Abstract
The targeting and organization of podocyte slit diaphragm proteins nephrin and neph1 is critical for development and maintenance of a functional glomerular filtration barrier. Myo1c is a non-muscle myosin motor protein that interacts directly with nephrin and neph1 and mediates their intracellular transport to the podocyte intercellular junction. Here we investigated the necessity of Myo1c in podocyte development using zebrafish as a model system. Immunofluorescence microscopy and in situ RNA hybridization analysis of zebrafish embryos showed that Myo1c is widely expressed in various tissues including the zebrafish glomerulus. Knockdown of the Myo1c gene in zebrafish using antisense morpholino derivatives resulted in an abnormal developmental phenotype that included pericardial edema and dilated renal tubules. Ultra-structural analysis of the glomerulus in Myo1c depleted zebrafish showed abnormal podocyte morphology and absence of the slit diaphragm. Consistent with these observations, the glomerular filter permeability appeared altered in zebrafish in which Myo1c expression was attenuated. The specificity of Myo1c knockdown was confirmed by a rescue experiment in which co-injection of Myo1c morpholino derivatives with orthologous Myo1c mRNA prepared from mouse cDNA lessened phenotypic abnormalities including edema in Myo1c morphants. Thus, our results demonstrate that Myo1c is necessary for podocyte morphogenesis.
INTRODUCTION
Myosins are actin-based molecular motors that participate in diverse cellular functions including maintenance of membrane tension, intracellular movement of secretory vesicles, (endocytosis and exocytosis) and promotion of cell adhesion and motility.1–5 The Myosin superfamily consists of more than 20 classes that are further subdivided into two major groups that include conventional and unconventional myosins.6, 7 The muscle myosins are mostly considered conventional, while all other myosins are referred as the unconventional myosins.7 The unconventional class I myosin family members include, Myo1a, b, c, d, e, f and g myosins that link the actin cytoskeleton with cell membranes.7, 8 These myosins associate with the actin-rich membrane structures including filopodia, lamellipodia and leading edges of the migrating cells,1, 9–11 and have been shown to be actively involved in membrane dynamics. Despite advances in the biochemical characterization of these myosins, their specific cellular functions remain poorly defined.
The primary structure of a myosin includes head or motor domain that binds ATP and can generate force, neck or regulatory domain that can bind calmodulin, and a C-terminal cargo binding domain.1, 12–14 Recent studies have documented the presence of several myosins in podocytes,15, 16 however, only the non-muscle myosin Myh9, and the unconventional myosins Myo1e and Myo1c have been shown to play a role in podocyte biology.16–20 In humans, genetic mutations in Myh9 are associated with the development of thrombocytopenia, nephritis and hearing loss; mutations in Myo1e are associated with childhood-onset glucocorticoid-resistant focal segmental glomerulosclerosis.17–19, 21–23 Myo1c mutations have been described in patients with hearing loss.24 While mutant mouse models of Myh9 and Myo1e demonstrate a kidney phenotype with altered glomerular filtration function,25, 26 the role of Myo1c in glomerular function is not known. This is primarily due to the lack of an appropriate Myo1c animal model. Although the Myo1c knockout mouse model has not been formally reported, construction of this mouse model was briefly discussed in a review by Gillespie et al, suggesting that these mice die at an early embryonic stage.27 This observation further highlights the need for an animal model system that provides insight into the physiological function of Myo1c.
The biochemical and functional analysis of Myo1c protein supports two prominent roles for Myo1c. First, studies in the stereocilia of hair cells suggest that it regulates the movement of hair cell adaptation complex.28, 29 Examination of Myo1c in adipocytes demonstrates its role in exocytosis of glucose transporter Glut4 containing vesicles.30, 31 A recent study describes the role of Myo1c in powering the asymmetric movement of actin filaments.32 Second, the role of Myo1c has been implicated in translocation of proteins and organelles to plasma membrane, and in regulating plasma membrane plasticity, cell motility and pathogen entry.5, 9, 16, 30, 31 We recently demonstrated that Myo1c is expressed in glomerular podocytes and localizes at their specialized intercellular junctions, commonly known as the “slit diaphragm”.16 We further demonstrated that in podocytes, Myo1c directly interacted with membrane proteins Nephrin and Neph1 and the depletion of Myo1c protein in cultured podocytes inhibited the localization of these slit diaphragm proteins at podocyte cell membrane.16 Since the localization of Nephrin and Neph1 at podocyte cell membrane is critical for podocyte development and maintenance,33, 34 it is likely that Myo1c dependent transport mechanisms play an important role in the organization and maintenance of the slit diaphragm. To further determine the in vivo significance of Myo1c in podocyte biology, we developed a zebrafish model system where Myo1c was selectively knocked down using specific morpholinos.
Zebrafish is a convenient model for examining the functional necessity of gene products in glomerular development and function. Zebrafish’s short life span, the transparency of zebrafish larvae, and its easy genetic manipulation contributes to this value. The morphological and functional analysis of zebrafish glomeruli suggests that it is similar to mammalian glomerulus.35, 36 In addition, injection of fluorescent dyes allows the study of filtration process in real time in live larvae.36, 37 Zebrafish are increasingly being utilized to study the function of various slit diaphragm proteins including Nephrin, Neph1 and podocin.37, 38 In these studies it has been shown that the depletion of either Nephrin, Neph1 or podocin proteins induced a renal phenotype with defects in glomerular filtration and organization of podocytes.37, 38 These changes are similar to the glomerular defects observed in mice with these same proteins selectively deleted from podocytes.39–43 Similarly, as shown in our study, the knockdown of Myo1c in zebrafish impaired glomerular permeability and altered podocyte morphology with loss of the slit diaphragm. These results strongly support the conclusion that Myo1c is an essential component of the slit diaphragm and is required for development of glomerular podocytes.
RESULTS
Myo1c is expressed in Zebrafish glomeruli
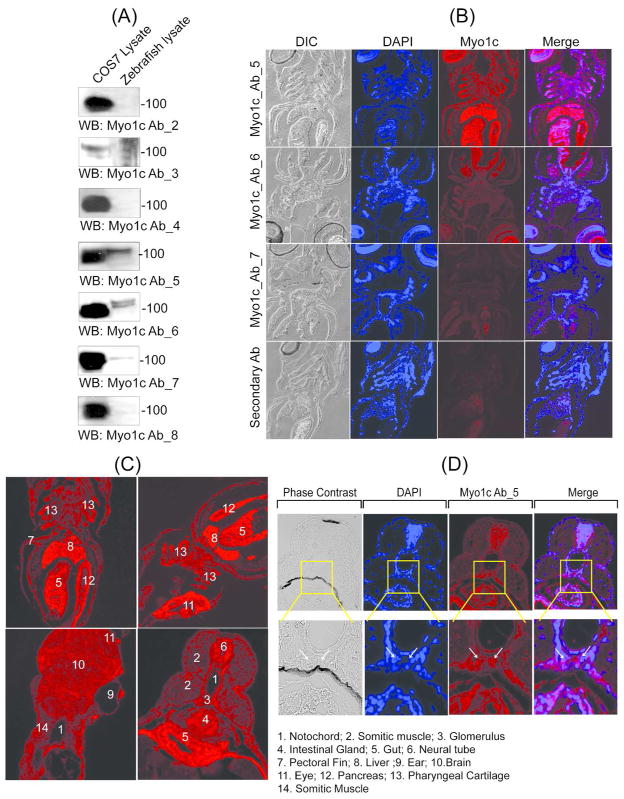
The expression of Myo1c has never been explored in zebrafish. To determine the expression of Myo1c in zebrafish, lysate was prepared from approximately 50 embryos obtained at 48hpf and examined for Myo1c protein expression using seven different Myo1c monoclonal antibodies raised against the N-terminal or the tail domain of Myo1c.11 Myo1c antibodies 2 and 3 were generated against the tail region of Myo1c, and monoclonal antibodies 4 through 8 were generated against the highly conserved motor/head domain of Myo1c. Among the 7 antibodies, only three antibodies (5, 6 and 7) detected Myo1c from the zebrafish lysate (figure 1A). These antibodies were used for immuno-histochemical analysis of zebrafish sections at 72hpf. Specific staining was observed only with the Myo1c 5 antibody (figure 1B). The specificity of this antibody was confirmed by performing an additional control experiment where Myo1c 5 antibody was pre-incubated with mouse Myo1c full length protein that completely blocked the staining (supplemental Figure 1). Zebrafish transverse and cross sections were stained with Myo1c 5 antibody and were analyzed by fluorescence microscopy. Staining analysis showed that Myo1c is expressed in the glomerular region (figure 1C) and an enlarged view of this region is presented in figure 1D, where arrows indicate the presumed position of glomerulus. In addition, the Myo1c staining was observed in various regions including somatic muscles, liver, gut, eye, intestinal gland, pancreas, pectoral fin, neural tube, and pharyngeal cartilages (figure 1C).
