Abstract
Patients exposed to organophosphate (OP) compounds demonstrate a central apnea. The Kölliker-fuse nuclei (KF) are cholinergic nuclei in the brainstem involved in central respiratory control. We hypothesize that exposure of the KF is both necessary and sufficient for OP induced central apnea. We performed an animal study of acute OP exposure. Anesthetized and spontaneously breathing Wistar rats (n=24) were exposed to a lethal dose of dichlorvos using three experimental models. Experiment 1 (n=8) involved systemic OP poisoning using subcutaneous (SQ) 2,2-dichlorovinyl dimethyl phosphate (dichlorvos) at 100mg/kg or 3x LD50. Experiment 2 (n=8) involved isolated poisoning of the KF using stereotactic microinjections of dichlorvos (625 micrograms in 50 microliters) into the KF. Experiment 3 (n=8) involved systemic OP poisoning with isolated protection of the KF using SQ dichlorvos (100mg/kg) and stereotactic microinjections of organophosphatase A (OpdA), an enzyme that degrades dichlorvos. Respiratory and cardiovascular parameters were recorded continuously. Animals were followed post exposure for 1 hour or until death. There was no difference in respiratory depression between animals with SQ dichlorvos and those with dichlorvos microinjected into the KF. Despite differences in amount of dichlorvos (100mg/kg vs 1.8mg/kg) and method of exposure (SQ vs CNS microinjection), 10 min following dichlorvos both groups (SQ vs microinjection respectively) demonstrated a similar percent decrease in respiratory rate (51.5 vs 72.2), minute ventilation (49.2 vs 68.8) and volume of expired gas (17.5 vs 0.0). Animals with OpdA exposure to the KF during systemic OP exposure demonstrated less respiratory depression, compared to SQ dichlorvos alone (p<0.04). No animals with SQ dichlorvos survived past 25 min post exposure, compared to 50% of animals with OpdA exposure to the KF. In conclusion, exposure of the KF is sufficient but not necessary for OP induced apnea. Protection of the KF during systemic OP exposure mitigates OP induced apnea.
Keywords: Organophosphate, Kolliker-fuse, Organophosphatase, Central apnea, Respiratory failure
1.0 Introduction
Organosphosphate (OP) exposure causes mortality through a number of mechanisms including profound pulmonary effects with pulmonary secretions, cardio-depressant effects and/or central apnea. OP exposures represent a considerable clinical challenge worldwide secondary to accidental or intention exposure to insecticides in rural areas, with estimates that 200,000 people die from OP exposure each year(Eddleston and Phillips, 2004). Patients that survive the initial exposure can spend days in intensive care on a ventilator with a mortality of over 15%(Eddleston, 2000). The treatment for patients with acute OP exposure remains a focus of research, as clinicians still do not know the optimal therapy(Eddleston et al. , 2008) and the exact mechanisms of the central apnea remain a mystery.
Central control of respiration involves a series of brainstem neural circuits that facilitate the initiation and modulation of respiratory activity. At its most basic level the respiratory control system includes a central respiratory oscillator with both feedback and feed-forward afferent pathways for modulation as well as efferent pathways connecting to components of the respiratory system. Many parts of this control system such as the ventral medullary surface and the Kölliker-Fuse (KF) nucleus contain cholinergic neurons that are likely affected during acute OP poisoning. The cholinergic KF nucleus is located in the pons and is primarily involved in aspects of central respiratory control. Among other actions, the KF nucleus has been implicated in respiratory phase shifting (switching from inspiratory to expiratory) and regulation of ventilator response to hypercapnia and hypoxia(Cohen, 1971, Saint John et al. , 1975). Considered an essential part of the pneumotaxic center (with the pontine respiratory group (PRG) and the medial parabrachial nucleus), the KF extends cholinergic connections to other areas involved in respiration(Fort et al. , 1989, Nitsos and Walker, 1999).
There has been limited research concerning the areas of the brain that are affected by acute OP exposure, but a few lines of evidence support the theory that KF exposure is involved in OP-induced central apnea. First, limited animal research indicates that brainstem exposure serves a central role in OP-induced central respiratory failure(Gaspari and Paydarfar, 2011). In this study OP exposure limited to the brainstem produces a central apnea similar to systemic exposure. Second, disruption of functioning KF nuclei can produce central respiratory failure (Song et al. , 2010). Song and colleagues demonstrated that bilateral lesions of the KF nucleus produce a complete apnea with total cessation of phrenic discharge in anesthetized rats.
