Abstract
Sleep spindles are characteristic electroencephalographic waveforms that may play functionally significant roles in sleep-dependent memory consolidation, cortical development, and neuropsychiatric disorders. Circumstantial evidence has connected endogenous progesterone and its metabolites to the production of sleep spindles, however, the effects of exogenous progestins on sleep spindles have not been described in women. We examined differences in sleep spindle frequency and morphology in a clinical sample of women (n=21) referred for polysomnography taking depot medroxyprogesterone acetate (MPA), relative to a matched comparison group. Consistent with our hypotheses, women taking MPA demonstrated significantly higher sleep spindle density and maximal amplitude relative to comparison patients. Our results suggest that progestins potentiate the generation of sleep spindles, which may have significant implications for research that examines the role of these waveforms in learning, development, and mental illness.
Keywords: medroxyprogesterone, sleep spindles, progestin, progesterone
Introduction
Sleep spindles are characteristic electroencephalographic (EEG) waveforms generated during non-rapid eye movement (NREM) sleep. These waxing-waning oscillations are thought to promote sleep maintenance, as well as play functionally significant roles in sleep-dependent memory consolidation and cortical development (Steriade, 2003; Khazipov et al., 2004; Fogel and Smith, 2011). In addition, topographic decrements in sleep spindles in schizophrenia may be a biomarker for the disorder and reflect thalamic reticular and/or thalamocortical deficits (Ferrarelli et al., 2007; Ferrarelli et al., 2010). Thus, understanding the endogenous and exogenous factors that affect sleep spindle generation is important for a broad range of disciplines, including research related to sleep, cognitive function, plasticity, and neuropsychiatric disorders.
There are several circumstantial lines of evidence that suggest progesterone and its metabolites may affect the production of sleep spindles in women. First, high frequency EEG spectral power that includes the spindle range (approximately 11–16 Hz) is elevated in the luteal phase of the menstrual cycle (in both healthy women and those with severe premenstrual symptoms), during which progesterone is elevated relative to the follicular phase (Driver et al., 1996; Baker et al., 2007; Baker et al., 2012). Additionally, the frequency of sleep spindles increases from the follicular to luteal phase, suggesting modulation by neurohormonal changes across the menstrual cycle (Ishizuka et al., 1994). Second, rats exposed to exogenous progesterone demonstrate increased spectral power from 10 to 25 Hz, which encompasses spindle range activity (Lancel et al., 1996). Third, recent data from our laboratory demonstrates women with major depressive disorder (MDD) have increased sleep spindles relative to age and sex-matched controls (Plante et al., 2013), which could potentially be due to increased progesterone during both the follicular and luteal phase in women with MDD (Hardoy et al., 2006; Holsen et al., 2011). However, because neither increased sleep spindles (Goetz et al., 1983; Reynolds et al., 1985; de Maertelaer et al., 1987; Lopez et al., 2010) nor increased progesterone (Young et al., 2000; Girdler et al., 2012) in women with MDD has been a universal finding across studies, this link is speculative, particularly since levels of progesterone and sleep spindles have not been correlated within a single MDD cohort. Fourth, metabolites of progesterone are allosteric modulators at the GABA-A receptor, which plays a crucial role in spindle formation in the thalamic reticular nucleus, the anatomic site of sleep spindle generation (De Gennaro and Ferrara, 2003; Belelli and Lambert, 2005). However, given myriad other neurohormonal changes that are associated with the menstrual cycle (e.g. changes in estradiol) and mood disorders (e.g. changes in the hypothalamic-pituitary–adrenal axis) (Cutler and García, 1980; Pariante and Miller, 2001), lines of inquiry that assess more specifically the effects of progestins on sleep spindles are required to advance the hypothesis that these steroids enhance the production of sleep spindles in women. Thus, the primary aim of this study was to examine changes in sleep spindles associated with use of medroxyprogesterone acetate (MPA), a synthetic form of human progesterone. Our primary hypothesis was that MPA use would be associated with increased spindle density and morphology (e.g. amplitude, duration) during NREM sleep.
