Abstract
Repeated acquisition and discrimination reversal tasks are often used to examine behavioral relations of, respectively, learning and cognitive flexibility. Surprisingly, despite their frequent use in cognitive neuroscience and behavioral pharmacology, variables that control performance under these two tasks have not been widely studied. The present studies were conducted to directly investigate the controlling variables in nonhuman primates. Squirrel monkeys were trained with a touchscreen variant of the repeated acquisition task in which a novel pair of S+/S− stimuli was presented daily. Subjects learned to discriminate the two stimuli (acquisition) and, subsequently, with the contingencies switched (reversal). Results indicate that rates of both acquisition and reversal learning increased across successive sessions, but that rate of reversal learning remained slower than acquisition learning, i.e., more trials were needed for mastery. Subsequent experiments showed this difference between the rate of learning novel discriminations and reversal was reliable for at least 5 days between acquisition and reversal and notwithstanding the interpolation of additional discriminations. Experimental analysis of the S+/S− elements of the tasks revealed that the difference in the rate of learning could not be attributed to a relatively aversive quality of the S− or to a relatively appetitive quality of the S+ but, rather, to contextual control by the S+/S− stimulus complex. Thus, if either element (S+ or S−) of the stimulus complex was replaced by a novel stimulus, the rate of acquisition approximated that expected with a novel stimulus pair. These results improve our understanding of fundamental features of discrimination acquisition and reversal.
Keywords: Repeated Acquisition, Discrimination Reversal, Touchscreen, Squirrel Monkey
INTRODUCTION
The ability to effectively discriminate among stimuli in the environment has obvious survival value (e.g., purple berries = nutrition, red berries = poison). Likewise, the cognitive flexibility needed to adapt to changing environmental conditions can be equally critical (e.g., color rules may change in a new berry patch). Such contingency-based capabilities appear to be fundamental across species, and the empirical investigation of their features and underlying neural mechanisms in laboratory animals has been central to understanding basic principles of learning-based performance. In this regard, the repeated acquisition task and discrimination reversal tasks are among the most commonly used assays of learning and cognitive flexibility, respectively.
The repeated acquisition task is used to assay basic behavioral building blocks of discrimination learning. In its typical arrangement, an experimental subject is presented with two concurrently-available stimuli – one arbitrarily designated as S+ (responses result in reinforcement) and the other as S− (responses result in timeout). Through trial-and-error, subjects eventually acquire the discrimination, indicated by exclusive S+ responding, after which another pair of stimuli is presented for discrimination (i.e., repeated acquisition). In seminal work with monkeys, Harlow (1949) discovered a reliable effect – the rate at which a subject acquires such discriminations increases as a function of repeated exposure to the task until very few trials are required to learn the discrimination. He called this phenomenon a learning set. The importance of this finding lies not simply in the observation of an ever-expanding discrimination repertoire of the subject, but, also, in the identification of higher-order behavioral processes created by cumulative learning histories.
A variant of discrimination learning also has been useful for exploring another behavioral dimension of learning, namely cognitive flexibility, that is, the adaptation of response strategies in the face of changing environmental conditions (Easton 2005; Mackintosh et al. 1968). In the discrimination reversal task, the initial phase is identical to the repeated acquisition task, however, after the discrimination is mastered, the designation of S+ and S− switch. That is, after the subject responds reliably to the S+, and not S−, stimulus, their programmed consequences are reversed. As with initial acquisition, the primary dependent measure is trials to mastery under these new conditions.
Because both tasks involve such fundamental cognitive behavior, the localization of function, neurobiological substrates, and neurochemical signaling involved in learning and flexibility continue to be active topics of investigation. For example, neural network studies in rats and monkeys suggest that the interconnected lateral prefrontal cortex and striatum of the basal ganglia play key roles in discrimination learning by encoding reward and error (e.g., Histed et al. 2009; Knowlton et al. 1996; Lee et al. 2012; Schultz et al. 1997; reviewed in Kehagia et al. 2010). On the other hand, the orbitofrontal cortex appears to be the primary neural substrate of reversal learning and flexibility, as established through highly-selective lesion studies in monkeys and rats (e.g., Butter 1969; Butter et al. 1963; Clarke et al. 2008; Chudasama and Robbins 2003; Fellows and Farah 2003; Iversen and Mishkin 1970; Izquierdo et al. 2004; Jones and Mishkin 1972; Kim and Ragozzino 2005; reviewed in Chudasama 2011). Additionally, both learning and flexibility are thought to involve modulation by the monoamine neurotransmitters dopamine (DA) and serotonin (5-hydroxytryptamine [5-HT]). In this regard, recent studies have shown that overexpression of striatal D2 receptors disrupts prefrontal function in discrimination learning (Bach et al. 2008). Moreover, highly selective depletion of 5-HT, but not DA, from the orbitofrontal cortex has been shown to obstruct reversal learning (Clarke et al. 2004; 2005; 2007; Walker et al. 2009; reviewed in Roberts 2011). Although the studies above suggest some neurochemical dissociations between learning and flexibility, the available data are not entirely consistent with this view, and others suggest overlap, or complementarity, in the roles of DA and 5-HT systems in these processes (reviewed in Olvera-Cortés et al. 2008).
