Abstract
To explore the hypothesis that alterations in cellular membrane lipids are present at the stage of preclinical Alzheimer’s disease (AD) (i.e., cognitively normal at death, but with AD neuropathology), we performed targeted shotgun lipidomics of lipid extracts from postmortem brains of subjects with preclinical AD. We found sulfatide levels were significantly lower in subjects with preclinical AD compared to those without AD neuropathology. We also found that the level of ethanolamine glycerophospholipid was marginally lower at this stage of AD, whereas changes of the ceramide levels were undetectable with the available samples. These results indicate that cellular membrane defects are present at the earliest stages of AD pathogenesis and also suggest that sulfatide loss is among the earliest events of AD development, while alterations in the levels of ethanolamine glycerophospholipid and ceramide occur relatively later in disease.
Keywords: Ceramide, membrane lipids, plasmalogen, preclinical Alzheimer’s disease, shotgun lipidomics, sulfatide
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized clinically by progressive cognitive impairment (Waldemar et al. 2007) and neuropathologically by the appearance of extracellular amyloid-beta (Aβ), neuritic plaques, and intraneuronal neurofibrillary tangles (Montine et al. 2012). The failures of several trials of disease-modifying therapeutics, including several targeting the amyloid precursor protein (APP) and its cleavage product Aβ, have highlighted our incomplete knowledge of both cognitive impairment and the pathogenesis of AD (Mangialasche et al. 2010, Salomone et al. 2012). Although the causes of sporadic AD are not known, there are profound biochemical alterations in multiple pathways in the AD brain including membrane lipid dysregulation and defects in synaptic neurotransmission in addition to the changes in Aβ metabolism and tau phosphorylation. Recent pathological, biochemical, and genetic studies have led to major insights into AD pathogenesis (Jones et al. 2010). Consistent with those genetic studies, the occurrence of alterations in cellular membrane lipids has been found at the earliest clinically recognizable stage of AD (Han 2005).
Although general cellular membrane defects occur in advanced AD (Han 2005), our lipidomics analysis has demonstrated the presence of subtle changes of sulfatide and ceramide, as well as of deficiency in ethanolamine plasmalogen (a subclass of ethanolamine glycerophospholipid (PE)) at the earliest clinically recognizable stage (i.e., MCI) of AD (Han et al. 2001, Han et al. 2002). These changes are specific relative to other classes of lipids (Han 2005) whereas depletion of sulfatides in AD is also specific relative to other neurological complications (Cheng et al. 2003). The levels of these lipids are also changed in plasma or cerebrospinal fluid of patients (Han et al. 2003b, Goodenowe et al. 2007, Han et al. 2011).
Our mechanistic study has demonstrated that sulfatide metabolism is regulated through the metabolism and trafficking of apolipoprotein E (apoE) in an isoform-dependent manner (Han et al. 2003a), while apoE is the major genetic risk factor for AD (Holtzman et al. 2012). The depletion of sulfatides is tightly associated with Aβ pathology (Han 2010) and may be linked to the white matter abnormality in AD (Bozzali et al. 2002, Yoshiura et al. 2006, Medina et al. 2006, Xie et al. 2006, Zhang et al. 2009) since sulfatide is exclusively synthesized in oligodendrocytes, is largely present in myelin sheath, and plays an essential role in myelin function in the CNS (Marcus et al. 2006, Takahashi & Suzuki 2012).
Deficiency in plasmalogen PE may affect synaptic function and structure, leading to cholinergic system dysfunction in AD since synaptic vesicles are very enriched in plasmalogen PE containing polyunsaturated fatty acids (i.e., over 60 mol% of plasmalogen PE in total PE) as previously discussed (Han et al. 2001). A recent study has further showed that plasmalogen may directly involve the processing of amyloid precursor protein metabolism by directly affecting γ-secretase activity, thereby resulting in a vicious cycle: Aβ reduces plasmalogen levels and reduced plasmalogen levels directly increase γ-secretase activity leading to an even stronger production of Aβ peptides (Rothhaar et al. 2012).
