Abstract
Despite accumulating evidence suggesting a positive correlation between leptin levels, obesity, post-menopause and breast cancer incidence, our current knowledge on the mechanisms involved in these relationships is still incomplete. Since the cloning of leptin in 1994 and its receptor (OB-R) 1 year later by Friedman’s laboratory (Zhang et al., 1994) and Tartaglia et al. (Tartaglia et al., 1995), respectively, more than 22,000 papers related to leptin functions in several biological systems have been published (Pubmed, 2012). The ob gene product, leptin, is an important circulating signal for the regulation of body weight. Additionally, leptin plays critical roles in the regulation of glucose homeostasis, reproduction, growth and the immune response. Supporting evidence for leptin roles in cancer has been shown in more than 1000 published papers, with almost 300 papers related to breast cancer (Pubmed, 2012). Specific leptin-induced signaling pathways are involved in the increased levels of inflammatory, mitogenic and pro-angiogenic factors in breast cancer. In obesity, a mild inflammatory condition, deregulated secretion of proinflammatory cytokines and adipokines such as IL-1, IL-6, TNF-α and leptin from adipose tissue, inflammatory and cancer cells could contribute to the onset and progression of cancer. We used an in silico software program, Pathway Studio 9, and found 4587 references citing these various interactions. Functional crosstalk between leptin, IL-1 and Notch signaling (NILCO) found in breast cancer cells could represent the integration of developmental, proinflammatory and pro-angiogenic signals critical for leptin-induced breast cancer cell proliferation/migration, tumor angiogenesis and breast cancer stem cells (BCSCs). Remarkably, the inhibition of leptin signaling via leptin peptide receptor antagonists (LPrAs) significantly reduced the establishment and growth of syngeneic, xenograft and carcinogen-induced breast cancer and, simultaneously decreased the levels of VEGF/VEGFR2, IL-1 and Notch. Inhibition of leptin–cytokine crosstalk might serve as a preventative or adjuvant measure to target breast cancer, particularly in obese women. This review is intended to present an update analysis of leptin actions in breast cancer, highlighting its crosstalk to inflammatory cytokines and growth fact ors essential for tumor development, angiogenesis and potential role in BCSC.
Keywords: Breast cancer, Leptin, Cytokines, NILCO, Obesity, Leptin peptide receptor antagonist
1. Introduction
Leptin, mainly secreted by adipose tissue, is the most studied adipokine since this protein was first cloned in 1994 (Zhang et al., 1994). Both leptin and its receptor, OB-R, are necessary for normal mammary gland development. However, leptin/OB-R are very low expressed in epithelial cells of normal human mammary glands (Jarde et al., 2008a). In contrast, leptin/OB-R are overexpressed in breast cancer cells. A complex crosstalk between leptin and pro-angiogenic, inflammatory and mitogenic factors occurs in breast cancer. Leptin actions would provide a link between proinflammatory and pro-angiogenic actions of leptin, IL-1, VEGF and macrophages in breast cancer progression (Guo et al., 2012a). However, the individual contributions of these factors to obesity-related cancers, including breast cancer, are not well understood.
Obesity-related cancer is a contemporary socio-epidemiological health problem. The World Health Organization (WHO) reported that more than 400 million people are obese and approximately 700 million will have this condition worldwide by 2015 (WHO, 2006). Obesity, mainly due to unhealthy diets and lifestyles, is a proven factor contributing to higher risk and poor prognosis of cancer. Studies from US populations show that death rates from all cancers combined among obese individuals were 52% higher (for men) and 62% higher (for women) than the rates in men and women of normal weight (Calle et al., 2003). How obesity is associated to cancer incidence is still an unexplainable or unanswered question (Prieto-Hontoria et al., 2011; Zhang et al., 2010).
Obesity is characterized by high levels of estrogen, which increases the growth of endocrine responsive of cancer, in particular, breast cancer. Obesity’s effects on postmenopausal breast cancer are linked to the tumor expression of estrogen and progesterone receptors. However, not all breast cancers respond to estrogens. In contrast, premenopausal obese women develop most often estrogen and progesterone receptor negative tumors. Ovarian hormones are more relevant for estrogen responsive than unresponsive and for lobular than for ductal tumor (Unkown, 2012). Therefore, estrogen and progesterone unresponsive breast cancers are mostly dependent on growth factors (i.e., insulin, insulin-like growth factor-I) and adipokines (i.e., leptin) (Rose and Vona-Davis, 2010).
Inflammatory cytokines are involved in the regulation of peripheral synthesis of estrogens, which is enhanced in obese or elderly subjects. In addition, elevated levels of cytokines induced by stress or immunosuppression may alter the risk of developing breast cancer (Reed and Purohit, 1997). Elevated lifetime estrogen exposure has been shown to be a major risk factor for cancer in hormone-de pendent organs, particularly in breast and endometrium (Clemons and Goss, 2001; Henderson et al., 1988). Estrogens are also important regulators of the development and progression of es***trogen receptor positive (ER+) breast carcinoma (Bernstein and Ross, 1993; Johnston, 2010). ERs are known to regulate a huge number of genes affecting cancer proliferation and vascular function (Mendelsohn and Karas, 1999; Welboren et al., 2007).
Earlier studies suggest that estrogen may influence leptin synthesis in a tissue- and cell type-specific fashion (Henson and Castracane, 2002). Jarde et al. examined the expression of leptin, OB-R and ER in human primary breast cancer and adjacent non-cancerous tissue. Their findings suggest that OB-R and ER are co-expressed in breast cancer indicating a possible interaction between leptin and estrogen systems to promote breast carcinogenesis (Jarde et al., 2008b). Antiestrogens may stimulate the synthesis and release of leptin in the adipocytes (Marttunen et al., 2000). A recent report has confirmed these previous findings (Chen et al., 2012). In addition, leptin can transactivate ER (Catalano et al., 2004) and increase the expression of aromatase in MCF-7 cells (Catalano et al., 2003). These leptin effects represent additional links between obesity and estrogen signaling that promotes the development of estrogen-responsive breast cancer (Guo et al., 2012a).
Obesity and markers of the metabolic syndrome (insulin, free/bioavailable IGF-1 and leptin) correlate to breast cancer (Protani et al., 2010). Hyperinsulinemia could induce breast cancer progression through leptin-dependent mechanisms (Bartella et al., 2008). However, the individual contributions of all these factors to obesity-related cancers are often contradictory and not well understood in diverse scenarios. Therefore, the causal mechanisms linking obesity and breast cancer are still unknown. Some studies suggest that there is no evidence that targeting obesity (reduction of body weight) after breast cancer diagnosis improves survival. These data would suggest that the additional trouble of weight loss on women already affected with breast cancer is unjustifiable (Protani et al., 2010). However, in sharp contrast with this idea is the fact that obesity and diabetes increase the likelihood of breast cancer recurrence, death and chemoresistance (Rose and Vona-Davis, 2010; Dowsett et al., 2010; Yager and Davidson, 2006).
Obesity is a state of chronic inflammation, where many cytokines show altered profiles (Lumeng et al., 2007a, 2007b). Among them, inflammatory cytokines [i.e., interleukin-1 (IL-1), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α, etc.] and leptin have been implied as positive effectors of obesity-induced changes in tumor and stroma cells (Ziccardi et al., 2002; Vendramini-Costa and Carvalho, 2012). Cytokines secreted by the adipose tissue have also pro-angiogenic effects, among these a non-inflammatory cytokine, the vascular endothelial growth factor (VEGF), has the more prominent role in angiogenesis. In addition, IL-1 (Coxon et al., 2002; Salven et al., 2002; Voronov et al., 2003), IL-6 (Guo et al., 2012c), hepatocyte growth factor (HGF) (Bussolino et al., 1992) and leptin (Bouloumie et al., 1998), also promote angiogenesis. Recruitment of inflammatory cells significantly contributes to adipose neovascularization and breast cancer inflammation and angiogenesis (Cao, 2007). Moreover, it has been suggested that the inflammatory cells and cytokines found in tumors are more likely to contribute to tumor growth, progression, and immunosuppression than they are to mount an effective host antitumor response (Balkwill and Mantovani, 2001).
Leptin is an immunomodulator whose levels are increased by infection and inflammation (Faggioni et al., 2001) can also modulates adaptive immune response (Fantuzzi and Faggioni, 2000). Within the breast cancer tissue, chemotactic factors are produced causing an influx of inflammatory cells. These cells can be activated within the tumor and secrete proinflammatory cytokines, IL-1, IL-6, IFN-γ, TNF-α and IL-17. Stimulation of the tumor cells within the breast by these cytokines induce the production of CCLL22, a chemokine that then recruits Tregs into the tumor (Watanabe et al., 2010). These Tregs are producers of cytotoxic T-Lymphocyte antigen 4 (CTLA-4), FoxP3 and glucocorticoid-induced TNFR-relate d protein (GITR), all immunosuppressive proteins that decrease the tumor cytotoxic capacity of CD8 + T cells. The increased presence of Tregs in tumors and peripheral blood of cancer patients is usually an indicator of poor prognosis in cancer outcomes (Watanabe et al., 2008). The Treg population may be increased through the decreased production of IL-1α by plasmacytoid dendritic cells (Sisirak et al., 2012).
2. Leptin and OB-R in breast cancer
In addition of the adipose tissue, leptin is synthesized and secreted in low quantities by various organs/tissues, viz. stomach (Bado et al., 1998), skeletal muscle (Wang et al., 1998), brain (Wiesner et al., 1999), placenta (Masuzaki et al., 1997) and endometrium at the time of embryo implantation (Gonzalez et al., 2000a). Leptin levels are higher in women compared to males (pre-menopausal females > post-menopausal females > males) even after correction by body weight (Rosenbaum et al., 1996). These gender differences in leptin levels could be related to subcutaneous synthesis and estrogen and androgen regulations (for review see Ahima and Osei, 2004).
Lean and normal weighted individuals are sensitive to leptin levels, which regulate appetite and size of adipose tissue through complex signaling actions at hypothalamic levels. Therefore, leptin was once considered “the silver bullet” to address the reduction of body overweight and decrease obesity rate. However, further investigations demonstrated that obesity is characterized by high levels of plasmatic leptin, which leads to the development of a non-responsive hypothalamic stage for the regulation of energy expenditure and appetite. Despite that obese individuals show high plasma levels of leptin, they are unable to control appetite via leptin-induced hypothalamic actions. Thus, obesity is the consequence of a leptin-resist ant state, which seems to be independent of aging (Gabriely et al., 2002). Conversely, high plasma levels of leptin have been linked to breast cancer development (Guo et al., 2012a).
Leptin exist as a unique protein with proinflammatory functions that belongs to the family of helical cytokines. Leptin is structurally similar to IL-6, IL-12, IL-15, prolactin, growth hormone (GH), granulocyte colony-stimulating factor (GCSF) and oncostatin M. Leptin is a small non-glycosilated protein (ob or LEP; molecular weight of 16 kDa; 167 amino acids) that has a high percentage of identity sequence among mammals. The N-terminal region (94 amino acids) of leptin is essential for both the biological and the receptor binding activities (Imagawa et al., 1998).
In contrast to leptin, at least six alternative spliced forms of the leptin receptor, OB-R, the product of the diabetic (db) gene, have been identified in humans: viz. a long isoform (OB-RL, OB-Rb or LEPR) with full intracellular signaling capabilities, shorter isoforms with less biological activity (OB-Rs or OB-Ra) (Wang et al., 1997) and a soluble leptin receptor (OB-Re or sOB-R) (Lewandowski et al., 1999; Maamra et al., 2001). The large extracellular domain of OB-R (816 amino acids) is common to all OB-R forms, and the variable length cytoplasmatic tail (300 amino acid residues) distinguishes the several isoforms. The extracellular region of cloned OB-R differs from that of many other cytokine receptors in that it contains several homologous segments representing potential ligand binding sites (Tartaglia et al., 1995). OB-RL and membrane-bound shorter isoforms of OB-R have a cytoplasmatic motif (Box 1) required for JAK (Janus kinases)-related activation of PI-3K (phosphatidylinositol-3-kinase) and MAPK (mitogen-activated protein kinase) pathways . However, only OB-RL has a docking site (cytoplasmatic tail motif; Box 2) essential for the activation of the JAK-STAT (signal transducers and activators of transcription) pathway. Induced mutations of the OB-Rb intracellular domain showed that Tyr 113s controls STAT3 (Blenis, 1993). In addition, the SH2 domains of SOCS (suppressor of cytokine signaling) bind the phosphorylated tyrosine residues on JAK2 regulating OB-Rb (Takahashi et al., 1997).
