Abstract
Objective
Constrictive extracellular matrix (ECM) remodeling contributes significantly to restenosis after arterial reconstruction but its molecular regulation is poorly defined. Hyaluronan (HA) accumulates within ECM at sites of injury where it is thought to facilitate smooth muscle cell (SMC) trafficking and collagen remodeling analogous to its role in cutaneous wound healing. SMC receptors for HA include receptor for hyaluronan-mediated motility (RHAMM), which mediates HA-induced migration. We hypothesized RHAMM would also mediate SMC-matrix interactions to alter extent of constrictive remodeling.
Methods
We studied the role of RHAMM in SMC attachment to collagen, migration, and contraction of collagen gels using blocking antibodies and SMC from RHAMM−/− knockout mice (rKO). We then determined the role of RHAMM in constrictive artery wall remodeling by comparing changes in wall geometry in rKO versus wild-type +/+ (WT) controls 1 month after carotid ligation.
Results
HA increased SMC attachment to collagen-coated plates but blocking RHAMM reduced adhesion (p=0.025). rKO SMC also demonstrated reduced adhesion (% adherent: 36.1±2.2 vs. 76.3±1.9, p< 0.05). SMC contraction of collagen gels was enhanced by HA and further increased by RHAMM blockade (p< 0.01) or knockout (gel diameter, mm: rKO, 6.7±0.1 vs. WT, 9.8±0.1, p=0.015). RHAMM promoted constrictive remodeling in vivo as carotid artery size was significantly larger in rKO mice 1 month after ligation. Neointimal thickening however was not affected in rKO (p=NS vs WT) but lumen size was significantly larger (lumen area, μm2: 52.4±1.4 × 103 vs. 10.4±1.8 × 103, p=0.01) because artery size constricted less (EEL area, μm2: rKO, 92.4±4.7×103 vs. WT, 51.3±5.9 × 103, p=0.015). Adventitial thickening and collagen deposition were also more extensive in ligated rKO carotids (adventitial thickness, μm: 218±12.2 vs. 109±7.9, p=0.01).
Conclusion
HA activation of RHAMM significantly impacts SMC-ECM adhesive interactions and contributes to constrictive artery wall remodeling in mice. Strategies to block RHAMM at sites of vessel injury may prove useful in the prevention of clinical restenosis.
Keywords: smooth muscle cells, constrictive remodeling, restenosis, RHAMM, hyaluronan
INTRODUCTION
Constrictive artery wall remodeling contributes to restenosis after angioplasty 1–5 and refers to change in vessel geometry from reorganization of wall components (cells and ECM) into a smaller caliber (“inward”, “negative” or “constrictive” remodeling). Remodeling is distinct from transient caliber changes from vasoconstriction. Small changes in wall caliber from remodeling after injury impact lumen size dramatically, independent of new cells or ECM from hyperplasia. Simply put, fitting a given wall cross-sectional area into a smaller caliber disproportionately narrows the lumen (Figure 1).
Figure 1.
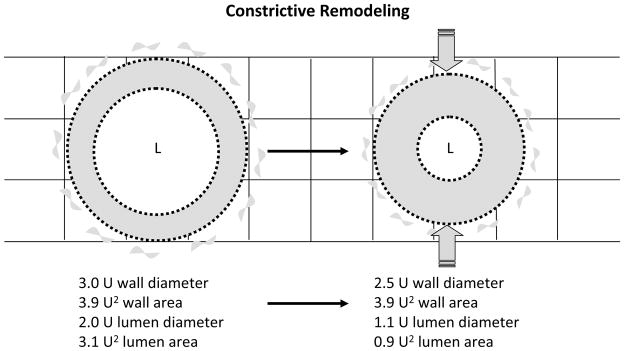
Constrictive remodeling following arterial reconstruction. In this example a 17% decrease in outer artery wall caliber from 3.0U to 2.5U, with cross-sectional wall area held constant, reduces lumen area by 68% from 3.1U2 to 0.9U2. L: lumen. U: arbitrary unit.
Constrictive remodeling by definition implies SMCs reorganize ECM but factors driving constrictive versus outward remodeling remain poorly defined. Wall shrinkage has parallels to cutaneous wound contraction and fibrosis.2 SMCs repopulate sites of vessel injury producing and remodeling ECM 1,2, which alters wall geometry just as myofibroblasts repopulate and contract wounds.6–8 ECM produced in response to arterial injury is distinct from normal wall.2,9–11 It mimics ECM in healing wounds which is initially rich in glycosaminoglycans (e.g. HA) and proteoglycans (e.g. versican), later replaced by collagen and other structural proteins.6,8,12 Changes in ECM composition can significantly alter SMC function and ECM remodeling critical to restenosis.2,13–16
A major component of ECM induced by artery injury is HA, a large hydrophilic disaccharide polymer (MW >106) important in tissue remodeling during embryogenesis, wound healing, and tumor metastasis.6,12,17–23 HA enhances collagen remodeling in wounds, limiting fibrosis and wound contraction.12,19,21,22 Small amounts of HA are found in normal arteries produced by intracellular hyaluronan synthases and secreted into the pericellular coat of SMC, adventitial fibroblasts and endothelial cells. Within days of injury (e.g. angioplasty) large quantities are deposited at sites of injury.2,10 HA promotes SMC migration and proliferation13–15 and collagen remodeling.16 SMC use specific HA receptors, including CD44 and RHAMM24–26, and also link to HA indirectly through other ECM components that bind HA (e.g. versican, aggrecan, fibrinogen).14,17–19 HA has pro-migratory effects on SMC mediated in part by binding to and activating its receptor, RHAMM13,14,26,27 which also influences SMC spreading and polarity.28,29 RHAMM expression increases following cutaneous wounding and is essential for fibroplasia and wound closure.30–32
These parallels prompted the hypothesis that HA alters SMC remodeling of ECM at sites of arterial injury by activating RHAMM. To test this hypothesis, we studied the effects of blocking or knocking out RHAMM, on SMC-matrix interactions in vitro and artery wall remodeling in mice. Our findings suggest a critical role for RHAMM in mediating adhesive interactions between SMC and ECM and constrictive artery wall remodeling in vivo.
MATERIALS AND METHODS
Reagents
Collagen-I was obtained from Advanced Biomatrix and high molecular weight HA (> 1 million mw) from Sigma. Polyclonal RHAMM antibody was from Santa Cruz and nonimmune IgG from PharMingen. DMEM and FBS were from Gibco and agarose from FMC Bioproducts.
