Abstract
Hepatic innate immune cells, in particular interstitial dendritic cells (DC), regulate inflammatory responses and may promote inherent liver tolerogenicity. Following tissue injury, adenosine triphosphate (ATP) is released and acts as a damage-associated molecular pattern that activates innate immune cells via pattern recognition receptors. CD39 (ectonucleoside triphosphate diphosphohydrolase-1) rapidly hydrolyzes extracellular ATP to maintain physiological levels. We hypothesized that CD39 expression on liver DC might contribute to regulation of their innate immune functions. Mouse liver conventional myeloid (m) DC were hyporesponsive to ATP compared with their splenic counterparts. This disparity was ascribed to more efficient hydrolysis of ATP by higher expression of CD39 on liver mDC. Human liver mDC expressed greater levels of CD39 than those from peripheral blood. The comparatively high expression of CD39 on liver mDC correlated strongly with both ATP hydrolysis and adenosine production. Notably, CD39-/- mouse liver mDC exhibited a more mature phenotype, greater responsiveness to Toll-like receptor 4 ligation, and stronger pro-inflammatory and immunostimulatory activity than wild-type (WT) liver mDC. To investigate the role of CD39 on liver mDC in vivo, we performed orthotopic liver transplantation with extended cold preservation using CD39-/- or WT donor mouse livers. Compared with WT liver grafts, CD39-/- grafts exhibited enhanced interstitial DC activation, elevated proinflammatory cytokine levels, and more severe tissue injury. Moreover, portal venous delivery of WT but not CD39-/- liver mDC to donor livers immediately post-transplant exerted a protective effect against graft injury in CD39-/- to CD39-/- liver transplantation. These data reveal that CD39 expression on conventional liver mDC limits their pro-inflammatory activity and confers protective properties on these important innate immune cells against liver transplant ischemia/reperfusion injury.
Keywords: ectonucleotidase activity, ATP hydrolysis, lipopolysaccharide, T cells
Introduction
The liver is regarded as a tolerogenic environment.1-3 Interstitial antigen (Ag)-presenting cells (APC) in the liver, in particular bone marrow-derived dendritic cells (DC), appear refractory to stimulation with microbe or danger-associated molecular patterns (MAMPs or DAMPs) compared with their counterparts in blood and secondary lymphoid tissues. There is also evidence that liver DC play important roles in regulation of hepatic injury4-6 and innate and adaptive immunity.3 Several mechanisms may contribute to negative regulation of liver DC maturation and their ability to suppress hepatic inflammation and immunity.3, 4, 7
Adenosine triphosphate (ATP) is an essential metabolic energy source in biological systems.8 Cells undergoing apoptosis or necrosis release ATP, which acts as a DAMP, with proinflammatory/immunostimulatory capacity. Thus, ATP can activate various immune cells, including DC.9, 10 ATP also recruits monocytes and neutrophils.11 The extracellular ATP concentration is strictly maintained by CD39, a member of the ecto-nucleoside triphosphate diphosphophydrolase (E-NTPDase) family that hydrolyzes ATP into AMP. The latter is degraded to adenosine, a potent anti-inflammatory molecule, via ecto-5’-nucleotidase (CD73),- another ecto-nucleotidase.12 CD39 is expressed on regulatory T cells (Treg) and its hydrolysis of ATP and production of adenosine are considered mechanisms of immune regulation by Treg.13 While expression of CD39 by epidermal Langerhans cells and blood monocyte-derived DC has also been reported and implicated in T cell activation,14, 15 its expression and function on other tissue-resident DC, in particular liver DC, has not been investigated.
Hepatic ischemia-reperfusion injury (IRI) remains an important clinical problem.16, 17 In transplantation, its significance is enhanced by the increased use of extended criteria donor organs. Oxygen deprivation induces death of hepatocytes, that release various DAMPs, such as high mobility group box B1 (HMGB-1), self-DNA, self-SNA and ATP. DAMPs stimulate innate immune mechanisms through cell-associated pattern recognition receptors, that include Toll-like receptors (TLRs), HMGB-1-like receptors, C-type lectin receptors, and nucleotide-binding domain leucine-rich repeats18 expressed on innate immune cells. Triggering of DC via these receptors induces their activation/maturation.19 DC have been implicated in the regulation of inflammation and tissue injury after liver IR,4, 20-22 with both inhibitory and enhancing effects being reported. While there is evidence for a protective role of CD39 in total hepatic warm ischemia23 and liver cold IRI24 based on studies using CD39-/- mice and CD39 overexpressing mice respectively, cold IRI is more clinically-relevant for assessing tissue injury during liver transplantation. Here, we examined the expression and function of CD39 on liver conventional mDC in vitro and using a cold liver IRI model in vivo. Our novel findings suggest that expression of CD39 on liver mDC attenuates their proinflammatory activity and exerts a protective affect against extended cold liver preservation injury.
Materials and Methods
Mice
Male C57BL/6 (B6;H-2b) and BALB/c (H-2d) mice (8- to 12-wk old) were purchased from The Jackson Laboratory, Bar Harbor, ME. CD39-/- mice (B6 background), were bred from pairs received from the Beth Israel Medical Center, Harvard University, Boston, MA. Animals were maintained in the specific pathogen-free Central Animal Facility of the University of Pittsburgh School of Medicine. Experiments were conducted under an institutional Animal Care and Use Committee-approved protocol and in accordance with criteria outlined in the National Institutes of Health “Guide for the Care and Use of Laboratory Animals”. Mice were fed a diet of Purina rodent chow (Ralston Purina, St. Louis, MO) and received tap water ad libitum.
Reagents
ATP was purchased from Sigma (St. Louis, MO) and E. coli lipopolysaccharide (LPS) was from InvivoGen (San Diego, CA).
Isolation of Mouse Liver, Spleen and Other Tissue DC
DC were isolated and purified as described.7, 25 Thus, livers, kidneys, and spleens were harvested from mice given recombinant human fms-like tyrosine kinase 3 ligand (Flt3L) (Amgen;10μg/day i.p.; 10 days) and digested in collagenase (Sigma). Plasmacytoid (p)DC were positively selected from the DC-enriched fraction using plasmacytoid DC Ag (PDCA)-1 immunomagnetic microbeads (Miltenyi Biotec, Auburn, CA), as described.26 Conventional myeloid (m)DC (CD11b+CD11c+NK1.1-mPDCA-1-) were isolated from the pDC-depleted, DC-enriched fraction using anti-CD11c microbeads (Miltenyi).7
Isolation of Human Liver and Blood DC
Human liver non-parenchymal cells were obtained from histologically normal surgical resection liver tissue as a by-product of hepatocyte isolation using a three-step collagenase perfusion technique27 and density gradient centrifugation. Liver and circulating mDC were isolated using human BDCA-1+(CD1+) DC isolation kits (Miltenyi).
Flow Cytometry
Mouse cell surface molecule and intracellular cytokine and FoxP3 staining was performed as described.26 Details of the mAbs used are in the Supplementary Methods. Human DC were also stained as described28 with the additional use of anti-human CD39 PE (eBioA1; eBioscience). Flow cytometric analysis was performed using an LSR II flow cytometer (BD Biosciences) and data were analyzed using FlowJo 7.6 (Tree Star, Ashland, OR).