Systemic OP exposure induces a central apnea in a rodent animal model of OP poisoning(Gaspari and Paydarfar, 2007), and in this manuscript we explore the affect of focal exposure to the KF in the same animal model. Because of its prominent role in respiratory control and its cholinergic nature, the KF has the potential to be a primary brainstem site involved in OP-induced central apnea. We hypothesize that OP exposure of the KF is both necessary and sufficient for OP induced central apnea.
2.0 Materials and Methods
This study was approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School. Animal housing and care protocols were in agreement with the guidelines of the National Institutes of Health.
2.1 Anesthesia and Surgical Procedures
Animals were obtained from Charles River Laboratories (Wilmington, MA), housed in pairs and maintained on a 12h light/dark cycle. Food and water was provided ad libitum. A total of 29 male Wistar rats 275-300g were anesthetized with isoflurane (Webster Veterinary, Sterling, MA) and kept on a continuous calibrated dose of 2.2% to maintain anesthesia. This dose was titrated to ensure sufficient sedation, which was tested periodically by a foot or tail pinch. A tracheostomy was performed to allow spontaneous breathing of 100% oxygen within the circuit during the study. The setup of this circuit has been previously described and has a system dead space of roughly 10% of the animals’ total tidal volume(Gaspari and Paydarfar, 2007). Femoral venous access was obtained under anesthesia using a cut-down procedure and visualization of the femoral vein and placement of PE50 tubing. For a breakdown of the animals used in each protocol, see Table 1.
Table 1.
Experimental Protocol Details
| Sub-cutaneous Injection | Micro-injection | Time to Euthanasia | |
|---|---|---|---|
| Protocol 1 (n=8) | Dichlorvos | None | 60 min |
| Protocol 2 (n=11) | None | Dichlorvos | 13 min |
| Protocol 3 (n=8) | Dichlorvos | OpdA | 60 min |
| Protocol 4 (n=2) | None | OpdA | 60 min |
*OpdA – Organophosphatase enzyme
2.2 Respiratory and Hemodynamic Recordings
Physiologic recordings were collected using a digital data acquisition system (PowerLab, ADInstruments, Inc., Colorado Springs, CO) and a Dell computer (Round Rock, Texas). These recordings included end-tidal CO2, respiratory rate, volume of expired gas (Ve), and pulse oxygenation. Signals were filtered and amplified using the CyberAmp (AutoMate Scientific, Berkeley, CA), and a non-invasive pulse oximeter (MouseOx, Starr Life Sciences Corp., Oakmont, PA) was attached to the right rear paw to measure arterial oxygen saturation. Two animals did not have pulse oximetry recordings due to technical issues with the equipment. The signals were collected and displayed for the duration of the study and stored for later analysis. In all animals, physiologic recording started prior to experimental exposure and continued until euthanasia.
2.3 Stereotaxic Setup
A stereotaxic restraint with digital display console (Small Animal Stereotaxic Instrument Model 942, Kopf Instruments, Tujunga, CA) was used to align the animal for accurate cannula placement and later infusion. Two 7.9mm Kopf Mounting Holders (Mounting Holder MH-325-G/7.9K, Plastics One Inc., Roanoke, VA) were attached to the stereotaxic manipulators with 22-gauge outer guide cannulas (Plastics One Inc, Roanoke, VA) with 2mm pedestals and 6mm inner injection cannulas. The cannulas were stereotactically placed so that the tip of the outer guide cannulas was 4mm above the correct final coordinates. Once the outer cannulas were in place, internal injection cannulas were inserted to project 4mm past the tip of the external guide cannula. The proximal ends of the injection cannulas were connected via PE200 tubing to an infusion pump (CMA 402, CMA Microdialysis, North Chelmsford, MA). The needles and tubing were pre-filled with the injection solution prior to placement. The location of the guide and insertion cannulas was not changed at any time once placed in the brain.
