Abstract
Objective
There have been few prospective, multicenter studies investigating the natural history of type 1 diabetes (T1D) from the time of diagnosis. The objective of this report from the Pediatric Diabetes Consortium (PDC) T1D New Onset (NeOn) study was to assess the natural history and clinical outcomes in children during the first year after diagnosis of T1D.
Research Design and Methods
Clinical measures from the first year following diagnosis were analyzed for 857 participants (mean age 9.1 years, 51% female, 66% non-Hispanic White) not participating in an intervention study who had a HbA1c result at 12 months.
Results
Mean HbA1c ± SD was 102 ± 25 mmol/mol (11.4 ± 2.3%) at diagnosis, 55 ± 12 mmol/mol (7.2 ± 1.1%) at 3 months, 56 ± 15 mmol/mol (7.3 ± 1.3%) at 6 months and 62 ± 16 mmol/mol (7.8 ± 1.5%) at 12 months from diagnosis. A severe hypoglycemic (SH) event occurred in 31 (4%) participants (44 events, 5.2 events per 100 person-years). Diabetic ketoacidosis (DKA) not including diagnosis occurred in 10 (1%) participants (13 events, 1.5 events per 100 person-years).
Conclusions
After onset of T1D, mean HbA1c reaches its nadir at 3–6 months with a gradual increase through 12 months. SH and DKA are uncommon but still occur during the first year with T1D. Data from large cohorts, such as the PDC T1D NeOn study, provide important insights into the course of T1D during the first year following diagnosis, which will help to inform the development of models to target future interventions.
Keywords: diabetes, children, HbA1c or glycemic control
Introduction
The Diabetes Control and Complications Trial (DCCT) and its follow-up Epidemiology of Diabetes Interventions and Complications (EDIC) study clearly demonstrated that patients with type 1 diabetes (T1D) should aim for blood glucose and A1c levels as close to normal as possible and as early in the course of T1D as possible to prevent or delay the vascular and neuropathic complications of the disease (1–4). While these goals of therapy were particularly difficult to achieve in youth with T1D in the DCCT (2), the introduction of rapid and long acting insulin analogs, improvements in insulin pump technology and the development of continuous glucose monitoring (CGM) devices have provided clinicians with better methods to achieve strict glycemic control. Despite these advances, treatment outcomes recently reported by leading pediatric diabetes treatment centers involved in the T1D Exchange Clinic Registry demonstrated that too many youth with established T1D fail to achieve target A1c levels and the rates of severe hypoglycemia (SH) and diabetic ketoacidosis (DKA) remain unacceptably high (5).
The early honeymoon period of T1D is the one phase of the disease where a substantial proportion of youth with new-onset T1D are able to achieve excellent glucose control with minimal risks of acute complications. Moreover, it has long been recognized that maintenance of strict metabolic control will help to prolong the honeymoon period by slowing the loss of residual endogenous insulin secretion (1). Nevertheless, there have been few recent studies in clinical practice settings that have evaluated the impact of therapy of youth with new-onset T1D on the outcomes of treatment during the early phases of the disease. To fill this void, the Pediatric Diabetes Consortium (PDC) T1D New Onset (NeOn) Study was undertaken by 7 pediatric diabetes centers in the United States caring for children and adolescents with T1D. This paper reports on the major clinical outcomes of this cohort during the first year following diagnosis.
Study Design and Methods
Patients
The PDC enrolled 1,052 patients diagnosed with T1D based on American Diabetes Association criteria (6) between July 2009 and April 2011. The protocol was approved by the Institutional Review Board (IRB) at each of the 7 participating centers. Informed consent was obtained from participants 18 years of age and from parents of those less than 18 years of age. Assent was obtained from participants <18 years of age as required by local IRB regulations. To be eligible for enrollment in the study, patients had to be <19 years of age and managed at one of the 7 participating PDC centers within 3 months of diagnosis. A detailed description of PDC and the design of the study have been published previously (7). The analyses herein included 857 of the 1,052 PDC participants: 163 were not included because a 1 year HbA1c measurement (319 to 455 days from diagnosis) was not available and 32 were excluded due to participation in an intervention study.
