Abstract
Chronic neuropathic pain is a frequent comorbidity following spinal cord injury (SCI) and often fails to respond to conventional pain management strategies. Preventive administration of docosahexaenoic acid (DHA) or consumption of a diet rich in omega-3 polyunsaturated fatty acids (O3PUFAs) confers potent prophylaxis against SCI and improves functional recovery. The present study examines whether this novel dietary strategy provides significant antinociceptive benefits in rats experiencing SCI-induced pain. Rats were fed control chow or chow enriched with O3PUFAs for 8 weeks before being subjected to sham or cord contusion surgeries, continuing the same diets after surgery for another 8 more weeks. The paw sensitivity to noxious heat was quantified for at least 8 weeks post-SCI using the Hargreaves test. We found that SCI rats consuming the preventive O3PUFA-enriched diet exhibited a significant reduction in thermal hyperalgesia compared to those consuming the normal diet. Functional neurometabolomic profiling revealed a distinctive deregulation in the metabolism of endocannabinoids (eCB) and related N-acyl ethanolamines (NAEs) at 8 weeks post-SCI. We found that O3PUFAs consumption led to a robust accumulation of novel NAE precursors, including the glycerophospho-containing docosahexaenoyl ethanolamine (DHEA), docosapentaenoyl ethanolamine (DPEA), and eicosapentaenoyl ethanolamine (EPEA). The tissue levels of these metabolites were significantly correlated with the antihyperalgesic phenotype. In addition, rats consuming the O3PUFA-rich diet showed reduced sprouting of nociceptive fibers containing CGRP and dorsal horn neuron p38 MAPK expression, well-established biomarkers of pain. The spinal cord levels of inositols were positively correlated with thermal hyperalgesia, supporting their role as biomarkers of chronic neuropathic pain. Notably, the O3PUFA-rich dietary intervention reduced the levels of these metabolites. Collectively, these results demonstrate the prophylactic value of dietary O3PUFA against SCI-mediated chronic pain.
Keywords: DHA, EPA, dietary fatty acids, endocannabinoid metabolome, spinal cord injury, chronic pain
Chronic neuropathic pain is one of the most important determinants in the perceived quality of life of spinal cord injury (SCI) patients (Anderson, 2004). Unfortunately, current therapeutics to treat this condition lack necessary efficacy and are limited in scope by unwanted side effects and poor tolerance. These shortcomings could be partly overcome with the use of preventive approaches that can provide resilience to damage prior to irreversible biochemical alterations have occurred in the perturbed cord. Trauma to the spinal cord triggers a robust secondary pathophysiological response, leading to cell death, inflammation, and dysfunction (Hulsebosch, 2002; Norenberg et al., 2004). Neuroinflammation is regarded as a hallmark mechanism underlying injury progression and pain processing (Christensen and Hulsebosch, 1997a; Hulsebosch et al., 2009), and thus represents an attractive target for therapeutic strategies (Kwon et al., 2011a; Kwon et al., 2011b).
Dietary-essential omega-3 polyunsaturated fatty acids (O3PUFAs), such as docosahexaenoic acid (DHA), are integral components of neural membrane phospholipids and play crucial roles in anti-inflammatory responses (Calder, 2008). Longstanding studies have demonstrated that dietary PUFAs are mediating factors in pain processing, as evidenced by increased threshold for thermal pain and neuropathic pain in rats fed with high omega-3 to omega-6 PUFA ratios (Yehuda and Carasso, 1993). Recent studies have shown that O3PUFAs and their derivatives can exert strong antinociceptive effects against thermal and chemical stimulation in various animal models (Nakamoto et al., 2010; Xu et al., 2010; Tokuyama and Nakamoto, 2011). Given this evidence, it would seem reasonable to consider that dietary O3PUFAs may also play important roles in SCI-induced pain.
In a recent report, we showed that SCI causes a robust PUFA deregulation and leads to a marked DHA deficiency, which was associated with impaired recovery and dysfunction (Figueroa et al., 2013). Notably, administration of O3PUFAs maintained the cord PUFA homeostasis, conferred neuroprotection, prevented dysfunction and facilitated recovery after acute and chronic SCI, even when administered in a prophylactic manner (Figueroa et al., 2012; Figueroa et al., 2013). These findings led us to hypothesize that a preventive diet enriched in O3PUFAs modulates behavioral responses implicated in pathological nociception in rats. This idea is supported by studies showing that the diet type at the time of injury can affect pain behaviors associated with nerve lesions (Shir and Seltzer, 2001; Alloui et al., 2003; Hargraves and Hentall, 2005; Li et al., 2005; Estebe et al., 2006). Despite this evidence, diet remains a largely unexplored therapeutic avenue to ameliorate pain in SCI.
This study is an initial attempt to assess the effects of dietary O3PUFAs on thermal pain stimuli in SCI rats. Because N-Acylated ethanolamines (NAE) and related endocannabinoids (eCBs) are bioactive lipids implicated in pain processing (Beltramo et al., 2006; Petrosino et al., 2007; Hama and Sagen, 2011), we focused on identifying the involvement of dietary O3PUFAs in their local modulation following SCI. Here, the eCB metabolome has been expanded to include the ethanolamines, glycerides, and metabolic precursors, intermediates, and derivatives. These metabolites have been implicated in regulating anti-inflammatory responses and can exert cannabimimetic actions as endogenous agonist of cannabinoid receptors (Devane et al., 1992; Hanus et al., 1993; Priller et al., 1995; Brown et al., 2010), but whether dietary PUFAs impact the levels of these bioactive lipids in SCI has not been comprehensively evaluated. To address this issue, we employed both LC/MS and GC/MS-based metabolomics on cord samples collected from sham and contusion SCI operated Sprague-Dawley rats that received either control or O3PUFA-enriched diets. Deciphering the neurometabolomic profile that distinguishes pain-like behaviors may have important clinical implications for pain management and allow for improved prognosis in SCI.
2. Experimental Procedures
2.1. Animals
All animal studies were performed in compliance with the Loma Linda University School of Medicine regulations and institutional guidelines consistent with the NIH Guide for the Care and Use of Laboratory Animals. Female Sprague-Dawley rats were received from Charles River Laboratories (Portage, MI) and housed in individual cages on alternating 12 h light/ dark cycles. It is worth noting that in this study we used two independent cohorts of animals. Although both cohorts received the same dietary and surgical interventions, and behavioral testing, there were differences in the time allowed for survival (cohort 1: at least 4 animals per diet group, allowed to survive until 12 weeks post-injury; cohort 2: at least 13 animals per diet group, allowed to survive until 8 weeks post-injury). Animals in cohort 2 were also used to determine the effect of dietary O3PUFAs in sensorimotor and autonomic dysfunction after SCI (Figueroa et al., 2013).
2.2. Diet Composition
Custom AIN-93-based diets were prepared with modifications to the fat composition as described previously (Figueroa et al., 2013). Briefly, dietary fats were approximately 6% of the pellets dry weight and were supplied as either soybean oil (control chow) or menhaden fish oil (O3PUFA-enriched chow: DHA = 12.82-gm and EPA = 6.91-gm per 100 gm of diet). Diets were matched for cholesterol content.
2.3. Surgical and Post-Operative Procedures
Eight weeks after the dietary pretreatment, animals were deeply anesthetized with a mixture of ketamine/xylazine (80 mg/kg and 10 mg/kg, respectively). The spinal cord injuries were generated using the well-characterized New York University (NYU) Impactor (Gruner, 1992). Notably, trauma caused using this device induces below-level pain that is well developed and longstanding, suggesting that the model is suitable for chronic pain research (Jung et al., 2008). To produce the contusion, the skin and the muscles overlying the spinal column were cut. A laminectomy was performed at the T9-T10 level and the T8 and T12 spinal processes were clamped to the Impactor, and the exposed dorsal surface of the cord was subjected to weight drop impact using a 10-g rod released from a height of 12.5-mm. Sham animals received only a laminectomy. The animals body temperature was maintained at 37°C during the procedure. After operation, muscle layers were sutured and skin layers closed. The bladders of injured rats were expressed using the Crede’s maneuver three times a day until voiding reflexes were restored. Cefazolin (Bristol Myers Squibb, New York, NY; 25 mg/kg, s.q.) and Buprenex® (buprenorphine; Reckett and Colman Pharmaceuticals, Inc. Richmond, VA; 0.05 mg/kg, s.c.) were given to all rats for 5 and 3 consecutive days, respectively. Animals were allowed to survive for 8 or 12 weeks post-operation and the spinal cord tissue dissected for metabolomics and immunohistochemical analyses, respectively.
2.4. Nociceptive Testing
Thermal hyperalgesia (TH) was assessed using the well-established Hargreaves withdrawal test to thermal noxious stimulus (Hargreaves et al., 1988). This behavior has been found to be a sensitive and reproducible behavioral test to investigate chronic neuropathic pain and is exhibited approximately 28 days following contusion injury (Resnick et al., 2004; DomBourian et al., 2006; Rajpal et al., 2007; Cramer et al., 2008). In the week prior to the baseline recordings, the animals were habituated to the behavioral testing apparatus by undergoing 5 different daily testing sessions. Once plantar paw placement was re-established, rats were evaluated weekly until animals were euthanized. Briefly, the animals were placed in a Plexiglas enclosure that rested on an elevated glass floor (Plantar Test, UGO BASILE, Biological Research Apparatus, Comerio, Italy). After allowing the animals to acclimate to the chamber for 30 min, a movable focused infrared emitter was placed under the animal’s paw. A photocell automatically turned the emitter off when the animal moved its paw and the latency time for the animal to withdraw its paw was recorded. Strength of stimulation was adjusted to produce hindpaw baseline latencies close to 12 seconds (approximately 50–60 °C). A safety cutoff of 20 sec was used to prevent prolonged exposure to the noxious heat. Five different trials were performed per paw with at least 5 min allowed between each trial. The instrument operators were blinded to the treatment assignations. Minimum and maximum latency values were excluded from each paw analysis at each time point.
It is well-recognized that the testing season, the climate (humidity and temperature), the time of day, the cage density, the animal weight, the locomotor behavior, the number of instrument operators, and order of testing have a significant impact on the results of pain studies in rodents (Mogil et al., 2000; Mogil and McCarson, 2000; Le Bars et al., 2001; Chesler et al., 2002). Furthermore, the repetitive nature of the Hargreaves test makes it very susceptible to learning phenomena (Kocevski and Tvrdeić, 2008). Dietary lipids, including DHA, are known modulators of learning and sensitization processes, which could introduce unwanted confounding effects and affect the outcome of sensory results (Ikemoto et al., 2001; Hashimoto et al., 2002; Lim et al., 2005; McNamara et al., 2008; Joseph et al., 2012). On the basis of this evidence and to facilitate data interpretation, the thermal latencies are represented as percent change from baseline and were normalized to changes observed in sham animals as previously reported (Figueroa et al., 2013). Briefly, the nociceptive phenotype was determined by the following equation:
| EQ.1 |
HWL% = hindpaw withdrawal latency percent change from baseline normalized to week-to-week changes in sham animals; ib = latency from injured animal in diet A at baseline; ix = latency from injured animal in diet A at time x; sb = averaged latency from all sham animals in diet A at baseline; sx = averaged latency from all sham animals in diet A at time x.
2.5. Metabolomic Profiling
Unbiased metabolic profiling was performed as previously described (Figueroa et al., 2013). Animals were deeply anesthetized and transcardially perfused with ice-cold PBS to limit blood contamination. Spinal cord samples (75–100 mg) were flash frozen in liquid nitrogen and immediately stored at −80°C. Samples were homogenized in water at the time of analyses. The protein was precipitated with methanol containing four standards to report on extraction efficiency. The resulting supernatant was split into equal aliquots for analysis on the three platforms. Aliquots were dried under nitrogen and vacuum-desiccated. The metabolomics profiling platform employed for this analysis was based on a combination of three independent platforms: ultrahigh performance liquid chromatography/tandem mass spectrometry (UHPLC/MS/MS2) optimized for basic species, UHPLC/MS/MS2 optimized for acidic species, and gas chromatography/mass spectrometry (GC/MS). Controls were analyzed concomitantly with the experimental samples. For instance, aliquots of a well-characterized human plasma pool served as technical replicates throughout the data set, extracted water samples served as process blanks, and a cocktail of standards spiked into every analyzed sample allowed instrument performance monitoring. Experimental samples and controls were randomized across platform run days. The metabolites were identified by automated comparison of the ion features in the experimental samples and compared to a reference library of chemical standard entries that included retention time, molecular weight (m/z), preferred adducts, and in-source fragments as well as associated MS spectra. The neurometabolomics features were curated by visual inspection for quality control using software developed at Metabolon (Dehaven et al., 2010). Archived mass spectrometry data from our previously reported study, which was curated for only identified ‘named’ compounds in Metabolon’s chemical reference library (Figueroa et al., 2013), was re-curated to further investigate the unidentified compounds that were detected in the study.