SMC culture
Aortas were harvested from mice and Sprauge Dawley rats and adventitia and endothelium removed. The media was minced into 1 mm pieces, plated onto 30 mm dishes, covered with DMEM-10% serum and cultured in 5% CO2 at 37°C. Outgrowth SMCs were expanded and typical morphology, growth, and staining for α-actin and sm-MHC verified.16 Cells were P2 to P5 in all assays. Absence of RHAMM expression was confirmed in rKO SMCs using RT-PCR.
SMC adhesion assay
48-well plates were precoated with collagen-I (50 μg/mL) with/without HA (25 μg/mL) at 4°C overnight then dried. SMCs (105cells/ml, 100 μl) were then seeded into wells and allowed to adhere for 1 h at 37°C. For blocking experiments, SMC were pretreated with anti-RHAMM antibody or control IgG (50 μg/ml) for 30 min at 37°C prior to seeding. After 1 hour, nonadherent cells were removed from wells by aspirating media and rinsing with additional PBS. Cells were then pelletted and resuspended in 1 ml media. Adherent cells were then released from each well with trypsin and similarly pelletted and resuspended. Aliquots were counted using a hemocytometer to calculate numbers of cells in each sample and their fraction of the total.
Migration assay
Scratch assays were performed as described13. SMCs were grown to confluence in 24-well plates with DMEM-10% FBS then serum-starved for 36 hrs. For blocking experiments, quiescent cells were pretreated for 30 min with anti-RHAMM antibody or non-immune IgG (50 μg/ml). A uniform scratch wound was created with a sterile pipet tip and wound area calculated from digital photomicrographs at 0 and 48 hours. Healing was calculated as % denuded area resurfaced by SMC after 48 hours from digital images (ImagePro, Media Cybernetics).
Gel contraction assay
ECM remodeling was measured after seeding SMCs into discs of collagen-I, with/without HA16. Collagen solution (2 mg/ml in DMEM) with/without HA (1 mg/ml) at pH 7.4 was mixed 1:1 with SMC (1.5 × 105 cells/ml). Using 24 well plates, 500 μl was added to each well and cast in the shape of the well-bottom by polymerizing at 37°C. Gels were then covered with media and diameter measured (mm) at 0, 4, 8, 12, and 18 hrs. For blocking experiments, SMC were pretreated with anti-RHAMM or control IgG (50 μg/ml) at 37°C for 30 minutes before creating gels.
Gels were fixed in formalin after contracting, embedded on-edge in paraffin, and adjacent sections were cut midgel and stained for cell morphology (H&E) or collagen (picrosirius red). Collagen fibers were imaged under polarized light and deposition at the SMC surface measured from digital images by thresholding pixel intensity (ImagePro). Labeled pixel number was expressed as percent of total pixels in a ROI extending 50 μm from each SMC plasma membrane. Fifty cells were assayed per gel and 10 gels per condition.
Mouse carotid ligation model
rKO mice and WT littermates (C57B6 background, age 10 weeks, mean weight 28.0 gm, n=17 and 16/group, respectively) were obtained from Dr. Eva Turley, University of Western Ontario, and have been previously characterized.31,32 Mice underwent unilateral carotid ligation as described.33–35 Anesthesia was induced with halothane then maintained with intraperitoneal ketamine (110 mg/kg) and xylazine (6.4 mg/kg). Carotid arteries were exposed through a midline neck incision and the left common carotid ligated at its bifurcation using 7-0 suture. Incisions were closed with absorbable suture and mice recovered then returned to group housing. All procedures followed the NIH guide for the Care and Use of Laboratory Animals and guidelines of the Institution Animal Care and Use Committee at Wake Forest.
Artery wall histology and morphometry
Animals were anesthetized on Day 28, heparinized, and vasculature rinsed with Ringer’s via the left ventricle and vented from the cava. Carotid arteries were fixed in situ with formalin then removed en bloc with surrounding tissues and embedded in paraffin. Serial cross-sections were cut at 50 μm intervals starting 800 μm proximal to the left common carotid ligature. Verhoeff-Van Gieson (VVG) stain was used to define the internal and external elastic lamina (IEL, EEL) and image analysis software (ImagePro) used to measure areas bounded by the outer adventitia, EEL (artery wall size), IEL, and lumen. Adventitial, medial, and neointimal areas were then calculated by subtraction. Neointimal cell density was measured in cross-sections of ligated carotids by counting nuclei in contiguous non-overlapping X400 fields and the average number of nuclei/μm2 recorded. Comparisons were then made between groups. Collagen deposition was detected in cross-sections by picrosirius red staining viewed with polarized light. HA was localized using biotinylated HA-binding protein (1:20 dilution) and the ABC reaction as described.2,10
Statistics
In vitro assays were run with multiple samples per condition and repeated at least three times to establish consistency. Data were averaged within and between experiments to determine a mean of means±SD for each endpoint. In vivo morphometric data were averaged from 5 cross sections/artery and mean of means±SD determined for each group. Differences between groups were compared using 2-tailed Student’s t test with significance assigned at p=0.05.
RESULTS
Arterial SMC culture and genotype
SMC cultured from rat and mouse aortas exhibited typical hill-and-valley morphology and expressed α-actin and sm-MHC. Growth rates and morphology were similar for rKO and WT SMC and loss of RHAMM expression confirmed in knockout lines (Figure 2).
Figure 2.
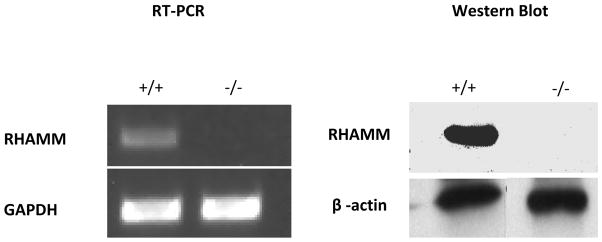
RHAMM mRNA and protein expression in aortic SMCs cultured from WT and rKO mice measured with reverse transcriptase polymerase chain reaction (RT-PCR) and western blot.