Data Collection
Demographic, socioeconomic and clinical characteristics, laboratory data, hospitalizations, severe hypoglycemic events, and diabetic ketoacidosis (DKA) events were collected from medical records and from interviews with the participant and/or parent at onset and at 1, 3, 6, 9 and 12 months from diagnosis. Follow-up visits were completed per usual care and all visits during the first year post-diagnosis were entered in the standardized electronic case report forms for the study. SH was defined as events that led to alterations in consciousness that were so severe that they required the assistance of another person to administer carbohydrate, glucagon, or other resuscitative actions. For those developmentally too young to independently recognize and treat hypoglycemia, hypoglycemia was considered severe if there were associated signs or symptoms of neuroglycopenia, including temporary impairment of cognition; incoherent, disoriented, and/or combative behavior; seizure; or coma. DKA was defined according to DCCT criteria (1) of pH <7.3 or HCO3 <15mEq/L and treatment in a healthcare facility. SH and DKA events were included in the analysis if they occurred between 7 and 365 days from diagnosis. Body mass index (BMI) was computed from the closest height and weight measured by the health care provider within 14 days of diagnosis. BMI percentile and standard deviation score for age and gender were calculated using the 2000 CDC population growth chart data (8).
The insulin dose adjusted HbA1c (IDAA1c) was defined as HbA1c % + [4 × insulin dose (units per kg per 24 h)] (9). Two separate criteria were used to assess a partial remission at each time point mentioned above: 1) IDAA1c ≤9.0% and 2) HbA1c <53 mmol/mol (7.0%) and total daily insulin dose <0.5 units per kg per day (10, 11).
Statistical Analyses
The cumulative incidences of SH, DKA, emergency room visits and hospitalizations were calculated with the Kaplan-Meier estimator. Event rates were determined as the total number of events divided by the total amount of follow up time scaled to events per 100 person-years. Follow-up times were based on the last visit within 365 days from diagnosis for each participant. Cox regression was used to determine the association of the following baseline factors with SH events in the first year: age, gender, race/ethnicity, health insurance status, parental education, family income, family structure and DKA at diagnosis. A baseline multivariate model was constructed using stepwise selection with p-values <0.10 required to be included in the model. Due to multiple comparisons, only factors with p-values <0.01 were considered statistically significant, although factors with p-values <0.10 were included in the model to adjust for potential confounding. All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
Results
The 857 participants included in the analysis had a mean age of 9.1 years (range 0.7 to 18.8 years); 51% were female; 66% were non-Hispanic White, 21% were Hispanic and 7% were non-Hispanic Black. The majority of participants had health insurance, at least some college for parent education and a family income above $50,000. At diagnosis, 11% of the participants were overweight (85%–<95% BMI percentile) and 9% obese (≥95% BMI percentile) and 33% presented in DKA (Table 1).
Table 1.
Participant Characteristics at Diagnosis (N=857a)
| N | % | |
|---|---|---|
| Age at Diagnosis | ||
| <5 years | 165 | 19% |
| 5–<12 years | 481 | 56% |
| 12–<19 years | 211 | 25% |
| mean ± SD | 9.1 ± 4.1 | |