OB-R has a helical structure that is similar to those of gp130, the common signal transducing receptor component for the IL-6 family of cytokines, G-CSF (granulocyte colony stimulating factor) and LIF (leukemia inhibitory factor) receptors (Baumann et al., 1996). OB-R is related to class I cytokine receptors, which includes the receptors of IL-1, IL-2, IL-6 and the growth hormone (Fruhbeck, 2006). This super-family of receptors lacks auto-phosphorylation capabilities and needs auxiliary kinases for activation. OB-R binding to leptin induces conformational changes that recruit JAKs, which in turn phosphorylates OB-R and activates STATs. SOCS-3 plays an important role as a negative regulator of leptin signaling. It seems SOCS-3 is activated by a feed-back induced by leptin. The over-expression of SOCS-3 inhibits leptin-induced tyrosine phosphorylation of JAK2 and ERK activation by binding to phosphorylated Tyr 985 of OB-Rb (Bjorbaek et al., 1999).
Leptin has very high specificity for binding to OB-R (Gonzalez and Leavis, 2003). However, there is a complete lack of structural data for the OB-R/leptin complex. To date the structure of this complex has been not resolved. A hypothetical model for the leptin-OB-R complex has been described based on the similarities between granulocyte-colony stimulator factor (G-CSF) and leptin and their receptors (Hiroike et al., 2000). In this model, leptin binds to OB-R in a 2:2 ratio as was found for the G-CSF/G-CSF receptor complex (Aritomi et al., 1999). More recently, a molecular model of the putative leptin-LBD (leptin binding domain) antibody complex revealed that the bound antibody blocked leptin binding through only a small overlap in their binding sites, and that leptin binding is likely to involve an induced fit mechanism (Carpenter et al., 2012). Strict biunivocal binding-affinity and activation of leptin/OB-R complex makes it a unique molecular target for prevention and treatment of breast cancer, particularly in obesity contexts (Guo et al., 2012a; Gonzalez and Leavis, 2003; Gillespie et al., 2012; Gonzalez et al., 2006; Rene Gonzalez et al., 2009). Nevertheless, the mechanism of OB-R activation via leptin binding remains poorly understood.
Spontaneous mutations in the leptin (ob) and OB-R (db) genes in C57Bl/6J mice lead to hyperphagia, morbid obesity, hyperinsulemia and infertility. A nonsense mutation in codon 105 causes the lack of protein synthesis in ob/ob mice (Zhang et al., 1994). A point mutation (G → T) in the genomic OB-R sequence induces the synthesis of truncated non-functional OB-RL in db/db mice (Chen et al., 1996). However, in humans ob or db mutations showed low penetration and scarce number of affected individuals (Paracchini et al., 2005).
2.1. Leptin signaling pathways and breast cancer
Leptin-induced intracellular signals comprise several pathways commonly triggered by many inflammatory cytokines (viz, JAK2/STAT; (MAPK)/extracellular regulated kinases 1 and 2 (ERK1/2) and PI-3K/AKT1 and, non-canonic al signaling pathways: protein kinase C (PKC), c-Jun NH(2)-terminal kinase (JNK) and p38 MAP kinase) (Guo et al., 2012a) (Fig. 1). Leptin can also induce adenosine monophosphate (AMP)-Activated Protein Kinase (AMPK) activation in some cells. Leptin selectively stimulates phosphorylation and activation of the alpha2 catalytic subunit of AMPK (alpha2 AMPK) in skeletal muscle. Leptin-activated AMPK inhibits the activity of acetyl coenzyme A carboxylase (ACC), which stimulates the oxidation of fatty acids and the uptake of glucose, and prevents the accumulation of lipids in nonadipose tissues (Minokoshi et al., 2002). Each of these leptin-induced signals is essential to its biological effects on food intake, energy balance, adiposity, immune and endocrine systems, as well as oncogenesis (Guo et al., 2012a).
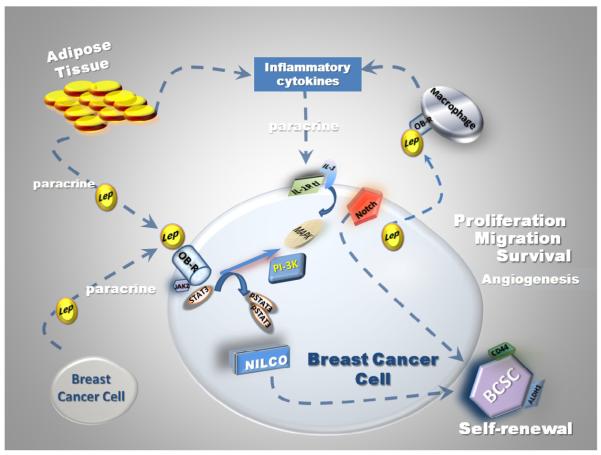
Fig. 1.
Role of leptin and inflammatory cytokine crosstalk in breast cancer. Progression of breast cancer is closely related to leptin and the actions of angiogenic and inflammatory cytokines. Breast cancer cells and associate stroma express an array of inflammatory cytokines in a simultaneous manner. Adipose tissue expresses tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6), which may cause obesity-related insulin resistance (Unkown, 2012; Kern et al., 2001). In primary breast cancer the expression of interleukin 1 (IL-1), IL-6 and TNF-α correlated to tumor associate macrophages (TAM) and VEGF (Ueno et al., 2000). Leptin crosstalk to cytokines in breast cancer is closely related to tumor progression (proliferation, migration and metastasis), which also impact on self-renewal of breast cancer stem cells and tumor angiogenesis (Guo et al., 2012a).
Compelling evidence for a role of leptin in breast cancer was provided by Dr. Cleary’s studies by showing that leptin signaling-deficient (ob/ob and db/db) mice do not develop mammary tumors. Moreover, the progeny from ob/ob or db/db mice crossed with MTTV-TGF-α mice (prone to develop mammary cancer), did not show mammary tumors (Cleary et al., 2003, 2004). These mice show, however, high levels of insulin/IGF-1, which have been suggested to play important roles in mammary carcinogenesis. Further, we have also provided solid evidence sustaining a role for leptin signaling in breast cancer, by demonstrating that the inhibition of leptin signaling in vivo significantly delays the onset and reduces the growth of syngeneic, xenograft and carcinogenic-induced mammary tumors in mice (Gillespie et al., 2012; Gonzalez et al., 2006; Rene Gonzalez et al., 2009).
2.2. Leptin and cytokine crosstalk in breast cancer
Leptin’s paracrine or autocrine actions can stimulate tumor cells to secrete inflammatory cytokines. Leptin can also stimulate the tumor-induced colonization of stroma, which leads to the stroma secretion of several growth factors and cytokines, etc. (Guo et al., 2012a). In turn, inflammatory cytokines can also modulate the synthesis and secretion of leptin from adipose and tumor cells (Faggioni et al., 1998; Sarraf et al., 1997). Leptin pro-angiogenic, inflammatory and mitogenic effects in breast cancer are eventually related to leptin crosstalk with several cytokines secreted by cancer and stromal cells (Guo et al., 2012a).
Groundbreaking data from immunohistochemistry studies conducted by Ishikawa et al. in normal breast and breast cancer tissues evidenced abnormally high levels of leptin/OB-R in malignant tissues (Ishikawa et al., 2004). Normal epithelial mammary cells showed weaker staining of leptin and OB-R compared to adipocytes in adjacent adipose tissue. In sharp contrast, leptin production was enhanced in breast cancer. Moreover, none of the normal mammary glands exhibited significant immunoreactivity to OB-R, whereas it was expressed in most of the carcinoma cells. Interestingly, distant metastasis was detected in 21 (34%) of 61 OB-R-positive tumors with leptin overexpression, but in none of the 15 tumors that lacked OB-R expression or leptin overexpression (p < 0.05) (Ishikawa et al., 2004). Further studies showed that leptin and OB-R were detected in 39–86% and 41–79% of breast cancer tissues, respectively. Data from these studies suggest that the expression of leptin in breast cancer was correlated to highly proliferative tumors and metastasic tissues (Kim, 2009; Garofalo et al., 2006). Leptin and OB-R mRNAs were virtually detected in all breast cancer using real-time RT-PCR. Interestingly, OB-RL and OB-Rs mRNA were inversely correlated with the expression of progesterone receptors and high OB-RL/OB-Rs ratios were associated with a shorter relapse-free survival (Revillion et al., 2006). Leptin and OB-R expression have also been reported in several breast cancer cell lines (see Table 1).
Table 1.
Expression of leptin/OB-R in breast cancer.
| Breast cancer |
Subtype | Leptin | OB-R | Techniques | Reference |
|---|---|---|---|---|---|
| Carcinoma | 92% | 83% | IHC | Ishikawa et al. (2004) | |
| DICa (80%; n = 417/517) | 39% (p = 0.02)b | 79% | IHC | Kim (2009) | |
| 24% of TNBC | (p = 0.05)b | ||||
| No TNBC | 36% | 80% | IHC | Kim (2009) | |
| Normal BMI | 43% | 74% | IHC | Kim (2009) | |
| Overweight/obese | 37% | 85% | IHC | Kim (2009) | |
| Primary tumor | 86% | 41% | IHC | Garofalo et al. (2006) | |
| Metastasis | 94% | 52% | IHC | Garofalo et al. (2006) | |
| Diverse subtypes (n = 322) | 99% | 100% | Real-time RT-PCR | Revillion et al. (2006) | |
| Diverse subtypes (n = 20) | 100% | Real-time RT-PCR | Laud (2002) | ||
| Cell lines | |||||
| Human | MCF-7 | 2.6 pg/ml/mg | (+) | ELISA, WB | Rene Gonzalez et al. (2009) |
| (0.15 pM)c | |||||
| MDA-MB-231 | 9.6 pg/ml/mg | (+) | |||
| (0.41 pM)c | |||||
| MCF-7 | (+) | IHC; real-time RT-PCR; in situ hybridization |
Laud (2002) and Cascio (2008) | ||
| MDA-MB231 | 10 pg/ml c | (+) | IHC, real-time RT-PCR | Soma (2008) and Bartella et al. (2008) | |
| SKBR-3 | (+) | IHC | Soma (2008) | ||
| T47D | (+) | IHC; real-time RT-PCR; in situ hybridization |
Laud (2002) | ||
| ZR75-1 and HTB-26 | (+) | RT-PCR | Frankenberry (2006) | ||
| Mouse | 4T1 | (+) | (+) | IHC, WB, ELISA |
Gonzalez et al. (2006), Gonzalez-Perez et al. (2010) and Zhou et al. (2011) |
| EMT6 | (+) | (+) | IHC, WB, ELISA | Gonzalez-Perez et al. (2010) and Zhou et al. (2011) | |
| MMT | (+) | (+) | IHC, WB, ELISA | Gonzalez-Perez et al. (2010) and Zhou et al. (2011) |
Ductal invasive carcinoma.
Compared to no TNBC.
Basal level.
Leptin pro-angiogenic, inflammatory and mitogenic effects in breast cancer are eventually related to its crosstalk with several cytokines secreted by cancer and stromal cells (Guo et al., 2012a). Leptin can stimulate the tumor-induced colonization of stroma, which leads to the secretion of several growth factors and cytokines (Guo et al., 2012a). In addition, paracrine or autocrine actions of leptin can stimulate tumor cells to secrete inflammatory cytokines. Steroid hormones including estrogen, progesterone and glucocorticoids and, insulin participate in the regulation of leptin metabolism (Lepercq et al., 1998). Leptin can also interact with other cytokines and growth factors. Leptin secretion and mRNA expression were modulated in response to IGF-I (Smith and Sheffield, 2002). Additionally, leptin synergistically stimulated FGF-2 and VEGF functions (Cao et al., 2001), and its secretion was down-regulated together with GM-CSF and IL-6 after inhibition of VEGF and platelet derived growth factor (PDGF) (Duhrsen et al., 2001). Providing a feed-back mechanism, in turn several inflammatory cytokines can modulate the synthesis and secretion of leptin from adipose and tumor cells (Faggioni et al., 1998; Sarraf et al., 1997). Indeed, leptin is regulated by IL-1 (Fanggioni et al., 1998; Janik et al., 1997), TNF-α (Finck et al., 1998) and TGF-β, linking leptin with the inflammatory response (Sarraf et al., 1997). Remarkably, STAT3 activation is a common feature of leptin and a variety of cytokines such as IL-6, G-CSF, epidermal growth factor (EGF), IL-1 and TNF -α (Finck et al., 1998; Takeda et al., 1998).