In addition to research to understand neural bases of these behavioral processes, the repeated acquisition and discrimination reversal tasks are also commonly used to determine the effects of psychoactive drugs on learning and cognitive flexibility. Thus, drugs known to have deleterious effects on cognition, for example, the monoaminergic stimulant methamphetamine and the primary psychoactive ingredient in marijuana Δ9-tetrahydrocannabinol produce dose-dependent decrements in the acquisition rate of novel discriminations (Galizio et al. 2009; Kangas and Bergman 2012; Nakamura-Palacios et al. 2000; Winsauer et al. 1999), whereas other drugs, for example, the attention enhancer methylphenidate, have been shown to increase the rate of discrimination reversals (Handley and Calhoun 1978). Overall, these assays provide a useful means to assess both the short-term and long-term adverse effects of abused drugs and the safety of novel therapeutics with regard to well-established learning processes.
Surprisingly, despite their common use in cognitive neuroscience and behavioral pharmacology, there is a paucity of research directly examining behavioral mechanisms that are critical to the acquisition of stimulus discrimination and reversal. More specifically, there is no information regarding experimental variables that may guide the development of performance under each task. Thus, the purpose of the present series of experiments was to address this gap by assessing similarities and differences of performance under these assays of learning and cognitive flexibility.
EXPERIMENT 1
The purpose of Experiment 1 was to characterize the repeated acquisition and discrimination reversal of visual discriminations with squirrel monkeys using a touchscreen apparatus. Conditions were programmed daily for the subject to learn a novel S+/S− discrimination using concurrently-presented digital photographs, first under repeated acquisition and, subsequently, under conditions of discrimination reversal. The rate at which novel discriminations and reversals were mastered served as the primary dependent variable.
METHOD
Subjects
Four experimentally naïve adult male squirrel monkeys (Saimiri sciureus) were individually housed in a temperature- and humidity-controlled vivarium with a 12-h light/dark cycle (7am–7pm). Subjects had unlimited access to water in the home cage and were maintained at approximate free-feeding weights by post-session feedings of a nutritionally balanced diet of high protein banana-flavored biscuits (Purina Monkey Chow, St. Louis, MO). In addition, fresh fruit and environmental enrichment were provided daily. Experimental sessions were conducted 5 days a week (Monday–Friday). The experimental protocol for the present studies was approved by the Institutional Animal Care and Use Committee at McLean Hospital. Subjects were maintained in a facility licensed by the U.S. Department of Agriculture and in accordance with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2003).
Apparatus
Details, schematics, and photographs of the touch-sensitive experimental chamber can be found in Kangas and Bergman (2012). Briefly, a custom-built Plexiglas™ chamber measuring 38 cm × 40 cm × 60 cm was situated in a custom-built 50 cm × 60 cm × 70 cm sound- and light-attenuating enclosure. A 17” touch-sensitive screen (1739L, ELO TouchSystems, Menlo Park, CA) was mounted on an inside wall of the enclosure and fit into a 34 cm × 27 cm cut-out in one wall of the chamber. An infusion pump (PHM-100-10, Med Associates, St. Albans, VT) outside the enclosure was used to deliver precise quantities of a milk solution (30% sweetened condensed milk, 70% water) into a custom-designed 5 cm × 3.5 cm × 1.27 cm Plexiglas receptacle, which contained a shallow well with a circle diameter of 2.5 cm. A speaker bar (NQ576AT, Hewlett-Packard, Palo Alto, CA) mounted to the top of the inside enclosure wall above the screen was used to emit an audible feedback click each time the subject touched a stimulus presented on the screen. All experimental events and data collection were programmed in E-Prime Professional 2.0 (Psychology Software Tools, Inc., Sharpsburg, PA).