T Cell Purification
Bulk T cells from spleens of BALB/c mice were incubated with a mAb cocktail consisting of anti-CD45R/B220 (RA3-6B2), anti-CD16/CD32 (2.4G2), anti-TER-119, anti-CD11b (M1/70), and anti-Ly6G (RB-8C5) (BD PharMingen, San Diego, CA) and non-T cells eliminated by negative selection using Dynabeads (InvitroGen, Grand Island, NY). Methods use to purify Treg and assess their function are in the Supplementary Methods.
Mixed Leukocyte Reaction (MLR)
Unstimulated or ATP-conditioned B6 DC were used as stimulators of bulk normal allogeneic BALB/c T cells (2×105/well) in 72 hr MLR as described.7
Cytokine Measurements
Cytokine levels were determined by cytometric bead array (BD Bioscience) (IL-6, tumor necrosis factor [TNF]α and monocyte chemotactic protein [MCP]-1) or ELISA (IL-12p40) (BioLegend).
Real-Time Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated and mRNA expression quantified as described7 by Fast SYBR Green real-time RT-PCR with an ABI-Prism 7000 Fast Sequence Detection System (Applied Biosystems, Carlsbad, CA) and with appropriate primers (all from Invitrogen) in triplicate. Primer sequences are provided in the Supplementary Methods. The expression of each gene was normalized to β-actin mRNA content and calculated with respect to normal liver tissue.
ATP Hydrolysis Assay
DC (1×105) were incubated with ATP (100μM) and supernatants were collected at multiple time points (0, 30, 60 and 90 min; 2 and 3 hr). ATP concentration was determined by luminescence assay (ATPlite, PerkinElmer) and the data expressed as the frequency of luminescent events (counts per second; cps). Adenosine concentrations were measured by mass spectrometric analysis.29
Liver Transplantation IRI
Orthotopic liver transplantation was performed as described30 with minor modifications.31 Liver grafts (B6) were perfused with 5 ml University of Wisconsin (UW) solution via the inferior vena cava, stored in UW solution for 24 hr at 4°C, then transplanted into normal wild-type (WT) B6 or CD39-/- B6 recipients.
Adoptive Transfer of DC to Liver Grafts
Purified liver mDC (3×106) syngeneic with the (B6) liver graft were infused intraportally in 50μl PBS using a 35G needle, immediately after graft implantation.
Assessment of Liver Injury
Liver enzymes (AST/ALT) were quantified in serum as described,31 and graft histopathology assessed on H&E-stained paraffin sections in a ‘blinded’ fashion. Areas of necrosis were quantified and Suzuki scores32 determined.
Statistical Analyses
Statistical significance was ascertained by unpaired Student’s ‘t’ test using Prism version 5.00 (Graphpad Software, San Diego, CA). p<0.05 was considered significant.
Results
Mouse Liver Conventional mDC are Less Responsive to ATP than Lymphoid Tissue mDC and Undergo Less ATP-Induced Maturation
To address its influence on liver and spleen conventional mDC purified from normal B6 mice, we stimulated freshly-isolated cells with ATP overnight. As shown in Fig. 1A, expression of MHC II, CD80, CD86 and B7-H1 increased significantly on a subpopulation of spleen mDC after ATP stimulation. Under identical culture conditions, the influence of ATP on liver mDC was minimal, and the relative expression of these molecules after ATP stimulation (compared with unstimulated cells) was significantly less on liver mDC compared with spleen mDC (Fig 1B). The extent of activation of the spleen and liver DC subpopulation by ATP was dose-dependent (Suppl Fig. 1A). Next, to test the functional maturation of the DC, we set up MLR, using ATP-stimulated B6 (H-2b) DC as stimulators and normal BALB/c (H-2d) bulk CD3+ T cells as responders. As shown in Fig. 1C, although both ATP-stimulated spleen and liver DC acquired increased ability to induce T cell proliferation, the influence of ATP on spleen DC was significantly greater (Fig. 1D). This was in keeping with the ability of ATP to enhance T cell costimulatory molecule expression on these APC (Fig. 1B). Moreover, while both spleen and liver DC secreted greater levels of proinflammatory cytokines after ATP stimulation, splenic DC produced greater amounts of IL-1β, IL-6 and IL-12p40 (Fig 1E). Taken together, these findings indicate that liver mDC are comparatively resistant to ATP stimulation.
Fig. 1.
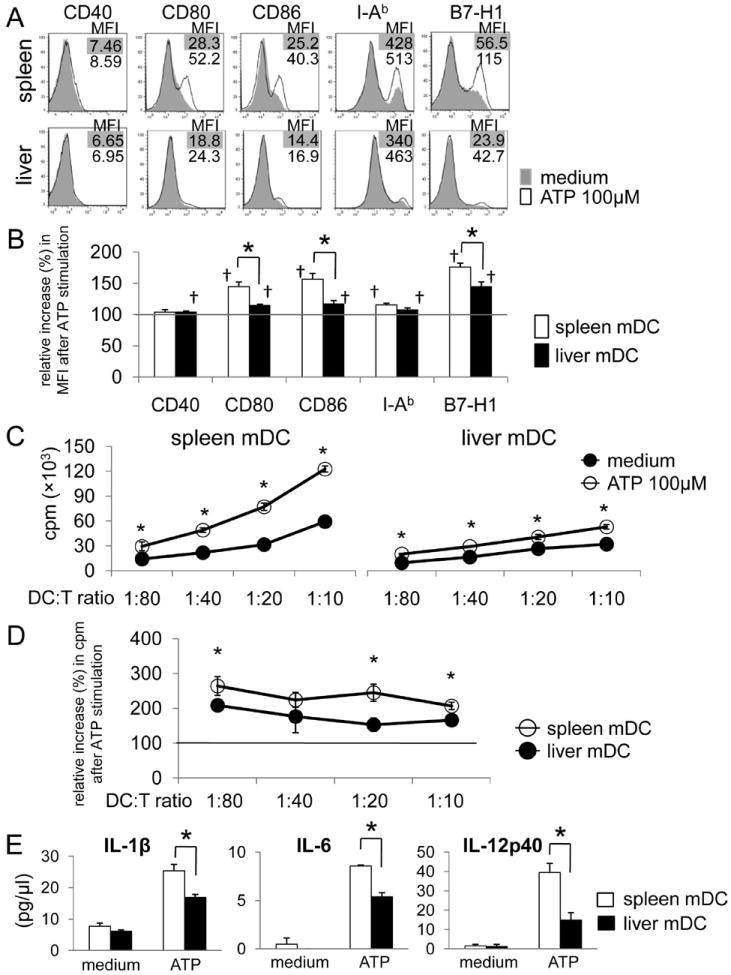
Mouse (B6) conventional liver myeloid (m) DC are hyporesponsiveness to ATP stimulation. Freshly-isolated spleen or liver mDC were stimulated with ATP for 18 hr. (A) Cell surface expression (MFI) of CD40, CD80, CD86, MHC class II (I-Ab) and B7-H1 was measured by flow cytometry. The relative increase in MFI from baseline (without ATP) for each molecule across multiple experiments (n=5) is shown in (B). †, p<0.05 compared with unstimulated condition; *, p<0.05 comparing spleen and liver mDC. (C) Mouse (B6) spleen or liver mDC were pre-stimulated with ATP for 3h, then co-cultured with normal allogeneic BALB/c (I-Ad) splenic T cells for 72 hr. Radioisotope (3H) incorporation during the final 18 hr of culture was determined using a beta scintillation counter. *, p<0.05. (D) Relative increase in cpm from baseline (without ATP stimulation).*, p<0.05. (E) Liver mDC secreted lower levels of pro-inflammatory cytokines in response to ATP stimulation than spleen mDC (n = 3 experiments). *, p<0.05
