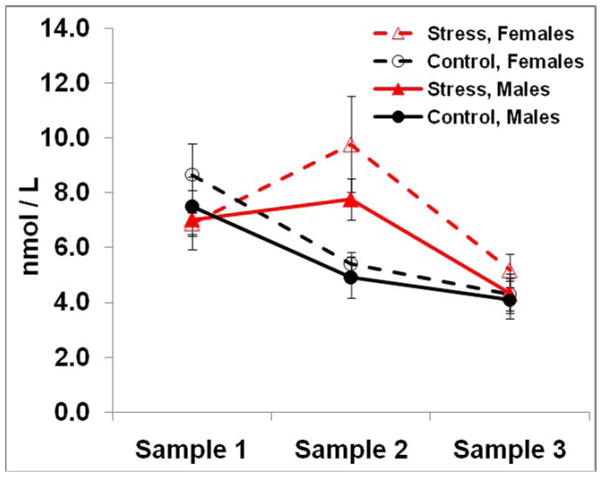
Analysis of the salivary cortisol data revealed that the cold-pressor test induced a cortisol response in the stress group (see Figure 3). There was a significant main effect of time (F(2, 66) = 14.29, MSe = 160.57, p < .001, ηp2 = .30) that was qualified by a significant stress by time interaction (F(2, 66) = 4.42, MSe = 49.65, p < .05, ηp2 = .12). Prior to encoding, concentrations of salivary cortisol did not differ between the stress (M = 8.90, SD = 4.99) and control groups (M = 8.65, SD = 5.71; t(35) = 0.14, p = .89); however, thirty minutes after the cold-pressor test, cortisol concentrations were significantly higher in the stress group (M = 9.50, SD = 5.01) compared to the control group (M = 5.29, SD = 2.19; t(35) = 3.34, p < .005). Prior to retrieval, there was no difference in cortisol concentrations between the stress (M = 4.98, SD = 2.27) and control groups (M = 4.27, SD = 2.01; t(35) = 1.01, p = .32). Thus, salivary cortisol was significantly higher in the stress group, relative to the control group, at sample 2.
Figure 3.
Mean salivary cortisol levels for stress (triangles) and control (circles) groups of males (solid lines) and females (dashed lines). Error bars represent SEs of the means. Sample 1 was taken just prior to encoding; sample 2 was taken twenty minutes after the cold-pressor test and sample 3 was taken one hour later, just prior to the first memory assessment.
Although there was no significant main effect of sex in the ANOVA (F(1, 33) = 1.04, MSe = 27.41, p > .05, ηp2 = .03) or significant interactions of sex (all F’s < 1.18), Figure 3 suggests that the stress-related cortisol response in the females was somewhat more variable than in the males. Further examination of individual subject measures indicated that only one male failed to show a substantial stress-related increase in cortisol, while almost half of the females failed to show such an increase.
3.2. Recognition Memory
Overall recognition performance can be assessed by visual examination of Figure 2, which reveals that stress (i.e., the functions plotted in red) increased males’ recognition accuracy for neutral and negative items relative to the non-stress control conditions (i.e., functions plotted in black). In contrast, for females, stress did not increase recognition, but rather it appeared to lead to a decrease in memory for the neutral materials. An analysis of overall recognition accuracy (Table 1), measured as d′ calculated at the midpoint of the ROC, revealed a significant interaction between stress and sex (F(1, 33) = 4.62, MSe = 1.96, p < .05, ηp2 = .12), indicating that stress differentially influenced males and females. In addition, there was a valence by sex interaction (F(1, 33) = 5.73, MSe = .47, p < .05, ηp2 = .15), and a main effect of valence (F(1, 33) = 4.55, MSe = .38, p < .05, ηp2 = .12). The latter effects reflected the fact that overall recognition was better for negative than neutral materials and this benefit was larger for males.
Table 1.