RHAMM promotes SMC adhesion to collagen and HA
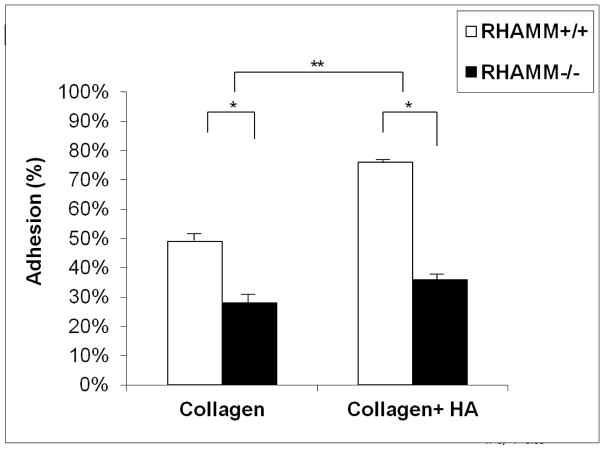
We established the timing of SMC adhesion to plastic pre-coated with collagen and found ~50% attached within one hour. HA significantly increased adhesion to collagen (% adherent: collagen+HA, 83.2±2.2% vs. collagen, 66.4±2.3, p=0.01, N=4) while blocking RHAMM with antibody significantly reduced adhesion (% adherent: 48.8±1.9 vs. 80.6±2.4, p= 0.025, N=4). This effect was mirrored by loss of RHAMM expression, which significantly inhibited adhesion to collagen with/without HA for rKO SMC (p< 0.05, Figure 3).
Figure 3.
Chart shows number of cells adherent to plastic coated with either type-I collagen alone or collagen with HA. Adherent cell number expressed as percent of total SMCs loaded per well. HA increased adhesion by 35.5±1.9% compared to collagen alone (**p=0.01,n=4) and knocking out RHAMM significantly reduced adhesion to both matrix coatings (n=4 independent experiments, * p<0.05).
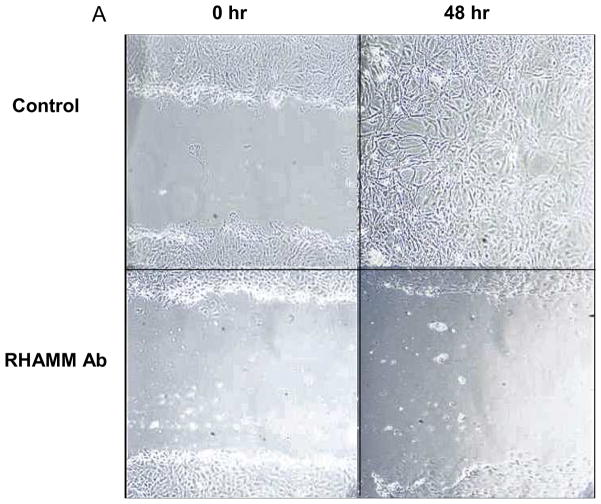
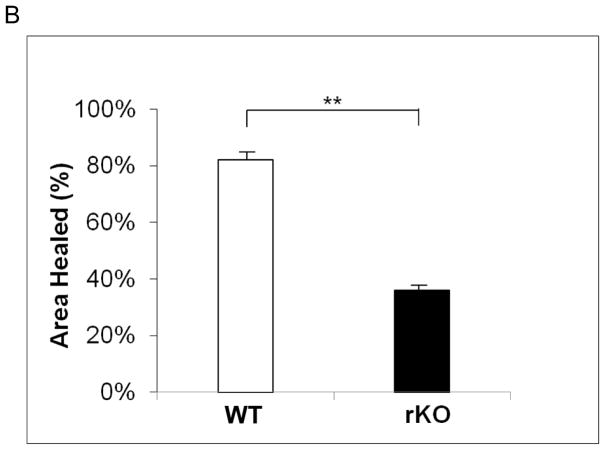
RHAMM stimulates SMC migration
HA signaling through RHAMM can promote SMC migration.26,27 We confirmed that blocking RHAMM inhibited migration, as scratch wound coverage at 48 hours was significantly reduced by blocking antibody (% wound area covered: 8.2±2.3 vs. 92.2±2.2, p=0.008, N=4). The impact of RHAMM deletion on SMC migration has not previously been described. WT SMCs migrated into and nearly completely resurfaced scratch wounds within 48 hours whereas migration was significantly less for rKO (% wound covered: 36.3±3.5 vs. 82.6±2.7, p=0.01, Figure 4).
Figure 4.
Scratch Wound Migration Assay. A. Photomicrographs show representative scratch wounds, acutely and then 48 hours later for SMC treated with control IgG (above) or anti-RHAMM blocking antibody (below). RHAMM blockade inhibited migration into scratch wounds whereas control wounds were nearly confluent at 48 hours. B. Bar chart showing % area healed by WT and rKO SMCs. Loss of RHAMM significantly reduced extent of migration into scratch wound of confluent SMC culture compared with WT controls at 48 hours. (** p=0.01,n=4).
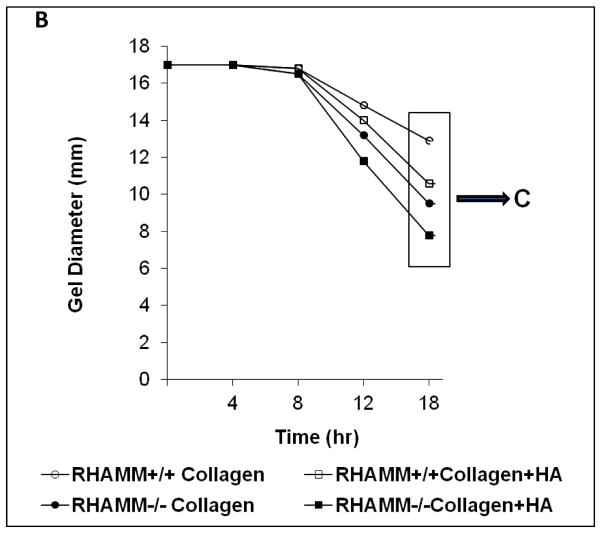
RHAMM modulates HA-induced collagen gel remodeling
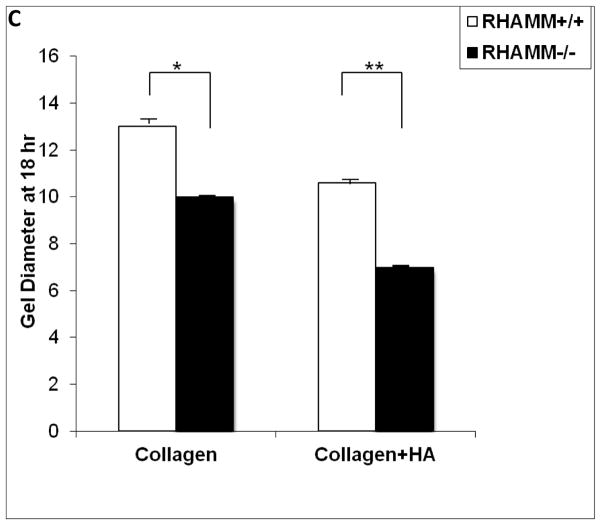
We previously reported HA enhances contraction of collagen gels by primate SMCs16. We confirmed a similar effect of HA on rat SMC (Figure 5A) and to address the contribution of RHAMM, SMC were pre-treated with blocking antibody or control IgG. Surprising to us, gel contraction increased after RHAMM blockade (gel diameter, mm: 6.8±0.1 vs. 10.0±0.1, p<0.01, N=6) and a consistent effect was observed for SMCs from rKO and WT mice. HA increased collagen contraction by WT SMC (gel diameter, mm: collagen, 12.8±0.2 vs. collagen-HA, 10.5±0.13, p=0.01, N=6, Figure 5A, B) at the end of 18 hrs and loss of RHAMM expression further increased the extent of gel contraction by rKO cells (gel diameter: collagen, 9.8±0.1 vs. collagen-HA, 6.7±0.1, p≤0.015 for both vs. WT, N=6, Figure 5B, C).