2.3. In silico analysis of leptin–cytokine interaction networks in breast cancer
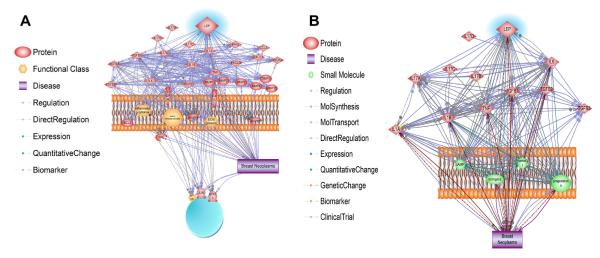
To verify leptin–cytokine interactions in breast cancer Pathway Studio 9 software (Ariadine Genomics, MD) was used. Fig. 2a depicts the various relationships between the proteins involved in breast cancer. Three hundred five relationships were detected and these results are included in the Supplementary material. This analysis proved to be too large and thus, only the cytokines, IL-6, IL-1, IL-17, TNF-α, and TGF-β were imported into the software and their relationships analyzed in terms of breast cancer (Fig. 2b). Ninety-eight references were found demonstrating breast neoplasms were up regulated by TNF, leptin, IL-6, IL-1α and TGF-β2 (see Supplementary material). An additional 89 papers reported how these cytokines regulated the expression of each other. Increases in estrogen, progesterone, TGF-β1, TGF-β2, TGF-β3, leptin, IL-6, TNF, IL-1β and cortisol were associated with breast neoplasms as described in 124 manuscripts . Genetic polymorphisms were reported in the TGF-β1, TGF-β2, leptin, IL-6, TNF, IL-1β and IL-1α genes that are found in breast cancers patients (101 papers). Potential biomarkers for breast cancers include TGF-β1, progesterone, leptin, TNF and IL-1α as described in 61 manuscripts. The results of this analysis reemphasizes the complexity of pathways, soluble mediators and cells involved in breast cancer and the need to elucidate the various interactions in order to develop new rational treatment regimens.
Fig. 2.
In silico analysis of leptin-cytokine interactions in breast cancer. (A) Interaction networks between leptin and proteins involved in breast cancer were analyzed using Pathway studio program (Ariadine Genomics, MD). Detailed description of relationships detected is included in the supplementary material, (S1). (B) Relationships between leptin and main inflammatory cytokines [IL-6, IL-1, interleukin 17, (IL-17), TNF-α, and transforming growth factor beta, (TGF-β)] were imported and analyzed in terms of breast cancer. Detailed description of interactions is shown in supplementary material (S2).
3. Inflammatory cytokines and breast cancer
Cytokines in the tumor microenvironment may play a role in the growth and metastatic nature of the cancer cells in the breast. Influx of inflammatory leukocytes into the tumor can produce different cytokine profiles dependent upon the type of cell that predominate the tumor. Cell populations from breast cancer and adjacent non-affected tissue were examined by flow cytometry. Women who received chemotherapy prior to surgery had an increase number of myeloid cells and CD8+/CD4 + T cell ratio when compared to women who did not receive chemotherapy (Ruffell et al., 2012). Differences in the cell types could affect the cytokine milieu thus altering the cytotoxicity capacity of cells within the tumor.
A decrease in the Th1 cytokines, IL-6, IL-21, IL-1β, and TNF-α increases the cytotoxic capacity of CD8 + T cells that can produce IFN-γ which decreases angiogenesis and enhances MHC expression and tumor recognition. An increase in theseTh1 cytokines can increase the production of IL-17 that stimulates angiogenesis and tumor growth (Murugaiyan and Saha, 2009). In addition, TGF-β and IL-6 inactivate cytotoxic CD8 + T cells which then produce more IL-17 (Liu et al., 2007).
Th17 cells are also increased in breast cancer tissue when compared to normal tissue and a corresponding increase in IL-6, IL-1β and IL-17 was found in the tissue (Yang et al., 2012). The cytokines, RANTES and MCP-1α, produced by breast cancer tumor cells and fibroblasts increased chemotaxis of Th17 cells (Su et al., 2010). IL-17 was also expressed by breast cancer macrophages and when various breast cancer cell lines were exposed to IL-17, their ability to migrate in a matrigel invasion assay increased (Zhu et al., 2008).
Poor outcome in breast cancer has been associated with a tumor cytokine profile showing increased expression of IL-1, IL-5, IL-6, IL-17, IFN-β and NFκB (Eiro et al., 2012). IL-1 is consistently found in breast cancer biopsies and can be derived from adipocytes or tumor infiltrating macrophages. In a MCF-7 mouse model, IL-1α enhanced tumor growth and wasting (Kumar et al., 2003), whereas, inhibition of SDF-1α and IL-1β significantly reduced tumor growth (Schmid et al., 2011).
Both innate and adaptive cells have leptin receptors and can respond to stimulation by the elicitation of cytokines. These cells include monocytes/macrophages, dendritic, natural killer, T and B cells as well as Tregs (Watanabe et al., 2010). When leptin binds to its receptor on these cells, proinflammatory cytokines are produced such as IL-1, IL-6, IL-12, TNF-α and IFN-γ (Iikuni et al., 2008).
3.1. Main inflammatory cytokines
Leptin and IL-1 synergic actions can activate NFκB, which increases VEGF (Gonzalez-Perez et al., 2010). Leptin-induced IL-1 could enhance the synthesis of macrophage chemoattractant protein macrophage (MCP-1) by breast cancer cells leading to the recruitment and activation of infiltrating TAM. MCP-1 also correlates to known angiogenic factors (i.e., VEGF, TNF-α, and IL-8) (Ueno et al., 2000). It is known that macrophages respond to leptin stimulation by secreting proinflammatory and pro-angiogenic cytokines, i.e. IL-1, TNFα, IL-1, IL-6, IL-12 and nitric oxide, and thus leptin may modulate macrophage phenotype: M1 (Th1 immunity-proinflammatory/cytotoxic) and M2 (suppress Th1/anti-inflammation/pro-angiogenesis) activation profiles (Loffreda et al., 1998). However, despite that breast cancer express many inflammatory and angiogenic cytokines only few reports show results from simultaneous determinations of cytokines and leptin within tumor tissue.
Cytokines have diverse effects in breast cancer. Some cytokines IL-1, IL-6, interleukin 11 (IL-11), transforming growth factor beta (TGF-β)] stimulate while others [interleukin 12 (IL-12), interleukin-18 (IL-18), interferons (IFNs)] inhibit breast cancer proliferation and/or invasion (Nicolini et al., 2006). In addition, the expression of interleukin 8 (IL-8) correlates with breast cancer angiogenesis, tumorigenicity, and metastasis (Waugh and Wilson, 2008). Therefore, many cytokines have been used as biomarkers of prognosis and outcomes of breast cancer. Several cytokines show altered levels in breast cancer. Table 2 shows the expression of main inflammatory cytokines (IL-1, IL-6 and TNF-α) linked to leptin in breast cancer tissues.
Table 2.
Expression of inflammatory cytokines in breast cancer.
| Breast cancer |
Subtype | IL-1 | IL-6 | TNF-α | Other CK | Technique | Reference |
|---|---|---|---|---|---|---|---|
| Carcinoma | Metastasic | 6.9 pg/ml; p < 0.0001 |
ELISA | Zhang et al. (1999) | |||
| No metastasic | 1.1 pg/ml (serum) |
||||||
| All tumors | 92% (+) [69/75) | ELISA | Yamashita et al. (1994) | ||||
| All tumors | ≈11 pg/ml/mg- p* |
||||||
| Stage IV tumors | 2859 pg/ml/mg | ||||||
| Poor differentiated tumors |
All components IL-1 system (+) |
IHC, ELISA, RT-PCR | Singer et al. (2006) | ||||
| All tumors (50% stage II) |
52–118 pg/ml (normal women 44–55 pg/ml) |
72–226 pg/ml (normal women 35–77 pg/ml) |
124–191 pg/ml (normal women 103–158 pg/ml) |
Multiplex bead array | Lyon et al. (2008) | ||
| Cell lines | |||||||
| MDA-MB-231 | >1000 pg/ml | MCP-1 ≈ 500 pg/ml | Multiplex (VersaMAP Development System; R&D System Inc.) |
Schmidt (2012) | |||
| IL-8 ≈ 1000 pg/ml | |||||||
| IL-2 ≈ 100–200 pg/ ml |
|||||||
| GM-CSF ≈ 1110 pg/ ml |
|||||||
| G-CSF ≈ 1000 pg/ml | |||||||
| MDA-MB-435 | IL-8 ≈10–20 pg/ml | Multiplex Development System; R&D System Inc.) |
Schmidt (2012) | ||||
| SK-BR-3 | MCP ≈ 1000 pg/ml | Multiplex (VersaMAP Development System; R&D System Inc.) |
Schmidt (2012) | ||||
| IL-2 ≈ 100 pg/ml | |||||||
| ZR-75-1 | IL-2 ≈ 100 pg/ml | Multiplex Development System; R&D System Inc.) |
Schmidt (2012) | ||||
| T47D | MCP-1 ≈100 pg/ml | Multiplex (VersaMAP Development System; R&D System Inc.) |
Schmidt (2012) | ||||
| IL-2 ≈ 100–200 pg/ ml |
|||||||
| MCF-7 | IL-2 ≈ 100–200 pg/ ml |
Multiplex Development System; R&D System Inc.) |
Schmidt (2012) | ||||
| IL-8 ≈ 50 pg/ml |
3.1.1. IL-1
Remarkably, all components of the IL-1 system (ligands IL-1α and β, antagonist IL-1Ra, and receptor IL-1R) are expressed in breast cancer. IL-1α is a member of the interleukin 1 family and is a proinflammatory cytokine induced in predominantly macrophages, neutrophils and endothelial cells. It synergistically reacts with TNF-α to promote inflammation and fever. IL-1β, another member of the IL-1 family, is produced by activated macrophages in response to stimulation as well as adipocytes . It is a proinflammatory cytokine that causes cell proliferation and is increased in a number of chronic inflammatory diseases. IL-1 is a pluripotent cytokine that promotes angiogenesis, tumor growth, and metastasis in experimental models and in several tumor types including non-small-cell lung carcinoma, colorectal adenocarcinoma, and melanoma tumor (Elaraj et al., 2006).
Adipocytes produce both leptin and IL-1 and high levels in breast cancer reflects poor prognosis (Singer et al., 2003). It was recently shown that leptin stimulates breast cancer cells to produce IL-1 through the VEGF/VEGFR2 signaling pathway thus perhaps modulating angiogenesis in breast cancer (Zhou et al., 2011).
The expression of IL-1 in breast cancer is associate d with aggressive tumor phenotype. Moreover, IL-1 gene is frequently expressed in metastases from patients with several types of human cancers. IL-1α was detected in both malignant and stroma cells and, correlated with poor differentiation . High level of IL-1R type I was also consistently found in breast cancer tissue. A fine balance of levels of IL-1 system components is probably required for cancer development (Singer et al., 2006). Indeed, activation of the IL-1/IL-1R cytokine family via autocrine and/or paracrine mechanisms leads to a cascade of secondary protumorigenic cytokines. IL-1 induces IL-8 expression in vitro in human breast cancer cell lines (Pantschenko et al., 2003a). Other report suggested that IL-6 and IL-1β stimulated the activity of aromatase (Honma et al., 2002) in triple negative (SK-BR3) and estrogen responsive (MCF-7) breast cancer cell lines Interestingly, it was earlier found that IL-1α levels correlated inversely with ER levels (p < 0.06), whereas IL-1Ra levels correlated directly with both ER levels (p < 0.009) and IL-1β levels (p < 0.06)(Miller et al., 2000). Later, it was confirmed that IL-1α inversely correlated to ERα (Pantschenko et al., 2003a). This suggests that the expression of IL-1 in poorly differentiated ERα (−) tumors could contribute to the malignant phenotype (Singer et al., 2006).
3.1.2. IL-6
IL-6 is a proinflammatory cytokine and an acute phase protein produced by T cells, macrophages, adipocytes, smooth muscle cells, osteoblasts and the liver. This cytokine is found within breast cancer tissue and adipocytes (Basolo et al., 1996). Breast cancer and stromal cells can also produce IL-6 (Dirat et al., 2011; Fantuzzi, 2005; Walter et al., 2009), which enhances tumor cell migration and invasion and contributes to tumor drug sensitivity (Conze et al., 2001). IL-6 acts as a differentiation factor of B cells, which are transformed into plasma secreting immunoglobulin cells. When IL-6 transcription is inhibited in a ERbB2 breast cancer mouse model, mammary cell tumors are significantly diminished (Rokavec et al., 2012). Obese women have higher circulating levels of leptin and IL-6, a poorer prognosis of breast cancer survival and IL-6 and leptin are detected in breast cancer tissue (Maccio et al., 2010).
Expression of IL-6 is closely associated with clinical stage in human breast cancer. However, patients without breast cancer show high levels of IL-6, which have significant relationships with obesity and insulin resistance (Kern et al., 2001). IL-6 was detectable in 92% of breast cancers (69/75) and correlated to breast cancer stage. IL-6 levels were significantly higher in bigger tumors and, stage IV than in stage I-III patients and, those with distant metastas is. However, no significant association was found between IL-6 concentration and age, histological type, histological grade, lymph node involvement, or hormone receptor status (Yamashita et al., 1994). Similarly, investigations in Japanese patients with metastatic breast cancer suggested that higher IL-6 serum levels correlated to significantly poor response to chemo-endocrine therapy and survival. Multivariate analysis revealed that IL-6, as well as disease-free interval, were independent prognostic factors of metastatic breast cancer (Zhang and Adachi, 1999).