Figure 1. Myo1c is expressed in the Zebrafish glomerulus.
(A) The lysates from COS7 cells (control) and zebrafish embryos ( 48hpf) were analyzed by western blotting to test the specificity of seven different Myo1c monoclonal antibodies raised against the tail region (antibodies 2, 3 and 4) and the highly conserved motor/head domain of Myo1c (5, 6. 7 and 8). Among these, only three antibodies (5, 6 and 7) detected Myo1c in zebrafish lysate. (B) Further immuno-histochemical analysis of 72hpf zebrafish sections using Myo1c 5, 6 and 7 antibodies showed specific staining with only Myo1c 5 antibody. (C) The staining of zebrafish sections with Myo1c 5 antibody showed expression of Myo1c in various regions including glomerulus, somatic muscles, liver, gut, eye, intestinal gland, pancreas, pectoral fin, and pharyngeal cartilages. (D) An enlarged view of the glomerular region (arrows indicate glomerulus) co-stained with DAPI and the Myo1c 5 antibody is shown.
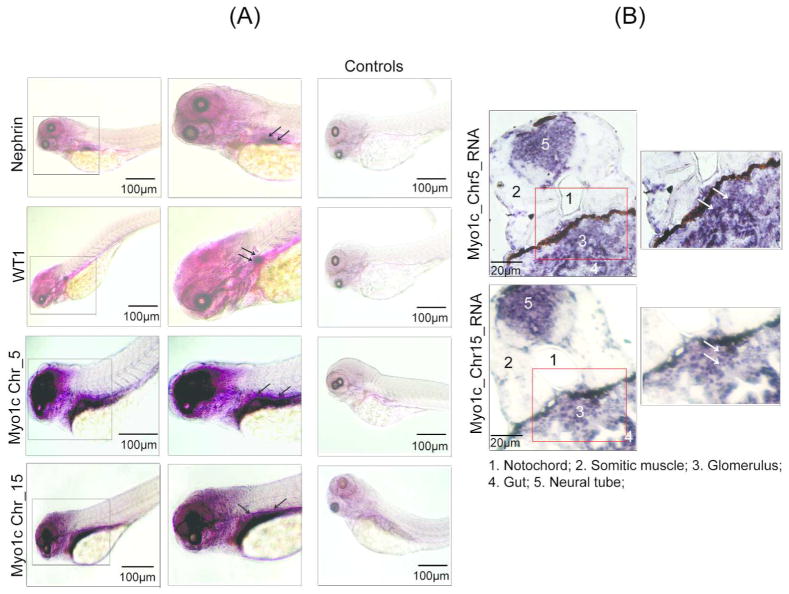
Analysis of the zebrafish genome indicates presence of two Myo1c homologs that are encoded by two separate genes localized on chromosomes 5 and 15. Nucleotide and protein sequences from both the Myo1c homologs display, respectively, 88% and 73% similarity. (data not shown). To determine the expression and specific localization of these Myo1c homologs, in situ hybridization was performed using 72hpf fish embryos with RNA probes specific for each Myo1c homolog (supplement figure 2). The expression pattern suggests that both homologs are expressed in the glomerular region (a later stage, representing fused glomerulus rather than a separated primordial glomerulus is shown) (figure 2A, supplemental figure 3A). The stained embryos were further sectioned and analyzed by light microscopy to confirm glomerular staining (figure 2A & supplement figure 3B). In addition, Myo1c expression was observed in other tissues including brain, the region surrounding eyes, gut, neural tube, pectoral fin, and somatic muscle (figure 2B, supplement figure 3B). Our further investigation remained focused on the zebrafish nephros. Staining of zebrafish glomerulus with Nephrin and WT1 probes was used as controls. Results presented in figures 2A are consistent with the published observations where expression of Nephrin and WT1 was specifically observed in zebrafish glomerulus.37, 44 Although Myo1c staining was visualized in the glomerular region, it was diffused in comparison to the Nephrin and WT1 staining, most likely due to staining of adjacent structures (figure 2A). Analysis of sections obtained from in situ RNA hybridization stained embryos further confirmed the expression of Myo1c in zebrafish glomerulus (figure 2B).
Figure 2. Both Myo1c homologs are expressed in zebrafish glomerulus.
(A) Whole mount in-situ hybridization of zebrafish embryos was performed using DIG-labeled probes against the two Myo1c homologs (Myo1c_Chr5 & 15). The arrows indicate glomerular region where each homolog is expressed. Nephrin and WT1 RNA probes were used as positive controls, whereas the sense RNA probes for each gene served as negative controls. (B) The stained embryos were sectioned and analyzed by light microscopy to confirm glomerular staining (arrows).