Methods
2.1. Subjects
All patients evaluated in this study were drawn from a clinical population of patients referred to a sleep disorders laboratory for polysomnography in accordance with a chart review protocol approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board. To identify individuals for inclusion, all diagnostic overnight polysomnograms performed at Wisconsin Sleep™ (the sleep laboratory associated with the University of Wisconsin School of Medicine and Public Health) from October 2, 2007 to March 1, 2013 were initially cross-referenced with MPA medication history in the electronic medical record (EpicCare, Epic Systems Incorporated, Verona WI). This list was further narrowed by removing patients with benzodiazepine and/or benzodiazepine receptor agonists in their medication history, due to known effects of these medications on sleep spindles (Suetsugi et al., 2001). Patients were excluded if they were on any other hormonal (estrogen and/or progestin) therapy besides MPA. Additional exclusion criteria based on information available in the medical record included the following: moderate or worse obstructive sleep apnea [apnea-hypopnea index (AHI) ≥15/hr], periodic limb movement disorder [periodic limb movement arousal index (PLMAI) ≥10/hr], total sleep time during sleep study <90 minutes, significant neurologic disorder (e.g. history of stroke), or psychotic disorder. To minimize risk of non-adherence or intermittent use of oral MPA influencing results, only patients taking depot (i.e. intramuscular) MPA at the time of their sleep study (i.e. injection within three months of study) were included.
Comparison patients were determined using a strategy to match subjects and minimize known potential confounders that might affect sleep spindles. For each MPA patient, all diagnostic polysomnograms performed in women not taking benzodiazepines or benzodiazepine receptor agonists who were within 3 years of age relative to the MPA patient were considered as potential matches. Patients with significant sleep disordered breathing, sleep-related movement disorder, neurologic, or psychotic disorder (using the above criteria), or use of any hormonal (including estrogen and/or progestin) therapy or contraceptive were excluded as potential matches. From this subset, further matching was performed based on presence or absence of concomitant antidepressant medication, to minimize potential confounders of depression and/or antidepressant therapy (Dotan et al., 2008; Plante et al., 2013). The final list of both MPA and comparison subjects was determined prior to visual inspection, processing, or analyses of the polysomnographic data.
2.2. EEG Recording and Analysis
All patients underwent routine overnight in-laboratory polysomnography (using identical amplifiers and other recording equipment) that utilized standard electroencephalogram (EEG), electrooculogram (EOG), sub-mental electromyogram (EMG), electrocardiogram (ECG), bilateral tibial EMG, respiratory inductance plethysmography, pulse oximetry, and a position sensor. A registered polysomnographic technologist scored sleep stages in 30 second epochs using Alice® Sleepware (Philips Respironics, Murrysville, PA) according to standard criteria (Iber et al., 2007). EEG signals were sampled at 200 Hz.
Data processing and analysis procedures were conducted using data from channel F3-A2. This derivation was selected a priori because prior investigations have utilized this channel for similar analyses (Lopez et al., 2010), and we did not have hypotheses regarding whether there would be between-group differences in slow (e.g. 11–13 Hz) or fast (e.g., 13–16 Hz) sleep spindles, which are more prominent in frontal and central regions, respectively (Schabus et al., 2007). F3 was judged to be the best channel to balance topographic differences in sleep spindles that vary depending on the frequency of the waveform because topographic data have suggested comparable spindle density of fast spindles in frontolateral (i.e. F3) and centrolateral (i.e. C3) channels (Ferrarelli et al., 2010; Plante et al., 2013), the density of fast sleep spindles decreases dramatically with lateral displacement from the midline, and the use of centrolateral EEG channels have missed pertinent between-group differences in fast sleep spindles (Ferrarelli et al., 2007).
Semi-automatic artifact rejection (performed blind to study group) was used to remove individual epochs with excessive artifact (e.g. muscle movement). To maintain congruence between spectral analysis and traditional sleep staging based on 30-second epochs, spectral analysis of NREM sleep was performed in consecutive 6-second epochs (Welch’s averaged modified periodogram with a Hamming window), resulting in a frequency resolution of 0.17 Hz, consistent with prior studies from our laboratory (Goldstein et al., 2012; Plante et al., 2012). Power spectra of artifact-free NREM epochs (stages N2 and N3) were compared for all 0.17 Hz frequency bins from 1 to 25 Hz between MPA and control groups. Significant between-group differences in EEG spectral power within the spindle range (approximately 11–16 Hz) were used to focus the spindle detection algorithm (Iber et al., 2007).
