Abstract
Tear proteins are potential biomarkers, drug targets, and even biotherapeutics. As a biotherapeutic, a recombinant tear protein might physiologically rescue the ocular surface when a deficiency is detected. Such a strategy pays more attention to the natural prosecretory and protective properties of the tear film and seeks to alleviate symptoms by addressing cause, rather than the current palliative, non-specific and temporary approaches. Only a handful of tear proteins appear to be selectively downregulated in dry eye, the most common eye disease. Lacritin and lipocalin-1 are two tear proteins selectively deficient in dry eye. Both proteins influence ocular surface health. Lacritin is a prosecretory mitogen that promotes basal tearing when applied topically. Levels of active monomeric lacritin are negatively regulated by tear tissue transglutaminase, whose expression is elevated in dry eye with ocular surface inflammation. Lipocalin-1 is the master lipid sponge of the ocular surface, without which residual lipids could interfere with epithelial wetting. It also is a carrier for vitamins and steroid hormones, and is a key endonuclease. Accumulation of DNA in tears is thought to be proinflammatory. Functions of these and other tear proteins may be influenced by protein-protein interactions. Here we discuss new advances in lacritin biology and provide an overview on lipocalin-1, and newly identified members of the tear proteome.
Keywords: lacritin, tear lipocalin, dry eye, tear proteome, cornea, lacrimal gland
1. Introduction
Tears accumulate on the avascular corneal epithelium, and vascularized conjunctiva, as a translucent film rich in proteins, lipids and metabolites. The tear proteome is estimated to comprise 1,543 proteins (Zhou et al., 2012), with over half designated as ‘intracellular’ (Table 1) by Gene Ontology, implying that cell death from normal epithelial renewal may be a contributor. Beyond its capacity to lubricate the lid, tears are essential for the refraction of light (Montés-Micó et al., 2007). Equally important and irreplaceable by drugs or drops is the role of tears in promoting corneal epithelial health for when tears are chronically insufficient the epithelium becomes stressed and releases inflammatory cytokines that further exacerbate the situation (Massingale et al., 2009). Dry eye affects 5 – 6% of the general population, rising to 6 – 9.8% and as high as 34%, respectively in postmenopausal women (Schaumberg et al., 2003) and the elderly (Lin et al., 2005).
TABLE 1.
Proteins in the normal human tear proteome that are predicted to be extracellular according to Gene Ontology (GO), with single and double underline indicating a respective decrease or increase in Dry Eye.
| Gene Symbol | Protein | Function (as per Locust Link, OMIM or Source) |
|---|---|---|
| (i) Angiogenesis | ||
| ANG | angiogenin, ribonuclease, RNase A family, 55 | promotes angiogenesis |
| AAMP | angio-associated migratory cell protein4,a | promotes angiogenesis |
| BAI3 | brain-specific angiogenesis inhibitor 33 | possible angiogenesis inhibitor |
| ECGF1 | endothelial cell growth factor 12 | promotes angiogenesis |
| EFEMP1 | fibulin 3 isoform 14,a | inhibits angiogenesis |
| SERPINF1 | serp. pep. inhib., cl. F (α-2 antiplas., PEDF), mem. 12 | promotes neurodifferent. and inhibits angiogenesis |
| (ii) Biosynthesis | ||
| ATP5A1 | ATP synth, H+ transp, mitoch F1 complex, α subunit 14,a | catalyzes ATP synthesis in mitochondrion |
| ATP5B | ATP synth, H+ transp., mitoch. F1 complex, βpolypep.2 | catalyzes ATP synthesis in mitochondrion |
| B4GALT1 | UDP-Gal:betaGlcNAc beta 1,4- galactosyltransferase2 | polypep, glycoconjugate and lactose biosynthesis |
| PDIA6 | protein disulfide isomerase family A, member 62 | predicted electron transport and protein folding roles |
| (III) Calcium | ||
| AHSG | alpha-2-HS-glycoprotein2 | calcification inhibitor |
| ANXA2 | annexin A2 Ca2+ depend. phospholip. binding prot.2 | osteoclast formation and bone resorption |
| ANXA5 | annexin A5 Ca2+ depend. phospholip. binding prot.2,7 | promotes Ca2+ channel activity |
| CALR | calreticulin2 | Ca2+ binding protein in ER and nucleus; SS assoc. |
| CALU | calumenin2 | Ca2+ binding protein in ER, protein folding/sorting |
| CALML5 | calmodulin like skin protein4,a | Ca2+ binding protein and keratinocyte differentiation |
| CANT1 | CANT12 | Ca2+ activated nucleotidase |
| GAS6 | growth arrest specific 64,a | Ca2+ channel regulator and cell communication |
| MCFD2 | multiple coagulation factor deficiency protein 24,a | Ca2+ binding protein and protein transport |
| NUCB1 | nucleobindin 12 | golgi and peripheral membrane Ca2+ binding protein |
| NUCB2 | nucleobindin 22 | peripheral membrane Ca2+ binding protein |
| PPIB | peptidylprolyl isomerase B (cyclophilin B)4,a | Ca2+ flux, ERK phosphorylation and chemotaxis |
| PPIC | peptidylprolyl isomerase C (cyclophilin C)2 | protein folding, binds cyclosporin A |
| (IV) Carbohydrate | ||
| AGL | amylo-1, 6-glucosidase, 4-alphaglucanotransferase2 | glycogen degradation |
| AMY1C | amylase alpha 1C4,a | starch degradation |
| B3GAT3 | beta-1,3-glucuronyltransferase 34,a | proteoglycan biosynthesis |
| CES1 | carboxylesterase 14,a | xenobiotic metabolism |
| CHI3L2 | chitinase 3-like 22 | glycan but not heparin binding |
| ENO1 | enolase 1, (alpha)2 | glycolytic enzyme |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase2,7 | carbohydrate metabolism |
| LGALS3 | lectin, galactoside-binding, soluble, 3, galectin2,7 | galactose-specific lectin |
| LGALS3BP | lectin, galactoside-binding, soluble, 3 binding protein2 | binds Mac-2 and galectin 1 |
| MANBA | mannosidase, beta A2 | lysosomal N-linked oligosaccharide catabolism |
| MDH2 | malate dehydrogenase mitochondrial4,a | NADPH oxidation |
| PKM2 | pyruvate kinase, muscle2 | carbohydrate degradat., binds bacterial Opa protein |
| (V) Carrier/Binding Protein/Seroid Assoc. | ||
| ALB | albumin*1,2,3 | carrier protein |
| AFM | afamin4,a | vitamin E binding protein |
| APCS | serum amyloid P-component4,a | protein complex assembly |
| ARTS-1 | type 1 TNFR shedding aminopeptidase regulator2 | binds TNFR1 to promote shedding |
| AZGP1 | alpha-2-glycoprotein 1#1,2,7 | zinc-binding, lipid degrad., cell adhesion |
| CD14 | CD14 molecule2 | binds LPS binding protein (LBP) and apoptotic cells |
| DMBT1 | deleted in malignant brain tumors 11,2,3,7 | scavenger receptor, binds surfactant protein D |
| DSP | desmoplakin2 | key component of desmosomes |
| GC | group-specific component (vitamin D binding protein)2 | carrier protein for vitamin D and metabolites |
| HBB | hemoglobin beta chain4,a | oxygen transport |
| HP | haptoglobin#1,2 | hemoglobin binding, turnover to diminish iron loss |
| HPX | hemopexin1,2 | heme binding, turnover |
| HSPG2 | heparan sulfate proteoglycan 2 (perlecan)2,7 | growth factor binding, filtration, matrix polymerization |
| IGFBP1 | insulin-like growth factor binding protein 15 | slows turnover of IGF s |
| IGFBP2 | insulin-like growth factor binding protein 25 | slows turnover of IGF s |
| KPNB1 | karyopherin (importin) beta 12 | nuclear transport |
| LCN1 | lipocalin 1 (tear prealbumin) Δ#$¥§1,2,13 | hydrophobic prot. binding, cyst. protease. inhibitor |
| M6PRBP1 | mannose-6-phosphate receptor binding protein 12 | endosome-to-golgi transport |
| PEBP4 | phosphatidylethanolamine-binding protein 42 | ? |
| PLIN3 | perilipin34,a | endosome-to-golgi transport |
| SCGB1D1 | secretoglobin, family 1D, member 1†#1,2,3,7 | in complex that binds steroids, including androgen |
| SCGB2A1 | secretoglobin, family 2A, member 1#1,2,3,7 | possibly binds steroids, including androgen |
| SCGB2A2 | secretoglobin, family 2A, member 2 (mammaglobin 2)# 7 | ? |
| TCN1 | transcobalamin I (vitamin B12 bind., R bind. family)#2,7 | binds and helps move vitamin B12 into cells |
| TF | transferrin1,2,3 | iron binding and transport to proliferating cells |
| TTR | transthyretin (prealbumin, amyloidosis type I)1,2 | thyroxine binding and transport |
| (vi) Cell Adhesion/Motility/Structure | ||
| ACTB | actin, beta1 | cell structure, motility |
| ACTA1 | actin, alpha 1, skeletal muscle7 | formation of filaments |
| CDH1 | E-cadherin4,a | cell- cell adhesion, migration and cell communication |
| CFL1 | cofilin 14,a | cytoskeleton organization |
| CSTA | stefin a4,a | cell - cell adhesion, keratin organization |
| DAG1 | dystroglycan4,a | cell- matrix adhesion and cell communication |
| FGA | fibrinogen alpha chain1,2 | cell adhesion, spreading, mitogenic, chemotactic |
| FGB | fibrinogen beta chain4,a | cell adhesion, spreading, mitogenic, chemotactic |
| FGG | fibrinogen gamma chain2 | cell adhesion, spreading, mitogenic, chemotactic |
| FLRT3 | fibronectin leucine rich transmembrane protein 33 | possibly cell adhesion, receptor signaling |
| FN1 | fibronectin 12,7 | cell adhesion, migration, blood coagulation |
| GSN | gelsolin (amyloidosis, Finnish type)2 | blocks actin monom. exchange or promotes nucleat. |
| ICAM1 | intercellular adhesion molecule 14,a | cell - cell adhe., positive regulator of vasoconstric. |
| IGFALS | insulin like growth fac. bind. protein, acid labile subunit4,a | cell adhesion and cell communication |
| JUP | catenin, gamma4,a | cell - cell adhesion, cell migration and proliferation |
| KRT17 | Keratin, type I cytoskeletal 174,a | + ve regul. of hair follicle dev. and inter. filament org. |
| KRT18 | keratin184,a | cytoskeletal intermediate filaments |
| LAMA3 | laminin, alpha 313 | cell adhesion and differentiation |
| LGALS9 | galectin 94,a | cell - cell adhesion and cell communication |
| LUM | lumican4,a | fibril organization and spacing |
| MFGE8 | milk fat globule-EGF factor 8 protein2 | cell adhesion, rotavirus binding/inhibition |
| MIF | macrophage migration inhibitory factor4,a | chemo attractant and cell communication |
| MMP10 | matrix metalloproteinase 104,a | cell migration |
| MSLN | mesothelin2,3 | possible cell adhesion activity |
| PFN1 | profilin 12 | regulator of actin polymerization and cytoskeleton |
| SERPINF2 | alpha 2 antiplasmin4,a | collagen fibril organization |
| SLIT3 | slit homolog 3 (Drosophila)2 | cell migration |
| THBS1 | thrombospondin 12 | cell-cell and cell-matrix adhesion |
| TLN1 | talin 12 | actin filament assembly and cell spreading |
| TNFAIP2 | tumor necrosis factor alpha induced protein 24,a | promotes cell migration |
| VIM | vimentin2 | cytoskeletal intermediate filaments |
| (vii) Cell Growth | ||
| ANGPTL1 | angiopoietin-like 12,7 | may inhibit cell growth |
| DKK4 | dickkopf homolog 44,a | negative regulator of WNT pathway |
| EHD4 | EH domain containing protein 44,a | protein trafficking |
| EGF | epidermal growth factor (beta-urogastrone)5 | prosecretory mitogen |
| FAM3C | predicted osteoblast protein4,a | cell communication and development |
| GDNF | glial cell derived neurotrophic factor5 | dopaminergic neuron survival, differentiation |
| GRN | granulin4,a | growth factor and cell signaling |
| HDGF | hepatoma derived growth factor4,a | mitogenic and cell communication |
| HGF | hepatocyte growth factor (hepapoietin A; scatter factor)5 | serine protease-activated mitogen |
| KRT8 | keratin 84,a | cell growth and maintenance |
| LACRT | lacritin *Δ†#£¥1,2,13,5,7 | prosecretory mitogen |
| MUC4 | mucin 42 | epithelial cell proliferation and differentiation |
| NTF3 | neurotrophin 35 | sensory neuron survival |
| NTF5 | neurotrophin 55 | peripheral sensory sympathetic neuron survival |
| PDAP1 | PDGF alpha associated protein 14,a | cell communication |
| PDGFC | platelet derived growth factor C4,a | cell proliferation and wound healing |
