SUMMARY
Nursing has important physiological and psychological consequences on mothers during the postpartum period. Tuberoinfundibular peptide of 39 residues (TIP39) may contribute to its effects on prolactin release and maternal motivation. Since TIP39-containing fibers and the receptor for TIP39, the parathyroid hormone 2 receptor (PTH2 receptor) are abundant in the arcuate nucleus and the medial preoptic area, we antagonized TIP39 action locally to reveal its actions. Mediobasal hypothalamic injection of a virus encoding an antagonist of the PTH2 receptor markedly decreased basal serum prolactin levels and the suckling-induced prolactin release. In contrast, injecting this virus into the preoptic area had no effect on prolactin levels, but did dampen maternal motivation judged by reduced time in a pup-associated cage during a place-preference test. In support of an effect of TIP39 on maternal motivation, we observed that TIP39 containing fibers and terminals had the same distribution within the preoptic area as neurons expressing Fos in response to suckling. Furthermore, TIP39 terminals closely apposed the plasma membrane of 82% of Fos-ir neurons. Retrograde tracer injected into the arcuate nucleus and the medial preoptic area labeled TIP39 neurons in the posterior intralaminar complex of the thalamus (PIL), indicating that these cells but not other groups of TIP39 neurons project to these hypothalamic regions. We also found that TIP39 mRNA levels in the PIL markedly increased around parturition and remained elevated throughout the lactation period, demonstrating the availability of the peptide in postpartum mothers. Furthermore, suckling, but not pup exposure without physical contact, increased Fos expression by PIL TIP39 neurons. These results indicate that suckling activates TIP39 neurons in the PIL that affect prolactin release and maternal motivation via projections to the arcuate nucleus and the preoptic area, respectively.
Keywords: maternal behavior, rat dams, suckling, prolactin release, brain circuitry, preoptic area, hypothalamus, ascending neuronal pathway
INTRODUCTION
Nursing plays a pivotal role in control of mothers motivation and lactation (Numan et al., 2006). Rat dams that ignore or even hurt pups without maternal sensitization, for instance, vigorously protect them after giving birth (Brunton and Russell, 2008). These abrupt shifts in motivation are also accompanied by metabolic and endocrine adaptations necessary for milk production (Russell et al., 2001; Woodside, 2007). To support lactation, prolactin levels increase enormously in rat dams, and pup suckling is an important stimulus for this (Neville, 2006). While oxytocin (Bosch and Neumann, 2012) and possibly prolactin, too, contribute to maternal motivation (Grattan et al., 2008), suckling can also directly activate specific neuronal pathways to brain centers for maternal behavior (Stern and Lonstein, 2001; Brunton and Russell, 2008). Bilateral lesion of the hypothalamic preoptic area, or the combination of a unilateral lesion with a coronal transection posterior to the preoptic area on the contralateral side of the brain lead to the cessation of maternal care (Olazabal et al., 2002; Numan and Woodside, 2010). Thus, while dopaminergic cells residing in the arcuate nucleus are responsible for suckling-induced prolactin release (Freeman et al., 2000), maternal behaviors are largely regulated by the preoptic area. Although it is known that ascending pathways regulate hypothalamic maternal centers, it has yet to be shown how information about suckling reaches the hypothalamus, and which neurotransmitters are involved in this information transfer.