3.1.3. IL-17
IL-17 is a proinflammatory cytokine induced by IL-23 in immune cells, CD4+, CD8+, γδ T cells and natural killer cells. It is a member of the IL-17 family, of which most prominent, IL-17α enhances the immune response to infections and can induce the production of IL-6. It synergistically works with TNF-α and IL-1β to cause many chronic inflammatory diseases including autoimmune disease. IL-17 potential actions in breast cancer were described few years ago by Zhu et al. in 2008. These authors reported that IL-17 protein is expressed in cell lines and had a potential role in breast cancer. IL-17 was found largely restricted to macrophages. IL-17 directly induced breast cancer cell invasion that was inhibited by MMP selective antagonists. However, MMP-2, MMP-3 or MMP-9 were not involved in IL-17 actions, raising the possibility of other classes of protease being involved (Zhu et al., 2008).
Leptin treated CD4+ cells expressed IL-17 (Won et al., 2011), while macrophages treated with leptin produced both IL-6 and TNF-α (Pantschenko et al., 2003b) and increased chemotaxis (Gruen et al., 2007). Dendritic cells treated with leptin increased production of IL-1β, IL-6, IL-12, TNF-α and MIP-1α (Mattioli et al., 2005). Human bone marrow-mesenchymal stem cells stimulated with IL-17 expressed leptin (Noh, 2012). Obese women have increased leptin in circulation that correlates with high levels of IL-17 (Sumarac-Dumanovic et al., 2009)(26).
3.1.4. TNF-α
TNF-α is a multifunctional cytokine involved in inflammation, cell survival and apoptosis acting via two receptors (TNFR1 and TNFR2). TNF-α is a T-lymphocyte differentiation factor mainly produced by activated macrophages, Tlymphocytes, and natural killer (NK) cells. In contrast, fibroblasts, smooth muscle cells, and tumor cells express low levels of TNF-α. There are some contradictory data published on the role of TNF-α in breast cancer. Low serum concentrations of TNF-α and lack of correlation to breast cancer clinico-pathological parameters have been reported (Zhang and Adachi, 1999). However, high levels of TNF-α have been suggested as a predictor biomarker of advanced disease in comparison to low levels found in breast cancer early disease stages (Panis et al., 2012).
Membrane-bound pro-TNF is activated by TNF-converting enzyme (TACE) (Bemelmans et al., 1996). TNF-α acts synergistically with cytostatic drugs. Therefore, TNF-α is used in the treatment of advanced soft tissue sarcomas and metastatic melanomas and other irresectable tumors. TNF-α targets the tumor-associated vasculature inducing hyperpermeability and destruction of the vascular lining. This results in an immediate effect of selective accumulation of cytostatic drugs inside the tumor and a late effect of destruction of the tumor vasculature (van Horssen et al., 2006). Novel potential therapies of mesenchymal pre-activated with bone marrow stem cells with TNF-α have been suggested to upregulate TRAIL, which has cancer apoptotic activity and inhibited the progression of lung tumors formed from TNBC (Lee et al., 2012).
TNF-α has been linked to breast cancer development. Bcl-2 has been found directly regulated by NFκB in response to TNF-α (Wang et al., 2012). In addition, KiSS1 (a tumor suppressor) abrogates TNFa-induced NF-kB pathway and RhoA activation, thereby inhibiting TNF-α induced breast cancer cell migration (Cho et al., 2009). TNF-α has also been suggested to lead estrogen metabolism into more hormonally active and carcinogenic products in estrogen responsive breast cancer cells, MCF-7. This may implicate a new possible explanation for inflammation associated breast cancer (Kamel et al., 2012). Remarkably, TNFR1 activation has dual opposite actions: apoptosis (via death receptor activation cascade) and survival signaling (via MAPK, PI-3K and NFκB pathways) (Wang et al., 2012) (Wang et al., 2012). Overall, the regulation of TNF-α opposite actions in breast cancer is not well understood.
Entangled associations between leptin and TNF-α in breast cancer have been reported. In vitro 3TL adipocytes downregulate leptin mRNA expression by actions of inflammatory cytokines, IL-1 and TNF-α (Loffreda et al., 1998). Moreover, TNF-α via TNFR1 inhibited the expression of leptin in human and mouse adipocytes (Yamaguchi et al., 1998). In contrast, TNF-α induced the secretion of leptin from an pre-existent adipocyte pool (Kirchgessner et al., 1997). In addition, in vivo responses of genetically leptin-deficient mice show an opposite situation under the effects of inflammatory cytokines. Moreover, leptin is upregulated by IL-1 and TNF-α, but leptin can also upregulate these molecules in macrophages (Gonzalez et al., 2000b). Tissue factor (TF), considered a hallmark of cancer progression, was upregulated by leptin via transcriptional regulation of TNF-α in breast cancer cells (Napoleone et al., 2012). Additionally, TNF-α, and leptin plasma levels were simultaneously decreased in female Sprague Dawley rats under regimens designed for limiting energy availability via diet or physical activity (Zhu et al., 2012).
3.2. Other inflammatory cytokines
CSF is a cytokine that promotes breast cancer, but it is also used to the prophylactic reduction of the severity and duration of neutropenia as well as the incidence of febrile neutropenia after cancer chemotherapy (Trueman, 2009).
IL-8 is an inflammatory and angiogenic cytokine that shows elevate levels in breast cancer. As mentioned, IL-1α and IL-1β induced IL-8 stimulation up to 104-fold in normal mammary epithelial cells, but increase IL-8 3–10-fold more in breast cancer cells unresponsive to estrogen. IL-8 is also upregulated by TNF-α and -β, but to lower levels (2- to 8-fold) in breast cancer cells unresponsive to estrogen and has no significant effects in breast cancer cells responsive to estrogens (Pantschenko et al., 2003b) .
Interleukin 4 (IL-4) was originally described as a B cell growth factor and a key cytokine for Th2 type immune reactions that induce immune-tolerance and angiogenesis. IL-4 upregulates adhesion molecules, inhibit cell proliferation, and mediate signal transduction in breast cancer cells. However, IL-4 has potent anti-tumor activity against various tumors, including breast cancer (Nagai and Toi, 2000) IL-4 receptors have been suggested as novel targets for cancer cytotoxin therapy (Kawakami et al., 2001). Interleukin-10 (IL-10) can also exert dual proliferative and inhibitory effect on breast tumor cells indicating a complex role of IL-10 in breast cancer initiation and progression. IL-10 induces immunotolerance, which allows breast cancer cells to escape from mechanisms of immune surveillance (Hamidullah et al., 2012).
Though, there is not consistent consensus for the clinical meaning of differential levels of cytokine expressions in breast cancer. Levels of cytokines were markedly different in women with breast cancer as compared with those in women who did not have breast cancer. For instance, G-CSF, and IL-17 showed high levels in women with breast cancer compared to women without breast cancer. Significant increased levels of IL-8 and macrophage inflammatory protein-1β (MIP-1) correlated to increased age in women without breast cancer (Lyon et al., 2008). However, high circulating levels of some cytokines seem to be adverse prognostic indicators (IL-1β, IL-6, IL-8, IL-10, IL-18, gp130). IL-2, IFNs (α, β, and γ), IL-6, IL-12 have been used for treatment of advanced breast cancer either to induce or increase hormone sensitivity and/or to stimulate cellular immunity (Nicolini et al., 2006). Cytokines actions are also related to breast cancer stem cells (BCSC) (Korkaya et al., 2011), which will be discussed later.
4. Leptin induces IL-1 system in breast cancer
Leptin, OB-R and IL-1 systems are co-expressed in breast cancer. The IL-1 system is composed of IL-1 two ligands (IL-1α and IL-1β; both 17 kDa), two receptor types I (IL-1 R tI, 80 kDa) and II (IL-1R tII, 60–68 kDa) and a receptor antagonist (IL-1 Ra, 25 kDa). These molecules play important roles in the regulation of inflammatory processes .
Tissue array analysis of breast cancer samples (n = 75) suggested that IL-1 and leptin systems are simultaneously expressed by cancer and stroma cells (Colbert et al., 2012). In addition, mice hosting human breast cancer xenografts derived from human MCF-7 (ER+) and MDA-MB231 cells (triple negative: TNBC; ER-, PR- and HER2-) and treated with PEG-LPrA2 (a potent and specific leptin signaling antagonist) (Gonzalez and Leavis, 2003; Gillespie et al., 2012; Gonzalez et al., 2006) showed effectively reduction of growth and expression of leptin, OB-R, and IL-1 receptor type I (Rene Gonzalez et al., 2009). Moreover, diet-induced-obesity (DIO)-mice hosting breast tumors induced by DMBA (a carcinogen, 7,12-dimethylbenz[a]anthracene) showed higher levels of IL-1, IL-1R tI and leptin/OB-R than normal mammary glands (Gillespie et al., 2012). Furthermore, PEG-LPrA2 treatment decreased the levels of IL-1R tI in mammary glands from DIO-mice (Gillespie et al., 2012). These data further assess that leptin and IL-1 system are closely related for the development of breast cancer.
We have previously found that leptin induces the in vitro expression of IL-1 system in breast cancer cells (Zhou et al., 2011). Leptin increased protein and mRNA levels of all components of the IL-1 system. IL-1 upregulation involved leptin activation of JAK2/STAT3, MAPK/ERK 1/2, PI-3K/AKT1, PKC, p38 and JNK. Moreover, in breast cancer cells leptin-induced phosphorylation of mTOR (mammalian target of Rapamycin)/4E-BP1 increased IL-1β and IL-1Ra expression, but downregulated IL-1α. Molecular analysis of leptin regulation of IL-1α promoter suggested that leptin-induced SP1 and NF-κB transcription factors were essential for IL-1α expression. In addition, leptin receptor (OB-Rb) was also upregulated by leptin in breast cancer cells (Zhou et al., 2011). These data strongly suggest that leptin and IL-1 systems are closely related in breast cancer.
It is probable that leptin induction of IL-1R tI is a general phenomenon in cancer cells. We have also found that leptin uses several signaling mechanisms to induce IL-1R tI in human endometrial cancer cells (i.e., An3Ca, SK-UT2 and Ishikawa cells) (Carino et al., 2008). However, IL-1β was only increased by leptin in benign primary epithelial endometrial cells. JAK2 and PI-3K activations were required for leptin increase of LIF, IL-1/IL-1R tI. Leptin-mediated activation of mTOR, mainly linked to MAPK, played a central role in leptin regulation of all cytokines and receptors (Carino et al., 2008). These results suggest that leptin’s effects are cell-specific and could confer a proliferative or cell survival advantage.
5. Leptin, cytokines and breast cancer angiogenesis
Leptin was earlier identified as a pro-angiogenic cytokine (Sierra-Honigrnann et al., 1998). Both leptin (Gonzalez-Perez et al., 2010) and IL-1 (Salven et al., 2002) upregulate VEGF, promote angiogenesis (Bouloumie et al., 1998; Gainsford et al., 1996) and are related to poor prognosis of breast cancer (Guo et al., 2012a). Leptin is as potent as VEGF in promoting tumor angiogenesis. In addition, leptin phosphorylates VEGFR-2 independent of VEGF in endothelial (Garonna et al., 2011) and breast cancer cells.
We have early identified that the inhibition of leptin signaling via PEG-LPrA2 impaired the expression of VEGF and VEGFR-2 in mouse 4T1-cell derived syngeneic mammary cancer (Gonzalez et al., 2006). In 4T1 cells leptin increases the expression of VEGF, VEGFR-2, and cyclin D1 through JAK2/STAT3, and/or PI-3K and ERK 1/2. In contrast to leptin-induced levels of cyclin D1 the changes in VEGF or VEGFR-2 were more dependent on specific signaling pathways. Moreover, incubation of 4T1 cells with anti-VEG-FR-2 antibody increased leptin-mediated VEGF expression suggesting an autocrine/paracrine loop (Gonzalez et al., 2006).
Similar results were found in human breast cancer xenografts hosted by severe immunodeficient mice (SCID–BALB/C). PEG-LPrA 2 more effectively reduced the growth of ER+ (>40-fold) than ER-BC (twofold) and expression of pro-angiogenic (VEGF/VEGFR2, leptin/OB-R, and IL-1 R tI and pro-proliferative molecules (proliferating cell nuclear antigen, PCNA, and cyclin D1) in ER+ than in ER− BC. Interestingly, mouse tumor stroma in ER + BC expressed high levels of VEGF and leptin that was induced by leptin signaling (Rene Gonzalez et al., 2009). Furthermore, comparable results were found in female C57BL/6J mice, which are unresponsive to DMBA-induced mammary tumors without hormonal stimulation (Gillespie et al., 2012). Remarkably, accelerated development of DMBA-mammary tumors was found in C57BL/6J mice treated with DMBA fed a high fat diet. Strikingly, PEG-LPrA2 inhibition of leptin signaling in these mice prevented the development of mammary tumors and reduced the levels of several molecules with angiogenic roles in breast cancer, including VEGF/VEGFR-2, OB-R, hypoxia inducible factor-1 alpha (HIF-1α), NFκB p105, IL-1R tI and Notch (Gillespie et al., 2012).