Figure 5.
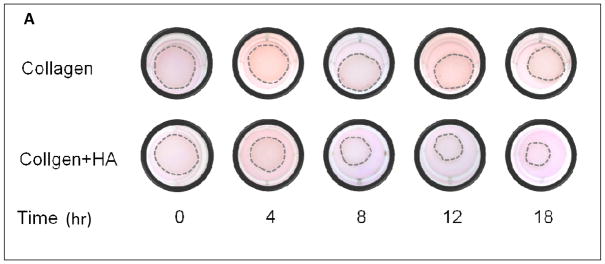
Collagen Gel Contraction Assay. A. Photograph of a series of collagen gels with and without HA floating in media (dashed outline). Collagen-I alone (top row) and with HA (bottom row) seeded with WT SMCs. HA enhanced contraction of collagen resulting in smaller gel diameter at 18 hr. B. Line chart summarizes mean gel diameter ± SD at each time point for WT SMC in collagen (○), versus collagen + HA (□), and for rKO SMC in collagen (●), versus collagen + HA (■). Data represent mean ± SD from 6 independent experiments. C. Bar chart compares final gel diameter at 18 hrs. Loss of RHAMM significantly increased contraction (smaller gel diameter, *p=0.015, **p<0.01,n=6).
RHAMM inhibits pericellular collagen condensation
SMCs accumulate collagen fibrils at the cell-surface as they contract collagen gels. This may result from traction applied via collagen receptors (e.g. β1 integrins) on the SMC surface or indirectly through traction on other ECM (e.g. HA) bound to collagen fibers. We previously demonstrated increased collagen condensation around SMC in gels containing HA, that paralleled HA-enhanced gel contraction.16 Consistent with this relationship, rKO cells showed more pericellular collagen than WT (pericellular collagen, % ROI: 78±3.2 vs. 47±2.9, p<0.05, Figure 6).
Figure 6.
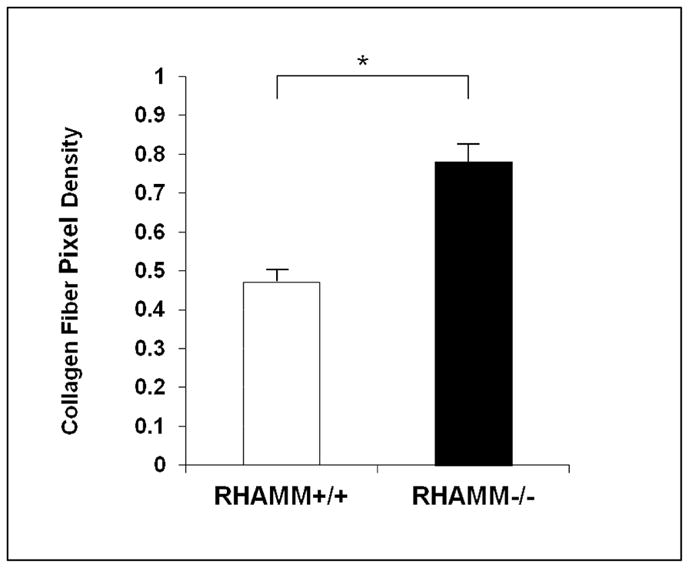
Pericellular Accumulation of Collagen Fibers. Collagen fibers at the surface of SMC was measured in gels at 18 hrs, expressed as image area occupied by picrosirius red-stained fibers viewed under polarized light. Condensation was enhanced around rKO compared with WT SMCs. Data are mean ± SD of 100 cells imaged from each condition. * p<0.05.
RHAMM promotes constrictive artery remodeling
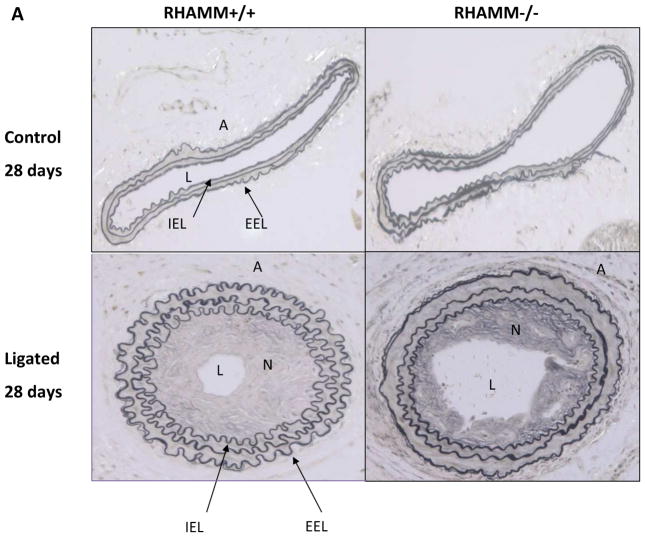
Three control mice and one knockout expired after surgery, leaving 13 and 16, respectively for analysis 28 days after ligation. Unligated arteries from both groups demonstrated normal histology with a thin loose adventitia, thin media, and an endothelial monolayer (Figure 7A). Morphometry showed no differences in lumen, media or adventitial areas or artery size (EEL area) between groups (p=NS). Following ligation, WT carotids showed significant constrictive remodeling with artery caliber decreasing 62±1.2% compared to unligated carotids (EEL area, μm2: 51.3±5.9×103 vs. 133.7±6.3×103, p=0.025). Ligation also induced neointimal hyperplasia and modest medial thickening (Figure 7). Constrictive remodeling and hyperplasia combined to reduce lumen area by 90±1.8% at 28 days in WT mice (lumen area, μm2: ligated, 10.4±1.8×103 vs. unligated, 113.4±5.5×103, p<0.05).