2.4 Experimental Models
Rats were exposed to 2,2-dichlorovinyl dimethyl phosphate (dichlorvos), a common organophosphate compound using one of three experimental models. Experiment 1 (systemic OP exposure, n=8) involved exposure to subcutaneous (SQ) dichlorvos at 100mg/kg, or 3x LD 50. Experiment 2 (isolated OP exposure of the Kölliker-Fuse nuclei, n=11) consisted of stereotactic microinjections of dichlorvos (12.5mg/cc at 5ul/min for 10 minutes, 1250 μg total bilaterally) into the KF. Experiment 3 (isolated protection of KF nuclei during systemic OP exposure, n=8) involved exposure to SQ dichlorvos at 100mg/kg simultaneous with stereotactic microinjections of the enzyme organophosphorous hydrolase A (OpdA) (3.5mg/ml, 5ul/min over 10 minutes, 350μg total bilaterally) into the KF nuclei. OpdA is an enzyme that rapidly degrades dichlorvos(Bird et al. , 2008). Experiment 4 (control, n=2) consisted of animals with OpdA microinjected into the KF without exposure to dichlorvos. (See Table 1 for review of exposure models)
The dose of dichlorvos used in experiment 2 was calculated to represent roughly 2x the dose following SQ exposure in experiment 1 based on previous experiments in our lab and other labs. The intravenous dose of dichlorvos used during previous experiments in our lab (10 mg/kg IV) produces a similar apnea as the SQ dose in this manuscript (100mg/kg SQ). This dichlorvos exposure produces a relative concentration of 0.6 mg/kg in brain tissue(Blair et al. , 1975). The cumulative dose of dichlorvos at 12 minutes in experiment 2 was 0.0625 mg or 1.33 mg/kg (assuming distribution to the medulla and pons). These calculations were performed assuming a ratio of body weight to brain weight of 200(2008), a ratio of brain weight to the weight of the medulla and pons of 6.67(Culley and Lineberger, 1968).
2.5 Experimental Protocol
Following sedation and tracheostomy, each animal was placed in the stereotaxic apparatus. The placement of the injection cannula into the KF was performed using the stereotaxic coordinates from a stereotactic atlas(Paxinos and Watson, 1986). An incision in the scalp and periosteum was made and the surrounding area was retracted and debrided down to bone. Access to the brain at the point of entry into the skill was obtained using a stereotacticaly manipulated drill (Microtorque II drill (Harvard Apparatus, Holliston, MA)). The guide cannulas and injection needles were inserted using the digital guidance (Kopf Instruments).
Animals were allowed to stabilize on anesthetic for at least 30 minutes prior to initiation of the experimental exposure. At time zero, animals were exposed to SQ injections (if appropriate), followed immediately by KF microinjections (if indicated by the experimental protocol). Microinjections lasted for roughly 10 minutes in all study groups. Upon completion of the microinjection, the animals were monitored for an additional 2-3 minutes or until apnea. Animals with no microinjection (i.e. subcutaneous dichlorvos alone) were followed for 60 minutes or until apnea.
In animals receiving microinjections, the injection needles were then removed at the end of the experiment (leaving the guide cannula) and a new injection needle pre-loaded with 1% aqueous Potassium Permanganate (99+%, Sigma Aldrich, St Louis, MO) was inserted. To histologically mark the tip of the needle, an injection of the Potassium Permanganate was given at a rate of 8.3ul/min for 7 minutes.
2.6 Confirmation of Injection location
The animals in protocols with microinjection were euthanized immediate following the completion of the potassium permanganate injection and perfused with 1X Dulbecco's phosphate buffered saline (Invitrogen, Carlsbad, CA) and 4% paraformaldehyde. The brains were then extracted and transected coronally every 1mm rostral to caudal to determine the location of tissue staining from potassium permanganate. Brain slices with tissue staining from potassium permanganate were referenced to a rat brain atlas to determine the exact location of the needle tip.
2.7 Statistical Analysis
Data is presented as mean ± standard deviation unless noted. Comparison between groups was performed using student's T-test or chi-square where appropriate. Comparison between groups over time was performed using a two-way ANOVA with a Bonferroni post-test comparison at individual time points.
3.0 Results
3.1 Experiment 1 – Systemic OP exposure
Animals in this experimental protocol demonstrated a marked respiratory depression starting an average of 5.1 (± 1.6) minutes after exposure, progressing to full apnea an average of 15.9 (± 4.9) minutes post-exposure. This is similar to previously published results from our lab(Gaspari and Paydarfar, 2007). In all animals at 10 minutes post exposure, respiratory rate had decreased by an average of 48.7% and Ve by an average of 14.9% with no animals surviving past 22 minutes. As described in our previous publication, animals demonstrated a gradual decline in blood pressure and pulse rate following apnea. Peripheral signs and symptoms of OP poisoning such as urination, defecation and prominent oral secretions were also seen in most animals. The average time to death for all animals was 18.3 min (± 6.2 min). Changes in respiratory parameters over time are depicted in Figures 1 and 2.