2.3. Spindle Range Activity and Detection
Spindle detection and analyses were performed similar to prior studies in our laboratory utilizing a customized spindle detection algorithm in MATLAB (MathWorks, Natick, MA) (Ferrarelli et al., 2007; Ferrarelli et al., 2010; Plante et al., 2013). NREM epochs were filtered within frequency bands for which between-group differences were observed, and rectified filtered signals were used as the time series. Spindle detection occurred when the mean signal amplitude exceeded an upper threshold (6 times the mean amplitude). The peak amplitude for each spindle was defined as the local maximum above the upper threshold. The beginning and end of the spindle occurred when the amplitude of the time series dropped below a lower threshold (two times the mean amplitude), occurring ≥0.25 seconds from the peak. Four spindle parameters were calculated for each patient: density (number of spindles detected divided by NREM sleep time), maximal amplitude (mean peak amplitude of all detected spindles), duration (mean duration of all detected spindles), and integrated spindle activity (ISA; integrated absolute amplitude values of each spindle divided by NREM sleep time) (Ferrarelli et al., 2010).
2.4. Statistics
Sleep architecture measures, spectral power, and spindle parameters were compared using unpaired two-tailed t-tests. Statistical analyses were performed using MATLAB. All values in the text are reported as mean±standard deviation.
Results
Twenty-one patients taking MPA who met inclusion/exclusion criteria were identified, along with 21 matched comparison patients. MPA and comparison groups demonstrated similar ages (range 16–54 years for MPA; 15–53 years for comparison patients) and body habitus (Table 1). Elevated body mass indices in both groups were consistent with obese populations frequently referred for polysomnography to evaluate for sleep-disordered breathing. In terms of medical co-morbidities, 1 MPA patient and 1 comparison patient had diabetes mellitus, 4 MPA patients and 1 comparison patient had hypertension, and two healthy comparison patients had treated hypothyroidism. There were no significant differences between groups in terms of sleep architecture variables, AHI, or PLMAI (Table 1).
Table 1.
Demographic, clinical, and polysomnographic data.
| MPA | CON | p* | |
|---|---|---|---|
| (N=21) | (N=21) | ||
| Age (years) | 34.1 (11.5) | 34.0 (11.8) | 0.98 |
| BMI (kg/m2) | 39.0 (9.5) | 37.0 (9.6) | 0.50 |
| TST (min) | 382.4 (99.8) | 415.6 (109.1) | 0.31 |
| WASO (min) | 73.8 (65.0) | 58.6 (39.6) | 0.36 |
| SE (%) | 79.2 (16.6) | 83.7 (9.0) | 0.28 |
| SOL (min) | 22.7 (29.6) | 19.6 (15.5) | 0.67 |
| N1 (%) | 11.8 (8.3) | 9.9 (8.4) | 0.48 |
| N2 (%) | 61.4 (10.8) | 62.2 (10.3) | 0.80 |
| N3 (%) | 12.1 (9.4) | 9.7 (7.3) | 0.36 |
| REM (%) | 14.7 (8.6) | 18.2 (7.8) | 0.18 |
| REML (min) | 137.9 (102.7) | 148.9 (85.9) | 0.71 |
| AHI (#/hr) | 3.3 (4.2) | 1.8 (3.0) | 0.19 |
| PLMAI (#/hr) | 3.3 (2.9) | 2.0 (1.6) | 0.09 |
MPA, (depot) medroxyprogesterone acetate; CON, control; BMI, body mass index (kg/m2), TST, total sleep time; WASO, wake after sleep onset; AI, arousal index; SE, sleep efficiency (TST/time in bed); SOL, sleep onset latency; N1/2/3, NREM stage 1/2/3 (% of TST); REM, stage REM (% of TST); REML, REM latency (time from sleep onset to first REM sleep epoch); AHI, apnea-hypopnea index; PLMAI, periodic limb movement arousal index.
Values are displayed as mean (standard deviation).
p-value derived using 2-tailed, independent samples t-tests.
Fifteen MPA patients and their matched comparison subjects were taking antidepressant medication(s) at the time of their sleep study, either alone or in combination. Among MPA patients, 9 were taking specific serotonin reuptake inhibitor/seritonin-norepinehprine reuptake inhibitors (SSRIs/SNRIs), 3 were taking tricyclic antidepressants (TCAs), and 5 were taking bupropion; among comparison patients, 14 were taking SSRIs/SNRIs, 2 were taking TCAs, and 2 were taking bupropion. Three MPA patients were also taking topirimate and one comparison subject was taking lamotrigine. These agents were not exclusionary because, to our knowledge, they have not been shown to alter sleep spindles. However, as antiepileptic drugs (AEDs), they could theoretically have effects on the GABA-A receptor and in turn, affect spindle activity (Greenfield, 2013). Otherwise, patients were not taking other CNS active agents known or theorized to potentially alter spindle activity.