Accumulating evidence indicates that AD neuropathologic changes are present in the brain several years before the onset of clinical symptoms (Price et al. 2009, Montine et al. 2012). In the presymptomatic stage of AD, biomarkers (CSF Aβ42 and pTau, and PET-amyloid imaging) may indicate the presence of AD neuropathologic change (Fagan et al. 2006). We hypothesized that alterations in sulfatide, plasmalogen PE, and ceramide, which are present at the earliest clinically recognizable stage of AD, could also exist at the stage of preclinical AD. In this study, we utilized multi-dimensional mass spectrometry-based shotgun lipidomics (MDMS-SL) (Yang et al. 2009, Han et al. 2012) to test the hypothesis and determine the mass levels of individual molecular species of these lipid classes with the available subjects. We found significant reduction of the mass levels of sulfatide species in subjects with preclinical AD, whereas plasmalogen PE and ceramide species were marginally changed or not changed at the stage. These results indicate that cellular membrane defects are present at the earliest stages of AD pathogenesis and that sulfatide depletion occurs prior to the other lipid abnormalities.
MATERIALS AND METHODS
Participants and clinical assessments
The participants came from a sample of community dwelling volunteers enrolled in a longitudinal study of healthy aging and AD conducted by the Washington University Alzheimer’s Disease Research Center (ADRC), St Louis, Missouri. Participants in this longitudinal study are 60 years or older and in good general health and have (other than AD) no neurologic, psychiatric, or systemic medical illness that could contribute substantially to dementia. All procedures were approved by the university’s Institutional Review Board (IRB), and written informed consent was obtained from the participants and their informants or collateral sources for participation in the longitudinal study and consent for autopsy. Briefly, at enrollment and annual follow-up, experienced clinicians determined the cognitive status of participants using a clinical battery that included the Clinical Dementia Rating (CDR) (Morris 1993).
Neuropathologic assessment
Brain tissue samples were obtained from the Knight ADRC Neuropathology Core, Washington University in St. Louis. At autopsy, the left hemibrain was preserved in 10% buffered formalin for neuropathologic assessment. The right cerebral hemisphere was sliced coronally at 1 cm intervals and snap frozen by contact with liquid nitrogen vapor-cooled Teflon-coated aluminum plates and stored in air-tight bags at −80°C until dissected. Formalin-fixed tissue samples from 15 standard cortical and subcortical regions were embedded in paraffin wax and sections cut at 7 μm as previously described (Cairns et al. 2010). Only brain tissue samples collected within the previous 5 years of the study were used. The cases were assessed using established rating scales and neuropathologic diagnostic criteria for AD (Braak & Braak 1991, Braak et al. 2006, Mirra et al. 1991, NIA-Reagan Institute 1997, Montine et al. 2012). The cases used in this study excluded any case with a clinical history of trauma or any neuropathologic evidence of chronic traumatic enecphalopathy or traumatic brain injury. Also, no case had evidence of neuroinflammation caused by any process other than the neurodegeneration of AD.
Preparation of lipid extracts from postmortem brain tissues
All brain samples (~ 1 g each) were dissected from superior frontal gyrus region and run blinded. A protein assay on the homogenate of each tissue (~ 25 mg) from individual dissected brain sample was performed with a bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA) using bovine serum albumin as standard. Lipids were extracted from individual homogenates after protein assay by a modified Bligh and Dyer technique (Cheng et al. 2010b) in the presence of internal standards. A portion of each individual lipid extract was treated with lithium methoxide and followed by being washed with hexane as previously described (Jiang et al. 2007). The treated lipid samples were used for the analysis of the sphingolipidome of each individual brain sample.
Control of neuronal cell population of samples
One of the concerns in lipidomic analysis of human brain samples is the consistency of the neuronal cell population of individual samples. Our previous studies demonstrated that such a consistency could be controlled through measurement of an ion intensity ratio of two PE species at m/z 726.5 (18:1-18:1 plasmalogen PE) and 790.4 (18:0-22:6 PE) in the negative-ion electrospray ionization mass spectra of brain PE species (Han et al. 2001). A ratio of not greater than 0.12 as a criterion in the current study on gray matter samples was used. In case of that a ratio of > 0.12 from any prepared sample was determined from the mass spectrum, a new piece of tissue (~ 20 mg) from the dissected superior frontal gyrus sample was taken and a lipid extraction was prepared again as described above. In addition, gray matter classification generally also meets the following criteria: (1) the mass content of the plasmalogen PE species at m/z 726 is lower than 3% of total PE content; and (2) the total plasmalogen PE mass content is not higher than 45% of the total PE content.