| range | 0.7–18.8 | |
| Gender | ||
| Female | 436 | 51% |
| Race/Ethnicity | ||
| White (non-Hispanic) | 549 | 66% |
| Hispanic or Latino | 175 | 21% |
| Black/African American | 59 | 7% |
| Other/Multiple Race | 54 | 6% |
| Health Insurance | ||
| Private | 576 | 68% |
| CHP or Other Gov’t Sponsored | 235 | 28% |
| Military | 16 | 2% |
| None | 15 | 2% |
| Parent Education | ||
| High School or Less | 225 | 32% |
| AA | 103 | 15% |
| BS/BA | 204 | 29% |
| MS/MA/Professional Degree | 162 | 23% |
| Family Income | ||
| <$25,000 | 75 | 13% |
| $25,000–$49,999 | 111 | 19% |
| $50,000–$74,999 | 97 | 17% |
| $75,000–$99,999 | 81 | 14% |
| ≥$100,000 | 213 | 37% |
| BMI Percentile | ||
| <85% | 401 | 81% |
| 85%–<95% | 52 | 10% |
| ≥95% | 43 | 9% |
| DKA at Diagnosis | 275 | 33% |
| HbA1c | ||
| 37–<86 mmol/mol (5.5–<10%) | 226 | 28% |
| 86–<119 mmol/mol (10–<13%) | 368 | 45% |
| ≥119 mmol/mol (≥13%) | 224 | 27% |
| mean ± SD | 102 ± 25 mmol/mol (11.4 ± 2.3%) | |
| range | 37–180 mmol/mol (5.5–18.6%) | |
Mean HbA1c ± SD was 102 ± 25 mmol/mol (11.4 ± 2.3%) at diagnosis and dropped to 74 ± 16 mmol/mol (8.9 ± 1.5%) one month later. HbA1c values continued to fall reaching a minimum mean value of 55 ± 12 mmol/mol (7.2 ± 1.1%) at 3 months and 56 ± 15 mmol/mol (7.3 ± 1.3%) at 6 months, and then rising to 62 ± 16 mmol/mol (7.8 ± 1.5%) 12 months from diagnosis (Table 2). HbA1c was ≤53 mmol/mol (7.0%) for 33% of participants at 12 months. The IDAA1c was 11.2 ± 2.1% one month from diagnosis, dropped to its lowest value at 3 and 6 months (mean 9.3%) and then rose to 10.4 ± 2.2% at 12 months. HbA1c and IDAA1c trajectories were similar when restricting analysis to participants with data at all 6 time points (HbA1c: n=216; IDAA1c: n=86; data not shown). More participants met the criterion for partial remission defined by IDAA1c ≤9% (24% at 12 months) than the alternate criterion of HbA1c <53 mmol/mol (7.0%) and insulin dose <0.5 u/kg/day (12% at 12 months; Table 2).
Table 2.
HbA1c and Insulin Dose Adjusted HbA1c (IDAA1c)
| Time from Diagnosis
|
||||||
|---|---|---|---|---|---|---|
| Baseline N=818 | 1 month N=551 | 3 month N=683 | 6 month N=705 | 9 month N=673 | 12 month N=857 | |
|
| ||||||
| HbA1c | ||||||
| mean ± SD (mmol/mol) | 102 ± 25 | 74 ± 16 | 55 ± 12 | 56 ± 15 | 59 ± 15 | 62 ± 16 |
| mean ± SD (%) | 11.4 ± 2.3 | 8.9 ± 1.5 | 7.2 ± 1.1 | 7.3 ± 1.3 | 7.6 ± 1.4 | 7.8 ± 1.5 |
| ≤53 mmol/mol (7.0%) | 3% | 7% | 50% | 50% | 39% | 33% |
| IDAA1c | N/A | N=479 | N=562 | N=588 | N=547 | N=732 |
| mean ± SD (%) | 11.2 ± 2.1 | 9.3 ± 1.8 | 9.3 ± 2.0 | 9.9 ± 2.1 | 10.4 ± 2.2 | |
| IDAA1c ≤9.0% | 11% | 51% | 50% | 35% | 24% | |
| HbA1c <53mmol/mol (7.0%) and insulin <0.5 u/kg/day | 4% | 27% | 28% | 16% | 12% | |
Participants initiated insulin injections at diagnosis with either multiple daily injections (55%), or a fixed dose (45%) where daily insulin doses remain the same with consistent amounts of carbohydrate eaten at each meal and an added insulin amount for adjustment for glucose value. By 12 months from onset, 29% of participants were using an insulin pump. Insulin dose was relatively steady over the first year of T1D and did not vary meaningfully by age or gender. Only 1% of participants were using a CGM at 12 months. The median number of self monitored blood glucose (SMBG) tests per day (5 tests/day) remained consistent throughout the first year of T1D. BMI percentiles increased from diagnosis to 1 month and then remained fairly stable for the remainder of the year (Table 3).
Table 3.