Figure 1. Early Respiratory Effects following different OP exposures.
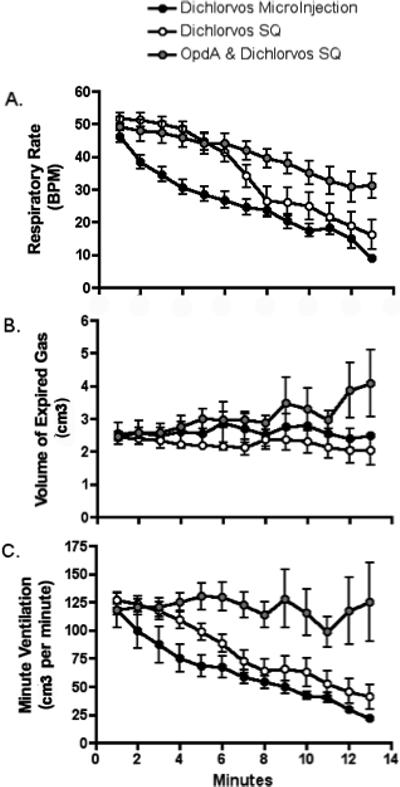
Graphs include data from three experimental protocols, subcutaneous dichlorvos alone, microinjection of dichlorvos into the KF alone, and subcutaneous dichlorvos with microinjection of Organophosphatase A enzyme (OpdA) into the KF. Data is presented as mean with standard deviation. Exposure occurs at time 0.
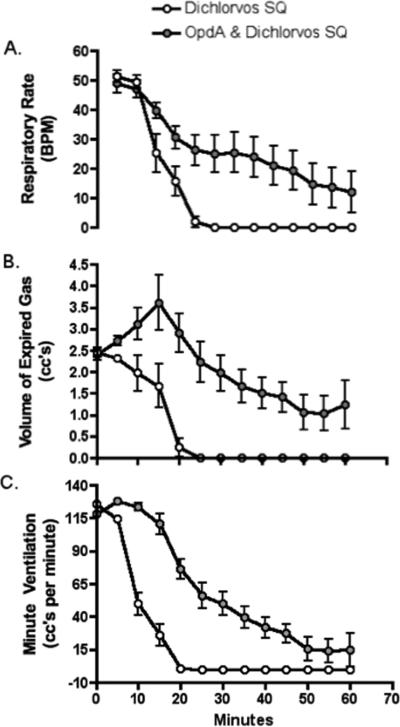
Figure 2. Later Respiratory Effects following Different OP exposures.
Graphs include data from two experimental protocols, subcutaneous dichlorvos alone, and subcutaneous dichlorvos with microinjection of Organophosphatase A enzyme (OpdA) into the KF. Data is presented as mean with standard deviation. Exposure occurs at time 0.
3.2 Experiment 2 – Isolated OP exposure to KF
Animals exposed to OP through microinjection into the KF demonstrated a steady decline in respiratory parameters starting immediately after the initiation of the exposure. At the termination of the experiment (13 min post microinjection) animals demonstrated a mean decline in respiratory rate and minute ventilation of 90.8% (± 20.1%) and 76.2% (± 21.1) respectively. Despite the differences in dose, animals with dichlorvos microinjection into the KF nuclei experienced a similar decrease in respiratory rate, volume of inspired gas and minute ventilation compared to animals exposed to systemic dichlorvos (see fig 1). In both groups there was a steady decline in respiratory rate and minute ventilation but no change in volume of inspired gas (over the initial 13 minutes of exposure).
Needle placement was within 5mm of the KF for most animals in experiment 2. Figure 3 demonstrates the location of the needle tips in relation to the KF nuclei.
Figure 3. Location of needle tip – OP microinjection.