Quantitative analyses of lipids
A triple-quadrupole mass spectrometer (Thermo Scientific TSQ Vantage, San Jose, CA, USA) equipped with an automated nanospray apparatus (i.e., Nanomate HD, Advion Bioscience Ltd., Ithaca, NY, USA) and Xcalibur system software were utilized in the study as described (Yang et al. 2009). The individual molecular species corresponding to each of the ion peaks was identified using MDMS through building block analyses (Yang et al. 2009). Plasmalogen PE species were identified as previously described (Yang et al. 2007). The identified species were quantified using a two-step approach as previously described (Yang et al. 2009, Yang & Han 2011). Matrix-assisted laser desorption/ionization-time-of-flight (MALDI-TOF) MS analysis of sulfatide species was performed by using a Bruker Microflex LRF mass analyzer (Bruker Daltonics Inc., Billerica, MA, USA) with 9-AA as matrix as described previously (Cheng et al. 2010a).
Statistical analysis
Quantitative data were normalized to protein content to minimize the effects of neuron and synapse loss upon quantitative analyses of phospholipids and were presented as the means ± SD. Differences between mean values were determined by a Mann Whitney test, where *p < 0.05 and **p < 0.01 compared to controls.
RESULTS
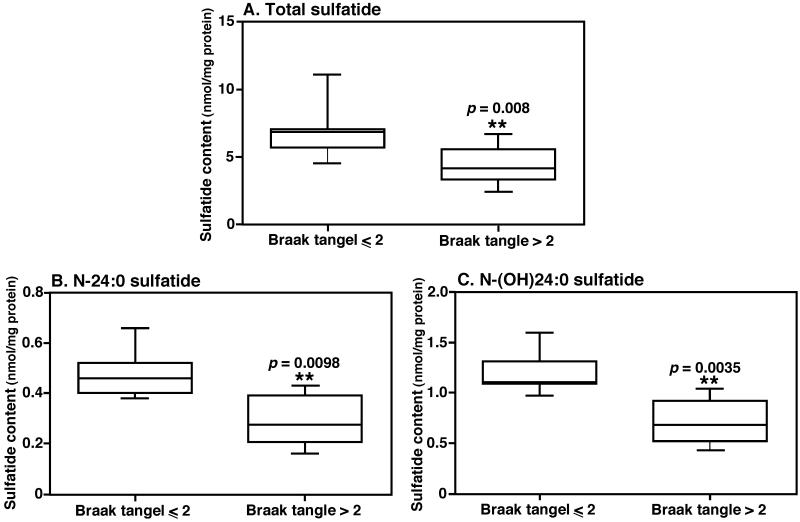
MALDI-TOF/MS analyses of lipid extracts from those with intermediate or high likelihood AD changes (Braak tangle stage > II) showed significant reduction of sulfatide levels in comparison to those who were with no or low likelihood AD changes (Braak tangle stage ≤ II) (Fig. 1). These results were further confirmed by MDMS-SL (Jiang et al. 2007). Specifically, the total sulfatide levels decreased from 6.79 ± 1.68 nmol/mg protein in controls to 4.39 ± 1.26 nmol/mg protein in those with preclinical AD (a reduction of 35%, p < 0.01) (Fig. 1a); the levels of the predominant sulfatide species (i.e., N-(OH)24:0 sulfatide, the ion at m/z 906.7) reduced from 1.19 ± 0.19 nmol/mg protein in controls to 0.71 ± 0.21 nmol/mg protein with preclinical AD (a reduction of 40%, p < 0.005) (Fig. 1c). The levels of other sulfatide species were also lower to a certain degree in samples of preclinical AD subjects (Fig. 1). To further determine the relationship of the sulfatide levels with Braak tangle stages, a linear regression analysis was performed. We found that sulfatide mass levels were correlated very well with Braak tangle stages with a correlation coefficient of −0.8286.
Figure 1.
Significant depletion of sulfatide in preclinical AD. The boxplots illustrate the levels of total sulfatide (A), N-24:0 sulfatide (B), and N-(OH)24:0 sulfatide (C), which are significantly lower in preclinical AD (Braak tangle > II, n = 6) compared to the controls (Braak tangle > II, n = 8). The levels of individual sulfatide species in lipid extract of brain samples were determined by MALDI-TOF/MS and presented after normalization to the protein content of each tissue sample.
