To The Editor
Adenosine deaminase (ADA) mutations can lead to severe combined immunodeficiency (SCID), profound T-, B- and NK-cell lymphopenia and the impairment of cellular and humoral immunity. Without intervention, patients with early-onset ADA-SCID can die of infection and failure to thrive within the first two years of life. Treatment options include bone marrow transplant (BMT), enzyme replacement therapy with pegylated ADA (PEG-ADA), and gene therapy (GT).1
Elevated IgE has been well described in delayed-onset ADA-SCID, but literature on similar phenotypes in early-onset ADA-SCID is limited, with little description of clinical allergy.2 We therefore decided to characterize the clinical and cellular allergic phenotypes in a cross-sectional cohort of 18 patients treated for early-onset ADA-SCID with BMT, GT, and/or PEG-ADA (Table I, E1).
Table I.
Patient characteristics of atopic and non-atopic groups.
| Overall | Atopic | Non-Atopic | Normal range | p value (significance) | |
|---|---|---|---|---|---|
| Number | 18 | 10 | 8 | ||
| Age (years) | 8 (2-31) | 13 (6-31) | 6 (2-13) | 0.03 (*) | |
| Age at diagnosis of ADA-SCID (months) | 5 (0-60) | 6 (2-60) | 2 (0-18) | 0.07 (ns) | |
| CD3 (cells/μl) | 305 (23-2455) | 261 (23-2455) | 368 (116-1047) | 714-2266 cells/μl | 0.46 (ns) |
| % CD4+ | 55.5 (6-83) | 56 (10-83) | 52 (6-64) | 27-59 | 0.93 (ns) |
| % CD8+ | 34 (4-86) | 28 (4-86) | 38 (22-86) | 13-33 | 0.51 (ns) |
| % CD4-CD8− | 6.5 (1-29) | 5 (1-21) | 9 (3-29) | 1.3-9.2 | 0.31 (ns) |
| CD4:CD8 ratio | 1.77 (0.07-5.29) | 2.08 (0.12-5.29) | 1.29 (0.07-2.88) | 1.11-5.17 | 0.57 (ns) |
| % HLA-DR+ | 42 (10-81) | 47 (22-81) | 34 (10-67) | 0-19.6 | 0.13 (ns) |
| IgE (IU/ml) | 230 (0-2808) | 879 (42-2808) | 28 (0-612) | 0-90 | 0.0009 (***) |
| Absolute eosinophil count (cells/μl) | 202 (10-1840) | 160 (60-1840) | 265 (10-1030) | 30-470 | 0.56 (ns) |
| Clinical Allergy | |||||
| Allergic rhinitis | 50.0% | 80.0% | 25% | ||
| Asthma | 22.2% | 40.0% | 0.0% | ||
| Atopic dermatitis | 11.1% | 10.0% | 12.5% | ||
| Food allergy | 11.1% | 20.0% | 0.0% | ||
| Urticaria | 11.1% | 20.0% | 0.0% | ||
| Number (%) with ≥1 positive allergen skin prick test | 6 of 9 (66.7%) | 6 of 7 (85.7%) | 0 of 2 (0%) | ||
| Number (%) with ≥1 positive allergen specific IgE | 7 of 13 (53.8%) | 7 of 8 (87.5%) | 0 of 5 (0%) | ||
| Current PEG-ADA use | 12 | 9 | 3 | ||
| PEG-ADA alone (No prior BMT or GT) | 2 | 1 | 1 | ||
| Post BMT, on PEG-ADA | 5 | 3 | 2 | ||
| Post GT, on PEG-ADA | 5 | 5 | 0 | ||
| No current PEG-ADA use | 6 | 1 | 5 | ||
| Post BMT, off PEG-ADA | 1 | 0 | 1 | ||
| Post GT, off PEG-ADA | 5 | 1 | 4 |
Age, lymphocyte subsets, IgE, and eosinophil count represent median (range) values. P values <0.05 are considered significant; ns=non-significant
Allergic histories, allergen skin prick testing (SPT), total and allergen-specific serum IgE (sIgE), and peripheral absolute eosinophil count (AEC) were measured in a subset of patients consenting to participation and where clinically feasible (Please see Methods and Table E1 in this article's Online Repository at www.jacionline.org). Atopy was defined as the presence of 1) clinical allergy (history of allergic rhinitis, food allergy, asthma, atopic dermatitis, or urticaria) and (2) elevated total serum IgE or evidence of allergic sensitization (positive allergen sIgE or positive SPT). Overall, atopy was present in 56% of patients (Table I). Not all laboratory measurements of allergic sensitization were completed in 9 of the 18 patients, two of whom had clinical symptoms consistent with allergy, thus this may represent an underestimate of the atopic prevalence in this population. The most common atopic manifestations were allergic rhinitis (50%) and asthma (22.2%), followed by food allergy (11.1%), mild atopic dermatitis (11.1%) and urticaria (11.1%); interestingly, severe atopic dermatitis was not a common feature. For further information regarding SPT and sIgE results, see Table E1.