Myo1c knockdown induces edema in zebrafish embryos
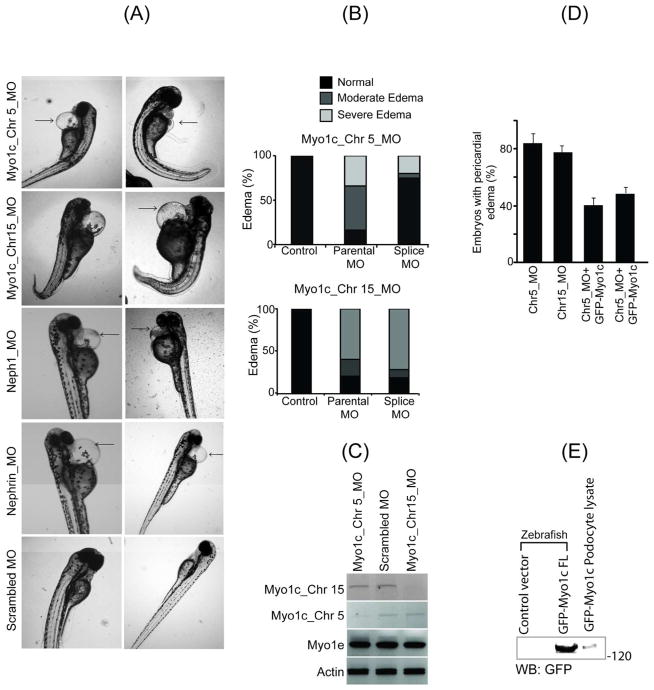
To investigate the function of Myo1c in zebrafish, Myo1c protein expression was knocked down using anti-sense morpholinos. Both the Myo1c homologs, Myo1c_Chr5_MO and Myo1c_CHr15_MO (Myo1c gene located on chromosome 5: Ensembl gene ID: ENSDARG00000061579 and Myo1c gene located on chromosome 15: Ensembl gene ID: ENSDARG00000020924, respectively) were knocked down individually. Morpholinos targeting the transcriptional initiation site (parental morpholinos) and splice targeting morpholinos (Splice morpholinos) were constructed and synthesized. The morpholinos that specifically knocked-down Nephrin and Neph1 proteins expression and control oligos were synthesized and used as positive and negative controls. Morpholinos were injected at one cell stage and phenotypic observations were recorded at 48hpf and 72hpf from three independent experiments. An optimal dose for each morpholino was selected that consistently produced a morphant survival rate of more than 80% (data not shown). Knockdown of both Myo1c homologs using either the parental or splice morpholinos (it is important to note that each Myo1c homolog was knocked down separately) showed varying but prominent embryonic pericardial edema and whole body edema (figure 3A and B). The edema was categorized as moderate (a prominent pericardial edema) and severe (whole body edema) as shown in supplemental figure 4A. Eighty-four percent and 25% of zebrafish larvae showed edema when treated with Myo1c_Chr5_MO parental and splice morpholinos, respectively, and 80% and 82% showed edema when treated with Myo1c_Chr15_MO parental and splice morpholinos, respectively (Figure 3A and B). Knockdown of both Myo1c homologs was confirmed by RT-PCR using cDNA derived from morphant embryos displaying moderate edema (figure 3C). Notably, the knockdown of one Myo1c homolog did not alter the expression of other homolog. Additionally, gene expression of another class I myosin family member motor protein Myo1e, remained unaffected (figure 3C).
Figure 3. Knockdown of zebrafish Myo1c by morpholino induces edema in zebrafish embryos.
(A) Knock down of both the Myo1c homologs (Myo1c_Chr5_MO and Myo1c_CHr15_MO) using specific morpholinos was analyzed at 72hpf and showed pericardial edema. Knockdowns of Nephrin and Neph1 in a similar fashion were used as positive controls, which also resulted in edemic phenotype. The scrambled morpholino was used as a negative control. (B) Morpholinos derived from the transcriptional initiation site (parental morpholinos) and the splice site (Splice morpholinos) were used in Myo1c knockdown experiments. Intensity of edema was categorized as moderate (prominent pericardial edema) and severe (whole body edema). The Myo1c_Chr5_MO knockdown showed 84% and 25% edema with parental and splice morpholinos respectively and Myo1c_Chr15_MO showed 80% and 82% edema with parental and splice morpholinos respectively. (C) Knockdown of both the Myo1c homologs was confirmed by RT-PCR. It is noted that specific knockdown of one homolog did not alter the expression of other homolog. Expression of β-Actin and another class I myosin family member motor protein Myo1e were also determined as controls. (D) Phenotypic rescue of Myo1c knockdown embryos using an orthologous mouse Myo1c mRNA. The capped mRNA from mouse Myo1c ortholog was prepared and co-injected with Myo1c morpholinos in one cell stage embryos and the phenotypic rescue was determined at 48–72hpf. Representative images from several independent experiments show that in addition to edema, the physical deformities are significantly reduced in rescued (Myo1c_Chr5/15_MO + GFP-Myo1c RNA) fish. The quantitative analysis of edematous phenotype in mutants and rescued embryos showed approximately 50% recovery. (E) The expression of mouse GFP-Myo1c protein in morphants lysate was confirmed by western blotting, using GFP monoclonal antibody.
The edematous phenotype in Myo1c knockdown embryo is rescued by over-expression of orthologous mouse Myo1c
To further determine if the phenotypic effects observed in Myo1c morphants are due to the specific knockdown of Myo1c, we attempted to rescue the observed pericardial edema through over-expression of mouse Myo1c ortholog in mutant embryos. Capped mRNA representing mouse Myo1c was prepared and co-injected with the Myo1c morpholinos into one cell stage embryos. Embryos were analyzed at 48hpf for the presence of edema. A quantitative analysis of the observed phenotype shows that approximately 80% of the embryos displayed an edematous phenotype when injected with either Myo1c_Chr5 or Myo1c_Chr15 morpholino (figure 3D and supplemental figure 4B). When these morpholinos were co-injected with the Myo1c ortholog, only 40% of morphants displayed edema (figure 3D and supplemental figure 4B), suggesting an incomplete penetrant rescue of the edematous phenotype. In addition to edema, morphant embryos appeared morphologically deformed and these deformities were mostly absent in mutant embryos co-expressing the Myo1c ortholog (supplemental figure 4B). Expression of mouse ortholog (GFP-Myo1c protein) was confirmed by western blot analysis of lysate obtained from 24hpf morphant embryos (figure 3E). These results suggest that knockdown of Myo1c induces severe morphological changes in zebrafish embryos that can be rescued by re-expressing a mouse Myo1c ortholog.
Myo1c knockdown induces glomerular phenotype
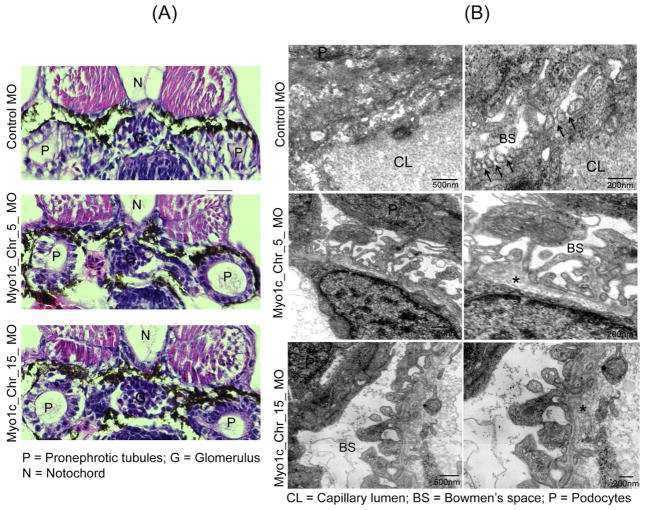
The effect of Myo1c knockdown on zebrafish kidney in 72hpf embryos was evaluated by histological and ultrastructural analyses using light microscopy and transmission electron microscopy (TEM) respectively. The histological analysis presented in figure 4A shows little alteration of the glomerulus but prominent dilation of pronephric tubules in the Myo1c_Chr5_MO and Myo1c_Chr15_MO knockdown fish. In addition, the microvilli of the proximal tubules of Myo1c morphants appeared abnormal. Further, consistent enlargement of the surrounding Bowman’s space and edema in the vicinity of gut region was also noted in Myo1c knockdown embryos but not in control embryos (figure 4A and supplemental figure 5). Ultrastructural analysis of control glomeruli showed well developed podocyte foot processes that appeared as beads on a string (figure 4B).37, 38 The magnified view demonstrates presence of the slit diaphragm observed as a thin membranous structure linking the foot processes (figure 4B arrows). In contrast, knockdown of both Myo1c homologs severely affected podocytes morphology with the absence of slit diaphragms (figure 4B). While control foot processes were regularly spaced, mutant embryo foot processes were irregularly shaped and lacked fine interdigitations (Figure 4B). In addition, thickening of the GBM (glomerular basement membrane) was also noted in knockdown embryos (marked by an asterisk in figure 4B. Collectively, these results demonstrate that Myo1c knockdown induces a glomerular phenotype.