Of particular importance, inositols features have been implicated as osmolytes and clinical metabolic markers of inflammation (Brand et al., 1993; Schuhmann et al., 2003), SCI-mediated chronic pain (Pattany et al., 2002), and recently as a marker of SCI progression (Erschbamer et al., 2011). Since SCI-induced edema and water disturbances may introduce bias in the quantification of osmolytes, the relative levels of Ins were quantified relative to creatine levels (Ins/Cr ratio).
2.6. Metabolomics Analyses
An estimate of the false discovery rate (FDR), which is given by the q-value, was calculated to take into account the multiple comparisons that normally occur in metabolomic-based studies as previously reported (Storey and Tibshirani, 2003b, a). For example, when analyzing 200 compounds, it is expected to see about 10 compounds meeting the p ≤ 0.05 cut-off by random chance. Thus, the q-value describes the false discovery rate; a low q-value (q < 0.10) is an indication of high confidence in a result.
The partial least square-discriminant analysis (PLS-DA) is a supervised method that uses multivariate regression techniques to extract via linear combination of original variables (X) the information that can predict the class membership (Y). This regression was performed using the plsr function provided by R pls package. The classification and cross-validation were performed using the corresponding wrapper function offered by the caret package. To assess the significance of class discrimination, prediction accuracy during training and the separation distance permutation test were performed. In each permutation, a PLS-DA model was built between the data (X) and the permuted class labels (Y) using the optimal number of components determined by cross validation for the model based on the original class assignment. The variable importance in projection (VIP), which is a weighted sum of squares of the PLS loadings and takes into account the amount of explained Y-variation in each dimension was used to measure the impact of each metabolite in the model. Generally, features with high impact have VIP values higher than 1.
2.7. Immunodetection
Immunofluorescence methods has been described previously (Figueroa et al., 2012). Briefly, spinal cord sections were dried at room temperature for 10–15 min, washed with PBS, and post-fixed with 4% PFA for 10 min. The sections were blocked and incubated at 4°C ON in 20% normal donkey serum with 0.1% Tween-20 with rabbit anti-phosphorylated-p38, p-p38 (1:200; R&D Systems, Minneapolis, MN) and mouse anti-NeuN monoclonal antibody (1:250; Millipore, Billerca, MA). Additional experiments used mouse anti-CD11b (OX42, 1:100; AbD Serotec, Raleigh, NC) to examine reactive microglial cells. Alternatively, sections were incubated with rabbit anti-GAP43 (1:500; Abcam, Cambridge, MA) and sheep polyclonal calcitonin gene-related peptide (CGRP; 1:500; Abcam). The sections were then washed with PBS and incubated in secondary antibodies [Alexa Fluor® 594-conjugated donkey anti–rabbit or donkey anti-sheep (1:500; Invitrogen, Carlsbad, CA) and Alexa Fluor® 488-conjugated donkey anti–mouse or donkey anti-rabbit (1:500; Invitrogen)]. Primary antibody omission and normal serum controls were used to confirm the specificity of the immunoreaction. Slides were examined with an Olympus Optical Fluoview FV1000 confocal microscope. Unbiased stereological methods were followed as previously reported (Figueroa et al., 2012). Two blinded observers quantified the immunoreactivity in lamina I to III, which were identified by superimposing photomicrographs with spinal cord diagrams from the Watson, Paxinos, and Kayalioglu spinal cord atlas. For each animal, the p-p38-positive neurons were counted manually in at least 4 randomly selected areas of the superficial dorsal horns. The mean number of p-p38-expressing neurons was then tabulated for each animal and group. For fiber sprouting analyses, the CGRP-GAP43 double labeling immunoreactivity was quantitated by inverting merged images into black (marker-positive) and white in the NIH Image J program for measurement of positive pixels/area in the dorsal horns laminae I to III. Results were obtained by averaging measurements made by blinded investigators.
2.8. Statistical Analysis
Statistical analyses were performed using SPSS version 20.0 (IBM: SPSS, Armonk, New York), Prism 5 software v5d (GraphPad Software Inc., San Diego, CA), the “R” program (http://cran.r-project.org/), and metaboanalyst (Xia et al., 2009; Xia et al., 2012). Two-Way Analysis of Variance (ANOVA) followed by Bonferroni post-hoc comparisons was used to determine the effect of the diet type, injury, and time on hindpaw thermal withdrawal latencies and differences within and between groups. To determine the antinociceptive effects of dietary O3PUFAs in time we calculated the area under the nociception versus time curve (AUC) and subjected AUCs to t-tests as previously described (Svensson et al., 2005; Buczynski et al., 2010; Morisseau et al., 2010). ANOVA contrasts were used to identify features that differed significantly between tested groups. All other data were assessed by Mann-Whitney U test. The Kolmogorov-Smirnov and Shapiro-Wilk normality tests together with the Grubbs’ method, also known as ESD (extreme studentized deviate; www.graphpad.com), were used to investigate outliers and spread. Spearman’s rank correlation tests were used to explore associations between detected metabolites and the sensory phenotype. Data are presented as mean ± SEM. Statistical differences were considered significant at p < 0.05 unless otherwise specified.
3. Results
3.1. General conditions and summary of previously published findings related to this study
Previously, we reported that experimental spinal cord injury (SCI) leads to a marked docosahexaenoic acid (DHA) deficiency and motor and autonomic deficits, which were corrected by dietary omega-3 polyunsaturated fatty acids (O3PUFAs) prophylaxis (Figueroa et al., 2013). Here, we hypothesized that this dietary prophylactic intervention would reduce thermal hypersensitivity in SCI. We characterized the antihyperalgesic effects of this dietary strategy and investigated the extent to which dietary O3PUFAs impact the levels of bioactive metabolites and cellular targets associated with nociception and inflammation in the injured cord.
3.2. Preventive dietary O3PUFAs attenuate the development of below-level thermal hyperalgesia after injury to the spinal cord
To examine the effect of dietary O3PUFAs on the onset and maintenance of neuropathic pain after SCI, we used the paw thermal sensitivity to noxious heat (Hargreaves testing). We found no differences in the average baseline hindpaw withdrawal latencies between groups (10.61 ± 0.59 s for control-fed animals and 11.34 ± 0.44 s for animals receiving O3PUFA diets; mean ± SEM, p > 0.05). Because of differences in survival times and missing data points between animal cohorts, we used two-way ANOVA to identify the effect of the diet type and operation in the thermal latencies differences. We found that both diet type and the surgical intervention were significant sources of variation in our data set [for the diet effect F(3,49253) = 40.22, p = 0.0001, n = 8–18 rats per group]. Post hoc analysis revealed significant differences in thermal thresholds between sham and injured animals receiving control diets from week 8 to week 12 post-operation (p < 0.05). A novel finding of this study is that the animals consuming diets rich in O3PUFAs showed no significant alterations in their hindpaw withdrawal latencies when compared to their sham counterparts (p > 0.05). Post hoc comparisons between injured groups revealed that the most significant differences in nociception occurred between after 7 wpi and hence the focus period of this current investigation (p < 0.05; Fig. 1A). Sham-operated rats receiving O3PUFA-rich diets did not show significant changes in their thermal thresholds when compared to animals being fed with control diets (p > 0.05).
Figure 1.
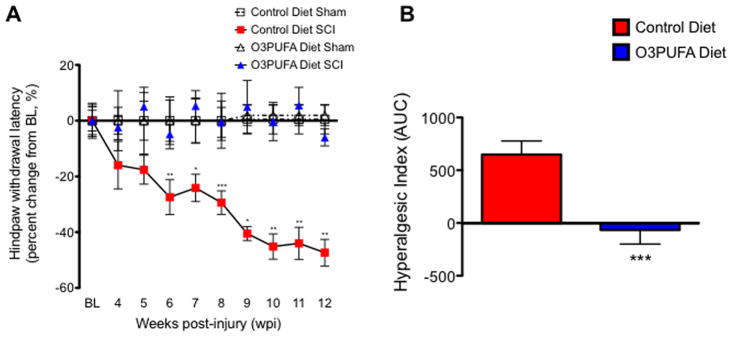
Responsiveness to thermal stimulation in animals receiving control and O3PUFA-enriched diets. (A) Thoracic contusion to the spinal cord leads to below-level thermal hyperalgesia in animals receiving control diets. Hindpaw withdrawal latencies (averaged percent change from baseline) are plotted versus time (weeks post-injury, wpi). For each timepoint, the individual latencies were adjusted to the percent change from baseline observed in sham animals receiving the same diet using equation 1 (see Materials and Methods section). No significant latency alterations were observed between sham animals. Notably, dietary O3PUFAs prevented the development of thermal hyperalgesia (p > 0.05 when compared to sham animals). TW-ANOVA identified the diet type and surgery as significant sources of variation [for diet/surgery F(3,49253) = 40.22, p = 0.0001, n = 8–18]. Bonferroni’s post hoc analyses showed significant thermal withdrawal latency changes when comparing injured animals receiving the different diet types (p < 0.05). (B) To investigate the overall effect of O3PUFA in thermal hindpaw sensitivities, the hyperalgesic index was generated using the area under the curve (AUC). Analyses of the AUC revealed that the O3PUFA diet had a significant antihyperalgesic effect (Mann Whitney U rank test; p < 0.001). Each bar represents mean ± SEM; n = 18.
Calculation of the area under the thermal withdrawal latency change versus time curve (AUC) showed a potent antihyperalgesic effect of dietary O3PUFAs in SCI rats (Fig. 1B; Mann Whitney U rank test p = 0.0008; n = 18).
3.3. Metabolomic profiling reveals distinctive endocannabinoid signatures associated with chronic SCI and dietary O3PUFAs
We used an untargeted metabolomics approach (Figueroa et al., 2013) to investigate the neurochemical consequences of O3PUFAs consumption on the endocannabinoid (eCB) metabolome. Figure 2A summarizes the four groups analyzed in this study and the metabolomic interactions between them. A total of 275 named metabolites and 76 unnamed biochemical were detected and analyzed. The diet rich in O3PUFA significantly changed more than 20% of the detected metabolic features (74 total altered features: 40 upregulated, 34 downregulated when compared to animals receiving control diets). Of significance, more than 40% of these altered metabolites were associated with the endocannabinoid metabolome and are the focus of the present study. In this study, data was protected against false positives using the false discovery rate q value. We first sorted the metabolomic data by the p-value and chose the cutoff for significance at p < 0.05. We found that the false discovery rate of various diet-derived metabolites with significant p values were extremely low (q < 0.1%) when comparing injured animals, validating the significance of our results (see below).
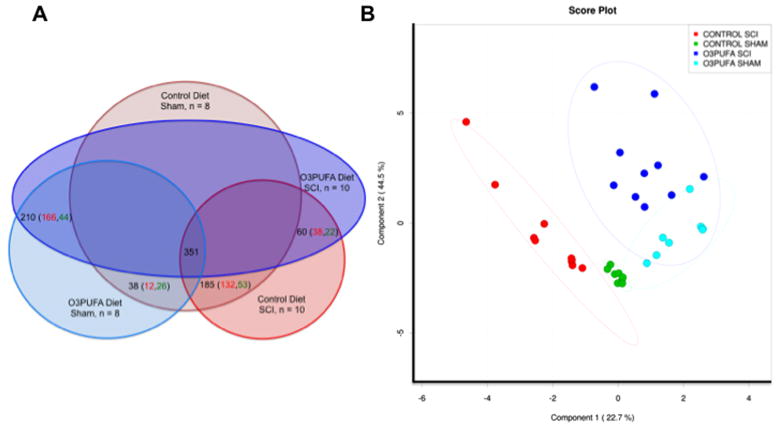
Figure 2.
SCI and dietary O3PUFAs modulate the endocannabinoid-related neurometabolome. (A) Venn diagram depicting the numerical interactions among data sets. ANOVA contrasts analyses were used to evaluate the regulation pattern differences between groups. The total number of features detected across 36 spinal cord tissue samples was 351 metabolites. Diagram illustrates the number of total metabolites significantly altered between groups (e.g., O3PUFA diet SCI/Control diet SCI contrast resulted in 60 significantly altered metabolites; 38 upregulated and 22 downregulated; p < 0.05). (B) Partial least square discriminant analysis (PLS-DA) distinguished subgroups based on dietary intake at 8 weeks post-injury (wpi). Model was constructed using scaled intensity peaks of the detected features associated with the endocannabinoids (eCBs) system: classic eCBs, eCBs glycerols, and related N-acyl ethanolamines (NAEs) and metabolites. Projections provided statistically significant separations between subgroups.