Additionally, leptin also increased the levels of VEGF and VEG-FR-2 in human endometrial cancer cells compared with benign endometrial cells. Leptin-induction of VEGF levels in endometrial cancer cells was related to the activation of MAPK/ERK1/2 and mTOR but not to PI-3K/AKT1 signaling pathway (Carino et al., 2008). In addition to its angiogenic actions in ECs, the VEGF/VEG-FR-2 signaling paracrine–autocrine loop functions as an important survival process in cancer cells (Guo et al., 2010).
We further described comprehensive mechanisms for leptin upregulation of VEGF/VEGFR-2 transcriptional expression in breast cancer cells (Gonzalez-Perez et al., 2010). Deletion analysis of VEGF promoter via transfection of VEGF-Luc reporters (full-length and transcription factor-binding deletions) and RNA knockdown showed that HIF-1α and NFκB were essentials for leptin regulation of VEGF. Leptin activation of HIF-1α was mainly linked to canonic (MAPK, PI-3K) and non-canonic (PKC, JNK and p38 MAP) signaling pathways. Leptin non-canonic signaling pathways (JNK, p38 MAP and to less extent PKC) were linked to NFκB activation. SP1 was involved in leptin regulation of VEGF. AP1 was not involved and AP2 repressed leptin-induced increase of VEGF. Overall, these data suggest that leptin signaling regulates VEGF in breast cancer mainly through HIF-1α and NFκB (Gonzalez-Perez et al., 2010). In addition, it was found that leptin-mediated upregulation of IL-1 system in breast cancer cells was closely related to VEGF (Zhou et al., 2011).
Notch is a known pro-angiogenic factor linked to poor prognosis of breast cancer (Guo et al., 2011). Leptin was identified as an inducer of Notch (ligands JAG1 and Dll-4; receptors Notch1–4 and targeted molecules, survivin and Hey2) in breast cancer (Guo and Gonzalez-Perez, 2011; Guo et al., 2011) and endothelial cells (Lanier et al., 2012). In breast cancer cells, RNA knockdown and pharmacological inhibitors of leptin signaling significantly abrogated activity of reporter gene-lucifer ase CSL promoter (RBP-Jk, an essential transcription factor involved in Notch transcriptional actions). These investigations showed that leptin regulation of CSL involved the activation of JAK2/STAT3, MAPK, PI-3K/mTOR, p38 and JNK signaling pathways. Leptin activated NFκB, Sp1 and HIF-1α increasing the expression of Notch (Guo and Gonzalez-Perez, 2011; Guo et al., 2011) and VEGF mRNA and protein (Gonzalez-Perez et al., 2010) in breast cancer cells under normoxic conditions. Interestingly, leptin upregulatory effects on cell proliferation/migration and pro-angiogenic factors Notch, IL-1 and VEGF/VEGFR-2 were abrogated by a γ-secretase inhibitor (an essential protease activating membrane-bound receptors), DAPT, as well as siRNA against CSL. Moreover, leptin upregulation of VEGF/VEGFR2 was impaired by IL-1 signaling blockade (Zhou et al., 2011). In addition, blockade of IL-1R tI inhibited leptin-induced Notch, Hey2 and survivin expression. These data suggest that leptin pro-angiogenic signature in breast cancer is linked to, or regulated, in part by IL-1 signaling. We show for the first time that a novel unveiled crosstalk between Notch, IL-1 and leptin (NILCO) occurs in breast cancer. These data reinforce the notion that leptin and IL-1 crosstalk is essential for leptin pro-angiogenic actions (Guo and Gonzalez-Perez, 2011). Leptin induction of proliferation/migration and upregulation of VEGF/VEGFR-2 in breast cancer cells were related to an intact Notch signaling axis. NILCO could represent the integration of developmental, proinflammatory and pro-angiogenic signals critical for leptin-induced cell proliferation/migration and regulation of VEGF/VEG-FR-2 in breast cancer.
Overall, leptin pro-angiogenic actions in breast cancer likely proceeds by mechanisms involving tumor endothelial (rapid response: leptin/OB-R mediated pVEGFR2 and mid-term response: leptin-induced Notch), breast cancer (leptin induces VEGF expression, which induces tumor angiogenesis) and stromal cells (leptin induces the secretion of pro-angiogenic factors). However, leptin pro-angiogenic actions in breast cancer are mediated by poorly characterized mechanisms.
6. Leptin, cytokines and breast cancer stem cells (BCSC)
BCSC are defined by their ability to undergo self-renewal, as well as tumor differentiation. BCSC initiate and drive carcinogenesis and differentiation contributing to tumor cellular heterogeneity through deregulation of the self-renewal process (Pang and Argyle, 2009). Thus, the population of BCSC may be a risk factor for carcinogenesis (Kakarala and Wicha, 2008). It has been suggested that breast cancer and stroma cells secreted cytokines and growth factors that eventually could enhance the proliferation and survival of BCSCs, induce angiogenesis, and recruit tumor-associated macrophages and other immune cells, which secrete additional growth factors, forming a positive feedback loop that promotes tumor cell invasion and metastasis (Korkaya et al., 2011).
BCSC are identified via molecular phenotypic markers (CD44+CD24−/ALDH1+) (Kakarala and Wicha, 2008). We have found that leptin induces the expression of CD44 and ALDH1 in several breast cancer cell lines (Guo et al., 2012b). Leptin is also involved in the regulation of factors associated to BCSC, i.e., HER2 (Korkaya et al., 2008; Korkaya and Wicha, 2009), Akt (Korkaya et al., 2009) as well as transcriptional factors, such as STAT3 (Zhou et al., 2007), NF-κB (Pratt et al., 2009). Notably, leptin (Kern et al., 2001; Yamashita et al., 1994), IL-6 (Iliopoulos et al., 2009; Liu et al., 2011) and, IL-8 (Ginestier et al., 2010) could mediate the regulation of BCSC renewal. In addition, leptin also activates the Notch signaling pathway (Guo and Gonzalez-Perez, 2011; Guo et al., 2011; Knight et al., 2011) that is important for the maintenance of BCSC through the inhibition of differentiation (Artavanis-Tsakonas et al., 1999; Leong and Karsan, 2006; Radtke and Raj, 2003).
The NF-κB signaling pathway, which can be triggered by leptin (Guo et al., 2012a), is essential for the regulation of IL-6 and IL-8 (Barnes and Karin, 1997). NF-κB was very early identified as a Notch target gene (Oswald et al., 1998), which mediate Notch angiogenic actions (Wang et al., 2007; Johnston et al., 2009). Notch signaling targets cyclin D1 (Ronchini and Capobianco, 2001) and cmyc (Artavanis-Tsakonas et al., 1999), which are also involved in angiogenesis (Ronchini and Capobianco, 2001; Weng et al., 2006). Then, leptin induction of Notch in breast cancer (Gruen et al., 2007; Mattioli et al., 2005) could be related to leptin upregulation of cyclin D1.Indeed, leptin was early identified as an inducer of the oncogenic protein cyclin D1 (Gonzalez et al., 2006; Rene Gonzalez et al., 2009).
Leptin upregulates several genes, including BCSC biomarkers (CD44 and ALDH1), Notch and Wnt, angiogenic factors, cell cycle activators, chromosome regulators and mesenchymal markers in breast cancer (Guo et al., 2012b). Leptin-induced effects correlated with the activation of STAT3 and upregulation of a functional VEG-FR-2/Notch axis, which was linked to the increased proliferation and migration of breast cancer cells. Therefore, we propose a novel mechanism for leptin induction of BCSC through a functional VEG-FR-2/Notch axis (Guo et al., 2012b).
Similarly, IL-6 promotes breast cancer bone metastasis via Jagged1 (JAG1)/Notch signaling (Sethi et al., 2011). Leptin deficiency is related to low levels of BCSC in murine mammary tumor virus (MMTV)-Wnt-1 transgenic mice (Zheng et al., 2011). Moreover, embryonic pluripotent stem cells exhibit sensitized responses to leptin, including the phosphorylation and activation of STAT3 and induction of Oct4 and Sox2, thereby establishing a self-reinforcing signaling module (Feldman et al., 2011).
BCSC are believed to be responsible for the development of drug resistance and relapse of breast cancer. Triple negative breast cancer (TNBC: ER-, PR- and HER2-) is an aggressive form of the disease that has an early onset, and is associated with poor survival and a resistance to common therapeutic treatments. We have recently found that leptin is a factor promoting survival of ER+ and TNBC. Moreover, leptin significantly decreased the chemotherapeutic effects of cisplatin on breast cancer cells. Leptin-induced chemotherapeutic resistance was related to the activation of a crosstalk between Notch and Wnt signaling pathways in MCF-7 (ER+) and MDA-MB231 (TNBC). Interestingly, leptin-induced effects were dependent of an intact Notch–Wnt axis in TNBC. The mechanisms of leptin-induced drug resistance activation were related to the activation of Wnt/β-catenin, Notch (Notch1–4 and JAG1/Dll-4 and targets survivin/Hey2), STAT3 and VEGF/VEGFR-2. This may imply that obesity, characterized by elevated leptin levels, could negatively affect the outcome of TNBC treatment. Taken together, this data supports the theory that inhibition of leptin signaling could be a novel way to prevent and treat TBNC, particularly in the context of obesity and abnormal Wnt and Notch signaling (McGlothen et al., 2011).
7. Conclusions and perspectives
Breast cancer progression and metastas is are closely related to the secretion, actions and crosstalk of an array of cytokines. Stroma and breast cancer cells modify their behavior through multiples signaling and crosstalks, being the leptin cytokine crosstalk a main mechanism for tumor development. Pandemic obesity and high levels of leptin could represent additional risks for breast cancer development. Actions of leptin–cytokine crosstalk in breast cancer and immune cells elicit tumor angiogenesis and increase the proliferation of BCSC, which could decrease therapy effectiveness and increase relapse. Targeting leptin–cytokine crosstalk could enhance therapy potential, especially in obese patients showing higher levels of leptin and poorer prognosis.
Supplementary Material
Acknowledgments
This work was partially funded by Grants from NIH/NCI 1SC1CA138658-05; and the Georgia Cancer Coalition Distinguished Cancer Scholar Award to R.R.G-P., U54 MSM/TU/UAB, NIH/2G12RR003034-26 and facilities and support services at Morehouse School of Medicine (NIH1C06 RR18386) and NIH/NCRR Grant 1G12RR026250-03.
Footnotes
Appendix A. Supplementary material Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.mce.2013.03.025.