In earlier studies, we identified a neuropeptide that we named ‘tuberoinfundibular peptide of 39 residues’ (TIP39) based on its abundance and that of its receptor, the parathyroid hormone 2 (PTH2) receptor, in the mediobasal hypothalamus (Usdin et al., 1999; Dobolyi et al., 2010). TIP39 neurons are present in three brain regions, the periventricular gray and the ‘posterior intralaminar complex’ (PIL) of the thalamus and the medial paralemniscal nucleus in the lateral pons (Dobolyi et al., 2002; Dobolyi et al., 2003). TIP39 levels decrease markedly in all three areas during early postnatal development (Dobolyi et al., 2006b; Brenner et al., 2008). We previously found that in postpartum day 9 dams TIP39 levels are dramatically elevated over that of non-lactating dams in the PIL and the medial paralemniscal nucleus, but not in the periventricular gray of the thalamus (Cservenak et al., 2010; Varga et al., 2012). We also observed that pup exposure induces Fos in TIP39 neurons of the PIL and the medial paralemniscal nucleus (Cservenak et al., 2010; Varga et al., 2012). In addition, the body weight of pups reared by dams lacking TIP39 signaling is reduced during the lactation period (Coutellier et al., 2011). Since TIP39 fibers and the PTH2 receptor are abundant in the preoptic area and arcuate nucleus (Faber et al., 2007), we have now addressed whether TIP39 neurons convey suckling information to the hypothalamus that regulates maternal motivation and elicit prolactin release. We previously showed that intracerebroventricular injection of a PTH2-R antagonist inhibited suckling stimulated prolactin release (Cservenak et al., 2010). In this study, to learn more about the potential roles of TIP39 during lactation and to clarify its site(s) of action, we antagonized TIP39 actions in the arcuate nucleus or in the preoptic area by means of a virus expressing an antagonist of the PTH2 receptor, and measured maternal motivation, behavior, and the prolactin release. To determine the origin of TIP39 fibers in the arcuate nucleus and the preoptic area, we injected retrograde tracer into these sites and examined the labeling of TIP39 neurons. We also evaluated the time course of TIP39 expression around and during the period of lactation. To test whether suckling itself is the proper signal that activates TIP39 neurons, we compared Fos activation in PIL TIP39 neurons of suckling mothers and mothers with only visual, auditory, and olfactory interaction with their pups.
MATERIALS AND METHODS
Animals
This study was approved by the Semmelweis University, Budapest, Animal Examination Ethical Council of the Animal Protection Advisory Board. Procedures involving rats were carried out in accordance with the Hungarian Ministry of Agriculture's Animal Hygiene and Food Control Department guidelines for experimental protocols and with EU Directive 2010/63/EU for animal experiments.
A total of 106 mother and 10 control female rats (Wistar; Charles Rivers Laboratories, Hungary) were used (12 for retrograde tracer studies, 25 for TIP39 in situ hybridization, 15 for Fos activation, 28 for prolactin measurement, and 26 mothers and 10 control females for the behavioral tests). All animals were 90-120 days old when sacrificed. Animals were kept under standard laboratory conditions with 12-h light, 12-h dark periods (lights on at 6.00 AM), and supplied with food and drinking water ad libitum. Pregnant and mother rats were housed individually in standard white cages (41 × 22 × 19 cm) or in blue cages (35 × 28 × 22 cm) during place preference conditioning. Mother rats delivered their pups on day 22 of pregnancy. Mothers who delivered fewer than 8 pups or whose pups died were excluded from the study. The number of pups was adjusted to 8 within 2 days of delivery. For surgery, perfusions, and dissections, rats were anesthetized with an intramuscular injection of anesthetic mix containing 0.2ml/300g body weight ketamine (100 mg/ml) and 0.2ml/300g body weight xylazine (20 mg/ml).
Virus preparation and injection
Two lentiviral vectors were prepared: a HYWH-GFP virus was designed to express a secreted form of histidine4, tyrosine5, tryptophan6, histidine7-TIP39 (HYWH-TIP39) an antagonist of the PTH2 receptor (Kuo and Usdin, 2007), plus GFP that remained within infected cells and allowed their visualization, while a control virus expressed GFP only. To produce th HYWH-GFP lentiviral vector, we used a combination of oligonucleotide synthesis, PCR, and conventional subcloning techniques. In the resulting construct, a strong mammalian promoter (EF-1α) drives expression of a fusion protein between the fibronectin leader sequence with signal peptide cleavage site and the HYWH-TIP39 sequence a PTH2 receptor antagonist (Kuo and Usdin, 2007). This is followed by an internal ribosome reentry site (IRES) and then enhanced green fluorescent protein (EGFP) sequence and a woodchuck hepatitis post-transcriptional regulatory element (WPRE) as described previously (Dimitrov et al., 2013). In brief, two oligonucleotides (ATGCTCAGGGGTCCGGGACCCGGGCGGCTGCTGCTGCTGGCAGTCCTGTGCCTGGGGACC and AGGACACGGACCCCTGGAGCCACGCGACGTGGCTTCGGCCCTTCTCGTTCTCCTCGGACCGCGTAATGACCGTAC) were annealed and extended to generate the fibronectin signal sequence and the 5’ end of HYWH-TIP39. A PCR reaction containing this product, plus the two following oligonucleotides: GCATGAGCTCGCCGCCACCATGCTCAGGGGTCCGGGA and GCATGGATCCTCAGGGCGCGTCCAGCA, plus a plasmid encoding HYWH-TIP39 produced a fibronectin signal sequence/HYWH-TIP39 fusion sequence that we then cloned into the PmeI site of the lentiviral vector pWPI (Addgene plasmid 12254, contributed by D. Trono, École Polytechnique Fédérale de Lausanne). Following sequencing in the NINDS intramural sequencing facility to determine correct sequence and orientation, viral particles were produced by calcium phosphate-mediated co-transfection of HEK293T cells with this plasmid, psPAX2 (Addgene plasmid 12260; D. Trono), and pMD2.G (Addgene plasmid 12259; D. Trono). A concentrated virus stock was produced by polyethylene glycol precipitation of tissue culture media followed by ultracentrifugation (Kutner et al., 2009). A titer of approximately 10e10 transducing units/ml was estimated by counting fluorescent cells 72h following infection of HEK293 cells. To produce virus encoding only GFP we processed the plasmid pFUGW-GFP (Addgene # 14883, contributed by D. Baltimore, California Inst Technology) and helper plasmids using the procedures described above for the production of HYWH-encoding virus (Lois et al., 2002).
