Abstract
Because of its essential role in gas exchange and oxygen delivery, the lung has evolved a variety of strategies to control inflammation and maintain homeostasis. Invasion of the lung by pathogens (and in some instances exposure to certain noninfectious particulates) disrupts this equilibrium and triggers a cascade of events aimed at preventing or limiting colonization (and more importantly infection) by pathogenic microorganisms. In this review we focus on viral infection of the lung and summarize recent advances in our understanding of the triggering of innate and adaptive immune responses to viral respiratory tract infection, mechanisms of viral clearance, and the well-recognized consequences of acute viral infection complicating underlying lung diseases, such as asthma.
Key words: Viral sensor molecules, innate immunity, adaptive immunity, T-cell immunity, B-cell immunity, viral clearance, tissue repair, stem cells
Abbreviations used: AIM2, Absent in melanoma 2; APC, Antigen-presenting cell; ASC, Apoptosis-associated speck-like protein containing CARD; CTL, Cytotoxic CD8+ T-cell; DAMP, Damage-associated molecular pattern; DC, Dendritic cell; GC, Germinal center; HMGB1, High-mobility group box 1; IAV, Influenza A virus; ILC, Innate lymphoid cell; ILC-II, Type II innate lymphoid cell; IRF, Interferon regulatory factor; LAPC, Late activator antigen-presenting cell; MAVS, Mitochondrial anti-viral signaling; MLN, Mediastinal lymph node; NK, Natural killer; NLRP3, Nod-like receptor family protein 3; PAMP, Pathogen-associated molecular pattern; PRR, Pattern recognition receptor; RIG-I, Retinoic acid–inducible gene I; RLR, RIG-I–like receptor; RSV, Respiratory syncytial virus; TFH, Follicular helper T; TLR, Toll-like receptor; Treg, Regulatory T
Information for Category 1 CME Credit
Credit can now be obtained, free for a limited time, by reading the review articles in this issue. Please note the following instructions.
Method of Physician Participation in Learning Process: The core material for these activities can be read in this issue of the Journal or online at the JACI Web site: www.jacionline.org. The accompanying tests may only be submitted online at www.jacionline.org. Fax or other copies will not be accepted.
Date of Original Release: December 2013. Credit may be obtained for these courses until November 30, 2014.
Copyright Statement: Copyright © 2013-2014. All rights reserved.
Overall Purpose/Goal: To provide excellent reviews on key aspects of allergic disease to those who research, treat, or manage allergic disease.
Target Audience: Physicians and researchers within the field of allergic disease.
Accreditation/Provider Statements and Credit Designation: The American Academy of Allergy, Asthma & Immunology (AAAAI) is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians. The AAAAI designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
List of Design Committee Members: Jae-Kwang Yoo, PhD, Taeg S. Kim, PhD, Matthew M. Hufford, PhD, and Thomas J. Braciale, MD, PhD
Activity Objectives
-
1.
To understand the involvement of the innate and adaptive immune responses during viral respiratory tract infections.
-
2.
To review the mechanisms of viral clearance.
-
3.
To recognize the consequences of acute viral infections.
-
4.
To provide evidence of the effect of viral infections in asthmatic patients.
Recognition of Commercial Support: This CME activity has not received external commercial support.
Disclosure of Significant Relationships with Relevant Commercial
Companies/Organizations: The authors declare that they have no relevant conflicts of interest.
Viewed as an organ, the lung is a highly compartmentalized structure with the primary function of gas exchange. Like other organs, such as the skin and gastrointestinal tract, that are exposed to the external environment, the lung possesses physical and chemical barriers to microbial invasion. Thus the conducting airway epithelial cells provide both mechanical (ie, ciliated epithelial movement and mucus production) and biochemical (ie, antimicrobial enzymes/peptides) barriers that inhibit colonization of the lungs by most microorganisms. However, many respiratory pathogens (and some commensal microbes), including viruses, have evolved to successfully colonize and replicate on or within the lung epithelial cells, occasionally causing life-threatening diseases.
The cellular constituents of the normal lung include cells of hematopoietic origin (CD45+), as well as stromal cells (CD45−). Among the stromal cell types, type I and II alveolar epithelial cells and several epithelial cell subtypes lining the conducting airway are of particular importance because they are the primary cell types targeted by certain respiratory tract viruses and importantly by the subsequent host immune response to infection. Cellular destruction produced by the virus, host response, or both can, if extensive enough, result in severely compromised pulmonary function. Therefore efficient suppression of early viral replication and minimization of immune-mediated injury are quintessential hallmarks of effective recovery from pulmonary viral infection.
In this review we will discuss recent developments in the process of innate immune recognition during viral respiratory tract infection, immune effector mechanisms involved in viral clearance and injury development, recovery from infection, and the effect of viral respiratory tract infection on underlying lung diseases.
Initiation phase
Initiation of antiviral innate immunity in the lung
The successful initiation of the host immune response to microbial invasion requires the recognition of pathogen-associated molecular patterns (PAMPs). This is achieved through recognition of microbial PAMPs by 1 or more of a variety of cellular receptors (pattern recognition receptors [PRRs]) for these PAMPs displayed by CD45− stromal cells, such as respiratory epithelial cells, as well as CD45+ cells, within the lung.1, 2, 3 Recent studies also emphasize the importance of host immune cell recognition of damage-associated danger signals (damage-associated molecular patterns [DAMPs]) typically composed of sequestered self-constituents released from infected cells, damaged cells, or both.4 These PAMP and DAMP molecular “red flags” can also activate the intracellular innate protein complex, the inflammasome, which also can play a key role in orchestrating both the innate and adaptive immune responses to viruses.5, 6 In addition, the role of complement in controlling early viral replication and in the initiation of innate and adaptive immunity is being increasingly appreciated. Thus an emerging view from recent investigations is that that the recognition and subsequent eradication of invading viral pathogens at mucosal sites, such as the airways, requires the concerted action of PRRs, as well as immune sensors of cellular stress/damage arising from viral infection.
Recognition of viral pathogen–associated molecular patterns
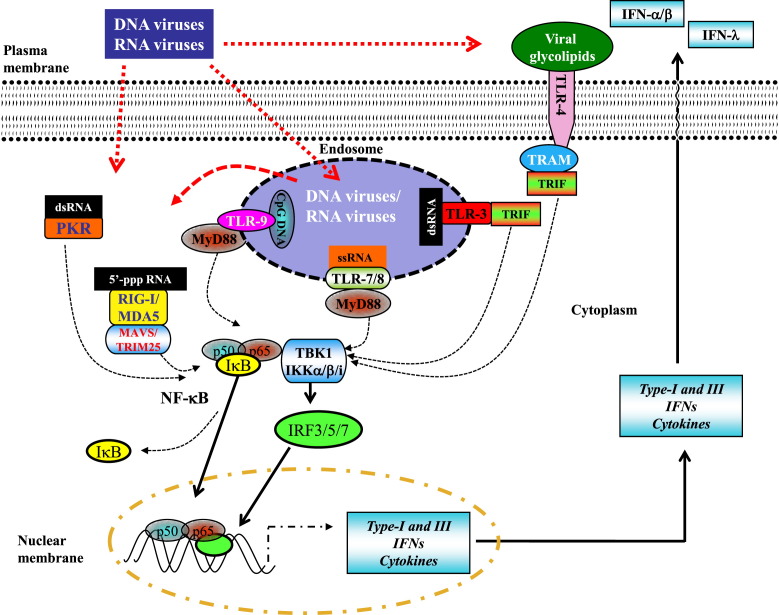
Viral infection of epithelial cells is first detected by a germline-encoded set of sensors expressed by epithelial cells and innate immune cells (ie, PRRs), which recognize PAMPs originating from the invading viral pathogens.3, 7, 8 PRR sensors include the Toll-like receptors (TLRs); RNA-sensing RIG-I–like receptors (RLRs), such as retinoic acid–inducible gene I (RIG-I) and melanoma differentiation-associated protein 5; and C-type lectin receptors (Fig 1 ). PRR recognition depends on the detection of evolutionarily conserved microbial ligands that are critical for -microbial structure/function, such as viral envelope proteins and nucleic acid motifs within the DNA or RNA genomes of the virus. Although RLRs sense microbial constituents in the cytosolic compartments, TLRs and C-type lectin receptors detect microbes on the cell surface and in endosomes.8
Fig 1.
Innate recognition of viral pathogen–associated pattern molecules. Interferon (types I and III) production in response to viral respiratory tract infection can be triggered by recognition of (1) double-stranded RNA (dsRNA) by the cytosolic receptors melanoma differentiation-associated protein 5 (MDA5) and retinoic acid-inducible gene I (RIG-I) or (2) dsDNA (B-DNA) by DAI or as yet unknown cytosolic DNA receptors (DNA-RX; not depicted). This recognition leads to the activation of interferon regulatory transcription factor (IRF)-3 through the kinase TANK-binding kinase (TBK)-1 (or IKKi) and stimulates the production of interferons (types I and III) at the site of infection. RIG-I is also triggered by 5′-pppRNA transcribed from dsDNA by using RNA polymerase III. In addition, ligation of TLR3, TLR4, TLR7, and TLR9 by respective viral molecules triggers type I interferon production by means of signaling through adaptor molecules, including MyD88, Toll-interleukin 1 receptor (TIR) domain containing adaptor protein (TIRAP), TRIF-related adaptor molecule (TRAM) and TIR-domain-containing adapter-inducing interferon-β (TRIF). The association of these adaptors with TBK1 ultimately results in the activation of the IRF family members (ie, IRF3/5/7) and, in some instances, nuclear factor κB, leading to the transcription of interferon genes (types I and III) and proinflammatory cytokines, such as pro–IL-1β, pro–IL-18, and IL-6. Of note, the production of interferons (types I and III) can be amplified by a positive feedback loop in which the interferons produced early trigger transcription in both autocrine and paracrine fashions.
RIG-I is the prototypical member of the RLR family of cytosolic PRRs that recognize nucleotide motifs displayed by RNA viruses (Fig 1). Its primary function is to activate interferon genes through the adaptor mitochondrial anti-viral signaling (MAVS), which in turn engages the interferon regulatory factor (IRF) 3/7 transcription factor signaling pathway.9 RIG-I signaling is also important for activation of the inflammasome and IL-1β production (see below). Infection of cells by vesicular stomatitis virus or transfection of cells by RNA activates the RIG-I pathway and leads to pro–IL-1β production through a MAVS–CARD9–nuclear factor κB signaling pathway. In parallel, RIG-I can also directly activate the inflammasome complex by binding the adaptor apoptosis-associated speck-like protein containing CARD (ASC) (see below).10, 11 Recently, the interferon-inducible protein absent in melanoma 2 (AIM2) has been identified as a novel sensor for cytosolic DNA, such as DNA virus genomes, through an HIN-200 DNA-binding domain.12 AIM2 can activate caspase-1 and the inflammasome in addition to inducing type I interferons.13 (Other potential cytosolic DNA recognition molecules, such as cyclic-GMP-AMP [cGAMP] synthase [cGAS] and DNA-dependent activator of IFN regulatory factors [DAI], have also been implicated as sensors triggering type I interferon responses.) Although RIG-I and AIM2 detect viral PAMPs in the cytosol, TLRs sense these PAMPs within endosomes (eg, TLR3, TLR7, and TLR9) or on the cell surface (eg, TLR2 and TLR4; Fig 1).