Figure 7.
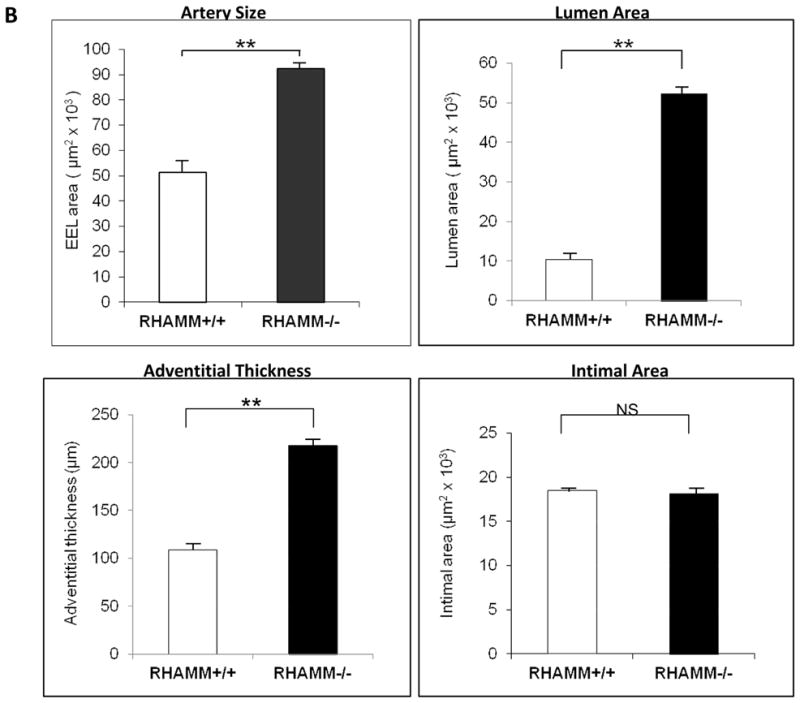
Carotid Artery Histology and Morphometry. A. Upper panel shows artery wall architecture (adventitia, media, intima) in unligated (control) carotid arteries of WT and rKO mice, which was normal and similar for both 28 days after contralateral carotid ligation. Bottom panel shows ligated carotid arteries for both groups. WT mice demonstrated more shrinkage of the ligated artery wall and lumen (constrictive remodeling) than rKO mice. B. Morphometry data for the ligated arteries from each group are summarized in bar charts. rKO mice (n=16) demonstrated significantly thicker adventitia, larger artery (EEL area), and larger lumen area but not difference in neointimal hyperplasia. A, adventitia; N, neointima; L, lumen; IEL, internal elastic lamina; EEL, external elastic lamina; **, p≤0.01. All panels, X200 magnification.
Loss of RHAMM expression did not significantly impact neointimal thickening (p=NS, Figure 7B) or neointimal cell density (nuclei/μm2×103: rKO, 4.0±0.7 vs. WT, 3.6±0.5, P=NS) 28 days post-ligation but wall caliber was much larger in rKO mice (EEL area, μm2: 92.4±4.7×103 vs. 51.3±5.9×103, p=0.015) indicating less wall constriction. As a result, lumen caliber was much larger than in WT controls (lumen area, μm2: 52.4±1.4×103 vs. 10.4±1.8×103, p=0.01, Figure 7).
Improved remodeling associated with adventitial thickening
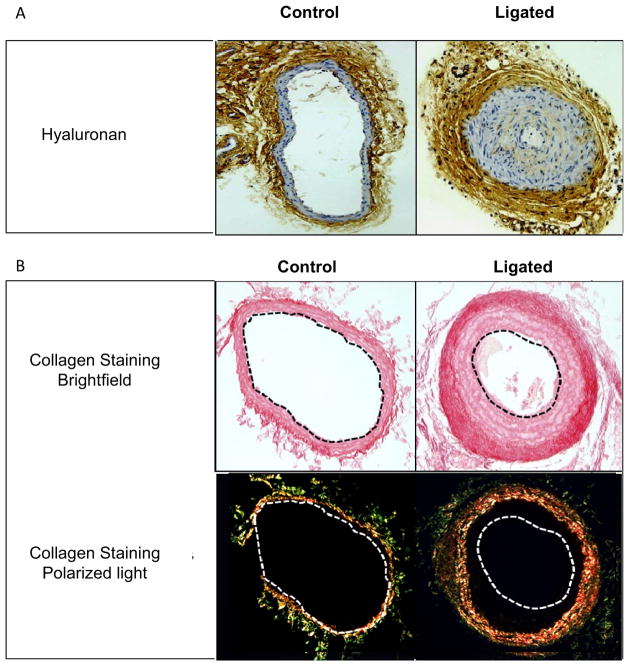
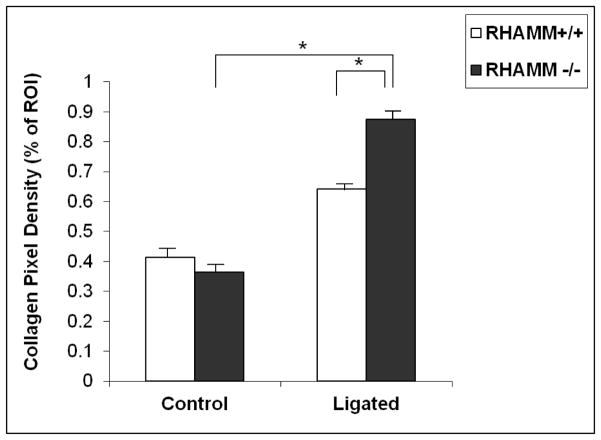
Adventitial thickening is common after angioplasty and investigators have speculated that adventitial fibrosis contributes to wall shrinkage. Ligation caused significant adventitial thickening, HA deposition, and collagen accumulation in both groups of mice (Figure 8). However, loss of RHAMM expression was associated with greater thickening (thickness, μm: rKO, 218.4±12.2 vs. WT, 109.2±7.9, p=0.01, Figure 7B) and increased collagen fiber density (labeled pixels, %ROI: rKO, 88.3±2.2 vs. WT, 62.6±2.3, p<0.05, Figure 9).
Figure 8.