Group means (and standard deviations) from initial memory assessment.
| Initial Test | ||||
|---|---|---|---|---|
| Recall | Recognition d′ | |||
| Neutral | Negative | Neutral | Negative | |
| Stress | ||||
| Males (n = 8) | 4.71 (1.89) | 10.86 (3.02) | 2.25 (0.48) | 2.54 (0.50) |
| Females (n = 10) | 4.00 (1.83) | 7.70 (2.98) | 2.01 (0.36) | 2.13 (0.45) |
| Control | ||||
| Males (n = 9) | 3.67 (2.60) | 9.00 (4.06) | 1.88 (0.75) | 2.28 (0.62) |
| Females (n = 10) | 4.80 (1.81) | 8.40 (3.50) | 2.39 (0.64) | 2.33 (0.66) |
| Recognition ROC Estimates | ||||
|---|---|---|---|---|
| Recollection | Familiarity | |||
| Neutral | Negative | Neutral | Negative | |
| Stress | ||||
| Males (n = 8) | 0.40 (0.09) | 0.54 (0.13) | 1.86 (0.60) | 1.70 (0.50) |
| Females (n = 10) | 0.42 (0.13) | 0.54 (0.12) | 1.25 (0.43) | 1.27 (0.58) |
| Control | ||||
| Males (n = 9) | 0.45 (0.27) | 0.52 (0.24) | 1.05 (0.69) | 1.34 (0.50) |
| Females (n = 10) | 0.57 (0.17) | 0.51 (0.24) | 1.46 (0.58) | 1.66 (0.40) |
| Recognition R/K Estimates | ||||
|---|---|---|---|---|
| Recollection | Familiarity | |||
| Neutral | Negative | Neutral | Negative | |
| Stress | ||||
| Males (n = 8) | 0.14 (0.10) | 0.24 (0.19) | 0.60 (0.14) | 0.61 (0.15) |
| Females (n = 10) | 0.20 (0.17) | 0.32 (0.16) | 0.50 (0.13) | 0.49 (0.11) |
| Control | ||||
| Males (n = 9) | 0.18 (0.19) | 0.21 (0.18) | 0.43 (0.20) | 0.60 (0.17) |
| Females (n = 10) | 0.27 (0.25) | 0.34 (0.26) | 0.59 (0.17) | 0.55 (0.17) |
Correlational analyses revealed no significant association between overall recognition performance (i.e., d′) and levels of salivary cortisol (as measured by the difference between sample 2 and sample 1). Nor were significant associations revealed when the analyses were limited to the stress group or to subjects who exhibited a stress-induced cortisol response.
3.3. Recollection and Familiarity
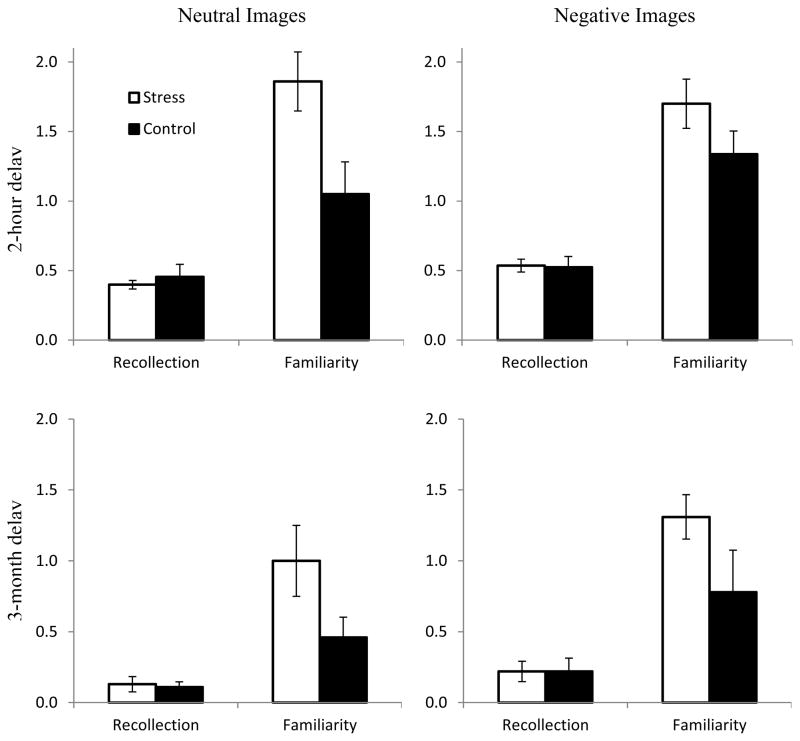
Subsequent analyses examined the effects of stress on estimates of familiarity and recollection. Estimates were derived on the basis of the ROC confidence analysis and remember/know reports, but because both led to similar conclusions we focus here on the ROC estimates (see Table 1 for estimates for both ROC and remember/know reports). An examination of familiarity estimates derived from the ROC analysis revealed a significant interaction between stress and sex (F(1, 33) = 5.07, MSe = 3.61, p < .05, ηp2 = .13), indicating that stress had different effects on familiarity in males and females. Follow-up analyses indicated that for the males there was a significant main effect of stress (F(1, 15) = 10.10, MSe = 2.91, p < .01, ηp2 = .40), and no stress by valence interaction (F < 1.0), indicating that stress increased males’ familiarity for neutral and negative items. In contrast, for females, there were no main effects or interactions, indicating that familiarity was not significantly affected by stress or valence.