Insulin Regimen, Dosage and CGM Use
| 1 month | 3 month | 6 month | 9 month | 12 month | |
|---|---|---|---|---|---|
|
| |||||
| Insulin Delivery | N=618 | N=687 | N=713 | N=673 | N=855 |
| Pump | 3 (<1%) | 58 (8%) | 118 (17%) | 171 (25%) | 282(33%) |
| Injections (fixed dose) | 255 (41%) | 153 (22%) | 123 (17%) | 91 (14%) | 97 (11%) |
| Injections (MDI) | 360 (58%) | 476 (69%) | 472 (66%) | 411 (61%) | 476 (56%) |
| N=587 | N=655 | N=679 | N=640 | N=812 | |
| CGM Users | 1 (<1%) | 1 (<1%) | 7 (1%) | 5 (1%) | 12 (1%) |
| CGM and Pump Users | 0 | 0 | 2 (<1%) | 3 (<1%) | 11 (1%) |
| Self Blood Glucose | |||||
| Monitoring (# tests/day) | N=406 | N=425 | N=436 | N=404 | N=497 |
| median (25th, 75th percentile) | 5 (4, 6) | 5 (4, 6) | 5 (4, 6) | 5 (4, 6) | 5 (4, 6) |
| Insulin Dosage (u/kg/day) | N=500 | N=562 | N=592 | N=552 | N=733 |
| mean (SD) | 0.6 (0.3) | 0.5 (0.3) | 0.5 (0.3) | 0.6 (0.3) | 0.7 (0.3) |
| median (25th, 75th percentile) | 0.5 (0.4, 0.7) | 0.5 (0.3, 0.6) | 0.5 (0.3, 0.7) | 0.5 (0.4, 0.7) | 0.6 (0.5, 0.8) |
| Mean Insulin Dosage (u/kg/day) by Age and Gender | |||||
| <5 years | |||||
| Female (N=71) | 0.4 | 0.5 | 0.5 | 0.7 | 0.7 |
| Male (N=89) | 0.5 | 0.5 | 0.5 | 0.6 | 0.6 |
| 5–<12 years | |||||
| Female (N=262) | 0.6 | 0.5 | 0.5 | 0.6 | 0.7 |
| Male (N=212) | 0.5 | 0.5 | 0.5 | 0.5 | 0.6 |
| 12–<19 years | |||||
| Female (N=94) | 0.7 | 0.6 | 0.6 | 0.6 | 0.7 |
| Male (N=115) | 0.7 | 0.6 | 0.6 | 0.6 | 0.7 |
| BMI Percentile | |||||
| <85% | 370 (70%) | 457 (71%) | 485 (72%) | 466 (73%) | 605 (72%) |
| 85%–<95% | 92 (17%) | 104 (16%) | 116 (17%) | 108 (17%) | 130 (16%) |
| ≥95% | 68 (13%) | 80 (12%) | 75 (11%) | 68 (11%) | 103 (12%) |
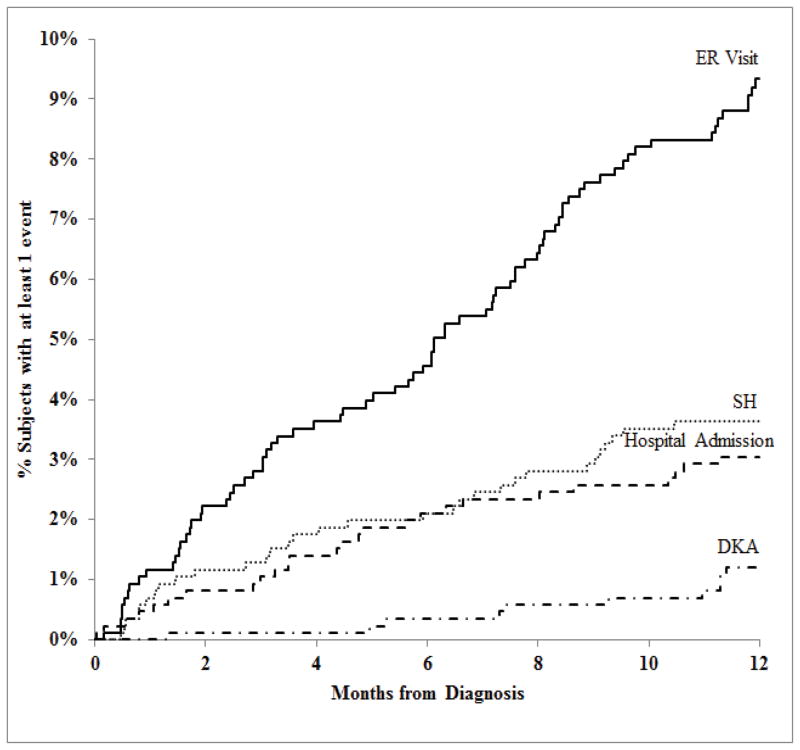
During the first year of T1D, SH occurred in 31 (4%) participants (44 events) for a rate of 5.2 events per 100 person-years; seizure or loss of consciousness occurred in 17 (39%) of the 44 events. DKA occurred in 10 (1%) participants (13 events) during the first year of T1D not including episodes at presentation for a rate of 1.5 events per 100 person-years. Table 4 shows the SH and DKA rates according to age and Figure 1 shows the frequencies during the first year. After adjusting for clinical site, SH events were more likely with younger age (7% for those <5 years old, 3% for 5–11 year olds, and 1% for 12–17 year olds; p-value=0.002) and with a history of DKA at diagnosis (6% versus 2%; p-value=0.01).
Table 4.