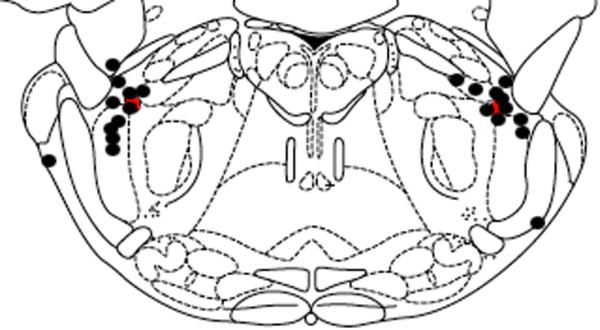
Microinjection needle tip locations (n=8) were marked using microinjection of KMnO4. The level of the brainstem slice depicted in the drawing is 3.3 mm caudal to intra-aural. The red oval represents the region of the KF. Black circles represent the tip of the microinjection needle. Animals with inadequate KMnO4 injection resulting in an insufficient lesion on the histological specimen were not included. The figure is adapted from a digital rat brain atlas (Paxinos and Watson, 1986).
3.3 Experiment 3 – Isolated protection of KF during OP exposure
Animals exposed to systemic dichlorvos simultaneous with OpdA microinjected into the KF experienced overall a less precipitous decline in respiratory rate and increase in volume of expired gas when compared to the other groups over the first 13 min post exposure (see fig 1). When compared to dichlorvos exposure alone, these animals also demonstrated a less precipitous decline over 1 hour (see fig 2). Animals with OpdA protection of the KF during systemic dichlorvos demonstrate significantly greater RR and TV at all time points after 10 minutes when compared to systemic exposure alone, with a significant decrease in mortality at 60min post exposure (50% vs 100%, p=0.023).
Needle placement was within 5mm of the KF for most animals in experiment 3. Figure 4 demonstrates the location of the needle tips in relation to the KF nuclei.
Figure 4. Location of Needle Tip - OpdA injection.
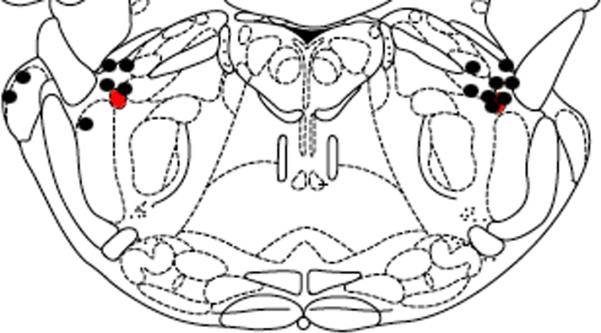
Microinjection needle tip locations (n=8) were marked using microinjection of KMnO4. The level of the brainstem slice depicted in the drawing is 3.3 mm caudal to intra-aural. The red oval represents the region of the KF. Black circles represent the tip of the microinjection needle. Animals with inadequate KMnO4 injection resulting in an insufficient lesion on the histological specimen were not included. The figure is adapted from a digital rat brain atlas(Paxinos and Watson, 1986). *OpdA = organophosphatase A enzyme.
3.4 Experiment 4 – OpdA KF microinjection alone
Animals demonstrated no significant change in respiratory rate, volume of expired gas or minute ventilation from baseline throughout the entire 13-minute experiment.
Respiratory rate decreased from 50bpm ± 0.7 (time 0) to 45 bpm ± 1.5 (time 12 min) while volume of expired gas increased from 3.0 (± 0.08) ccs to 3.23 (± 0.05) ccs.
4.0 Limitations
One limitation of this manuscript relates to the microinjection methodology used to expose the KF. An inherent limitation of microinjection involves diffusion to sites remote from the injection site. However, our injections are well clustered around the KF as demonstrated in Figure 3 and 4. In addition, the diffusion following microinjection would mimic the natural progression of OP through the brain. In addition, all of the animal models used in this manuscript involve anesthetized animals. Isoflurane produces a respiratory depression(Dardai and Heavner, 1989) that introduces a confounding element to our results.
Another limitation involves the differences in amount of time that the animals were monitored post-exposure between the dichlorvos microinjection (followed for 13 min) and the dichlorvos SQ (followed for 1 hour). The rationale behind limiting the time following microinjection relates to potential diffusion to adjacent brainstem sites. Microinjection introduces a compound to a specific location within the brainstem, but over time these compounds will diffuse into adjacent tissue and blood vessels, making it difficult to determine the exact location of the exposure. Animals with systemic exposure were followed for 1 hour based on previously published methodology(Gaspari and Paydarfar, 2007).