Figure 4. Zebrafish Myo1c knockdown induces a renal phenotype.
(A) The histological analysis of 72hpf Myo1c_Chr5_MO and Myo1c_Chr15_MO knockdown embryo sections using Hematoxylin and Eosin (H&E) showed, little alterations of glomerulus but prominent dilation of the pronephric tubules. (B) Ultra-structural analysis of the 96hpf control and morphants glomeruli by TEM showed well developed foot processes in control that appeared as beads on a string, in contrast, knockdown of both the Myo1c homologs showed loss of fine interdigitations and altered foot processes with loss of slit diaphragm (indicated by arrows in the control). Additionally, thickening of GBM was also noted in the morphant embryos (indicated by asterisk).
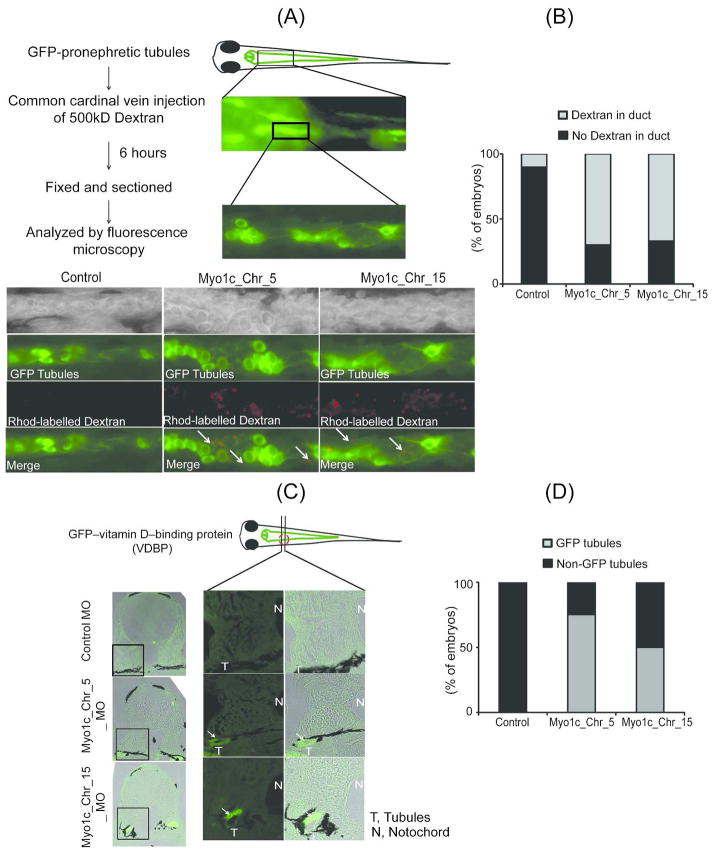
Loss of Myo1c results in an impaired glomerular filtration barrier
Two different transgenic fish lines were used to evaluate the glomerular filtration function in Myo1c morphants. In the first glomerular filtration assay model, a transgenic fish expressing eGFP specifically in the renal tubules using a NaK-ATPase alpha1A4 promoter was used.45 Rhodamine labeled dextran was injected into the control and Myo1c knockdown embryos through common cardinal vein at 96hpf when glomeruli are fully developed and functional.37, 38 Following the injection of 500kDa Rhodamine-labeled dextran, embryos were incubated in growth medium for 6hr, then anesthetized, fixed, sectioned and imaged by epifluorescence microscopy to evaluate the presence of Rhodamine in pronephric tubules. It is to noted that embryos displaying milder edematous phenotype were selected for this assay to ensure the optimal blood flow that is critical for this assay. Increased uptake of Rhodamine-labeled dextran was observed in the pronephric duct of Myo1c knockdown fish (figure 5A, supplement figure 6 arrows). In contrast, very little or no Rhodamine dextran was detected in the lumen of tubules from control fish. Quantitative analysis of these results (ten embryos from control and Myo1c_Chr5_MO knockdown, whereas nine from the Myo1c_Chr15_MO knockdown zebrafish were analyzed) suggests that more than 70% of the morphant embryos displayed a leaky phenotype when compared to controls (Figure 5B). In the second assay, we used a recently developed transgenic zebrafish expressing eGFP-tagged vitamin D-binding protein (VDBP) as a tracer for evaluating glomerular leakage or proteinuria.46 The eGFP-VDBP transgenic embryos were injected with Myo1c and control morpholinos and the embryos were fixed at 96hpf and cross sectioned to visualize leakage of eGFP-VDBP in the pronephric tubules (figure 5C). As shown in figure 5C and D, more than 50% of the embryos showed presence of GFP in the tubules whereas no GFP was observed in the controls (six fishes in each group were analyzed). The results from both transgenic models are consistent with our earlier histological observations and suggest that attenuation of Myo1c expression results in failure of podocyte development and impaired glomerular filter function.
Figure 5. Zebrafish Myo1c knockdown results in an impaired glomerular filtration barrier.
(A) The Myo1c knockdown and control embryos (96hpf) were generated on a transgenic background where renal tubules were marked by the expression of GFP and were injected with 500kD rhodamine labeled dextran though common cardinal vein. Presence of red dextran in the green tubules of coronally sectioned embryos is indicated by the arrows. (B) Quantification of the observed phenotype showed that dextran was detected in ~70% of morphant embryos, indicating an impaired glomerular function. (C) Transverse (rostral to caudal) sections of control and Myo1c knockdown embryos of GFP-VDBP transgenic zebrafish after 96hpf were analyzed that further confirm accumulation of GFP-VDBP in the pronephric tubules (indicated with arrows) of Myo1c knockdown embryos. (D) The quantitative analysis shows that GFP-VDBP was detected in the tubules of ~ 50% Morphant embryos.
DISCUSSION
This is the first study to investigate in vivo function of motor protein Myo1c in vertebrates. We found that Myo1c, an actin-based motor, is necessary for normal podocyte development in zebrafish. Actin dynamics plays a major role in defining changes in the podocyte morphology during development and in disease, when podocytes undergo remodeling to produce effacement, including alteration of podocyte intercellular junction with loss of the slit diaphragm. These changes were observed in diverse glomerular disorders including minimal change disease, congenital nephrotic syndrome and diabetic nephropathy.47–49 A number of podocyte proteins that are localized at the slit diaphragm including Nephrin and Neph1 regulate actin dynamics by assembling a signaling cascade at the podocyte intercellular junction.33, 34, 48 Recent reports including ours, have highlighted changes in the localization of these proteins--where they are displaced from the slit diaphragm--in response to various glomerular injuries.50–52 However, the mechanisms that mediate movement of slit diaphragm proteins are not understood. We recently demonstrated that Myo1c participates in determining the localization of Nephrin and Neph1 at podocyte cell membrane via direct interaction with these proteins.16 Because the role of Myo1c has been implicated in regulating actin dynamics,53, 54 and the intracellular movement of Nephrin and Neph1,16 it may not be surprising that its deletion results in failure of the development of a cell that is so dependent on actin dynamics in determining its structure.