References
- Ahima RS, Osei SY. Leptin signaling. Physiol. Behav. 2004;81:223–241. doi: 10.1016/j.physbeh.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Aritomi M, Kunishima N, Okamoto T, Kuroki R, Ota Y, Morikawa K. Atomic structure of the GCSF-receptor complex showing a new cytokine–receptor recognition scheme. Nature. 1999;401:713–717. doi: 10.1038/44394. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y, Lewin MJ. The stomach is a source of leptin. Nature. 1998;394:790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- Bartella V, Cascio S, Fiorio E, Auriemma A, Russo A, Surmacz E. Insulin-dependent leptin expression in breast cancer cells. Cancer Res. 2008;68:4919–4927. doi: 10.1158/0008-5472.CAN-08-0642. [DOI] [PubMed] [Google Scholar]
- Basolo F, Fiore L, Fontanini G, Conaldi PG, Calvo S, Falcone V, Toniolo A. Expression of and response to interleukin 6 (IL6) in human mammary tumors. Cancer Res. 1996;56:3118–3122. [PubMed] [Google Scholar]
- Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, Lai CF, Tartaglia LA. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc. Natl. Acad. Sci. USA. 1996;93:8374–8378. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemelmans MH, van Tits LJ, Buurman WA. Tumor necrosis factor: function, release and clearance. Crit. Rev. Immunol. 1996;16:1–11. doi: 10.1615/critrevimmunol.v16.i1.10. [DOI] [PubMed] [Google Scholar]
- Bernstein L, Ross RK. Endogenous hormones and breast cancer risk. Epidemiol. Rev. 1993;15:48–65. doi: 10.1093/oxfordjournals.epirev.a036116. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J. Biol. Chem. 1999;274:30059–30065. doi: 10.1074/jbc.274.42.30059. [DOI] [PubMed] [Google Scholar]
- Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc. Natl. Acad. Sci. USA. 1993;90:5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouloumie A, Drexler HC, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ. Res. 1998;83:1059–1066. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J. Cell Biol. 1992;119:629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- Cao Y. Angiogenesis modulates adipogenesis and obesity. J. Clin. Invest. 2007;117:2362–2368. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc. Natl. Acad. Sci. USA. 2001;98:6390–6395. doi: 10.1073/pnas.101564798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carino C, Olawaiye AB, Cherfils S, Serikawa T, Lynch MP, Rueda BR, Gonzalez RR. Leptin regulation of proangiogenic molecules in benign and cancerous endometrial cells. Int. J. Cancer. 2008;123:2782–2790. doi: 10.1002/ijc.23887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter B, Hemsworth GR, Wu Z, Maamra M, Strasburger CJ, Ross RJ, Artymiuk PJ. Structure of the human obesity receptor leptin-binding domain reveals the mechanism of leptin antagonism by a monoclonal antibody. Structure. 2012;20:487–497. doi: 10.1016/j.str.2012.01.019. [DOI] [PubMed] [Google Scholar]
- Catalano S, Marsico S, Giordano C, Mauro L, Rizza P, Panno ML, Ando S. Leptin enhances, via AP-1, expression of aromatase in the MCF-7 cell line. J. Biol. Chem. 2003;278:28668–28676. doi: 10.1074/jbc.M301695200. [DOI] [PubMed] [Google Scholar]
- Catalano S, Mauro L, Marsico S, Giordano C, Rizza P, Rago V, Montanaro D, Maggiolini M, Panno ML, Ando S. Leptin induces, via ERK1/ERK2 signal, functional activation of estrogen receptor alpha in MCF-7 cells. J. Biol. Chem. 2004;279:19908–19915. doi: 10.1074/jbc.M313191200. [DOI] [PubMed] [Google Scholar]
- Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- Chen X, Zha X, Chen W, Zhu T, Qiu J, Roe OD, Li J, Wang Z, Yin Y. Leptin attenuates the anti-estrogen effect of tamoxifen in breast cancer. Biomed. Pharmacother. 2012 doi: 10.1016/j.biopha.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Cho SG, Li D, Stafford LJ, Luo J, Rodriguez-Villanueva M, Wang Y, Liu M. KiSS1 suppresses TNFalpha-induced breast cancer cell invasion via an inhibition of RhoA-mediated NF-kappaB activation. J. Cell. Biochem. 2009;107:1139–1149. doi: 10.1002/jcb.22216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary MP, Phillips FC, Getzin SC, Jacobson TL, Jacobson MK, Christensen TA, Juneja SC, Grande JP, Maihle NJ. Genetically obese MMTV-TGF-alpha/Lep(ob)Lep(ob) female mice do not develop mammary tumors. Breast Cancer Res. Treat. 2003;77:205–215. doi: 10.1023/a:1021891825399. [DOI] [PubMed] [Google Scholar]
- Cleary MP, Juneja SC, Phillips FC, Hu X, Grande JP, Maihle NJ. Leptin receptor-deficient MMTV-TGF-alpha/Lepr(db)Lepr(db) female mice do not develop oncogene-induced mammary tumors. Exp. Biol. Med. (Maywood) 2004;229:182–193. doi: 10.1177/153537020422900207. [DOI] [PubMed] [Google Scholar]
- Clemons M, Goss P. Estrogen and the risk of breast cancer. N. Engl. J. Med. 2001;344:276–285. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- Colbert L, McGlothen T, Wilson K, Gillespie C, Dickson T, Guo S, Gonzalez-Perez RR. Differential expression of NILCO reveals pathogenesis of human breast cancer. Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research. AACR; Chicago, IL, USA. 2012. [Google Scholar]
- Conze D, Weiss L, Regen PS, Bhushan A, Weaver D, Johnson P, Rincon M. Autocrine production of interleukin 6 causes multidrug resistance in breast cancer cells. Cancer Res. 2001;61:8851–8858. [PubMed] [Google Scholar]
- Coxon A, Bolon B, Estrada J, Kaufman S, Scully S, Rattan A, Duryea D, Hu YL, Rex K, Pacheco E, Van G, Zack D, Feige U. Inhibition of interleukin-1 but not tumor necrosis factor suppresses neovascularization in rat models of corneal angiogenesis and adjuvant arthritis. Arthritis Rheum. 2002;46:2604–2612. doi: 10.1002/art.10546. [DOI] [PubMed] [Google Scholar]
- Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, Garrido I, Escourrou G, Valet P, Muller C. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, Buyse M, Baum M, Buzdar A, Colleoni M, Coombes C, Snowdon C, Gnant M, Jakesz R, Kaufmann M, Boccardo F, Godwin J, Davies C, Peto R. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J. Clin. Oncol. 2010;28:509–518. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- Duhrsen U, Martinez T, Vohwinkel G, Ergun S, Sun L, McMahon G, Durig J, Hossfeld DK, Fiedler W. Effects of vascular endothelial and platelet-derived growth factor receptor inhibitors on long-term cultures from normal human bone marrow. Growth Factors. 2001;19:1–17. doi: 10.3109/08977190109001072. [DOI] [PubMed] [Google Scholar]
- Eiro N, Gonzalez L, Gonzalez LO, Fernandez-Garcia B, Lamelas ML, Marin L, Gonzalez-Reyes S, Del Casar JM, Vizoso FJ. Relationship between the inflammatory molecular profile of breast carcinomas and distant metastasis development. PloS One. 2012;7:e49047. doi: 10.1371/journal.pone.0049047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elaraj DM, Weinreich DM, Varghese S, Puhlmann M, Hewitt SM, Carroll NM, Feldman ED, Turner EM, Alexander HR. The role of interleukin 1 in growth and metastasis of human cancer xenografts. Clin. Cancer Res. 2006;12:1088–1096. doi: 10.1158/1078-0432.CCR-05-1603. [DOI] [PubMed] [Google Scholar]
- Faggioni R, Fantuzzi G, Fuller J, Dinarello CA, Feingold KR, Grunfeld C. IL-1 beta mediates leptin induction during inflammation. Am. J. Physiol. 1998;274:R204–8. doi: 10.1152/ajpregu.1998.274.1.R204. [DOI] [PubMed] [Google Scholar]
- Faggioni R, Feingold KR, Grunfeld C. Leptin regulation of the immune response and the immunodeficiency of malnutrition. FASEB J. 2001;15:2565–2571. doi: 10.1096/fj.01-0431rev. [DOI] [PubMed] [Google Scholar]
- Fanggioni R, Fantuzzi G, Fuller J, et al. IL-1 beta mediates leptin induction during inflammation. Am. J. Physiol. 1998;274:R204–R208. doi: 10.1152/ajpregu.1998.274.1.R204. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. (quiz 920) [DOI] [PubMed] [Google Scholar]
- Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J. Leukoc. Biol. 2000;68:437–446. [PubMed] [Google Scholar]
- Feldman DE, Chen C, Punj V, Tsukamoto H, Machida K. Pluripotency factor-mediated expression of the leptin receptor (OB-R) links obesity to oncogenesis through tumor-initiating stem cells. Proc. Natl. Acad. Sci. USA. 2011 doi: 10.1073/pnas.1114438109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck BN, Kelley KW, Dantzer R, Johnson RW. In vivo and in vitro evidences for the involvement of tumor necrosis factor-alpha in the induction of leptin by lipopolysaccharide. Endocrinology. 1998;139:2278–2283. doi: 10.1210/endo.139.5.6012. [DOI] [PubMed] [Google Scholar]
- Fruhbeck G. Intracellular signalling pathways activated by leptin. Biochem. J. 2006;393:7–20. doi: 10.1042/BJ20051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriely I, Ma XH, Yang XM, Rossetti L, Barzilai N. Leptin resistance during aging is independent of fat mass. Diabetes. 2002;51:1016–1021. doi: 10.2337/diabetes.51.4.1016. [DOI] [PubMed] [Google Scholar]
- Gainsford T, Willson TA, Metcalf D, et al. Leptin can induce proliferation, and functional activation of hemopoietic cells. Proc. Natl. Acad. Sci. USA. 1996;93:14564–14568. doi: 10.1073/pnas.93.25.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, Russo A, Sulkowski S, Surmacz E. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin. Cancer Res. 2006;12:1447–1453. doi: 10.1158/1078-0432.CCR-05-1913. [DOI] [PubMed] [Google Scholar]
- Garonna E, Botham KM, Birdsey GM, Randi AM, Gonzalez-Perez RR, Wheeler-Jones CP. Vascular endothelial growth factor receptor-2 couples cyclo-oxygenase-2 with pro-angiogenic actions of leptin on human endothelial cells. Plos One. 2011;6:e18823. doi: 10.1371/journal.pone.0018823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie C, Quarshie A, Penichet M, Gonzalez-Perez RR. Potential role of leptin signaling in DMBA-induced mammary tumors by non-responsive C57BL/6J mice fed a high-fat diet. J. Carcinogene Mutagene. 2012:3. [Google Scholar]
- Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum D, Guan JL, Dontu G, Wicha MS. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J. Clin. Invest. 2010;120:485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez RR, Leavis PC. A peptide derived from the human leptin molecule is a potent inhibitor of the leptin receptor function in rabbit endometrial cells. Endocrine. 2003;21:185–195. doi: 10.1385/ENDO:21:2:185. [DOI] [PubMed] [Google Scholar]
- Gonzalez RR, Caballero-Campo P, Jasper M, Mercader A, Devoto L, Pellicer A, Simon C. Leptin and leptin receptor are expressed in the human endometrium and endometrial leptin secretion is regulated by the human blastocyst. J. Clin. Endocrinol. Metab. 2000a;85:4883–4888. doi: 10.1210/jcem.85.12.7060. [DOI] [PubMed] [Google Scholar]
- Gonzalez RR, Simon C, Caballero-Campo P, Norman R, Chardonnens D, Devoto L, Bischof P. Leptin and reproduction. Hum. Reprod. Update. 2000b;6:290–300. doi: 10.1093/humupd/6.3.290. [DOI] [PubMed] [Google Scholar]
- Gonzalez RR, Cherfils S, Escobar M, Yoo JH, Carino C, Styer AK, Sullivan BT, Sakamoto H, Olawaiye A, Serikawa T, Lynch MP, Rueda BR. Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2) J. Biol. Chem. 2006;281:26320–26328. doi: 10.1074/jbc.M601991200. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Perez RR, Xu Y, Guo S, Watters A, Zhou W, Leibovich SJ. Leptin upregulates VEGF in breast cancer via canonic and non-canonical signalling pathways and NFkappaB/HIF-1alpha activation. Cell Signal. 2010;22:1350–1362. doi: 10.1016/j.cellsig.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruen ML, Hao M, Piston DW, Hasty AH. Leptin requires canonical migratory signaling pathways for induction of monocyte and macrophage chemotaxis. Am. J. Physiol. Cell Physiol. 2007;293:C1481–8. doi: 10.1152/ajpcell.00062.2007. [DOI] [PubMed] [Google Scholar]
- Guo S, Gonzalez-Perez RR. Notch, IL-1 and leptin crosstalk outcome (NILCO) is critical for leptin-induced proliferation, migration and VEGF/VEGFR-2 expression in breast cancer. PloS One. 2011;6:e21467. doi: 10.1371/journal.pone.0021467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Colbert LS, Fuller M, Zhang Y, Gonzalez-Perez RR. Vascular endothelial growth factor receptor-2 in breast cancer. Biochim. Biophys. Acta. 2010;1806:108–121. doi: 10.1016/j.bbcan.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Liu M, Gonzalez-Perez RR. Role of Notch and its oncogenic signaling crosstalk in breast cancer. Biochim. Biophys. Acta. 2011;1815:197–213. doi: 10.1016/j.bbcan.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Liu M, Wang G, Torroella-Kouri M, Gonzalez-Perez RR. Oncogenic role and therapeutic target of leptin signaling in breast cancer and cancer stem cells. Biochim. Biophys. Acta. 2012a;1825:207–222. doi: 10.1016/j.bbcan.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Gillespie C, Liu M, Gonzalez-Perez RR. Autocrine stimulation of VEGFR-2 by leptin is associated with Notch signaling pathway and cancer stem cell marker expression. Cancer Res. 2012b;72:5297. [Google Scholar]