5.0 Discussion
OP exposure of the KF nuclei alone was sufficient to induce a central apnea, with similar characteristics when compared to systemic exposure of the same agent. Following either SQ exposure or microinjection, animals demonstrated a decrease in all respiratory parameters (respiratory rate, volume of expired gas, minute ventilation). Despite differences in dosing and timing, the decline in respiratory rate for both systemic exposure (100mg/kg SQ) and isolated KF exposure (1250 μg CNS microinjection) was similar over the initial 13 minutes. Animals exposed to KF microinjection of OP were not followed beyond 13 minutes, so it remains unclear if the later declines in respiratory parameters seen in the systemic exposure would have occurred following isolated KF exposure. Interestingly, there was no significant delay or change in the magnitude of the initial respiratory rate decline. These arguments support the theory that the mechanism for OP-induced respiratory rate depression involves exposure to the KF.
Isolated exposure of the KF nuclei to OpdA during systemic OP exposure mitigated the OP-induced apnea. Animals with OpdA microinjected into bilateral KF nuclei during systemic OP exposure demonstrated an increase in survival when compared to animals with systemic OP exposure alone. However, OpdA exposure of the KF during acute OP exposure did not completely eliminate OP-induced decreases in respiratory rate. This implies that the decrease in respiratory rate following OP exposure is related to effects at alternative brainstem sites. Previous studies of OpdA have demonstrated that a small amount rapidly degrades dichlorvos so it is unlikely that the respiratory effects are related to insufficient degradation at the site of the microinjection(Bird, Sutherland, 2008). Animals with OpdA exposure to the KF nuclei demonstrated a transient increase in volume of expired gas when compared to animals with exposure of the KF (either isolated exposure or systemic exposure), supporting the theory that the volume of expired gas decrease following OP exposure is related to exposure to the KF nuclei.
An analysis of the data does not eliminate the possibility of a time-based effect of OP compounds on the KF nuclei. Both SQ dichlorvos groups with or without OpdA microinjection demonstrate a similar rate of decline in respiratory rate from 10-20 minutes post exposure, albeit with a difference in magnitude. It could be argued that the similarities in respiratory rate and minute ventilation changes are related to differences in the threshold of inhibition. In other words, OpdA exposure that introduces a thresholding effect of cholinesterase inhibition could help explain the apparent delay in the rapid drop in respiratory rate observed groups.
The contribution of KF exposure during OP intoxication to OP-induced central apnea may depend on exposure to other respiratory control centers. For example, it is well known that brainstem acetylcholine circuits contribute to the control of breathing and their contributions differ depending on sleep state, which in turn can be affected through pontine cholinergic circuits. Previous researchers have shown that exposure of the KF nuclei to the anti-cholinergic agent atropine at night produces a decrease in respiratory rate and tidal volume, but has no effect during daytime(Bonis et al. , 2010b). Interestingly, exposure of the pontine reticular formation (PRF) to a cholinergic agonist induces a sleep-like state and respiratory depression characterized by a decrease in respiratory rate but not tidal volume(Horner and Kubin, 1999, Taguchi et al. , 1992). It is possible that OP exposure to the KF results in post-synaptic inhibition of afferent pathways projecting from the PRF, producing increased acetylcholine in the KF and respiratory depression.
OP effects in areas of the pons with afferent input into the KF could help explain our findings. Animals with OP exposure to the KF demonstrate respiratory depression through an increase in previously described cholinergic afferent inputs. Animals with OpdA exposure to the KF would have no acetylcholine increase related to direct OP exposure to the KF, but exposure to the PRF could produce some increased acetylcholine related to synaptic release. Increased afferent input from the PRF would disproportionally suppress respiratory rate over Ve. However, it is not clear what inputs are responsible for the increase in Ve.
Systemic OP exposure introduces cholinergic effects “downstream” from the central respiratory control centers that may also contribute to respiratory failure. Respiratory muscle fatigue secondary to fasciculations could introduce decreased respiratory effort and tidal volumes, but this would result in an increase in respiratory rate that was not seen in our studies. Increased pulmonary secretions could decrease oxygenation and lessen the effective diameter of the smaller airways, producing an increase in respiratory effort(Lekeux et al. , 1986). Increased pulmonary interstitial fluid(Kehrer et al. , 1986) would increase lung “stiffness” producing decreased tidal volumes. These effects likely contribute only slightly to the respiratory failure demonstrated in our animal model.