Mutations in the Myo1c gene have been shown to associate with loss of hearing in humans.24 Since patients with renal disorders were excluded from this study 24, the analysis from this study is limited to hearing loss and therefore, even though Myo1c is a widely expressed motor protein, its significance in systems other than hearing remains known.24 In addition to Nephrin and Neph1, Myo1c is necessary for the translocation of glucose transporter Glut4, implicating its role in diabetes. However, many of the physiological roles of Myo1c still remain unclear,30, 31 largely because appropriate animal models where Myo1c is either completely or selectively deleted don’t exist. To determine the role of Myo1c in maintaining a functional glomerular filtration barrier, we used a rapidly developing zebrafish model system where Myo1c protein was depleted through morpholino injection. While loss of Myo1c in zebrafish larvae induces a glomerular developmental phenotype, other Myo1c related defects may exist.
The characterization of Myo1c expression in zebrafish embryos confirms its widespread expression in various tissues including somatic muscle, glomerulus, intestine, liver, brain, pancreas, pectoral fin, and pharyngeal cartilages. Since the head domain of Myo1c is highly conserved,11 it is not surprising that the Myo1c antibody (Myo1c ab_5) that reacts with head region recognized zebrafish Myo1c protein. However, this antibody cannot differentiate between various Myo1c isoforms or homologs. Analysis of the zebrafish genome identified two Myo1c homologs encoded by two separate genes, one located on chromosome 5 (Myo1c_chr5) and the other on chromosome 15 (Myo1c_chr15) that may result from a duplication event occurred during evolution.55 Specific staining of zebrafish embryos by in situ RNA hybridization, using DIG labeled probes for each Myo1c homolog, further demonstrated that Myo1c is expressed in glomeruli. However, unlike Nephrin and WT1 that are specifically localized in glomeruli, the distribution of Myo1c in surrounding tissues suggests a widespread expression of this protein. Analysis of serial sections through zebrafish glomeruli confirmed the presence of both Myo1c homologs in zebrafish glomerulus.
Phenotypic analysis of Myo1c knockdown embryos showed prominent pericardial and whole body edema that was more pronounced in embryos where maternal Myo1c signal was blocked. This observation is consistent with the disruption of glomerular filtration barrier as might be expected, given the interaction of Myo1c with Nephrin and Neph1, which have a well-defined role in regulating podocyte actin cytoskeletal dynamics.37, 38 Interestingly, the knockdown of another myosin Myh9 that was recently reported also showed a similar edemic phenotype in zebrafish.56 This analysis is supported by the observation that glomerular ultrastructure is severely damaged in these morphants.37, 38, 56 However, given the widespread expression of Neph1, Myo1c and Myh9, the role of alternate mechanisms contributing to the edematous phenotype cannot be ruled out. It is especially noteworthy that the co-expression of an ortholog of Myo1c, derived from mouse cDNA, rescued this phenotype. This further suggests that edematous phenotype observed in these zebrafish embryos is primarily due to the loss of Myo1c protein expression.
Every slit diaphragm protein that has been knocked down in zebrafish demonstrates severe alterations to the glomerular architecture associated with tubular dilation, podocyte effacement and loss of the slit diaphragm.37, 38 In agreement with these observations, histological analysis of Myo1c knockdown embryos also showed significant dilation of pronephric tubules (figure 5A); however, changes in the glomerular tuft were less obvious by light microscopy. Remarkably, dilation of renal proximal tubules was also observed in genetic knockout mouse models of Nephrin.39–41 It has been suggested that the loss of slit diaphragm proteins alters glomerular functionality leading to a leaky filter in these morphants, thus increasing luminal pressure resulting in tubular distension.37 In addition to tubular dilation, the ultrastructural analysis of Myo1c morphants also displayed foot process effacement, loss of fine digitations, thickening of GBM and increased volume of Bowman’s space, which is consistent with the knockdown of other slit diaphragm proteins.37, 56 Another morphological alteration, the microvillus-like protrusions in the Bowman’s space that is commonly observed in proteinuric condition in mammals 33, 57 was also visible in the Myo1c morphants (figure 4B). Similar changes in the podocyte morphology have been described with knockdown of non-muscle myosin Myh9 gene that is strongly associated with glomerular diseases.56 These morphological changes in podocytes were previously attributed to the loss of podocyte polarity in the Myh9 study,56 and, whether Myo1c depletion also affects podocyte polarity, will require further investigation. Since Nephrin and Neph1 have been shown to function as guide posts in other in vivo systems,40, 42, 51 it is likely that loss of Myo1c induces mislocalization of these slit diaphragm proteins, leading to abnormal development of podocytes in these morphants.
Recent advances in understanding the functional significance of slit diaphragm proteins in zebrafish model system have led to the development of an in vivo glomerular filter permeability assay, where a transgenic fish is used to determine the clearance of labeled dextran or BSA from renal system.37, 38 A similar assay using two different transgenic zebrafish lines expressing either eGFP in pronephric tubules,45 or eGFP-tagged vitamin D–binding protein (VDBP) as a tracer for proteinuria, 46 were used in this study to demonstrate the leakiness of glomerular filter in Myo1c morphants. Comparative analysis of control and mutant embryos in each transgenic model showed significant loss of glomerular filtration function in Myo1c morphants. This permeability assay further demonstrated that alterations in podocyte structure directly correlated with the loss of glomerular function in Myo1c morphants. Present findings are consistent with our previous in vitro study, where we demonstrated that loss of Myo1c in cultured podocytes decreased rate of cell migration and transepithelial resistance, with an increase in the permeability of BSA.16 Similar findings were recently reported in the mouse knockout model of another myosin family member Myo1e, where knockout of Myo1e, specifically in podocytes, induced a glomerular phenotype with altered glomerular filtration function.20 A similar approach, where Myo1c is selectively deleted from the mouse podocytes will further highlight the significance of Myo1c in podocyte development and function. In addition, this model will allow us to establish the role of Myo1c in regulating localization of slit diaphragm proteins during podocyte development and in response to glomerular injury. The development of this model is currently under progress.
Since many reports including ours now show the presence of multiple class I myosins in podocytes 57,15, it is plausible that one myosin may substitute for the loss of other myosin. However, biochemical studies suggest that these myosins contain distinct kinetic parameters that further suggests that they may regulate different cellular processes 58. The intracellular trafficking of podocyte proteins including Nephrin and Neph1 requires multiple steps ranging from internalization, partitioning into endosomes and lysosomes and then recycling at the membrane which may involve separate steps of docking and fusion at the membrane. Based on the published observations it is likely that Myo1c may play a role in the recycling process 5. In contrast, Myo1e literature indicates the role of Myo1e in endocytosis 59. Since trafficking involves various steps, it is possible that each step requires input from a different myosin to regulate the trafficking of podocyte proteins. However, understanding the trafficking mechanisms for podocyte proteins is still in its infancy and significant work needs to be done before a working hypothesis for the role of myosins in podocytes can be proposed. Nevertheless, results from this study strongly indicate that Myo1c participates in the development of podocytes and assembly of podocyte intercellular junction and is critical for glomerular filtration function.