The partial least square-discriminant analysis (PLS-DA) score plot was obtained using the variation scores of the first two principal components, component 1 (22.7%) and component 2 (44.5%). These analyses revealed distinctive endocannabinoid-related profiles between groups (Fig. 2B). Each plot mark corresponds to an animal subject and the variability in relative metabolite levels detected for that animal. Hotelling’s T2 confidence ellipse, at a significance level of 0.05, showed no outliers. Permutation analyses validated the class discrimination and neurometabolomic separation (observed test statistic p < 0.01). Consequently, more than 67% (component 1 + component 2) of the metabolomics differences can be explained with certainty by the generated PLS model. For simplicity, only the prediction accuracy during training result is shown (Fig. 3A).
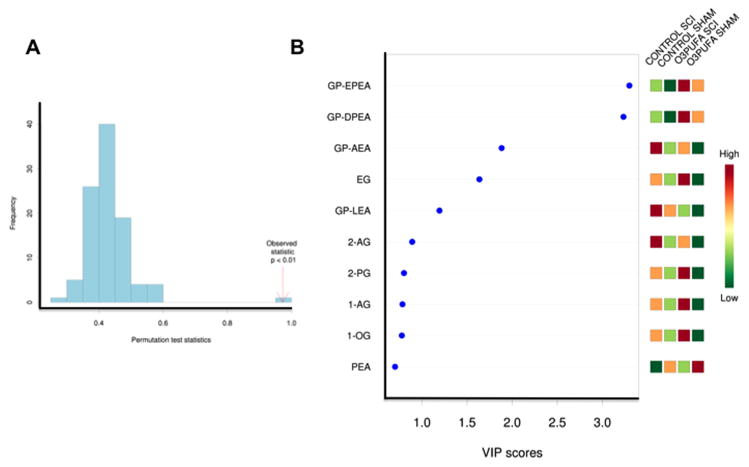
Figure 3.
PLS-DA model validation and metabolite impact. (A) Prediction accuracy training permutation test validate the PLS-DA model by showing a significant observed statistic (p < 0.01). (B) The variable influence on projection (VIP) analyses, which reflect the importance of metabolites showed the significant contribution of selective NAEs, endocannabinoids, endocannabinoid glycerols, and O3PUFA-derived glycerophospho ethanolamines (GP-NAEs) in our PLS model. The colored boxes on the right indicate the relative concentrations of the corresponding metabolite in each group under study. Abbreviations: GP, glycerophospho; EPEA, eicosapentaenoyl ethanolamine; DPEA, docosapentaenoyl ethanolamine; AEA, arachidonoyl ethanolamine; EG, eicosenoyl glycerol; LEA, linoleoyl ethanolamine; 2-AG, 2-arachidonoyl glycerol; 2-PG, 2-palmitoyl glycerol; 1-OG, 1-oleoyl glycerol; PEA, palmitoyl ethanolamine.
We found that both chronic SCI and the preventive diet enriched in O3PUFAs had a significant impact in the levels of acyl glycerol class endocannabinoids and in the metabolism of N-acyl ethanolamines (Figure 3B). Also, the cord of injured animals receiving the O3PUFAs showed higher levels of eicosenoyl, palmitoyl, arachidonoyl, and oleoyl glycerols when compared to control fed injured animals. In general, the diet rich in O3PUFAs skewed the metabolomic profile towards increased levels of long-chain N-acyl ethanolamines. In particular, we identified a selective group of glycerophospho-containing N-acyl ethanolamines (GP-NAEs). These molecules showed the strongest influence (highest variable importance in projection, VIP, values) to the observed metabolomics differences between groups.
3.4. Dietary O3PUFA leads to a marked accumulation of diet-derived N-acyl ethanolamine (NAEs) precursors
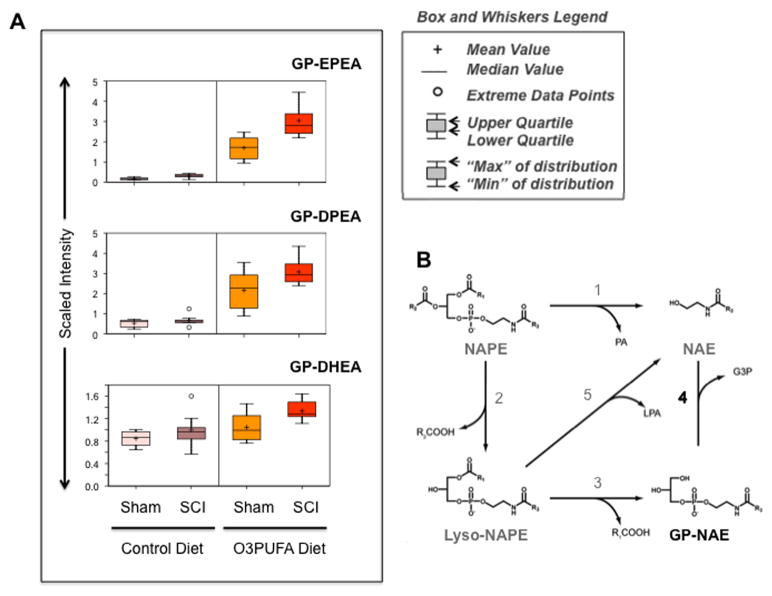
The beneficial neurological effects of O3PUFAs are partly related to their anti-inflammatory properties, however the exact mechanisms behind these actions are unknown. A putative mechanism could be via their conversion to related N-acyl ethanolamines (NAEs). Here, we present new evidence demonstrating the significant impact of diet in the regulation of these bioactive lipids after chronic SCI. In agreement with previous studies(Wood et al., 2010), we found that dietary O3PUFAs lead to a robust accumulation N-acyl ethanolamines glycerophospholipids containing DHA, DPA, and EPA fatty acids (Fig. 4A). Interestingly, metabolomic analysis revealed very low abundance of these lipids in the cord of animals receiving control diets.
Figure 4.
Chronic O3PUFAs consumption leads to a robust accumulation of diet-derived glycerophospho ethanolamines in the spinal cord. (A) Box and whiskers graphs illustrate a marked increase in the levels of O3PUFA-dervied GP-NAEs (relative to the median metabolite levels in each group). (B) Potential metabolic pathways for the biosynthesis of NAEs. This illustration is adapted and modified from previous reports (Simon and Cravatt, 2006, 2008). Reaction 1 is mediated by an NAPE-selective phospholipase D (PLD). Pathways 2-3-4 and 2–5 are NAPE-PLD independent. Recent studies suggest the involvement of a novel phospholipase A/B, named Abh4, α-β-hydrolase 4 in the NAPE conversion to GP-NAE (reaction 2 and 3). The secretory PLA2 can also release fatty acid from sn-2 position of NAPE (reaction 2). Reaction 4 is catalyzed by a new glycerophosphodiesterase, GDE1. Lyso-PLD catalyzes reaction 5. Abbreviations: DHEA, docosahexaenoyl ethanolamine; DPEA, docosapentaenoyl ethanolamine; EPEA, eicosapentaenoyl ethanolamine; GP, glycerophospho, NAE, N-acyl ethanolamine; NAPE, N-acyl phosphatidyl ethanolamine; G3P, glycerol-3-phosphate; LPA, lyso-phosphatidic acid; PA, phosphatidic acid.
Figure 4B illustrates the current knowledge on the NAEs biosynthetic pathways (Simon and Cravatt, 2006, 2008). Briefly, it has been proposed that NAEs are biosynthesized from their corresponding N-acyl phosphatidyl ethanolamines (NAPEs) occur through a single NAPE-PLD-dependent pathway (NAPE-PLD). Alternatively, NAPE-PLD-independent multi-step processes have been recently reported and involve alpha/beta-hydrolase 4 (ABDH4 or Abh4) and the glycerophosphodiesterase, GDE1. The results presented herein suggest a marked activation of NAPE-PLD-independent pathways following chronic SCI.
The effects of the diet and chronic SCI on the detected metabolites associated with the endocannabinoid and related NAEs are summarized in Table 1. ANOVA contrasts were performed to determine statistical differences in metabolite relative amounts between groups (differences were considered significant when p < 0.05; n = at least 8 rats per group). The false discovery rate showed very low q-values for features associated with the diet-derived O3PUFAs (EPEA = 8 × 10−15; DPEA = 2.40 × 10−9; DHEA = 0.012), supporting the significance of the observations.
Table 1.
The endocannabinoid (eCB) metabolome is altered following chronic SCI and influenced by dietary O3PUFAs. ANOVA contrasts were performed to determine statistical differences in metabolite relative amounts (differences were considered significant when p < 0.05; n = 8 rats per sham group and 10 rats per injury group). Comparisons were made between the four studied groups: (1) sham control diet, (2) SCI control diet, (3) sham O3PUFA-rich diet, and (4) SCI O3PUFA-rich diet. Notably, in spinal cord injured animals, the O3PUFA diet decreased the levels of the glycerophospho 2-LEA and 2-AEA and dramatically increased the levels of O3PUFA-derived GP-NAEs. Numbers represent fold of change. Heat map color legend: red, significant increase; green, significant decrease; light red and green represent marginal statistical significance. Abbreviations: LEA, linoleyl ethanolamine; AEA, arachidonoyl ethanolamine; GP-NAEs, glycerophospho n-acyl ethanolamines.