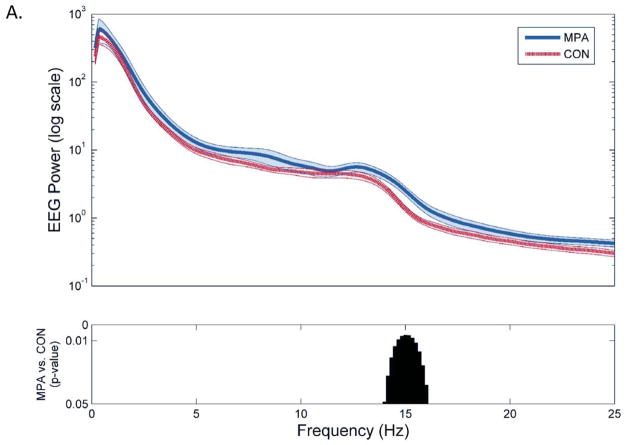
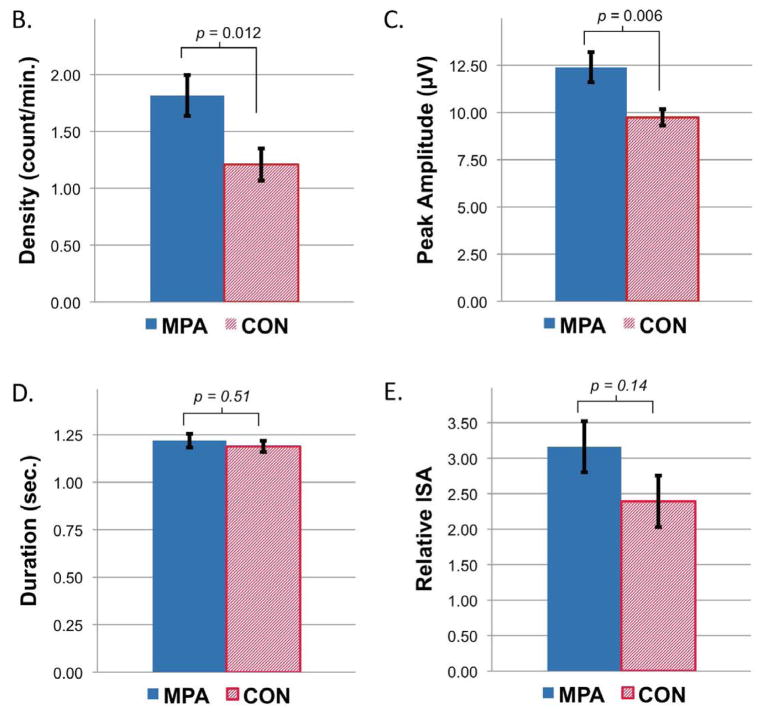
Semi-automated artifact rejection retained greater than 90% of NREM epochs in each group for analyses, and the percent of retained NREM epochs did not differ between groups (90.7±2.9% among MPA vs. 91.1±2.1% among comparison patients, p=0.57). Spectral analysis demonstrated significant increases in the high spindle range (14.0–16.0 Hz) in MPA relative to comparison groups, with no other significant differences in spectral power (Figure 1a). Spindle detection and analyses within this 14.0–16.0 Hz frequency range demonstrated significantly greater spindle density (1.82±0.83 vs. 1.21±0.65 counts/min, p=0.012) and peak amplitude (12.40±3.65 vs. 9.74±1.97 μV, p=0.006) among MPA relative to control groups, without significant differences in spindle duration (1.22±0.16 vs. 1.19±0.14 seconds, p=0.51) or ISA (3.16±1.66 vs. 2.39±1.66, p=0.14) (Figure 1b–e).
Figure 1.
Differences in sleep spindle parameters between women taking depot medroxyprogesterone acetate (n=21) and matched controls (n=21) from F3-A2. A) EEG spectral power averaged across NREM sleep and statistical group comparison of individual 0.17 Hz bins from 1 to 25 Hz, B) spindle density, C) peak amplitude, D) duration, and E) integrated spindle activity between MPA and control groups. All data plotted ± SEM.
Post hoc exploratory analyses using data extracted from C3-A2 demonstrated a similar increase in spectral power among MPA patients relative to controls that was isolated to a 15.0–16.5 Hz band, with no other significant differences in spectral power from 1–25 Hz (data not shown). Similar to data from F3-A2, MPA patients demonstrated increased spindle density (1.19±0.63 vs. 0.73±0.50 counts/min, p=0.012) and maximal amplitude (9.31±2.09 vs. 7.35±1.89 μV, p=0.003) within this frequency range. In addition, MPA patients demonstrated a significantly greater duration of sleep spindles (1.53±0.12 vs. 1.45±0.09 seconds, p=0.014) and a trend towards greater ISA (2.94±1.26 vs. 2.18±1.51, p=0.086) relative to comparison subjects.