| PHGDH | phosphoglycerate dehydrogenase4,a | cell proliferation, metabolism, energy pathways |
| PTPRF | protein tyrosine phosphatase, receptor type, F4,a | pro apoptotic |
| QSOX1 | (QSCN6) quiescin Q6 sulfhydryl oxidase 12 | growth regulation |
| RNASET2 | ribonuclease 64,a | tumor suppressor |
| SERPINB5 | serpin peptidase inhibit., clade B (ovalbum), member 52 | blocks mammary tumor growth |
| TES | testin4,a | tumor supp., cytoskel. and adhe. complex organi. |
| TFF1 | trefoil factor 14,a | growth factor and protein binding |
| TPM3 | tropomyosin 34,a | muscle dynamics and cell growth |
| (viii) Cytoprotective/Anti-Apoptotic | ||
| CASP14 | caspase 144,a | apoptosis induction |
| CLU | clusterin1,2,3,7 | inhibits apoptosis |
| MUC16 | mucin 162 | cytoprotective, hydrophillic |
| MUC5AC | mucin 5AC2 | mucus/gel-forming, cytoprotective, hydrophilic |
| NAMPT | pre b cell colony enhancing factor 14,a | anti-apoptotic |
| PARK7 | oncogene DJ14,a | nucleotide metabolism, oxidat. stress, transformation |
| PHB | prohibitin4,a | energy metab., fat utili., anti apop. and cell commu. |
| PIP | prolactin-induced protein*$#§1,2,3,7 | inhibitor of T-cell apoptosis, aspartyl protease (?) |
| PRB1 | proline-rich protein BstNI subfamily 17 | ? |
| PROL1 | proline rich, lacrimal 1#1,2,13,3,7 | possible ocular protective function |
| PRR4 | proline rich 4 (lacrimal)Δ†#1,2,13,3,7 | possible ocular protective function |
| SERPINB2 | urokinase inhibitor4,a | regulates proteolysis, wound healing, anti-apoptotic |
| TFF3 | trefoil factor 34,a | chemotaxis, anti apoptosis, migration |
| TPT1 | tumor protein, translationally-controlled 14,a | anti-apoptotic and stem cell maintenance |
| (IX) Extracellular Matrix | ||
| COL6A1 | collagen, type VI, alpha 12 | microfibril component |
| FBLN1 | fibulin 14,a | extracellular matrix organization |
| MUCL1 | mucin-like 13 | ? |
| SPARCL1 | SPARC-like 1 (mast9, hevin)2,7 | reg. of collagen assembly and decorin secretion |
| TGFBI | transforming growth factor, beta-induced, 68kDa variant4,a | reg. of cell adh. and extracellular matrix organi. |
| (x) Immune | ||
| ATRN | attractin2 | receptor or clustering of immune cells |
| C1S | complement component 1, subcomponent S4,a | complement activation and innate immunity |
| C1QB | complement C1q subcomponent, B chain4,a | complement activation and innate immunity |
| C1QC | complement C1q subcomponent subunit C4,a | negative regul. of granul. and macrop. differentiation |
| C1R | complement component 1, subcomponent R4,a | complement activation and innate immunity |
| C3 | complement component 3#1,2,7 | complement activation |
| C4A | complement component 4A (Rodgers blood group)2 | cleaved to a trimer for complement activation |
| C4B | complement component 4 B4,a | similar and greater activity than C4A |
| C8A | complement component C8 alpha4,a | complement activation and innate immunity |
| C8B | complement component C8 beta4,a | complement activation and innate immunity |
| C8G | complement component C8 gamma4,a | complement activation and innate immunity |
| CCL2 | chemokine (C-C motif) ligand 2€5 | monocyte, basophil specific chemotaxis |
| CCL4 | chemokine (C-C motif) ligand 45 | Inflammatory, chemokinetic |
| CCL8 | chemokine (C-C motif) ligand 85 | monocyte, basophil, eosinphil, lympho. chemotaxis |
| CCL11 | chemokine (C-C motif) ligand 115 | eosinophil specific chemotaxis |
| CCL22 | chemokine (C-C motif) ligand 225 | NK cell, dendritic, monocyte chemotaxis |
| CCL24 | chemokine (C-C motif) ligand 245 | resting T cell chemotaxis |
| CD55 | decay accelerating factor for complement4,a | complement activation and innate immunity |
| CD59 | CD59 glycoprotein4,a | protects leukocytes from homolog. complement |
| CFI | complement component I4,a | complement activation and innate immunity |
| CFB | complement factor B1,2 | CFD cleaved to: prolif. serine protease & antiprolif. |
| CFH | complement factor H1,2,7 | restricts complement activation to microbial defense |
| CSF1 | colony stimulating factor 1 (macrophage)5 | prod n, different, function of macrophages |
| CSF2 | colony stimulating factor 2 (granulocyte-macrophage)5 | prod n, different, function of granulocytes, macroph. |
| CSF3 | colony stimulating factor 3 (granulocyte)5 | prod n, different, function of granulocytes, macroph. |
| CXCL5 | chemokine (C-X-C motif) ligand 5€5 | inflammatory cytokine, neutrophil activation |
| CXCL10 | chemokine (C-X-C motif) ligand 105 | T cell, monocyte chemotaxis |
| CXCL1 | chemokine (C-X-C motif) ligand 1 (MGS activity, alpha)5 | neutrophil chemotaxis |
| ELANE | neutrophil elastase4,a | phagocytosis and leukocyte migration |
| FAM3B | fam3b4,a | ? cytokine |
| FCRL5 | Fc receptor-like 513 | possible mature B cell inhibitory co-receptor |
| HLA-B | hla-B4,a | MHC, class I, receptor activity |
| HLA-G | hla-G4,a | MHC, class I, receptor activity |
| HRG | histidine rich glycoprotein4,a | inhibit T cell proliferation, complement activation |
| HSPD1 | 60 kDa heat shock protein, mitochondrial4,a | B cell, T cell and macroph. activ. and protein fold. |
| IGHD | FLJ00382 protein4,a | +ve regul. of B cell prolif. and immune respon. |
| IL6 | interleukin 6 (interferon, beta 2)6 | B cell, nerve cell differentiation |
| IL8 | interleukin 85,6 | inflammatory response mediator, angiogenic |
| IL10 | interleukin 105 | inhibit activated macrophage cytokine synthesis |
| IL12 | interleukin 12€5 | activated T, NK cell mitogen |
| IL15 | interleukin 155 | T cell proliferation |
| IL16 | interleukin 16 (lymphocyte chemoattractant factor) €5 | CD4+ lymphocyte, monocyte, eosinophil migration |
| IL18 | interleukin 184,a | regul. angiog., cell adhe. and immune respon. |
| IFNG | interferon, gamma5 | immune-regulatory, antiviral |
| LGALS8 | putative uncharacterized protein LGALS84,a | plasma cell differentiation and T cell stimulation |
| ORM1 | orosomucoid 11,2 | apparent modulator of acute-phase immune activity |
| ORM2 | orosomucoid 24,a | apparent modulator of acute-phase immune activity |
| PGLYRP2 | peptidoglycan recognition protein 24,a | innate immunity |
| PPBP | pro-platelet basic prot. (chemokine (C-X-C motif) lig. 7)5 | neutrophil chemoattractant and prosecretory |
| SERPING1 | serpin peptid. inhibitor, clade G (C1 inhib), member 12 | complement activation regulator |
| TGFB2 | transforming growth factor, beta 25 | suppress IL-2 T cell growth |
| TNF | tumor necrosis factor (TNF superfamily, member 2)5 | autoimmune, pyrogen, mitogen, differentiation |
| TNFSF13 | tumor necrosis factor ligand superfamily, member 134,a | cell proliferation and immune response |
| (XI) Lipid/Cholesterol | ||
| ANPEP | alanyl (membrane) aminopeptidase2 | aminoprotease |
| ANXA1 | annexin A1§1 | regulate phospholipase a2 activity |
| APOA1 | apolipoprotein A-I1 | cholesterol transport |
| APOA2 | apolipoprotein A-II2 | in high density lipoprotein particles |
| APOA4 | apolipoprotein A-IV1 | in HDL and chylomicrons |
| APOB | apolipoprotein B2 | in chylomicrons and low density lipoproteins |
| APOC2 | apolipoprotein C-II4,a | lipoprotein trans., -ve regulator of VLDL clearance |
| APOC3 | apolipoprotein C-III variant 14,a | lipid transport |
| APOD | apolipoprotein D4,a | VLDL transport |
| APOE | apolipoprotein E4,a | VLDL transport |
| APOH | apolipoprotein H1,2 | lipoprotein metabolism, coagulation |
| APOL1 | apolipoprotein L14,a | lipid transport |
| APOM | apolipoprotein M4,a | HDL particle assembly and clearance |
| GM2A | GM2 ganglioside activator protein4,a | lipid metabolism |
| PAFAH1B2 | PAFAH beta subunit4,a | lipid metabolism |
| PAM | Isof. 1 of peptidyl-glyc. α-amidating monooxy. 4,a | protein and fatty acid metabolism |
| PLA2G2A | phospholipase A2, group IIA3 | membrane phospholipid metabolism |
| PLTP | phospholipid transfer protein2,3 | cholesterol metabolism |
| PON1 | paraoxonase 14,a | HDL trans. and cholesterol metab. and antioxidant |
| PSAP | prosaposin2,7 | enzyme stimulator in glycosphingolipid metabolism |
| SAA4 | serum amyloid A44,a | HDL transport |
| (XII) Other/Uknown | ||
| AGT | angiotensinogen2 | cleaved to angiotensin I, blood pressure |
| APOA1BP | apolipoprotein A-1 binding protein4,a | ? |
| A1BG | alpha 1b glycoprotein4,a | ? |
| B2M | beta-2-microglobulin1,2,3,7 | loss assoc. with hypercatabolic hypoproteinemia |
| BTD | biotinidase4,a | nitrogen compounds metabolism |
| CLEC3B | tetranectin4,a | stimulates activation plasminogen to plasmin |
| COCH | cochlin4,a | ? |
| EVC2 | Ellis van Creveld syndrome 2 (limbin)13 | mutation assoc. wdith Ellis-van Creveld syndrome |
| F12 | coagulation factor xii4,a | blood coagulation regulator |
| GPX3 | glutathione peroxidase 34,a | regulates oxidative-reductive process |
| HSPA4 | heat shock 70kDa protein 42 | heat shock protein |
| HSP90AA1 | heat shock protein HSP 90-alpha4,a | protein folding |
| KNG1 | kininogen4,a | blood coagulation regulator |
| LIN7C | protein lin-7 homolog C4,a | neurotransmitter secretion |
| LRG1 | leucine-rich alpha-2-glycoprotein 12 | ? |
| QDPR | quinoid dihydropteridine reductase4,a | tetrahydrobiopterin metabolism |
| RNASE4 | ribonuclease 44,a | nucleotide and nucleic acid metabolism |
| RPL7A | ribosomal protein L7A4,a | ribosome biogenesis |
| SFRP1 | secreted frizzled-related protein 12 | possible role in polarity of retinal photoreceptor cells |
| SMR3A | submax. gland androg. reg. prot. 3 homol. A (mouse)1,3 | ? |
| SMR3B | submax. gland androg. reg. prot. 3 hom. B (mouse)1,3,7 | ? |
| SOD1 | superoxide dismutase 14,a | antioxidant |
| SOD3 | superoxide dismutase 34,a | binds heparin sulphate, anti oxidant |
| SULF2 | isoform 2 of extracellular sulfatase Sulf-24,a | sulfur compound metabolism |
| TJP1 | isoform short of tight junction protein ZO-14,a | blastocyst formation |
| TXNRD1 | thioredoxin reductase 14,a | cell redox homeostasis |
| VMO1 | vitelline membrane outer layer protein 1 homolog4,a | vitelline membrane formation |
| VTN | vitronectin 4,a | cytolysis and blood coagulation regulator |
| YARS | tyrosyl-tRNA synthetase4,a | protein metabolism |
| XDH | xanthene dehydrogenase4,a | purine metabolism |
| (xiii) Phosphatase/Kinase/GTPase/Other Enzyme | ||
| ACPP | acid phosphatase2 | phosphatase |
| ARHGAP1 | Rho GTPase activating protein2 | GTPase activator of Rho, Rac and Cdc42 |
| CA2 | carbonic anhydrase II2 | hydration of carbon dioxide |
| CP | ceruloplasmin (ferroxidase)1,2,7 | peroxidation of Fe(II)transferrin, binds copper |
| F2 | coagulation factor II (thrombin)1 | fibrinogen to fibrin conversion |
| F5 | coagulation factor V2 | prothrombin/thrombin conversion with coag. factor X |
| GNPTG | N-acetylglucosamine-1-phosphate transf., γsubunit2 | targeting of lysosomal hydrolases to lysosomes |
| HTRA1 | HtrA serine peptidase 12 | cleaves IGF-binding proteins |
| MMP9 | matrix metallopeptidase 92 | matrix collagen IV and V degradation |
| NME1 | nucleoside diphosphate kinase A4,a | cell different., develop. and metastasis suppres. |
| NENF | neudesin4,a | positive regulator of MAPK cascade |
| S100A8 | S100 calcium binding protein A8 $§1,2 | possible cytokine and inhibitor of casein kinase |
| S100A9 | S100 calcium binding protein A9 $§1,2 | possible inhibitor of casein kinase |
| TGM2 | transglutaminase 22 | protein crosslinker |
| TXN | thioredoxin2 | catalyzes dithiol-disulfide exchange and redox rxns |
| USP5 | ubiquitin specific peptidase 5 (isopeptidase T)2 | cellular protein degradation |
| (xiv) Protease/Inhibitor/Antimicrobial | ||
| A2M | alpha-2-macroglobulin2,7 | protease inhibitor, cytokine transporter |
| AMBP | alpha-1-microglobulin/bikunin precursor2 | inhibits trypsin, plasmin, elastase |
| ARG1 | arginase4,a | hydrolysis of L-arginine |