- Guo Y, Xu F, Lu T, Duan Z, Zhang Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat. Rev. 2012c;38:904–910. doi: 10.1016/j.ctrv.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Hamidullah, Changkija B, Konwar R. Role of interleukin-10 in breast cancer. Breast Cancer Res. Treat. 2012;133:11–21. doi: 10.1007/s10549-011-1855-x. [DOI] [PubMed] [Google Scholar]
- Henderson BE, Ross R, Bernstein L. Estrogens as a cause of human cancer: the Richard and Hinda Rosenthal Foundation award lecture. Cancer Res. 1988;48:246–253. [PubMed] [Google Scholar]
- Henson MC, Castracane VD. Leptin: roles and regulation in primate pregnancy. Semin. Reprod. Med. 2002;20:113–122. doi: 10.1055/s-2002-32502. [DOI] [PubMed] [Google Scholar]
- Hiroike T, Higo J, Jingami H, Toh H. Homology modeling of human leptin/leptin receptor complex. Biochem. Biophys. Res. Commun. 2000;275:154–158. doi: 10.1006/bbrc.2000.3275. [DOI] [PubMed] [Google Scholar]
- Honma S, Shimodaira K, Shimizu Y, Tsuchiya N, Saito H, Yanaihara T, Okai T. The influence of inflammatory cytokines on estrogen production and cell proliferation in human breast cancer cells. Endocr. J. 2002;49:371–377. doi: 10.1507/endocrj.49.371. [DOI] [PubMed] [Google Scholar]
- Iikuni N, Lam QL, Lu L, Matarese G, La Cava A. Leptin and Inflammation. Curr. Immunol. Rev. 2008;4:70–79. doi: 10.2174/157339508784325046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NFkappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa K, Numata Y, Katsuura G, Sakaguchi I, Morita A, Kikuoka S, Matumoto Y, Tsuji T, Tamaki M, Sasakura K, Teraoka H, Hosoda K, Ogawa Y, Nakao K. Structure-function studies of human leptin. J. Biol. Chem. 1998;273:35245–35249. doi: 10.1074/jbc.273.52.35245. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Kitayama J, Nagawa H. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin. Cancer Res. 2004;10:4325–4331. doi: 10.1158/1078-0432.CCR-03-0749. [DOI] [PubMed] [Google Scholar]
- Janik JE, Curti BD, Considine RV, et al. Interleukin-1 alpha increases serum leptin concentrations in humans. J. Clin. Endocrinol. Metab. 1997;82:3084–3086. doi: 10.1210/jcem.82.9.4214. [DOI] [PubMed] [Google Scholar]
- Jarde T, Caldefie-Chezet F, Damez M, Mishellany F, Perrone D, Penault-Llorca F, Guillot J, Vasson MP. Adiponectin and leptin expression in primary ductal breast cancer and in adjacent healthy epithelial and myoepithelial tissue. Histopathology. 2008a;53:484–487. doi: 10.1111/j.1365-2559.2008.03121.x. [DOI] [PubMed] [Google Scholar]
- Jarde T, Caldefie-Chezet F, Damez M, Mishellany F, Penault-Llorca F, Guillot J, Vasson MP. Leptin and leptin receptor involvement in cancer development: a study on human primary breast carcinoma. Oncol. Rep. 2008b;19:905–911. [PubMed] [Google Scholar]
- Johnston SR. New strategies in estrogen receptor-positive breast cancer. Clin. Cancer Res. 2010;16:1979–1987. doi: 10.1158/1078-0432.CCR-09-1823. [DOI] [PubMed] [Google Scholar]
- Johnston DA, Dong B, Hughes CC. TNF induction of jagged-1 in endothelial cells is NFkappaB-dependent. Gene. 2009;435:36–44. doi: 10.1016/j.gene.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakarala M, Wicha MS. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J. Clin. Oncol. 2008;26:2813–2820. doi: 10.1200/JCO.2008.16.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel M, Shouman S, El-Merzebany M, Kilic G, Veenstra T, Saeed M, Wagih M, Diaz-Arrastia C, Patel D, Salama S. Effect of tumour necrosis factor-alpha on estrogen metabolic pathways in breast cancer cells. J. Cancer. 2012;3:310–321. doi: 10.7150/jca.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K, Kawakami M, Puri RK. Overexpressed cell surface interleukin-4 receptor molecules can be successfully targeted for antitumor cytotoxin therapy. Crit. Rev. Immunol. 2001;21:299–310. [PubMed] [Google Scholar]
- Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2001;280:E745–51. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- Kim HS. Leptin and leptin receptor expression in breast cancer. Cancer Res. Treat. 2009;41:155–163. doi: 10.4143/crt.2009.41.3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchgessner TG, Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Tumor necrosis factor-alpha contributes to obesity-related hyperleptinemia by regulating leptin release from adipocytes. J. Clin. Invest. 1997;100:2777–2782. doi: 10.1172/JCI119824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight BB, Oprea-Ilies GM, Nagalingam A, Yang L, Cohen C, Saxena NK, Sharma D. Survivin upregulation, dependent on leptin-EGFR-Notch1 axis, is essential for leptin induced migration of breast carcinoma cells. Endocr. Relat. Cancer. 2011 doi: 10.1530/ERC-11-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkaya H, Wicha MS. HER-2, notch, and breast cancer stem cells: targeting an axis of evil. Clin. Cancer Res. 2009;15:1845–1847. doi: 10.1158/1078-0432.CCR-08-3087. [DOI] [PubMed] [Google Scholar]
- Korkaya H, Paulson A, Iovino F, Wicha MS. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27:6120–6130. doi: 10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, Clouthier SG, Wicha MS. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009;7:e1000121. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J. Clin. Invest. 2011;121:3804–3809. doi: 10.1172/JCI57099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Kishimoto H, Chua HL, Badve S, Miller KD, Bigsby RM, Nakshatri H. Interleukin-1 alpha promotes tumor growth and cachexia in MCF-7 xenograft model of breast cancer. Am. J. Pathol. 2003;163:2531–2541. doi: 10.1016/s0002-9440(10)63608-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier V, Gillespie C, McGlothen T, Dickson T, Guo S, Gonzalez.-Perez RR. Leptin induces Notch in endothelial cells: role of VEGFR-2 transactivation. Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research. AACR; Chicago, IL, USA. 2012. [Google Scholar]
- Lee RH, Yoon N, Reneau JC, Prockop DJ. Preactivation of human MSCs with TNF-alpha enhances tumor-suppressive activity. Cell Stem Cell. 2012 doi: 10.1016/j.stem.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Leong KG, Karsan A. Recent insights into the role of Notch signaling in tumorigenesis. Blood. 2006;107:2223–2233. doi: 10.1182/blood-2005-08-3329. [DOI] [PubMed] [Google Scholar]
- Lepercq J, Cauzac M, Lahlou N, Timsit J, Girard J, Auwerx J, Hauguel-de Mouzon S. Overexpression of placental leptin in diabetic pregnancy: a critical role for insulin. Diabetes. 1998;47:847–850. doi: 10.2337/diabetes.47.5.847. [DOI] [PubMed] [Google Scholar]
- Lewandowski K, Horn R, O’Callaghan CJ, Dunlop D, Medley GF, O’Hare P, Brabant G. Free leptin, bound leptin, and soluble leptin receptor in normal and diabetic pregnancies. J. Clin. Endocrinol. Metab. 1999;84:300–306. doi: 10.1210/jcem.84.1.5401. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Tsai JP, Shen CR, Sher YP, Hsieh CL, Yeh YC, Chou AH, Chang SR, Hsiao KN, Yu FW, Chen HW. Induction of a distinct CD8 Tnc17 subset by transforming growth factor-beta and interleukin-6. J. Leukoc. Biol. 2007;82:354–360. doi: 10.1189/jlb.0207111. [DOI] [PubMed] [Google Scholar]
- Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F, Korkaya H, Heath A, Dutcher J, Kleer CG, Jung Y, Dontu G, Taichman R, Wicha MS. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71:614–624. doi: 10.1158/0008-5472.CAN-10-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Klein AS, Bulkley GB, Bao C, Noble PW, Lane MD, Diehl AM. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 2007a;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007b;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- Lyon DE, McCain NL, Walter J, Schubert C. Cytokine comparisons between women with breast cancer and women with a negative breast biopsy. Nurs. Res. 2008;57:51–58. doi: 10.1097/01.NNR.0000280655.58266.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maamra M, Bidlingmaier M, Postel-Vinay MC, Wu Z, Strasburger CJ, Ross RJ. Generation of human soluble leptin receptor by proteolytic cleavage of membrane-anchored receptors. Endocrinology. 2001;142:4389–4393. doi: 10.1210/endo.142.10.8442. [DOI] [PubMed] [Google Scholar]
- Maccio A, Madeddu C, Gramignano G, Mulas C, Floris C, Massa D, Astara G, Chessa P, Mantovani G. Correlation of body mass index and leptin with tumor size and stage of disease in hormone-dependent postmenopausal breast cancer: preliminary results and therapeutic implications. J. Mol. Med. (Berl.) 2010;88:677–686. doi: 10.1007/s00109-010-0611-8. [DOI] [PubMed] [Google Scholar]
- Marttunen MB, Andersson S, Hietanen P, Karonen SL, Koistinen HA, Koivisto VA, Tiitinen A, Ylikorkala O. Antiestrogenic tamoxifen and toremifene increase serum leptin levels in postmenopausal breast cancer patients. Maturitas. 2000;35:175–179. doi: 10.1016/s0378-5122(00)00121-3. [DOI] [PubMed] [Google Scholar]
- Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, Mise H, Nishimura H, Yoshimasa Y, Tanaka I, Mori T, Nakao K. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat. Med. 1997;3:1029–1033. doi: 10.1038/nm0997-1029. [DOI] [PubMed] [Google Scholar]
- Mattioli B, Straface E, Quaranta MG, Giordani L, Viora M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J. Immunol. 2005;174:6820–6828. doi: 10.4049/jimmunol.174.11.6820. [DOI] [PubMed] [Google Scholar]
- McGlothen TZ, Gillespie C, Colbert L, Blaylock-Hogans D, Guo S, Gonzalez-Perez RR. Leptin signaling impacts Notch and Wnt crosstalk in breast cancer. Cancer Res. 2011:71. [Google Scholar]
- Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N. Engl. J. Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- Miller LJ, Kurtzman SH, Anderson K, Wang Y, Stankus M, Renna M, Lindquist R, Barrows G, Kreutzer DL. Interleukin-1 family expression in human breast cancer: interleukin-1 receptor antagonist. Cancer Invest. 2000;18:293–302. doi: 10.3109/07357900009012171. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J. Immunol. 2009;183:4169–4175. doi: 10.4049/jimmunol.0901017. [DOI] [PubMed] [Google Scholar]
- Nagai S, Toi M. Interleukin-4 and breast cancer. Breast Cancer. 2000;7:181–186. doi: 10.1007/BF02967457. [DOI] [PubMed] [Google Scholar]
- Napoleone E, Cutrone A, Cugino D, Latella MC, Zurlo F, Iacoviello L, de Gaetano G, Donati MB, Lorenzet R. Leptin upregulates tissue factor expression in human breast cancer MCF-7 cells. Thromb. Res. 2012;129:641–647. doi: 10.1016/j.thromres.2011.07.037. [DOI] [PubMed] [Google Scholar]
- Nicolini A, Carpi A, Rossi G. Cytokines in breast cancer. Cytokine Growth Factor Rev. 2006;17:325–337. doi: 10.1016/j.cytogfr.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Noh M. Interleukin-17A increases leptin production in human bone marrow mesenchymal stem cells. Biochem. Pharmacol. 2012;83:661–670. doi: 10.1016/j.bcp.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Oswald F, Liptay S, Adler G, Schmid RM. NF-kappaB2 is a putative target gene of activated Notch-1 via RBP-Jkappa. Mol. Cell. Biol. 1998;18:2077–2088. doi: 10.1128/mcb.18.4.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang LY, Argyle DJ. Using naturally occurring tumours in dogs and cats to study telomerase and cancer stem cell biology. Biochim. Biophys. Acta. 2009;1792:380–391. doi: 10.1016/j.bbadis.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Panis C, Pizzatti L, Cristina Herrera A, Cecchini R, Abdelhay E. Putative circulating markers of the early and advanced stages of breast cancer identified by high-resolution label-free proteomics. Cancer Lett. 2012 doi: 10.1016/j.canlet.2012.11.020. [DOI] [PubMed] [Google Scholar]
- Pantschenko AG, Pushkar I, Anderson KH, Wang Y, Miller LJ, Kurtzman SH, Barrows G, Kreutzer DL. The interleukin-1 family of cytokines and receptors in human breast cancer: implications for tumor progression. Int. J. Oncol. 2003a;23:269–284. [PubMed] [Google Scholar]
- Pantschenko AG, Pushkar I, Miller LJ, Wang YP, Anderson K, Peled Z, Kurtzman SH, Kreutzer DL. In vitro demonstration of breast cancer tumor cell sub-populations based on interleukin-1/tumor necrosis factor induction of interleukin-8 expression. Oncol. Rep. 2003b;10:1011–1017. [PubMed] [Google Scholar]
- Paracchini V, Pedotti P, Taioli E. Genetics of leptin and obesity: a HuGE review. Am. J. Epidemiol. 2005;162:101–114. doi: 10.1093/aje/kwi174. [DOI] [PubMed] [Google Scholar]
- Pratt MA, Tibbo E, Robertson SJ, Jansson D, Hurst K, Perez-Iratxeta C, Lau R, Niu MY. The canonical NF-kappaB pathway is required for formation of luminal mammary neoplasias and is activated in the mammary progenitor population. Oncogene. 2009;28:2710–2722. doi: 10.1038/onc.2009.131. [DOI] [PubMed] [Google Scholar]
- Prieto-Hontoria PL, Perez-Matute P, Fernandez-Galilea M, Bustos M, Martinez JA, Moreno-Aliaga MJ. Role of obesity-associated dysfunctional adipose tissue in cancer: a molecular nutrition approach. Biochim. Biophys. Acta. 2011;1807:664–678. doi: 10.1016/j.bbabio.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res. Treat. 2010;123:627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- Pubmed, s. 2012 < http://www.ncbi.nlm.nih.gov/pubmed?term=leptin>.