Additional post hoc investigation in which patients taking non-exclusionary AEDs were removed from analyses did not alter statistical significance of any reported findings.
Discussion
Our findings demonstrate that use of depot medroxyprogesterone acetate (MPA) is associated with increases in spindle range activity, as well as sleep spindle density and maximal amplitude during NREM sleep in women. Our results corroborate prior circumstantial research that has suggested a role of progesterone in the production of sleep spindles, and provides more substantive evidence that progestins potentiate the generation of sleep spindles.
These results have important implications for research that examines the role of sleep spindles in several areas of neuroscientific inquiry, particularly sleep-dependent memory consolidation and psychotic illness. Recent work by Genzel and colleagues has demonstrated a sex and menstrual cycle effect on memory performance following naps in healthy subjects, with women demonstrating both improvement and increased spindle activity only during the mid-luteal phase of their menstrual cycle (Genzel et al., 2012). Intriguingly, estrogen levels correlated with offline change in declarative memory, and progesterone with motor learning, suggesting a complex relationship between sex steroids, sleep spindles, and type of memory consolidation in healthy women. Moreover, both animal models (Phillips et al., 2012) and patients with schizophrenia have demonstrated decreases in sleep spindles (Ferrarelli et al., 2007; Ferrarelli et al., 2010). Schizophrenia patients have also shown impairment of sleep-dependent memory consolidation of motor learning (Wamsley et al., 2012), as well as alterations of steroids in the progesterone metabolic pathway (Bicikova et al., 2013), suggesting further research that clarifies the relationships between these variables may be a fruitful avenue of research. The results of our study suggest that MPA, which has limited affinity for estrogen receptors and antiestrogenic activity (Schindler et al., 2008), may be a useful pharmacologic probe to help disentangle the complex, but important relationships between sleep-dependent memory consolidation, sleep spindles, and sex steroids, both in healthy subjects and patients with psychiatric disorders.
Prior investigations that have examined changes in spectral power in men administered exogenous progesterone have demonstrated increases in spectral power above 15 Hz isolated to the later portions of the night, without significant increases observed when averaged across all NREM sleep (Friess et al., 1997). Our findings of increased spindle range activity among women taking MPA, that were derived from all-night NREM data, suggest the effect of exogenous progestins on sleep spindles may be enhanced in women relative to men. Moreover, it is interesting that our results suggest changes in spectral power associated with MPA are isolated to the spindle range, and are not associated with higher frequency beta activity (e.g. >17 Hz). Thus, although speculative, increases in beta activity above the spindle range observed in prior studies that have compared the luteal to follicular phase of the menstrual cycle (Baker et al., 2007), may be due to other factors associated with transitions between menstrual phase, beyond changes in progesterone.
Although this study was not designed to determine the biological mechanism through which MPA enhances the production of sleep spindles, the observed effects are most likely to be mediated through interaction with GABA-A receptors in the thalamic reticular nucleus. Animal models have demonstrated MPA increases central allopregnanolone (Bernardi et al., 2006), which is also synthesized in the brain from endogenous progesterone by 5p-reductase type I and 3p-hydroxysteroid dehydrogenase, both of which are co-expressed in GABAergic reticular thalamic nuclei neurons (Agís-Balboa et al., 2006). Allopregnanolone is a potent allosteric modulator of GABA action at GABA-A receptors, which play a crucial role in the generation of sleep spindles in the reticular nucleus (De Gennaro and Ferrara, 2003; Belelli and Lambert, 2005). However, because MPA is also an agonist at both androgen and glucocorticoid receptors (although with substantially less affinity than progesterone receptors) (Schindler et al., 2008), it is possible that increases in sleep spindles observed in this study may be mediated by some other neurohormonal pathway. An alternative mechanism through which progesterone may potentiate sleep spindles is via increased temperature, since oral contraceptives (containing both estrogen and progestins) have been demonstrated to increase core body temperature (Baker et al., 2001), which may in turn, alter spindle range activity (Deboer, 1998). Because our study is not able to clarify the pathway through which MPA potentiates sleep spindles, further research is indicated to elucidate the direct mechanism through which MPA enhances sleep spindles in vivo.