MS analysis of sulfatide profiles present in superior frontal gyrus revealed two obvious differences in comparison to those of middle frontal gyrus, which are previously analyzed (Han et al. 2002). First, the sulfatide species containing hydroxy fatty amide chain (e.g., the ion clusters at m/z 906.67 and 932.68) were more abundant in the superior frontal gyrus samples compared to those from the middle frontal gyrus as reported previously (Han et al. 2002). Second, the levels of sulfatides in the superior frontal lobe region (Fig. 1a) were much lower than those in the middle frontal region (Han et al. 2002) (i.e., 6.79 ± 1.68 vs. 39.2 ± 10.4 nmol/mg protein), which was consistent with the greater abundance of axons in the latter.
PE and ceramide were not significantly changed with preclinical AD
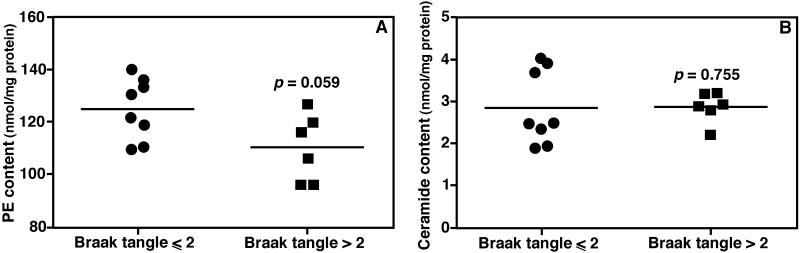
In contrast to the reduction in the levels of sulfatide species in subjects with preclinical AD, we only detected the levels of three PE species reduced significantly by MDMS-SL, including 18:1-18:1 plasmalogen PE (p = 0.041), and 18:0-22:5 and 18:0-22:6 diacyl PE (p = 0.018 and 0.020, respectively). Nevertheless, there was a tendency towards reduced levels of the entire PE class in subjects with preclinical AD (p = 0.059) (Fig. 2a), largely contributed from the plasmalogen PE species.
Figure 2.
Comparison of total ethanolamine glycerophospholipid and ceramide content in brain samples of subjects between controls and those at preclinical AD. The total levels of PE (Panel A) or ceramide (Panel B) in brain samples of subjects of controls or at preclinical AD were determined by MDMS-SL. The levels were normalized to the protein content of each tissue sample and are individually presented. Each bar represents the mean value of the levels of the group.
Our previous study demonstrated that the ceramide mass levels of subjects at the earliest clinically recognizable stages of AD (MCI) were at the highest in comparison to other stages (Han et al. 2002). We predicted that the mass levels of ceramides could even be higher at the preclinical AD stage than the MCI stage of AD. In contrast, MDMS-SL analysis of ceramide species did not detect the level of any ceramide species in the brain region from the subjects with preclinical AD as higher than that of controls (p = 0.755) (Fig. 2b).
To further confirm the specificity of sulfatide deficiency relative to other lipid classes as previously observed (Han et al. 2002, Han 2005), we also determined the levels of other lipid classes including cholesterol, sphingomyelin, cerebroside, and phosphatidylcholine. We did not find any significant differences of the mass levels of these lipid classes between the groups.
DISCUSSION
To explore the hypothesis that alterations in lipid content in neural cellular membranes and signaling might occur as one of the earliest events in AD pathogenesis, we performed a targeted determination of the mass levels of sulfatide, plasmalogen PE, and ceramide species in postmortem gray matter of superior frontal gyrus from subjects with preclinical AD since we have previously revealed that the mass levels of these lipid classes were substantially and specifically altered at the earliest clinically-recognizable stages of AD (Han et al. 2001, Han et al. 2002). We found that sulfatide mass levels were significantly lower in subjects with preclinical AD compared to controls. We also found that the mass levels of PE tended to be lower at this stage of AD while changes of the ceramide levels were undetectable with this size of samples. This study is significant in two respects: (1) significant brain cellular membrane changes occur in cognitively normal individuals with AD neuropathology, and (2) sulfatide depletion occurs earlier than the changes in plasmalogen PE and ceramide.