Using stereotaxic injections (as described below for tracer injections), we targeted these viral vectors bilaterally into the mediobasal hypothalamus immediately lateral to the arcuate nucleus and also into the medial preoptic area, of female rats. Glass micropipettes of 15–20-μm internal diameter were backfilled with virus suspended in sterile saline. Once the pipette was in place, 300 nl of the virus was pressure injected. Pipettes were left in place for 10 min. After virus injections, animals were allowed to recover for 2 weeks before the start of mating. After the experiments, the animals were perfused and the position of the virus injection was determined based on the fluorescent visualization of GFP in the infected cells. Based on the design of the virus, it expresses the PTH2 receptor antagonist HYWH-TIP39 and GFP as long es the infected cell is viable. Functional evidence for the in vivo effectiveness of the virus has also been recently provided (Dimitrov et al., 2013).
Measurement of suckling-induced prolactin release
Implantation of jugular cannulae
Jugular catheters were placed on days 9-10 postpartum under gas anesthesia, one day before blood sampling began. Dams received 25-mm-long sterile polyethylene jugular cannulae (Plastics One). The right common jugular vein was exposed, a cannula was inserted into the vessel, secured in place with suture, and pulled through incisions on the skin between the scapulae. The inserted cannulae were filled with heparinized saline and sealed with metal pins.
Blood sampling
Each rat was handled before the blood sampling for 5 m per day for 3 days before the procedure. On the day of the experiment (postpartum days 10-11) at 08.00 h, a first blood sample was taken, then dams were separated from their pups for 4 hours. After 4 h separation, a second blood sample was obtained 5 min before the pups were returned to their mothers. Suckling usually started immediately, and never longer than 10 m after the return of the pups. Blood samples were taken at 5, 15, 30, and 60 min after pups were reunited with their mother. At each time point, 200 μl blood was obtained and an equal amount of sterile saline was injected into the circulation through the same canullas. Plasma was separated and stored at −20°C until assayed for prolactin.
Prolactin assay
Prolactin was measured with radioimmunoassay kits kindly provided by Dr. Alfred F. Parlow (National Hormone and Peptide Program, Harbor UCLA Medical Center, Torrance, CA, USA). As described previously, our procedure differed slightly from instructions supplied with the kit (Bodnar et al., 2005). The chloramine-T method was used for iodination, and protein A (BactASorb, Human Rt, Gödöllő, Hungary) was used to separate bound and free hormone. LKB Clinigamma software was used for data collection and calculations for curve fitting. Within-assay variance was 10%. Between-assay variance was 14%. The sensitivity of the prolactin assay was 0.5 ng/ml rat plasma (or 25 pg prolactin). All samples were analyzed in duplicate using 50 μl of plasma for each measurement.
Statistical analysis of the prolactin assay
Statistical analyses were performed using Prism 5 for Windows (GraphPad Software, Inc., La Jolla, CA). Basal plasma prolactin levels between the 2 groups (rats injected with PTH2 receptor antagonist expressing virus and rats injected with control virus) before taking away the pups were compared using Student's t-test. For the suckling experiment, plasma prolactin levels of the 2 groups were compared using two-way repeated measures ANOVA to evaluate whether the expression of the antagonist had an effect on the prolactin level. To determine at which time points the antagonist injection was effective, Bonferroni Post-Tests for posthoc comparisons were used.