Procedure
Repeated Acquisition
During experimental sessions, subjects were placed within the experimental chamber and were not restrained or restricted in their movement. Each session began with concurrent presentation of two 7 cm × 7 cm digital photographs, each in a different randomly-selected quadrant of the screen. A response to one initiated a 0.15 ml milk delivery (S+) paired with an 880 ms yellow screen flash, followed by a 10 s intertrial interval (ITI) blackout, whereas a touch to the other stimulus immediately initiated the 10 s ITI (S−). The same two stimuli were presented during each of 200 trials comprising each session. Subjects were presented with a new S+/S− pair each session, and photographs for each session were randomly selected from our laboratory bank of >10,000 images. Thus, the subject was required to learn a new S+/S− discrimination each session based on distinguishing features of two visual stimuli that had not been previously viewed. The primary dependent variable was the number of trials to acquire discrimination; the criterion for mastery was responses to S+ in 9 of 10 consecutive trials. Data for each subject were collected across 30 sessions of the repeated acquisition task.
Discrimination Reversal
Beginning on Session 31, experimental conditions were altered to incorporate a discrimination reversal task. Under the new conditions, the first 100 trials were conducted exactly as described above. However, on Trial 101 the relationship between S+ and S− was reversed without signal. That is, during Trials 101–200, the stimulus that was initially S+ was made S−, and vice versa. As before, the primary dependent measure was trials to mastery of the new relationship (i.e., number of trials until ≥90% accuracy across 10 consecutive trials was achieved). This discrimination reversal condition was also conducted for 30 sessions.
RESULTS AND DISCUSSION
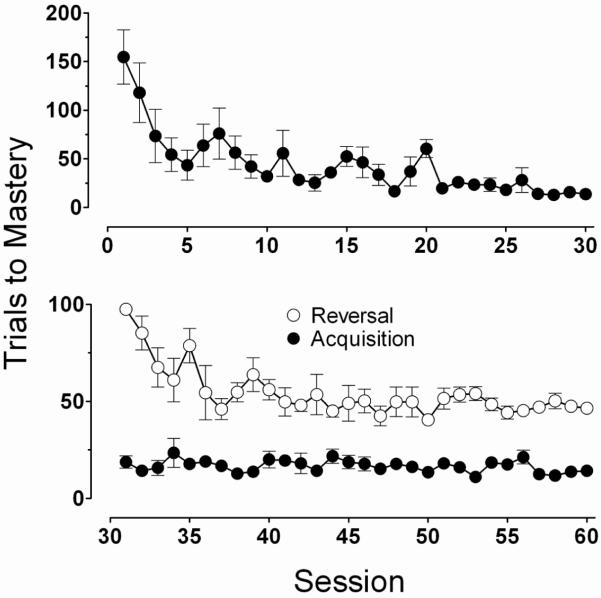
The upper panel of Figure 1 presents the group average for the number of trials required to acquire the discrimination across each of the first 30 successive sessions under the repeated acquisition task. Overall, the rate of acquisition increased across successive sessions until subjects acquired the discrimination quickly each session. This was evident in the number of trials to reach mastery which, as shown in the upper panel of Figure 1, decreased steadily until achieving a plateau within approximately the first 20 trials. Group average acquisition data generally represent findings that were consistent among subjects. There was some variability in acquisition rate across subjects, notably during the first 20 sessions but, as the error bars indicate, variance diminished across sessions. These data confirm that squirrel monkeys can effectively learn new discriminations with complex visual stimuli each session, and that the manner in which the rate of acquisition changes across experimental sessions (i.e., learning set) is similar to that observed in traditional approaches with 3-dimensional stimuli that are manually presented to the monkey (cf. Harlow 1949; Warren 1965).
Figure 1.
Upper Panel: Mean number of trials (±S.E.M.) to discrimination mastery (90% correct) during the first 30 sessions of Repeated Acquisition.
Lower panel: Mean number of trials (±S.E.M.) to mastery (90% correct) during first 30 sessions of discrimination reversal (open symbols) conducted during Trials 101–200 following discrimination mastery (filled symbols) acquired during Trials 1–100.
The lower panel of Figure 1 presents group average data from the first set of discrimination reversal experiments. During sessions in this condition, the S+/S− contingency was reversed in an unsignaled manner on Trial 101, and this new contingency was in effect from Trial 101–200. The filled symbols indicate the number of trials to mastery of the initial discrimination. As shown, novel discriminations continued to be mastered each session at a rapid and stable rate of acquisition. The open symbols represent the number of trials to mastery of the reversal beginning in Trial 101. As the data series indicates, a similar decrease across sessions in the number of trials to mastery of the reversal was observed. However, the rate of reversal learning reached a plateau at approximately 40–50 trials to mastery, which was much slower than observed with initial acquisition (approximately 15–20 trials to mastery). Here again, mean performance was generally representative of the individual subject across the condition. Inspection of within-session responding indicates that some of this difference in trials to mastery can be reliably accounted for by an initial response perseverance to the stimulus that was initially S+ (but now S−) at the onset of reversal for approximately 10 trials, which is not surprising because reversals were unsignaled. Perseverance was typically followed by a longer period of mixed response allocation than was observed in the initial discrimination acquisition (data not shown).