The type I interferons (IFN-α and IFN-β) are under tight transcriptional regulation and are induced after recognition of pathogen components during infection by various host PRRs (Fig 1).14, 15 The type I interferons are responsible for inducing transcription of a large group of genes that play a role in host resistance to viral infections, as well as activating key components of the innate and adaptive immune systems, including antigen presentation and production of cytokines involved in activation of T cells, B cells, and natural killer (NK) cells.15 Plasmacytoid dendritic cells (DCs) are well recognized as the cell type specialized for the production of large amounts of type I interferons.14 In addition, type III interferons, consisting of 3 IFN-λ molecules called IFN-λ1, IFN-λ2, and IFN-λ3 (also called IL-29, IL-28A, and IL-28B, respectively), have been recently identified and classified as an interferon family.16 IFN-λs signal through a receptor heterodimer complex consisting of IL-10 receptor β and IFN-λR1 (also known as IL-28RA). Despite the distinct receptor complexes used by type I (ie, IFNAR-1 and IFNAR-2) and type III interferons, they trigger similar intracellular signaling pathways in a wide variety of target cells, resulting in many of the same biological activities, including antiviral activity. Intriguingly, unlike type I interferon receptors, which are widely expressed on many cell types, including leukocytes, the receptors for IFN-λs are largely restricted to cells of epithelial origin. Therefore the IFN-λ ligands and their IFN-λ receptors represent a potential novel antiviral therapeutic target.
Although the importance of recognition of viral PAMPs by PRRs has been well established in vitro, the relevance of host recognition of viral PAMPs by PRR types in vivo to innate and adaptive immunity is less clear. Neither the absence of TLR-317 nor the absence of the RIG-I signaling adaptor MAVS18 diminishes viral clearance and the adaptive immunity to influenza A virus (IAV) infection. Similarly, Tlr7 −/− or Tlr7 −/− Mavs −/− mice are able to mount an effective CD8+ T-cell response and efficiently clear IAV.18, 19, 20 These studies suggest that in vivo there might be considerable redundancy among different PRRs in their ability to support the early antiviral sensor roles necessary for the induction of innate and adaptive immune responses.
Damage-associated signal recognition
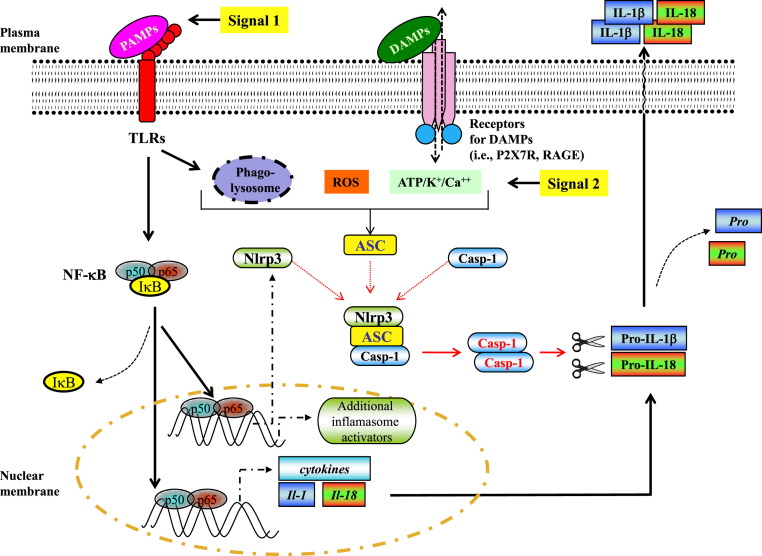
As noted above, along with recognition of microbial products, such as viral nucleic acids and proteins, by PRRs, infection also results in the release of host cell constituents from damaged/dying cells, as well as intact cells located within sites of infection. These damage-associated signals produced as a result of viral replication are, along with viral PAMPs, believed to play a crucial role in activating the inflammasome and controlling the magnitude and quality of the subsequent innate and adaptive immune response to viral infection (Fig 2 ).4, 5, 6 Damage-associated signals are molecules, such as nucleotides (ie, ATP/uridine triphosphate), heat shock proteins, nuclear proteins (ie, high-mobility group box 1 [HMGB1]), mitochondrial DNA, cytokines, and reactive oxygen species, that leak from or are actively released from intracellular compartments after cellular stress or damage. In addition, extracellular molecules, such as extracellular ATP, extracellular matrix components, and uric acids, can also serve as damage-associated signals. These released molecules are then recognized by sensors expressed by surrounding cells, such as antigen-presenting cells (APCs), involved in the initiation of the innate and adaptive immune response (Fig 2). The signals transduced in responding cells by these released self-constituents result in the activation of the cellular protease caspase-1, which in turn catalyzes the maturation and release of active IL-1β and IL-18. Caspase-1 activation is dependent on the assembly of an intracellular inflammasome sensor complex, which is made up of Nod-like receptor family protein 3 (NLRP3), apoptosis-associated speck-like domain containing a caspase-recruitment domain,21 and procaspase-1.5, 6
Fig 2.
Proposed pathway for NLRP3 inflammasome activation during viral respiratory tract infection. Respiratory tract viruses can trigger both signal 1 and signal 2 for NLRP3 inflammasome activation. Sensing of viral pathogen–associated pattern molecules induces the transcription of pro–IL-1β/pro–IL-18 and NLRP3 along with additional proinflammatory cytokines. The purinergic receptors, such as P2X7 receptor, an ATP-gated ion channel that causes potassium (K+) efflux when activated, are partially required for M2-induced inflammasome activation. In the case of influenza virus infection, virus-encoded M2 ion channel protein transports protons (H+) out of the lumen and triggers M2-mediated inflammasome activation. Phagolysosomal maturation and the activity of reactive oxygen species (ROS) and cathepsin B also play a role in virus-induced inflammasome activation, although the underlying mechanisms remain to be explored. The activation of the inflammasome in DCs and macrophages leads to the activation of caspase-1, which mediates the processing of pro–IL-1β/pro–IL-18 to mature IL-1β/IL-18 and its subsequent release into the extracellular space. RAGE, Receptor for advanced glycation end-products.
The inflammasome
Evidence of inflammasome activation by respiratory tract viruses were initially obtained in primary macrophages responding in vitro to infection with Sendai virus or IAV.22, 23 Subsequently, it was demonstrated that products of respiratory tract viruses, such as purified single-stranded RNA from IAV, could, in an NLPR3-dependent manner, activate the inflammasome complex in a variety of cell types, including airway epithelial cells, THP-1 cells, and bone marrow–derived DCs or macrophages.24, 25, 26 It is also noteworthy that the IAV-M2 viral protein, a proton-selective ion channel involved in viral uncoating, can serve as an ionophore to promote nucleotide transport into the cell cytosol and directly trigger caspase-1 activation.27 Respiratory syncytial virus (RSV) also induces the expression of caspase-1 and IL-1β, as well as secretion of IL-1β from neonatal human monocytes, suggesting a potential role for inflammasome signaling in RSV infection.28 Likewise, viral DNA from adenovirus also activates NLRP3, at least in vitro.29
The in vivo significance of inflammasome activation in host defense to viral infection is best illustrated by experimental models of influenza virus infection in mice. Mice deficient in inflammasome complex–associated molecules, such as NLPR3, IL-1 receptor, caspase-1, or ASC, are more susceptible to severe IAV infection than control infected animals with an intact inflammasome complex, as evidenced by increased viral titers, reduced infiltration of neutrophils and monocytes to the infected lung, impaired adaptive immune responses, and reduced cytokine/chemokine levels (IL-1β, IL-6, IL-18, TNF-α, and keratinocyte-derived cytokine).24, 25, 30 More recent evidence further suggests that IL-1 receptor–deficient mice exhibit a reduction in the extent of migration of lung DCs, in particular CD103+ lung DCs, to the draining lymph nodes in response to IAV infection.20 This diminished DC migration was associated with a reduction in the total accumulation of inflammatory/immune cells into the draining lymph nodes and the subsequent impairment of the antiviral CD8+ and CD4+ T-cell response in the infected lungs, which is consistent with the critical role of migrant CD103+ lung DCs in the initiation of adaptive immune responses to viral respiratory tract infection. Although reports differ in the extent to which inflammasome impairment affects the host response to viral respiratory tract infection, overall, the accumulating data strongly suggest that activation of the NLRP3 inflammasome and ensuing IL-1–dependent, IL-1 receptor–mediated signaling are critical for the establishment of antiviral innate and adaptive immune defenses, as well as resolution and repair of tissue damage, at least for experimental IAV infection.
It is increasingly clear that signaling through receptors sensing cellular damage produced by viral infection also plays an important role in activating the innate immune response. As noted above, this occurs, at least in part, through inflammasome activation. Accordingly, intracellular molecules (ie, ATP and HMGB1) serving as DAMPs are released from the infected cells, most often as a consequence of infection-induced apoptosis, necrosis, or pyroptosis,31 and accumulate in the extracellular space at a high concentration during viral infection, where these DAMPs can modulate antiviral immunity.32, 33, 34 For example, during adenovirus infection in the lungs, ATP-mediated signaling through the purinergic receptor P2X7 receptor (P2X7R) appears to be required for inflammasome-dependent induction of inflammatory mediators because inhibition or deficiency of P2X7R (or the inflammasome-dependent pro–IL-1–activating protease caspase-1) significantly reduced IL-1β secretion and neutrophil infiltration.35 Similarly, P2Y2R purinergic receptor–deficient mice exhibit increased morbidity and mortality with diminished viral clearance and increased neutrophil infiltration in the lung after pneumonia virus of mice infection.36 However, recognition of products of cellular damage need not always result in an enhanced innate host response and accelerated viral clearance. For example, recognition of HMGB1 through the DAMP receptor known as receptor for advanced glycation end-products reduces the host resistance to IAV infection.37 Although the contribution of the inflammasome pathway during viral respiratory tract infection has not been fully explored, the observations to date suggest that an array of damage-associated signals released during viral infection modulates, to varying degrees (ie, in a pathogen-dependent manner), the host antiviral immune response and thereby susceptibility to acute viral respiratory tract infection.