The KF contributes to respiratory control during normal physiologic conditions, and the loss of this normal function may contribute to respiratory collapse and delayed recovery following acute OP intoxication. The KF contributes to hypoxic ventilatory response in awake animals through cholinergic mechanisms. Control animals (goats) exposed to hypoxia (10.8% oxygen) demonstrate a normal hyperventilation response with an increase in heart rate but in goats with atropine exposure to bilateral KF there was an attenuation of both the hypoxic hyperventilation response and the reflexive tachycardia(Bonis et al. , 2010a). OP exposure may induce a similar response (i.e. loss of hypoxic respiratory drive). This may help to explain previous findings from our lab regarding recovery of respiratory control following acute OP exposure. Rats with a central apnea following dichlorvos exposure demonstrated a recovery in respiratory activity only if they did not experience hypoxia during the poisoning(Gaspari and Paydarfar, 2012). Regardless, it is unlikely that animals acutely intoxicated with OP agents demonstrate a normally functioning KF.
Our results explore the mechanistic underpinnings of OP induced respiratory failure, an area that has not received a great deal of attention in the scientific literature. Current research efforts into OP countermeasures would benefit from a greater understanding of the fundamental mechanisms of OP poisoning, especially in relation to the central effects. In addition, a better understanding of how respiratory areas of the brain are affected following OP exposure will allow for more complete and precise mechanistic studies in the future.
6.0 Conclusion
In summary, animals with isolated exposure of the KF nuclei demonstrated a similar change in respiratory rate and Ve when compared to systemic exposure. In other words, KF exposure alone was sufficient to generate OP-induced respiratory depression. Protection of the KF using OpdA during systemic OP exposure appears to mitigate but not eliminate the respiratory depressant effects of dichlorvos. Our results support the theory that a distributed exposure of multiple sites in the brainstem is required for OP-induced apnea, but the KF is one of the brainstem sites that is disrupted following acute OP exposure.
Highlights.
Organophosphate (OP) exposure to the Kölliker-fuse nuclei alone is sufficient for central apnea
OP induced respiratory failure was similar for animals exposed subcutaneously and injection into the Kölliker-fuse nuclei
OpdA enzyme protection of the KF mitigates organophosphate induced central apnea
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Romolo J Gaspari, Department of Emergency Medicine University of Massachusetts Worcester, MA 01655.
Courtney Dunn, Department of Emergency Medicine University of Massachusetts Worcester, MA 01655 Courtney.Dunn@umassmed.edu.
Bibliography
- Brain Facts and Figures. 2008.
- Bird SB, Sutherland TD, Gresham C, Oakeshott J, Scott C, Eddleston M. OpdA, a bacterial organophosphorus hydrolase, prevents lethality in rats after poisoning with highly toxic organophosphorus pesticides. Toxicology. 2008;247:88–92. doi: 10.1016/j.tox.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair D, Hoadley C, Hutson DH. The distribution of dichlorvos in the tissues of mammals after its inhalation or intravenous administration. Toxicology and applied pharmacology. 1975;31:243–53. doi: 10.1016/0041-008x(75)90160-x. [DOI] [PubMed] [Google Scholar]
- Bonis JM, Neumueller SE, Krause KL, Kiner T, Smith A, Marshall BD, et al. The pontine respiratory group, particularly the Kolliker-Fuse nucleus, mediates phases of the hypoxic ventilatory response in unanesthetized goats. Journal of applied physiology. 2010a;108:1321–35. doi: 10.1152/japplphysiol.00935.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonis JM, Neumueller SE, Krause KL, Kiner T, Smith A, Marshall BD, et al. A role for the Kolliker-Fuse nucleus in cholinergic modulation of breathing at night during wakefulness and NREM sleep. Journal of applied physiology. 2010b;109:159–70. doi: 10.1152/japplphysiol.00933.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MI. Switching of the respiratory phases and evoked phrenic responses produced by rostral pontine electrical stimulation. The Journal of physiology. 1971;217:133–58. doi: 10.1113/jphysiol.1971.sp009563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culley WJ, Lineberger RO. Effect of undernutrition on the size and composition of the rat brain. The Journal of nutrition. 1968;96:375–81. doi: 10.1093/jn/96.3.375. [DOI] [PubMed] [Google Scholar]