Collagen and HA deposition 28 days after carotid ligation. A. HA (brown staining) accumulated in neointima and adventitia of ligated compared to unligated carotid arteries (control). B. Picrosirius red staining for collagen fibers viewed under brightfield (red staining, top) and polarized light (darkfield, bottom) in control and ligated carotid arteries of WT mice shows significant collagen fiber condensation in the adventitia of ligated arteries after 28 days. All panels, X200 magnification.
Figure 9.
Picrosirius staining of adventitial collagen in both WT and rKO arteries 28 days following ligation, quantified using thresholding of digital images viewed under polarized light. Collagen fiber density was higher in rKO mice (n=16) compared with WT controls (n=13). *p<0.05, ROI- region of interest.
DISCUSSION
Presently there are no pharmacological approaches to prevent constrictive wall remodeling following arterial reconstruction as the molecular regulation of wall shrinkage is incompletely defined.1,2,5, Expression profiling shows marked up-regulation of ECM genes after angioplasty that overlaps substantially with programs of gene expression induced during wound healing.2,9,11 For example, HA is prominent in cutaneous wounds where it promotes wound closure and collagen remodeling and limits fibrosis.6,17,19,21,22,31,32 HA also increases dramatically in the injured artery where it colocalizes with new collagen.2,10 HA increases collagen contraction by SMC and adventitial fibroblasts, suggesting a role for HA in regulating constrictive remodeling after injury.16
SMCs bind HA in the ECM using specific receptors, predominantly RHAMM and CD44.24–26 We focused on RHAMM because of its importance in regulating cell movement and shape, wound healing and fibrosis.13,26–32 SMC upregulate RHAMM after injury where it controls cytoskeletal polarity, formation of lamellapodia, and migration.13,26,29 Changes in shape, movement, and matrix remodeling are interrelated so we hypothesized HA-enhanced collagen remodeling by arterial SMC is mediated by RHAMM, contributing to constrictive remodeling in vivo.
Our key findings were that blocking or deleting RHAMM inhibited SMC attachment to collagen and inhibited migration, but promoted contraction of collagen gels. Further, loss of RHAMM reduced constrictive artery wall remodeling in knockout mice and improved lumen caliber without altering neointimal thickening. These findings suggest that HA works through RHAMM to enhance SMC adhesion and migration in culture and to promote constrictive artery wall remodeling and lumen stenosis in vivo.
Inhibition of migration is the archetypal response to RHAMM-blockade in the literature.13,26–28 We confirmed a polyclonal RHAMM antibody nearly completely abolished migration of SMC into scratch wounds, presumably by preventing extracellular HA from ligating and activating RHAMM at the cell surface. Activation of intracellular RHAMM could induce distinct signaling, so it was important to determine whether complete loss of RHAMM similarly affected migration. This is the first report to document that complete loss of RHAMM expression similarly impairs SMC migration.
Matrix remodeling depends in part on direct cell-ECM adhesive interactions and the impact of HA on initial SMC attachment has not previously been reported. We found HA consistently increased SMC attachment to plates pre-coated with collagen and that blocking or deleting RHAMM impaired SMC adhesion to collagen with or without exogenous HA, suggesting endogenous HA production is sufficient to activate RHAMM. While effects of RHAMM inhibition on SMC adhesion has not previously been reported, others have addressed the role of HA on adhesion of skin fibroblasts and endothelial cells.36,37 Attachment of these cell to dishes pre-coated with HA was less efficient than to dishes pre-coated with collagen or Matrigel alone. However, these experiments differed from our approach by not combining HA with collagen.
HA promoted collagen gel contraction in vitro but this effect is likely mediated by other HA receptors, as blocking RHAMM did not block contraction but rather increased gel shrinkage suggesting a potential release of inhibition. The effect was mirrored by results with rKO SMC, which contracted gels more than WT expressing RHAMM. This is the first study to suggest RHAMM may temper HA-enhanced remodeling of collagen by vascular SMC. We previously reported an opposite effect for CD4416, where blocking antibody inhibited gel contraction and pericellar collagen condensation. Together, these data suggest distinct HA signaling pathways balance the extent of collagen remodeling. Blocking RHAMM may have shifted the balance toward contraction mediated by CD44. In contrast, Bagli et al reported that a peptide targeting HA binding sites for RHAMM reduced collagen gel contraction by bladder SMC.38 Differences in experimental design likely explain this paradox, as we manipulated RHAMM directly while they targeted RHAMM binding sites on the HA backbone. Also, they SMCs seeded onto the surface of collagen that was already polymerized, which may require that SMCs first migrate into the gel interior to induce contraction. If so, any inhibitory effect of the peptide on migration could indirectly limit contraction. We distributed SMC throughout the collagen before polymerization, likely limiting anti-migratory impact of treatment on collagen remodeling.
Blocking or deleting RHAMM also increased accumulation of collagen at the SMC surface. If loss of RHAMM results in a compensatory increase CD44 activity, internalization of HA through CD44 could increase, pulling any collagen fibers attached to the HA backbone to the cell surface where it can be condensed into fibers. Other investigators have documented crosstalk between CD44 and RHAMM establishing a precedent for co-modulation of receptor activity.32
This is the first report suggesting RHAMM promotes constrictive remodeling and lumen narrowing in vivo as loss of RHAMM significantly increased vessel wall and lumen caliber. Others have speculated that blocking RHAMM might reduce restenosis by inhibiting neointimal hyperplasia.39 Migration contributes to neointimal formation so we were surprised that loss of RHAMM had no impact on neointimal area after ligation. However, we did not directly measure SMC migration in the mouse artery wall so future experiments are warranted to determine whether SMC migration is in fact impaired in rKO mice in vivo. This underscores a potential disconnect between in vitro observations and in vivo results and the importance of confirmatory studies in appropriate pre-clinical models.
While loss of RHAMM did not alter neointimal area, we documented a significant increase in adventitial thickening from collagen deposition. Adventitial fibrosis is common at sites of angioplasty and we and others have speculated “belt-like” constriction from adventitial fibroblasts could contribute to restenosis.1,2,40 It was surprising then that fibrosis was greater in the group with the least constrictive remodeling. We observed a similar paradox in monkey iliac arteries one month following angioplasty where adventitial thickness and collagen deposition were greater in arteries that maintained caliber versus constriction (RL Geary, unpublished observations). These findings challenge our understanding of the role of adventitia in artery wall repair and it remains unclear whether the increase in adventitial area in rKO arteries helped to inhibit wall constriction.