Interestingly, although not investigated or addressed directly, inspection of baseline data in previous studies show similar differences in acquisition and reversal rates with other nonhuman primates, including rhesus macaques (e.g., Porter et al. 2011), baboons (e.g., Zürcher et al. 2010), and marmosets (e.g., Spinelli et al. 2004). In contrast, several studies with rats and pigeons have demonstrated rapid reversal learning and even some anticipatory errors when reversal occurred at the midpoint of each session (e.g., Rayburn-Reeves et al., 2011; 2012). However, these rapid reversals were observed under conditions in which the same discrimination was reversed daily (red and green stimulus lights), which leaves unclear whether this represents a species difference or a fundamental methodological difference between studies that strictly alternate the same stimuli and those that reverse novel discriminations daily.
EXPERIMENT 2
The observed difference in rates of acquisition and reversal raises some interesting considerations. For example, can this difference be attributed to differing learning processes, in agreement with the idea that learning a novel discrimination is fundamentally different than mastering the reversal (e.g., learning vs. cognitive flexibility)? In addition, given the clear evidence of high savings, that is, improved performance across sessions for initial acquisition (i.e., learning set), why did reversal performance not improve to the same degree? Reasonably, the invariability and, therefore, high predictability of daily stimulus reversal at Trial 101, though otherwise unsignaled, might have facilitated the adjustment, especially across numerous successive reversals.
The purpose of Experiment 2 was to assess the reliability of this differential learning rate of acquisition and reversal following two manipulations predicted to weaken the initial learning history prior to reversal. First, we examined several temporal delays between discrimination acquisition and reversal. Second, we examined whether learning additional novel discriminations would affect the rate of reversal of a previously learned discrimination. That is, if reversal mastery requires additional trials because of the strength of the initial discrimination, would the passage of time or learning additional discriminations, which presumably weaken that strength, increase the rate of reversal learning?
METHOD
Subjects and Apparatus
Same as Experiment 1.
Procedure
To assess the effects of the passage of time on rate of reversal learning, six intervals between the acquisition of a novel discrimination and the reversal of that discrimination were evaluated. The effects of interposing intervals of 0 (immediate unsignaled reversal at Trial 101 as described in Exp 1), 1, 24, 48, 72, and 96 hours were determined twice and examined in a random sequence across subjects. The subject spent the intervals longer than 1 hr in their home cage located within the facility vivarium. In addition, during the 96 hour interval test, in which the novel discrimination was learned on a Monday and reversed on the following Friday, 3 additional novel discriminations were learned, one each day, on Tuesday, Wednesday, and Thursday. This was designed to determine whether learning additional discriminations following initial acquisition might interfere with rates of reversal learning.
RESULTS AND DISCUSSION
Figure 2 presents the effects of temporal intervals between initial acquisition and reversal. The filled symbols indicate the number of trials to acquisition mastery and the open symbols indicate the number of trials to reversal mastery when assessed following the time interval plotted on the abscissa. As the figure shows, no systematic changes in the rate of reversal were observed following any of the intervals tested, suggesting no effect of the passage of time (i.e., up to 96 hrs) between learning a discrimination and reversal. No change in reversal rate following the 96 hour test suggested that, in addition, the acquisition of 3 novel discriminations during the 3 intervening days produced no decrement or improvement in learning the reversal of the discrimination acquired 96 hours earlier. Taken together, these data indicate that the passage of time and the acquisition of additional novel discriminations prior to reversal had no effect on the rate of reversal learning, at least within the time-frame examined. Although one may hypothesize longer intervals of time and/or learning additional discriminations may increase the rate of reversal learning by making the stimuli essentially novel again, no evidence or trend in that direction was observed under the present conditions. On a cautionary note, the present findings do not bear on the related question of whether the features and dimensions of the stimuli that control the discrimination performance can be overshadowed or forgotten. In summary, data from the present experiment indicate considerable stability over time in the difference in the rates of learning a novel discrimination and its reversal.
Figure 2.