| AZU1 | azurocidin 1 (cationic antimicrobial protein 37)2 | antibacterial and monocyte chemoattractant |
| CLN5 | ceroid-lipofuscinosis neuronal protein 54,a | lysosome organization |
| CST1 | cystatin SN§1,2,3 | cysteine protease inhibitor |
| CST2 | cystatin SA3 | thiol protease inhibitor |
| CST3 | cystatin C1,3 | abundant cysteine protease inhibitor |
| CST4 | cystatin S #§1,2,3,7 | cysteine protease inhibitor |
| CST5 | cystatin D3 | cysteine protease inhibitor |
| CST6 | cystatin M4,a | cysteine protease inhibitor |
| CTSB | cathepsin B2 | lysosomal cysteine protease |
| CTSD | cathepsin D2 | lysosomal aspartyl protease |
| CTSG | cathepsin G2 | chymotrypsin C-like protease, antimicrobial |
| CTSS | cathepsin S4,a | hydrolase and protease activity |
| DCD | dermcidin2 | C-terminal antibacterial, N-terminal prosurvival |
| DEFA3 | defensin, alpha 3, neutrophil-specific1 | anti-bacterial, -viral, -fungal |
| DPP4 | dipeptidyl-peptidase 4 (CD26)2 | intrinsic membrane serine exoprotease |
| ELA2 | elastase 2, neutrophil1 | matrix hydrolysis, antibacterial |
| GBP1 | interferon-induced guanylate-binding protein 14,a | anti-viral |
| HABP2 | hyaluronan-binding protein 2 isoform 24,a | hyaluronan binding |
| IDE | insulin degrading enzyme4,a | insulin degradation |
| IGHA1 | immunoglobulin heavy constant alpha 11,2,13,3,7 | microbial and foreign antigen defense |
| IGHA2 | immunoglob. heavy constant alpha 2 (A2m marker)3 | microbial and foreign antigen defense |
| IGHM | immunoglobulin heavy constant mu1,2,3 | microbial and foreign antigen defense |
| IGJ | immunoglobulin J polypeptide1,3 | microbial and foreign antigen defense |
| IGKC | immunoglobulin kappa constant 3 | microbial and foreign antigen defense |
| IGLC2 | immunoglobulin lambda constant 2 #3 | microbial and foreign antigen defense |
| IGLV1-40 | immunoglobulin lambda variable 1–402 | microbial and foreign antigen defense |
| ITIH1 | inter-alpha (globulin) inhibitor H12 | hyaluronan bp/carrier, pred. serine protease inhib. |
| ITIH2 | inter-alpha (globulin) inhibitor H22 | hyaluronan bp/carrier, pred. serine protease inhib. |
| ITIH3 | inter alpha trypsin inhibitor, heavy chain 34,a | hyaluronan metabolism |
| ITIH4 | inter-alpha (globulin) inhibitor H42 | predicted serine protease inhibitor |
| KLKB1 | plasma kallikrein4,a | fibrinolysis and proteolysis |
| LCN2 | lipocalin 2 (oncogene 24p3)2 | MMP9 binding, bacteriostatic, growth factor-like |
| LPO | lactoperoxidase2,7 | antibacterial |
| LTF | lactotransferrin $#§1,2,13,3,7 | iron metabolism, antibacterial |
| LYZ | lysozyme (renal amyloidosis) *$#1,2,13,3,7 | hydrolase, antibacterial |
| MPO | myeloperoxidase2 | antimicrobial |
| MUC7 | mucin 7, secreted1 | antibacterial, antifungal |
| PH4B | protein disulfide isomerase4,a | breaking and formation of disulphide bonds |
| PI3 | elafin4,a | elastase inhibitor |
| PIGR | polymeric immunoglobulin receptor #1,2,3,7 | antibacterial, polymeric Ig transcellular transport |
| PLG | plasminogen2 | activates urokin.-type plasmin. activat., collagenas., |
| PRB4 | proline-rich protein BstNI subfamily 43 | possible bacterial binding (lost in point mutant) |
| RNPEP | arginyl aminopeptidase4,a | exoprotease (removes arginine and/or lysine) |
| S100A7 | S100 calcium binding protein A74,a | chemotactic, anti-bacterial |
| SCPEP1 | reinoid-inducible serine carboxypeptidase4,a | carboxyprotease activity |
| SERPINA1 | serpin peptid. inhibit., clade A (alpha-1), member 1*1,2 | serine protease inhibitor, anti-inflammatory |
| SERPINA3 | serpin peptidase inhibit., clade A (alpha-1), member 32 | serine protease inhibitor |
| SERPINA4 | kallistatin4,a | kallikrein inhibitor |
| SERPINB8 | serpin B84,a | prohormone convertase inhibitor |
| SERPINA6 | corticosteroid binding globulin4,a | serine protease inhib. and cortisol binding and trans. |
| SERPINA7 | thyroxine binding globulin4,a | hormone binding and serine protease inhibitor |
| SERPINC1 | serpin peptid. inhibit., clade C (antithrom.), member 11,2 | blood coagulation cascade regulator |
| SLPI | secretory leukocyte peptidase inhibitor1,3,7 | acid-stable protease inhib., antibacterial |
| SPINT1 | serine peptidase inhibitor, kunitz type 14,a | protease inhib. and extra cellular matrix organi. |
| TIMP1 | TIMP metallopeptidase inhibitor 15,2 | metalloprotease inhibitor |
| TIMP2 | TIMP metallopeptidase inhibitor 15 | metalloprotease inhibitor, inhib. endothelial prolif. |
| WFDC2 | WAP four disulfide core domain 24,a | serine protease inhibitor |
| (XV) Receptor/Channel/Transport | ||
| ATP5J | ATP synth.-coup. factor 6, mitochon. isoform B precursor4,a | proton and ion transport |
| CLIC2 | chloride intracellular channel 23 | potential chloride ion channel |
| MFI2 | melanoma associated antigen p974,a | iron ion transport and homeostasis |
| MT1X | metallothionein4,a | cellular metal ion homeostasis |
| PRSS8 | prostasin4,a | channel regulator and protease |
| RBP4 | retinol binding protein 44,a | retinol binding and transport |
| SLC7A4 | solute carrier family 7, member 43 | cationic amino acid transport |
| SLC12A2 | solute carrier family 12, member 24,a | ion transport |
| STX7 | syntaxin 74,a | vesicle mediated transport |
The list is derived from published reflex tear (1, 4), closed eye tear (2), open and closed eye tear (3), Meibomian gland secretion (14), open and closed eye tear capture ELISA or antibody array (5, 6) and lacrimal gland EST (7) analyses. Not listed are the numerous cytoplasmic proteins that are also detected in tears (2, 4).
*,Δ,†,$,#,€,£,¥,§ suggested to be less or more than normal in tears from patients suffering from *blepharitis (8), $#€ dry eye (9, 10,11), ΔSjögren’s syndrome(12), †contact lens related dry eye (13) £climatic droplet keratopathy (15), ¥fusarium keratitis (16) or §dry eye and meibomian gland dysfunction (17).
(1- Zhou et al., 2004; 2- de Souza et al., 2006; 3- Sack et al., 2007; 4- Zhou et al., 2012; 5- Sack et al., 2005; 6- Ozyildirim et al., 2005; 7- Green-Church et al., 2008; 8- Koo et al., 2005; 9- Zhou et al., 2009; 10- Srinivasan et al., 2012; 11- Na et al., 2012; 12-Kitagawa et al., 2007; 13- Nicholas et al., 2009; 14- Tsai et al., 2006; 15-Lei et al., 2007; 16-Ananthi et al., 2013; 17-Soria et al., 2013)
Updated from Table 1 of “Laurie GW, Olsakovsky LA, Conway BP, McKown RL, Kitagawa K, Nichols JJ. Dry eye and designer ophthalmics. Optom Vis Sci 2008;85:643–52. ©The American Academy of Optometry 2008.”
Relatively few tear proteins appear to be selectively down- or upregulated in dry eye (Table 1). Appreciating which are bioactive and at what molar levels would be insightful. The only growth factor-like molecule downregulated in mild to severe aqueous deficiency was lacritin (Srinivasan et al., 2012). Lacritin promotes basal tearing when added topically in rabbits (Samudre et al., 2011). Also decreased was lipocalin-1 (Srinivasan et al., 2012), that cleanses the ocular surface of lipids that would otherwise interfere with ocular surface wetting (Glasgow and Gasymov, 2011). Lacritin was the most severely downregulated protein in contact lens-related dry eye (Nichols and Green-Church, 2009) - perhaps in part because it is readily adsorbed on contact lenses (Green-Church and Nichols, 2008). It is also deficient in blepharitis (Koo et al., 2005), a common inflammation of the eyelid, associated with evaporative dry eye (Mathers et al., 1993). Two studies did not note any lacritin change in dry eye using mass spectrometry coupled with liquid chromatographic cationic separation followed by reverse phase separation (Zhou et al., 2009; Boehm et al., 2013), although one observed a decrease of lipocalin-1 (Zhou et al., 2009). 2-D SDS PAGE prior to mass spectrometry (Koo et al., 2005; Nichols and Green-Church, 2009; Srinivasan et al., 2012) is necessary to distinguish monomeric lacritin from inactive multimeric lacritin (Velez et al., 2013), and likely also the inactive lacritin-c splice variant (McKown et al., 2009). Exploration of lacritin cell targeting and signaling mechanisms has revealed a network of interdependent molecules, each necessary for lacritin activity. New evidence is suggesting that some of these are also decreased in dry eye. Here we review recent advances in our understanding of lacritin, and provide an overview of lipocalin-1 whose eye specific expression parallels that of lacritin. We also update our current understanding of the tear proteome.
2. Lacritin
2.1. Structure and expression
The discovery of lacritin indirectly emerged from a screen for novel factors capable of promoting tear protein secretion, with cDNA cloning out of a human lacrimal gland library (Sanghi et al., 2001). The lacritin gene, LACRT, is one of the most eye specific (Sanghi et al., 2001) and resides on 12q13, within ~1.24 Mb of the AAAS gene associated with alacrima (Kumar et al., 2002). Human lacritin is coded by a 417 bp open reading frame that translates as a 14.3 kDa hydrophilic protein with a 19 amino acid signal peptide resulting in a secreted protein with a predicted molecular mass of 12.3 kDa (Fig. 1). Mobility in SDS PAGE gels is ~18 kDa for recombinant lacritin generated in E. coli (Wang et al., 2006), and ~23 – 25 kDa with glycosylation in tears (Seifert et al., 2012). Such aberrant mobility may be attributable to lacritin’s C-terminal amphipathic α-helix that supports lacritin cell surface targeting of the heparan sulfate proteoglycan syndecan-1 (Fig. 1), as confirmed by circular dichroism (Wang et al., 2006; Zhang et al., 2013) and point mutagenesis (Zhang et al., 2013). Lacking the C-terminal amphipathic α-helix and inactive (Wang et al., 2013) is splice variant lacritin-c (McKown et al., 2009). PSIPRED (v3.3) predicts three other C-terminal half α-helices (Fig. 1) in secreted lacritin. The C-terminal half is thus ordered, whereas the N-terminal half is largely diisordered (Fig. 1; McKown et al, ’09). Thirteen sites of O-glycosylation (NetOGlyc 3.1) and one N-glycosylation site (NetNGlyc 1.0) are predicted (McKown et al., 2009), with O-glycosylation restricted to the disordered region predicted by PONDR (‘Predictor of Naturally Disordered Regions’) in the N-terminal half (McKown et al., 2009). The N-glycosylation site flanks the syndecan-1 binding domain, but is not generally conserved among orthologs. Patients with climatic droplet keratopathy display decreased N-linked glycosylation (Lei et al., 2009), however non-glycosylated lacritin is active in prosecretory, mitogenic, cytoprotective and syndecan-1 binding assays. It is possible that glycosylation enhances stability.
Fig. 1.

Linear diagram of secreted lacritin. PSIPRED (v3.3) predicted α-helices are indicated by rectangles; the arrow indicates a short predicted β-strand. The most C-terminal α-helix is amphipathic and targets the cell surface proteoglycan syndecan-1 after heparanase modification (Wang et al., 2006). Both the hydrophobic face (L108/L109/F112) and the cationic face (Q103/K107 and K111) of lacritin are involved (Zhang et al., 2013). These target three syndecan-1 elements (Zhang et al., 2013): (i) the conserved hydrophobic sequence GAGAL, and (ii) chondroitin-4-sulfated and (iii) heparanase-cleaved 3-O-sulfated heparan sulfated chains on N-terminal serines 15, 23 and 25 (human syndecan-1 numbering excludes the signal peptide). α-helicity of lacritin’s C-terminal a-helix has been validated by circular dichroism (Wang et al., 2006; Zhang et al., ’13). Syndecan-1 has a short transmembrane domain known only for cytoskeletal signaling. Rapid lacritin signaling appears to be attributable to an associated G-protein coupled receptor, as first implied by pertussis toxin inhibitable mitogenic signaling (Wang et al., 2006).