In view of the potential role of inflammasomes in viral infection, it is not unexpected that viruses would devise mechanisms to interfere with inflammasome activation. For example, the NS1 nonstructural protein of the H1N1 subtype IAV (eg, A/PR/8/34) is capable of blocking caspase-1 activation, IL-1β maturation, and apoptosis.38 This inflammasome inhibitory action by NS1 is dependent on the N-terminal domain of the NS1 protein, and IAV lacking the N-terminus of NS1 is attenuated in cell culture and induces higher levels of IL-1β and accelerated apoptosis. However, the caspase-1 inhibitory effect of NS1 might be IAV strain specific because NS1 from highly pathogenic avian H5N1 activates caspases and induces apoptosis.39 Thus highly pathogenic IAV virus strains, such as H5N1, might not need to use caspase-1 inhibition as a strategy to suppress inflammasome activation–associated inflammation because these viruses might directly downregulate expression of NLRP3 inflammasome components on infection.40
Complement system
The complement system is an essential component of innate immunity and has evolved as an important bridge between the innate and adaptive immune systems.41, 42 Pulmonary viral infection can result in complement activation both locally (in the lungs) and systemically. Complement deficiency (ie, in components C3, C3a, and C5a) in mice causes markedly reduced T-cell responses to severe acute respiratory syndrome coronavirus43, 44 and IAV infection,45, 46 resulting in a reduced survival rate. In a recent study complement deficiency had no effect on the ability of DCs to trigger T-cell responses after influenza infection but instead resulted in diminished lung DC migration and accumulation into the lung draining lymph nodes (mediastinal lymph node [MLN]),46 suggesting a potential mechanism for the aforementioned impaired anti-IAV T-cell response in infected lungs. In this connection it is noteworthy that the CD103+ lung DC subset, which plays a prominent role in the induction of the adaptive immune T-cell response to viral respiratory tract infection in the MLNs, is now recognized to have the unique capacity to both sense and produce complement, thereby controlling both its own migration and the migration of CD11b+ respiratory DCs from the infected lung into the MLNs. Intriguingly, emerging evidence suggests that certain complement components (eg, C1q) can also either negatively or positively regulate inflammasome activation, depending on the disease.47, 48 Thus components of the complement pathway might, like viral PAMPs and cellular DAMPs, provide additional immunologic cues to regulate host immunity to viral infection.
Resolution phase
Viral clearance and the process of recovery from primary viral respiratory tract infection (as well as resistance to reinfection) are primarily mediated by the adaptive host immune response, which represents the cellular and humoral immune responses acting directly to orchestrate viral clearance or in consort with cells and products of the innate immune system.2, 49, 50, 51, 52, 53 In animal models of RSV infection, both cytotoxic CD8+ T-cell (CTL) and antibody responses play a pivotal role in RSV clearance from the lung.49, 51, 53 In experimental influenza virus infection, CTLs likewise contribute to viral clearance from the infected lungs, and in the case of IAV infection, the CTL response is primarily directed against conserved viral internal proteins, including the acid subunit of the polymerase, matrix, and nucleoprotein shared by most IAV strains independent of subtype. CTLs might confer a modest degree of protection from reinfection and potentially provide a degree of heterosubtypic immunity, which is immunity against IAV strains of different subtypes.
An effective B-cell response to viral respiratory tract infection is typically associated with the clearance of infectious virus at the site of infection and classically is reflected in the generation of serum antibody capable of neutralizing the virus in standard assays of viral neutralization in vitro, such as plaque reduction, although antibodies lacking the capacity to neutralize virus in vitro can still be effective in vivo. In the case of influenza virus infection, an effective B-cell response typically reflects the production of antibody directed to the viral glycoproteins hemagglutinin and neuraminidase. The production of these antibodies is believed to be important for the ultimate elimination of infectious virions from the lungs after primary viral infection and essential to preventing reinfection with viral strains of the same subtype in the case of IAV.2, 50, 52, 54
Cellular immunity
In viral respiratory tract infections cellular immune (ie, T-cell) responses can also play an important role in viral clearance from the lungs. In immunocompetent infants infected with RSV, viral clearance is typically achieved within 3 weeks of the onset of infection. However, RSV virus can persist for several months or more in infants with defective cellular immunity.55, 56, 57, 58 As noted above, respiratory DCs play an important role as initiators of the antiviral T-cell response. In the mouse model of IAV infection, langerin+CD103+CD11b− DCs within the conducting and terminal airways have been demonstrated to take up viral antigens and subsequently migrate from the site of infection into the MLNs. Here they act as APCs to trigger the activation of viral antigen–specific native T cells (both CD4+ and CD8+ T cells).59 CD103−CD11b+Ly6C+ inflammatory DCs also infiltrate into IAV-infected conducting airways and lung parenchyma. Within hours of the onset of infection, these DCs also migrate into the MLNs carrying viral antigens.60, 61, 62 Although lymph node–resident CD8α+ DCs are also capable of stimulating CD8+ T cells by cross-presenting viral antigens after uptake, they are less efficient than migrant CD103+ respiratory DCs as APCs for CD8+ T cells and cannot induce CD4+ T-cell responses.60, 63 Ex vivo sorted CD103+CD11b− DCs derived from MLNs of IAV-infected mice are the most potent at inducing CD8+ T-cell responses.64 Moreover, in vivo depletion of CD103+CD11b− DCs before IAV infection by diphtheria toxin administration into mice expressing diphtheria toxin receptor selectively in lung CD103+CD11b− DCs resulted in a diminished antiviral CD8+ T-cell response and delayed viral clearance from the infected lungs.59 These results and comparable findings in other models of viral infection suggested that among the DC subsets present in the MLNs, the migrant langerin+CD103+CD11b− respiratory DCs are the most potent APCs for the activation of naive antiviral T cells after viral lung infection.65, 66
After stimulation by DCs, the now activated naive T cells undergo multiple rounds of cell division in the MLNs and primarily differentiate into antiviral effector T cells. These freshly activated effector T cells emigrate out of the MLNs to the site of infection (ie, the infected lungs), where they can further interact with recently recruited viral antigen–displaying inflammatory DCs.67, 68, 69 This second round of interaction with DCs in the infected lung tissue might be crucial for an efficient antiviral T-cell response because in vivo depletion of lung DCs during infection led to significantly reduced effector CD8+ T-cell expansion and therefore defective viral clearance in the lung.67 This can be partially explained by the finding that in the IAV-infected lungs CD11chi DCs provide the T-cell survival factor IL-15 to virus-specific effector CD8+ T cells through transpresentation of IL-15 by using DC-expressed IL-15Rα.68 Therefore in patients with pulmonary viral infection, DCs might play an important role in modulating antiviral T-cell responses not only in the MLNs but also at the site of infection. However, this interaction might not always be beneficial because onsite restimulation of antiviral CD8+ effector T cells by CD11c+ DCs elicits not only the cytotoxic response, which is crucial for viral clearance, but also proinflammatory cytokine production, including IFN-γ, directly by the responding T cells (or indirectly through cytokines produced by infiltrating innate immune effectors cells). The excess production of proinflammatory cytokines can lead to enhanced inflammation and injury, resulting in immunopathology during pulmonary viral infection.70
As with the initiation phase of the adaptive immune response (ie, the activation of naive antiviral T cells), available evidence suggests that the production of proinflammatory and regulatory cytokines by activated effector T cells in the infected lungs is dependent on costimulation (ie, recognition of costimulatory ligands, such as CD80, CD86, and CD70) displayed on the surface of myeloid lineage CD45+ inflammatory cells infiltrating the infected lungs recognized by costimulatory receptors on the antiviral effector T cells. For example, the in vivo blockade of CD80 and CD86 in experimental murine IAV infection markedly diminishes IFN-γ production by anti-IAV CTLs in the lung but has no effect on viral clearance from the lungs.70 In IAV-infected lungs some of the CD45− lung parenchymal cell types, including type II alveolar epithelial cells, present viral antigens in the context of MHC-II molecules, which can be recognized and potentially trigger a cytotoxic response by effector CD4+ T cells.71 However, at present, the contribution of CD4+ T-cell cytotoxicity to control viral replication and viral clearance in vivo in patients with IAV infection is not certain.72, 73 Rather, results from experimental IAV infection studies suggest that the primary role of antiviral CD4+ T cells is to support antiviral B-cell response.74, 75
Humoral immunity
In patients infected with RSV, there is an inverse correlation between the frequency of lower respiratory tract infection caused by RSV and anti-RSV neutralizing antibody responses. Analyses in experimental models of RSV infection demonstrate a similar correlation.76, 77, 78 In the animal model of IAV infection, the humoral response to primary IAV infection consists of contributions from innate-like B-1 B cells, as well as virus-specific adaptive B cells localized to the extrafollicular (marginal zone) and follicular regions of the MLNs.52 On secondary exposure to virus, B cells present in inflammation-induced bronchial associated lymphoid tissue can also provide a local contribution to this antibody response. After activation by antigens, antigen-specific B cells undergo proliferative expansion and a series of differentiation events, resulting in the formation of germinal centers (GCs) within the lymphoid follicles, where B-cell receptor affinity maturation and memory B-cell formation occur.79, 80 The efficient activation and differentiation of both extrafollicular and follicular B cells have been demonstrated to be dependent on CD4+ T-cell help.81, 82, 83, 84
A distinct subpopulation of CD4+ T cells, T follicular helper T (TFH) cells, have been implicated as the major provider of T-cell help for B-cell activation/differentiation and, particularly based on studies both in human subjects and rodents, for the GC response and the generation of GC B cells.21, 85, 86, 87, 88
Recently, in the mouse model of IAV infection, a novel migratory CD45+ mononuclear cell type has been identified: late activator antigen-presenting cells (LAPCs).89, 90 LAPCs migrate from the IAV-infected lungs into the MLNs late in the infection cycle (ie, between 6 and 12 days after infection). On migration to MLNs, LAPCs promote the differentiation of viral antigen–primed CD4+ T cells into TFH cells through inducible costimulator/inducible costimulator ligand–mediated stimulation.54 In ex vivo coculture experiments neither B cells nor DCs isolated from the MLNs of IAV-infected mice efficiently induce TFH differentiation by antigen-primed CD4+ T cells. Therefore because of the late migration of LAPCs from the infected respiratory tract to the site of CD4+ T-cell activation and differentiation, LAPCs might be uniquely positioned to monitor the extent of microbial replication in the respiratory tract after the initial induction of T-cell responses by DCs in the MLNs and thereby regulate the balance between tissue-migrating TH1-type effector T cells and the MLN-resident antibody-supporting TFH differentiation.
Regulation of pulmonary inflammation during viral respiratory tract infections
The response of both innate and adaptive immune cells to pulmonary viral infections facilitates viral clearance but at the same time can, as noted above, produce excessive pulmonary inflammation resulting in tissue damage.91 Multiple cell types and molecules play a role in maintaining pulmonary homeostasis during pulmonary viral infections.92 Lung epithelial cells express CD200, which, after its engagement by a receptor (CD200R) displayed by alveolar macrophages under homeostatic conditions and inflammatory macrophages during IAV infection, inhibits the proinflammatory activity of these mononuclear cell populations in the lungs.93 Lung epithelial cells also constitutively express TGF-β1, which maintains immune homeostasis in the normal (uninfected) lung tissue.92 The in vivo blockade of TGF-β1 during IAV infection results in lethal lung tissue injury.94 Innate immune cells can also play a role in controlling excessive inflammation during viral respiratory tract infections. In the mouse model of RSV infection, both plasmacytoid DCs and alternatively activated (M2) macrophages have been suggested to play a crucial role in restricting excessive T cell–mediated inflammation through an unknown mechanism.95, 96 Adaptive immune cells also play a role in tissue homeostasis: forkhead box protein 3+ regulatory T (Treg) cells97 have an important role in maintaining immune homeostasis by suppressing inflammation in naive hosts. After viral respiratory tract infection, Treg cells in the lungs have been shown to produce the anti-inflammatory cytokine IL-10 through a BLIMP1 (transcription regulator B lymphocyte–induced maturation protein 1, also known as PRDM1)–dependent mechanism and control excessive immune responses.98, 99, 100 In the mouse model of RSV infection, the depletion of Treg cells leads to excessive pulmonary inflammation and lung injury during the course of infection.97, 101, 102 Recently, not only Treg cells but also conventional effector T cells (both CD4+ and CD8+ T cells) have been shown to exert a “regulatory” function in the lung through the production of IL-10 during simian virus 5, IAV, or RSV infection.100, 103, 104 The in vivo blockade of T cell–derived IL-10 results in excessive pulmonary inflammation characterized by increased accumulation of CD45+ inflammatory cells and enhanced production of proinflammatory cytokines, especially during the course of IAV or RSV infection.100, 104, 105 These findings reinforce the view that there are multiple layers of immunoregulation at both the cellular and molecular levels, which play an essential role in maintaining lung tissue homeostasis and preventing excess inflammation during the resolution phase of viral respiratory tract infections.