Mouse Liver mDC Express P2X7 and P2Y14 and Higher Levels of CD39 Compared with DC from Other Tissues
To explore the basis of ATP resistance of liver mDC, we examined expression of extracellular nucleotide plasma membrane P2 purinergic receptors for ATP on freshly-isolated cells by RT-PCR. While liver mDC expressed several P2 receptors at the mRNA level, P2X7 and P2Y14 were the most highly expressed (Suppl Fig. 1B). We confirmed the expression of P2X7 and P2Y14 on liver mDC by flow cytometry. Expression of both P2X7 (especially) and P2Y14 was enhanced after 18hr ATP stimulation. Mature liver mDC (CD86hi), however, did not express P2X7 or P2Y14 (Suppl Fig. 1C), suggesting that these receptors were negatively regulated upon cell activation. Expression of P2X733, 34 was similar on freshly-isolated mDC from spleen, bone marrow, blood, liver and kidney (Fig. 2A). Since CD39 is the key molecule that hydrolyzes ATP and regulates ATP concentration,34 we considered that the resistance of liver DC to ATP might be due to ATP hydrolysis by cell surface-expressed CD39. However, while > 95% of mDC from each tissue expressed CD39 (data not shown), liver mDC displayed significantly higher levels (MFI) than mDC from lymphoid and other non-lymphoid tissues, including kidney mDC (Fig. 2B). CD39 was not detected on mouse hepatocytes (Fig. 2C). Interestingly, liver mDC, but not liver plasmacytoid (p) DC (that represent a comparatively high proportion of liver DC compared with spleen DC35) expressed greater levels of CD39 than other liver and spleen innate and adaptive immune cells (Fig. 2D). Liver mDC also expressed CD73 (Fig. 2E, F) that contributes to adenosine generation.
Fig. 2.
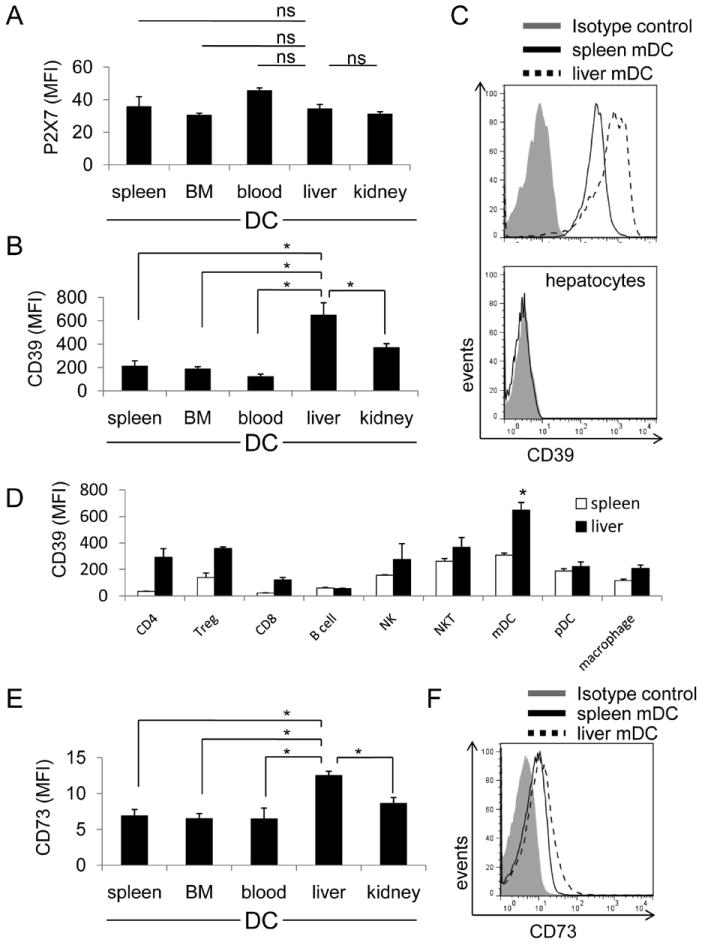
Mouse (B6) liver mDC express the highest level of cell surface CD39 among DCs from selected non-lymphoid and lymphoid tissues. Expression of CD39 and its principal receptor P2X7, on freshly-isolated liver, spleen, peripheral blood, bone marrow and kidney mDC, as well as hepatocytes was examined by flow cytometry. (A) Expression of P2X7 on each DC population determined by flow cytometry (n=4 independent experiments). (B) Intensity (MFI) of CD39 expression on each DC population; n=4 independent experiments. (C) Histogram showing representative data comparing CD39 expression on spleen and liver mDC and the absence of CD39 on hepatocytes. Bars indicate means + 1SD (n=4 independent experiments). ns = not significant. *; p<0.05. (D) CD39 expression on liver and spleen leukocytes; n=6 independent experiments.*; significantly higher than all other liver and spleen cell populations. (E) Expression of CD73 on DC isolated from various tissues; n=4 independent experiments. (F) Representative data (n=4 experiments) of CD73 expression on spleen and liver mDC.
CD39 on Liver DC Enhances ATP Hydrolysis and Adenosine Production
Freshly-isolated DC were cultured in ATP-containing medium and ATP concentration determined at various times by luminescence assay. As shown in Fig. 3, the ATP concentration decreased progressively (approx 80%) over 120 min in the presence of liver mDC from WT B6 mice. Initially (first 30 min), liver and spleen mDC from WT mice hydrolyzed ATP at similar rates, but only liver mDC continued to reduce ATP levels over the ensuing 120 min (Fig. 3A). By contrast, an equivalent number of DC from CD39-/- mice failed to hydrolyze ATP. As expected, the extent of ATP hydrolysis mediated by liver versus splenic mDC was consistent with their different levels of CD39 expression (Fig. 2 B,C). However, the expression levels of other ectoenzymes,- CD39L1 and CD39L3, was similar on spleen and liver mDC (Fig. 3C). ATP stimulation (120 min) did not alter CD39 expression on spleen or liver mDC (Fig. 3C). Production of adenosine (Fig. 3B) also correlated with the differential levels of CD39 and CD73 expression on liver and spleen mDC (Fig. 2E, F). These data indicate that the superior ability of liver mDC to hydrolyze ATP results from their comparatively high CD39 expression. To confirm the processing of ATP by CD39 on liver mDC, we pre-cultured WT or CD39-/- liver mDC in ATP-containing medium for 3 hr, then applied the cell-free culture supernatant to WT liver mDC for 18 hr, together with LPS stimulation. As expected, the medium from WT compared with CD39-/- DC cultured with ATP induced less IAb, costimulatory molecule and B7-H1 expression (Fig. 3D).