There are limitations of this study that merit discussion. First, the number of available sleep studies that met inclusion/exclusion criteria determined sample size, and thus we may have had inadequate power to detect differences in spindle duration and/or ISA. Second, we do not know if comparison patients were in the follicular or luteal phase of their menstrual cycle, which may confound results and affect the magnitude of between-group differences. In the same vein, we do not have measurements of serum levels of progesterone or medroxyprogesterone, which would strengthen our results if these were to correlate with measures of spindle density and/or morphology. Also, primary analyses were performed using a single EEG channel, and thus further research that examines the topographic effects of MPA on sleep spindles across the cortex is warranted, particularly given differences in the regulation of slow and fast spindles (Schabus et al., 2007; Ayoub et al., 2013), and recent evidence that sleep spindles are localized phenomena (Nir et al., 2011). Additionally, despite careful matching of comparison patients and the use of depot MPA which limited potential confounds (medication non-adherence, age, etc.), because these data were derived from a clinical population, differences between groups may have been due to some other factor that was not controlled for in our study design. In particular, the use of antidepressant medication as a surrogate marker for depression has inherent limitations, and ideally, formal evaluation for the presence or absence of a personal (or family) history of a mood disorder, as well as assessment of active symptomatology at the time of the study, would have been preferred, but was not possible given the constraints and accuracy of available data. Similarly, we do not know menopausal status or the reasons patients were taking MPA, and thus, the conditions for which MPA was prescribed could theoretically underlie the observed differences between groups. Conversely, the fact that the hypothesized increases in sleep spindle density and peak amplitude were observed between potentially heterogeneous patient groups suggests that the effects of MPA may be quite robust, since statistical significance was evident despite the increased variability inherent to real world patient populations. Finally, this study was retrospective rather than prospective, and thus future research employing placebo controlled randomized trials would be more suitable to demonstrate a causal relationship between MPA and increases in sleep spindles.
In conclusion, we have demonstrated depot medroxyprogesterone acetate is associated with increased sleep spindle density and maximal amplitude during NREM sleep in a clinical sample of women referred for overnight polysomnography. These findings provide evidence that progestins play a role in the potentiation of sleep spindles. Further research that explores the connections between progestins, cognitive functioning, neuropsychiatric disease, and sleep spindles is warranted to clarify the functional significance of these findings.
Acknowledgments
We thank Mr. Brian Hamm, who provided assistance with search queries of the electronic medical record, and Drs. Meredith Rumble and Ruth Benca, who provided oversight and maintenance of the IRB protocol under which this study was conducted. Dr. Plante is supported by NIMH (K23MH099234), the Brain and Behavior Research Foundation, and the American Sleep Medicine Foundation.
ROLE OF FUNDING SOURCE
No funding source played a role in the study design, data collection, analysis and interpretation of the data, and the decision to submit the paper for publication.
Footnotes
CONFLICTS OF INTEREST
Dr. Plante has owned stock in Pfizer and received royalties from Cambridge University Press.
Mr. Goldstein declares no conflicts of interest.
CONTRIBUTORS
Dr. Plante designed the study, performed chart reviews, managed literature searches and analyses, and wrote the first draft of the manuscript. Mr. Goldstein performed EEG processing and analyses, conducted statistical analyses, and designed figures/tables. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agís-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci USA. 2006;103 (39):14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub A, Aumann D, Hörschelmann A, Kouchekmanesch A, Paul P, Born J, Marshall L. Differential effects on fast and slow spindle activity, and the sleep slow oscillation in humans with carbamazepine and flunarizine to antagonize voltage-dependent na(+) and ca(2+) channel activity. Sleep. 2013;36 (6):905–911. doi: 10.5665/sleep.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker FC, Kahan TL, Trinder J, Colrain IM. Sleep quality and the sleep electroencephalogram in women with severe premenstrual syndrome. Sleep. 2007;30 (10):1283–1291. doi: 10.1093/sleep/30.10.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker FC, Mitchell D, Driver HS. Oral contraceptives alter sleep and raise body temperature in young women. Pflugers Arch. 2001;442 (5):729–737. doi: 10.1007/s004240100582. [DOI] [PubMed] [Google Scholar]