| Fold of Change | |||||
|---|---|---|---|---|---|
| ANOVA Contrasts | |||||
| Biochemical Name | Ctrl-SCI Ctrl-Sham | O3PUFA-SCI O3PUFA-Sham | O3PUFA-Sham Ctrl-Sham | O3PUFA-SCI Ctrl-SCI | |
| PUFAs | eicosapentaenoate (EPA; 20:5) | 5.09 | 4.08 | 6.71 | 5.37 |
| docosahexaenoate (DHA; 22:6) | 2.87 | 3.30 | 1.20 | 1.38 | |
| arachidonate (AA; 20:4) | 1.14 | 1.03 | −1.14 | −1.25 | |
| eCBs | oleic ethanolamide (OEA) | −1.41 | −1.33 | −1.03 | 1.02 |
| palmitoyl ethanolamide (PEA) | −1.92 | −1.61 | 1.05 | 1.24 | |
| 2-arachidonoyl glycerol (2-AG) | 2.21 | 2.37 | −1.18 | −1.10 | |
| 2-oleoylglycerol (2-OG) | 1.66 | 2.07 | −1.30 | −1.03 | |
| Precursors, Derivatives, Isomers, Candidates | ethanolamine | 1.34 | 1.69 | 1.08 | 1.36 |
| phosphoethanolamine | 1.92 | 2.47 | −1.19 | 1.08 | |
| glycerophosphoethanolamine | 1.12 | 1.93 | −1.04 | 1.64 | |
| glycerol | 1.02 | 1.01 | −1.04 | −1.04 | |
| glycerol 3-phosphate (G3P) | 1.41 | 1.90 | 0.94 | 1.26 | |
| 1-palmitoyl glycerophosphoethanolamine | 1.60 | 1.18 | 1.38 | 1.01 | |
| 2-palmitoyl glycerophosphoethanolamine | 1.10 | −1.04 | 1.05 | −1.09 | |
| 1-palmitoleoyl glycerophosphoethanolamine | 3.26 | 3.77 | 1.20 | 1.38 | |
| 2-palmitoleoyl glycerophosphoethanolamine | 1.43 | 1.44 | 1.24 | 1.25 | |
| 1-stearoyl glycerophosphoethanolamine | 1.59 | 1.40 | 1.22 | 1.07 | |
| 1-oleoyl glycerophosphoethanolamine | 2.33 | 2.36 | 1.08 | 1.10 | |
| 2-oleoyl glycerophosphoethanolamine | −1.12 | −1.20 | 1.10 | 1.02 | |
| 2-linoleoyl glycerophosphoethanolamine | 1.35 | 1.02 | −1.47 | −1.96 | |
| 1-arachidonoyl glycerophosphoethanolamine | 4.52 | 4.06 | −1.15 | −1.28 | |
| 2-arachidonoyl glycerophosphoethanolamine | 1.20 | 1.06 | −1.16 | −1.32 | |
| 2-docosapentaenoyl glycerophosphoethanolamine (GP-DPEA) | 1.26 | 1.42 | 4.05 | 4.56 | |
| 2-docosahexaenoyl glycerophosphoethanolamine (GP-DHEA) | 1.18 | 1.28 | 1.24 | 1.34 | |
| elcosapentaenoyl glycerophosphoethanolamine (GP-EPEA) | 1.83 | 1.8 | 10.27 | 10.15 | |
| 1-palmitoyl plasmenylethanolamine | 2.28 | 1.78 | 1.48 | 1.15 | |
| 1-palmitoyl glycerol (1-monopalmitin) | 2.53 | 2.53 | 1.10 | 1.10 | |
| 2-palmitoyl glycerol (2-monopalmitin) | 1.89 | 2.27 | −1.27 | −1.04 | |
| 1-stearoyl glycerol (1-monostearin) | 1.94 | 2.11 | 1.01 | 1.10 | |
| 1-oleoyl glycerol (1-monoolein) | 2.42 | 2.58 | −1.03 | 1.04 | |
| 1-arachidonyl glycerol | 1.70 | 2.64 | −1.56 | −1.01 | |
| eicosenoyl glycerol (monoeicosenoin) | 3.70 | 3.22 | −1.06 | −1.22 | |
 Green: indicates significant difference (p≤0.05) between the groups shown, metabolite ratio of < 1.00
Green: indicates significant difference (p≤0.05) between the groups shown, metabolite ratio of < 1.00
 Light Green: narrowly missed statistical cutoff for significance 0.05<p<0.10, metabolite ratio of < 1.00
Light Green: narrowly missed statistical cutoff for significance 0.05<p<0.10, metabolite ratio of < 1.00
 Red: indicates significant difference (p≤0.05) between the groups shown: metabolite ratio of ≥ 1.00
Red: indicates significant difference (p≤0.05) between the groups shown: metabolite ratio of ≥ 1.00
 Light Red: narrowly missed statistical cutoff for significance 0.05<p<0.10, metabolite ratio of ≥ 1.00
Light Red: narrowly missed statistical cutoff for significance 0.05<p<0.10, metabolite ratio of ≥ 1.00
 Non-colored text and cell: mean values are not significantly different for that comparison
Non-colored text and cell: mean values are not significantly different for that comparison
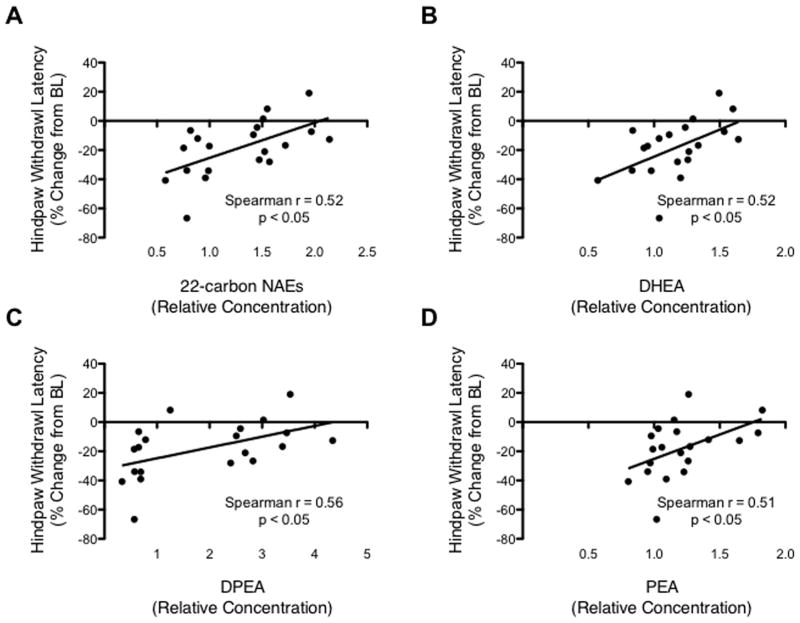
Spearman’s correlation analyses were used to explore the linear trends between cord metabolite levels and changes in thermal thresholds. The relative levels of NAEs containing 22 carbons N-acyl chains and the glycerophospho NAEs of O3PUFAs were positively correlated with reduced thermal withdrawal latency changes (Fig. 5A–D; Spearman r values > 0.50; p < 0.05). We also found a significant positive correlation between the relative levels of palmitoyl ethanolamine (PEA) and non-hyperalgesic responses, supporting its anti-inflammatory roles in SCI (Esposito et al., 2011; Esposito and Cuzzocrea, 2012). Together, our findings suggest that these diet-derived metabolites may play significant roles in antinociception.
Figure 5.
Metabolic features correlated with pain-like phenotypes. Scatter plot shows the significant relationship between the levels of (A) ethanolamines containing 22-carbon N-acyl chains, (B) GP-DHEA, (C) GP-DPEA, (D) PEA and the hindpaw thermal withdrawal latency change from baseline. For every correlation, the Spearman r was higher than 0.50, with a p < 0.05, XY = 20 pairs.
3.5. Functional metabolomics implicate the NAEs biosynthetic pathways in SCI-induced neuropathic pain
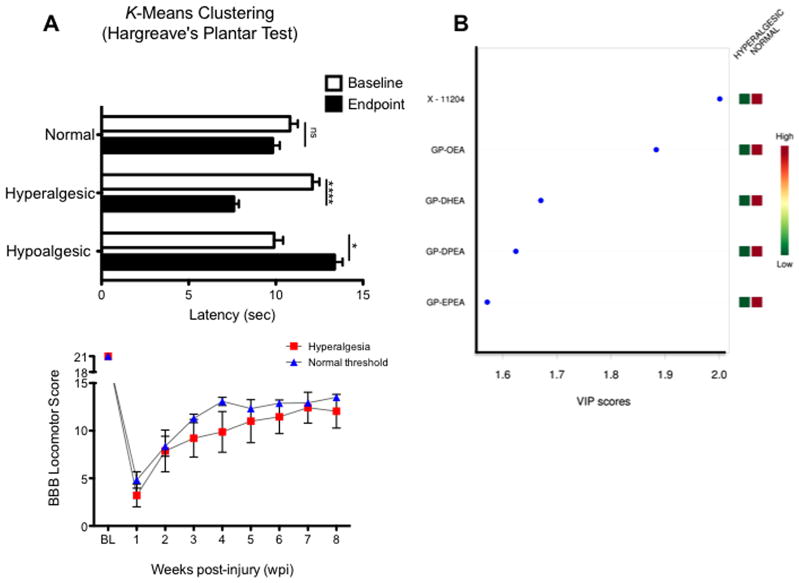
The biochemical basis and etiology underlying chronic neuropathic pain remains poorly understood and has limited the development of effective interventions. To characterize the unique lipidomic changes underlying neuropathic pain-like behaviors after contusive SCI, we first used the K-means clustering method to assign the animals to three groups according to their thermal threshold changes (Fig. 6A). This partitioning method identified a group of animals that showed no significant alterations in their thermal threshold (normal behavior, non-hyperalgesic; p > 0.05). Another cluster was shown to exhibit significantly reduced latencies when compared to their baseline values (hyperalgesic behavior; p < 0.05). The algorithm also identified animals with increased thermal latencies at 8 wpi when compared to baseline values (hypoalgesic behavior; p < 0.05). The mean baseline values did not differ significantly between clustered groups (p > 0.05), demonstrating that the different phenotypes developed after SCI.
Figure 6.
(A) K-means clustering divided animal based on their nociceptive behavior (Δ latency = latencyendpoint - latencybaseline). A group of animals exhibited no significant Δ latency changes at endpoint (“normal” or non-hyperalgesic cluster). The clustering algorithm identified two additional groups: hyperalgesic rats (significant Δ latency decrease at endpoint) and hypoalgesic (increased Δ latency at endpoint). Data is presented as mean ± SEM. Data was analyzed by TW-ANOVA followed by Bonferroni’s post hoc: ns, not significant; (*) = p < 0.05; (****) = p <0.0001. (B) Metabolomic analyses using the normal and hyperalgesic clusters confirmed the potential role of the NAE metabolism in pain processing after SCI. In particular, the O3PUFA-derived NAEs were shown to have a significant impact in the metabolic differences observed between thermal pain behaviors. Notably, the animals showing hyperalgesic phenotypes exhibited reduced levels of these metabolites. Abbreviations: X-11204, unnamed compound which has been tentatively identified as an unsaturated hydroxyl fatty acid with an empirical formula of C13H24O3; GP, glycerophospho; OEA, oleoyl ethanolamine; DHEA, docosahexanoyl ethanolamine; DPEA, docosapentaenoyl ethanolamine; EPEA, eicosapentaenoyl ethanolamine. (C) Basso, Beattie and Bresnahan locomotor scores measured in hyperalgesic (n = 6) and non-hyperalgesic (n = 13) rats. Repeated measures ANOVA did not identify pain classification (non-hyperalgesia vs. hyperalgesia) as a significant contributor of the total variance (p > 0.05). Pain classification accounted for 1.54% of the total variance (after adjusting for matching) with a p value of 0.2669. F statistics (1,66.88) = 1.318, indicating that the extent of the injury was similar in both groups. To determine whether the mean differences were more apparent, the data was analyzed using the Mann-Whitney U t-test at the time points showing greatest difference. The analysis revealed a p value of 0.2005 at 4 weeks post-injury, which can be interpreted as no reason to reject the null hypothesis. In other words, the locomotor score distributions of both dietary groups are identical at 4 wpi. This observation proposes underlying neurochemical mechanisms that may be independent of the injury severity and to the amount of spared tissue. Data represents mean ± SEM.
Notably, the glycerophospho N-acyl ethanolamines (GP-NAEs) derived from the O3PUFA-rich diet were the most relevant metabolites for explaining the differences between non-hyperalgesic and hyperalgesic animals (Fig. 6B). The metabolomics analyses revealed decreased levels of the O3PUFA-derived GP-NAEs in the hyperalgesic animals, suggesting a potential role for these in neuropathic pain.
Although additional factors contribute to the development of pain following SCI, it is well established that the extent of cord damage is a major determinant (Lindsey et al., 2000; Yezierski, 2000). To determine the relative contribution of tissue spared to the observed metabolomics differences, we assessed the cord damage between the animals exhibiting thermal hyperalgesia and those showing no significant differences in paw withdrawal thresholds when compared to baseline values (p > 0.05). Because the spinal cords were used for metabolomics studies, we could not determine the extent of injury using stereological techniques. However, the Basso, Beattie and Bresnahan (BBB) locomotor score provides a reliable indirect measure of the injury magnitude (Basso et al., 1996). We found that the BBB locomotor scores were not significantly different between clustered groups, indicating that the extent of injury (and repair) was similar (p > 0.05; Fig. 6C). This observation validates that additional processes such as chronic neuroinflammation and hyperexcitability play major roles in pathological nociception after SCI.
3.6. Animals fed with a diet rich in O3PUFAs exhibit reduced levels of p38 MAPK expression in dorsal horn neurons following SCI
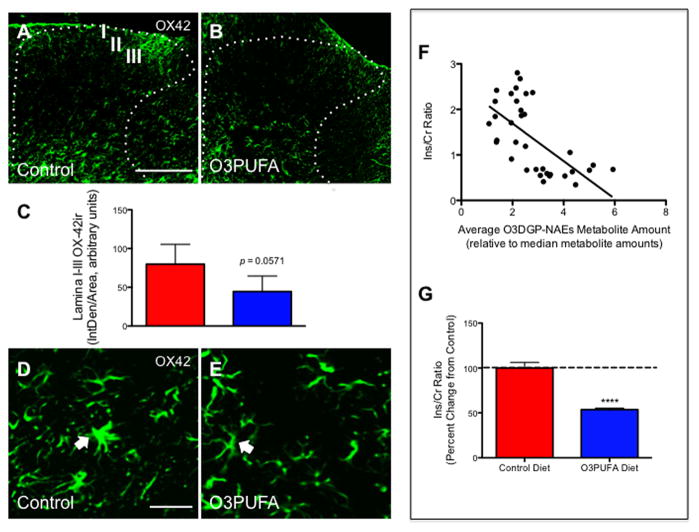
To examine the anti-inflammatory effects of the O3PUFA-enriched diet, we determined the mRNA levels of key cytokines, chemokines, and receptors that have been associated with inflammatory pain (e.g., IL-1β, IL-6, TNF-α, CCL2, CCR2, CX3CL1, and CX3CR1). We found that the O3PUFA diet did not reduce the mRNA levels of these pro-inflammatory factors in below-level cord segments when compared to control-fed animals at 8 wpi (p > 0.05; data not shown). Histological analyses showed no significant differences in the dorsal horn immunoreactivity (IR) to OX42 between treatment groups (Fig. 7A–C). These results do not contradict the well-established roles of dietary O3PUFAs but rather suggest that these anti-inflammatory effects may occur during the initial inflammatory trigger in a time- and context-dependent manner. This observation also points to the involvement of additional mechanisms. For instance, although we did not observe significant differences in the expression of these biomarkers, qualitative differences were noticeable in cell morphology. In particular, we found microglia with morphological features typically implicated in activated states in the spinal cords of animals receiving control chow (e.g., hypertrophied cell bodies and thick processes) (Fig. 7D). Interestingly, a few animals receiving the preventive dietary intervention rich in O3PUFAs exhibited microglial cells with small soma containing thin and radially projecting processes (Fig. 7E), confirming previous observations on the DHA-elicited immunomodulatory effects in microglia (Ebert et al., 2009).