Our previous mechanistic studies have not found any significant dysregulation of sulfatide biosynthesis and degradation in AD subjects, but have identified the tight association of sulfatide metabolism with apoE metabolism depending on the expression levels, turnover rates, and isoforms of apoE (Han et al. 2002, Han et al. 2003a, Han 2007, Cheng et al. 2010b, Kiebish et al. 2012). Thus, we performed some targeted studies on apoE metabolism in the current study. We attempted to determine the relationship of the apoE isoforms with sulfatide changes and found the correlation was insignificant, possibly due to the small number of samples. We also determined the levels of apoE in the brain samples by Western blot analysis but could not detect significant difference between the groups. Unfortunately, it is not feasible to perform apoE turnover studies with postmortem brain samples. Collectively, the efforts to identify mechanisms leading to sulfatide loss at the preclinical AD stage were inconclusive. However, enhanced apoE metabolism and Aβ clearance during the development of AD neuropathology is a likely early mechanism we previously proposed (Han 2010).
In summary, we performed a targeted lipidomics analysis of clinically and neuropathologically well-characterized brain tissue from cognitively normal participants with or without AD neuropathology and found that sulfatide levels were significantly lower in those with AD neuropathology. The results provide strong evidence that membrane defects are an early event in AD pathogenesis and that sulfatide may play an important role. Theses data indicate that sulfatide may be a potential biomarker of disease and a potential target for therapeutic intervention.
Table 1.
Clinical and demographic characteristics of subjects
| Characteristics | Control | Preclinical AD |
|---|---|---|
|
| ||
| Clinical dementia rating | 0 | 0 |
| Cases (Sex) | 8 (2 M, 6 F) | 6 (2 M, 4 F) |
| Age at death (mean ± SD (range) (years)) | 90.5 ± 3.9 (85 - 96) | 89.7 ± 8.2 (78 - 104) |
| Post-mortem interval (mean ± SD (range) (hours)) | 11.6 ± 5.1 (6 - 20) | 11.2 ± 2.7 (7 - 15) |
| AD neuropathologic changes* | No AD (5), low likelihood AD (3) |
Intermediate (5), high likelihood AD (1) |
| Neurofibrillary tangle stage** | 2 (0; 1 M, 1 F), 2 (I; 1 M, 1 F), 4 (II; 4 F) |
3 (III; 1 M, 2 F), 1 (IV; 1 F), 2 (V; 1 M, 1 F) |
| Ab plaque stage** | 5 (0; 2 M, 3 F), 2 (A; 2 F), 1 (C; 1 F) |
3 (B; 3 F), 3 (C; 2 M, 1 F) |
Note: Criteria for the neuropathologic diagnosis of AD (low, intermediate, and high) (NIA-Reagan Institute 1997)
Neuropathologic staging of AD lesions: Braak neurofibrillary tangle stages: 0, I to VI and Aβ plaque stages: 0, A to C (Braak et al. 2006).
Acknowledgements
We thank the participants and their families for making brain donations to facilitate AD research. The authors are grateful for the technical assistance of the staff of the Knight ADRC Neuropathology Core, Washington University School of Medicine. This work was supported by grants from the National Institutes of Health National Institute on Aging (R01 AG31675 to XH, and P01-AG03991 (Neuropathology Core) and P50-AG05681 (Neuropathology Core) to NJC). There are no actual or potential conflicts of interest with other people or organizations within 3 years of beginning the work submitted that could inappropriately influence their work.
REFERENCES
- Bozzali M, Falini A, Franceschi M, Cercignani M, Zuffi M, Scotti G, Comi G, Filippi M. White matter damage in Alzheimer’s disease assessed in vivo using diffusion tensor magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2002;72:742–746. doi: 10.1136/jnnp.72.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Taylor-Reinwald L, Morris JC. Autopsy consent, brain collection, and standardized neuropathologic assessment of ADNI participants: the essential role of the neuropathology core. Alzheimers Dement. 2010;6:274–279. doi: 10.1016/j.jalz.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Sun G, Yang K, Gross RW, Han X. Selective desorption/ionization of sulfatides by MALDI-MS facilitated using 9-aminoacridine as matrix. J. Lipid Res. 2010a;51:1599–1609. doi: 10.1194/jlr.D004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Xu J, McKeel DW, Jr., Han X. Specificity and potential mechanism of sulfatide deficiency in Alzheimer’s disease: An electrospray ionization mass spectrometric study. Cell. Mol. Biol. 2003;49:809–818. [PubMed] [Google Scholar]