Restoration phase
Repair processes associated with viral respiratory tract infections
Once virus and virus-infected cells are cleared and associated pulmonary inflammation is controlled, the repair response to viral respiratory tract infection (the restoration phase) ensues. The lung's ability to quickly and appropriately regenerate the epithelium damaged during infection will determine whether normal pulmonary function is regained or complications occur. Unfortunately, the repair processes after acute lung injury are not well understood and largely based on chemically induced injury models,106, 107 which might not completely reflect the repair processes after a viral infection.108
Restoration of the respiratory epithelial barrier after injury (chemical or viral) can be divided into 3 overlapping stages: (1) coverage by neighboring epithelial cells of the denuded area through local spreading and migration, (2) migration and proliferation of progenitor cells to reconstitute the epithelium, and (3) differentiation of epithelial cells/progenitors into defined cell types to restore barrier and respiratory function.107 The sheer variety of cell types and soluble mediators involved is compounded by the complex organization of the lung itself.106, 107 However, these repair processes are rapid. The lung barrier can be largely, if not fully, reconstituted and lung respiratory function can be restored at least partially within days after viral clearance, depending on the severity of the infection and the extent of lung involvement.
Epithelial restoration is initiated locally as epithelial cells cover the space created by the loss of necrotic and apoptotic tissue neighboring the residue of cellular viral infection, host response to infection, or both. Dead cells are eliminated by the mucosal-ciliary elevator or removed by professional phagocytes and epithelial cells themselves.109, 110 Neighboring cells proliferate and in some cases (eg, type II alveolar epithelial cells and Clara cells) differentiate into another epithelial cell type to re-establish epithelial integrity. The degree to which these cell types can differentiate and contribute to the formation of the epithelial cell barrier might be limited. The migration of bona fide progenitor cells to inflamed sites is required to fully restore epithelial cell types.
The human lung contains stem cells capable of forming functional bronchioles, alveoli, and pulmonary vessels after tissue damage.111 To date, a number of specific progenitor cells have been identified in human subjects and mice, notably bronchial associated stem cells and tracheal basal cells.112, 113, 114, 115, 116 Some progenitors might also arrive from the bloodstream.117 Even these progenitors are limited in their ability to differentiate into various epithelial cell types. For example, bronchial associated stem cells appear unable to reconstitute alveoli.118 Thus multiple pools of progenitor cells are likely to be required to appropriately repair the damaged lung tissue. Different stem cell progenitors might also be induced (or recruited) based on the type of lung injury. During a murine IAV infection, tracheal basal cells assemble into Krt5+ pods and display a number of markers indicating they are reconstituting and in many cases generating alveoli.119 Interestingly, these cells were not present in a bleomycin injury model, suggesting the lung possesses specific repair processes rather than a generalized repair process for all types of injury.119
Although immune cells are mediating viral clearance and inducing inflammation, recent research has highlighted their function in promoting tissue repair. Particular interest has been directed to a family of innate lymphoid cells (ILCs), which is composed of rare, although important, lymphocytes devoid of traditional lineage markers (eg, CD3 and CD19) but dependent developmentally on Id2.120 These cell populations have been noted in mucosal and lymphoid tissue in both rodents and human subjects.121, 122, 123, 124, 125
One particular ILC type has been implicated in respiratory tract disease and repair: type II ILCs (ILC-IIs), which are also termed nuocytes, multipotent progenitors, and natural helper cells, depending on surface marker expression and location within the mouse.123, 124, 125 Similar cells have been demonstrated in human subjects.121, 122 On stimulation with IL-25, IL-33, or both,121, 122, 125, 126 ILC-IIs are potent producers of type II immune response (TH2) cytokines (IL-5 and IL-13 but not IL-4), extracellular matrix proteins, and growth factors. These products implicate ILC-IIs in the recovery processes after lung tissue damage. During IAV infection, depletion of ILC-IIs led to thermodysregulation, impaired epithelial regeneration, and deregulated lung function, which was independent of viral control.122 In particular, ILC-II–derived amphiregulin, an epithelial cell growth factor, was important in restoring epithelial integrity after viral clearance. It remains to be determined whether the other products of ILC-IIs are important for recovery from infection and restoration of barrier and respiratory function, although there is strong evidence that some ILC-II products can contribute to the exacerbation of asthma (discussed below).
ILCs can produce significant quantities of IL-22, which protects epithelial cells from apoptosis and triggers proliferation. IL-22 has been demonstrated to restore epithelial function in the gastrointestinal tract.127 Although IL-22–producing ILCs have not been identified in the lung, murine NKp46+ conventional NK cells are a significant source of IL-22 during IAV infection.128, 129 On the basis of analyses with IL-22–deficient mice and cells, NK cell–derived IL-22 has been reported to contribute to respiratory epithelial repair, induce IL-10 production, and exert antiviral effects.129 However, some investigators have noted no physiologic role of IL-22 during recovery from influenza infection when the cytokine is acutely depleted during infection by in vivo treatment of infected animals with neutralizing anti–IL-22 antibodies.122, 128 Thus it remains to be determined whether IL-22 is a bona fide cytokine aiding lung repair in experimental or human viral respiratory tract infection.
After viral clearance, the host immune response in the lung transitions from that of a proinflammatory type I immune response (TH1) and begins to acquire the characteristics of a type II immune response, the latter having been previously implicated in tissue repair.108, 130 For example, mice deficient in IL-4 receptor α (one of the 2 chains of the IL-4 receptor), which mediates IL-4 and IL-13 signaling, exhibit increased and sustained inflammation and impaired wound-repair responses.131, 132 Although IL-13 can stimulate lung epithelial cell proliferation and provisional matrix deposition, neutralization of IL-13 during murine IAV infection did not affect gross lung function.122 On the other hand, IL-4 and IL-13 signaling enhances the generation of alternatively activated macrophages (ie, M2 macrophages), which are anti-inflammatory and can be involved in tissue repair.133 During RSV infection, for example, the absence of M2 macrophages correlated with enhanced inflammation, and M2 macrophage transfer into IL-4 receptor α–deficient animals reduced lung pathology.95
Respiratory tract virus–associated sequelae
After infection with certain respiratory tract viruses, subjects can exhibit manifestations or sequelae of infection outside of the lung. For example, influenza infection in human subjects has been linked to myositis, myopathy, myocarditis, and central nervous system inflammation.134, 135 The mechanistic basis for these sequelae is unclear, and potential mechanisms are described elsewhere.136, 137, 138
Although secondary bacterial infections are associated with a number of respiratory tract viruses, including RSV and rhinoviruses,139, 140, 141 their most significant association is with IAV. Along with exacerbation of pre-existing conditions (eg, cardiovascular disease and asthma), bacterial coinfections are the primary cause of mortality in influenza-infected patients.142 IAV replication in the respiratory epithelium leads to impairment of mucosal-ciliary clearance and increased bacterial colonization.143, 144 Secondary infections primarily coincide with resolution of inflammation and the onset of reparative responses rather than early viral replication,142, 145, 146 indicating host responses also have a significant effect on the susceptibility to bacterial superinfection. In experimental models the release of type I interferons and IFN-γ during viral clearance from the lung negatively affects the ability of lung-infiltrating neutrophils and macrophages, respectively, to control bacterial infection.147, 148 In addition, the desensitization of TLRs, as well as the upregulation of anti-inflammatory molecules, such as IL-10, during viral clearance from the lung might serve to suppress immune responses to bacterial infection.149, 150, 151 Thus a loss of epithelial barrier function and the antiviral immune response might paradoxically create an environment that supports enhanced bacterial colonization, resulting in bacterial superinfection and ultimately bacterial pneumonia.
Viral infections of the lung are a recognized risk factor in the development of asthma and, more importantly, a major inducer of asthma exacerbation. Most forms of asthma (including asthma with the onset in young adults) have their origins in infancy.152 Viral respiratory tract infections in the first 2 years of life, particularly severe lung infection, are strong prognosticators for eventual asthma development.153, 154, 155 The highest risk for eventual asthma development is in children who experience a lung infection and become atopic to aeroallergens in the first 1 to 2 years of life.153, 156, 157, 158, 159 Intriguingly, aeroallergen sensitization alone is rarely associated with asthma throughout life.160 These observations imply that virus- and allergen-induced inflammation might act synergistically to disrupt normal lung function at a point in life when the lung is growing and experiencing significant anatomic changes.
RSV and rhinovirus infections, in particular, are associated with asthma risk. Severe cases of infant RSV infections have been associated with the development of childhood wheezing, as well as early adult asthma.153, 161, 162, 163 However, there are conflicting reports on whether RSV-associated childhood wheeze decreases with age.162, 163 Recently, greater emphasis has been placed on rhinovirus infection and its connection with asthma development. For example, rhinovirus is a 3 times greater disease burden for symptomatic lung infection than RSV in infants.164 It is unclear whether rhinovirus replication is occurring within the lower airways during infection because there are certain impediments for rhinovirus to replicate in this location,165 but population studies have demonstrated a strong link between severe rhinovirus-related infections in infants and eventual asthma development.153, 154, 157
Better documented but still poorly understood is the strong connection between viral respiratory tract infection and asthma exacerbation in subjects with pre-existing allergic disease and airway hyperresponsiveness. Asthma exacerbations have been documented after infection with rhinoviruses, seasonal and pandemic influenza, adenoviruses, and coronaviruses.166, 167, 168, 169 Eighty percent of children requiring hospitalization after an acute asthma attack had a viral infection at the time of entry.167
The mechanisms by which viral respiratory tract infection might contribute to asthma exacerbation are unclear, but current evidence demonstrates a skewed immune response in infected children. Wheezing children with symptomatic rhinovirus infection have diminished type I immune responses (eg, IFN-γ and IL-12).168 In contrast, type II cytokine levels were increased in young patients infected with rhinovirus, likely from a T-cell source. Furthermore, as discussed above, the contribution of type II cytokines produced by ILC-II responding to viral lung infection now needs to be considered. In a T cell–independent model of allergen sensitization, IL-5– and IL-13–producing ILC-IIs were critical for the development of lung eosinophilia and mucus hypersecretion.170 In a mouse model of IAV infection, airway hyperreactivity after infection was regulated by IL-13 production derived from ILC-IIs.171 Transfer of ILC-IIs into IL-13–deficient animals resulted in the exacerbation of asthma symptoms.