Figure 7.
Dietary O3PUFA did not reduce microglial cell immunoreactivity in superficial dorsal horns following chronic SCI. Representative images from OX42 immunoreacted spinal cord injured caudal sections from animals receiving control (A) or O3PUFA (B) diets. Quantitative analyses showed no significant changes in inflammatory markers immunoreactivity between treatment groups in the dorsal horn laminae I-III at 12 weeks post-injury (C). Closer examination revealed that following chronic SCI, spinal microglia displayed hypertrophied cell bodies and thick processes, which are characteristic of their activated state (D). Interestingly, some animals treated with dietary O3PUFA showed microglial cells with small soma containing thin and radial projecting processes (resting state of microglia, E). Scale bar = A-B, 200 μm; D–E, 20 μm. The arrows indicate OX42-positive cell somata. Dietary O3PUFA-derived GP-NAEs levels are potentially implicated in anti-inflammatory responses. (F) A scatter plot shows the relationship between the levels of the O3PUFA-derived GP-NAEs (O3DGP-NAEs) and the total inositol-to-creatine levels (Ins/Cr). The Spearman r = −0.68, CI(−0.83 to −0.44), p = 0.0001, XY = 18 pairs includes sham and injury-operated animals fed with control diet. (G) Preventive administration of dietary O3PUFAs resulted in a significant reduction in the Ins levels (Mann-Whitney U test, **** p < 0.0001, n = 10). Data represents mean ± SEM.
Because LC/MS and GC/MS-based metabolomics provide a more sensitive method to assess inflammatory biomarkers, we measured the levels of inositol (Ins; see Experimental Procedures). Interestingly, the averaged relative levels of the major omega-3 PUFA-derived GP-NAEs were negatively associated with Ins relative levels (Spearman r = −0.68, p < 0.0001; Fig. 7A). Further, dietary O3PUFAs significantly reduced the cord Ins levels (Fig. 7B; p < 0.0001, n = 10). Altogether, these data support an anti-inflammatory and antinociceptive role for O3PUFAs in chronic SCI.
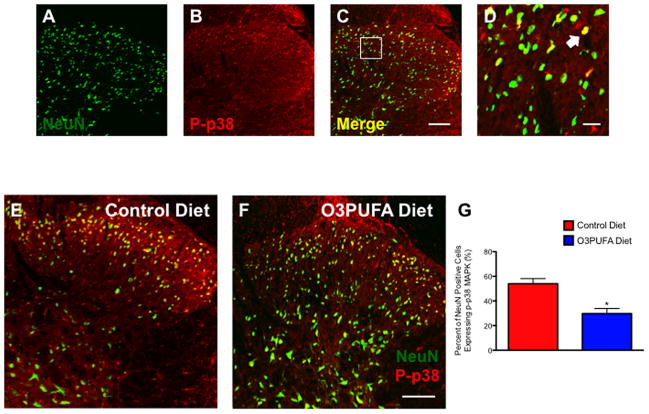
A number of pharmacological studies implicate the spinal p38 mitogen-activated protein kinase, p38 MAPK, as one important underlying mechanism of nociception and neuronal hyperexcitability in SCI (Crown et al., 2008; Gwak et al., 2009). Here, we used immunohistochemistry to examine the expression of this established pain biomarker in rats treated with dietary O3PUFAs relative to animals receiving control diets. We found that the cords of animals receiving O3PUFAs had decreased phosphorylated p38-positive neurons in the superficial dorsal horns relative to controls at 12 wpi (Fig. 8A–G; p < 0.05; n = at least 4 animals). Not surprisingly, we found a significant positive correlation between the expression levels of neuronal p-p38 MAPK and the hyperalgesic behaviors (data not shown).
Figure 8.
Preventive dietary O3PUFAs reduce the expression of phosphorylated p38 in below-level dorsal horn neurons. At 12 weeks post-injury, laser confocal microscopic evaluation revealed dorsal NeuN-positive neurons (A) containing the phosphorylated p38 MAPK (B). Merged photomicrographs (C) and inset (D) show distinctive neuronal subpopulations expressing this inflammatory marker after chronic SCI. Dorsal horn photomicrographs show noticeable differences in the number of p-p38-containing neurons when comparing dietary groups (control = E vs. O3PUFA = F). (G) Manual cell counts confirmed that the dietary O3PUFA intervention significantly reduced the percent of NeuN-positive cells expressing p-p38 MAPK in below-level dorsal horn superficial laminae (p < 0.05; n = at least 4 animals). Scale bars = A–C and E–F, 100 μm; D, 20 μm.
3.7. Dietary O3PUFA reduces the sprouting of CGRP-containing fibers in chronic SCI
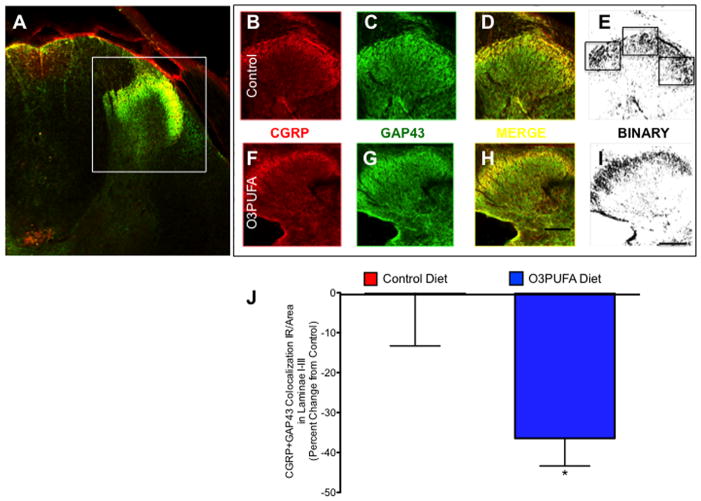
The growth-associated protein 43 (GAP43) and calcitonin gene-related peptide (CGRP) have been widely used as biomarkers of nociceptive fiber sprouting and neuropathic pain (Nahin et al., 1994; Christensen and Hulsebosch, 1997b; Ondarza et al., 2003). Thus, we tested whether the preventive O3PUFA-enriched diet reduces the sprouting of CGRP-containing primary afferents following chronic SCI. Laser confocal microscopy showed CGRP and GAP43 colocalization in spinal cord sections obtained from regions 3–5 mm caudal to the injury site (Fig. 9A). CGRP (Fig. 9B, F) and GAP43 (Fig. 9C, G) labeled photomicrographs from animals receiving control chow (Fig. 9B–E) or O3PUFA-rich (Fig. 9F–I) diets were merged (Fig. 9D, H) and subsequently converted to binary format to facilitate automated analyses (Fig. 9E, I). Quantitative double labeling immunofluerescence revealed decreased sprouting of CGRP-positive primary afferents at 12 wpi (Mann Whitney U test p < 0.05, n = at least 4 animals) (Fig. 9J).
Figure 9.
Dietary O3PUFA-pretreatment reduces nociceptive fiber sprouting following chronic SCI. (A) Double labeled spinal cord section showing calcitonin gene-related peptide (CGRP) and growth-associated protein 43 (GAP43) immunoreactivity (IR) in spinal cord. Confocal photomicrographs showing CGRP (B) and GAP43 (C) immunoreactivity in control chow-fed rat. Images were merged to quantify the immunoreactivity of CGRP-containing sprouting afferent fibers (D). Optical density was most intense in laminae I to III of the dorsal horns. Sections were morphometrically analyzed using stereological methods after thresholding binary images using ImageJ software (E). Representative image from CGRP (F) and GAP43 (G) immunoreactivity in an animal fed O3PUFA-enriched diets. Merged (H) and binary (I) images depict CGRP-containing GAP43+ fibers in the dorsal horn (H). Boxes represent areas showing colocalization. Scale bar = 100 μm. (I) Results from quantification of binary particle counts of dorsal horn superficial laminae. Analysis showed that O3PUFA-enriched diet significantly reduced the colocalization of GAP43 and CGRP, suggesting a reduction in nociceptive fiber sprouting. Bars represent means ± standard error of the mean; Mann Whitney U test *p < 0.05, n = at least 4 rats.
4. Discussion
This study shows that a preventive diet rich in omega-3 polyunsaturated fatty acids (O3PUFAs) reduces thermal hyperalgesia in rats experiencing chronic spinal cord injury (SCI). This antihyperalgesic effect was directly correlated with the levels of a series of novel glycerophospho ethanolamines containing O3PUFA acyl chains. The anti-inflammatory effects of this O3PUFA-enriched diet were evident by a significant reduction in levels of inflammatory biomarkers, including cord inositols and the phosphorylated p38 MAPK in dorsal horn neurons.
Chronic neuropathic pain is a debilitating co-morbidity associated with SCI and persists even at the later stages of recovery and rehabilitation. This condition often manifest as evoked pain, including hyperalgesia (amplified pain response to noxious stimuli) and/or allodynia (painful response to innocuous stimuli). The intensity and frequency of neuropathic pain is particularly influenced by trauma-induced neurochemical and neuroanatomical changes in synaptic circuitry and dorsal horn neuron hyperexcitability (Crown et al., 2008; Gwak et al., 2009; Gwak et al., 2010). Current approaches to treat neuropathic pain include behavioral, pharmacological, and surgical modalities, however, none of these interventions are regarded as highly effective. This could be partly due to patients being treated after considerable and perhaps irreversible changes have developed. There is thus a need to develop preventive approaches to build resilience to damage. Complementary with current strategies, this type of approach may be particularly important in individuals at high risk of traumatic injuries like those actively participating in contact sports, selected surgeries, first responders, and our men and women in the military service. The promise of using preventive approaches to treat chronic neuropathic pain is supported by studies showing that central pain can be prevented by pre-administration of opiates (Dickenson and Sullivan, 1987), anti-inflammatory molecules (Plunkett et al., 2001), or by prophylactic cell transplantation strategies (Brewer and Yezierski, 1998; Yu et al., 1998). Although it may seem unreasonable to use these approaches in individuals undergoing SCI due to potential and unwanted side effects, prophylactic treatment with dietary O3PUFAs offers an excellent profile of clinical safety and may be beneficial in preventing pain and dysesthesias in individuals at risk (Bays, 2006; Mayer et al., 2006).
O3PUFAs, such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), are ubiquitous lipid messengers that regulate crucial neural processes in health and disease. We recently reported that dietary O3PUFAs exert a stringent control over phospholipid production and are principal determinants of the cord lipid composition following chronic SCI (Figueroa et al., 2013). Further, when the spinal cord is enriched with O3PUFAs before SCI, these lipids mediate robust neuroprotection, recovery, and activate pro-restorative responses (Figueroa et al., 2012; Figueroa et al., 2013; Lim et al., 2013). Several recent studies have shown the importance of PUFAs in nociception, supporting the hypothesis that dietary lipids are key elements of the nociceptive pathways (Yehuda and Carasso, 1993; Nakamoto et al., 2010; Xu et al., 2010; Tokuyama and Nakamoto, 2011; Veigas et al., 2011). Consistent with this idea, the present study shows that consumption of a diet rich in O3PUFAs produces significant thermal anti-hyperalgesia when compared to animals receiving control diets after contusion SCI. In contrast, thoracic contusion to the cord resulted in a significant reduction in below-level thermal withdrawal latencies in animals receiving control chow. Importantly, we reported that control-fed injured rats exhibited significant sensory deficits to mechanical stimulation on their hindpaws after SCI (Figueroa et al., 2013). Together, these findings discard the idea that thermal hyperalgesia was due to a hyperreflexic responses, a common occurrence in contusive SCI (Christensen and Hulsebosch, 1997a). This observation further suggests that both neurodegenerative and pro-inflammatory responses may play important roles in the development of neuropathic pain after SCI (Krassioukov et al., 1999; Baumgärtner et al., 2002).