Severe Hypoglycemica,c (SH), Diabetic Ketoacidosisc(DKA), ER Visitsb and Hospital Admissionsb (N=857)
| Rate (total # events) | |
|---|---|
|
| |
| SH Events per 100 person-years | 5.2 (44) |
| SH Rate by Age at Diagnosis | |
| <5 years | 10.5 (17) |
| 5–<12 years | 4.0 (19) |
| 12–<19 years | 3.8 (8) |
| SH Impairment by Age at Diagnosis | Frequency (% of SH events) |
| Seizure/Loss Conscious | 17 (39%) |
| <5 years | 10 (59%) |
| 5–<12 years | 7 (37%) |
| 12–<19 years | 0 |
| Required Assistanced | 25 (57%) |
| <5 years | 6 (35%) |
| 5–<12 years | 11 (58%) |
| 12–<19 years | 8 (100%) |
| SH Treatmente | |
| Glucagon Given | 19 (43%) |
| ER Visit | 18 (41%) |
| Hospital Admission | 2 (5%) |
| Ambulance | 9 (20%) |
| Emergency Medical Technician Assist | 9 (20%) |
| SH Outcome | |
| Fully Recovered | 44 (100%) |
| Rate (total # events) | |
| DKA Events per 100 person-years | 1.5 (13) |
| DKA Rate by Age at Diagnosis | |
| <5 years | 2.5 (4) |
| 5–<12 years | 1.1 (5) |
| 12–<19 years | 1.9 (4) |
| # Events/Patients | |
| ER Visits | 96/79 |
| Hospital Admissions | 29/26 |
| Floorf Only | 20/19 |
| ICU and Floorf | 6/4 |
| ICU only | 3/3 |
A severe hypoglycemia event is defined as a hypoglycemic event that required the assistance of another person due to altered consciousness to actively administer carbohydrate, glucagon, or other resuscitative actions. Multiple episodes of SH events on the same day counted as one episode and any episode on a separate calendar date was counted as a new event.
ER visits and Hospital admissions due to reasons other than SH or DKA.
24 participants had one SH event, 5 participants had two SH events, 1 participant had four SH events and 1 participant had six SH events. All 44 events occurred when participant was not wearing a CGM sensor or had unknown status of sensor wearing. 7 participants had one DKA event and 3 participants had two DKA events.
Required assistance without seizure or loss conscious.
Same participant can have more than one treatment.
“Floor” is a non-ICU inpatient admission.
Figure 1. Cumulative Incidences of SH, DKA, ER Visits and Hospital Admissions within the First Year of T1D (N=857).
Hospital admissions and ER visits due to reasons other than SH or DKA.
For reasons other than SH or DKA, there were 96 trips to the emergency room (ER) by 79 (9%) and 29 hospitalizations unrelated to SH or DKA for 26 (3%) participants. The frequencies for these are also shown in Figure 1.