Type II immune responses during viral infections can also enhance the transition of macrophages into an M2 phenotype.172 M2 macrophages can produce additional type II cytokines, enhance mucous cell metaplasia, and augment airway hyperactivity.172 Similarly, respiratory DCs were demonstrated to upregulate FcεRIα in a type I interferon–dependent fashion after viral clearance in an experimental murine model.173 FcεRIα+ DCs augmented the recruitment of TH2 cells and mucous cell metaplasia.173 IgE levels are a significant risk factor for asthma development, and treatment of children and young adults with chronic asthma with omalizumab (anti-IgE) can significantly reduce the incidence of asthma exacerbation.174, 175, 176
As noted above, the transition to a type II response profile after viral infection is associated with lung repair processes. One intriguing hypothesis linking early viral respiratory tract infection and subsequent asthma development in infants and very young children (in which the lungs are in the process of maturing) is the possibility that viral respiratory tract infection results in a deregulated repair response affecting lung development and function, which in turn predisposes subjects to the subsequent development of airway hyperreactivity and asthma.
The epithelium in asthmatic patients is also fundamentally changed.177 Bronchial epithelial cells, as well as bronchial lavage cells, are more sensitive to viral replication.168, 178, 179 There is evidence that asthmatic epithelial cells are more sensitive to rhinovirus infection because of inherent deficiencies in type I and type III interferon production in response to infection.178, 179 However, these observations might be dependent on the strain of rhinovirus.177
Conclusions
Emerging evidence from a variety of experimental models of viral infection of the lung, as well as data from human studies, suggest that viral infection and particularly the host response to infection, although occurring on a continuum, can be viewed as occurring in 3 stages (Table I ).
Table I.
Role of innate and adaptive immune cells during pulmonary virus infection
| Cell type | Site of action | Pulmonary viral infection phases |
||
|---|---|---|---|---|
| Initiation phase∗ | Resolution phase† | Restoration phase‡ | ||
| Respiratory epithelial cells | Lung | Initial target of viral replication | Targets of effector immune cells; inhibit inflammatory macrophage activities | Regeneration source of epithelial barrier; can eliminate dying/dead cells |
| Lung fibroblasts | Lung | Unknown | Unknown | Produce growth factors and matrix proteins to facilitate recovery |
| Vascular endothelial cells | Lung | Initiation of cytokine storm; recruitment of innate immune cells | Unknown | Unknown |
| Migratory lung DCs | ||||
| Langerin+CD103+CD11b− DCs | MLN/lung | Induction of antiviral T-cell responses (CD8+ > CD4+ T cells) | Trigger the activation of antiviral T cells (both CD4+ and CD8+ T cells) | Unknown |
| CD103−CD11b+ DCs | MLN/lung | Induction of antiviral T-cell responses (CD8+ < CD4+ T cells) | Trigger the activation of antiviral T cells (both CD4+ and CD8+ T cells) | Unknown |
| Nonmigratory DCs | ||||
| CD8α+ DCs | MLN | Cross-presentation to antiviral CD8 T cells | Trigger the activation of viral antigen-specific T cells (CD8+ T cells) | Unknown |
| Alveolar macrophage | Lung | Initiation of innate immune responses | Unknown | Can eliminate dying/dead cells; can modify ILC activities |
| Alternatively activated macrophage | Lung | Unknown | Restrict excessive T cell–mediated inflammation | Can eliminate dying/dead cells; might aid tissue repair |
| Inflammatory monocytes | Lung | Amplification of inflammatory response | Provide T-cell survival signals; restimulate T cells | Can eliminate dying/dead cells |
| Neutrophils | Lung | Amplification of inflammatory response | Restimulate T cells | Can eliminate dying/dead cells |
| Eosinophils | Lung | Unknown | Unknown | Accumulate after infection; function unknown |
| ILCs | Lung | Unknown | Unknown | Produce growth factors, matrix proteins, and TH2 cytokines to facilitate recovery |
| NK cells | Lung | DC activation; augmenting inflammation | Unknown | Source of IL-22; might facilitate epithelial repair |
| NKT cells | Lung | Augmenting inflammation | Unknown | Can modify ILC activities (Braciale, unpublished observations) |
| LAPCs | MLN | Unknown | Induce antiviral B-cell response through promoting TFH cell differentiation | Unknown |
| Effector T cells | ||||
| TH1 cells | Lung | Not applicable | Proinflammatory cytokine secretion | Unknown |
| TH2 cells | Lung | Not applicable | Unknown | Source of TH2 cytokine that might facilitate recovery |
| CTL cells | Lung | Not applicable | Proinflammatory cytokine secretion; crucial for viral clearance in RS through cytotoxicity | Unknown |
| TFH cells | MLN | Not applicable | Induce both antiviral extrafollicular and follicular B-cell responses | Unknown |
| Treg cells | MLN/lung | Unknown | Control excessive immune response | Unknown |
| B cells | MLN | Unknown | Crucial for viral clearance in RS through neutralizing virions | Unknown |
NKT, Natural killer T; RS, respiratory system.
The initiation phase includes viral entry, replication/amplification, and initiation of innate and adaptive immunity, typically before the arrival of antiviral effector T cells to the lung.
The resolution phase encompasses the clearance of infectious virions through the actions of mainly, but not exclusively, effector T and B cells.
The restoration phase includes the repair/regeneration of respiratory epithelial cells and a return to homeostatic pulmonary function.
First is the initiation phase, in which CD45− stromal cell constituents, as well as resident and newly recruited CD45+ cells, represent the innate immune sentinels that initially respond to viral invasion. The response of these cells established an inflammatory milieu in which subsequent events occur and also serves to initiate the adaptive immune response.
Second is the resolution phase, in which cells of the adaptive immune system are recruited into the infected lung and the cells and their soluble products (eg, cytokines and antibody) serve the primary function of eliminating infectious virus and virus-infected cells, as well as orchestrating the recruitment and function of the innate immune cells involved in viral elimination. The adaptive immune cells, along with the CD45+ innate immune cells and the CD45− lung stromal cells, begin the process of restoring the normal structure and function to the infected lung.
Third is the restoration phase, in which the cellular elements making up the host response to infection, as well as resident and newly recruited stem cells, continue the process of restoration of lung barrier integrity and essential cellular functions (eg, gas exchange), as well as the elimination of cellular debris associated with infection. It is during this phase that lung “remodeling,” which is associated with either normal restoration of pulmonary structural integrity or airway and parenchymal changes associated with pulmonary disease, occurs. Over the past decades, we have seen remarkable advances in our understanding of the first 2 phases of the response to viral infection in the respiratory tract. A major challenge for the future is to elucidate the mechanisms that underlie the repair processes resulting in the restoration of normal pulmonary function and structure.
What do we know?
-
•
PAMPs trigger a wide range of intracellular signaling pathways and initiate optimal antiviral immunity in the infected lung.
-
•
Damage-associated host-derived components play a significant role in orchestrating host antiviral immune responses.
-
•
The host adaptive immune response, including the CTL-mediated response and B cell–mediated humoral responses, is crucial to clear virus in the infected lung.
-
•
The host's respiratory epithelium and immune response cooperate to restore lung function and homeostasis after viral infection.
-
•
The general idea of the stem cells responsible for restoring respiratory epithelial complexity is known.
-
•
ILCs are potent producers of type II cytokines, which might have implications for tissue repair and asthma.
-
•
Viral infections result in a number of disease maladies not necessarily dependent on active viral replication.
-
•
Childhood viral infections are strongly linked to eventual asthma development.
What is still unknown?
-
•
Not all cell types are equally created: cell type–specific (ie, epithelial cells vs DCs) innate immune responses to PAMPs and DAMPs
-
•
Regulation of the production of damage-associated molecules
-
•
Qualitative and quantitative immune responses to different damage-associated molecules
-
•
Identification of cellular receptors and signaling pathways for many DAMPs
-
•
Detailed mechanisms by which the host repairs the lung and restores function
-
•
Mechanisms regulating stem cell migration and differentiation in vivo after viral clearance
-
•
The location of innate lymphoid cells in the lung, potential roles during viral pathogenesis, and the extent to which they regulate tissue repair after injury
-
•
The mechanisms determining the transition from an antiviral type 1 response to a reparative type 2 response
-
•
Why some subjects have sequelae after viral infection and others do not
-
•
Whether childhood viral infections are a cause of eventual asthma development
-
•
Why viral infections, a nominal potent trigger of TH1 responses, could augment TH2 responses in certain children and asthmatic subjects
Footnotes
Series editors: Donald Y. M. Leung, MD, PhD, and Dennis K. Ledford, MD
References
- 1.Holt P.G., Strickland D.H., Wikstrom M.E., Jahnsen F.L. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol. 2008;8:142–152. doi: 10.1038/nri2236. [DOI] [PubMed] [Google Scholar]
- 2.Braciale T.J., Sun J., Kim T.S. Regulating the adaptive immune response to respiratory virus infection. Nat Rev Immunol. 2012;12:295–305. doi: 10.1038/nri3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeuchi O., Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi Y., Evans J.E., Rock K.L. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 5.Lamkanfi M., Dixit V.M. The inflammasomes. PLoS Pathog. 2009;5:e1000510. doi: 10.1371/journal.ppat.1000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pang I.K., Iwasaki A. Inflammasomes as mediators of immunity against influenza virus. Trends Immunol. 2011;32:34–41. doi: 10.1016/j.it.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Creagh E.M., O'Neill L.A. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 2006;27:352–357. doi: 10.1016/j.it.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Wilkins C., Gale M., Jr. Recognition of viruses by cytoplasmic sensors. Curr Opin Immunol. 2010;22:41–47. doi: 10.1016/j.coi.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoneyama M., Fujita T. RNA recognition and signal transduction by RIG-I-like receptors. Immunol Rev. 2009;227:54–65. doi: 10.1111/j.1600-065X.2008.00727.x. [DOI] [PubMed] [Google Scholar]
- 10.Poeck H., Bscheider M., Gross O., Finger K., Roth S., Rebsamen M. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nat Immunol. 2010;11:63–69. doi: 10.1038/ni.1824. [DOI] [PubMed] [Google Scholar]
- 11.Rintahaka J., Wiik D., Kovanen P.E., Alenius H., Matikainen S. Cytosolic antiviral RNA recognition pathway activates caspases 1 and 3. J Immunol. 2008;180:1749–1757. doi: 10.4049/jimmunol.180.3.1749. [DOI] [PubMed] [Google Scholar]