Median serum IgE was 879 IU/ml in the atopic patients and 28 IU/ml in the non-atopic patients (normal 0-90 IU/ml) (Table I) while the median AEC was within the normal range in both groups. The degree of lymphopenia did not differ between the groups. Patients had a marked increase in CD3+HLA-DR+ T cells which was even greater in atopic patients (47% vs, 34%), suggesting a high degree of immune activation. Atopic patients were significantly older (median 13 years) than those without atopy (median 6 years) (Table I).
T-helper 2 (Th2) cytokines IL-4, IL-5, and IL-13, are critical for the development of IgE-mediated hypersensitivity and eosinophilic inflammation.3 By intracellular cytokine staining, significantly higher percentages of ex vivo central memory and effector memory CD4+ cells produced IL-4 and IL-13 in ADA-SCID patients compared to healthy donors (Figure 1A, E1A), with no difference seen in the percent IFN-γ+ cells, (Figure E1C). No statistically significant differences were found in Th2-cytokine producing cells between the atopic and non-atopic groups (Figure 1B, E1B).
Figure 1.
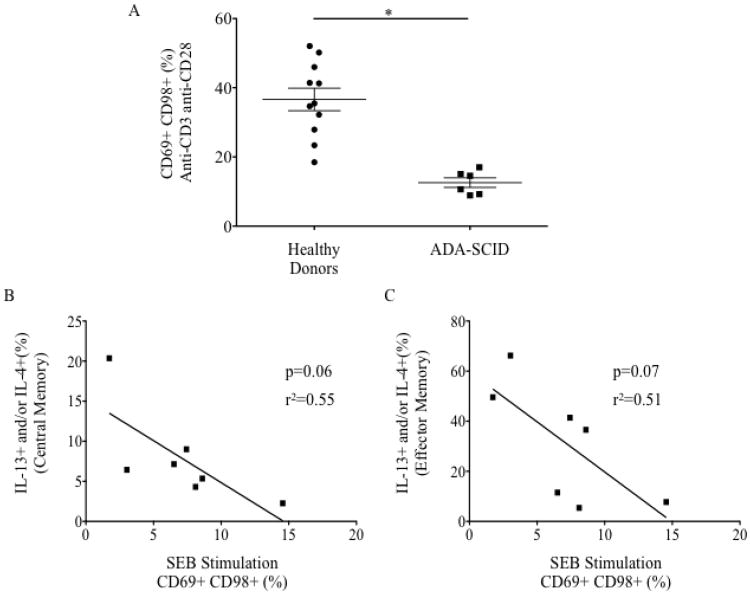
Percentage IL-4+ or IL-13+ central memory CD4+ cells from (A) healthy donors (n=16) versus ADA-SCID (n=12) (B) atopic (n=7) versus non-atopic (n=5) ADA-SCID patients. (C) Percentage CD69+CD98+CD4+ cells following stimulation with staphylococcal enterotoxin B (SEB) in healthy donors (n=14) versus ADA-SCID (n=7). ns non-significant, *p<0.05, **p<0.01, ***p<0.001
One previous study attributed the Th2 phenotype in a patient with ADA-SCID who had undergone GT to Th2 cytokines produced by transduced CD8+ cells.4 Only tbree of twelve patients in our cohort had increased percentage of IL4+ or IL-13+ CD8+ cells compared to healthy donors (Figure E1D), only one of whom had undergone GT, making it unlikely that the observed Th2 cytokine production by CD8+ T cells is a direct effect of transduction.
Eight of the nine atopic patients in our cohort were currently being treated with PEG-ADA. However, PEG-ADA treated patients were also older and most had undergone prior BMT or GT making it difficult to establish a direct link between PEG-ADA and atopy (Table I).
While defects in regulatory T cell (Treg) number and function are associated with a variety of allergic and autoimmune diseases , we found a similar percentage of CD4+CD45RO+FoxP3+ Tregs in our cohort as a whole compared to healthy controls (Figure E2A). Patients who were atopic paradoxically had a higher percentage of Tregs compared to non-atopic patients (Figure E2B). Aberrant cytokine production by Tregs, a hallmark of Treg dysfunction, was not seen in atopic or non-atopic ADA-SCID patients (Figure E2C), however it is still possible that functional suppression is impaired.
Weak TCR signaling is associated with Th2 differentiation and atopy 5, and evidence exists for abnormal TCR signaling in untreated ADA-SCID in cell lines and in murine models.6, 7 Short-term PBMC activation was markedly impaired in ADA-SCID patients (Figure 1C, E3A) but did not correlate with atopic status. There was a trend toward an inverse correlation between activation to staphylococcal enterotoxin B (SEB) and Th2 cytokine production (Figure E3B-C). This dysfunction could be due to intrinsic signaling defects but also could reflect in vitro anergy due to chronic in vivo T-cell activation.