In contrast, analysis of recollection estimates revealed a significant main effect of valence (F(1, 33) = 4.57, MSe = .08, p < .05, ηp2 = .12), such that recollection was greater for negative than for neutral pictures. In addition, for recollection, there were no significant effects of stress or sex, nor were there any significant interactions.
Thus, the ROC results indicated that for males stress led to an increase in familiarity based recognition for both negative and neutral materials, but did not impact recollection (see Figure 4). In contrast, for females, stress did not appear to impact familiarity or recollection. Finally, in contrast to the effects of stress on familiarity, negative materials led to higher estimates of recollection than did neutral materials, and this effect was not modulated by stress or sex.
Figure 4.
Mean estimates of recollection and familiarity for males. Error bars represent the SEs of the means.
Recall
Initial analysis of recall data (Table 1) revealed a main effect of valence (F(1, 33) = 58.07, MSe = 376.99, p < .001, ηp2 = .64) reflecting the fact that participants recalled more negative (M = 8.78, SD = 3.42) than neutral pictures (M = 4.32, SD = 2.03). However, there was no effect of stress, nor any higher-order interactions. Planned comparisons examining the effects of stress in each of the four conditions indicated there were no significant effects of stress (all t’s < 1.1). Thus, overall recall was sensitive to valence, but not to stress. Analysis of the number of details recalled revealed a main effect of valence (F(1, 33) = 10.84, MSe = 13.29, p < .005, ηp2 = .24), reflecting recall of more details for negative (M = 5.5, SD = 1.7) than neutral (M = 4.6, SD = 2.0) pictures. There were no effects of stress, nor any higher-order interactions on the number of details recalled. To the extent that recall relies on processes similar to recollection, the results converge with the recognition results which showed that recollection was greater for negative than neutral materials and that recollection was not increased by stress.
3.4. Delayed Recognition and Recall
Approximately three months after the initial test session participants were recruited to participate in a follow-up study (n = 21). The results of the recognition and recall tests were similar to what was seen in the initial test phase (see Tables 1 and 2, Figures 2 and 4). For overall recognition (d′) there was an interaction between stress and sex (F(1, 17) = 4.84, MSe = 1.70, p < .05, ηp2 = .22), reflecting that fact the stress led to an increase in recognition in the males, but not in the females. In addition, there was a main effect of valence (F(1, 17) = 18.34, MSe = 1.25, p < .001, ηp2 = .52) indicating that recognition was generally better for negative than neutral items.
Table 2.
Group means (and standard deviations) from delayed memory assessment.
| Delayed Test | ||||
|---|---|---|---|---|
| Recall | Recognition d′ | |||
| Neutral | Negative | Neutral | Negative | |
| Stress | ||||
| Males (n = 5) | 0.75(0.96) | 6.25 (3.76) | 1.32 (0.43) | 1.58 (0.40) |
| Females (n = 6) | 3.00(1.27) | 5.83 (2.04) | 1.01 (0.38) | 1.38 (0.46) |
| Control | ||||
| Males (n = 5) | 0.60(0.89) | 5.20 (3.90) | 0.61 (0.45) | 1.03 (0.65) |
| Females (n = 5) | 3.00(2.45) | 6.50 (2.38) | 1.20 (0.38) | 1.54 (0.46) |
| Recognition ROC Estimates | ||||
|---|---|---|---|---|
| Recollection | Familiarity | |||
| Neutral | Negative | Neutral | Negative | |
| Stress | ||||
| Males (n = 5) | 0.13(0.12) | 0.22 (0.16) | 1.00 (0.56) | 1.31 (0.35) |
| Females (n = 6) | 0.14(0.16) | 0.15 (0.17) | 0.75 (0.18) | 1.18 (0.52) |
| Control | ||||
| Males (n = 5) | 0.11(0.08) | 0.22 (0.21) | 0.46 (0.32) | 0.78 (0.66) |
| Females (n = 5) | 0.28(0.15) | 0.17 (0.19) | 0.58 (0.36) | 1.41 (0.42) |
| Recognition R/K Estimates | ||||
|---|---|---|---|---|
| Recollection | Familiarity | |||
| Neutral | Negative | Neutral | Negative | |
| Stress | ||||
| Males (n = 5) | 0.05(0.06) | 0.14 (0.09) | 0.43 (0.14) | 0.45 (0.09) |
| Females (n = 6) | 0.09(0.08) | 0.23 (0.18) | 0.30 (0.12) | 0.41 (0.15) |
| Control | ||||
| Males (n = 5) | 0.05(0.07) | 0.21 (0.21) | 0.16 (0.16) | 0.27 (0.19) |
| Females (n = 5) | 0.02(0.03) | 0.06 (0.06) | 0.38 (0.09) | 0.49 (0.13) |