Discussion
The PDC T1D NeOn study is comprised of a large cohort of children and adolescents in the United States followed from the onset of T1D. Compared with the SEARCH for Diabetes in Youth Study, the percentages of Hispanic and African-American participants in the NeOn cohort are slightly higher and gender distributions are similar (12). Mean HbA1c values at diagnosis were similar to those reported in other studies (13–15). The majority of patients were from well-educated, non-Hispanic white families with relatively high socio-economic status. The 19% prevalence of overweight and obesity at diagnosis indicates that the pediatric population with T1D is not immune to the obesity epidemic.
The temporal changes in total daily insulin doses and HbA1c levels over the first year of treatment of T1D provide insights regarding the partial remission phase of diabetes as it is currently being observed in clinical practice settings. The lowest mean total daily insulin doses were rapidly achieved by 1–3 months of treatment, reflecting the start of the remission phase during the first few months of treatment, whereas reductions in HbA1c values lagged behind and did not reach nadir levels until 3 to 6 months. Mean HbA1c levels subsequently increased at 9 and 12 months while total daily insulin doses changed little suggesting that as the honeymoon phase was waning, insufficient attention was paid to the need to increase insulin doses. Nevertheless, the mean HbA1c of 62 mmol/mol (7.8%) in the T1D NeOn cohort compares favorably to mean HbA1c levels between 64–75 mmol/mol (8.0–9.0%) at 1 year post-diagnosis that have been reported in other pediatric cohorts (16–19). The proportion of patients who switched to pump therapy rose sharply during the second six months of treatment.
A variety of metrics have been used by clinicians as rules of thumb to identify patients in the honeymoon phase, including HbA1c levels <53 mmol/mol (7.0%), total daily insulin <0.5 U/kg/day or, more commonly, the combination of both (10, 11). A retrospective chart review of a smaller group of subjects from a pediatric diabetes center in the US reported similar mean HbA1c levels at 6 months followed by higher values at 9 and 12 months as compared to our data. While the percent of subjects in partial remission at 6 months after diagnosis was comparable to our group, it was lower at 12 months despite the fact that partial remission was defined as total daily insulin dose<0.5 units/kg body weight/day with a relatively higher HbA1C of <64 mmol/mol (8%) (17). More recently, Mortensen and colleagues (9) described and validated a new metric that they termed the Insulin Dose Adjusted A1c (IDAA1C) defined as equal to the HbA1c level + 4 × total daily insulin dose (u/kg/day). They demonstrated that an IDAA1C value ≤9.0 corresponds to a predicted stimulated C-peptide >300 pmol/L. Rather than using arbitrary cut points for HbA1c and daily insulin dose, IDAA1C is a continuous variable that accounts for patients who might have mild elevations in HbA1c levels above 53 mmol/mol (7.0%) in the face of very low total daily insulin doses. Thus it is noteworthy that the percentage of participants in remission at 6 (50% vs. 44%) and 12 months (24% vs. 18%) in our study is comparable with Mortensen et al.’s findings (9) and that IDAA1c identified nearly twice as many patients in partial remission than the older combination cut point method (10, 11).
With respect to glucose monitoring, self-monitoring blood glucose measurements averaged 4–6 tests per day during the entire year of study. CGM was prescribed infrequently during the first year. Approximately half of the severe hypoglycemic events occurred during the first half of the year when insulin sensitivity has been shown to increase (20, 21), while DKA events clustered in the second half of the year when HbA1c levels were rising.
The NeON Study data provide relevant information to inform pediatric clinical care of T1D. The findings indicate that the 6-month visit may be an ideal time to proactively counsel families that they will need to increase their vigilance in recognizing glucose trends and in increasing insulin doses over the next few months to compensate for waning of endogenous insulin secretion. The 6 and 9 month visits may also be good times to review sick day management rules and protocols to prevent ketoacidosis. The findings of this study can also help inform the design and sample size estimates of future beta cell preservation trials for new-onset patients by providing data on the outcomes that might be expected in the control group that will receive standard diabetes management.