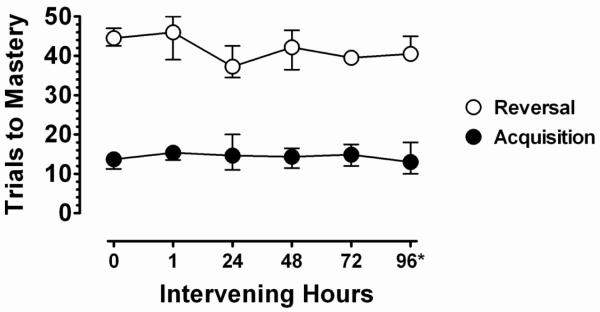
Mean number of trials (±S.E.M.) to discrimination mastery (90% correct) of initial acquisition (filled symbols) and reversal (open symbols) plotted as a function of the intervening temporal interval between acquisition and reversal. The asterisk at the 96 hour interval signifies that 3 additional novel discriminations were also learned during intervening time between acquisition and reversal. See text for additional details.
EXPERIMENT 3
Experiments 1 and 2 showed large and reliable differences in the rate of learning a novel discrimination and the rate of reversal. As discussed above, some of this difference can be accounted for by the initial response perseverance to the stimulus that was initially S+ (but now S−) at the onset of reversal. This is not surprising because reversals were unsignaled. What is unclear, however, is why initial perseverance is followed by a longer period of mixed response allocation than was observed in the initial discrimination acquisition. Possibly, at least initially during reversal, the subject responds to what was S+ and/or away from what was S−. That is, the reinforcement history gained during initial discrimination acquisition, in addition to conditioning appetitive properties to the original S+ stimulus, also could have conditioned aversive properties to the original S− stimulus, making responses to that stimulus during reversal difficult (i.e., aversive). Both types of reinforcement history reasonably might contribute to a lower rate of reversal learning. To evaluate this possibility, Experiment 3 was conducted to determine whether the S+ and S− stimulus gained, respectively, conditioned appetitive and aversive properties during initial acquisition. Thus, after acquisition, either S+ or S− was replaced by a novel (i.e., neutral) stimulus for reversal learning.
METHOD
Subjects and Apparatus
Same as Experiment 1.
Procedure
Test for Conditioned Aversive Properties of S−
Test sessions began with the acquisition of a novel discrimination during Trials 1–100 as described in Experiment 1. However, during discrimination reversal beginning on Trial 101, the stimulus that was S− changed to S+, but the stimulus that was S+ was replaced by a novel stimulus. This was done to test for potential conditioned aversive properties of the initially S− stimulus. Response allocation during the first 10 trials was scrutinized closely. If S− had gained conditioned aversive properties during initial discrimination acquisition (i.e., first 100 trials), subjects might be expected, at least initially, to respond away from what was S− (now S+) and instead respond to the novel (neutral) stimulus. This test was conducted 3 times with each subject.
Test for Conditioned Appetitive Properties of S+
Test sessions also began with the acquisition of a novel discrimination during Trials 1–100 as described in Experiment 1. However, during the reversal trials beginning on Trial 101, the stimulus that was S+ changed to S−, and the stimulus that was S− was replaced by a novel stimulus. This was designed to test for potential conditioned appetitive properties of the initially S+ stimulus. Again, response allocation during the first 10 trials received close scrutiny. If S+ had gained conditioned appetitive properties during the initial discrimination acquisition (i.e., first 100 trials), subjects might be expected, at least initially, to respond to what was S+ (now S−) despite the fact that the novel (neutral) stimulus was now S+. This test was also conducted 3 times with each subject.
RESULTS AND DISCUSSION
Figure 3 presents results of tests for conditioned aversive and appetitive properties. The gray bar shows the average number of the first 10 trials in which the subject responded to the novel stimulus during the test for conditioned aversive properties. As discussed above, aversive properties presumably would be reflected in the subject's responding away from that stimulus and, instead, to the novel (neutral) stimulus. However, during these tests, responses to the novel stimulus were not significantly different than chance (two-tailed binomial probability test, p=.36). This split in response allocation, evident in all subjects during the first 10 trials, suggests that the original S− stimulus did not gain strong conditioned aversive properties.
Figure 3.