Note that the mean study-test delay for the control group was 115 days (SD = 62, range = 34–170) which was slightly longer than the mean delay for the stress group which was 89 days (SD = 43, range = 27–165). This difference was not significant (t < 1). Nonetheless, to verify that the stress effects were not impacted by the difference in retention interval we repeated the analysis but removed the subject with the longest delay and the subject with the shortest, such that the average delay in the control and stress conditions were similar (M = 105 and M = 95 days, respectively), and the results were unchanged, indicating that the stress effects were not produced by differences in retention interval. There was an interaction between stress and sex that approached significance (F(1, 15) = 4.06, MSe = 1.61, p = .06, ηp2 = .21), and a main effect of valence (F(1, 15) = 22.80, MSe = 1.28, p < .001, ηp2 = .60).
An analysis of ROC familiarity estimates revealed a main effect of valence (F(1, 17) = 12.10, MSe = 2.28, p < .005, ηp2 = .42), indicating that familiarity was generally higher for negative than neutral materials. In addition, there was a marginally significant interaction of stress and sex (F(1, 17) = 4.16, MSe = 0.84, p = .057, ηp2 = .20) indicating that stress had different effects on males and females. Although the interaction was not quite significant, to characterize the observed effects further and to facilitate comparison to previous studies (e.g., Yonelinas et al., 2011), separate analyses were performed on males’ and females’ delayed recognition performance, despite the small sample size. The analysis indicated that stress led to an increase in familiarity estimates in the males (F(1, 8) = 5.76, MSe = 1.44, p < .05, ηp2 = .42), but not in the females (p > .1). In contrast to familiarity, there were no significant effects on recollection in males or females. Examination of delayed recall performance (Table 2) revealed a main effect of valence (F(1, 15) = 32.45, MSe = 155.81, p < .001, ηp2 = .684), indicating that participants recalled more negative (M = 5.89, SD = 2.85) than neutral pictures (M = 1.89, SD = 1.79). There were no main effects of stress or sex, nor any higher-order interactions on recall, replicating the results of the initial recall test.
Thus, the results of the delayed memory tests largely replicated the main results seen in the initial tests (see Figure 4). Namely, stress led to an increase in familiarity for neutral and negative items in males but did not impact recollection or recall. Thus, the protective effects of stress on familiarity appear stable across a delay of as long as three months.
4. Discussion
The current results show that post-encoding stress induced by the cold-pressor test enhances familiarity-based recognition memory in males, but does not impact recollection. The beneficial effects of stress were observed for negative as well as neutral materials, and were seen when memory was tested two hours after encoding, as well as when memory was tested as much as 3 months later. Stress was found to selectively increase familiarity-based recognition, as measured using the confidence ROC method and the remember/know method. In contrast, recollection-based recognition was not influenced by stress, nor was free recall performance. These results suggest that stress protects memory from the effects of forgetting by protecting the strength of the underlying memory representation, rather than impacting the search processes involved in free recall or recollection-based recognition.
The current study replicates and extends the results of the one other published study that has examined the effects of stress on recognition (Yonelinas et al., 2011), which found that post-encoding skydiving led to an increase in familiarity-based recognition. The current results indicate that the beneficial effects of stress are not limited to skydiving, but also arise when using a laboratory stressor (i.e., the cold-pressor test). In addition, the current study shows that the effects are observed not only over a short retention interval (of 2 hours) but are also maintained over 3 months. Finally, in Yonelinas et al. (2011), stress improved recognition for neutral but not negative materials, but it was not clear if the lack of an effect on negative materials was due to high levels of performance in that study. The current study showed that when performance was decreased using faster encoding rates, and by examining performance after a longer delay, stress had beneficial effects on both negative and neutral materials.