METHODS
Zebrafish lines
Wild-type and transgenic Zebrafish embryos were obtained from Zebrafish core facility of the university, where they are maintained and bred for experimental purpose. Embryos were grown in egg medium (E3) at 28.5°C. EGFP Na+-K+-ATPase transgenic fish that were used in labeled dextran assays were provided by Dr. Michael Peck (director of the zebrafish core facility at University of Pennsylvania). The transgenic zebrafish expressing green fluorescent protein (GFP)–tagged vitamin D–binding protein (VDBP) was obtained from Dr. Weibin Zhou (University of Michigan Medical School). For in situ hybridization experiments, dechorionated embryos were grown in E3 solution containing 0.0045% PTU (1-Phenyl-2-thiourea, Sigma) after 10hpf, to suppress pigmentation. 60
Western Blotting
Zebrafish embryos were dechorionated using 10Pl pronase (30mg/ml) for 24hours. They were then anesthetized using 3% Tricane and transferred into 1.7ml micro-tubes, followed by addition of protein sample buffer (4.5 ml Distilled water, 1.0ml 0.5M Tris-HCl, pH 6.8, 0.8 ml Glycerol, 1.6ml 10% (w/v) Sodium Dodecyl Sulfate (SDS), 0.4ml β-Mercaptoethanol, 0.02% Bromphenol Blue and a cocktail of protease inhibitors (PI)) and manual homogenization. Approximately 50 embryos were used for preparing lysate for western blotting. Western blotting was performed using seven different Myo1c monoclonal antibodies that were all generated against purified Myo1c and react with either tail (antibodies 2 and 3) or the highly conserved head region of Myo1c (antibodies 4–8).11
Immuno-histochemistry
Dechorionated zebarfish embryos at 72 hpf were anesthetized, fixed with 4% paraformaldehyde, paraffin-embedded and sectioned (5μm). Indirect immunofluorescence was performed as previously described,16 with slight modifications. Antigen retrieval was performed by incubating the sections at 60°C overnight in Tris-EDTA buffer (pH 9.0). Staining with primary antibodies pre-incubated with the full length Myo1c protein and secondary antibody alone were used as negative controls. Sections were mounted with antifade reagent, containing 4′,6-diamidino-2-phenylindole (DAPI), and stored overnight in dark before microscopy was performed. Images were collected at 10X, 25X and 63X magnification on a Zeiss Inverted fluorescence Microscope.
In situ hybridization
Whole-mount in situ hybridization of embryos was performed as described,60 with slight modifications. The DIG-labeled RNA probes used in this procedure were prepared using DIG RNA Labeling Kit (SP6/T7) (Cat No. 11175025910). Briefly, mRNA was prepared from embryos using mRNA isolation kit (Qiagen) and was converted to cDNA using a reverse transcriptase kit (Invitrogen, Cat No. 11904 018). As described,60 the target cDNA was amplified using specific primers containing T7 promoter sequence (5′ TAATACGACTCACTATAGGG-3′) at the 5′ end. Primer sequences used for the synthesis of Nephrin, Myo1c, WT1 and control probes are described in supplement table 1. Purified PCR products were used to synthesize DIG-labeled RNA using DIG RNA labeling mix (UTP) (Roche, cat. no. 1277073) according to the manufacturer’s protocol. Labeling of RNA was determined using DIG-High Prime DNA Labeling and Detection Starter Kit II (11585614910). Further characterization of labeled RNA probes is provided in the supplemental figure 2. Hybridization of embryos with DIG labeled probes was performed as described.60 The labeled embryos and their sections were imaged using a dissecting scope fitted with a color camera.
Morpholino designing and injection
Genetic analysis of the zebrafish Myo1c gene suggests that two different isoforms of Myo1c are expressed that are localized on two separate genes [one on Chromosome 5 (Ensembl gene ID: ENSDARG00000061579) and the other on Chromosome 15 (Ensembl gene ID: ENSDARG00000020924)]. Morpholinos against both the gene products were designed from Gene Tools, LLC, Philomath, OR. Morpholinos targeted against the translational and splice blocking sites were designed and synthesized (stock concentration of 300nmol). Morpholino sequences for Myo1c chromosome 5 were GCG CTC TCC ATC ATT AAC CGT ACA C (parental) and GTA TGC ATC TTA CAT AAA TGA GGC C (splice), and for Myo1c chromosome 15 were TGG CTG TCA GGG CAC TCT CCA TCA T (parental) and CAT GGG TAA TAA AGA TGG CTT ACA T (splice). Standard negative control and scrambled morpholinos were also purchased from Gene-tool. In addition, the morpholinos targeted against Nephrin and Neph1 zebrafish proteins were also synthesized and used as positive controls. The Nephrin morpholino sequence has previously been described,37 and for Neph1 (Ensembl gene ID: ENSDART00000146804) knockdown the morpholino sequence used was GGC AGG TCA TGC TGA AGC CCA TCT C. Morpholinos were dissolved in sterile distilled water (1mM stock) and 1–2 nl of each morpholino was injected per embryo. For each morpholino, 100–200 one-stage cell embryos were injected and each experiment was repeated at least three times to obtain statistically relevant data. All experimental procedures were carried out at the Zebrafish core facility in accordance with the University of Pennsylvania guidelines.
Morpholino mediated knockdown was validated by RT-PCR using mRNA derived from 72hpf zebrafish embryos. Specific primers that were used for amplification of the target sequences are as follows: for Myo1c chromosome 5, forward 5′GAA AAC CTG CGC CGG CGG TAC3′ and reverse 5′TCT CCC AGC CTT CGG CCT CA3′ primers were used and for Myo1c chromosome 15, forward 5′TGA GAA CCT CCG CAA ACG C3′ and reverse 5′GTG CAC GAC GCG GGA TTT CT3′ primers were used. For controls, beta-Actin primers as described by Stoll et al,61 were used and Myo1e primers, 5′ CGG GGA CAC TAC CGG TAC CAC TG 3′ (forward) and 5′ TTG CCG TCC ATC TTT CGG CTG G 3′ (reverse) were used. The specificity of PCR products was also verified by DNA sequencing.
Phenotypic rescue using Myo1c ortholog
To determine the specificity of Myo1c knockdown, and to rescue the edemic phenotype, a recovery experiment was performed using mRNA derived from mouse ortholog of Myo1c. Following forward 5′ TAA TAC GAC TCA CTA TAG GGA CTA TGG AGA GCG CCT TGA CT 3′ and reverse 5′ TTA CTT GTA CAG CTC GTC CAT GCC GAG 3′ primers were used to amplify the mouse GFP-Myo1c full length cDNA, which was then used to synthesize capped mRNA using mMESSAGE mMACHINE® T7 Kit (Ambion, Cat No. AM1344). 100 ng of the purified mRNA was co-injected with morpholinos to induce recovery and the embryos were imaged at 48hpf.
Transmission electron microscopy (TEM)
96hpf zebrafish embryos were used for TEM. Embryos were fixed in a mixture of glutaraldehyde and 4% paraformaldehyde solution for overnight. Following fixation, embryos were sectioned and imaged at the Biomedical Imaging Core/Electron Microscopy Resource Laboratory of University of Pennsylvania.
Glomerular filtration assay
Two different zebrafish transgenic lines were employed. The transgenic zebrafish line described previously, 37, 38 in which GFP is specifically expressed in pronephric tubules at 24hpf, was used as the first model. The basic procedure has been described previously.37, 38 Briefly, 500kD dextran, labeled with Rhodamine (NANOCS, USA, Catalog number, DX500-RB-1) was injected into the common cardinal vein (CCV) of 96 hpf embryos, anesthetized with 3% Tricane (Sigma). Six hours post- injection, these embryos were fixed and sectioned. Only embryos with visible blood circulation (evaluated by sufficiently moving blood cells) were used to determine the uptake of Rhodamine labeled dextran by pronephric ducts using fluorescence microscopy. It should be noted that the fluorescently labeled dextran was dialyzed against PBS prior to its injection in the embryos to remove any low molecular weight contaminants. In the second model, zebrafish expressing eGFP–tagged vitamin D–binding protein (VDBP) were injected with Myo1c and control morpholinos. The embryos were fixed at 96hpf and cross sectioned and processed as described earlier 46. Accumulation of GFP-VDBP in the renal tubules was analyzed using fluorescence microscopy.