- Baker FC, Sassoon SA, Kahan T, Palaniappan L, Nicholas CL, Trinder J, Colrain IM. Perceived poor sleep quality in the absence of polysomnographic sleep disturbance in women with severe premenstrual syndrome. J Sleep Res. 2012;21 (5):535–545. doi: 10.1111/j.1365-2869.2012.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6 (7):565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Bernardi F, Pluchino N, Pieri M, Begliuomini S, Lenzi E, Puccetti S, Casarosa E, Luisi M, Genazzani AR. Progesterone and medroxyprogesterone acetate effects on central and peripheral allopregnanolone and beta-endorphin levels. Neuroendocrinology. 2006;83 (5–6):348–359. doi: 10.1159/000095400. [DOI] [PubMed] [Google Scholar]
- Bicikova M, Hill M, Ripova D, Mohr P, Hampl R. Determination of steroid metabolome as a possible tool for laboratory diagnosis of schizophrenia. J Steroid Biochem Mol Biol. 2013;133:77–83. doi: 10.1016/j.jsbmb.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Cutler WB, García CR. The psychoneuroendocrinology of the ovulatory cycle of woman: a review. Psychoneuroendocrinology. 1980;5 (2):89–111. doi: 10.1016/0306-4530(80)90013-x. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7(5):423–440. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- de Maertelaer V, Hoffman G, Lemaire M, Mendlewicz J. Sleep spindle activity changes in patients with affective disorders. Sleep. 1987;10 (5):443–451. doi: 10.1093/sleep/10.5.443. [DOI] [PubMed] [Google Scholar]
- Deboer T. Brain temperature dependent changes in the electroencephalogram power spectrum of humans and animals. J Sleep Res. 1998;7 (4):254–262. doi: 10.1046/j.1365-2869.1998.00125.x. [DOI] [PubMed] [Google Scholar]
- Dotan Y, Suraiya S, Pillar G. Sleep spindles in post traumatic stress disorder: significant importance of selective serotonin reuptake inhibitors. Harefuah. 2008;147 (10):763–767. 839–740. [PubMed] [Google Scholar]
- Driver HS, Dijk DJ, Werth E, Biedermann K, Borbely AA. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metabol. 1996;81 (2):728–735. doi: 10.1210/jcem.81.2.8636295. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, Watson A, Bria P, Tononi G. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164 (3):483–492. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Peterson MJ, Sarasso S, Riedner BA, Murphy MJ, Benca RM, Bria P, Kalin NH, Tononi G. Thalamic dysfunction in schizophrenia suggested by whole-night deficits in slow and fast spindles. Am J Psychiatry. 2010;167 (11):1339–1348. doi: 10.1176/appi.ajp.2010.09121731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel SM, Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011;35 (5):1154–1165. doi: 10.1016/j.neubiorev.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Friess E, Tagaya H, Trachsel L, Holsboer F, Rupprecht R. Progesterone-induced changes in sleep in male subjects. Am J Physiol. 1997;272 (5 Pt 1):E885–891. doi: 10.1152/ajpendo.1997.272.5.E885. [DOI] [PubMed] [Google Scholar]
- Genzel L, Kiefer T, Renner L, Wehrle R, Kluge M, Grözinger M, Steiger A, Dresler M. Sex and modulatory menstrual cycle effects on sleep related memory consolidation. Psychoneuroendocrinology. 2012;37 (7):987–998. doi: 10.1016/j.psyneuen.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Lindgren M, Porcu P, Rubinow DR, Johnson JL, Morrow AL. A history of depression in women is associated with an altered GABAergic neuroactive steroid profile. Psychoneuroendocrinology. 2012;37 (4):543–553. doi: 10.1016/j.psyneuen.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz RR, Goetz DM, Hanlon C, Davies M, Weitzman ED, Puig-Antich J. Spindle characteristics in prepubertal major depressives during an episode and after sustained recovery: a controlled study. Sleep. 1983;6 (4):369–375. doi: 10.1093/sleep/6.4.369. [DOI] [PubMed] [Google Scholar]