Lacritin mRNA and protein are highly expressed in human lacrimal gland as the sixth most common mRNA (Ozylidirim et al., 2005), with detection in lacrimal acinar cell secretory granules (Sanghi et al., 2001). Lacritin protein also appears to be expressed by the human meibomian gland (Tsai et al., 2006), and was recently detected in the gland of Wolfring (Ubels et al., 2012). In monkey, lacritin mRNA has been detected also in conjunctiva and corneal epithelium, and at low levels in other eye tissues including retinal and lens epithelia (Nakajima et al., 2007). A single retinal hit has been noted in Human Proteinpedia (HuPA_00710). In non-ocular tissues, lacritin is highly expressed in an apparent ductal-like cell in human submandibular and parotid glands - but not in acinar cells. Lacritin also appears to be produced at low levels in thyroid (Sanghi et al., 2001), and has been noted by RT-PCR in normal breast and invasive breast cancer tumors (Weigelt et al., 2003) and by proteomics in saliva (Human Proteinpedia [HuPA_00047]), lung lavage (HuPA_00022) and plasma (Schenk et al., 2008). No other expressing tissues were noted in a fifty tissue RNA dot blot and by tissue microarray of seventy-five different human organs (Sanghi et al., 2001). Release is apical from acinar cells into ducts that carry lacritin onto the surface of the eye (Morimoto-Tochigi et al., 2010), where lacritin is detected in tears (Sanghi et al., 2001; Nakajima et al., 2007; Seifert et al., 2012).
2.2. Predicted and demonstrated orthologs
We extracted LACRT aligned genomic sequences from over twenty-one species in Ensembl (release 70), guided by AceView defined exon boundaries for LACRT (Supplementary Fig. 1; see method in Laurie et al., 2012). Exon sequences were spliced (Supplementary Fig. 2) and then translated (Supplementary Fig. 3), with some displaying incomplete sequence. No complete mouse or rat ortholog was apparent although other rodents are represented, an observation possibly related to the predicted telomeric location or alternatively reflective of significant differences in lacrimal specific gene expression between mouse and human (Ozyildirim et al., 2005). Non-primate lacritins display on average 25% amino acid identity with human lacritin (vs 75% for primates), although bushbaby lacritin is only 41% identical (Table 2). Analysis of each by PSIPRED (version 3.3) displayed a predominance of predicted α-helices in the C-terminal half (Fig. 2).
Table 2.
Human lacritin and orthologs (numbering includes signal peptide)
| Species | Nucleotide # | Amino acid # | Nucleotide identity (%) | Amino acid identity (%) |
|---|---|---|---|---|
| Human [H. sapiens] | 417 | 138 | ||
| Bushbaby [O. garnettii] | 540 | 138 | 71 | 41 |
| Cat [Felis catus] | 356 | 119 | 72 | 38 |
| Chimpanzee [P. troglodytes] | 414 | 137 | 99 | 99 |
| Cow [B. Taurus] | 423 | 126 | 69 | 4 |
| Dog [Canis lupus familiaris] | 368 | 111 | 64 | 35 |
| Elephant [L. Africana] | 427 | 136 | 68 | 7 |
| Gibbon [N. leucogenys] | 331 | 108 | 93 | 67 |
| Gorilla [G. gorilla gorilla] | 417 | 137 | 99 | 97 |
| Guinea pig [C. porcellus] | 422 | 100 | 62 | 11 |
| Horse [E. caballus] | 336 | 111 | 75 | 45 |
| Lesser hedgehog tenec [E. telfairi] | 391 | 120 | 66 | 4 |
| Macaque [M. Mulatta] | 417 | 140 | 93 | 89 |
| Marmoset [C. jacchus] | 424 | 137 | 87 | 74 |
| Microbat [M. lucifugus] | 417 | 111 | 72 | 35 |
| Mouse lemur [M. murinus] | 417 | 135 | 70 | *42 |
| Orangutan [P. abelii] | 417 | 138 | 97 | 94 |
| Panda [A. melanoleuca] | 433 | 126 | 68 | 37 |
| Rabbit [O. cuniculus] | 443 | 122 | 63 | 23 |
| Shrew [S. araneus] | 503 | 165 | 62 | *32 |
| Squirrel [L. tridecemlineatus] | 514 | 143 | 61 | 26 |
| Tree shrew [T. belangen] | 437 | 139 | 64 | *30 |
Values were determined by extraction of nucleotide sequence from Ensembl (release 70) genomic alignments with exon boundaries guided by AceView.
Identities differ slightly from those indicated by Ensembl.
Fig. 2.
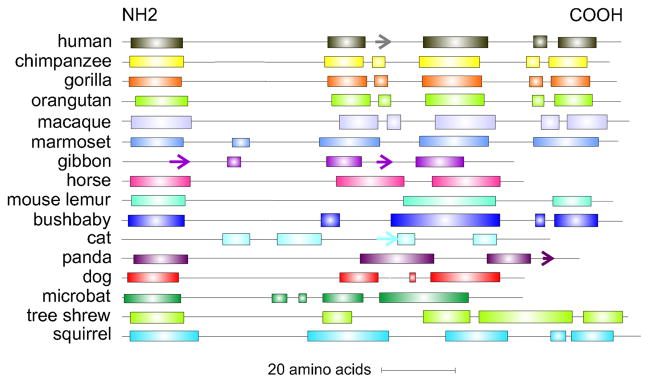
Predicted and demonstrated lacritin orthologs. Shown are secondary structure predictions by PSIPRED (v3.3). Rectangles and arrow respectively indicate predicted α-helices and a β-strand. The N-terminal α-helix is the signal peptide. The most C-terminal α-helix in human lacritin regulates many of lacritin’s activities via an amphipathic structure.
Predicted identity of horse lacritin with human lacritin (45%) is greater than any other known non-primate ortholog (Table 2). Blotting of horse tears with anti-C vs anti-N terminal domain specific antibodies revealed that horse lacritin was mainly represented as a ~13 kDa C-terminal-half fragment with a predicted amphipathic αhelix at the C-terminus (Laurie et al., 2012).
2.3. Tools and manufacture
The 363 bp coding region without signal peptide coding sequence of human lacritin was subcloned into pTYB1 (New England Biolabs, Inc; Ipswich MA) as ‘pLAC’ for expression of lacritin-intein fusion protein (Wang et al., 2006). Intein serves as a convenient affinity tag for purification that is retained on the chitin affinity column upon elution with β-mercaptoethanol. Eluted lacritin is concentrated, dialyzed versus PBS and then is further purified on DEAE (diethylaminoethanol) covalently linked to Sepharose. DEAE removes contaminating endotoxin. Also binding to DEAE is a C-terminal lacritin cleavage fragment, and the E. coli chaperone DnaK (McKown et al., 2013). DnaK is likely involved in lacritin synthesis.
Although lacritin’s low nanomolar prosecretory (Sanghi et al., 2001), mitogenic (Wang et al., 2006) and cytoprotective (Wang et al, 2013) activities require only 1 or 10 nM lacritin, optimizing production from current ~0.1 g/l yields is desirable for cost efficiency - particularly for scale up. C-terminal hydrophobic residues might promote some misfolding. Misfolded molecules aggregate and become secluded in inclusion bodies. Single or double point mutagenesis to serine of I68, I68/I78, V69, I73, V91, I98, F104, L108, L109 or F112 enhanced yields two or three times that of unaltered lacritin (McKown et al., 2013). Some are inactivating, but lacritins V69S, I73S, L108S and L109S retain activity (Wang et al, 2013). Salt bridges can also promote misfolding, although many are stabilizing. We mutated several. Increases in yield were noted, particularly with lacritins K66S/E70S, K66S/E70S/E103S/K107S and E103S/K107S (McKown et al., 2013), of which lacritin K66S/E70S retains activity. Another approach is to address codon usage. Some common human codons are rare or uncommon in E. coli such that tRNA’s necessary for recombinant protein production are insufficient. Thirteen synonymous mutants were generated, however yield increases were low. It is possible that rare to common codon mutagenesis of lacritins V69S, I73S, L108S, L109S or K66S/E70S may increase yields (McKown et al., 2013).
2.4. Cell targeting
A deglycanated form of syndecan-1 was discovered to be the main cell surface binding protein for lacritin by mass spectrometric sequencing cell surface proteins bound to lacritin columns in buffer containing 100 mM NaCl. Validation was by affinity precipitation (Ma et al., 2006). Syndecan-1 is a widely expressed cell surface heparan sulfate proteoglycan with a carboxy terminal end anchored in the plasma membrane with short cytoplasmic tail, and an ectodomain substituted proximally with chondroitin sulfate chain(s) at serines 184 and 194 (human syndecan-1; numbering excludes the signal peptide), and distally with up to three heparan sulfate chains (serines 15, 23, 25) without or with a short chondroitin sulfate chain (Kokenyesi and Bernfield, 1994). Lacritin’s C-terminal α-helix (Wang et al., 2006) bound a domain within syndecan-1 amino acids 1 – 50, with binding dependent on prior heparanase cleavage of heparan sulfate (Ma et al., 2006). SiRNA knockdown of syndecan-1 abrogated lacritin dependent mitogenic activity, as did depletion of heparanase (but not heparanase-2), but could be rescued by addition of exogenous heparanase or with bacterial heparitinase (Ma et al., 2006). Recently, we narrowed the binding domain to hydrophobic amino acids 20 – 30 that when reduced to synthetic peptide GAGAL enhanced lacritin C-terminal α-helicity (Zhang et al., 2013). Binding is equally dependent on substitution of S23 and S25 (and possibly S15) with both heparan sulfate and chondroitin sulfate as a novel hybrid domain of hydrophobic core protein, heparanase cleaved heparan sulfate and adjacent chondroitin sulfate (Zhang et al., 2013). Heparanase is not widely expressed, but is detected in tears by Western blotting (Ma, Wang and Laurie, unpublished), although at levels too low yet for proteomic detection.
With each modification essential for lacritin binding, cell targeting by lacritin is very selective. Contributing to cell selectivity is a signaling receptor(s). Signaling is initiated within seconds (Wang et al, 2006). A candidate G-protein coupled receptor has been identified (Zimmerman and Laurie, unpublished). SiRNA knockdown in cells abrogates lacritin dependent mitogenesis (Ma and Laurie, unpublished). Lacritin targets rat (Sanghi et al., 2001) and monkey (Fujii et al., 2013) primary lacrimal acinar cells in secretion assays, and primary human corneal epithelial (Wang et al., 2013) and HCE-T (Wang et al., 2006, 2013) human corneal epithelial (SV40-large T) cells in cytoprotection assays. Lacritin is mitogenic for HCE-T, HCE, human salivary HSG/HeLa and embryonic kidney (HEK293) cells, but not for human epidermal (A431), cervical (HeLa), foreskin fibroblast (HS68), fibrosarcoma (HT1080), erythroleukemia (K-562), breast carcinoma (MCF7), melanoma (SK-MEL, WM-164), Leydig (TM4), Sertoli (TM3), glioma (U-1242-MG, U251-MG) and mouse fibroblast (NIH3T3) (Wang et al., 2006). No fibrosis, angiogenesis, or inflammation has been observed in eyes of lacritin treated rabbits (Samudre et al., 2011), monkeys or rats (unpublished).
2.5. Prosecretory and protearing activities
Our original discovery screen employed primary cultures of rat lacrimal acinar cells (Sanghi et al., 2001) in a 96-well assay that monitored tear peroxidase release normalized to cellular DNA (Chen et al., 1998). 0.8 – 13 nM of recombinant human lacritin stimulated peroxidase secretion in a dose-dependent manner, without affecting carbachol/VIP stimulated secretion (Sanghi et al., 2001). Similarly, lacritin purified from monkey tears triggered protein secretion from monkey lacrimal acinar cells even under conditions of stress from the inflammatory cytokines interferon-γ and tumor necrosis factor that abrogated carbachol stimulated secretion (Fuji et al., 2013). Low level tear stimulation, induced in part by lacritin (Samudre et al, 2011), is responsible for continually wetting the ocular surface (Dartt, 2009) with basal tears.
3.2 nM recombinant human lacritin triggers calcium signaling by cultured human corneal epithelial cells (Sanghi et al., 2001). The corneal epithelium is intimately associated with sensory nerves that penetrate and form neuro/epithelial junctional complexes (Muller et al., 1996). Treating normal rabbits with 0.8 – 8 μM recombinant lacritin promoted tearing within 60 min - the earliest time point assayed, and lasted at least 240 min (Samudre et al., 2011). Tearing was measured via Schirmer strips 10 min after proparacaine anesthesia to minimize the inclusion of reflex tears. To test for toxicity, eyes were treated with 4 μM lacritin three times daily for two weeks, or alternatively with 1.1 μM lacritin truncation C-25 that lacks the syndecan-1 binding site. Lacritin steadily enhanced basal tearing without toxicity. One week after washout, basal tearing remained elevated, whereas C-25 had no effect (Samudre et al., 2011). These observations highlight the potential of lacritin as a tear secretagogue alone or in combination with other agonists.