- Radtke F, Raj K. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat. Rev. Cancer. 2003;3:756–767. doi: 10.1038/nrc1186. [DOI] [PubMed] [Google Scholar]
- Reed MJ, Purohit A. Breast cancer and the role of cytokines in regulating estrogen synthesis: an emerging hypothesis. Endocr. Rev. 1997;18:701–715. doi: 10.1210/edrv.18.5.0314. [DOI] [PubMed] [Google Scholar]
- Rene Gonzalez R, Watters A, Xu Y, Singh UP, Mann DR, Rueda BR, Penichet ML. Leptin-signaling inhibition results in efficient anti-tumor activity in estrogen receptor positive or negative breast cancer. Breast Cancer Res. 2009;11:R36. doi: 10.1186/bcr2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revillion F, Charlier M, Lhotellier V, Hornez L, Giard S, Baranzelli MC, Djiane J, Peyrat JP. Messenger RNA expression of leptin and leptin receptors and their prognostic value in 322 human primary breast cancers. Clin. Cancer Res. 2006;12:2088–2094. doi: 10.1158/1078-0432.CCR-05-1904. [DOI] [PubMed] [Google Scholar]
- Rokavec M, Wu W, Luo JL. IL6-mediated suppression of miR-200c directs constitutive activation of inflammatory signaling circuit driving transformation and tumorigenesis. Mol. Cell. 2012;45:777–789. doi: 10.1016/j.molcel.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronchini C, Capobianco AJ. Induction of cyclin D1 transcription and CDK2 activity by Notch(ic): implication for cell cycle disruption in transformation by Notch(ic) Mol. Cell. Biol. 2001;21:5925–5934. doi: 10.1128/MCB.21.17.5925-5934.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose DP, Vona-Davis L. Interaction between menopausal status and obesity in affecting breast cancer risk. Maturitas. 2010;66:33–38. doi: 10.1016/j.maturitas.2010.01.019. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, Leibel RL. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J. Clin. Endocrinol. Metab. 1996;81:3424–3427. doi: 10.1210/jcem.81.9.8784109. [DOI] [PubMed] [Google Scholar]
- Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc. Natl. Acad. Sci. USA. 2012;109:2796–2801. doi: 10.1073/pnas.1104303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salven P, Hattori K, Heissig B, Rafii S. Interleukin-1alpha promotes angiogenesis in vivo via VEGFR-2 pathway by inducing inflammatory cell VEGF synthesis and secretion. FASEB J. 2002;16:1471–1473. doi: 10.1096/fj.02-0134fje. [DOI] [PubMed] [Google Scholar]
- Sarraf P, Frederich RC, Turner EM, Ma G, Jaskowiak NT, Rivet DJ, 3rd, Flier JS, Lowell BB, Fraker DL, Alexander HR. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J. Exp. Med. 1997;185:171–175. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid MC, Avraamides CJ, Foubert P, Shaked Y, Kang SW, Kerbel RS, Varner JA. Combined blockade of integrin-alpha4beta1 plus cytokines SDF-1alpha or IL-1beta potently inhibits tumor inflammation and growth. Cancer Res. 2011;71:6965–6975. doi: 10.1158/0008-5472.CAN-11-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi N, Dai X, Winter CG, Kang Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell. 2011;19:192–205. doi: 10.1016/j.ccr.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Honigrnann MR, Nath AK, Murakami C, Garcia-Cardena G, Papetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ, Flores-Riveros JR. Biological action of leptin as an angiogenic factor. Science. 1998;282:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- Singer CF, Kronsteiner N, Hudelist G, Marton E, Walter I, Kubista M, Czerwenka K, Schreiber M, Seifert M, Kubista E. Interleukin 1 system and sex steroid receptor expression in human breast cancer: interleukin 1alpha protein secretion is correlated with malignant phenotype. Clin. Cancer Res. 2003;9:4877–4883. [PubMed] [Google Scholar]
- Singer CF, Hudelist G, Gschwantler-Kaulich D, Fink-Retter A, Mueller R, Walter I, Czerwenka K, Kubista E. Interleukin-1alpha protein secretion in breast cancer is associated with poor differentiation and estrogen receptor alpha negativity. Int. J. Gynecol. Cancer. 2006;16(Suppl 2):556–559. doi: 10.1111/j.1525-1438.2006.00695.x. [DOI] [PubMed] [Google Scholar]
- Sisirak V, Faget, Julien, Gobert, Michael, Goutagny, Nadège, Vey, Nelly, Treilleux, Isabelle, Renaudineau, Sarah, Poyet, Gaelle, Labidi-Galy, Sana Intidhar, Goddard-Leon, Sophie, Durand, Isabelle, Mercier, Le, Isabelle, Bajard, Agathe, Bachelot, Thomas, Puisieux, Alain, Puisieux, Isabelle, Blay, Yves, Jean, Mountier-Caux, Christine, Caux, Christophe, Bendriss-Vermare, Nathalie Impaired IFN-α production by plasmacytoid dendritic cells favors regulatory T-cell expansion that may contribute to breast cancer progression. Cancer Res. 2012;72:6. doi: 10.1158/0008-5472.CAN-11-3468. [DOI] [PubMed] [Google Scholar]
- Smith JL, Sheffield LG. Production and regulation of leptin in bovine mammary epithelial cells. Domest. Anim. Endocrinol. 2002;22:145–154. doi: 10.1016/s0739-7240(02)00121-2. [DOI] [PubMed] [Google Scholar]
- Su X, Ye J, Hsueh EC, Zhang Y, Hoft DF, Peng G. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J. Immunol. 2010;184:1630–1641. doi: 10.4049/jimmunol.0902813. [DOI] [PubMed] [Google Scholar]
- Sumarac-Dumanovic M, Stevanovic D, Ljubic A, Jorga J, Simic M, Stamenkovic-Pejkovic D, Starcevic V, Trajkovic V, Micic D. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int. J. Obes. (Lond.) 2009;33:151–156. doi: 10.1038/ijo.2008.216. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Okimura Y, Mizuno I, Iida K, Takahashi T, Kaji H, Abe H, Chihara K. Leptin induces mitogen-activated protein kinase-dependent proliferation of C3H10T1/2 cells. J. Biol. Chem. 1997;272:12897–12900. doi: 10.1074/jbc.272.20.12897. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S. Stat 3 activation is responsible for IL-6 dependent cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J. Immunol. 1998;16:4652–4660. [PubMed] [Google Scholar]
- Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Trueman P. Prophylactic G-CSF in patients with early-stage breast cancer: a health economic review. Br. J. Cancer. 2009;101(Suppl. 1):S15–7. doi: 10.1038/sj.bjc.6605271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, Koike M, Inadera H, Matsushima K. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin. Cancer Res. 2000;6:3282–3289. [PubMed] [Google Scholar]
- Unkown, C.G.o.H.F.i.B.C. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13:1141–1151. doi: 10.1016/S1470-2045(12)70425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Horssen R, Ten Hagen TL, Eggermont AM. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist. 2006;11:397–408. doi: 10.1634/theoncologist.11-4-397. [DOI] [PubMed] [Google Scholar]
- Vendramini-Costa DB, Carvalho JE. Molecular link mechanisms between inflammation and cancer. Curr. Pharm. Des. 2012;18:3831–3852. doi: 10.2174/138161212802083707. [DOI] [PubMed] [Google Scholar]
- Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proc. Natl. Acad. Sci. USA. 2003;100:2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Liang S, Ghosh S, Hornsby PJ, Li R. Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene. 2009;28:2745–2755. doi: 10.1038/onc.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kuropatwinski KK, White DW, Hawley TS, Hawley RG, Tartaglia LA, Baumann H. Leptin receptor action in hepatic cells. J. Biol. Chem. 1997;272:16216–16223. doi: 10.1074/jbc.272.26.16216. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu R, Hawkins M, Barzilai N, Rossetti L. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature. 1998;393:684–688. doi: 10.1038/31474. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kong D, Banerjee S, Li Y, Adsay NV, Abbruzzese J, Sarkar FH. Down-regulation of platelet-derived growth factor-D inhibits cell growth and angiogenesis through inactivation of Notch-1 and nuclear factor-kappaB signaling. Cancer Res. 2007;67:11377–11385. doi: 10.1158/0008-5472.CAN-07-2803. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang X, Zhao H, Liang B, Du Q. Clusterin confers resistance to TNF-alpha-induced apoptosis in breast cancer cells through NF-kappaB activation and Bcl-2 overexpression. J. Chemother. 2012;24:348–357. doi: 10.1179/1973947812Y.0000000049. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Yaginuma R, Ikejima K, Miyazaki A. Liver diseases and metabolic syndrome. J. Gastroenterol. 2008;43:509–518. doi: 10.1007/s00535-008-2193-6. [DOI] [PubMed] [Google Scholar]
- Watanabe MA, Oda JM, Amarante MK, Cesar Voltarelli J. Regulatory T cells and breast cancer: implications for immunopathogenesis. Cancer Metastasis Rev. 2010;29:569–579. doi: 10.1007/s10555-010-9247-y. [DOI] [PubMed] [Google Scholar]
- Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- Welboren WJ, Stunnenberg HG, Sweep FC, Span PN. Identifying estrogen receptor target genes. Mol. Oncol. 2007;1:138–143. doi: 10.1016/j.molonc.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng AP, Millholland JM, Yashiro-Ohtani Y, Arcangeli ML, Lau A, Wai C, Del Bianco C, Rodriguez CG, Sai H, Tobias J, Li Y, Wolfe MS, Shachaf C, Felsher D, Blacklow SC, Pear WS, Aster JC. C-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev. 2006;20:2096–2109. doi: 10.1101/gad.1450406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO World Health Organization Fact Sheet for World Wide Prevalence of Obesity. 2006 < http://www.who.int/mediacentre/factsheets/fs311/en/index.html >.
- Wiesner G, Vaz M, Collier G, Seals D, Kaye D, Jennings G, Lambert G, Wilkinson D, Esler M. Leptin is released from the human brain: influence of adiposity and gender. J. Clin. Endocrinol. Metab. 1999;84:2270–2274. doi: 10.1210/jcem.84.7.5854. [DOI] [PubMed] [Google Scholar]
- Won HY, Lee JA, Park ZS, Song JS, Kim HY, Jang SM, Yoo SE, Rhee Y, Hwang ES, Bae MA. Prominent bone loss mediated by RANKL and IL-17 produced by CD4+ T cells in TallyHo/JngJ mice. PloS One. 2011;6:e18168. doi: 10.1371/journal.pone.0018168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Murakami T, Tomimatsu T, Nishio Y, Mitsuda N, Kanzaki T, Kurachi H, Shima K, Aono T, Murata Y. Autocrine inhibition of leptin production by tumor necrosis factor-alpha (TNF-alpha) through TNF-alpha type-I receptor in vitro. Biochem. Biophys. Res. Commun. 1998;244:30–34. doi: 10.1006/bbrc.1998.8199. [DOI] [PubMed] [Google Scholar]
- Yamashita J, Hideshima T, Shirakusa T, Ogawa M. Primary tumor levels of interleukin-6 in relation to tumor burden in human breast-cancer. Oncol. Rep. 1994;1:1185–1187. doi: 10.3892/or.1.6.1185. [DOI] [PubMed] [Google Scholar]
- Yang L, Qi Y, Hu J, Tang L, Zhao S, Shan B. Expression of Th17 cells in breast cancer tissue and its association with clinical parameters. Cell Biochem. Biophys. 2012;62:153–159. doi: 10.1007/s12013-011-9276-3. [DOI] [PubMed] [Google Scholar]
- Zhang GJ, Adachi I. Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res. 1999;19:1427–1432. [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bellows CF, Kolonin MG. Adipose tissue-derived progenitor cells and cancer. World J. Stem Cells. 2010;2:103–113. doi: 10.4252/wjsc.v2.i5.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, Dunlap SM, Zhu J, Downs-Kelly E, Rich JN, Hursting SD, Berger NA, Reizes O. Leptin deficiency suppresses MMTV-Wnt-1 mammary tumor growth and abrogates tumor initiating cell survival. Endocr. Relat. Cancer. 2011 doi: 10.1530/ERC-11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang Y, Deng J, Margolick JB, Liotta LA, Petricoin E, 3rd, Zhang Y. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc. Natl. Acad. Sci. USA. 2007;104:16158–16163. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Guo S, Gonzalez-Perez RR. Leptin pro-angiogenic signature in breast cancer is linked to IL-1 signalling. Br. J. Cancer. 2011;104:128–137. doi: 10.1038/sj.bjc.6606013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Mulcahy LA, Mohammed RA, Lee AH, Franks HA, Kilpatrick L, Yilmazer A, Paish EC, Ellis IO, Patel PM, Jackson AM. IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res. 2008;10:R95. doi: 10.1186/bcr2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Jiang W, Zacher JH, Neil ES, McGinley JN, Thompson HJ. Effects of energy restriction and wheel running on mammary carcinogenesis and host systemic factors in a rat model. Cancer Prev. Res. (Phila) 2012;5:414–422. doi: 10.1158/1940-6207.CAPR-11-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziccardi P, Nappo F, Giugliano G, Esposito K, Marfella R, Cioffi M, D’Andrea F, Molinari AM, Giugliano D. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002;105:804–809. doi: 10.1161/hc0702.104279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.