In agreement with several previous studies, the beneficial effects of stress on memory were limited primarily to males (e.g., Andreano & Cahill, 2006; Yonelinas et al., 2011). It is not clear why stress effects are more consistently observed in males than females, although it has been suggested that this may be related to variations in hormonal levels across the estrus cycle (see Andreano, Arjomandi, & Cahill, 2008). In line with this explanation, compared to males, there was much greater variability in females’ salivary cortisol levels after the stress manipulation, and there were many more female non-responders in the stress group (i.e., females who did not show an increase in salivary cortisol after stress). Thus, the null effects of stress on females’ memory in the current study may be due to a failure of the cold-pressor test to reliably induce a cortisol response in females.
In the current study, free recall performance was greater for negative than neutral materials, but recall performance was not influenced by stress. These findings are consistent with the earlier skydiving study (Yonelinas et al., 2011), but they contrast with several studies that have shown that post-encoding stress can improve free recall (e.g., Cahill et al., 2003; Smeets et al., 2008). The current results might be attributed to lack of statistical power in the recall test, but arguing against this is the fact that recall was significantly increased for negative compared to neutral materials in both the immediate and delayed tests. The different effects of stress on recall seen across different studies may be due to differences in the specific procedures used in those studies. For example, the current study and the Yonelinas et al. study used a long list of neutral and negative photos, whereas studies that have found effects of stress on recall have used short sets of encoding materials (e.g., Cahill et al., 2003) or encoding materials that formed a well-organized storyline (e.g., Beckner et al., 2006).
It is possible that with short study lists or semantically-organized materials, stress may facilitate search strategies that impact recall as well as influencing memory strength. For example, familiarity may have been useful in supporting recall performance in those earlier studies. That is, although familiarity is not expected to contribute to free recall performance under most conditions (Mandler, 1980; Yonelinas, 2002) there is evidence that for semantically-related word pairs, familiarity can support cued recall by leading related words to come to mind readily and to appear familiar (e.g., Gruneberg & Monks, 1974; McCabe, Roediger, & Karpicke, 2011). Thus, with semantically organized materials or with very short word lists, recall performance may be supported at least in part by familiarity, and so may be influenced by stress. Future studies examining this possibility will be important.
The mechanism responsible for producing stress-related increases in recognition is not fully understood, but the current results provide support for the idea that post-encoding stress helps to consolidate memory traces after they have been encoded (e.g., McGaugh, 2000; Joëls et al., 2006; Schwabe et al., 2012). The finding that only familiarity was influenced by stress is consistent with this hypothesis, in the sense that the results indicate that stress led to a strengthening of the memory traces rather than an increase in the recall or recollection of those memories. In addition, to the extent that consolidation reflects a process of strengthening the cortical representation of an event, the consolidation account would be consistent with prior results implicating the medial temporal lobe cortex as being important for familiarity rather than the hippocampus per se (for a review, see Eichenbaum, Yonelinas & Ranganath, 2007).
Recent models of stress-related enhancement of memory emphasize the importance of interactions between two stress response systems, the hypothalamic-pituitary-adrenal (HPA) axis and the adrenergic response of the sympathetic nervous system, which are thought to modulate functional interactions between the hippocampus and basolateral amygdala (Schwabe et al., 2012). In the current study we did not observe a significant correlation between memory and cortisol, which is impacted by activation of the HPA axis, but this could reflect the limited sample size or a nonlinear stress/memory relationship (see Andreano & Cahill, 2006). Future studies of memory examining cortisol as well as measures of adrenergic responses such as salivary alpha amylase or autonomic responses (e.g., blood pressure changes) will be useful.
In sum, the current results add to the growing body of evidence showing that post-encoding stress can have beneficial effects on memory. In addition, the results indicate that these effects arise because stress protects the familiarity strength of the underlying memories rather than simply increasing the likelihood that subjects are able to recall or recollect items or details. These effects appear to occur for stress produced by either skydiving or the cold-pressor test, they can be observed for negative as well as neutral materials, and they persist for periods up to three months.
Highlights.