2.6. Promitogenic activity and signaling
Early studies also noted that recombinant lacritin promoted HSG/HeLa cell proliferation over a 0.2 – 0.8 nM dose range that approximated the serum positive control (Sanghi et al., 2001), suggesting that lacritin was a pleiotropic tear factor that might contribute to regulation of epithelial renewal as it flowed downstream from lacrimal acinar cells onto the eye (Wang et al., 2006). Over a broader dose range, lacritin displayed a biphasic dose response with an optimum of 1 or 10 nM. Lacritin truncations lacking 15 – 49 amino acids from the C-terminus were inactive, whereas truncation of 5 or 10 C-terminal, or 24 N-terminal amino acids had no effect (Wang et al., 2006). Mitogenic signaling is initiated within seconds and proceeds through the G proteins Gαi or Gαo to the phosphatase PP2A (Karnati and Laurie, unpublished), leading to rapid dephosphorylation of PKCα. Dephosphorylated protein kinase C-α (PKCα) translocates to the perinuclear Golgi region where it activates phospholipase D1 (PLD1) and phospholipase Cγ2 (PLCγ2) to generate IP3. IP3 triggered release of calcium into the cytoplasm activates the phosphatase calcineurin to in turn dephosphorylate the transcription factor NFATC1 (nuclear factor of activated T cells, calceneurin dependent 1) that translocates into the nucleus (Wang et al., 2006) to co-regulate the transcription of genes involved in cell growth and secretion (Heit et al., 2006). Via a parallel pathway, Gαi or Gαo/PKCα/PLC activates PLD1 and in tun mTOR that also promotes proliferation in a manner synergistic with NFATC1 (Wang et al., 2006).
2.7. Cytoprotective activity
Lacritin promotes the survival of human corneal epithelial cells stressed with interferon-γ and tumor necrosis factor (Wang et al., 2013). As a simple assay, we monitored the nuclear translocation of ‘Forkhead box O3’ (FOXO3). FOXO3 is nuclear in stressed or dying cells and cytoplasmic when cells are healthy. When interferon-γ/tumor necrosis factor stressed human corneal epithelial cells were treated 10 mM lacritin, FOXO was cytoplasmic - but remained nuclear with 10 nM C-25 (Wang et al., 2013). The same assay monitored manipulations with normal and dry eye tears. Normal, but not dry eye, tears are protective (cytoplasmic FOXO3), however protective activity is lost when lacritin is completely immunodepleted from tears (nuclear FOXO3). Similarly, spiking 10 nM lacritin, but not C-25, into dry eye tears restores protective activity (Wang et al., 2013).
To begin to discern how lacritin is prosurvival, we monitored the cleavage of caspases 3 and 9 in the absence or presence of lacritin. Lacritin had no effect on caspase cleavage and no DNA fragmentation was apparent, suggesting that interferon-γ/tumor necrosis factor treatment to induce stress did not trigger apoptosis and that the lacritin survival mechanism is not anti-apoptotic. However changes were observed in the lipidation of autophagy marker microtubule-associated protein 1 light chain 3 (LC3) (Wang et al., 2013). Autophagy removes stress damaged proteins and organelles from cells by enclosure and capture in lipidated LC3 covered autophagosomes. We monitored the process by transducing interferon-γ/tumor necrosis factor stressed human corneal epithelial cells with an LC3 construct double tagged with low pH sensitive green fluorescent protein and pH insensitive mCherry (‘LC3RG’) such that time dependent transition of LC3RG isolation membranes (double membrane that begins the enclosure of damaged proteins or organelles) to autophagosomes (enclosure completed and fused) and then fusion with lysosomes (low pH) could be followed. We discovered that lacritin, but not C-25, promoted the acceleration of autophagy for ~60 min, and then ceased. This was sufficient to restore oxidative phosphorylation, promote both mitochondrial fusion and cell survival, and trigger changes in 29 metabolites, including the suppression of kynurenine (Wang et al., 2013). Kynurenine is elevated in sera of patients with primary Sjögren’s syndrome (Pertovaara et al., 2005), rheumatoid arthritis and other autoimmune diseases (Katz et al., 2008). When instead of interferon-γ/tumor necrosis factor stress, LC3RG cells were co-transduced with a cyan fluorescent protein-labeled huntingtin construct (Htt103Q) that is cell toxic, the same autophagic pulse was triggered by lacritin, but not C-25. No autophagic pulse was apparent when cells were co-transduced with a non-toxic huntingtin construct (Htt25Q) (Wang et al., 2013). Thus stress is a prerequisite for lacritin stimulated autophagy.
Unlike mitogenic signaling, survival signaling is mediated by FOXO3 and FOXO1. By 1 min, lacritin promotes the acetylation (and phosphorylation) of FOXO3 - a modification coincident with ligation of autophagy-related protein 101 and acceleration of autophagy (Wang et al., 2013). We also observed that stress promotes the immediate acetylation of FOXO1, as first reported by Zhao et al., (2010) in cancer cells, but unlike Zhao et al., (2010), stress was insufficient to promote ligation of acetylated FOXO1 with autophagy-related protein 7. Instead, lacritin is required and does so 5 – 15 min after administration (Wang et al., 2013). The autophagic pulse mechanism appears to be well-suited for the stressed ocular surface as each new bolus of lacritin is delivered to the eye, to then drain away into the nasolacrimal duct. Maintaining all elements of this system may be required for ocular surface health.
2.8. Molar levels in tears
Systematically discerning the bioactivity and molar variation of individual tear proteins in the entire tear proteome could open up a new era of ocular diagnosis and treatment. To gain information on lacritin levels in tears, a ‘lacritin’ ELISA sold by USCN (#E2576Hu, L091229053) was tested. Unfortunately, it failed to detect recombinant human lacritin and the positive control migrated in SDS-PAGE as a broad smear (McKown and Laurie, unpublished). We therefore established an ELISA with N-terminal-specific anti-lacritin antibody ‘anti-Pep Lac N-Term’ that detects lacritin in tears without interference from other tear proteins (Seifert et al., 2012). Basal tear samples from 66 individuals aged 18 – 52 years were individually tested over six replicates revealing a mean of 4.2 ± 1.17 ng/100 ng of total tear protein with little difference by gender, although lacritin may be greater in female reflex tears (Ananthi et al., 2011). Assuming an estimated basal tear protein concentration of ~8 mg/ml (Sitaramamma et al., 1998), lacritin molar levels are 18 – 27 μM. In contrast, lysozyme is ~20 ng/100 ng total tear protein (Seifert et al., 2012), or ~100 μM - in agreement with others (Sen and Sain, 1982 [80 μM], Velos et al., 1985 [86 μM]) with estimates to 300 μM (Eylan et al., 1977). To assess whether time of day influences lacritin levels, we collected tears from 34 additional individuals at 7:30 – 8:30 am (0 hr), 11:30 am – 12:30 pm (4 hr), 4:30 – 5:30 pm (8 hr) and 7:30 – 8:30 am the following day (24 hr). No differences were observed.
18 – 27 μM lacritin is more than one-thousand fold greater than the accepted dose optimum of 1 – 10 nM (Wang et al., 2006), although 0.8 – 8 μM was effective when applied topically in rabbits (Samudre et al., 2011). Perhaps the availability of epithelial bioactive lacritin is restricted by an unknown ocular surface or secretory mechanism. Present in all anti-lacritin tear blots was the ~25 kDa lacritin band, and a ~13 kDa fragment. Also present, but overlooked were ~50 kDa, ~75 kDa and occasionally even higher molecular weight bands. Secondary antibodies showed nor cross-reactivity with tears. With new anti-lacritin monoclonal antibody 1F5 displaying preference for the 75 kDa band, we explored the putatively larger species in more detail (Velez et al., 2013) and discovered that tissue transglutaminase in tears (Table 1) generates lacritin multimers. Multimers are largely inactive (Velez et al., 2013).
Exogenous tissue transglutaminase (1.5 μM) from guinea pig promoted crosslinking between deprotonated lacritin lysines 82 or 85 with acceptor glutamine 106 that was initiated within 1 min, and completed 40 – 90 min later. No crosslinking was observed in the presence of EDTA, or after prior denaturation of tissue transglutaminase by boiling, or when tissue transglutaminase was replaced by an inactive recombinant human tissue transglutaminase (Velez et al., 2013). We then immunodepleted all lacritin monomer, multimer and fragment from human tears. Recombinant lacritin spiked into immunodepleted tears formed dimers, trimers and tetramers after overnight incubation at 37°C. In the negative control without tears, a small amount of dimer formed (Velez et al., 2013). Since glutamine 106 resides within the lacritin mitogenic domain (amino acids 100 – 109) that targets syndecan-1, we wondered whether lacritin activity was affected by cross-linking, and discovered that syndecan-1 binding was substantially decreased. Also suppressed was lacritin cytoprotective activity (Velez et al., 2013) since tissue transglutaminase cross-linked lacritin was substantially less effective at rescuing interferon-γ/tumor necrosis factor stressed human corneal epithelial cells. Blotting suggests that normal human tears contain 0.6 μM tissue transglutaminase, that thus appears to act as a negative regulator of monomeric (epithelially active) lacritin. Primary human corneal epithelial cells express both transglutaminase 1 and tissue transglutaminase mRNAs. mRNA expression of both increases with hyperosmolar stress, particularly transglutaminase 1 (Chen et al., 2008), however transglutaminase 1 has not been detected in tears. Thus, lacritin may be subjected to enhanced cross-linking and deactivation in dry eye.
3. Lipocalin-1
3.1. Structure and expression
Tear lipocalin (LCN1; recently reviewed by Glasgow BJ and Gasymov, 2011; Dartt, 2011) was originally noted as an unknown band in early electrophoretic separations of human tears and named tear pre-albumin (Erickson et al., 1956), based on its paper electrophoretic mobility near serum albumin proximal to the anode. Lack of immunological cross-reactivity in normal human tears suggested that the two were distinct (Josephson and Lockwood, 1964), in keeping with earlier studies by Fleming suggesting lack of serum immunoreactivity by rabbit anti-human tear antibodies (Fleming and Allison, 1925). Others confirmed this observation by gel electrophoresis, immunabsorption, gel fractionation and analytical ultracentrifugation, that also distinguished the substantially differing molecular weights of the two (Bonavida et al., 1969). In 1987, Pervaiz and Brew proposed the family name ‘lipocalin’ to describe a secondary or tertiary structurally homologous group of proteins with affinity for lipophilic ligands (Pervaiz and Brew, 1987). Subsequent cDNA cloning (Redl et al., 1992) from N-terminal tear albumin protein sequence out of a human lacrimal gland cDNA library identified a further member of the lipocalin family that was 58% identical to von Ebner’s gland protein from rat, and identical to human von Ebner’s gland protein, and was later designated as tear lipocalin with gene symbol LCN1. Tear lipocalin is a prominent component of tears with an estimated concentration of ~75 μM (Fullard et al., 1991).
Tear lipocalin runs as a ~18 kDa band in SDS PAGE (Millar et al., 2009). O-linked glysylation of C-terminal residues threonine 170 and serines 172 and 175 is weakly predicted by NetOGlyc 3.1 (numbering includes signal peptide). No N-linked glycosylation sites are predicted (NetNGlyc 1.0). Tear lipocaln forms an eight stranded antiparallel β barrel structure with C-terminal α-helix that creates a ~15 Å deep central cavity with positively charged bottom surface for ligand binding. Hydrophobic residues line the cavity (Gasymov et al., 2001; Breusted et al., 2005), that best accommodates fatty acids of 18 (22.5 Å) to 24 carbons (Abduragimov et al., 2000). The disulphide bond between cysteines 79 and 171 restricts retinol binding in preference to native lipids (Glasgow et al., 1998). Tear lipocalin also binds lysozyme and lactoferrin through electrostatic interactions (Gasymov et al., 1999), in keeping with the possibility that the three might be co-secreted as a complex.
Like LACRT, LCN1 is one of the most highly expressed human lacrimal gland genes and is equally eye specific (Ozyildirim et al., 2005). Western blotting has detected tear lipocalin in tears, saliva, sweat, and nasal mucus (Redl et al., 1992). Some expression in other tissues has been observed, including lung (Redl et al., 1998), pituitary gland (Wojnar et al., 2002), prostate (Holzfeind et al., 1996), plasma (Schenk et al., 2008) and semen (Pilch and Mann, 2006).
3.2. Orthologs
Twenty-eight tear non-overlapping orthologs are currently listed by Ensembl (release 70). Sequence identity of human tear lipocalin with primate and non-primate tear lipocalins is respectively 82 and 44%.
3.3. Tools and manufacture
Recombinant tear lipocalin has been largely produced in E. coli, although mammalian recombinant protein is commercially available. A large collection of point mutated tear lipocalins have been generated by the Glasgow group for structural studies (for example, Gasymov et al., 2002).