We employed the mouse carotid ligation model because changes in artery geometry (constrictive remodeling) are more consistent than after wire or balloon injury33 and ligation also induces HA and collagen deposition within the artery wall as after balloon injury. This model has been widely employed by others,33–35 first developed in response to inconsistent neointimal lesions resulting from balloon or filament denudation in mice.34 Nonetheless, we recognize ligation is not a typical injury and leads to nonphysiological flow (oscillating). Other limitations include that rKO mice lack RHAMM in all cell-types, raising the possibility that cell-types contributed to favorable wall remodeling. Leukocytes are important in artery wall injury and loss of RHAMM may affect leukocyte activation and recruitment.41
In summary, multiple lines of evidence suggest RHAMM and HA mediate SMC interactions with collagen and changes in SMC shape, polarity and movement. Our findings support the potential for pharmacological inhibitors of RHAMM to improve clinical outcomes by reducing restenosis from constrictive remodeling. Additional studies are needed to confirm these results in relevant preclinical models.
Clinical Relevance.
Constrictive (inward) remodeling is a critical factor in the pathogenesis of restenosis following artery injury. Presently, no effective treatment has been developed to prevent constrictive remodeling in patients except a metal stent. In this study we demonstrated that RHAMM interacts with HA and regulates SMC attachment, motility and remodeling of extracellular matrix in vitro. In a mouse carotid ligation model, we found that RHAMM promotes constrictive artery wall remodeling and lumen narrowing as knockout of RHAMM significantly improves wall caliber and prevents lumen stenosis. These results suggested that pharmacological inhibitors of RHAMM or approaches to block RHAMM signaling could be effective components of a multi-modal strategy to prevent restenosis following arterial reconstruction.
Acknowledgments
This work was supported by NIH grant R01HL57557 (RLG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jukema JW, Verschuren JJ, Ahmed TA, Quax PH. Restenosis after PCI. Part 1: pathophysiology and risk factors. Nat Rev Cardiol. 2011;13:53–62. doi: 10.1038/nrcardio.2011.132. [DOI] [PubMed] [Google Scholar]
- 2.Geary RL, Nikkari ST, Wagner WD, Williams JK, Adams MR, Dean RH. Wound healing: a paradigm for lumen narrowing after arterial reconstruction. J Vasc Surg. 1998;7:96–106. doi: 10.1016/s0741-5214(98)70296-4. [DOI] [PubMed] [Google Scholar]
- 3.Mintz GS, Popma JJ, Pichard AD, Kent KM, Satler LF, Wong SC, Hong MK, Kovach JA, Leon MB. Arterial remodeling after coronary angioplasty. A serial intravascular ultrasound study. Circulation. 1996;94:35–43. doi: 10.1161/01.cir.94.1.35. [DOI] [PubMed] [Google Scholar]
- 4.Mondy JS, Williams JK, Adams MR, Dean RH, Geary RL. Structural determinants of lumen narrowing after angioplasty in atherosclerotic nonhuman primates. J Vasc Surg. 1997;26:875–83. doi: 10.1016/s0741-5214(97)70103-4. [DOI] [PubMed] [Google Scholar]
- 5.Pasterkamp G, de Kleijn DP, Borst C. Arterial remodeling in atherosclerosis, restenosis and after alteration of blood flow: potential mechanisms and clinical implications. Cardiovasc Res. 2000;45:843–52. doi: 10.1016/s0008-6363(99)00377-6. [DOI] [PubMed] [Google Scholar]
- 6.Oksala O, Salo T, Tammi R, Häkkinen L, Jalkanen M, Inki P, Larjava H. Expression of proteoglycans and hyaluronan during wound healing. J Histochem Cytochem. 1995;43:125–35. doi: 10.1177/43.2.7529785. [DOI] [PubMed] [Google Scholar]
- 7.Gabbiani G, Hirshcel BJ, Ryan GB, Statkov PR, Majno G. Granulation tissue as a contractile organ. J Exp Med. 1972;135:719–33. doi: 10.1084/jem.135.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott PG, Dodd CM, Tredget EE, Ghahary A, Rahemtulla F. Immunohistochemical localization of the proteoglycans decorin, biglycan and versican and transforming growth factor-β in human post-burn hypertrophic and mature scars. Histopathology. 1995;26:423–31. doi: 10.1111/j.1365-2559.1995.tb00249.x. [DOI] [PubMed] [Google Scholar]
- 9.Nikkari ST, Geary RL, Hatsukami T, Ferguson M, Forough R, Alpers CE, Clowes AW. Expression of collagen and interstitial collagenase in restenosis after carotid endarterectomy. Am J Pathol. 1996;148:777–83. [PMC free article] [PubMed] [Google Scholar]
- 10.Riessen R, Wight TN, Pastore C, Henley C, Isner JM. Distribution of hyaluronan during extracellular matrix remodeling in human restenotic arteries and balloon-injured rat carotid arteries. Circulation. 1996;93:1141–7. doi: 10.1161/01.cir.93.6.1141. [DOI] [PubMed] [Google Scholar]
- 11.Geary RL, Wong JM, Rossini A, Schwartz SM, Adams LD. Expression profiling identifies 147 genes contributing to a unique primate neointimal smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2002;22:2010–16. doi: 10.1161/01.atv.0000038147.93527.35. [DOI] [PubMed] [Google Scholar]
- 12.Martin P. Wound healing-aiming for perfect skin regeneration. Science. 1997;4:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 13.Savani RC, Wang C, Yang B, Zhang S, Kinsella MG, Wight TN, Stern R, Nance DM, Turley EA. Migration of bovine aortic smooth muscle cells after wounding injury. The role of hyaluronan and RHAMM. J Clin Invest. 1995;95:1158–68. doi: 10.1172/JCI117764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evanko SP, Angello JC, Wight TN. Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:1004–13. doi: 10.1161/01.atv.19.4.1004. [DOI] [PubMed] [Google Scholar]
- 15.Evanko SP, Tammi MI, Tammi RH, Wight TN. Hyaluronan-dependent pericellular matrix. Adv Drug Deliv Rev. 2007;59:1351–65. doi: 10.1016/j.addr.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Travis JA, Hughes MG, Wong JM, Wagner WD, Geary RL. Hyaluronan enhances contraction of collagen by smooth muscle cells and adventitial fibroblasts: Role of CD44 and implications for constrictive remodeling. Circ Res. 2001;88:77–83. doi: 10.1161/01.res.88.1.77. [DOI] [PubMed] [Google Scholar]