Supplementary Material
Acknowledgments
This work was supported in whole or in part by the NIH and NIDDK Grants, RO1 1R01DK087956 to D. N., DK080751 to L.B.H., the center grant P30 DK050306 to the center for molecular studies in digestive and liver diseases and ASN Carl W. Gottschalk fellowship to W. Z. The authors also thank the NephCure foundation (NCF) for the NephCure Postdoctoral Grant, 2012-RFP-001 to E. A. We thank zebrafish core facility of the university and technical assistance provided by Jie He with our zebrafish experiments. The technical support provided by Raymond Meade (technical Director of the Biomedical Imaging Core Facility of the University of Pennsylvania) for zebrafish EM studies is also acknowledged. Dr. Kawakami from National Institute of Genetics, Japan is duly acknowledged for the Tol2 system that was used in the construction of VDBP-EGFP transgenic zebrafish.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Barylko B, Jung G, Albanesi JP. Structure, function, and regulation of myosin 1C. Acta Biochim Pol. 2005;52:373–380. [PubMed] [Google Scholar]
- 2.Fath KR, Burgess DR. Golgi-derived vesicles from developing epithelial cells bind actin filaments and possess myosin-I as a cytoplasmically oriented peripheral membrane protein. J Cell Biol. 1993;120:117–127. doi: 10.1083/jcb.120.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heintzelman MB, Hasson T, Mooseker MS. Multiple unconventional myosin domains of the intestinal brush border cytoskeleton. J Cell Sci. 1994;107 ( Pt 12):3535–3543. doi: 10.1242/jcs.107.12.3535. [DOI] [PubMed] [Google Scholar]
- 4.McConnell RE, Tyska MJ. Leveraging the membrane - cytoskeleton interface with myosin-1. Trends Cell Biol. 2010;20:418–426. doi: 10.1016/j.tcb.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandstaetter H, Kendrick-Jones J, Buss F. Myo1c regulates lipid raft recycling to control cell spreading, migration and Salmonella invasion. J Cell Sci. 2012;125:1991–2003. doi: 10.1242/jcs.097212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foth BJ, Goedecke MC, Soldati D. New insights into myosin evolution and classification. Proc Natl Acad Sci U S A. 2006;103:3681–3686. doi: 10.1073/pnas.0506307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woolner S, Bement WM. Unconventional myosins acting unconventionally. Trends Cell Biol. 2009;19:245–252. doi: 10.1016/j.tcb.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillespie PG, Albanesi JP, Bahler M, et al. Myosin-I nomenclature. J Cell Biol. 2001;155:703–704. doi: 10.1083/jcb.200110032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bose A, Robida S, Furcinitti PS, et al. Unconventional myosin Myo1c promotes membrane fusion in a regulated exocytic pathway. Mol Cell Biol. 2004;24:5447–5458. doi: 10.1128/MCB.24.12.5447-5458.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruppert C, Godel J, Muller RT, et al. Localization of the rat myosin I molecules myr 1 and myr 2 and in vivo targeting of their tail domains. J Cell Sci. 1995;108 ( Pt 12):3775–3786. doi: 10.1242/jcs.108.12.3775. [DOI] [PubMed] [Google Scholar]
- 11.Wagner MC, Barylko B, Albanesi JP. Tissue distribution and subcellular localization of mammalian myosin I. J Cell Biol. 1992;119:163–170. doi: 10.1083/jcb.119.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coluccio LM. Myosin I. Am J Physiol. 1997;273:C347–359. doi: 10.1152/ajpcell.1997.273.2.C347. [DOI] [PubMed] [Google Scholar]
- 13.Tang N, Lin T, Ostap EM. Dynamics of myo1c (myosin-ibeta ) lipid binding and dissociation. J Biol Chem. 2002;277:42763–42768. doi: 10.1074/jbc.M206388200. [DOI] [PubMed] [Google Scholar]
- 14.Hokanson DE, Ostap EM. Myo1c binds tightly and specifically to phosphatidylinositol 4,5-bisphosphate and inositol 1,4,5-trisphosphate. Proc Natl Acad Sci U S A. 2006;103:3118–3123. doi: 10.1073/pnas.0505685103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierchala BA, Munoz MR, Tsui CC. Proteomic analysis of the slit diaphragm complex: CLIC5 is a protein critical for podocyte morphology and function. Kidney Int. 2010;78:868–882. doi: 10.1038/ki.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arif E, Wagner MC, Johnstone DB, et al. Motor protein Myo1c is a podocyte protein that facilitates the transport of slit diaphragm protein Neph1 to the podocyte membrane. Mol Cell Biol. 2011;31:2134–2150. doi: 10.1128/MCB.05051-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kao WH, Klag MJ, Meoni LA, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008;40:1185–1192. doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopp JB, Smith MW, Nelson GW, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008;40:1175–1184. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mele C, Iatropoulos P, Donadelli R, et al. MYO1E mutations and childhood familial focal segmental glomerulosclerosis. N Engl J Med. 2011;365:295–306. doi: 10.1056/NEJMoa1101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krendel M, Kim SV, Willinger T, et al. Disruption of Myosin 1e promotes podocyte injury. J Am Soc Nephrol. 2009;20:86–94. doi: 10.1681/ASN.2007111172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong F, Li S, Pujol-Moix N, et al. Genotype-phenotype correlation in MYH9-related thrombocytopenia. Br J Haematol. 2005;130:620–627. doi: 10.1111/j.1365-2141.2005.05658.x. [DOI] [PubMed] [Google Scholar]
- 22.Kunishima S, Saito H. Advances in the understanding of MYH9 disorders. Curr Opin Hematol. 2010;17:405–410. doi: 10.1097/MOH.0b013e32833c069c. [DOI] [PubMed] [Google Scholar]
- 23.Hao J, Kunishima S, Guo X, et al. A large family with MYH9 disorder caused by E1841K mutation suffering from serious kidney and hearing impairment and cataracts. Ann Hematol. 2012;91:1147–1148. doi: 10.1007/s00277-011-1370-5. [DOI] [PubMed] [Google Scholar]
- 24.Zadro C, Alemanno MS, Bellacchio E, et al. Are MYO1C and MYO1F associated with hearing loss? Biochim Biophys Acta. 2009;1792:27–32. doi: 10.1016/j.bbadis.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Johnstone DB, Zhang J, George B, et al. Podocyte-specific deletion of Myh9 encoding nonmuscle myosin heavy chain 2A predisposes mice to glomerulopathy. Molecular and cellular biology. 2011;31:2162–2170. doi: 10.1128/MCB.05234-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chase SE, Encina CV, Stolzenburg LR, et al. Podocyte-specific knockout of myosin 1e disrupts glomerular filtration. Am J Physiol Renal Physiol. 2012 doi: 10.1152/ajprenal.00251.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillespie PG. Myosin I and adaptation of mechanical transduction by the inner ear. Philos Trans R Soc Lond B Biol Sci. 2004;359:1945–1951. doi: 10.1098/rstb.2004.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gillespie PG, Wagner MC, Hudspeth AJ. Identification of a 120 kd hair-bundle myosin located near stereociliary tips. Neuron. 1993;11:581–594. doi: 10.1016/0896-6273(93)90071-x. [DOI] [PubMed] [Google Scholar]