Several lines of evidence demonstrate that the level of spinal cord damage play significant roles in the development and maintenance of pain-like behaviors (Lindsey et al., 2000; Yezierski, 2000; Park et al., 2007), suggesting that pain may be amenable to neuroprotective approaches. In agreement with others, evidence from our lab has shown that O3PUFAs confer potent neuroprotection against SCI (Figueroa et al., 2012; Figueroa et al., 2013). Together, these data suggest that neuroprotection may be an underlying mechanism involved in the anti-nociceptive responses mediated by the dietary O3PUFAs. However, neuroprotective approaches do not always result in anti-nociceptive responses (Mills et al., 2002). We found comparable open-field locomotor scores when animals were grouped based on their thermal withdrawal phenotypes. While neurodegenerative differences between clusters may be subtle or undetectable as measured by the BBB locomotor scores, we interpret this finding as evidence supporting additional underlying causes of neuropathic pain.
The neurometabolomic etiology of neuropathic pain remains poorly understood and this gap in the literature has limited the development of effective therapeutics. N-Acylated ethanolamines (NAE) and related endocannabinoids (eCBs) are a large class of naturally occurring lipids that exhibit diverse bioactivities, including neuroprotective (Jackson et al., 2005) and antinociceptive (Beltramo et al., 2006; Petrosino et al., 2007; Hama and Sagen, 2011) responses. Notably, it has been shown that SCI rats exhibit profound alterations in the metabolic pathways associated with these lipids (Garcia-Ovejero et al., 2009). These lipids are produced on demand from membrane phospholipids and glycerophospho-linked precursors by a series of intracellular enzymatic reactions, followed by immediate signaling and metabolism (Berger et al., 2001; Artmann et al., 2008; Wang and Ueda, 2009; Banni and Di Marzo, 2010; Wood et al., 2010; Brown et al., 2012). The findings reported in the present study support and expand on these observations by showing that chronic SCI results in a marked deregulation in the metabolic pathways of NAEs and related eCBs. In particular, we found reduced levels of palmitoyl ethanolamine (PEA) in the chronically injured cord, which were correlated with hyperalgesic behaviors in SCI rats. PEA has been implicated in anti-inflammatory responses and functional recovery after SCI (Genovese et al., 2008; Esposito et al., 2011; Esposito and Cuzzocrea, 2012) and reduces pain-like behaviors in experimental models of neuropathic pain (Costa et al., 2008; Buczynski et al., 2010). Notably, dietary prophylaxis with O3PUFAs sustained the levels of PEA after SCI. Further, the O3PUFA-derived NAEs identified in this study are precursors of docosahexaenoyl ethanolamine (DHEA; synaptamide) and eicosapentaenoyl ethanolamine (EPEA), which bind to cannabinoid receptors in rats (Sheskin et al., 1997) and may contribute to the beneficial effects mediated by dietary O3PFAs (Balvers et al., 2010; Meijerink et al., 2011; Shinohara et al., 2012). Although the metabolic pathways involved in NAE biosynthesis remain unclear, our results strongly suggest that NAPE-PLD-independent (N-acyl phosphatidyl ethanolamine phospholipase D) pathways are activated in chronic SCI and represent a promising therapeutic target (Tsuboi et al., 2013). Because NAEs can modify the response to nociceptive stimuli and are tightly regulated by diet, O3PUFAs could have important implications for chronic pain management.
There is now strong evidence linking the neuroinflammatory milieu after SCI to changes in sensory electrical activity and pain-related behaviors (Hulsebosch, 2008). For instance, accumulating evidence implicates the activation of the spinal p38 mitogen-activated protein kinase, p38 MAPK, as a molecular mechanism underlying neuronal hyperexcitability and pain after SCI (Crown et al., 2006; Crown et al., 2008; Gwak et al., 2009). Remarkably, animals consuming dietary O3PUFAs exhibited reduced numbers of dorsal neurons expressing p-p38, indicating a potential molecular link between dietary lipids and pain. This finding is supported by studies demonstrating that both DHA and EPA alone attenuate the activation of p38 in endothelial cells stimulated by TNF-α (Xue et al., 2006). More recent studies showed that DHA also impairs p38 MAPK signaling in microglial cultures (Antonietta Ajmone-Cat et al., 2011). In agreement with these findings on the potential anti-inflammatory and antinociceptive roles of dietary O3PUFAs, this study shows a marked reduction in the levels of inositols and CGRP-positive sprouting fibers in the chronically injured cord. Importantly, the p38 MAPK has been linked to the regulation of inositol levels in human peripheral blood monocytes and macrophages (Denkert et al., 1998) and to the expression of calcitonin gene-related peptide in rats (Wang et al., 2011). This study supports the dynamic interplay among these biomarkers of the nociceptive system. Further studies are necessary to evaluate these molecular interactions and to investigate their potential as useful biomarkers to discriminate pain severity in SCI-related neuropathic pain.
Although experimental contusion SCI is a well-established and validated animal model for evaluating the development of pain and analgesic strategies (Resnick et al., 2004; DomBourian et al., 2006; Rajpal et al., 2007; Cramer et al., 2008; Jung et al., 2008), there are limitations in extrapolating findings from experimental animal models and to humans. It has been suggested that the mechanisms underlying evoked responses in SCI animals may differ from those of underlying spontaneous neuropathic pain in patients (Craig, 2009). The proper interpretation of animal pain-like behaviors thus remains a challenge and warrants further research. Given the significant contribution of supraspinal structures in pain processing (King et al., 2003; Baastrup et al., 2010; Davoody et al., 2011), futures studies should employ integrated pain-associated behaviors to facilitate data interpretation and to gain better understanding of the antinociceptive targets of dietary O3PUFAs.
4.1. Conclusions
In summary, this study shows for the first time that dietary O3PUFAs prophylaxis attenuates the development of thermal hyperalgesia following SCI, possibly by providing a better bioavailability for anti-inflammatory lipid mediators. Even though recent advances in pain research suggest that combinatorial strategies to both prevent and combat chronic neuropathic pain are a feasible goal (Hama and Sagen, 2012), identifying targets with the intention of preventing pain is an enormous conceptual challenge that has so far stymied drug discovery. The study presented herein supports the use of preventive alternative approaches in individuals at risk of developing neuropathic pain and identifies diet as a potential risk factor for poor outcome. Although further research is needed, our findings have remarkable public health implications to reduce the burden of pain, particularly in populations at risk. Because dietary O3PUFAs are safe, well tolerated, and confer robust protection against experimental SCI they should be favored for early pain management in human SCI.
Highlights.
Thermal hyperalgesia was reduced in SCI rats consuming O3PUFA-rich diets.
O3PUFA-derived ethanolamines are associated with the antihyperalgesic behaviors.
Dietary O3PUFAs reduced the levels of pain biomarkers in the injured spinal cord.
Acknowledgments
We thank our lab staff for providing advice and helpful discussions during the preparation of this manuscript. We would like to thank Dr. Pappan from Metabolon® for his technical assistance with the global metabolomic profiling. The authors are indebted to Dr. Manuel Montero and the animal care facility personnel for their excellent support with animal care. We also thank Lorena Salto for her editorial assistance. This work was supported in part by NIH grants 5R25GM060507 and 1P20MD006988
Abbreviations
- SCI
Spinal cord injury
- DHA
docosahexaenoic acid
- O3PUFAs
omega-3 polyunsaturated fatty acids
- eCBs
endocannabinoids
- NAEs
N-acyl ethanolamines
- DHEA
docosahexaenoyl ethanolamine/synaptamide
- DPEA
docosapentaenoyl ethanolamine
- EPEA
eicosapentaenoyl ethanolamine
- TH
thermal hyperalgesia
- HWL
hindpaw withdrawal latency
- UHPLC/MS/MS2
ultrahigh performance liquid chromatography/tandem mass spectrometry
- GC/MS
gas chromatography/mass spectrometry
- GAP43
growth-associated protein 43
- CGRP
calcitonin gene-related peptide
- PLS-DA
partial least square-discriminant analysis
- GP-NAEs
glycerophospho-containing N-acyl ethanolamines
- BBB
Basso, Beattie and Bresnahan
- LEA
linoleyl ethanolamine
- AEA
arachidonoyl ethanolamine
- EG
eicosenoyl glycerol
- 2-AG
2-arachidonoyl glycerol
- 2-PG
2-palmitoyl glycerol
- 1-OG
1-oleoyl glycerol
- PEA
palmitoyl ethanolamine
- PLD
phospholipase D
- Abh4
phospholipase A/B or α-β-hydrolase 4
- GDE1
glycerophosphodiesterase
- NAPE
N-acyl phosphatidyl ethanolamine
- G3P
glycerol-3-phosphate
- LPA
lyso-phosphatidic acid
- PA
phosphatidic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alloui A, Begon S, Chassaing C, Eschalier A, Gueux E, Rayssiguier Y, Dubray C. Does Mg2+ deficiency induce a long-term sensitization of the central nociceptive pathways? European journal of pharmacology. 2003;469:65–69. doi: 10.1016/s0014-2999(03)01719-9. [DOI] [PubMed] [Google Scholar]
- Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. Journal of Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Antonietta Ajmone-Cat M, Lavinia Salvatori M, De Simone R, Mancini M, Biagioni S, Bernardo A, Cacci E, Minghetti L. Docosahexaenoic acid modulates inflammatory and antineurogenic functions of activated microglial cells. J Neurosci Res. 2011 doi: 10.1002/jnr.22783. [DOI] [PubMed] [Google Scholar]
- Artmann A, Petersen G, Hellgren LI, Boberg J, Skonberg C, Nellemann C, Hansen SH, Hansen HS. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochimica et biophysica acta. 2008;1781:200–212. doi: 10.1016/j.bbalip.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Baastrup C, Maersk-Moller CC, Nyengaard JR, Jensen TS, Finnerup NB. Spinal-, brainstem- and cerebrally mediated responses at- and below-level of a spinal cord contusion in rats: evaluation of pain-like behavior. Pain. 2010;151:670–679. doi: 10.1016/j.pain.2010.08.024. [DOI] [PubMed] [Google Scholar]
- Balvers MGJ, Verhoeckx KCM, Plastina P, Wortelboer HM, Meijerink J, Witkamp RF. Docosahexaenoic acid and eicosapentaenoic acid are converted by 3T3-L1 adipocytes to N-acyl ethanolamines with anti-inflammatory properties. Biochimica et biophysica acta. 2010;1801:1107–1114. doi: 10.1016/j.bbalip.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Banni S, Di Marzo V. Effect of dietary fat on endocannabinoids and related mediators: consequences on energy homeostasis, inflammation and mood. Molecular nutrition & food research. 2010;54:82–92. doi: 10.1002/mnfr.200900516. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Experimental neurology. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Baumgärtner U, Magerl W, Klein T, Hopf HC, Treede RD. Neurogenic hyperalgesia versus painful hypoalgesia: two distinct mechanisms of neuropathic pain. Pain. 2002;96:141–151. doi: 10.1016/s0304-3959(01)00438-9. [DOI] [PubMed] [Google Scholar]
- Bays H. Clinical overview of Omacor: a concentrated formulation of omega-3 polyunsaturated fatty acids. The American journal of cardiology. 2006;98:71i–76i. doi: 10.1016/j.amjcard.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fredduzzi S, Reggiani A. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. The European journal of neuroscience. 2006;23:1530–1538. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- Berger A, Crozier G, Bisogno T, Cavaliere P, Innis S, Di Marzo V. Anandamide and diet: inclusion of dietary arachidonate and docosahexaenoate leads to increased brain levels of the corresponding N-acylethanolamines in piglets. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6402–6406. doi: 10.1073/pnas.101119098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Developmental neuroscience. 1993;15:289–298. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- Brewer KL, Yezierski RP. Effects of adrenal medullary transplants on pain-related behaviors following excitotoxic spinal cord injury. Brain research. 1998;798:83–92. doi: 10.1016/s0006-8993(98)00398-9. [DOI] [PubMed] [Google Scholar]