Fig. 3.
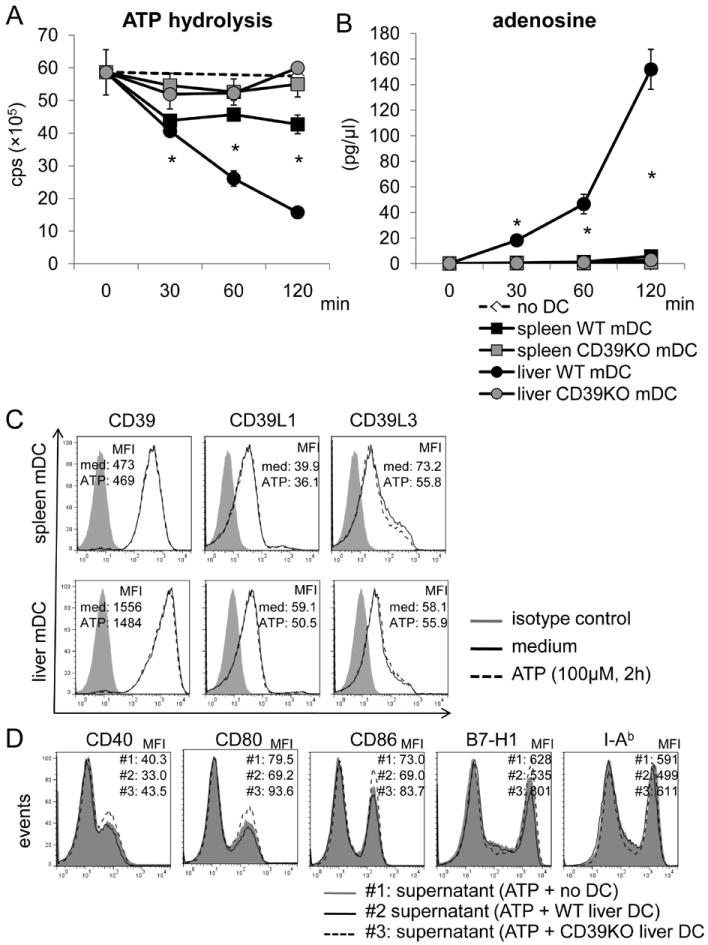
WT, but not CD39-/- B6 mouse liver mDC, hydrolyze ATP to a greater extent than WT spleen DC. WT or CD39-/- liver or spleen mDC (1×105) were cultured in ATP-containing medium (100nM) for the specified time. Supernatants were collected at each time point. (A) ATP concentration was determined by luminescence assay; cps=counts per second. (B) Adenosine concentration was measured by mass spectrometric analysis. n=3 experiments. *, p<0.05 comparing spleen and liver WT mDC. (C) Expression of CD39, CD39L1 and CD39L3 was determined by flow cytometry on liver and spleen mDC with or without ATP stimulation (100 μM) for 120 min. (D) WT or CD39-/- liver DC were cultured in ATP-containing medium for 3 hr. The cell-free culture medium was then transferred to WT liver mDC cultures that were stimulated with LPS for 18 hr. Liver mDC phenotype was determined by flow cytometry. Data in C and D are representative of 2 independent experiments.
Human Liver mDC Also Express Comparatively High Levels of CD39 and Hydrolyze ATP Faster Than Blood-Borne mDC
We assessed CD39 expression on liver DC freshly-isolated from histologically normal surgical resection tissue. Human liver and circulating mDC were gated on CD45+, lineage (CD3,CD14,CD19,CD20)-, BDCA-1+ cells, as described.28, 36 Similarly to mice (Fig. 2B), human liver mDC expressed significantly higher levels of CD39 than blood-borne mDC (Fig. 4A,B) and hydrolyzed ATP faster and produced more adenosine than circulating mDC (Fig. 4 C,D).
Fig. 4.
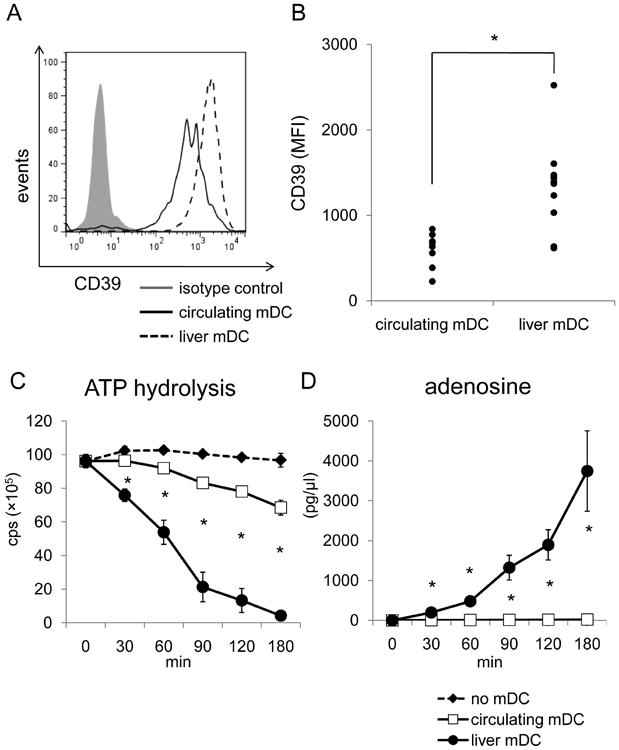
Freshly-isolated human liver mDC express higher levels of CD39 and hydrolyze ATP faster than circulating mDC. (A) The expression of CD39 by 7 individual human purified liver and peripheral blood mDC preparations was measured by flow cytometry, as described in the Materials and Methods. Liver and circulating mDC were defined by gating on CD45+, lineage (CD3, CD14, CD19, CD20)-, BDCA-1+cells. (B) Intensity (MFI) of CD39 expression was compared between human liver interstitial and blood-borne mDC. Each dot represents one individual (n=8 for circulating DC; n=10 for liver mDC). *, p<0.05. (C) Human liver mDC and circulating mDC were also purified using immunomagnetic beads and ATP hydrolysis assay conducted. (D) Adenosine concentrations were measured at each time point by mass spectrometric analysis. *, p<0.05 comparing circulating and liver mDC.
CD39 Regulates Liver mDC Responses to ATP Following TLR4 Ligation
We next tested the responses of liver mDC from WT or CD39-/- B6 mice to ATP, in the absence or presence of the TLR4 ligand LPS,- a MAMP to which liver-resident APC are exposed continually under steady-state conditions. LPS stimulation and combined ATP + LPS stimulation, modestly upregulated MHC II and co-regulatory molecule expression on liver mDC from WT and especially those from CD39-/- mice (Fig. 5A). Moreover, CD39-/- liver mDC secreted significantly greater quantities of pro-inflammatory cytokines in response to LPS ± ATP stimulation compared with WT liver DC (Fig. 5B). CD39-/- liver mDC also exhibited stronger naive T cell allostimulatory ability and induced more IFNγ+CD8+ T cells in MLR (Fig. 5C, D). These data suggest that CD39 contributes to the immune regulatory function of liver mDC.