Acknowledgments
The Pediatric Diabetes Consortium and its activities are supported by the Jaeb Center for Health Research Foundation through an unrestricted grant from Novo Nordisk. The University of Michigan Consortium center is supported by the Michigan Diabetes Research and Training Center from the National Institute of Diabetes and Digestive and Kidney Diseases (DK020572).
Abbreviations
- TID
type 1 diabetes
- PDC
Pediatric Diabetes Consortium
- NeOn
New Onset Study
- HbA1c
Hemoglobin A1c
- SD
standard deviation
- SH
severe hypoglycemic event
- DKA
diabetic ketoacidosis
- DCCT
the Diabetes Control and Complications Trial
- EDIC
Epidemiology of Diabetes Interventions and Complications
- CGM
continuous glucose monitoring
- IRB
institutional review board
- BMI
body mass index
- CDC
Centers for Disease Control and Prevention
- IDAA1c
insulin dose adjusted HbA1c
- SMBG
self-monitored blood glucose
- ER
emergency room
The Pediatric Diabetes Consortium Study Group
Clinical Centers: (Listed clinical center name, city, and state. Personnel are listed as (PI) for Principal Investigator, (I) for co-Investigator and (C) for Coordinators.) (1) Baylor College of Medicine, Houston, TX: Morey Haymond, MD (PI); Maria J. Redondo, MD, PhD (I); Krishna Hassan, MD (C); Kathy Shippy, RN, CCRP (C); Chris George (C); Mariam Pontifes (C); (2) Children’s Hospital of Los Angeles, Los Angeles, CA: Jamie Wood, MD (PI); Brian Ichihara, BA (C); Megan Lipton, MA, CCRP (C); Marisa Cohen, MPH (C); (3) Stanford University, Stanford, CA: Bruce Buckingham, MD (PI); Breanne Harris, BS (C); Satya Shanmugham, BS (C); (4) Barbara Davis Center for Childhood Diabetes, University of Colorado School of Medicine, Aurora, CO: Georgeanna J. Klingensmith, MD (PI); Eric Cruz, BA (C); Heidi Haro, BA, BS (C); Maria King, BA (C); Katherine Manseau (C); (5) University of Florida, Gainesville, FL: Desmond Schatz, MD (PI); Janet Silverstein, MD (I); Michael J. Haller, MD (I); Erica Dougherty, BS (C); (6) Yale University, New Haven, CT: William V. Tamborlane, MD (I); Eda Cengiz, MD (PI); Melody Martin, CCRP (C); Amy Steffen, BA (C); Lori Carria, MS (C); Darryll Cappiello (C); (7) University of Michigan, Ann Arbor, MI: Joyce M. Lee, MD, MPH (PI); Surair Bashir (C); Ashley Eason (C); Coordinating Center: Jaeb Center for Health Research, Tampa, FL: Roy W. Beck, MD, PhD; Katrina J. Ruedy, MSPH; Craig Kollman, PhD; Crystal G. Connor, MS, MPH; Beth Stevens.