- Brown I, Cascio MG, Rotondo D, Pertwee RG, Heys SD, Wahle KWJ. Cannabinoids and Omega-3/6 Endocannabinoids as Cell Death and Anticancer Modulators. Progress in lipid research. 2012 doi: 10.1016/j.plipres.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Brown I, Cascio MG, Wahle KW, Smoum R, Mechoulam R, Ross RA, Pertwee RG, Heys SD. Cannabinoid receptor-dependent and -independent anti-proliferative effects of omega-3 ethanolamides in androgen receptor-positive and -negative prostate cancer cell lines. Carcinogenesis. 2010;31:1584–1591. doi: 10.1093/carcin/bgq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczynski MW, Svensson CI, Dumlao DS, Fitzsimmons BL, Shim J-H, Scherbart TJ, Jacobsen FE, Hua X-Y, Yaksh TL, Dennis EA. Inflammatory hyperalgesia induces essential bioactive lipid production in the spinal cord. Journal of neurochemistry. 2010;114:981–993. doi: 10.1111/j.1471-4159.2010.06815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fatty Acids. 2008;79:101–108. doi: 10.1016/j.plefa.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, Mogil JS. Identification and ranking of genetic and laboratory environment factors influencing a behavioral trait, thermal nociception, via computational analysis of a large data archive. Neuroscience and biobehavioral reviews. 2002;26:907–923. doi: 10.1016/s0149-7634(02)00103-3. [DOI] [PubMed] [Google Scholar]
- Christensen MD, Hulsebosch CE. Chronic central pain after spinal cord injury. Journal of Neurotrauma. 1997a;14:517–537. doi: 10.1089/neu.1997.14.517. [DOI] [PubMed] [Google Scholar]
- Christensen MD, Hulsebosch CE. Spinal cord injury and anti-NGF treatment results in changes in CGRP density and distribution in the dorsal horn in the rat. Experimental neurology. 1997b;147:463–475. doi: 10.1006/exnr.1997.6608. [DOI] [PubMed] [Google Scholar]
- Costa B, Comelli F, Bettoni I, Colleoni M, Giagnoni G. The endogenous fatty acid amide, palmitoylethanolamide, has anti-allodynic and anti-hyperalgesic effects in a murine model of neuropathic pain: involvement of CB(1), TRPV1 and PPARgamma receptors and neurotrophic factors. Pain. 2008;139:541–550. doi: 10.1016/j.pain.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Craig AD. A rat is not a monkey is not a human: comment on Mogil (Nature Rev. Neurosci. 10, 283–294 (2009)) Nature reviews Neuroscience. 2009;10:466. doi: 10.1038/nrn2606-c1. [DOI] [PubMed] [Google Scholar]
- Cramer SW, Baggott C, Cain J, Tilghman J, Allcock B, Miranpuri G, Rajpal S, Sun D, Resnick D. The role of cation-dependent chloride transporters in neuropathic pain following spinal cord injury. Molecular pain. 2008;4:36. doi: 10.1186/1744-8069-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crown ED, Gwak YS, Ye Z, Johnson KM, Hulsebosch CE. Activation of p38 MAP kinase is involved in central neuropathic pain following spinal cord injury. Experimental neurology. 2008;213:257–267. doi: 10.1016/j.expneurol.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crown ED, Ye Z, Johnson KM, Xu GY, McAdoo DJ, Hulsebosch CE. Increases in the activated forms of ERK 1/2, p38 MAPK, and CREB are correlated with the expression of at-level mechanical allodynia following spinal cord injury. Experimental neurology. 2006;199:397–407. doi: 10.1016/j.expneurol.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Davoody L, Quiton RL, Lucas JM, Ji Y, Keller A, Masri R. Conditioned place preference reveals tonic pain in an animal model of central pain. The journal of pain: official journal of the American Pain Society. 2011;12:868–874. doi: 10.1016/j.jpain.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaven CD, Evans AM, Dai H, Lawton KA. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. Journal of cheminformatics. 2010;2:9. doi: 10.1186/1758-2946-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkert C, Warskulat U, Hensel F, Haussinger D. Osmolyte strategy in human monocytes and macrophages: involvement of p38MAPK in hyperosmotic induction of betaine and myoinositol transporters. Archives of biochemistry and biophysics. 1998;354:172–180. doi: 10.1006/abbi.1998.0661. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Sullivan AF. Subcutaneous formalin-induced activity of dorsal horn neurones in the rat: differential response to an intrathecal opiate administered pre or post formalin. Pain. 1987;30:349–360. doi: 10.1016/0304-3959(87)90023-6. [DOI] [PubMed] [Google Scholar]
- DomBourian MG, Turner NA, Gerovac TA, Vemuganti R, Miranpuri GS, Tureyen K, Satriotomo I, Miletic V, Resnick DK. B1 and TRPV-1 receptor genes and their relationship to hyperalgesia following spinal cord injury. Spine. 2006;31:2778–2782. doi: 10.1097/01.brs.0000245865.97424.b4. [DOI] [PubMed] [Google Scholar]
- Ebert S, Weigelt K, Walczak Y, Drobnik W, Mauerer R, Hume DA, Weber BHF, Langmann T. Docosahexaenoic acid attenuates microglial activation and delays early retinal degeneration. Journal of neurochemistry. 2009;110:1863–1875. doi: 10.1111/j.1471-4159.2009.06286.x. [DOI] [PubMed] [Google Scholar]
- Erschbamer M, Oberg J, Westman E, Sitnikov R, Olson L, Spenger C. 1H-MRS in spinal cord injury: acute and chronic metabolite alterations in rat brain and lumbar spinal cord. The European journal of neuroscience. 2011;33:678–688. doi: 10.1111/j.1460-9568.2010.07562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito E, Cuzzocrea S. Palmitoylethanolamide is a new possible pharmacological treatment for the inflammation associated with trauma. Mini reviews in medicinal chemistry. 2012 [PubMed] [Google Scholar]
- Esposito E, Paterniti I, Mazzon E, Genovese T, Di Paola R, Galuppo M, Cuzzocrea S. Effects of palmitoylethanolamide on release of mast cell peptidases and neurotrophic factors after spinal cord injury. Brain, behavior, and immunity. 2011;25:1099–1112. doi: 10.1016/j.bbi.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Estebe JP, Legay F, Gentili M, Wodey E, Leduc C, Ecoffey C, Moulinoux JP. An evaluation of a polyamine-deficient diet for the treatment of inflammatory pain. Anesthesia and analgesia. 2006;102:1781–1788. doi: 10.1213/01.ane.0000205755.43562.2b. [DOI] [PubMed] [Google Scholar]
- Figueroa JD, Cordero K, Baledosingh K, Torrado AI, Walker RL, Miranda JD, De Leon MA. Docosahexaenoic acid pretreatment confers protection and functional improvements after acute spinal cord injury in adult rats. J Neurotrauma. 2012;29:551–566. doi: 10.1089/neu.2011.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa JD, Cordero K, Illan MS, De Leon M. Dietary omega-3 polyunsaturated fatty acids improve the neurolipidome and restore the DHA status while promoting functional recovery after experimental spinal cord injury. Journal of Neurotrauma. 2013;30:853–868. doi: 10.1089/neu.2012.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ovejero D, Arevalo-Martin A, Petrosino S, Docagne F, Hagen C, Bisogno T, Watanabe M, Guaza C, Di Marzo V, Molina-Holgado E. The endocannabinoid system is modulated in response to spinal cord injury in rats. Neurobiology of disease. 2009;33:57–71. doi: 10.1016/j.nbd.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Genovese T, Esposito E, Mazzon E, Di Paola R, Meli R, Bramanti P, Piomelli D, Calignano A, Cuzzocrea S. Effects of palmitoylethanolamide on signaling pathways implicated in the development of spinal cord injury. The Journal of pharmacology and experimental therapeutics. 2008;326:12–23. doi: 10.1124/jpet.108.136903. [DOI] [PubMed] [Google Scholar]
- Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma. 1992;9:123–126. doi: 10.1089/neu.1992.9.123. discussion 126–128. [DOI] [PubMed] [Google Scholar]
- Gwak YS, Kim HK, Kim HY, Leem JW. Bilateral hyperexcitability of thalamic VPL neurons following unilateral spinal injury in rats. The journal of physiological sciences: JPS. 2010;60:59–66. doi: 10.1007/s12576-009-0066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak YS, Unabia GC, Hulsebosch CE. Activation of p-38alpha MAPK contributes to neuronal hyperexcitability in caudal regions remote from spinal cord injury. Experimental neurology. 2009;220:154–161. doi: 10.1016/j.expneurol.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama A, Sagen J. Activation of spinal and supraspinal cannabinoid-1 receptors leads to antinociception in a rat model of neuropathic spinal cord injury pain. Brain research. 2011;1412:44–54. doi: 10.1016/j.brainres.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama A, Sagen J. Combination Drug Therapy for Pain following Chronic Spinal Cord Injury. Pain Res Treat. 2012;2012:840486. doi: 10.1155/2012/840486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus L, Gopher A, Almog S, Mechoulam R. Two new unsaturated fatty acid ethanolamides in brain that bind to the cannabinoid receptor. Journal of medicinal chemistry. 1993;36:3032–3034. doi: 10.1021/jm00072a026. [DOI] [PubMed] [Google Scholar]
- Hargraves WA, Hentall ID. Analgesic effects of dietary caloric restriction in adult mice. Pain. 2005;114:455–461. doi: 10.1016/j.pain.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Tanabe Y, Fujii Y, Hagiwara R, Yamasaki H, Shido O. Mechanism of improvement of spatial cognition with dietary docosahexaenoic acid. Nippon Yakurigaku Zasshi. 2002;120:54P–56P. [PubMed] [Google Scholar]
- Hulsebosch CE. Recent advances in pathophysiology and treatment of spinal cord injury. Advances in physiology education. 2002;26:238–255. doi: 10.1152/advan.00039.2002. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE. Gliopathy ensures persistent inflammation and chronic pain after spinal cord injury. Experimental neurology. 2008;214:6–9. doi: 10.1016/j.expneurol.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain research reviews. 2009;60:202–213. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto A, Ohishi M, Sato Y, Hata N, Misawa Y, Fujii Y, Okuyama H. Reversibility of n-3 fatty acid deficiency-induced alterations of learning behavior in the rat: level of n-6 fatty acids as another critical factor. Journal of lipid research. 2001;42:1655–1663. [PubMed] [Google Scholar]
- Jackson SJ, Pryce G, Diemel LT, Cuzner ML, Baker D. Cannabinoid-receptor 1 null mice are susceptible to neurofilament damage and caspase 3 activation. Neuroscience. 2005;134:261–268. doi: 10.1016/j.neuroscience.2005.02.045. [DOI] [PubMed] [Google Scholar]
- Joseph MS, Ying Z, Zhuang Y, Zhong H, Wu A, Bhatia HS, Cruz R, Tillakaratne NJK, Roy RR, Edgerton VR, Gomez-Pinilla F. Effects of Diet and/or Exercise in Enhancing Spinal Cord Sensorimotor Learning. PloS one. 2012;7:e41288. doi: 10.1371/journal.pone.0041288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J-I, Kim J, Hong SK, Yoon YW. Long-term Follow-up of Cutaneous Hypersensitivity in Rats with a Spinal Cord Contusion. Korean J Physiol Pharmacol. 2008;12:299–306. doi: 10.4196/kjpp.2008.12.6.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CD, Devine DP, Vierck CJ, Rodgers J, Yezierski RP. Differential effects of stress on escape and reflex responses to nociceptive thermal stimuli in the rat. Brain research. 2003;987:214–222. doi: 10.1016/s0006-8993(03)03339-0. [DOI] [PubMed] [Google Scholar]
- Kocevski D, Tvrdeić A. The effect of repeated daily measurements on paw withdrawal latencies in Hargreaves test. Coll Antropol. 2008;32(Suppl 1):93–97. [PubMed] [Google Scholar]