Fig. 5.
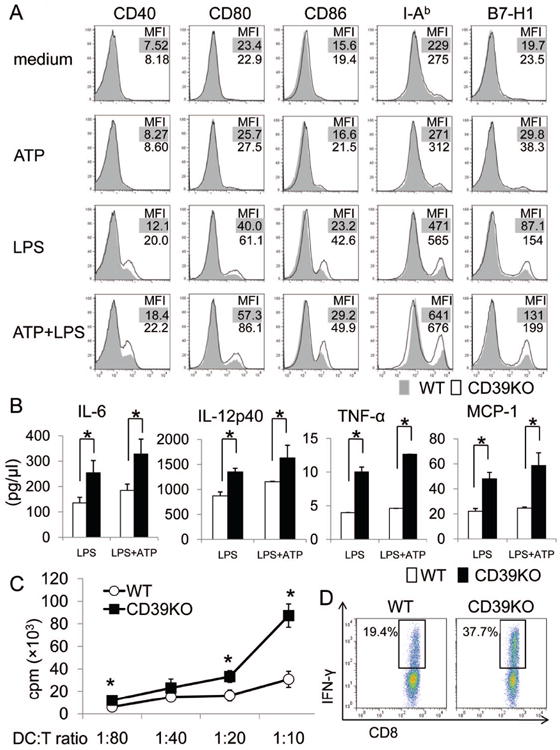
CD39 regulates the proinflammatory phenotype of liver mDC. Liver mDC were isolated from WT or CD39-/- B6 mice. (A) Expression of CD40, CD80, CD86, MHC class II (IAb) and B7-H1 on liver mDC, with or without ATP ± LPS stimulation for 18 hr (ATP: 100nM; LPS: 1μg/ml) was determined by flow cytometry. (B) Pro-inflammatory cytokine concentrations in culture medium were measured using cytometric bead array (IL-6, TNF-α and MCP-1) or by ELISA (IL-12p40). (C, D) WT or CD39-/- liver DC were cultured with normal allogeneic (BALB/c) T cells for 72 hr. (C) Radioisotope (3H) incorporation during the last 18 hr of culture was measured by scintillation counter. n = 3 experiments.*; p<0.05. (D) Incidences of CD8+IFNγ+ T cells in MLR were determined by flow cytometry. Data are representative of 3 independent experiments.
Leukocyte Populations in CD39-/- Livers
Absolute numbers of liver mDC and all other liver and spleen leukocyte populations examined were preserved in CD39-/- mice (Suppl Table 1). There was also no significant difference between WT and CD39-/- CD4 and CD8 T cells in their expression of cell surface activation markers (Suppl Fig. 2A) or their proliferative capacity after anti-CD3/CD28 bead or allogeneic DC stimulation (Suppl Fig. 2B). However, compared with those from WT mice, splenic Treg from CD39-/- mice exhibited a reduced suppressive function on effector T cell proliferation (Suppl Fig. 2C).
CD39-/- Liver grafts Exhibit Enhanced Cold IRI that is attenuated by WT Liver mDC
To examine the in vivo functional significance of CD39 in liver transplant-associated cold IRI, CD39-/- or WT livers were transplanted into syngeneic (B6) WT recipients with 24 hr cold preservation, as described.37 CD39-/- liver grafts elicited significantly higher levels of serum ALT and AST than WT grafts after 6 hr reperfusion (Fig. 6A). Histological analysis confirmed more extensive areas of necrosis and elevated Suzuki scores in CD39-/- liver grafts (Fig. 6B, C). Circulating IL-6, IL-12p40, and TNFα levels were all significantly higher in mice with CD39-/- grafts (Fig. 6D), correlating with higher levels of production of these cytokines by CD39-/- liver mDC in vitro (Fig. 5B). Freshly-isolated mDC from CD39-/- grafts (6 hr post-transplant) expressed higher levels of cell surface maturation markers and lower levels of co-inhibitory B7-H1 (PD-L1) compared with DC from WT liver grafts (Fig. 6E). Moreover, increased levels of pro-inflammatory cytokines were observed in grafts from CD39-/- donors (Fig. 6F). These results suggest that, due to the absence of CD39, unhydrolyzed ATP activated liver mDC and exacerbated cold I/R injury. To verify a protective role of CD39 on liver mDC in vivo, we also examined cold IRI in CD39-/- recipients of CD39-/- liver grafts that received WT or CD39-/- liver mDC intraportally, immediately after liver implantation. In this experiment, only the adoptively-transferred WT liver mDC could serve as the source of CD39 for ATP hydrolysis. When 3×106 WT (CD39+/+) but not CD39-/- liver mDC were infused into the CD39-/- liver grafts, the extent of liver IRI was reduced significantly (Fig. 7). These data demonstrate that CD39 on liver mDC can protect against liver transplant I/R injury.
Fig. 6.
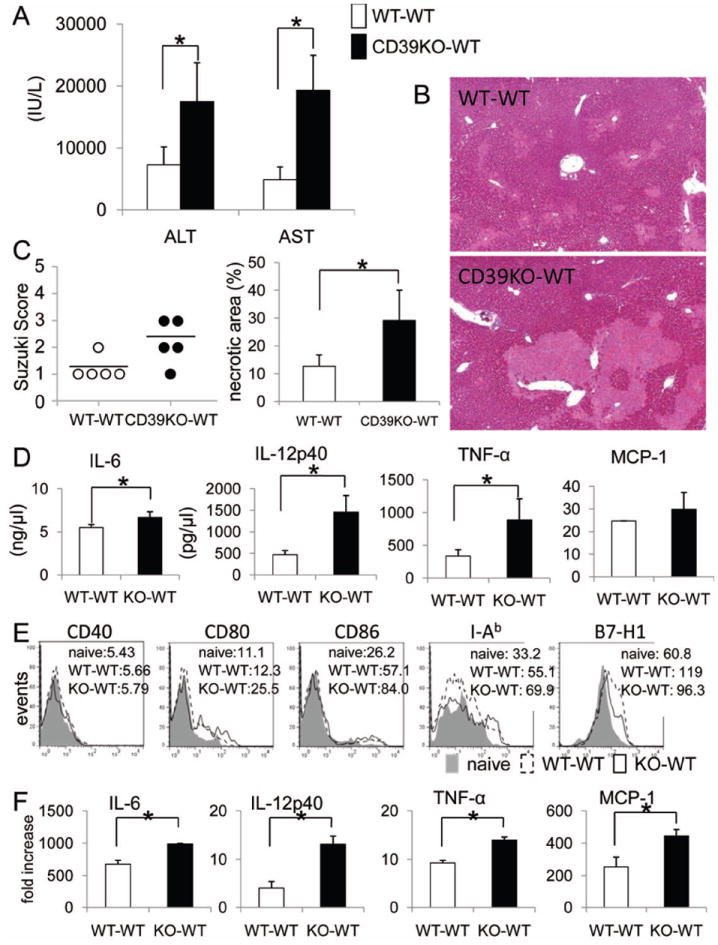
CD39 deficiency exacerbates cold liver IRI and the systemic inflammatory response associated with enhanced intragraft DC maturation after orthotopic liver transplantation (CD39KO → WT B6, versus WT B6 → WT B6) with 24 hr graft preservation. (A) Serum ALT and AST levels were measured 6 hr after liver transplantation. (B) Histological assessment of liver graft injury 6 hr after liver transplantation (hematoxylin and eosin staining; ×40). (C) Liver damage was assessed by Suzuki score (congestion, vacuolization and necrosis) and necrotic areas were quantified. (D) Systemic (serum) IL-6, TNF-α, and MCP-1 levels were measured by cytokine bead array and serum IL-12p40 levels by ELISA. (E) Liver graft mDC cell surface phenotype was determined by flow cytometry, 6 hr after liver transplantation. (F), Cytokine expression by liver graft mDC, 6 hr after liver transplantation. Liver DC were gated on CD45+CD11c+B220- cells. n=5 transplants per group. *, p<0.05
Fig. 7.