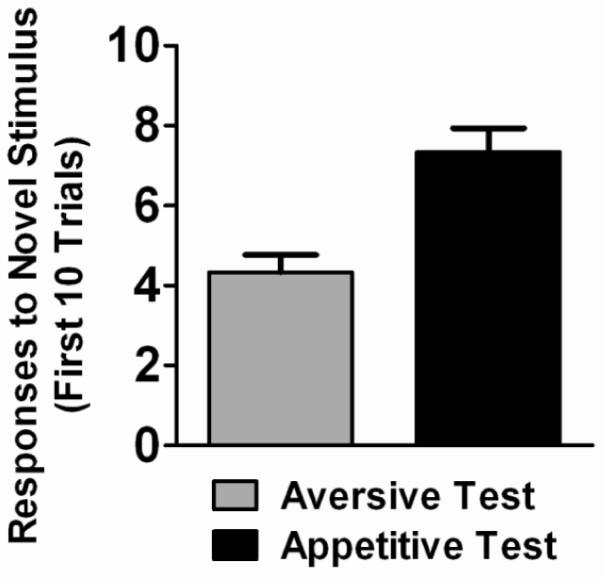
Results of tests for conditioned aversive (gray bar) and appetitive (black bar) properties. Bars show the mean number (±S.E.M.) of the first 10 trials in which the subject responded to the novel stimulus.
The black bar of Figure 3 shows the average number of the first 10 trials in which the subject responded to the novel stimulus during the test for conditioned appetitive properties. During these tests, subjects responded to the novel stimulus significantly more than chance (two-tailed binomial probability test, p=.0004). This result, observed in all subjects, was quite surprising. Again, the stimulus that was S+ during discrimination acquisition was made S− and what was S− was replaced by a novel stimulus. As discussed above, responding to what previously was S+ typically perseveres during the early trials after reversal, and was predicted here. The only difference between the present conditions and that of standard discrimination reversal was the replacement of S− with a novel stimulus. Thus, the novel S+ stimulus quickly controlled responding, even though the original S+ stimulus (now S−) was still available.
The results of Experiment 3 do not support the idea that the slower mastery of reversal learning can be attributed to lingering conditioning attributes of the S+ and S− stimuli. Thus, the mixed response allocation observed during the conditioned aversive properties tests indicates that S− was not particularly aversive at the onset of reversal, and, interestingly, the results from the conditioned appetitive properties tests suggest that the appetitive property of the earlier S+ was not apparent when the new S+ was introduced. Yet another interpretation of these data emerges when analyzed using the trials to mastery measures of Experiments 1 and 2. Figure 4 presents the trials to mastery across the 4 conditions of the present series of studies. The first bar shows the average number of trials to acquire a novel discrimination. The second bar shows the average number of trials to mastery following a reversal. The third and fourth bars show the average number of trials to learn the reversal during the conditioned aversive and appetitive tests, respectively. As the figure indicates, regardless of whether S+ was replaced by a novel stimulus (aversive test) or S− was replaced by a novel stimulus (appetitive test) during reversal, the number of trials to master the new relation were significantly fewer than those needed to master a typical reversal (p=.0019, p=.0017, respectively). Moreover, the number of trials to mastery during the aversive test and appetitive test did not differ significantly from the values for acquiring a novel discrimination each day (p=.11, p=.48, respectively). This suggests that replacing either S+ or S− with a novel stimulus essentially makes the discrimination a new problem. From another perspective, these data indicate that the subject does not simply associate either or both the S+ or S− through trial-and-error conditioning, with responding thereafter to or away from individual stimuli. Rather, as changing either element (S+ or S−) makes the stimulus pair functionally novel, the subject must be responding to the integrated `stimulus complex'. Thus, contextual stimulus control plays a critical role, at least in regard to the ease with which the new discrimination is mastered.
Figure 4.
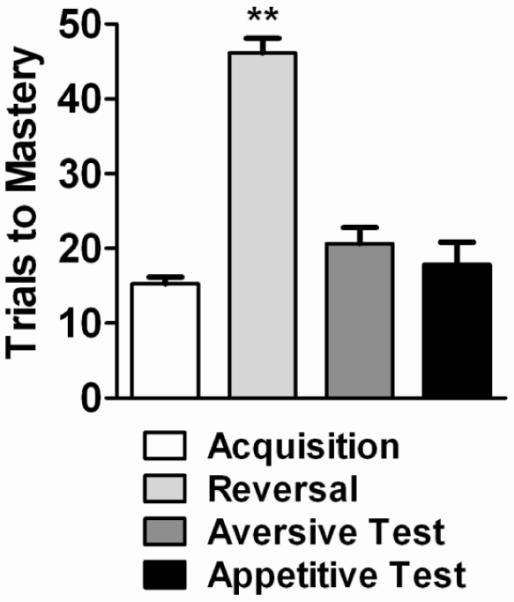
Mean number of trials (±S.E.M.) to mastery across 4 conditions. The first bar represents trials to acquire a novel discrimination. The second bar represents trials to mastery following a reversal. The third and fourth bars represent trials to learn the reversal during the conditioned aversive and appetitive tests, respectively. (**p<.01)
GENERAL DISCUSSION
The present series of studies systematically replicated repeated acquisition effects using a modern touchscreen variant of the task (cf. Harlow 1949; Warren 1965). In these studies, the rate of acquisition increased across successive sessions, confirming the development over time of what may be described as a learning set. During discrimination reversal sessions in which the stimulus designation of S+ and S− switched, the rate of reversal learning also increased; however, acquisition after reversal was slower, i.e., more trials were needed to master the discrimination. This difference in the rate of learning novel discriminations and reversals was reliable across time and following interposed distractions. Although the rate differences between acquisition and reversal may provide some suggestive evidence that different neural systems are involved (as reviewed above), neural activity was not measured here precluding definitive conclusions.