- Cheng H, Zhou Y, Holtzman DM, Han X. Apolipoprotein E mediates sulfatide depletion in amyloid precursor protein transgenic animal models of Alzheimer’s disease. Neurobiol. Aging. 2010b;31:1188–1196. doi: 10.1016/j.neurobiolaging.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, LaRossa GN, Spinner ML, Klunk WE, Mathis CA, DeKosky ST, Morris JC, Holtzman DM. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann. Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- Goodenowe DB, Cook LL, Liu J, Lu Y, Jayasinghe DA, Ahiahonu PW, Heath D, Yamazaki Y, Flax J, Krenitsky KF, Sparks DL, Lerner A, Friedland RP, Kudo T, Kamino K, Morihara T, Takeda M, Wood PL. Peripheral ethanolamine plasmalogen deficiency: a logical causative factor in Alzheimer’s disease and dementia. J. Lipid Res. 2007;48:2485–2498. doi: 10.1194/jlr.P700023-JLR200. [DOI] [PubMed] [Google Scholar]
- Han X. Lipid alterations in the earliest clinically recognizable stage of Alzheimer’s disease: Implication of the role of lipids in the pathogenesis of Alzheimer’s disease. Curr. Alz. Res. 2005;2:65–77. doi: 10.2174/1567205052772786. [DOI] [PubMed] [Google Scholar]
- Han X. Potential mechanisms contributing to sulfatide depletion at the earliest clinically recognizable stages of Alzheimer’s disease: a tale of shotgun lipidomics. J. Neurochem. 2007;103(s1):171–179. doi: 10.1111/j.1471-4159.2007.04708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X. The pathogenic implication of abnormal interaction between apolipoprotein E isoforms, amyloid-beta peptides, and sulfatides in Alzheimer’s disease. Mol. Neurobiol. 2010;41:97–106. doi: 10.1007/s12035-009-8092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Cheng H, Fryer JD, Fagan AM, Holtzman DM. Novel role for apolipoprotein E in the central nervous system: Modulation of sulfatide content. J. Biol. Chem. 2003a;278:8043–8051. doi: 10.1074/jbc.M212340200. [DOI] [PubMed] [Google Scholar]
- Han X, Fagan AM, Cheng H, Morris JC, Xiong C, Holtzman DM. Cerebrospinal fluid sulfatide is decreased in subjects with incipient dementia. Ann. Neurol. 2003b;54:115–119. doi: 10.1002/ana.10618. [DOI] [PubMed] [Google Scholar]
- Han X, Holtzman DM, McKeel DW., Jr. Plasmalogen deficiency in early Alzheimer’s disease subjects and in animal models: molecular characterization using electrospray ionization mass spectrometry. J. Neurochem. 2001;77:1168–1180. doi: 10.1046/j.1471-4159.2001.00332.x. [DOI] [PubMed] [Google Scholar]
- Han X, Holtzman DM, McKeel DW, Jr., Kelley J, Morris JC. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer’s disease: potential role in disease pathogenesis. J. Neurochem. 2002;82:809–818. doi: 10.1046/j.1471-4159.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- Han X, Rozen S, Boyle S, Hellegers C, Cheng H, Burke JR, Welsh-Bohmer KA, Doraiswamy PM, Kaddurah-Daouk R. Metabolomics in early Alzheimer’s disease: Identification of altered plasma sphingolipidome using shotgun lipidomics. PLoS ONE. 2011;6:e21643. doi: 10.1371/journal.pone.0021643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Yang K, Gross RW. Multi-dimensional mass spectrometry-based shotgun lipidomics and novel strategies for lipidomic analyses. Mass Spectrom. Rev. 2012;31:134–178. doi: 10.1002/mas.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Herz J, Bu G. Apolipoprotein e and apolipoprotein e receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006312. doi: 10.1101/cshperspect.a006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Cheng H, Yang K, Gross RW, Han X. Alkaline methanolysis of lipid extracts extends shotgun lipidomics analyses to the low abundance regime of cellular sphingolipids. Anal. Biochem. 2007;371:135–145. doi: 10.1016/j.ab.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Harold D, Williams J. Genetic evidence for the involvement of lipid metabolism in Alzheimer’s disease. Biochim. Biophys. Acta. 2010;1801:754–761. doi: 10.1016/j.bbalip.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Kiebish MA, Young DM, Lehman JJ, Han X. Chronic caloric restriction attenuates a loss of sulfatide content in the PGC-1α−/− mouse cortex: A potential lipidomic role of PGC-1α in neurodegeneration. J. Lipid Res. 2012;53:273–281. doi: 10.1194/jlr.M020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M. Alzheimer’s disease: clinical trials and drug development. Lancet Neurol. 2010;9:702–716. doi: 10.1016/S1474-4422(10)70119-8. [DOI] [PubMed] [Google Scholar]