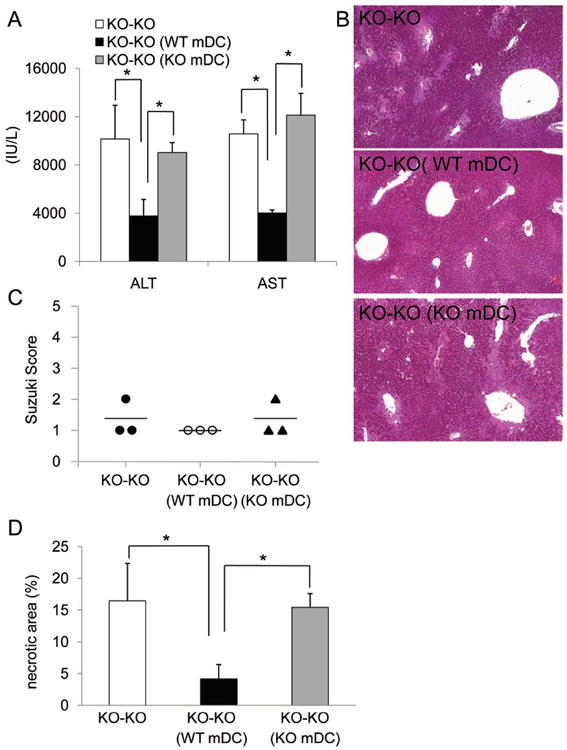
CD39 expression by liver mDC is important in regulation of extended cold IRI associated with mouse liver transplantation. Freshly-isolated WT or CD39KO liver mDC (3×106) were adoptively transferred via the portal vein immediately after orthotropic (CD39KO→CD39KO) liver transplantation. (A) Serum ALT and AST levels were measured 6 hr after liver transplantation. (B), Representative graft histology showing reduced necrotic damage in graft recipients given WT CD39+/+ liver mDC. (C), liver damage was assessed by Suzuki score and (D) quantitation of area of necrosis. n=3 transplants per group. *, p<0.05.
Discussion
Given their high rate of constitutive exposure to dietary antigens and MAMPs, it is important that liver DC maintain a tolerogenic state under normal physiological conditions in order to avoid inflammation.3 Several mechanisms have been proposed to restrain conventional liver mDC activation/maturation that may also contribute to their inherent tolerogenicity. These include expression of negative regulators of TLR signalling,7 and production of IL-10.4, 7 Here, we show for the first time, that resistance of mouse liver mDC to maturation induced by ATP is associated with significantly higher constitutive levels of CD39 on these cells compared with mDC from secondary lymphoid tissue or kidney. To what extent expression of CD39 in the cis or trans position might govern the responsiveness of the entire DC population in these tissues was not investigated. The higher levels of cell surface CD39 on liver mDC correlated with superior ability of these DC to hydrolyze ATP, a property that was absent from CD39-/- liver mDC. Our findings also show that the enhanced cold I/R injury and systemic inflammation observed following orthotopic liver transplantation from CD39-/- compared with WT donors is associated with increased activation/maturation of liver interstitial mDC. Moreover, WT but not CD39-/- liver mDC exerted a protective effect against transplant-induced liver IRI when adoptively transferred to CD39-/- liver grafts, implicating CD39 expression on liver mDC in the regulation of liver transplant IRI.
Innate immune cells, such as NK cells or DC, respond acutely in injury models by virtue of inherent cytotoxic properties and/or release of cytokines. Recently, deletion of CD39, the dominant ectonucleotidase on NK cells, has been shown to attenuate partial warm liver IRI,38 while adoptive cell transfer studies have supported a role of CD39 on NK cells in liver injury.38 Studies on NKT cells (that express both CD39 and CD73) have suggested a role for purinergic signaling in NKT cell-mediated mechanisms that result in liver immune injury.39 Activated NKT cells appear to be important in warm hepatic IRI,40 although less so in cold liver IRI.24 Thus it seems unlikely that NKT cells contributed in any major way to the enhanced liver damage associated with CD39 deficiency in the present liver transplant IRI studies. Pommey et al24 have shown that mice that over-express CD39 exhibit CD4+ T cell lymphopenia and impaired CD4+ T cell function that afford protection against liver IRI. Here we found that CD4+ and CD8+ T cell number and function were preserved in CD39-/- mice. CD39 deficiency promoted liver mDC maturation and their ability to induce T cell proliferation and IFNγ production. Treg also express CD39 and CD73, and CD39-/- Treg have impaired suppressive function13 as we have confirmed (for splenic Treg) in this study. Treg were rarer in liver than in spleen in both WT and CD39-/- mice, and while a role of Treg in cold liver IRI has not been established, we cannot completely discount a possible contribution of impaired Treg function to enhanced injury in CD39-/- livers.
Only limited studies have addressed the role of CD39 on DC. DC are a heterogenous population of innate immune cells comprising multiple subsets that exhibit considerable phenotypic diversity and functional plasticity. CD39 on mouse epidermal Langerhans cells (LC) (a distinct DC lineage from conventional, tissue-resident mDC) is the dominant LC-associated ecto-NTPDase, with diverse modulatory roles in cutaneous inflammation and immunity.14 In these studies, epidermal CD39-/- DC showed impaired Ag-presenting capacity, whereas in the present report, CD39-/- liver mDC displayed enhanced pro-inflammatory and T cell stimulatory ability. It has also been reported that CD39 is highly expressed on human immune regulatory DC generated with IL-10/TGFβ41,- both anti-inflammatory cytokines produced by several liver cell populations in the steady-state, and in response to inflammation. This property of the liver microenvironment may serve to upregulate CD39 expression on liver DC.