3.4. Lipid binding activity
Tear lipocalin is the main lipid binding protein in tears (Glasgow et al., 1995). It copurifies out of human tears with stearic (5.9 μM), palmitic (4.4 μM) and lauric (0.4 μM) acids - levels in keeping with the relative affinities of each for tear lipocalin as determined by displacement of the fatty acid analog DAUDA (Gasymov et al., 1999). By scavenging lipids, tear lipocalin is thought to help clear the ocular surface of sloughed cellular debris from epithelial turnover that might interfere with wetting. It also stabilizes the tear film (Schoenwald et al., 1998), and with ligand is itself stabilized (Tsukamoto et al., 2009).
3.5. Clearance of lipids coupled to tear lipocalin
Cell surface ‘lipocalin-1 interacting membrane receptor’ (LBR1L) captures tear lipocalin for endocytosis. Endocytic internalization of FITC-labeled tear lipocalin (Wojnar et al., 2003) or β-lactoglobulin (Fluckinger et al., 2008) in NT2 neuronal cells was abrogated by antisense knockdown of LMBR1L. LMBR1L is a plasma membrane protein with nine predicted transmembrane domains (Wojnar et al., 2001), discovered in a phage display screen of tear lipocalin binding proteins (Wojnar et al., 2001). LMBR1L is widely expressed, but is not listed in corneal or lacrimal gland EST databases (NEIBank) suggesting either that it is absent, or more likely that expression is low (as is common for receptor proteins).
3.6. Cysteine protease inhibition activity
Recombinant tear lipocalin (5 – 10 μM) and tear lipocalin synthetic peptide (50 – 150 μM) inhibited papain activity with similar activity as cystatin C (CST3), indicating that tear lipocalin is a cysteine protease inhibitor (van’t Hof et al., 1997). Tear lipocalin contains amino acid motifs similar to papain binding domains of family 2 cystatins. Leucine residues in the first cystatin like motif are necessary for protease inhibitor activity (Wojnar et al., 2001).
3.7. Bacterial growth inhibitory activity
Tear lipocalin (5 μM) inhibits the growth of E. coli in an FeCl3 reversible manner by capturing secreted bacterial siderphores with an affinity similar to stearic acid - suggesting that it is physiologically relevant. Growth assays were performed in M9 minimal medium with a NaCl concentration of 10 mM (Fluckinger et al., 2004). Siderphores deliver extracellular iron necessary for bacterial growth in minimal medium.
3.8. Endonuclease activity
Homology of two tear lipocalin sequence motifs with an Mg2+ dependent endonuclease from gram negative Serratia marcescens was rationale for studies demonstrating that tear lipocalin displays endonuclease activity, although 1355 fold less active than DNase I (Yusifov et al., 2000). Human reflex tears contain 714 ng/ml DNA, and endonuclease activity. The activity largely co-fractionates with tear lipocalin, and is Mg2+ dependent and partially NaCl sensitive (Yusifov et al., 2008). Strands of DNA of increased length in dry eye tears have been detected on Schirmer strips, coincident with inflammation, and decreased tear nuclease activity - the latter thought to be contributed by tear lipocalin and DNase I (Sonawane et al., 2012).
4. New additions to the tear proteome
We previously assembled all tear proteomic data into a single table, restricting entry to proteins designated as ‘extracellular’ or ‘plasma membrane’ in their primary or alternative location (Laurie et al., 2008). Now updated with 139 new entries from Zhou et al., (2012), the additions supplement tears with proangiogenic, anti-angiogenic, retinal survival, epithelial repair, cysteine protease inhibitor, immunosuppressive, and immunostimulatory activities (Table 1). Thirteen are highlighted below.
4.1. Angiogenesis
Exclusion of blood vessels from the cornea is essential for transparency (Ambati et al., 2006). Yet, tears contain both stimulators and inhibitors of angiogenesis. Now identified in tears are the stimulator angio-associated migratory cell protein (AAMP; 52 kDa) and the inhibitor fibulin 3 isoform 1 (EFEMP1; ~55 kDa). Antibody inhibition and antisense knockdown studies provide indirect evidence for the possibility that Angio-associated migratory cell protein is required for endothelial tube formation in co-culture with astrocytes (Beckner et al., 2002).
0.2 – 0.9 μM recombinant fibulin 3 isoform 1 inhibits sprouting of endothelial cells grown on collagen gels (Albig et al., 2006). Fibulin 3 isoform 1 binds the C-terminus of TIMP3 and, together with TIMP3, is a disease gene for macular degeneration (Klenotic et al., 2004).
4.2. Growth-like factors and epithelial biology
Tears contain growth factors. New tear growth factors (Zhou et al., 2012) are granulin (GRN; also known as epithelin), hepatoma derived growth factor (HDGF) and platelet derived growth factor C (PDGFC). Precursor progranulin (88 kDa) is processed to granulin (epithelin) 1 and 2. 0.8 – 3 nM granulin 1 enhances colony formation by normal rat kidney cells in agar in a manner that is opposed by 83 nM granulin 2 (Plowman et al., 1992).
Intraocular injection of 36 μM hepatoma derived growth factor (~28 kDa) after ocular nerve excision in rats increases the survival of retinal ganglion cells - in part via PI3K-Akt and MAP kinase signaling (Hollander et al., 2012).
Platelet derived growth factor C is secreted in inactive form in the vitreous, where it is activated by plasmin and thought to be involved in proliferative vitreoretinopathy (Lei et al., 2008). Platelet derived growth factor C is mitogenic for fibroblasts over a dose range of ~0.08 – 0.8 nM (Li et al., 2000).
Other interesting epithelial effectors now identified in tears include: trefoil factor 3 (TFF3; 7 – 12 kDa), cystatin-M (CST6; ~16.5 kDa) and growth arrest specific 6 (GAS6; ~75 kDa). Trefoil factor 3 plays an important role in epithelial repair. It is upregulated in injured cornea, and promotes the healing of NaOH wounded mouse corneas over a 7 – 400 μM dose range (Paulsen et al., 2008).
Cystatin-M is a cysteine protease inhibitor. Mice lacking cystatin-M develop metaplasia and keratitis of cornea (Zeeuwen et al., 2010).
Recombinant growth arrest specific 6 promotes photoreceptor outer segment phagocytosis with a dose optimum of 100 nM over a biphasic dose response (Hall et al., 2001). Anti-growth arrest specific 6 antibodies inhibit phagocytosis by retinal pigment epithelial cells (Karl et al., 2008).
4.3. Inflammation
Several new proteins are immunosuppressive (peptidylprolyl isomerise B [PPIB, also known as cyclophilin B], galectin 9 [LGALS9]), or immunostimulatory (pre B cell colony enhancing factor [NAMPT; also known as visfatin], arginase [ARG1]). Peptidylprolyl isomerise B (~24 kDa) binds cyclosporine A with high affinity (Kd of 9.8 nM; Husi et al., 1994) to together inhibit the phosphatase calcineurin within cells (Arber et al., 1992). Calcineurin is activated by calcium, as a downstream mediator of calcium signaling. Tear peptidylprolyl isomerise B would be expected to interact with topical cyclosporine A.
Galectin 9 (~40 kDa) is an S-type lectin with affinity for β-galactosides. Galectin 9 (025 – 0.75 μM) binds HAVCR2 (Tim-3) via carbohydrate recognition domain residues R64 and R238 to promote the death of TH1-, but not TH2-, CD4+ and CD8+ T cells, since HAVCR2 is a TH1-specific cell surface protein. Galectin 9 is therefore involved in T cell suppression making it a potential therapeutic candidate for treatment of autoimmune diseases (Zhu et al., 2005).
Pre B cell colony enhancing factor (~56 kDa) is a proinflammatory adipokine. 9 – 45 nM pre B cell colony enhancing factor promotes the production of cytokines by CD14(+) monocytes (Moschen et al., 2007) and by rheumatoid arthritis synovial fibroblasts (Meier et al., 2012), including IL-6, TNF and IL1β.
Arginase (~35 kDa) is a manganese metalloenzyme that successfully competes for the substrate L-arginine with nitric oxide synthase, thereby reducing levels of immunoregulatory nitric oxide. Mice lacking arginase display little lipopolysaccharide induced uveitis (Zhang et al., 2009).
4.4. Innate defense
Tears contain the small leucine-rich keratan sulfate proteoglycan lumican (LUM; ~75 kDa), that is abundant in the cornea as a modulator of collagen fibril formation (Chakravarti et al., 1998), and deficient in macular corneal dystrophy in mature form (Hassell et al., 1980). P. aeruginosa infection in lumican null versus mice suggests that lumican contributes to the innate response and clearance of bacteria (Shao et al., 2013).
5. Conclusions
Advantage should be taken of tear proteins as potential biomarkers, drug targets, and biotherapeutics. Tear-based biotherapeutics have considerable potential, particularly with the relatively small number of tear proteins that appear to be selectively downregulated in dry eye. Rather than simply alleviating symptoms, causes of ocular surface diseases may be addressable. Lacritin- and lipocalin-1-based therapeutics offer a platform to initiate this approach. Newly identified members of the tear proteome expand our appreciation of the functional capacity of the thin, but functionally dynamic tear film.
Supplementary Material
Highlights.
We review lacritin and tear lipocalin, and update the extracellular tear proteome.
We propose that advantage be taken of tear proteins as potential biomarkers.
Tear proteins might also serve as drug targets or therapeutics in dry eye.
With such an approach, causes of ocular surface diseases may be addressable.