- Goldstein MR, Plante DT, Hulse BK, Sarasso S, Landsness EC, Tononi G, Benca RM. Overnight changes in waking auditory evoked potential amplitude reflect altered sleep homeostasis in major depression. Acta Psychiatr Scand. 2012;125 (6):468–477. doi: 10.1111/j.1600-0447.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield LJ. Molecular mechanisms of antiseizure drug activity at GABAA receptors. Seizure. 2013 doi: 10.1016/j.seizure.2013.04.015. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardoy MC, Serra M, Carta MG, Contu P, Pisu MG, Biggio G. Increased neuroactive steroid concentrations in women with bipolar disorder or major depressive disorder. J Clin Psychopharmacol. 2006;26 (4):379–384. doi: 10.1097/01.jcp.0000229483.52955.ec. [DOI] [PubMed] [Google Scholar]
- Holsen LM, Spaeth SB, Lee JH, Ogden LA, Klibanski A, Whitfield-Gabrieli S, Goldstein JM. Stress response circuitry hypoactivation related to hormonal dysfunction in women with major depression. J Affect Disord. 2011;131 (1–3):379–387. doi: 10.1016/j.jad.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. American Academy of Sleep Medicine; Westchester, Illinois: 2007. [Google Scholar]
- Ishizuka Y, Pollak CP, Shirakawa S, Kakuma T, Azumi K, Usui A, Shiraishi K, Fukuzawa H, Kariya T. Sleep spindle frequency changes during the menstrual cycle. J Sleep Res. 1994;3 (1):26–29. doi: 10.1111/j.1365-2869.1994.tb00100.x. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsaki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432 (7018):758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- Lancel M, Faulhaber J, Holsboer F, Rupprecht R. Progesterone induces changes in sleep comparable to those of agonistic GABAA receptor modulators. Am J Physiol. 1996;271 (4 Pt 1):E763–772. doi: 10.1152/ajpendo.1996.271.4.E763. [DOI] [PubMed] [Google Scholar]
- Lopez J, Hoffmann R, Armitage R. Reduced sleep spindle activity in early-onset and elevated risk for depression. J Am Acad Child Adol Psychiatry. 2010;49 (9):934–943. doi: 10.1016/j.jaac.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y, Staba RJ, Andrillon T, Vyazovskiy VV, Cirelli C, Fried I, Tononi G. Regional slow waves and spindles in human sleep. Neuron. 2011;70 (1):153–169. doi: 10.1016/j.neuron.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49 (5):391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Phillips KG, Bartsch U, McCarthy AP, Edgar DM, Tricklebank MD, Wafford KA, Jones MW. Decoupling of sleep-dependent cortical and hippocampal interactions in a neurodevelopmental model of schizophrenia. Neuron. 2012;76 (3):526–533. doi: 10.1016/j.neuron.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante DT, Goldstein MR, Landsness EC, Peterson MJ, Riedner BA, Ferrarelli F, Wanger T, Guokas JJ, Tononi G, Benca RM. Topographic and sex-related differences in sleep spindles in major depressive disorder: a high-density EEG investigation. J Affect Disord. 2013;146 (1):120–125. doi: 10.1016/j.jad.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante DT, Landsness EC, Peterson MJ, Goldstein MR, Riedner BA, Wanger T, Guokas JJ, Tononi G, Benca RM. Sex-related differences in sleep slow wave activity in major depressive disorder: a high-density EEG investigation. BMC Psychiatry. 2012;12:146. doi: 10.1186/1471-244X-12-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CF, 3rd, Kupfer DJ, Taska LS, Hoch CC, Spiker DG, Sewitch DE, Zimmer B, Marin RS, Nelson JP, Martin D. EEG sleep in elderly depressed, demented, and healthy subjects. Biol Psychiatry. 1985;20 (4):431–442. doi: 10.1016/0006-3223(85)90045-9. [DOI] [PubMed] [Google Scholar]
- Schabus M, Dang-Vu TT, Albouy G, Balteau E, Boly M, Carrier J, Darsaud A, Degueldre C, Desseilles M, Gais S, Phillips C, Rauchs G, Schnakers C, Sterpenich V, Vandewalle G, Luxen A, Maquet P. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc Natl Acad Sci USA. 2007;104 (32):13164–13169. doi: 10.1073/pnas.0703084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH. Classification and pharmacology of progestins. Maturitas. 2008;61 (1–2):171–180. doi: 10.1016/j.maturitas.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Steriade M. Neuronal Substrates of Sleep and Epilepsy. Cambridge University Press; Cambridge, United Kingdom: 2003. [Google Scholar]
- Suetsugi M, Mizuki Y, Ushijima I, Kobayashi T, Watanabe Y. The effects of diazepam on sleep spindles: a qualitative and quantitative analysis. Neuropsychobiology. 2001;43 (1):49–53. doi: 10.1159/000054865. [DOI] [PubMed] [Google Scholar]
- Wamsley EJ, Tucker MA, Shinn AK, Ono KE, McKinley SK, Ely AV, Goff DC, Stickgold R, Manoach DS. Reduced sleep spindles and spindle coherence in schizophrenia: mechanisms of impaired memory consolidation? Biol Psychiatry. 2012;71 (2):154–161. doi: 10.1016/j.biopsych.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Midgley AR, Carlson NE, Brown MB. Alteration in the hypothalamic-pituitary-ovarian axis in depressed women. Arch Gen Psychiatry. 2000;57 (12):1157–1162. doi: 10.1001/archpsyc.57.12.1157. [DOI] [PubMed] [Google Scholar]