- Dardai E, Heavner JE. Comparison of respiratory and cardiovascular effects of halothane, isoflurane, and enflurane delivered via the Jackson-Rees breathing system in rats. New anaesthesia model for small animal surgery. Zeitschrift fur experimentelle Chirurgie, Transplantation, und kunstliche Organe : Organ der Sektion Experimentelle Chirurgie der Gesellschaft fur Chirurgie der DDR. 1989;22:50–4. [PubMed] [Google Scholar]
- Eddleston M. Patterns and problems of deliberate self-poisoning in the developing world. QJM : monthly journal of the Association of Physicians. 2000;93:715–31. doi: 10.1093/qjmed/93.11.715. [DOI] [PubMed] [Google Scholar]
- Eddleston M, Buckley NA, Eyer P, Dawson AH. Management of acute organophosphorus pesticide poisoning. Lancet. 2008;371:597–607. doi: 10.1016/S0140-6736(07)61202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddleston M, Phillips MR. Self poisoning with pesticides. BMJ. 2004;328:42–4. doi: 10.1136/bmj.328.7430.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort P, Sakai K, Luppi PH, Salvert D, Jouvet M. Monoaminergic, peptidergic, and cholinergic afferents to the cat facial nucleus as evidenced by a double immunostaining method with unconjugated cholera toxin as a retrograde tracer. The Journal of comparative neurology. 1989;283:285–302. doi: 10.1002/cne.902830209. [DOI] [PubMed] [Google Scholar]
- Gaspari RJ, Paydarfar D. Pathophysiology of respiratory failure following acute dichlorvos poisoning in a rodent model. Neurotoxicology. 2007;28:664–71. doi: 10.1016/j.neuro.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspari RJ, Paydarfar D. Dichlorvos-induced central apnea: effects of selective brainstem exposure in the rat. Neurotoxicology. 2011;32:206–14. doi: 10.1016/j.neuro.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspari RJ, Paydarfar D. Respiratory recovery following organophosphate poisoning in a rat model is suppressed by isolated hypoxia at the point of apnea. Toxicology. 2012;302:242–7. doi: 10.1016/j.tox.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner RL, Kubin L. Pontine carbachol elicits multiple rapid eye movement sleep-like neural events in urethane-anaesthetized rats. Neuroscience. 1999;93:215–26. doi: 10.1016/s0306-4522(99)00126-8. [DOI] [PubMed] [Google Scholar]
- Kehrer JP, Klein-Szanto AJ, Thurston DE, Lindenschmidt RC, Witschi HR. O,S,S,-trimethyl phosphorodithioate-induced lung damage in rats and mice. Toxicology and applied pharmacology. 1986;84:480–92. doi: 10.1016/0041-008x(86)90253-x. [DOI] [PubMed] [Google Scholar]
- Lekeux P, Kyavu A, Clercx C, Ansay M. Pulmonary function changes induced by experimental dichlorvos toxicosis in calves. Research in veterinary science. 1986;40:318–21. [PubMed] [Google Scholar]
- Nitsos I, Walker DW. Characterization of pontine neurons which respond to hypoxia in fetal sheep. Neuroscience letters. 1999;266:33–6. doi: 10.1016/s0304-3940(99)00249-9. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain: In Sterrotaxic Coordinates. 2nd Edition Academic Press; 1986. [Google Scholar]
- Saint John WM, Bond GC, Pasley JN. Integration of chemoreceptor stimuli by rostral brainstem respiratory areas. Journal of applied physiology. 1975;39:209–14. doi: 10.1152/jappl.1975.39.2.209. [DOI] [PubMed] [Google Scholar]
- Song G, Tin C, Poon CS. Bilateral lesions of pontine Kolliker-Fuse nuclei provoke apnea instead of apneusis in anesthetized adult rats. Advances in experimental medicine and biology. 2010;669:185–8. doi: 10.1007/978-1-4419-5692-7_37. [DOI] [PubMed] [Google Scholar]
- Taguchi O, Kubin L, Pack AI. Evocation of postural atonia and respiratory depression by pontine carbachol in the decerebrate rat. Brain research. 1992;595:107–15. doi: 10.1016/0006-8993(92)91458-q. [DOI] [PubMed] [Google Scholar]