- 12.Burckstummer T., Baumann C., Bluml S., Dixit E., Durnberger G., Jahn H. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 13.Hornung V., Ablasser A., Charrel-Dennis M., Bauernfeind F., Horvath G., Caffrey D.R. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asselin-Paturel C., Boonstra A., Dalod M., Durand I., Yessaad N., Dezutter-Dambuyant C. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2:1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 15.Platanias L.C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 16.Donnelly R.P., Kotenko S.V. Interferon-lambda: a new addition to an old family. J Interferon Cytokine Res. 2010;30:555–564. doi: 10.1089/jir.2010.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Goffic R., Balloy V., Lagranderie M., Alexopoulou L., Escriou N., Flavell R. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006;2:e53. doi: 10.1371/journal.ppat.0020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koyama S., Ishii K.J., Kumar H., Tanimoto T., Coban C., Uematsu S. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J Immunol. 2007;179:4711–4720. doi: 10.4049/jimmunol.179.7.4711. [DOI] [PubMed] [Google Scholar]
- 19.Heer A.K., Shamshiev A., Donda A., Uematsu S., Akira S., Kopf M. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J Immunol. 2007;178:2182–2191. doi: 10.4049/jimmunol.178.4.2182. [DOI] [PubMed] [Google Scholar]
- 20.Pang I.K., Ichinohe T., Iwasaki A. IL-1R signaling in dendritic cells replaces pattern-recognition receptors in promoting CD8(+) T cell responses to influenza A virus. Nat Immunol. 2013;14:246–253. doi: 10.1038/ni.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morita R., Schmitt N., Bentebibel S.E., Ranganathan R., Bourdery L., Zurawski G. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pirhonen J., Sareneva T., Kurimoto M., Julkunen I., Matikainen S. Virus infection activates IL-1 beta and IL-18 production in human macrophages by a caspase-1-dependent pathway. J Immunol. 1999;162:7322–7329. [PubMed] [Google Scholar]
- 23.Pirhonen J., Sareneva T., Julkunen I., Matikainen S. Virus infection induces proteolytic processing of IL-18 in human macrophages via caspase-1 and caspase-3 activation. Eur J Immunol. 2001;31:726–733. doi: 10.1002/1521-4141(200103)31:3<726::aid-immu726>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 24.Ichinohe T., Lee H.K., Ogura Y., Flavell R., Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen I.C., Scull M.A., Moore C.B., Holl E.K., McElvania-TeKippe E., Taxman D.J. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanneganti T.D., Body-Malapel M., Amer A., Park J.H., Whitfield J., Franchi L. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006;281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- 27.Ichinohe T., Pang I.K., Iwasaki A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat Immunol. 2010;11:404–410. doi: 10.1038/ni.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeuchi R., Tsutsumi H., Osaki M., Haseyama K., Mizue N., Chiba S. Respiratory syncytial virus infection of human alveolar epithelial cells enhances interferon regulatory factor 1 and interleukin-1beta-converting enzyme gene expression but does not cause apoptosis. J Virol. 1998;72:4498–4502. doi: 10.1128/jvi.72.5.4498-4502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muruve D.A., Petrilli V., Zaiss A.K., White L.R., Clark S.A., Ross P.J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 30.Thomas P.G., Dash P., Aldridge J.R., Jr., Ellebedy A.H., Reynolds C., Funk A.J. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fink S.L., Cookson B.T. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallucci S., Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–119. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 33.Trautmann A. Extracellular ATP in the immune system: more than just a “danger signal”. Sci Signal. 2009;2:pe6. doi: 10.1126/scisignal.256pe6. [DOI] [PubMed] [Google Scholar]
- 34.Bianchi M.E. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 35.Lee B.H., Hwang D.M., Palaniyar N., Grinstein S., Philpott D.J., Hu J. Activation of P2X(7) receptor by ATP plays an important role in regulating inflammatory responses during acute viral infection. PLoS One. 2012;7:e35812. doi: 10.1371/journal.pone.0035812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanderstocken G., Van de Paar E., Robaye B., di Pietrantonio L., Bondue B., Boeynaems J.M. Protective role of P2Y2 receptor against lung infection induced by pneumonia virus of mice. PLoS One. 2012;7:e50385. doi: 10.1371/journal.pone.0050385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Zoelen M.A., van der Sluijs K.F., Achouiti A., Florquin S., Braun-Pater J.M., Yang H. Receptor for advanced glycation end products is detrimental during influenza A virus pneumonia. Virology. 2009;391:265–273. doi: 10.1016/j.virol.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stasakova J., Ferko B., Kittel C., Sereinig S., Romanova J., Katinger H. Influenza A mutant viruses with altered NS1 protein function provoke caspase-1 activation in primary human macrophages, resulting in fast apoptosis and release of high levels of interleukins 1beta and 18. J Gen Virol. 2005;86:185–195. doi: 10.1099/vir.0.80422-0. [DOI] [PubMed] [Google Scholar]
- 39.Lam W.Y., Tang J.W., Yeung A.C., Chiu L.C., Sung J.J., Chan P.K. Avian influenza virus A/HK/483/97(H5N1) NS1 protein induces apoptosis in human airway epithelial cells. J Virol. 2008;82:2741–2751. doi: 10.1128/JVI.01712-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cilloniz C., Shinya K., Peng X., Korth M.J., Proll S.C., Aicher L.D. Lethal influenza virus infection in macaques is associated with early dysregulation of inflammatory related genes. PLoS Pathog. 2009;5:e1000604. doi: 10.1371/journal.ppat.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carroll M.C. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5:981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- 42.Ricklin D., Hajishengallis G., Yang K., Lambris J.D. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cameron M.J., Kelvin A.A., Leon A.J., Cameron C.M., Ran L., Xu L. Lack of innate interferon responses during SARS coronavirus infection in a vaccination and reinfection ferret model. PLoS One. 2012;7:e45842. doi: 10.1371/journal.pone.0045842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stoermer K.A., Morrison T.E. Complement and viral pathogenesis. Virology. 2011;411:362–373. doi: 10.1016/j.virol.2010.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kopf M., Abel B., Gallimore A., Carroll M., Bachmann M.F. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat Med. 2002;8:373–378. doi: 10.1038/nm0402-373. [DOI] [PubMed] [Google Scholar]
- 46.Kandasamy M., Ying P.C., Ho A.W., Sumatoh H.R., Schlitzer A., Hughes T.R. Complement mediated signaling on pulmonary CD103(+) dendritic cells is critical for their migratory function in response to influenza infection. PLoS Pathog. 2013;9:e1003115. doi: 10.1371/journal.ppat.1003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doyle S.L., Campbell M., Ozaki E., Salomon R.G., Mori A., Kenna P.F. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med. 2012;18:791–798. doi: 10.1038/nm.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benoit M.E., Clarke E.V., Morgado P., Fraser D.A., Tenner A.J. Complement protein C1q directs macrophage polarization and limits inflammasome activity during the uptake of apoptotic cells. J Immunol. 2012;188:5682–5693. doi: 10.4049/jimmunol.1103760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chanock R.M., Kim H.W., Vargosko A.J., Deleva A., Johnson K.M., Cumming C. Respiratory syncytial virus. I. Virus recovery and other observations during 1960 outbreak of bronchiolitis, pneumonia, and minor respiratory diseases in children. JAMA. 1961;176:647–653. [PubMed] [Google Scholar]
- 50.Graham M.B., Braciale T.J. Resistance to and recovery from lethal influenza virus infection in B lymphocyte-deficient mice. J Exp Med. 1997;186:2063–2068. doi: 10.1084/jem.186.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McIntosh K., Masters H.B., Orr I., Chao R.K., Barkin R.M. The immunologic response to infection with respiratory syncytial virus in infants. J Infect Dis. 1978;138:24–32. doi: 10.1093/infdis/138.1.24. [DOI] [PubMed] [Google Scholar]
- 52.Waffarn E.E., Baumgarth N. Protective B cell responses to flu—no fluke! J Immunol. 2011;186:3823–3829. doi: 10.4049/jimmunol.1002090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watt P.J., Zardis M., Lambden P.R. Age related IgG subclass response to respiratory syncytial virus fusion protein in infected infants. Clin Exp Immunol. 1986;64:503–509. [PMC free article] [PubMed] [Google Scholar]
- 54.Yoo J.K., Fish E.N., Braciale T.J. LAPCs promote follicular helper T cell differentiation of Ag-primed CD4+ T cells during respiratory virus infection. J Exp Med. 2012;209:1853–1867. doi: 10.1084/jem.20112256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bangham C.R., Cannon M.J., Karzon D.T., Askonas B.A. Cytotoxic T-cell response to respiratory syncytial virus in mice. J Virol. 1985;56:55–59. doi: 10.1128/jvi.56.1.55-59.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cannon M.J., Openshaw P.J., Askonas B.A. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988;168:1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fishaut M., Tubergen D., McIntosh K. Cellular response to respiratory viruses with particular reference to children with disorders of cell-mediated immunity. J Pediatr. 1980;96:179–186. doi: 10.1016/s0022-3476(80)80799-2. [DOI] [PubMed] [Google Scholar]
- 58.Hall C.B., Powell K.R., MacDonald N.E., Gala C.L., Menegus M.E., Suffin S.C. Respiratory syncytial viral infection in children with compromised immune function. N Engl J Med. 1986;315:77–81. doi: 10.1056/NEJM198607103150201. [DOI] [PubMed] [Google Scholar]
- 59.GeurtsvanKessel C.H., Willart M.A., van Rijt L.S., Muskens F., Kool M., Baas C. Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells. J Exp Med. 2008;205:1621–1634. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Belz G.T., Smith C.M., Kleinert L., Reading P., Brooks A., Shortman K. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc Natl Acad Sci U S A. 2004;101:8670–8675. doi: 10.1073/pnas.0402644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.GeurtsvanKessel C.H., Bergen I.M., Muskens F., Boon L., Hoogsteden H.C., Osterhaus A.D. Both conventional and interferon killer dendritic cells have antigen-presenting capacity during influenza virus infection. PLoS One. 2009;4:e7187. doi: 10.1371/journal.pone.0007187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin K.L., Suzuki Y., Nakano H., Ramsburg E., Gunn M.D. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol. 2008;180:2562–2572. doi: 10.4049/jimmunol.180.4.2562. [DOI] [PubMed] [Google Scholar]