Acknowledgments
Grant information: NIH RO1EY013143, RO1EY018222 (GWL); SR/FT/LS-157/2012 (RK)
GWL is supported by R01 EY013143 and EY018222. RK is supported by SR/FT/LS-157/2012 (RK). The authors acknowlege the multi-institutional Lacritin Consortium for help with much of the lacritin work reviewed, particularly the development of lacritin and syndecan-1 constructs by Ron Raab and Robert McKown at James Madison University, the supply of human tears by Denise Ryan (Walter Reed Army Medical Center), animal studies by Pat Williams’ group (Eastern Virginia Medical School), and mechanistic studies by members of the Laurie lab.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abduragimov AR, Gasymov OK, Yusifov TN, Glasgow BJ. Functional cavity dimensions of tear lipocalin. Curr Eye Res. 2000;21:824–832. doi: 10.1076/ceyr.21.4.824.5551. [DOI] [PubMed] [Google Scholar]
- Albig AR, Neil JR, Schiemann WP. Fibulins 3 and 5 antagonize tumor angiogenesis in vivo. Cancer Res. 2006;66:2621–2629. doi: 10.1158/0008-5472.CAN-04-4096. [DOI] [PubMed] [Google Scholar]
- Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, Albuquerque RJ, Richter E, Sakurai E, Newcomb MT, Kleinman ME, Caldwell RB, Lin Q, Ogura Y, Orecchia A, Samuelson DA, Agnew DW, St Leger J, Green WR, Mahasreshti PJ, Curiel DT, Kwan D, Marsh H, Ikeda S, Leiper LJ, Collinson JM, Bogdanovich S, Khurana TS, Shibuya M, Baldwin ME, Ferrara N, Gerber HP, De Falco S, Witta J, Baffi JZ, Raisler BJ, Ambati J. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthi S, Santhosh RS, Nila MV, Prajna NV, Lalitha P, Dharmalingam K. Comparative proteomics of human male and female tears by two-dimensional electrophoresis. Exp Eye Res. 2011;92:454–463. doi: 10.1016/j.exer.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Ananthi S, Venkatesh Prajna N, Lalitha P, Valarnila M, Dharmalingam K. Pathogen induced changes in the protein profile of human tears from Fusarium keratitis patients. PLoS One. 2013 doi: 10.1371/journal.pone.0053018. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber S, Krause KH, Caroni P. s-cyclophilin is retained intracellularly via a unique COOH-terminal sequence and colocalizes with the calcium storage protein calreticulin. J Cell Biol. 1992;116:113–125. doi: 10.1083/jcb.116.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckner ME, Jagannathan S, Peterson VA. Extracellular angio-associated migratory cell protein plays a positive role in angiogenesis and is regulated by astrocytes in coculture. Microvasc Res. 2002;63:259–269. doi: 10.1006/mvre.2001.2384. [DOI] [PubMed] [Google Scholar]
- Boehm N, Funke S, Wiegand M, Wehrwein N, Pfeiffer N, Grus FH. Alterations in the tear proteome of dry-eye patients - a matter of the clinical phenotype. Invest Ophthalmol Vis Sci. 2013 doi: 10.1167/iovs.11-8751. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Bonavida B, Sapse AT, Sercarz EE. Specific tear prealbumin: a unique lachrymal protein absent from serum and other secretions. Nature. 1969;221:375–376. doi: 10.1038/221375a0. [DOI] [PubMed] [Google Scholar]
- Breustedt DA, Korndörfer IP, Redl B, Skerra A. The 1.8-A crystal structure of human tear lipocalin reveals an extended branched cavity with capacity for multiple ligands. J Biol Chem. 2005;280:484–493. doi: 10.1074/jbc.M410466200. [DOI] [PubMed] [Google Scholar]
- Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, Carroll H. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Cell Biol. 1998;141:1277–1286. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Glass JD, Walton SC, Laurie GW. Role of laminin-1, collagen IV, and an autocrine factor(s) in regulated secretion by lacrimal acinar cells. Am J Physiol. 1998;275:278–284. doi: 10.1152/ajpcell.1998.275.1.C278. [DOI] [PubMed] [Google Scholar]
- Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009;28:155–177. doi: 10.1016/j.preteyeres.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartt DA. Tear lipocalin: structure and function. Ocul Surf. 2011;9:126–138. doi: 10.1016/s1542-0124(11)70022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza GA, Godoy LM, Mann M. Identification of 491 proteins in the tear fluid proteome reveals a large number of proteases and protease inhibitors. Genome Biol. 2006;7:R72. doi: 10.1186/gb-2006-7-8-r72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson OF, Feeney L, McEwen WK. Filter-paper electrophoresis of tears. II. Animal tears and the presence of a slow-moving lysozyme. AMA Arch Ophthalmol. 1956;55:800–806. [PubMed] [Google Scholar]
- Eylan E, Ronen D, Romano A, Smetana O. Lysozyme tear level in patients with herpes simplex virus eye infection. Invest Ophthalmol Vis Sci. 1977;16:850–853. [PubMed] [Google Scholar]
- Fleming A, Allison VD. On specificity of the protein of human tears. Brit J Exp Path. 1925;6:87–90. [Google Scholar]
- Fluckinger M, Haas H, Merschak P, Glasgow BJ, Redl B. Human tear lipocalin exhibits antimicrobial activity by scavenging microbial siderophores. Antimicrob Agents Chemother. 2004;48:3367–3372. doi: 10.1128/AAC.48.9.3367-3372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluckinger M, Merschak P, Hermann M, Haertlé T, Redl B. Lipocalin- interacting-membrane-receptor (LIMR) mediates cellular internalization of beta-lactoglobulin. Biochim Biophys Acta. 2008;1778:342–347. doi: 10.1016/j.bbamem.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Fujii A, Morimoto-Tochigi A, Walkup RD, Shearer TR, Azuma M. Lacritin-induced secretion of tear proteins from cultured monkey lacrimal acinar cells. Invest Ophthalmol Vis Sci. 2013;54:2533–2540. doi: 10.1167/iovs.12-10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullard RJ, Kissner DM. Purification of the isoforms of tear specific prealbumin. Curr Eye Res. 1991;10:613–628. doi: 10.3109/02713689109013853. [DOI] [PubMed] [Google Scholar]
- Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Interaction of tear lipocalin with lysozyme and lactoferrin. Biochem Biophys Res Commun. 1999;265:322–325. doi: 10.1006/bbrc.1999.1668. [DOI] [PubMed] [Google Scholar]
- Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Site-directed tryptophan fluorescence reveals the solution structure of tear lipocalin: evidence for features that confer promiscuity in ligand binding. Biochem. 2001;40:14754–14762. doi: 10.1021/bi0110342. [DOI] [PubMed] [Google Scholar]
- Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Binding studies of tear lipocalin: the role of the conserved tryptophan in maintaining structure, stability and ligand affinity. Biochim Biophys Acta. 1999;1433:307–320. doi: 10.1016/s0167-4838(99)00133-8. [DOI] [PubMed] [Google Scholar]
- Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Relaxation of beta-structure in tear lipocalin and enhancement of retinoid binding. Invest Ophthalmol Vis Sci. 2002;43:3165–3173. [PubMed] [Google Scholar]
- Glasgow BJ, Abduragimov AR, Farahbakhsh ZT, Faull KF, Hubbell WL. Tear lipocalins bind a broad array of lipid ligands. Curr Eye Res. 1995;14:363–372. doi: 10.3109/02713689508999934. [DOI] [PubMed] [Google Scholar]
- Glasgow BJ, Gasymov OK. Focus on molecules: tear lipocalin. Exp Eye Res. 2011;92:242–243. doi: 10.1016/j.exer.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green-Church KB, Nichols JJ. Mass spectrometry-based proteomic analyses of contact lens deposition. Mol Vis. 2008;14:291–297. [PMC free article] [PubMed] [Google Scholar]
- Green-Church KB, Nichols KK, Kleinholz NM, Zhang L, Nichols JJ. Investigation of the human tear film proteome using multiple proteomic approaches. Mol Vis. 2008;14:456–470. [PMC free article] [PubMed] [Google Scholar]
- Hall MO, Prieto AL, Obin MS, Abrams TA, Burgess BL, Heeb MJ, Agnew BJ. Outer segment phagocytosis by cultured retinal pigment epithelial cells requires Gas6. Exp Eye Res. 2001;73:509–520. doi: 10.1006/exer.2001.1062. [DOI] [PubMed] [Google Scholar]
- Hassell JR, Newsome DA, Krachmer JH, Rodrigues MM. Macular corneal dystrophy: failure to synthesize a mature keratan sulfate proteoglycan. Proc Natl Acad Sci US A. 1980;77:3705–3709. doi: 10.1073/pnas.77.6.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heit JJ, Apelqvist AA, Gu X, Winslow MM, Neilson JR, Crabtree GR, Kim SK. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature. 2006;443:345–349. doi: 10.1038/nature05097. [DOI] [PubMed] [Google Scholar]
- Hollander A, D’Onofrio PM, Magharious MM, Lysko MD, Koeberle PD. Quantitative retinal protein analysis after optic nerve transection reveals a neuroprotective role for hepatoma-derived growth factor on injured retinal ganglion cells. Invest Ophthalmol Vis Sci. 2012;53:3973–3989. doi: 10.1167/iovs.11-8350. [DOI] [PubMed] [Google Scholar]
- Holzfeind P, Merschak P, Rogatsch H, Culig Z, Feichtinger H, Klocker H, Redl B. Expression of the gene for tear lipocalin/von Ebner’s gland protein in human prostate. FEBS Lett. 1996;395:95–98. doi: 10.1016/0014-5793(96)01008-3. [DOI] [PubMed] [Google Scholar]
- Husi H, Zurini MG. Comparative binding studies of cyclophilins to cyclosporin A and derivatives by fluorescence measurements. Anal Biochem. 1994;222:251–255. doi: 10.1006/abio.1994.1481. [DOI] [PubMed] [Google Scholar]
- Josephson AS, Lockwood DW. Immunoelectrophoretic studies of the protein components of normal tears. J Immunol. 1964;93:532–539. [PubMed] [Google Scholar]
- Karl MO, Kroeger W, Wimmers S, Milenkovic VM, Valtink M, Engelmann K, Strauss O. Endogenous Gas6 and Ca2+-channel activation modulate phagocytosis by retinal pigment epithelium. Cell Signal. 2008;20:1159–1168. doi: 10.1016/j.cellsig.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. 2008;222:206–221. doi: 10.1111/j.1600-065X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- Klenotic PA, Munier FL, Marmorstein LY, Anand-Apte B. Tissue inhibitor of metalloproteinases-3 (TIMP-3) is a binding partner of epithelial growth factor-containing fibulin-like extracellular matrix protein 1 (EFEMP1). Implications for macular degenerations. J Biol Chem. 2004;279:30469–30473. doi: 10.1074/jbc.M403026200. [DOI] [PubMed] [Google Scholar]
- Kokenyesi R, Bernfield M. Core protein structure and sequence determine the site and presence of heparan sulfate and chondroitin sulfate on syndecan-1. J Biol Chem. 1994;269:12304–12309. [PubMed] [Google Scholar]
- Koo BS, Lee DY, Ha HS, Kim JC, Kim CW. Comparative analysis of the tear protein expression in blepharitis patients using two-dimensional electrophoresis. J Proteome Res. 2005;4:719–724. doi: 10.1021/pr0498133. [DOI] [PubMed] [Google Scholar]
- Kumar R, Huebner A, Laurie GW. Genetic separation of the human lacritin gene (“LACRT”) and triple A (Allgrove) syndrome on 12q13. Adv Exp Med Biol. 2002;506:167–174. doi: 10.1007/978-1-4615-0717-8_22. [DOI] [PubMed] [Google Scholar]
- Laurie DE, Splan RK, Green K, Still KM, McKown RL, Laurie GW. Detection of prosecretory mitogen lacritin in nonprimate tears primarily as a C-terminal-like fragment. Invest Ophthalmol Vis Sci. 2012;53:6130–6136. doi: 10.1167/iovs.11-8567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie GW, Olsakovsky LA, Conway BP, McKown RL, Kitagawa K, Nichols JJ. Dry eye and designer ophthalmics. OptomVis Sci. 2008;85:643–652. doi: 10.1097/OPX.0b013e318181ae73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H, Velez G, Hovland P, Hirose T, Kazlauskas A. Plasmin is the major protease responsible for processing PDGF-C in the vitreous of patients with proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2008;49:42–48. doi: 10.1167/iovs.07-0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Z, Beuerman RW, Chew AP, Koh SK, Cafaro TA, Urrets-Zavalia EA, Urrets-Zavalia JA, Li SF, Serra HM. Quantitative analysis of N-linked glycoproteins in tear fluid of climatic droplet keratopathy by glycopeptide capture and iTRAQ. J Proteome Res. 2009;8:1992–2003. doi: 10.1021/pr800962q. [DOI] [PubMed] [Google Scholar]
- Li X, Pontén A, Aase K, Karlsson L, Abramsson A, Uutela M, Bäckström G, Hellström M, Boström H, Li H, Soriano P, Betsholtz C, Heldin CH, Alitalo K, Ostman A, Eriksson U. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat Cell Biol. 2000;2:302–309. doi: 10.1038/35010579. [DOI] [PubMed] [Google Scholar]
- Lin PY, Cheng CY, Hsu WM, Tsai SY, Lin MW, Liu JH, Chou P. Association between symptoms and signs of dry eye among an elderly Chinese population in Taiwan: the Shihpai Eye Study. Invest Ophthalmol Vis Sci. 2005;46:1593–1598. doi: 10.1167/iovs.04-0864. [DOI] [PubMed] [Google Scholar]
- Ma P, Beck SL, Raab RW, McKown RL, Coffman GL, Utani A, Chirico WJ, Rapraeger AC, Laurie GW. Heparanase deglycanation of syndecan-1 is required for binding of the epithelial-restricted prosecretory mitogen lacritin. J Cell Biol. 2006;174:1097–1106. doi: 10.1083/jcb.200511134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009;28:1023–1027. doi: 10.1097/ICO.0b013e3181a16578. [DOI] [PubMed] [Google Scholar]
- Mathers WD. Ocular evaporation in meibomian gland dysfunction and dry eye. Ophthalmol. 1993;100:347–351. doi: 10.1016/s0161-6420(93)31643-x. [DOI] [PubMed] [Google Scholar]