- Krassioukov A, Wolfe DL, Hsieh JT, Hayes KC, Durham CE. Quantitative sensory testing in patients with incomplete spinal cord injury. Archives of physical medicine and rehabilitation. 1999;80:1258–1263. doi: 10.1016/s0003-9993(99)90026-6. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Okon E, Hillyer J, Mann C, Baptiste D, Weaver LC, Fehlings MG, Tetzlaff W. A systematic review of non-invasive pharmacologic neuroprotective treatments for acute spinal cord injury. Journal of Neurotrauma. 2011a;28:1545–1588. doi: 10.1089/neu.2009.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon BK, Okon EB, Plunet W, Baptiste D, Fouad K, Hillyer J, Weaver LC, Fehlings MG, Tetzlaff W. A systematic review of directly applied biologic therapies for acute spinal cord injury. Journal of Neurotrauma. 2011b;28:1589–1610. doi: 10.1089/neu.2009.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- Li F, Obrosova IG, Abatan O, Tian D, Larkin D, Stuenkel EL, Stevens MJ. Taurine replacement attenuates hyperalgesia and abnormal calcium signaling in sensory neurons of STZ-D rats. American journal of physiology Endocrinology and metabolism. 2005;288:E29–36. doi: 10.1152/ajpendo.00168.2004. [DOI] [PubMed] [Google Scholar]
- Lim S-Y, Hoshiba J, Salem N. An extraordinary degree of structural specificity is required in neural phospholipids for optimal brain function: n-6 docosapentaenoic acid substitution for docosahexaenoic acid leads to a loss in spatial task performance. Journal of neurochemistry. 2005;95:848–857. doi: 10.1111/j.1471-4159.2005.03427.x. [DOI] [PubMed] [Google Scholar]
- Lim SN, Gladman SJ, Dyall SC, Patel U, Virani N, Kang JX, Priestley JV, Michael-Titus AT. Transgenic mice with high endogenous omega-3 fatty acids are protected from spinal cord injury. Neurobiology of disease. 2013;51:104–112. doi: 10.1016/j.nbd.2012.10.021. [DOI] [PubMed] [Google Scholar]
- Lindsey AE, LoVerso RL, Tovar CA, Hill CE, Beattie MS, Bresnahan JC. An analysis of changes in sensory thresholds to mild tactile and cold stimuli after experimental spinal cord injury in the rat. Neurorehabilitation and neural repair. 2000;14:287–300. doi: 10.1177/154596830001400405. [DOI] [PubMed] [Google Scholar]
- Mayer K, Schaefer MB, Seeger W. Fish oil in the critically ill: from experimental to clinical data. Current opinion in clinical nutrition and metabolic care. 2006;9:140–148. doi: 10.1097/01.mco.0000214573.75062.0a. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Sullivan J, Richtand NM, Jandacek R, Rider T, Tso P, Campbell N, Lipton J. Omega-3 fatty acid deficiency augments amphetamine-induced behavioral sensitization in adult DBA/2J mice: relationship with ventral striatum dopamine concentrations. Synapse. 2008;62:725–735. doi: 10.1002/syn.20542. [DOI] [PubMed] [Google Scholar]
- Meijerink J, Plastina P, Vincken J-P, Poland M, Attya M, Balvers M, Gruppen H, Gabriele B, Witkamp RF. The ethanolamide metabolite of DHA, docosahexaenoylethanolamine, shows immunomodulating effects in mouse peritoneal and RAW264.7 macrophages: evidence for a new link between fish oil and inflammation. Br J Nutr. 2011:1–10. doi: 10.1017/S0007114510005635. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Chesler EJ, Wilson SG, Juraska JM, Sternberg WF. Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neuroscience and biobehavioral reviews. 2000;24:375–389. doi: 10.1016/s0149-7634(00)00015-4. [DOI] [PubMed] [Google Scholar]
- Mogil JS, McCarson KE. Identifying pain genes: bottom-up and top-down approaches. The journal of pain: official journal of the American Pain Society. 2000;1:66–80. doi: 10.1054/jpai.2000.9821. [DOI] [PubMed] [Google Scholar]
- Morisseau C, Inceoglu B, Schmelzer K, Tsai H-J, Jinks SL, Hegedus CM, Hammock BD. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. Journal of lipid research. 2010;51:3481–3490. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahin RL, Ren K, De León M, Ruda M. Primary sensory neurons exhibit altered gene expression in a rat model of neuropathic pain. Pain. 1994;58:95–108. doi: 10.1016/0304-3959(94)90189-9. [DOI] [PubMed] [Google Scholar]
- Nakamoto K, Nishinaka T, Mankura M, Fujita-Hamabe W, Tokuyama S. Antinociceptive effects of docosahexaenoic acid against various pain stimuli in mice. Biological & pharmaceutical bulletin. 2010;33:1070–1072. doi: 10.1248/bpb.33.1070. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. Journal of Neurotrauma. 2004;21:429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- Ondarza AB, Ye Z, Hulsebosch CE. Direct evidence of primary afferent sprouting in distant segments following spinal cord injury in the rat: colocalization of GAP-43 and CGRP. Experimental neurology. 2003;184:373–380. doi: 10.1016/j.expneurol.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Park SW, Yi JH, Miranpuri G, Satriotomo I, Bowen K, Resnick DK, Vemuganti R. Thiazolidinedione class of peroxisome proliferator-activated receptor gamma agonists prevents neuronal damage, motor dysfunction, myelin loss, neuropathic pain, and inflammation after spinal cord injury in adult rats. The Journal of pharmacology and experimental therapeutics. 2007;320:1002–1012. doi: 10.1124/jpet.106.113472. [DOI] [PubMed] [Google Scholar]
- Pattany PM, Yezierski RP, Widerström-Noga EG, Bowen BC, Martinez-Arizala A, Garcia BR, Quencer RM. Proton magnetic resonance spectroscopy of the thalamus in patients with chronic neuropathic pain after spinal cord injury. AJNR Am J Neuroradiol. 2002;23:901–905. [PMC free article] [PubMed] [Google Scholar]
- Petrosino S, Palazzo E, de Novellis V, Bisogno T, Rossi F, Maione S, Di Marzo V. Changes in spinal and supraspinal endocannabinoid levels in neuropathic rats. Neuropharmacology. 2007;52:415–422. doi: 10.1016/j.neuropharm.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Plunkett JA, Yu CG, Easton JM, Bethea JR, Yezierski RP. Effects of interleukin-10 (IL-10) on pain behavior and gene expression following excitotoxic spinal cord injury in the rat. Experimental neurology. 2001;168:144–154. doi: 10.1006/exnr.2000.7604. [DOI] [PubMed] [Google Scholar]
- Priller J, Briley EM, Mansouri J, Devane WA, Mackie K, Felder CC. Mead ethanolamide, a novel eicosanoid, is an agonist for the central (CB1) and peripheral (CB2) cannabinoid receptors. Molecular pharmacology. 1995;48:288–292. [PubMed] [Google Scholar]
- Rajpal S, Gerovac TA, Turner NA, Tilghman JI, Allcock BK, McChesney SL, Miranpuri GS, Park SW, Resnick DK. Antihyperalgesic effects of vanilloid-1 and bradykinin-1 receptor antagonists following spinal cord injury in rats. Journal of neurosurgery Spine. 2007;6:420–424. doi: 10.3171/spi.2007.6.5.420. [DOI] [PubMed] [Google Scholar]
- Resnick DK, Schmitt C, Miranpuri GS, Dhodda VK, Isaacson J, Vemuganti R. Molecular evidence of repair and plasticity following spinal cord injury. Neuroreport. 2004;15:837–839. doi: 10.1097/00001756-200404090-00020. [DOI] [PubMed] [Google Scholar]
- Schuhmann MU, Stiller D, Skardelly M, Bernarding J, Klinge PM, Samii A, Samii M, Brinker T. Metabolic changes in the vicinity of brain contusions: a proton magnetic resonance spectroscopy and histology study. J Neurotrauma. 2003;20:725–743. doi: 10.1089/089771503767869962. [DOI] [PubMed] [Google Scholar]
- Sheskin T, Hanus L, Slager J, Vogel Z, Mechoulam R. Structural requirements for binding of anandamide-type compounds to the brain cannabinoid receptor. Journal of medicinal chemistry. 1997;40:659–667. doi: 10.1021/jm960752x. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Mirakaj V, Serhan CN. Functional Metabolomics Reveals Novel Active Products in the DHA Metabolome. Front Immunol. 2012;3:81. doi: 10.3389/fimmu.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shir Y, Seltzer Z. Heat hyperalgesia following partial sciatic ligation in rats: interacting nature and nurture. Neuroreport. 2001;12:809–813. doi: 10.1097/00001756-200103260-00038. [DOI] [PubMed] [Google Scholar]
- Simon GM, Cravatt BF. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for alpha/beta-hydrolase 4 in this pathway. The Journal of biological chemistry. 2006;281:26465–26472. doi: 10.1074/jbc.M604660200. [DOI] [PubMed] [Google Scholar]
- Simon GM, Cravatt BF. Anandamide biosynthesis catalyzed by the phosphodiesterase GDE1 and detection of glycerophospho-N-acyl ethanolamine precursors in mouse brain. The Journal of biological chemistry. 2008;283:9341–9349. doi: 10.1074/jbc.M707807200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey JD, Tibshirani R. Statistical methods for identifying differentially expressed genes in DNA microarrays. Methods in molecular biology. 2003a;224:149–157. doi: 10.1385/1-59259-364-X:149. [DOI] [PubMed] [Google Scholar]
- Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proceedings of the National Academy of Sciences of the United States of America. 2003b;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson CI, Fitzsimmons B, Azizi S, Powell HC, Hua X-Y, Yaksh TL. Spinal p38beta isoform mediates tissue injury-induced hyperalgesia and spinal sensitization. Journal of neurochemistry. 2005;92:1508–1520. doi: 10.1111/j.1471-4159.2004.02996.x. [DOI] [PubMed] [Google Scholar]
- Tokuyama S, Nakamoto K. Unsaturated fatty acids and pain. Biological & pharmaceutical bulletin. 2011;34:1174–1178. doi: 10.1248/bpb.34.1174. [DOI] [PubMed] [Google Scholar]
- Tsuboi K, Ikematsu N, Uyama T, Deutsch DG, Tokumura A, Ueda N. Biosynthetic pathways of bioactive N-acylethanolamines in brain. CNS & neurological disorders drug targets. 2013;12:7–16. doi: 10.2174/1871527311312010005. [DOI] [PubMed] [Google Scholar]
- Veigas JM, Williams PJ, Halade G, Rahman MM, Yoneda T, Fernandes G. Fish oil concentrate delays sensitivity to thermal nociception in mice. Pharmacological research: the official journal of the Italian Pharmacological Society. 2011;63:377–382. doi: 10.1016/j.phrs.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ueda N. Biology of endocannabinoid synthesis system. Prostaglandins & other lipid mediators. 2009;89:112–119. doi: 10.1016/j.prostaglandins.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Wang Z, Chabot J-G, Quirion R. On the possible role of ERK, p38 and CaMKII in the regulation of CGRP expression in morphine-tolerant rats. Molecular pain. 2011;7:68. doi: 10.1186/1744-8069-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JT, Williams JS, Pandarinathan L, Janero DR, Lammi-Keefe CJ, Makriyannis A. Dietary docosahexaenoic acid supplementation alters select physiological endocannabinoid-system metabolites in brain and plasma. Journal of lipid research. 2010;51:1416–1423. doi: 10.1194/jlr.M002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Mandal R, Sinelnikov IV, Broadhurst D, Wishart DS. MetaboAnalyst 2.0--a comprehensive server for metabolomic data analysis. Nucleic acids research. 2012;40:W127–133. doi: 10.1093/nar/gks374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Psychogios N, Young N, Wishart DS. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic acids research. 2009;37:W652–660. doi: 10.1093/nar/gkp356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z-Z, Zhang L, Liu T, Park JY, Berta T, Yang R, Serhan CN, Ji R-R. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:592–597. 591. doi: 10.1038/nm.2123. following 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue H, Wan M, Song D, Li Y, Li J. Eicosapentaenoic acid and docosahexaenoic acid modulate mitogen-activated protein kinase activity in endothelium. Vascul Pharmacol. 2006;44:434–439. doi: 10.1016/j.vph.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Yehuda S, Carasso RL. Modulation of learning, pain thresholds, and thermoregulation in the rat by preparations of free purified alpha-linolenic and linoleic acids: determination of the optimal omega 3-to-omega 6 ratio. Proc Natl Acad Sci USA. 1993;90:10345–10349. doi: 10.1073/pnas.90.21.10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yezierski RP. Pain following spinal cord injury: pathophysiology and central mechanisms. Progress in brain research. 2000;129:429–449. doi: 10.1016/S0079-6123(00)29033-X. [DOI] [PubMed] [Google Scholar]
- Yu W, Hao JX, Xu XJ, Saydoff J, Haegerstrand A, Hokfelt T, Wiesenfeld-Hallin Z. Long-term alleviation of allodynia-like behaviors by intrathecal implantation of bovine chromaffin cells in rats with spinal cord injury. Pain. 1998;74:115–122. doi: 10.1016/s0304-3959(97)00204-2. [DOI] [PubMed] [Google Scholar]