Our liver cold I/R data show that mDC in CD39-/- liver grafts exhibit a more mature phenotype and that these grafts express more pro-inflammatory cytokines. This suggests that activation of liver mDC by unhydrolyzed ATP due to CD39 deficiency elicits enhanced production of pro-inflammatory cytokines, induces stronger T cell responses, and exacerbates CD39-/- liver damage after cold I/R. Our data further show that CD39-/- to CD39-/- liver transplantation results in less IRI compared with CD39-/- to WT cold I/R. High concentrations of ATP induce apoptosis. Thus CD39-/- T cells, NKT cells, macrophages and mDC are more susceptible to apoptosis induced by ATP. Because of lack of ATP hydrolysis, ATP concentrations remain high and immune cells, including liver mDC, may undergo apoptosis in the CD39KO to CD39KO liver transplant cold I/R model. On the other hand, not only is ATP hydrolyzed by recipient immune cells, but these cells are more resistant to ATP-related apoptosis due to their expression of CD39 in the CD39-/- to WT liver transplant cold I/R model.
ATP usually activates DC through P2X7.33, 42 We show here that liver mDC express comparatively high levels of P2X7 and P2Y14. Compared with other P2 receptors, P2X7 requires high levels of ATP (>100 μM) for activation and thus plays an important role under pathological conditions. The expression level of P2X7 was similar between mouse spleen and liver mDC and increased markedly after 18 hr ATP stimulation, but that the effect of ATP was less on liver mDC. Interestingly, in the present study, mDC expressed the highest level of CD39 amongst liver immune cells, and a higher level of CD39 than on mDC from other hematopoietic or parenchymal organs, again suggesting that local microenvironmental factors may affect CD39 expression on mDC. Our confirmation, in humans, that CD39 is expressed at greater levels on liver mDC compared with circulating mDC, and that, as in mice, human liver mDC hydrolyze ATP much more effectively than blood mDC, underscores the physiological relevance of our findings.
In conclusion, our data demonstrate that CD39 is a key molecule in regulation of liver mDC responses to the danger signal ATP, with ability to attenuate pro-inflammatory cytokine production and extended hepatic cold IRI associated with mouse liver transplantation. Improved understanding of how CD39 influences DC regulatory functions may promote the development of novel therapeutic strategies to impact inflammatory and also immune-mediated disorders, including those affecting liver transplantation.
Supplementary Material
Acknowledgments
Financial support: Supported by National Institutes of Health (NIH) grants P01AI81678 (AWT) and R01 HL094400 (SCR) and by grant #87429717 from the Roche Organ Transplantation Research Foundation (AWT). OY is the recipient of an American Society of Transplantation Basic Science Fellowship and an NIH postdoctoral fellowship (T32 AI74490).
Abbreviations
- Ag
antigen
- DAMP
danger-associated molecular pattern
- DC
dendritic cell(s)
- APC
antigen-presenting cell(s)
- MAMP
microbe-associated molecular pattern
- MHC
major histocompatibility complex
- ATP
adenosine triphosphate
- Treg
regulatory T cell(s)
- IR(I)
ischemia reperfusion (injury)
- LPS
lipopolysaccharide
- TLR
Toll-like receptor(s)
- DAMP
danger-associated molecular pattern
- Ab
antibody
- MLR
mixed leukocyte reaction
- WT
wild-type
Footnotes
Conflict of interest: The authors have nothing to disclose regarding funding or conflict of interest with respect to this article.
Contributor Information
Osamu Yoshida, Email: yoshidao@upmc.edu.
Shoko Kimura, Email: kimuras@upmc.edu.
Edwin K. Jackson, Email: edj@pitt.edu.
Simon C. Robson, Email: srobson@bidmc.harvard.edu.
David A. Geller, Email: gellerda@upmc.edu.
Noriko Murase, Email: murasen2@upmc.edu.
Angus W. Thomson, Email: thomsonaw@upmc.edu.
References
- 1.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 2.Benseler V, McCaughan GW, Schlitt HJ, Bishop GA, Bowen DG, Bertolino P. The liver: a special case in transplantation tolerance. Semin Liver Dis. 2007;27:194–213. doi: 10.1055/s-2007-979471. [DOI] [PubMed] [Google Scholar]
- 3.Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753–766. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 4.Bamboat ZM, Ocuin LM, Balachandran VP, Obaid H, Plitas G, Dematteo RP. Conventional DCs reduce liver ischemia/reperfusion injury in mice via IL-10 secretion. J Clin Invest. 2010;120:559–569. doi: 10.1172/JCI40008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiao J, Sastre D, Fiel MI, Lee UE, Ghiassi-Nejad Z, Ginhoux F, Vivier E, et al. Dendritic cell regulation of carbon tetrachloride-induced murine liver fibrosis regression. Hepatology. 2012;55:244–255. doi: 10.1002/hep.24621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang M, Ueki S, Kimura S, Yoshida O, Castellaneta A, Ozaki KS, Demetris AJ, et al. Roles of dendritic cells in murine hepatic warm and liver transplantation-induced cold ischemia/reperfusion injury. Hepatology. 2013;57:1585–1596. doi: 10.1002/hep.26129. [DOI] [PubMed] [Google Scholar]
- 7.Sumpter TL, Packiam V, Turnquist HR, Castellaneta A, Yoshida O, Thomson AW. DAP12 promotes IRAK-M expression and IL-10 production by liver myeloid dendritic cells and restrains their T cell allostimulatory ability. J Immunol. 2011;186:1970–1980. doi: 10.4049/jimmunol.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwiebert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta. 2003;1615:7–32. doi: 10.1016/s0005-2736(03)00210-4. [DOI] [PubMed] [Google Scholar]
- 9.Schnurr M, Then F, Galambos P, Scholz C, Siegmund B, Endres S, Eigler A. Extracellular ATP and TNF-alpha synergize in the activation and maturation of human dendritic cells. J Immunol. 2000;165:4704–4709. doi: 10.4049/jimmunol.165.8.4704. [DOI] [PubMed] [Google Scholar]
- 10.Wilkin F, Duhant X, Bruyns C, Suarez-Huerta N, Boeynaems JM, Robaye B. The P2Y11 receptor mediates the ATP-induced maturation of human monocyte-derived dendritic cells. J Immunol. 2001;166:7172–7177. doi: 10.4049/jimmunol.166.12.7172. [DOI] [PubMed] [Google Scholar]
- 11.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 13.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizumoto N, Kumamoto T, Robson SC, Sevigny J, Matsue H, Enjyoji K, Takashima A. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat Med. 2002;8:358–365. doi: 10.1038/nm0402-358. [DOI] [PubMed] [Google Scholar]
- 15.Berchtold S, Ogilvie AL, Bogdan C, Muhl-Zurbes P, Ogilvie A, Schuler G, Steinkasserer A. Human monocyte derived dendritic cells express functional P2X and P2Y receptors as well as ecto-nucleotidases. FEBS Lett. 1999;458:424–428. doi: 10.1016/s0014-5793(99)01197-7. [DOI] [PubMed] [Google Scholar]