References
- 1.Diabetes Control and Complications Trial Research Group. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group. J Pediatr. 1994;125:177–188. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 3.Martin CL, Albers J, Herman WH, et al. Neuropathy Among the Diabetes Control and Complications Trial Cohort 8 Years After Trial Completion. Diabetes Care. 2006;29:340–344. doi: 10.2337/diacare.29.02.06.dc05-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metzger M, Leibowitz G, Wainstein J, Glaser B, Raz I. Reproducibility of Glucose Measurements Using the Glucose Sensor. Diabetes Care. 2002;25:1185–1191. doi: 10.2337/diacare.25.7.1185. [DOI] [PubMed] [Google Scholar]
- 5.Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, DuBose SN, Hall CA. The T1D Exchange Clinic Registry. J Clin Endocrinol Metab. 2012;97:4383–4389. doi: 10.1210/jc.2012-1561. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34 (Suppl 1):S62–69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pediatric Diabetes Consortium. The Pediatric Diabetes Consortium: Improving Care of Children with Type 1 Diabetes Through Collaborative Research. Diabetes Technol Ther. 2010;12:685–688. doi: 10.1089/dia.2010.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuczmarski R, Ogden C, Grummer-Strawn L, et al. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 9.Mortensen HB, Hougaard P, Swift P, et al. New Definition for the Partial Remission Period in Children and Adolescents With Type 1 Diabetes. Diabetes Care. 2009;32:1384–1390. doi: 10.2337/dc08-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Couper JDK. Phases of diabetes in children and adolescents. Pediatr Diabetes. 2009 Sep 10;:13–16. doi: 10.1111/j.1399-5448.2009.00574.x. [DOI] [PubMed] [Google Scholar]
- 11.Lombardo FVM, Wasniewska M, Messina MF, Ruggeri C, Arrigo T, De Luca F. Two-year prospective evaluation of the factors affecting honeymoon frequency and duration in children with insulin dependent diabetes mellitus: the key-role of age at diagnosis. Diabetes Nutr Metab. 2002;15:246–251. [PubMed] [Google Scholar]
- 12.Dabelea D, Bell R, et al. Writing Group for the SEARCH for Diabetes in Youth Study Group. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 13.Mortensen HB, Swift PG, Holl RW, et al. Multinational study in children and adolescents with newly diagnosed type 1 diabetes: association of age, ketoacidosis, HLA status, and autoantibodies on residual beta-cell function and glycemic control 12 months after diagnosis. Pediatr Diabetes. 2010;11:218–226. doi: 10.1111/j.1399-5448.2009.00566.x. [DOI] [PubMed] [Google Scholar]
- 14.Ramchandani N, Ellis MK, Jain S, et al. Basal insulin requirements on continuous subcutaneous insulin infusion during the first 12 months after diagnosis of type 1 diabetes mellitus. J Diabetes Sci Technol. 2010;4:610–614. doi: 10.1177/193229681000400315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salemyr J, Bang P, Örtqvist E. Lower HbA1c after 1 year, in children with type 1 diabetes treated with insulin glargine vs. NPH insulin from diagnosis: a retrospective study. Pediatr Diabetes. 2011;12:501–505. doi: 10.1111/j.1399-5448.2010.00723.x. [DOI] [PubMed] [Google Scholar]
- 16.Beck JK, Lewis TV, Logan KJ, Harrison DL, Gardner AW, Copeland KC. Intensive vs. conventional insulin management initiated at diagnosis in children with diabetes: Should payer source influence the choice of therapy? Pediatr Diabetes. 2009;10:368–373. doi: 10.1111/j.1399-5448.2009.00538.x. [DOI] [PubMed] [Google Scholar]
- 17.Bowden SA, Duck MM, Hoffman RP. Young children (<5 yr) and adolescents (>12 yr) with type 1 diabetes mellitus have low rate of partial remission: diabetic ketoacidosis is an important risk factor. Pediatr Diabetes. 2008;9:197–201. doi: 10.1111/j.1399-5448.2008.00376.x. [DOI] [PubMed] [Google Scholar]
- 18.Chase HP, MacKenzie TA, Burdick J, et al. Redefining the clinical remission period in children with type 1 diabetes. Pediatr Diabetes. 2004;5:16–19. doi: 10.1111/j.1399-543X.2004.00034.x. [DOI] [PubMed] [Google Scholar]
- 19.Palta M, Shen G, Allen C, Klein R, D’Lessio D the Wisconsin Diabetes Registry. Longitudinal Patterns of Glycemic Control and Diabetes Care from Diagnosis in a Population-based Cohort with Type 1 Diabetes. Am J Epidemiol. 1996;144:954–961. doi: 10.1093/oxfordjournals.aje.a008865. [DOI] [PubMed] [Google Scholar]
- 20.Yki-Järvinen H, Koivisto V. Insulin sensitivity in newly diagnosed type 1 diabetics after ketoacidosis and after three months of insulin therapy. J Clin Endocrinol Metab. 1984;59:371–378. doi: 10.1210/jcem-59-3-371. [DOI] [PubMed] [Google Scholar]
- 21.Yki-Järvinen H, Koivisto V. Natural Course of Insulin Resistance in Type I Diabetes. N Engl J Med. 1986;315:224–230. doi: 10.1056/NEJM198607243150404. [DOI] [PubMed] [Google Scholar]