Evaluation of the S+/S− elements of the tasks revealed that S− was not particularly aversive following initial discrimination acquisition, nor was S+ particularly appetitive. That is, subjects did not specifically respond to S+ or away from S−. Rather, subsequent analyses showed that if either element (S+ or S−) of the stimulus complex was replaced by a novel stimulus, the rate of acquisition approximated that of a novel stimulus pair. Thus, contextual stimulus control appeared to govern responses in the presence of the integrated S+/S− stimulus complex. Taken together, these studies indicate that the behavioral mechanisms of discrimination learning and reversal involve contextual stimulus control of an integrative relationship between S+ and S− rather than control by specific elements of a stimulus.
ACKNOWLEDGEMENTS
The authors thank Rachel N. Cassidy for helpful suggestions, Rajeev I. Desai for comments on a previous version of this manuscript, and Michael Z. Leonard for assistance conducting this study. This research was supported by grants K01-DA035974 (B.D.K.) and R37-DA023142 (J.B.) from the National Institute on Drug Abuse.
REFERENCES
- Bach ME, Simpson EH, Kahn L, Marshall JJ, Kandel ER, Kellendonk C. Transient and selective overexpression of D2 receptors in the striatum causes persistent deficits in conditional associative learning. P Natl Acad Sci USA. 2008;105:16027–16032. doi: 10.1073/pnas.0807746105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butter CM. Preservation in extinction and in discrimination reversal tasks following selective frontal ablations in Macaca mulatta. Physiol Behav. 1969;4:163–171. [Google Scholar]
- Butter CM, Mishkin M, Rosvold HE. Conditioning and extinction of a food-rewarded response after selective ablations of frontal cortex in rhesus monkeys. Exp Neurol. 1963;7:65–75. doi: 10.1016/0014-4886(63)90094-3. [DOI] [PubMed] [Google Scholar]
- Chudasama Y. Animal models of prefrontal-executive function. Behav Neurosci. 2011;125:327–343. doi: 10.1037/a0023766. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to Pavlovian autoshaping and discrimination reversal learning: Further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Robbins TW, Roberts AC. Lesions of the medial striatum in monkeys produce perseverative impairments during reversal learning similar to those produced by lesions of the orbitofrontal cortex. J Neurosci. 2008;28:10972–10982. doi: 10.1523/JNEUROSCI.1521-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, Roberts AC. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J Neurosci. 2005;25:532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- Easton A. Behavioural flexibility, social learning, and the frontal cortex. In: Easton A, Emery NJ, editors. The cognitive neuroscience of social behavior. Psychology Press; New York: 2005. pp. 59–80. [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: Evidence from a reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Galizio M, McKinney P, Cerutti DT, Pitts RC. Effects of MDMA methamphetamine and methylphenidate on repeated acquisition and performance in rats. Pharmacol Biochem Be. 2009;94:305–311. doi: 10.1016/j.pbb.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley GW, Calhoun WH. The effects of methylphenidate on repeated acquisition of serial discrimination reversals. Psychopharmacology. 1978;57:115–117. doi: 10.1007/BF00426967. [DOI] [PubMed] [Google Scholar]
- Harlow HF. The formation of learning sets. Psychol Rev. 1949;56:51–65. doi: 10.1037/h0062474. [DOI] [PubMed] [Google Scholar]
- Histed MH, Pasupathy A, Miller EK. Learning substrates in the primate prefrontal cortex and striatum: sustained activity related to successful actions. Neuron. 2009;63:244–253. doi: 10.1016/j.neuron.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the interior prefrontal convexity. Exp Brain Res. 1970;11:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Opposing effects of amygdala and orbital prefrontal cortex lesions on the extinction of instrumental responding in macaque monkeys. Eur J Neurosci. 2005;22:2341–2346. doi: 10.1111/j.1460-9568.2005.04434.x. [DOI] [PubMed] [Google Scholar]