- 16.Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg. 2001;181:160–166. doi: 10.1016/s0002-9610(00)00573-0. [DOI] [PubMed] [Google Scholar]
- 17.Carini R, Albano E. Recent insights on the mechanisms of liver preconditioning. Gastroenterology. 2003;125:1480–1491. doi: 10.1016/j.gastro.2003.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Abu-Amara M, Yang SY, Tapuria N, Fuller B, Davidson B, Seifalian A. Liver ischemia/reperfusion injury: processes in inflammatory networks--a review. Liver Transpl. 2010;16:1016–1032. doi: 10.1002/lt.22117. [DOI] [PubMed] [Google Scholar]
- 19.Manfredi AA, Capobianco A, Bianchi ME, Rovere-Querini P. Regulation of dendritic- and T-cell fate by injury-associated endogenous signals. Crit Rev Immunol. 2009;29:69–86. doi: 10.1615/critrevimmunol.v29.i1.30. [DOI] [PubMed] [Google Scholar]
- 20.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou T, Sun GZ, Zhang MJ, Chen JL, Zhang DQ, Hu QS, Chen YY, et al. Role of adhesion molecules and dendritic cells in rat hepatic/renal ischemia-reperfusion injury and anti-adhesive intervention with anti-P-selectin lectin-EGF domain monoclonal antibody. World J Gastroenterol. 2005;11:1005–1010. doi: 10.3748/wjg.v11.i7.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsung A, Zheng N, Jeyabalan G, Izuishi K, Klune JR, Geller DA, Lotze MT, et al. Increasing numbers of hepatic dendritic cells promote HMGB1-mediated ischemia-reperfusion injury. J Leukoc Biol. 2007;81:119–128. doi: 10.1189/jlb.0706468. [DOI] [PubMed] [Google Scholar]
- 23.Sun X, Imai M, Nowak-Machen M, Guckelberger O, Enjyoji K, Wu Y, Khalpey Z, et al. Liver damage and systemic inflammatory responses are exacerbated by the genetic deletion of CD39 in total hepatic ischemia. Purinergic Signal. 2011;7:427–434. doi: 10.1007/s11302-011-9239-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pommey S, Lu B, McRae J, Stagg J, Hill P, Salvaris E, Robson SC, et al. Liver grafts from CD39-overexpressing rodents are protected from ischemia reperfusion injury due to reduced numbers of resident CD4(+) T cells. Hepatology. 2013;57:1597–1606. doi: 10.1002/hep.25985. [DOI] [PubMed] [Google Scholar]
- 25.Castellaneta A, Sumpter TL, Chen L, Tokita D, Thomson AW. NOD2 ligation subverts IFN-alpha production by liver plasmacytoid dendritic cells and inhibits their T cell allostimulatory activity via B7-H1 up-regulation. J Immunol. 2009;183:6922–6932. doi: 10.4049/jimmunol.0900582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matta BM, Raimondi G, Rosborough BR, Sumpter TL, Thomson AW. IL-27 production and STAT3-dependent upregulation of B7-H1 mediate immune regulatory functions of liver plasmacytoid dendritic cells. J Immunol. 2012;188:5227–5237. doi: 10.4049/jimmunol.1103382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gramignoli R, Green ML, Tahan V, Dorko K, Skvorak KJ, Marongiu F, Zao W, et al. Development and application of purified tissue dissociation enzyme mixtures for human hepatocyte isolation. Cell Transplantation. 2012;21:1245–1260. doi: 10.3727/096368911X600939. [DOI] [PubMed] [Google Scholar]
- 28.Castellaneta A, Mazariegos GV, Nayyar N, Zeevi A, Thomson AW. HLA-G Level on Monocytoid Dendritic Cells Correlates With Regulatory T-Cell Foxp3 Expression in Liver Transplant Tolerance. Transplantation. 2011;91:1132–1140. doi: 10.1097/TP.0b013e31821414c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandapathil M, Hilldorfer B, Szczepanski MJ, Czystowska M, Szajnik M, Ren J, Lang S, et al. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J Biol Chem. 2010;285:7176–7186. doi: 10.1074/jbc.M109.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian SG, Fung JJ, Demetris AJ, Ildstad ST, Starzl TE. Orthotopic liver transplantation in the mouse. Transplantation. 1991;52:562–564. doi: 10.1097/00007890-199109000-00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueki S, Castellaneta A, Yoshida O, Ozaki K, Zhang M, Kimura S, Isse K, et al. Hepatic B7 homolog 1 expression is essential for controlling cold ischemia/reperfusion injury after mouse liver transplantation. Hepatology. 2011;54:216–228. doi: 10.1002/hep.24360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Baroni M, Pizzirani C, Pinotti M, Ferrari D, Adinolfi E, Calzavarini S, Caruso P, et al. Stimulation of P2 (P2X7) receptors in human dendritic cells induces the release of tissue factor-bearing microparticles. FASEB J. 2007;21:1926–1933. doi: 10.1096/fj.06-7238com. [DOI] [PubMed] [Google Scholar]
- 34.Dwyer KM, Deaglio S, Gao W, Friedman D, Strom TB, Robson SC. CD39 and control of cellular immune responses. Purinergic Signal. 2007;3:171–180. doi: 10.1007/s11302-006-9050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol. 2004;172:1009–1017. doi: 10.4049/jimmunol.172.2.1009. [DOI] [PubMed] [Google Scholar]
- 36.Tokita D, Mazariegos GV, Zahorchak AF, Chien N, Abe M, Raimondi G, Thomson AW. High PD-L1/CD86 ratio on plasmacytoid dendritic cells correlates with elevated T-regulatory cells in liver transplant tolerance. Transplantation. 2008;85:369–377. doi: 10.1097/TP.0b013e3181612ded. [DOI] [PubMed] [Google Scholar]
- 37.Ueki S, Dhupar R, Cardinal J, Tsung A, Yoshida J, Ozaki KS, Klune JR, et al. Critical role of interferon regulatory factor-1 in murine liver transplant ischemia reperfusion injury. Hepatology. 2010;51:1692–1701. doi: 10.1002/hep.23501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beldi G, Banz Y, Kroemer A, Sun X, Wu Y, Graubardt N, Rellstab A, et al. Deletion of CD39 on natural killer cells attenuates hepatic ischemia/reperfusion injury in mice. Hepatology. 2010;51:1702–1711. doi: 10.1002/hep.23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beldi G, Wu Y, Banz Y, Nowak M, Miller L, Enjyoji K, Haschemi A, et al. Natural killer T cell dysfunction in CD39-null mice protects against concanavalin A-induced hepatitis. Hepatology. 2008;48:841–852. doi: 10.1002/hep.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torres-Aguilar H, Sanchez-Torres C, Jara LJ, Blank M, Shoenfeld Y. IL-10/TGF-beta-treated dendritic cells, pulsed with insulin, specifically reduce the response to insulin of CD4+ effector/memory T cells from type 1 diabetic individuals. J Clin Immunol. 2010;30:659–668. doi: 10.1007/s10875-010-9430-5. [DOI] [PubMed] [Google Scholar]
- 42.Mutini C, Falzoni S, Ferrari D, Chiozzi P, Morelli A, Baricordi OR, Collo G, et al. Mouse dendritic cells express the P2X7 purinergic receptor: characterization and possible participation in antigen presentation. J Immunol. 1999;163:1958–1965. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