- 17.Fraser JRE, Laurent TC, Laurent UBG. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 18.Itano N. Simple primary structure, complex turnover regulation and multiple roles of hyaluronan. J Biochem. 2008;144:131–7. doi: 10.1093/jb/mvn046. [DOI] [PubMed] [Google Scholar]
- 19.Knudson CB, Knudson W. Hyaluronan-binding proteins in development, tissue homeostasis, and disease. FASEB J. 1993;7:1233–1241. [PubMed] [Google Scholar]
- 20.Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB J. 2006;20:9–22. doi: 10.1096/fj.05-4682rev. [DOI] [PubMed] [Google Scholar]
- 21.Buchanan EP, Longaker MT, Lorenz HP. Fetal skin wound healing. Adv Clin Chem. 2009;48:137–61. doi: 10.1016/s0065-2423(09)48006-5. [DOI] [PubMed] [Google Scholar]
- 22.Gao F, Liu Y, He Y, Yang C, Wang Y, Shi X, Wei G. Hyaluronan oligosaccharides promote excisional wound healing through enhanced angiogenesis. Matrix Biol. 2010;29:107–16. doi: 10.1016/j.matbio.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Gerdin B, Hällgren R. Dynamic role of hyaluronan (HYA) in connective tissue activation and inflammation. J Intern Med. 1997;242:49–55. doi: 10.1046/j.1365-2796.1997.00173.x. [DOI] [PubMed] [Google Scholar]
- 24.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–13. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 25.Slevin M, Krupinski J, Gaffney J, Matou S, West D, Delisser H, Savani RC, Kumar S. Hyaluronan mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol. 2007;26:58–68. doi: 10.1016/j.matbio.2006.08.261. [DOI] [PubMed] [Google Scholar]
- 26.Gouëffic Y, Guilluy C, Guérin P, Patra P, Pacaud P, Loirand G. Hyaluronan induces vascular smooth muscle cell migration through RHAMM-mediated PI3K-dependent Rac activation. Cardiovasc Res. 2006;72:339–48. doi: 10.1016/j.cardiores.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 27.Hall CL, Lange LA, Prober DA, Zhang S, Turley EA. pp60(c-src) is required for cell locomotion regulated by the hyaluronan receptor RHAMM. Oncogene. 1996;13:2213–24. [PubMed] [Google Scholar]
- 28.Turley EA. Purification of a hyaluronate-binding protein fraction that modifies cell social behavior. Biochem Biophys Res Commun. 1982;108:1016–24. doi: 10.1016/0006-291x(82)92101-5. [DOI] [PubMed] [Google Scholar]
- 29.Silverman-Gavrila R, Silverman-Gavrila L, Hou G, Zhang M, Charlton M, Bendeck MP. Rear polarization of the microtubule-organizing center in neointimal smooth muscle cells depends on PKCα, ARPC5, and RHAMM. Am J Pathol. 2011;178:895–910. doi: 10.1016/j.ajpath.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lovvorn HN, 3rd, Cass DL, Sylvester KG, Yang EY, Crombleholme TM, Adzick NS, Savani RC. Hyaluronan receptor expression increases in fetal excisional skin wounds and correlates with fibroplasia. J Pediatr Surg. 1998;33:1062–9. doi: 10.1016/s0022-3468(98)90532-2. [DOI] [PubMed] [Google Scholar]
- 31.Tolg C, Poon R, Fodde R, Turley EA, Alman BA. Genetic deletion of receptor for hyaluronan-mediated motility (Rhamm) attenuates the formation of aggressive fibromatosis (desmoid tumor) Oncogene. 2003;22:6873–82. doi: 10.1038/sj.onc.1206811. [DOI] [PubMed] [Google Scholar]
- 32.Tolg C, Hamilton SR, Nakrieko KA, Kooshesh F, Walton P, McCarthy JB, Bissell MJ, Turley EA. Rhamm−/− fibroblasts are defective in CD44-mediated ERK1,2 motogenic signaling, leading to defective skin wound repair. J Cell Biol. 2006;175:1017–28. doi: 10.1083/jcb.200511027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearce JD, Li J, Edwards MS, English WP, Geary RL. Differential effects of Rho-kinase inhibition on artery wall mass and remodeling. J Vasc Surg. 2004;39:223–8. doi: 10.1016/s0741-5214(03)01037-1. [DOI] [PubMed] [Google Scholar]
- 34.Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol. 1997;17:2238–44. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- 35.Godin D, Ivan E, Johnson C, Magid R, Galis Z. Remodeling of carotid artery is associated with increased expression of matrix metalloproteinase in mouse blood flow cessation model. Circulation. 2000;102:2861–66. doi: 10.1161/01.cir.102.23.2861. [DOI] [PubMed] [Google Scholar]
- 36.Savani RC, Cao G, Pooler PM, Zaman A, Zhou Z, DeLisser HM. Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J Biol Chem. 2001;276:36770–8. doi: 10.1074/jbc.M102273200. [DOI] [PubMed] [Google Scholar]
- 37.Lokeshwar VB, Selzer MG. Differences in hyaluronic acid-mediated functions and signaling in arterial, microvessel, and vein-derived human endothelial cells. J Biol Chem. 2000;275:27641–9. doi: 10.1074/jbc.M003084200. [DOI] [PubMed] [Google Scholar]
- 38.Bägli DJ, Joyner BD, Mahoney SR, McCulloch L. The hyaluronic acid receptor RHAMM is induced by stretch injury of rat bladder in vivo and influences smooth muscle cell contraction in vitro. J Urol. 1999;162:832–40. doi: 10.1097/00005392-199909010-00071. [DOI] [PubMed] [Google Scholar]
- 39.Savani RC, Turley EA. The role of hyaluronan and its receptors in restenosis after balloon angioplasty: development of a potential therapy. Int J Tiss Reac. 1995;17:141–151. [PubMed] [Google Scholar]
- 40.Okamoto E, Couse T, De Leon H, Vinten-Johansen J, Goodman RB, Scott NA, Wilcox JN. Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation. 2001;104:2228–35. doi: 10.1161/hc4301.097195. [DOI] [PubMed] [Google Scholar]
- 41.Krupinski J, Ethirajan P, Font MA, Turu MM, Gaffney J, Kumar P, Slevin M. Changes in Hyaluronan Metabolism and RHAMM Receptor expression accompany formation of complicated carotid lesions and may be pro-angiogenic mediators of intimal neovessel growth. Biomark Insights. 2007;2:361–7. [PMC free article] [PubMed] [Google Scholar]