- Jones B, Mishkin M. Limbic lesions and the problem of stimulus-reinforcement associations. Exp Neurol. 1972;36:362–377. doi: 10.1016/0014-4886(72)90030-1. [DOI] [PubMed] [Google Scholar]
- Kangas BD, Bergman J. A novel touch-sensitive apparatus for behavioral studies in unrestrained squirrel monkeys. J Neurosci Meth. 2012;209:331–336. doi: 10.1016/j.jneumeth.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehagia AA, Murray GK, Robbins TW. Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Curr Opin Neurobiol. 2010;20:199–204. doi: 10.1016/j.conb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Kim J, Ragozzino ME. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiol Learn Mem. 2005;83:125–133. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Lee D, Seo H, Jung MW. Neural basis of reinforcement learning and decision making. Annu Rev Neurosci. 2012;35:287–308. doi: 10.1146/annurev-neuro-062111-150512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh NJ, McGonigle B, Holgate V, Vanderver V. Factors underlying improvement in serial reversal learning. Can J Psychology. 1968;22:85–95. doi: 10.1037/h0082753. [DOI] [PubMed] [Google Scholar]
- Nakamura-Palacios EM, Winsauer PJ, Moerschbaecher JM. Effects of cannabinoid ligand SR141716A alone or in combination with Δ9-tetrahrdrocannabinol or scopolamine on learning in squirrel monkeys. Behav Pharmacol. 2000;11:377–386. doi: 10.1097/00008877-200008000-00003. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. National Academies Press; Washington DC: 2003. [PubMed] [Google Scholar]
- Olvera-Cortés ME, Anguiano-Rodríguez P, López-Vázquez MA, Alfaro JM. Serotonin/dopamine interaction in learning. Prog Brain Res. 2008;172:567–602. doi: 10.1016/S0079-6123(08)00927-8. [DOI] [PubMed] [Google Scholar]
- Porter JN, Olsen AS, Gurnsey K, Dugan BP, Jedema HP, Bradberry CW. Chronic cocaine self-administration in rhesus monkeys: impact on associative learning, cognitive control, and working memory. J Neurosci. 2011;31:4926–4934. doi: 10.1523/JNEUROSCI.5426-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayburn-Reeves RM, Molet M, Zentall TR. Simultaneous discrimination reversal learning in pigeons and humans: anticipatory and perseverative errors. Learn Behav. 2011;39:125–37. doi: 10.3758/s13420-010-0011-5. [DOI] [PubMed] [Google Scholar]
- Rayburn-Reeves RM, Stagner JP, Kirk CR, Zentall TR. Reversal Learning in Rats (Rattus norvegicus) and Pigeons (Columba livia): Qualitative Differences in Behavioral Flexibility. J Comp Psychol Mar. 2012;19 doi: 10.1037/a0026311. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Roberts AC. The importance of serotonin for orbitofrontal function. Biol Psychiat. 2011;69:1185–1191. doi: 10.1016/j.biopsych.2010.12.037. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Pennanen L, Dettling AC, Feldon J, Higgins GA, Pryce CR. Performance of the marmoset monkey on computerized tasks of attention and working memory. Cognitive Brain Res. 2004;19:123–137. doi: 10.1016/j.cogbrainres.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Walker SC, Robbins TW, Roberts AC. Differential contributions of dopamine and serotonin to orbitofrontal cortex function in the marmoset. Cereb Cortex. 2009;19:889–898. doi: 10.1093/cercor/bhn136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JM. Primate learning in comparative perspective. In: Schrier AM, Harlow HF, Stollnitz F, editors. Behavior of nonhuman primates. Vol. 1. Academic; New York: 1965. pp. 249–281. [Google Scholar]
- Winsauer PJ, Lambert P, Moerschbaecher JM. Cannabinoid ligands and their effects on learning performance in rhesus monkeys. Behav Pharmacol. 1999;10:497–511. doi: 10.1097/00008877-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Zürcher NR, Rodriguez JS, Jenkins SL, Keenan K, Bartlett TQ, McDonald TJ, et al. Performance of juvenile baboons on neuropsychological tests assessing associative learning, motivation and attention. J Neurosci Meth. 2010;188:219–225. doi: 10.1016/j.jneumeth.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]