- 29.Holt JR, Gillespie SK, Provance DW, et al. A chemical-genetic strategy implicates myosin-1c in adaptation by hair cells. Cell. 2002;108:371–381. doi: 10.1016/s0092-8674(02)00629-3. [DOI] [PubMed] [Google Scholar]
- 30.Bose A, Guilherme A, Robida SI, et al. Glucose transporter recycling in response to insulin is facilitated by myosin Myo1c. Nature. 2002;420:821–824. doi: 10.1038/nature01246. [DOI] [PubMed] [Google Scholar]
- 31.Chen XW, Leto D, Chiang SH, et al. Activation of RalA is required for insulin-stimulated Glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Dev Cell. 2007;13:391–404. doi: 10.1016/j.devcel.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Fan Y, Eswarappa SM, Hitomi M, et al. Myo1c facilitates G-actin transport to the leading edge of migrating endothelial cells. J Cell Biol. 2012;198:47–55. doi: 10.1083/jcb.201111088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 34.Tryggvason K, Patrakka J, Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med. 2006;354:1387–1401. doi: 10.1056/NEJMra052131. [DOI] [PubMed] [Google Scholar]
- 35.Drummond IA. Kidney development and disease in the zebrafish. J Am Soc Nephrol. 2005;16:299–304. doi: 10.1681/ASN.2004090754. [DOI] [PubMed] [Google Scholar]
- 36.Wingert RA, Davidson AJ. The zebrafish pronephros: a model to study nephron segmentation. Kidney Int. 2008;73:1120–1127. doi: 10.1038/ki.2008.37. [DOI] [PubMed] [Google Scholar]
- 37.Kramer-Zucker AG, Wiessner S, Jensen AM, et al. Organization of the pronephric filtration apparatus in zebrafish requires Nephrin, Podocin and the FERM domain protein Mosaic eyes. Dev Biol. 2005;285:316–329. doi: 10.1016/j.ydbio.2005.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neumann-Haefelin E, Kramer-Zucker A, Slanchev K, et al. A model organism approach: defining the role of Neph proteins as regulators of neuron and kidney morphogenesis. Hum Mol Genet. 2010;19:2347–2359. doi: 10.1093/hmg/ddq108. [DOI] [PubMed] [Google Scholar]
- 39.Putaala H, Soininen R, Kilpelainen P, et al. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet. 2001;10:1–8. doi: 10.1093/hmg/10.1.1. [DOI] [PubMed] [Google Scholar]
- 40.Rantanen M, Palmen T, Patari A, et al. Nephrin TRAP mice lack slit diaphragms and show fibrotic glomeruli and cystic tubular lesions. J Am Soc Nephrol. 2002;13:1586–1594. doi: 10.1097/01.asn.0000016142.29721.22. [DOI] [PubMed] [Google Scholar]
- 41.Hamano Y, Grunkemeyer JA, Sudhakar A, et al. Determinants of vascular permeability in the kidney glomerulus. J Biol Chem. 2002;277:31154–31162. doi: 10.1074/jbc.M204806200. [DOI] [PubMed] [Google Scholar]
- 42.Donoviel DB, Freed DD, Vogel H, et al. Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Molecular and cellular biology. 2001;21:4829–4836. doi: 10.1128/MCB.21.14.4829-4836.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roselli S, Heidet L, Sich M, et al. Early glomerular filtration defect and severe renal disease in podocin-deficient mice. Mol Cell Biol. 2004;24:550–560. doi: 10.1128/MCB.24.2.550-560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anzenberger U, Bit-Avragim N, Rohr S, et al. Elucidation of megalin/LRP2-dependent endocytic transport processes in the larval zebrafish pronephros. J Cell Sci. 2006;119:2127–2137. doi: 10.1242/jcs.02954. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Pathak N, Kramer-Zucker A, et al. Notch signaling controls the differentiation of transporting epithelia and multiciliated cells in the zebrafish pronephros. Development. 2007;134:1111–1122. doi: 10.1242/dev.02806. [DOI] [PubMed] [Google Scholar]
- 46.Zhou W, Hildebrandt F. Inducible podocyte injury and proteinuria in transgenic zebrafish. J Am Soc Nephrol. 2012;23:1039–1047. doi: 10.1681/ASN.2011080776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnstone DB, Holzman LB. Clinical impact of research on the podocyte slit diaphragm. Nat Clin Pract Nephrol. 2006;2:271–282. doi: 10.1038/ncpneph0180. [DOI] [PubMed] [Google Scholar]
- 48.Shankland SJ. The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- 49.Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99:342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doublier S, Ruotsalainen V, Salvidio G, et al. Nephrin redistribution on podocytes is a potential mechanism for proteinuria in patients with primary acquired nephrotic syndrome. Am J Pathol. 2001;158:1723–1731. doi: 10.1016/S0002-9440(10)64128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Otaki Y, Miyauchi N, Higa M, et al. Dissociation of NEPH1 from nephrin is involved in development of a rat model of focal segmental glomerulosclerosis. Am J Physiol Renal Physiol. 2008;295:F1376–1387. doi: 10.1152/ajprenal.00075.2008. [DOI] [PubMed] [Google Scholar]
- 52.Wernerson A, Duner F, Pettersson E, et al. Altered ultrastructural distribution of nephrin in minimal change nephrotic syndrome. Nephrol Dial Transplant. 2003;18:70–76. doi: 10.1093/ndt/18.1.70. [DOI] [PubMed] [Google Scholar]
- 53.Brandstaetter H, Kendrick-Jones J, Buss F. Myo1c regulates lipid raft recycling to control cell spreading, migration and Salmonella invasion. J Cell Sci. 125:1991–2003. doi: 10.1242/jcs.097212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fan Y, Eswarappa SM, Hitomi M, et al. Myo1c facilitates G-actin transport to the leading edge of migrating endothelial cells. J Cell Biol. 198:47–55. doi: 10.1083/jcb.201111088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whitfield TT. Zebrafish as a model for hearing and deafness. J Neurobiol. 2002;53:157–171. doi: 10.1002/neu.10123. [DOI] [PubMed] [Google Scholar]
- 56.Muller T, Rumpel E, Hradetzky S, et al. Non-muscle myosin IIA is required for the development of the zebrafish glomerulus. Kidney Int. 2011;80:1055–1063. doi: 10.1038/ki.2011.256. [DOI] [PubMed] [Google Scholar]
- 57.Wagner MC, Rhodes G, Wang E, et al. Ischemic injury to kidney induces glomerular podocyte effacement and dissociation of slit diaphragm proteins Neph1 and ZO-1. J Biol Chem. 2008;283:35579–35589. doi: 10.1074/jbc.M805507200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greenberg MJ, Ostap EM. Regulation and control of myosin-I by the motor and light chain-binding domains. Trends Cell Biol. 2013;23:81–89. doi: 10.1016/j.tcb.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krendel M, Osterweil EK, Mooseker MS. Myosin 1E interacts with synaptojanin-1 and dynamin and is involved in endocytosis. FEBS Lett. 2007;581:644–650. doi: 10.1016/j.febslet.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- 61.Stoll SJ, Bartsch S, Augustin HG, et al. The transcription factor HOXC9 regulates endothelial cell quiescence and vascular morphogenesis in zebrafish via inhibition of interleukin 8. Circ Res. 2011;108:1367–1377. doi: 10.1161/CIRCRESAHA.111.244095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.