- Marcus J, Honigbaum S, Shroff S, Honke K, Rosenbluth J, Dupree JL. Sulfatide is essential for the maintenance of CNS myelin and axon structure. Glia. 2006;53:372–381. doi: 10.1002/glia.20292. [DOI] [PubMed] [Google Scholar]
- Medina D, DeToledo-Morrell L, Urresta F, Gabrieli JD, Moseley M, Fleischman D, Bennett DA, Leurgans S, Turner DA, Stebbins GT. White matter changes in mild cognitive impairment and AD: A diffusion tensor imaging study. Neurobiol. Aging. 2006;27:663–672. doi: 10.1016/j.neurobiolaging.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, Vinters HV, Hyman BT. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- NIA-Reagan Institute Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. Neurobiol. Aging. 1997;18(suppl):S1–S2. [PubMed] [Google Scholar]
- Price JL, McKeel DW, Jr., Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, Smith CD, Davis DG, Schmitt FA, Markesbery WR, Kaye J, Kurlan R, Hulette C, Kurland BF, Higdon R, Kukull W, Morris JC. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol. Aging. 2009;30:1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhaar TL, Grosgen S, Haupenthal VJ, Burg VK, Hundsdorfer B, Mett J, Riemenschneider M, Grimm HS, Hartmann T, Grimm MO. Plasmalogens inhibit APP processing by directly affecting gamma-secretase activity in Alzheimer’s disease. ScientificWorldJournal. 2012;2012:141240. doi: 10.1100/2012/141240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomone S, Caraci F, Leggio GM, Fedotova J, Drago F. New pharmacological strategies for treatment of Alzheimer’s disease: focus on disease modifying drugs. Br J Clin Pharmacol. 2012;73:504–517. doi: 10.1111/j.1365-2125.2011.04134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Suzuki T. Role of sulfatide in normal and pathological cells and tissues. J. Lipid Res. 2012;53:1437–1450. doi: 10.1194/jlr.R026682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldemar G, Dubois B, Emre M, Georges J, McKeith IG, Rossor M, Scheltens P, Tariska P, Winblad B. Recommendations for the diagnosis and management of Alzheimer’s disease and other disorders associated with dementia: EFNS guideline. Eur J Neurol. 2007;14:e1–26. doi: 10.1111/j.1468-1331.2006.01605.x. [DOI] [PubMed] [Google Scholar]
- Xie S, Xiao JX, Gong GL, Zang YF, Wang YH, Wu HK, Jiang XX. Voxel-based detection of white matter abnormalities in mild Alzheimer disease. Neurology. 2006;66:1845–1849. doi: 10.1212/01.wnl.0000219625.77625.aa. [DOI] [PubMed] [Google Scholar]
- Yang K, Cheng H, Gross RW, Han X. Automated lipid identification and quantification by multi-dimensional mass spectrometry-based shotgun lipidomics. Anal. Chem. 2009;81:4356–4368. doi: 10.1021/ac900241u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Han X. Accurate Quantification of Lipid Species by Electrospray Ionization Mass Spectrometry — Meets a Key Challenge in Lipidomics. Metabolites. 2011;1:21–40. doi: 10.3390/metabo1010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Zhao Z, Gross RW, Han X. Shotgun lipidomics identifies a paired rule for the presence of isomeric ether phospholipid molecular species. PLoS ONE. 2007;2:e1368. doi: 10.1371/journal.pone.0001368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiura T, Mihara F, Koga H, Ohyagi Y, Noguchi T, Togao O, Ogomori K, Miyoshi K, Yamasaki T, Kaneko K, Ichimiya A, Kanba S, Honda H. Mapping of subcortical white matter abnormality in Alzheimer’s disease using diffusion-weighted magnetic resonance imaging. Acad Radiol. 2006;13:1460–1464. doi: 10.1016/j.acra.2006.09.042. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schuff N, Du AT, Rosen HJ, Kramer JH, Gorno-Tempini ML, Miller BL, Weiner MW. White matter damage in frontotemporal dementia and Alzheimer’s disease measured by diffusion MRI. Brain. 2009;132:2579–2592. doi: 10.1093/brain/awp071. [DOI] [PMC free article] [PubMed] [Google Scholar]