- 63.Suarez-Ramirez J.E., Wu T., Lee Y.T., Aguila C.C., Bouchard K.R., Cauley L.S. Division of labor between subsets of lymph node dendritic cells determines the specificity of the CD8(+) T-cell recall response to influenza infection. Eur J Immunol. 2011;41:2632–2641. doi: 10.1002/eji.201141546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim T.S., Braciale T.J. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS One. 2009;4:e4204. doi: 10.1371/journal.pone.0004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beauchamp N.M., Busick R.Y., Alexander-Miller M.A. Functional divergence among CD103+ dendritic cell subpopulations following pulmonary poxvirus infection. J Virol. 2010;84:10191–10199. doi: 10.1128/JVI.00892-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Edelson B.T., Kc W., Juang R., Kohyama M., Benoit L.A., Klekotka P.A. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med. 2010;207:823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McGill J., Van Rooijen N., Legge K.L. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J Exp Med. 2008;205:1635–1646. doi: 10.1084/jem.20080314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McGill J., Van Rooijen N., Legge K.L. IL-15 trans-presentation by pulmonary dendritic cells promotes effector CD8 T cell survival during influenza virus infection. J Exp Med. 2010;207:521–534. doi: 10.1084/jem.20091711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shen C.H., Talay O., Mahajan V.S., Leskov I.B., Eisen H.N., Chen J. Antigen-bearing dendritic cells regulate the diverse pattern of memory CD8 T-cell development in different tissues. Proc Natl Acad Sci U S A. 2010;107:22587–22592. doi: 10.1073/pnas.1016350108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hufford M.M., Kim T.S., Sun J., Braciale T.J. Antiviral CD8+ T cell effector activities in situ are regulated by target cell type. J Exp Med. 2011;208:167–180. doi: 10.1084/jem.20101850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Debbabi H., Ghosh S., Kamath A.B., Alt J., Demello D.E., Dunsmore S. Primary type II alveolar epithelial cells present microbial antigens to antigen-specific CD4+ T cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L274–L279. doi: 10.1152/ajplung.00004.2005. [DOI] [PubMed] [Google Scholar]
- 72.Brown D.M., Dilzer A.M., Meents D.L., Swain S.L. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J Immunol. 2006;177:2888–2898. doi: 10.4049/jimmunol.177.5.2888. [DOI] [PubMed] [Google Scholar]
- 73.Graham M.B., Braciale V.L., Braciale T.J. Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J Exp Med. 1994;180:1273–1282. doi: 10.1084/jem.180.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Topham D.J., Doherty P.C. Clearance of an influenza A virus by CD4+ T cells is inefficient in the absence of B cells. J Virol. 1998;72:882–885. doi: 10.1128/jvi.72.1.882-885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Topham D.J., Tripp R.A., Hamilton-Easton A.M., Sarawar S.R., Doherty P.C. Quantitative analysis of the influenza virus-specific CD4+ T cell memory in the absence of B cells and Ig. J Immunol. 1996;157:2947–2952. [PubMed] [Google Scholar]
- 76.Hemming V.G., Prince G.A., Horswood R.L., London W.J., Murphy B.R., Walsh E.E. Studies of passive immunotherapy for infections of respiratory syncytial virus in the respiratory tract of a primate model. J Infect Dis. 1985;152:1083–1087. doi: 10.1093/infdis/152.5.1083. [DOI] [PubMed] [Google Scholar]
- 77.Prince G.A., Hemming V.G., Horswood R.L., Chanock R.M. Immunoprophylaxis and immunotherapy of respiratory syncytial virus infection in the cotton rat. Virus Res. 1985;3:193–206. doi: 10.1016/0168-1702(85)90045-0. [DOI] [PubMed] [Google Scholar]
- 78.Suffin S.C., Prince G.A., Muck K.B., Porter D.D. Immunoprophylaxis of respiratory syncytial virus infection in the infant ferret. J Immunol. 1979;123:10–14. [PubMed] [Google Scholar]
- 79.Vinuesa C.G., Tangye S.G., Moser B., Mackay C.R. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 80.Linterman M.A., Vinuesa C.G. Signals that influence T follicular helper cell differentiation and function. Semin Immunopathol. 2010;32:183–196. doi: 10.1007/s00281-009-0194-z. [DOI] [PubMed] [Google Scholar]
- 81.Vogelzang A., McGuire H.M., Yu D., Sprent J., Mackay C.R., King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 82.Nurieva R.I., Chung Y., Hwang D., Yang X.O., Kang H.S., Ma L. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee S.K., Rigby R.J., Zotos D., Tsai L.M., Kawamoto S., Marshall J.L. B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. J Exp Med. 2011;208:1377–1388. doi: 10.1084/jem.20102065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Choi Y.S., Kageyama R., Eto D., Escobar T.C., Johnston R.J., Monticelli L. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Breitfeld D., Ohl L., Kremmer E., Ellwart J., Sallusto F., Lipp M. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schaerli P., Willimann K., Lang A.B., Lipp M., Loetscher P., Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim C.H., Rott L.S., Clark-Lewis I., Campbell D.J., Wu L., Butcher E.C. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med. 2001;193:1373–1381. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 89.Yoo J.K., Baker D.P., Fish E.N. Interferon-beta modulates type 1 immunity during influenza virus infection. Antiviral Res. 2010;88:64–71. doi: 10.1016/j.antiviral.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 90.Yoo J.K., Galligan C.L., Virtanen C., Fish E.N. Identification of a novel antigen-presenting cell population modulating antiinfluenza type 2 immunity. J Exp Med. 2010;207:1435–1451. doi: 10.1084/jem.20091373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.La Gruta N.L., Kedzierska K., Stambas J., Doherty P.C. A question of self-preservation: immunopathology in influenza virus infection. Immunol Cell Biol. 2007;85:85–92. doi: 10.1038/sj.icb.7100026. [DOI] [PubMed] [Google Scholar]
- 92.Snelgrove R.J., Godlee A., Hussell T. Airway immune homeostasis and implications for influenza-induced inflammation. Trends Immunol. 2011;32:328–334. doi: 10.1016/j.it.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 93.Snelgrove R.J., Goulding J., Didierlaurent A.M., Lyonga D., Vekaria S., Edwards L. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol. 2008;9:1074–1083. doi: 10.1038/ni.1637. [DOI] [PubMed] [Google Scholar]
- 94.Carlson C.M., Turpin E.A., Moser L.A., O'Brien K.B., Cline T.D., Jones J.C. Transforming growth factor-beta: activation by neuraminidase and role in highly pathogenic H5N1 influenza pathogenesis. PLoS Pathog. 2010;6:e1001136. doi: 10.1371/journal.ppat.1001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shirey K.A., Pletneva L.M., Puche A.C., Keegan A.D., Prince G.A., Blanco J.C. Control of RSV-induced lung injury by alternatively activated macrophages is IL-4R alpha-, TLR4-, and IFN-beta-dependent. Mucosal Immunol. 2010;3:291–300. doi: 10.1038/mi.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smit J.J., Rudd B.D., Lukacs N.W. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J Exp Med. 2006;203:1153–1159. doi: 10.1084/jem.20052359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee D.C., Harker J.A., Tregoning J.S., Atabani S.F., Johansson C., Schwarze J. CD25+ natural regulatory T cells are critical in limiting innate and adaptive immunity and resolving disease following respiratory syncytial virus infection. J Virol. 2010;84:8790–8798. doi: 10.1128/JVI.00796-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Antunes I., Kassiotis G. Suppression of innate immune pathology by regulatory T cells during Influenza A virus infection of immunodeficient mice. J Virol. 2010;84:12564–12575. doi: 10.1128/JVI.01559-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cretney E., Xin A., Shi W., Minnich M., Masson F., Miasari M. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat Immunol. 2011;12:304–311. doi: 10.1038/ni.2006. [DOI] [PubMed] [Google Scholar]
- 100.Sun J., Madan R., Karp C.L., Braciale T.J. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fulton R.B., Meyerholz D.K., Varga S.M. Foxp3+ CD4 regulatory T cells limit pulmonary immunopathology by modulating the CD8 T cell response during respiratory syncytial virus infection. J Immunol. 2010;185:2382–2392. doi: 10.4049/jimmunol.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ruckwardt T.J., Bonaparte K.L., Nason M.C., Graham B.S. Regulatory T cells promote early influx of CD8+ T cells in the lungs of respiratory syncytial virus-infected mice and diminish immunodominance disparities. J Virol. 2009;83:3019–3028. doi: 10.1128/JVI.00036-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Palmer E.M., Holbrook B.C., Arimilli S., Parks G.D., Alexander-Miller M.A. IFNgamma-producing, virus-specific CD8+ effector cells acquire the ability to produce IL-10 as a result of entry into the infected lung environment. Virology. 2010;404:225–230. doi: 10.1016/j.virol.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sun J., Cardani A., Sharma A.K., Laubach V.E., Jack R.S., Muller W. Autocrine regulation of pulmonary inflammation by effector T-cell derived IL-10 during infection with respiratory syncytial virus. PLoS Pathog. 2011;7:e1002173. doi: 10.1371/journal.ppat.1002173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weiss K.A., Christiaansen A.F., Fulton R.B., Meyerholz D.K., Varga S.M. Multiple CD4+ T cell subsets produce immunomodulatory IL-10 during respiratory syncytial virus infection. J Immunol. 2011;187:3145–3154. doi: 10.4049/jimmunol.1100764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Beers M.F., Morrisey E.E. The three R's of lung health and disease: repair, remodeling, and regeneration. J Clin Invest. 2011;121:2065–2073. doi: 10.1172/JCI45961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Crosby L.M., Waters C.M. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol. 2010;298:L715–L731. doi: 10.1152/ajplung.00361.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gorski S.A., Hufford M.M., Braciale T.J. Recent insights into pulmonary repair following virus-induced inflammation of the respiratory tract. Curr Opin Virol. 2012;2:233–241. doi: 10.1016/j.coviro.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hashimoto Y., Moki T., Takizawa T., Shiratsuchi A., Nakanishi Y. Evidence for phagocytosis of influenza virus-infected, apoptotic cells by neutrophils and macrophages in mice. J Immunol. 2007;178:2448–2457. doi: 10.4049/jimmunol.178.4.2448. [DOI] [PubMed] [Google Scholar]
- 110.Juncadella I.J., Kadl A., Sharma A.K., Shim Y.M., Hochreiter-Hufford A., Borish L. Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature. 2013;493:547–551. doi: 10.1038/nature11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kajstura J., Rota M., Hall S.R., Hosoda T., D'Amario D., Sanada F. Evidence for human lung stem cells. N Engl J Med. 2011;364:1795–1806. doi: 10.1056/NEJMoa1101324. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 112.Cole B.B., Smith R.W., Jenkins K.M., Graham B.B., Reynolds P.R., Reynolds S.D. Tracheal basal cells: a facultative progenitor cell pool. Am J Pathol. 2010;177:362–376. doi: 10.2353/ajpath.2010.090870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Giangreco A., Arwert E.N., Rosewell I.R., Snyder J., Watt F.M., Stripp B.R. Stem cells are dispensable for lung homeostasis but restore airways after injury. Proc Natl Acad Sci U S A. 2009;106:9286–9291. doi: 10.1073/pnas.0900668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Giangreco A., Reynolds S.D., Stripp B.R. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol. 2002;161:173–182. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim C.F., Jackson E.L., Woolfenden A.E., Lawrence S., Babar I., Vogel S. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 116.Rock J.R., Onaitis M.W., Rawlins E.L., Lu Y., Clark C.P., Xue Y. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Krause D.S. Bone marrow-derived cells and stem cells in lung repair. Proc Am Thorac Soc. 2008;5:323–327. doi: 10.1513/pats.200712-169DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rawlins E.L., Okubo T., Xue Y., Brass D.M., Auten R.L., Hasegawa H. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kumar P.A., Hu Y., Yamamoto Y., Hoe N.B., Wei T.S., Mu D. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Spits H., Di Santo J.P. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 121.Mjosberg J.M., Trifari S., Crellin N.K., Peters C.P., van Drunen C.M., Piet B. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 122.Monticelli L.A., Sonnenberg G.F., Abt M.C., Alenghat T., Ziegler C.G., Doering T.A. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Moro K., Yamada T., Tanabe M., Takeuchi T., Ikawa T., Kawamoto H. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 124.Neill D.R., Wong S.H., Bellosi A., Flynn R.J., Daly M., Langford T.K. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Price A.E., Liang H.E., Sullivan B.M., Reinhardt R.L., Eisley C.J., Erle D.J. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bartemes K.R., Iijima K., Kobayashi T., Kephart G.M., McKenzie A.N., Kita H. IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2011;188:1503–1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zheng Y., Valdez P.A., Danilenko D.M., Hu Y., Sa S.M., Gong Q. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 128.Guo H., Topham D.J. Interleukin-22 (IL-22) production by pulmonary Natural Killer cells and the potential role of IL-22 during primary influenza virus infection. J Virol. 2010;84:7750–7759. doi: 10.1128/JVI.00187-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kumar P., Thakar M.S., Ouyang W., Malarkannan S. IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunol. 2013;6:69–82. doi: 10.1038/mi.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Allen J.E., Wynn T.A. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog. 2011;7:e1002003. doi: 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen F., Liu Z., Wu W., Rozo C., Bowdridge S., Millman A. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med. 2012;18:260–266. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Loke P., Gallagher I., Nair M.G., Zang X., Brombacher F., Mohrs M. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J Immunol. 2007;179:3926–3936. doi: 10.4049/jimmunol.179.6.3926. [DOI] [PubMed] [Google Scholar]