We examined the effects of post-encoding stress on recognition memory
Memory for negative and neutral pictures was tested at two intervals
Cold-press stress improved familiarity-based recognition in males, but not recollection or free recall
Cold-press stress did not affect females’ recognition or recall performance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreano JM, Arjomandi H, Cahill L. Menstrual cycle modulation of the relationship between cortisol and long-term memory. Psychoneuroendocrinology. 2008;33:874–882. doi: 10.1016/j.psyneuen.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Glucocorticoid release and memory consolidation in men and women. Psychological Science. 2006;17(6):466–70. doi: 10.1111/j.1467-9280.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learning & Memory. 2009;16(4):248–66. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- Beckner VE, Tucker DM, Delville Y, Mohr DC. Stress facilitates consolidation of verbal memory for a film but does not affect retrieval. Behavioral Neuroscience. 2006;120(3):518–27. doi: 10.1037/0735-7044.120.3.518. [DOI] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learning & Memory. 2003;10(4):270–4. doi: 10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazakoff BN, Johnson KJ, Howland JG. Converging effects of acute stress on spatial and recognition memory in rodents: A review of recent behavioural and pharmacological findings. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34:733–741. doi: 10.1016/j.pnpbp.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman JS, Wright RL, Korol DL. Acute stress impairs spatial memory in male but not female rats: Influence of estrous cycle. Pharmacology Biochemistry and Behavior. 2004;78(3):569–579. doi: 10.1016/j.pbb.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30(1):123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruneberg MM, Monks J. “Feeling of knowing” and cued recall. Acta Psychologica. 1974;38(4):257–265. [Google Scholar]
- Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: How does it work? Trends in Cognitive Sciences. 2006;10(4):152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-8. 2008. International affective picture system (IAPS): Affective ratings of pictures and instructional manual. [Google Scholar]
- Lommer D, Distler A, Nast HP, Sinterhauf K, Walter U, Wolff HP, Sieler K. Tagesprofile von Plasmaaldosteron, -Cortisol, -Renin, -Angiotensinogen, und -Angiotensinasen bio Normalpersonen. Klinische Wochen-schrift. 1976;54:123–130. doi: 10.1007/BF01468789. [DOI] [PubMed] [Google Scholar]
- Lupien S, McEwen B. The acute effects of corticosteroids on cognition: Integration of animal and human model studies. Brain Research Reviews. 1997;24(1):1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection theory: A user’s guide. 2. Mahwah, NJ: Lawrence Earlbaum Associates; 2005. [Google Scholar]
- Mandler G. Recognizing: The judgment of previous occurrence. Psychological Review. 1980;87:252–271. [Google Scholar]
- McCabe DP, Roediger HL, Karpicke JD. Automatic processing influence free recall: Converging evidence from the process dissociation procedure and remember-know judgments. Memory & Cognition. 2011;39(3):389–402. doi: 10.3758/s13421-010-0040-5. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Sapolsky RM. Stress and cognitive function. Current Opinion in Neurobiology. 1995;5(2):205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory: A century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Current Opinion in Neurobiology. 2002;12(2):205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT, Oitzl MS. Memory formation under stress: Quality and quantity. Neuroscience and Biobehavioral Reviews. 2010;34(4):584–591. doi: 10.1016/j.neubiorev.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Joëls M, Roozendaal B, Wolf OT, Oitzl MS. Stress effects on memory: An update and integration. Neuroscience and Biobehavioral Reviews. 2012;36:1740–1749. doi: 10.1016/j.neubiorev.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Smeets T, Otgaar H, Candel I, Wolf OT. True or false? Memory is differentially affected by stress-induced cortisol elevations and sympathetic activity at consolidation and retrieval. Psychoneuroendocrinology. 2008;33(10):1378–1386. doi: 10.1016/j.psyneuen.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Receiver-operating characteristics in recognition memory: Evidence for a dual-process model. Journal of Experimental Psychology. Learning, Memory, and Cognition. 1994;20(6):1341–54. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Consciousness, control, and confidence: The 3 Cs of recognition memory. Journal of Experimental Psychology: General. 2001;130(3):361–379. doi: 10.1037//0096-3445.130.3.361. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Parks CM, Koen JD, Jorgenson J, Mendoza SP. The effects of post-encoding stress on recognition memory: Examining the impact of skydiving in young men and women. Stress: The International Journal on the Biology of Stress. 2011;14(2):136–144. doi: 10.3109/10253890.2010.520376. [DOI] [PMC free article] [PubMed] [Google Scholar]