- McKown RL, Raab RW, Kachelries P, Caldwell S, Laurie GW. Conserved Regional 3’ Grouping of Rare Codons in the Coding Sequence of Ocular Prosecretory Mitogen Lacritin. Invest Ophthalmol Vis Sci. 2013;54:1979–1987. doi: 10.1167/iovs.12-10740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKown RL, Wang N, Raab RW, Karnati R, Zhang Y, Williams PB, Laurie GW. Lacritin and other new proteins of the lacrimal functional unit. Exp Eye Res. 2009;88:848–858. doi: 10.1016/j.exer.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier FM, Frommer KW, Peters MA, Brentano F, Lefèvre S, Schröder D, Kyburz D, Steinmeyer J, Rehart S, Gay S, Müller-Ladner U, Neumann E. Visfatin/pre-B-cell colony-enhancing factor (PBEF), a proinflammatory and cell motility-changing factor in rheumatoid arthritis. J Biol Chem. 2012;287:28378–28385. doi: 10.1074/jbc.M111.312884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar TJ, Mudgil P, Butovich IA, Palaniappan CK. Adsorption of human tear lipocalin to human meibomian lipid films. Invest Ophthalmol Vis Sci. 2008;50:140–151. doi: 10.1167/iovs.08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montés-Micó R. Role of the tear film in the optical quality of the human eye. J Cataract Refract Surg. 2007;33:1631–1635. doi: 10.1016/j.jcrs.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Morimoto-Tochigi A, Walkup RD, Nakajima E, Shearer TR, Azuma M. Mechanism for carbachol-induced secretion of lacritin in cultured monkey lacrimal acinar cells. Invest Ophthalmol Vis Sci. 2010;51:4395–4406. doi: 10.1167/iovs.09-4573. [DOI] [PubMed] [Google Scholar]
- Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, Tilg H. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178:1748–1758. doi: 10.4049/jimmunol.178.3.1748. [DOI] [PubMed] [Google Scholar]
- Müller LJ, Pels L, Vrensen GF. Ultrastructural organization of human corneal nerves. Invest Ophthalmol Vis Sci. 1996;37:476–488. [PubMed] [Google Scholar]
- Na KS, Mok JW, Kim JY, Rho CR, Joo CK. Correlations between tear cytokines, chemokines, and soluble receptors and clinical severity of dry eye disease. Invest Ophthalmol Vis Sci. 2012;53:5443–5450. doi: 10.1167/iovs.11-9417. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Walkup RD, Tochigi A, Shearer TR, Azuma M. Establishment of an appropriate animal model for lacritin studies: cloning and characterization of lacritin in monkey eyes. Exp Eye Res. 2007;85:651–658. doi: 10.1016/j.exer.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Nichols JJ, Green-Church KB. Mass spectrometry-based proteomic analyses in contact lens-related dry eye. Cornea. 2009;28:1109–1117. doi: 10.1097/ICO.0b013e3181a2ad81. [DOI] [PubMed] [Google Scholar]
- Ozyildirim AM, Wistow GJ, Gao J, Wang J, Dickinson DP, Frierson HF, Jr, Laurie GW. The lacrimal gland transcriptome is an unusually rich source of rare and poorly characterized gene transcripts. Invest Ophthalmol Vis Sci. 2005;46:1572–1580. doi: 10.1167/iovs.04-1380. [DOI] [PubMed] [Google Scholar]
- Paulsen FP, Woon CW, Varoga D, Jansen A, Garreis F, Jager K, Amm M, Podolsky DK, Steven P, Barker NP, Sel S. Intestinal trefoil factor/TFF3 promotes re-epithelialization of corneal wounds. J Biol Chem. 2008;283:13418–13427. doi: 10.1074/jbc.M800177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertovaara M, Raitala A, Uusitalo H, Pukander J, Helin H, Oja SS, Hurme M. Mechanisms dependent on tryptophan catabolism regulate immune responses in primary Sjogren’s syndrome. Clin Exp Immunol. 2005;142:155–161. doi: 10.1111/j.1365-2249.2005.02889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervaiz S, Brew K. Homology and structure-function correlations between alpha 1-acid glycoprotein and serum retinol-binding protein and its relatives. FASEB J. 1987;1:209–214. doi: 10.1096/fasebj.1.3.3622999. [DOI] [PubMed] [Google Scholar]
- Pilch B, Mann M. Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biol. 2006;7:R40. doi: 10.1186/gb-2006-7-5-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowman GD, Green JM, Neubauer MG, Buckley SD, McDonald VL, Todaro GJ, Shoyab M. The epithelin precursor encodes two proteins with opposing activities on epithelial cell growth. J Biol Chem. 1992;267:13073–13078. [PubMed] [Google Scholar]
- Redl B, Holzfeind P, Lottspeich F. cDNA cloning and sequencing reveals human tear prealbumin to be a member of the lipophilic-ligand carrier protein superfamily. J Biol Chem. 1992;267:20282–20287. [PubMed] [Google Scholar]
- Redl B, Wojnar P, Ellemunter H, Feichtinger H. Identification of a lipocalin in mucosal glands of the human tracheobronchial tree and its enhanced secretion in cystic fibrosis. Lab Invest. 1998;78:1121–1129. [PubMed] [Google Scholar]
- Sack R, Conradi L, Beaton A, Sathe S, McNamara N, Leonardi A. Antibody array characterization of inflammatory mediators in allergic and normal tears in the open and closed eye environments. Exp Eye Res. 2007;85:528–538. doi: 10.1016/j.exer.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Sack RA, Conradi L, Krumholz D, Beaton A, Sathe S, Morris C. Membrane array characterization of 80 chemokines, cytokines, and growth factors in open- and closed-eye tears: angiogenin and other defense system constituents. Invest Ophthalmol Vis Sci. 2005;46:1228–1238. doi: 10.1167/iovs.04-0760. [DOI] [PubMed] [Google Scholar]
- Samudre S, Lattanzio FA, Jr, Lossen V, Hosseini A, Sheppard JD, Jr, McKown RL, Laurie GW, Williams PB. Lacritin, a novel human tear glycoprotein, promotes sustained basal tearing and is well tolerated. Invest Ophthalmol Vis Sci. 2011;52:6265–6270. doi: 10.1167/iovs.10-6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghi S, Kumar R, Lumsden A, Dickinson D, Klepeis V, Trinkaus-Randall V, Frierson HF, Jr, Laurie GW. cDNA and genomic cloning of lacritin, a novel secretion enhancing factor from the human lacrimal gland. J Mol Biol. 2001;310:127–139. doi: 10.1006/jmbi.2001.4748. [DOI] [PubMed] [Google Scholar]
- Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318–326. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- Schenk S, Schoenhals GJ, de Souza G, Mann M. A high confidence, manually validated human blood plasma protein reference set. BMC Med Genomics. 2008;15:1–41. doi: 10.1186/1755-8794-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert K, Gandia NC, Wilburn JK, Bower KS, Sia RK, Ryan DS, Deaton ML, Still KM, Vassilev VC, Laurie GW, McKown RL. Tear lacritin levels by age, sex, and time of day in healthy adults. Invest Ophthalmol Vis Sci. 2012;53:6610–6616. doi: 10.1167/iovs.11-8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao H, Scott SG, Nakata C, Hamad AR, Chakravarti S. Extracellular Matrix Protein Lumican Promotes Clearance and Resolution of Pseudomonas aeruginosa Keratitis in a Mouse Model. PLoS One. 2013 doi: 10.1371/journal.pone.0054765. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenwald RD, Vidvauns S, Wurster DE, Barfknecht CF. The influence of tear proteins on the film stability of rabbit tear extracts. J Ocul Pharmacol Ther. 1998;14:15–29. doi: 10.1089/jop.1998.14.15. [DOI] [PubMed] [Google Scholar]
- Sen DK, Sarin GS. Immunoassay of tear lysozyme in conjunctival diseases. Br J Ophthalmol. 1982;66:732–735. doi: 10.1136/bjo.66.11.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaramamma T, Shivaji S, Rao GN. Effect of storage on protein concentration of tear samples. Curr Eye Res. 1998;17:1027–1035. doi: 10.1076/ceyr.17.10.1027.5241. [DOI] [PubMed] [Google Scholar]
- Sonawane S, Khanolkar V, Namavari A, Chaudhary S, Gandhi S, Tibrewal S, Jassim SH, Shaheen B, Hallak J, Horner JH, Newcomb M, Sarkar J, Jain S. Ocular surface extracellular DNA and nuclease activity imbalance: a new paradigm for inflammation in dry eye disease. Invest Ophthalmol Vis Sci. 2012;53:8253–8263. doi: 10.1167/iovs.12-10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria J, Durán JA, Etxebarria J, Merayo J, González N, Reigada R, García I, Acera A, Suárez T. Tear proteome and protein network analyses reveal a novel pentamarker panel for tear film characterization in dry eye and meibomian gland dysfunction. J Proteomics. 2013;78:94–112. doi: 10.1016/j.jprot.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Thangavelu M, Zhang L, Green KB, Nichols KK. iTRAQ quantitative proteomics in the analysis of tears in dry eye patients. Invest Ophthalmol Vis Sci. 2012;53:5052–5059. doi: 10.1167/iovs.11-9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PS, Evans JE, Green KM, Sullivan RM, Schaumberg DA, Richards SM, Dana MR, Sullivan DA. Proteomic analysis of human meibomian gland secretions. Br J Ophthalmol. 2006;90:372–377. doi: 10.1136/bjo.2005.080846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto S, Fujiwara K, Ikeguchi M. Fatty acids bound to recombinant tear lipocalin and their role in structural stabilization. J Biochem. 2009;146:343–350. doi: 10.1093/jb/mvp076. [DOI] [PubMed] [Google Scholar]
- Ubels JL, Gipson IK, Spurr-Michaud SJ, Tisdale AS, Van Dyken RE, Hatton MP. Gene expression in human accessory lacrimal glands of Wolfring. Invest Ophthalmol Vis Sci. 2012;53:6738–6747. doi: 10.1167/iovs.12-10750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van’t Hof W, Blankenvoorde MF, Veerman EC, Amerongen AV. The salivary lipocalin von Ebner’s gland protein is a cysteine proteinase inhibitor. J Biol Chem. 1997;272:1837–1841. doi: 10.1074/jbc.272.3.1837. [DOI] [PubMed] [Google Scholar]
- Velez VF, Romano JA, McKown RL, Green K, Zhang L, Raab RW, Ryan DS, Hutnik CM, Frierson HF, Jr, Laurie GW. Tissue Transglutaminase is a negative regulator of monomeric lacritin bioactivity. Invest Ophthalmol Vis Sci. 2013;54:2123–2132. doi: 10.1167/iovs.12-11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velos P, Cherry PM, Miller D. An improved method for measuring human tear lysozyme concentration. Arch Ophthalmol. 1985;103:31–33. doi: 10.1001/archopht.1985.01050010035012. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang N, Xie J, Walton SC, McKown RL, Raab RW, Ma P, Beck SL, Coffman GL, Hussaini IM, Laurie GW. Restricted epithelial proliferation by lacritin via PKCalpha-dependent NFAT and mTOR pathways. J Cell Biol. 2006;174:689–700. doi: 10.1083/jcb.200605140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Zimmerman K, Raab RW, McKown RL, Hutnik CM, Talla V, Tyler MF, Lee JK, Laurie GW. Lacritin rescues stressed epithelia via rapid FOXO3 associated autophagy that restores metabolism. J Biol Chem. 2013 doi: 10.1074/jbc.M112.436584. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigelt B, Bosma AJ, van ‘t Veer LJ. Expression of a novel lacrimal gland gene lacritin in human breast tissues. J Cancer Res Clin Onco. 2003;129:735–736. doi: 10.1007/s00432-003-0514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnar P, Lechner M, Redl B. Antisense down-regulation of lipocalin-interacting membrane receptor expression inhibits cellular internalization of lipocalin-1 in human NT2 cells. J Biol Chem. 2003;278:16209–16215. doi: 10.1074/jbc.M210922200. [DOI] [PubMed] [Google Scholar]
- Wojnar P, Dirnhofer S, Ladurner P, Berger P, Redl B. Human lipocalin-1, a physiological scavenger of lipophilic compounds, is produced by corticotrophs of the pituitary gland. J Histochem Cytochem. 2002;50:433–435. doi: 10.1177/002215540205000314. [DOI] [PubMed] [Google Scholar]
- Wojnar P, Lechner M, Merschak P, Redl B. Molecular cloning of a novel lipocalin-1 interacting human cell membrane receptor using phage display. J Biol Chem. 2001;276:20206–20212. doi: 10.1074/jbc.M101762200. [DOI] [PubMed] [Google Scholar]
- Yusifov TN, Abduragimov AR, Gasymov OK, Glasgow BJ. Endonuclease activity in lipocalins. Biochem J. 2000;347:815–819. [PMC free article] [PubMed] [Google Scholar]
- Yusifov TN, Abduragimov AR, Narsinh K, Gasymov OK, Glasgow BJ. Tear lipocalin is the major endonuclease in tears. Mol Vis. 2008;14:180–188. [PMC free article] [PubMed] [Google Scholar]
- Zeeuwen PL, van Vlijmen-Willems IM, Cheng T, Rodijk-Olthuis D, Hitomi K, Hara-Nishimura I, John S, Smyth N, Reinheckel T, Hendriks WJ, Schalkwijk J. The cystatin M/E-cathepsin L balance is essential for tissue homeostasis in epidermis, hair follicles, and cornea. FASEB J. 2010;24:3744–3755. doi: 10.1096/fj.10-155879. [DOI] [PubMed] [Google Scholar]
- Zhang W, Baban B, Rojas M, Tofigh S, Virmani SK, Patel C, Behzadian MA, Romero MJ, Caldwell RW, Caldwell RB. Arginase activity mediates retinal inflammation in endotoxin-induced uveitis. Am J Pathol. 2009;175:891–902. doi: 10.2353/ajpath.2009.081115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang N, Raab RW, McKown RL, Irwin JA, Kwon I, van Kuppevelt TH, Laurie GW. Targeting of heparanase-modified syndecan-1 by prosecretory mitogen lacritin requires conserved core GAGAL plus heparan and chondroitin sulfate as a novel hybrid binding site that enhances selectivity. J Biol Chem. 2013;288:12090–12101. doi: 10.1074/jbc.M112.422717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Yang J, Liao W, Liu X, Zhang H, Wang S, Wang D, Feng J, Yu L, Zhu WG. Cytosolic FOXO1 is essential for the induction of autophagy and tumour suppressor activity. Nat Cell Biol. 2010;12:665–675. doi: 10.1038/ncb2069. [DOI] [PubMed] [Google Scholar]
- Zhou L, Beuerman RW, Chan CM, Zhao SZ, Li XR, Yang H, Tong L, Liu S, Stern ME, Tan D. Identification of tear fluid biomarkers in dry eye syndrome using iTRAQ quantitative proteomics. J Proteome Res. 2009;8:4889–4905. doi: 10.1021/pr900686s. [DOI] [PubMed] [Google Scholar]
- Zhou L, Huang LQ, Beuerman RW, Grigg ME, Li SF, Chew FT, Ang L, Stern ME, Tan D. Proteomic analysis of human tears: defensin expression after ocular surface surgery. J Proteome Res. 2004;3:410–416. doi: 10.1021/pr034065n. [DOI] [PubMed] [Google Scholar]
- Zhou L, Zhao SZ, Koh SK, Chen L, Vaz C, Tanavde V, Li XR, Beuerman RW. In-depth analysis of the human tear proteome. J Proteomics. 2012;75:3877–3885. doi: 10.1016/j.jprot.2012.04.053. [DOI] [PubMed] [Google Scholar]
- Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