- 133.Varin A., Gordon S. Alternative activation of macrophages: immune function and cellular biology. Immunobiology. 2009;214:630–641. doi: 10.1016/j.imbio.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 134.Fraaij P.L., Heikkinen T. Seasonal influenza: the burden of disease in children. Vaccine. 2011;29:7524–7528. doi: 10.1016/j.vaccine.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 135.Kuiken T., Taubenberger J.K. Pathology of human influenza revisited. Vaccine. 2008;26(Suppl 4):D59–D66. doi: 10.1016/j.vaccine.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mamas M.A., Fraser D., Neyses L. Cardiovascular manifestations associated with influenza virus infection. Int J Cardiol. 2008;130:304–309. doi: 10.1016/j.ijcard.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 137.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang G.F., Li W., Li K. Acute encephalopathy and encephalitis caused by influenza virus infection. Curr Opin Neurol. 2010;23:305–311. doi: 10.1097/wco.0b013e328338f6c9. [DOI] [PubMed] [Google Scholar]
- 139.Jennings L.C., Anderson T.P., Beynon K.A., Chua A., Laing R.T., Werno A.M. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax. 2008;63:42–48. doi: 10.1136/thx.2006.075077. [DOI] [PubMed] [Google Scholar]
- 140.Talbot T.R., Poehling K.A., Hartert T.V., Arbogast P.G., Halasa N.B., Edwards K.M. Seasonality of invasive pneumococcal disease: temporal relation to documented influenza and respiratory syncytial viral circulation. Am J Med. 2005;118:285–291. doi: 10.1016/j.amjmed.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 141.Templeton K.E., Scheltinga S.A., van den Eeden W.C., Graffelman A.W., van den Broek P.J., Claas E.C. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase chain reaction. Clin Infect Dis. 2005;41:345–351. doi: 10.1086/431588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.van der Sluijs K.F., van der Poll T., Lutter R., Juffermans N.P., Schultz M.J. Bench-to-bedside review: bacterial pneumonia with influenza−pathogenesis and clinical implications. Crit Care. 2010;14:219. doi: 10.1186/cc8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pittet L.A., Hall-Stoodley L., Rutkowski M.R., Harmsen A.G. Influenza virus infection decreases tracheal mucociliary velocity and clearance of Streptococcus pneumoniae. Am J Respir Cell Mol Biol. 2010;42:450–460. doi: 10.1165/rcmb.2007-0417OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Plotkowski M.C., Puchelle E., Beck G., Jacquot J., Hannoun C. Adherence of type I Streptococcus pneumoniae to tracheal epithelium of mice infected with influenza A/PR8 virus. Am Rev Respir Dis. 1986;134:1040–1044. doi: 10.1164/arrd.1986.134.5.1040. [DOI] [PubMed] [Google Scholar]
- 145.Boyd M., Clezy K., Lindley R., Pearce R. Pandemic influenza: clinical issues. Med J Aust. 2006;185(suppl):S44–S47. doi: 10.5694/j.1326-5377.2006.tb00706.x. [DOI] [PubMed] [Google Scholar]
- 146.Madhi S.A., Klugman K.P. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med. 2004;10:811–813. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shahangian A., Chow E.K., Tian X., Kang J.R., Ghaffari A., Liu S.Y. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J Clin Invest. 2009;119:1910–1920. doi: 10.1172/JCI35412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sun K., Metzger D.W. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med. 2008;14:558–564. doi: 10.1038/nm1765. [DOI] [PubMed] [Google Scholar]
- 149.Didierlaurent A., Goulding J., Patel S., Snelgrove R., Low L., Bebien M. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J Exp Med. 2008;205:323–329. doi: 10.1084/jem.20070891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Smith M.W., Schmidt J.E., Rehg J.E., Orihuela C.J., McCullers J.A. Induction of pro- and anti-inflammatory molecules in a mouse model of pneumococcal pneumonia after influenza. Comp Med. 2007;57:82–89. [PMC free article] [PubMed] [Google Scholar]
- 151.van der Sluijs K.F., van Elden L.J., Nijhuis M., Schuurman R., Pater J.M., Florquin S. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J Immunol. 2004;172:7603–7609. doi: 10.4049/jimmunol.172.12.7603. [DOI] [PubMed] [Google Scholar]
- 152.Stern D.A., Morgan W.J., Halonen M., Wright A.L., Martinez F.D. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008;372:1058–1064. doi: 10.1016/S0140-6736(08)61447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kusel M.M., de Klerk N.H., Kebadze T., Vohma V., Holt P.G., Johnston S.L. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lemanske R.F., Jr., Jackson D.J., Gangnon R.E., Evans M.D., Li Z., Shult P.A. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 155.Oddy W.H., de Klerk N.H., Sly P.D., Holt P.G. The effects of respiratory infections, atopy, and breastfeeding on childhood asthma. Eur Respir J. 2002;19:899–905. doi: 10.1183/09031936.02.00103602. [DOI] [PubMed] [Google Scholar]
- 156.Holt P.G., Rowe J., Kusel M., Parsons F., Hollams E.M., Bosco A. Toward improved prediction of risk for atopy and asthma among preschoolers: a prospective cohort study. J Allergy Clin Immunol. 2010;125:653–659. doi: 10.1016/j.jaci.2009.12.018. e1-9. [DOI] [PubMed] [Google Scholar]
- 157.Jackson D.J., Gangnon R.E., Evans M.D., Roberg K.A., Anderson E.L., Pappas T.E. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Martinez F.D., Stern D.A., Wright A.L., Taussig L.M., Halonen M. Differential immune responses to acute lower respiratory illness in early life and subsequent development of persistent wheezing and asthma. J Allergy Clin Immunol. 1998;102:915–920. doi: 10.1016/s0091-6749(98)70328-8. [DOI] [PubMed] [Google Scholar]
- 159.Sigurs N., Bjarnason R., Sigurbergsson F., Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161:1501–1507. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 160.Holt P.G., Sly P.D. Viral infections and atopy in asthma pathogenesis: new rationales for asthma prevention and treatment. Nat Med. 2012;18:726–735. doi: 10.1038/nm.2768. [DOI] [PubMed] [Google Scholar]
- 161.Pullan C.R., Hey E.N. Wheezing, asthma, and pulmonary dysfunction 10 years after infection with respiratory syncytial virus in infancy. Br Med J (Clin Res Ed) 1982;284:1665–1669. doi: 10.1136/bmj.284.6330.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sigurs N., Aljassim F., Kjellman B., Robinson P.D., Sigurbergsson F., Bjarnason R. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65:1045–1052. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 163.Stein R.T., Sherrill D., Morgan W.J., Holberg C.J., Halonen M., Taussig L.M. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 164.Kusel M.M., de Klerk N.H., Holt P.G., Kebadze T., Johnston S.L., Sly P.D. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr Infect Dis J. 2006;25:680–686. doi: 10.1097/01.inf.0000226912.88900.a3. [DOI] [PubMed] [Google Scholar]
- 165.Kennedy J.L., Turner R.B., Braciale T., Heymann P.W., Borish L. Pathogenesis of rhinovirus infection. Curr Opin Virol. 2012;2:287–293. doi: 10.1016/j.coviro.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hasegawa S., Hirano R., Hashimoto K., Haneda Y., Shirabe K., Ichiyama T. Characteristics of atopic children with pandemic H1N1 influenza viral infection: pandemic H1N1 influenza reveals ‘occult’ asthma of childhood. Pediatr Allergy Immunol. 2011;22:e119–e123. doi: 10.1111/j.1399-3038.2010.01090.x. [DOI] [PubMed] [Google Scholar]
- 167.Johnston S.L., Pattemore P.K., Sanderson G., Smith S., Lampe F., Josephs L. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Message S.D., Laza-Stanca V., Mallia P., Parker H.L., Zhu J., Kebadze T. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A. 2008;105:13562–13567. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Olenec J.P., Kim W.K., Lee W.M., Vang F., Pappas T.E., Salazar L.E. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy Clin Immunol. 2010;125:1001–1006.e1. doi: 10.1016/j.jaci.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Halim T.Y., Krauss R.H., Sun A.C., Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 171.Chang Y.J., Kim H.Y., Albacker L.A., Baumgarth N., McKenzie A.N., Smith D.E. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kim E.Y., Battaile J.T., Patel A.C., You Y., Agapov E., Grayson M.H. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med. 2008;14:633–640. doi: 10.1038/nm1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Grayson M.H., Cheung D., Rohlfing M.M., Kitchens R., Spiegel D.E., Tucker J. Induction of high-affinity IgE receptor on lung dendritic cells during viral infection leads to mucous cell metaplasia. J Exp Med. 2007;204:2759–2769. doi: 10.1084/jem.20070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Busse W.W., Morgan W.J., Gergen P.J., Mitchell H.E., Gern J.E., Liu A.H. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–1015. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hollams E.M., Deverell M., Serralha M., Suriyaarachchi D., Parsons F., Zhang G. Elucidation of asthma phenotypes in atopic teenagers through parallel immunophenotypic and clinical profiling. J Allergy Clin Immunol. 2009;124:463–470. doi: 10.1016/j.jaci.2009.06.019. e1-16. [DOI] [PubMed] [Google Scholar]
- 176.Subrata L.S., Bizzintino J., Mamessier E., Bosco A., McKenna K.L., Wikstrom M.E. Interactions between innate antiviral and atopic immunoinflammatory pathways precipitate and sustain asthma exacerbations in children. J Immunol. 2009;183:2793–2800. doi: 10.4049/jimmunol.0900695. [DOI] [PubMed] [Google Scholar]
- 177.Bochkov Y.A., Hanson K.M., Keles S., Brockman-Schneider R.A., Jarjour N.N., Gern J.E. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol. 2010;3:69–80. doi: 10.1038/mi.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Contoli M., Message S.D., Laza-Stanca V., Edwards M.R., Wark P.A., Bartlett N.W. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 179.Wark P.A., Johnston S.L., Bucchieri F., Powell R., Puddicombe S., Laza-Stanca V. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]