Patients with early-onset ADA-SCID have a high incidence of atopy. They have elevated IgE, manifest clinical symptoms, particularly allergic rhinitis and asthma, and even in the absence of clinical allergy, have increased CD4+ Th2 cytokine production. Because non-atopic patients were much younger in our cohort, it is quite likely that many of them will go on to develop allergic disease as well. Clinicians caring for patients with both early-and delayed-onset ADA-SCID should be attuned to this allergic predisposition and consider referral for allergic evaluation and appropriate pharmacologic treatment in addition to managing the infectious phenotypes.
While complete abrogation of T-cell function usually leads to immunodeficiency, hypomorphic T-cell function can lead to autoimmune disease or allergy, with Omenn syndrome (OS) as an extreme example.2, 8, 9 We propose that patients with early onset ADA-SCID shift from a virtually complete lack of T cell mediated immune response prior to treatment, to an intermediate level of T cell function following treatment with BMT, PEG-ADA or GT which allows for the development of allergy with a phenotype less severe than OS.
This study represents a description of the cellular and clinical allergic phenotypes of patients treated for ADA-SCID. However, longitudinal assessments of T cell differentiation, cytokine production, and clinical atopy of ADA-SCID patients at diagnosis, and during treatment, are necessary to explain the correlation between T cell function, development of a Th2 milieu and the manifestation of allergic disease.
Supplementary Material
Acknowledgments
We thank the patients and their families for their commitment to clinical research, and the members of the Milner lab for their helpful discussions.
Funding: This project has been funded in whole or in part with federal funds from the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases (NIAID) and the Division of Intramural Research of the National Human Genome Research Institute (NHGRI).
3This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
3This research was supported [in part] by the National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gaspar HB, Aiuti A, Porta F, Candotti F, Hershfield MS, Notarangelo LD. How I treat ADA deficiency. Blood. 2009 Oct 22;114(17):3524–32. doi: 10.1182/blood-2009-06-189209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felgentreff K, Perez-Becker R, Speckmann C, Schwarz K, Kalwak K, Markelj G, et al. Clinical and immunological manifestations of patients with atypical severe combined immunodeficiency. Clin Immunol. 2011 Oct;141(1):73–82. doi: 10.1016/j.clim.2011.05.007. Epub 2011/06/15. eng. [DOI] [PubMed] [Google Scholar]
- 3.Romagnani S. The role of lymphocytes in allergic disease. The Journal of allergy and clinical immunology. 2000 Mar;105(3):399–408. doi: 10.1067/mai.2000.104575. [DOI] [PubMed] [Google Scholar]
- 4.Kawamura N, Ariga T, Ohtsu M, Yamada M, Tame A, Furuta H, et al. Elevation of serum IgE level and peripheral eosinophil count during T lymphocyte-directed gene therapy for ADA deficiency: implication of Tc2-like cells after gene transduction procedure. Immunology letters. 1998 Nov;64(1):49–53. doi: 10.1016/s0165-2478(98)00083-2. Epub 1998/12/29. eng. [DOI] [PubMed] [Google Scholar]
- 5.Yamane H, Paul WE. Early signaling events that underlie fate decisions of naive CD4(+) T cells toward distinct T-helper cell subsets. Immunological reviews. 2013 Mar;252(1):12–23. doi: 10.1111/imr.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apasov SG, Blackburn MR, Kellems RE, Smith PT, Sitkovsky MV. Adenosine deaminase deficiency increases thymic apoptosis and causes defective T cell receptor signaling. The Journal of clinical investigation. 2001 Jul;108(1):131–41. doi: 10.1172/JCI10360. Epub 2001/07/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassani B, Mirolo M, Cattaneo F, Benninghoff U, Hershfield M, Carlucci F, et al. Altered intracellular and extracellular signaling leads to impaired T-cell functions in ADA-SCID patients. Blood. 2008 Apr 15;111(8):4209–19. doi: 10.1182/blood-2007-05-092429. Epub 2008/01/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Datta S, Milner JD. Altered T-cell receptor signaling in the pathogenesis of allergic disease. The Journal of allergy and clinical immunology. 2011 Feb;127(2):351–4. doi: 10.1016/j.jaci.2010.11.033. Epub 2011/02/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avila EM, Uzel G, Hsu A, Milner JD, Turner ML, Pittaluga S, et al. Highly variable clinical phenotypes of hypomorphic RAG1 mutations. Pediatrics. 2010 Nov;126(5):e1248–52. doi: 10.1542/peds.2009-3171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.