Pup retrieval test
The 26 mother rats injected with virus into the preoptic area were used for testing. On postnatal day 6-7, all pups were separated from their mothers for 10 min. Subsequently, 3 pups were returned to the mother's cage in 3 different corners of the cage. The mother was visually observed for 3 min. The time required for the mother to retrieve the first, second, and third pup to the nest was recorded.
Conditioned place preference test
The procedure applied is a modification of previously described conditioned place preference tests used to investigate maternal motivation (Mattson et al., 2003; Seip and Morrell, 2009). On or about the 16th day of pregnancy, 13 control virus and 13 PTH2 receptor antagonist expressing virus injected pregnant rats were moved from their standard white cages to similar size blue cages. As an additional contextual clue for conditioning to pup association, a thick-walled oval orange tray (15 and 20 cm diameters) was placed permanently, with the litter, in the mother's blue cage on postpartum day 6-7.
Three days later, the mothers were deprived of their pups for 2h and then tested. The experimental apparatus consisted of freshly washed white and blue cages connected by a 20 cm-long tube 12 cm in diameter. The rat mothers were free to move about within the apparatus. A tray similar to the orange one used for conditioning was placed in the blue cage while a thin-walled black triangular tray (13 × 15 cm) was placed in the white cage. The illumination was the same in the 2 cages, but the tube was considerably darker. The position of the dams was monitored for 1h and the time spent in each compartment calculated.
A similar experiment was performed with non-maternal virgin female rats (5 control virus and 5 PTH2 receptor antagonist expressing virus injected) to evaluate their preference for the 2 different cages. The rats were moved from their standard white cages into the blue cages with the contextual clue for 3 days (without pups), after which they were tested with the same experimental apparatus as the mothers.
Statistical analysis of the conditioned place preference test
First, the test was validated with the analysis of time that the control virus injected animals spent in the different compartments using one-way repeated measures ANOVA followed by Bonferroni Post-Tests for posthoc comparisons. To evaluate the time the rats spent in the pup-associated vs. control cage, a time preference index was calculated as 100 * (time spent in the pup associated cage – time spent in the control cage) / (time spent in the pup associated cage + time spent in the control cage). The time index is zero if the rats spend the same amount of time in the two cages. The time preference indices of the 2 groups (rats injected with PTH2 receptor antagonist expressing virus and rats injected with control virus) were compared using Student's t-test. In an additional analysis that focused on the preference of individual animals, spending greater than 20% more time in one cage than the other was used as the cutoff for identifying cage preference. Between-group cage preference comparisons were examined using Chi-square test of independence and Fisher's exact test.
Retrograde tracer experiments
Injections of the retrograde tracer ‘cholera toxin B subunit’ (CTB from List Biological Laboratories, Campbell, CA) were targeted to the medial preoptic area (n = 6) and the arcuate nucleus (n=6), unilaterally. The relation of injection sites to the position of TIP39 fibers was evaluated by double labeling. For stereotaxic injections, rats were positioned in a stereotaxic apparatus with the incisor bar set at −3.3 mm. Holes of about 2-mm diameter were drilled into the skull above the target coordinates. Glass micropipettes of 15–20-μm internal diameter were filled with 0.25% CTB dissolved in 0.1 M phosphate buffer at pH 7.4 (PB) and lowered to the following stereotaxic coordinates (Paxinos and Watson, 2007): AP =−0.5 mm, L = 0.5 mm, V = 7.8 mm for the medial preoptic area, and AP =−2.8 mm, L = 0.2 mm, V = 9.3 mm for the arcuate nucleus. Once the pipette was in place, the CTB was injected by iontophoresis using a constant current source (51413 Precision Current Source, Stoelting, Wood Dale, IL) that delivered of +6 μA current, which was pulsed on for 7 seconds and off for 7 seconds for 15 min. Following injection, the pipette was left in place for 10 minutes with no current, was then withdrawn under negative current. Animals were sacrificed 7 days following tracer injection.
Histological analysis
Tissue collection
Rats were deeply anesthetized and perfused transcardially with 150 ml saline followed by 300 ml of ice-cold 4% paraformaldehyde prepared in PB. Brains were removed and postfixed in 4% paraformaldehyde for 24h and then transferred to PB containing 20% sucrose for 2 days. Serial coronal sections were cut at 50 μm on a sliding microtome between 1.0 and −15.0 mm bregma levels. Sections were collected in PB containing 0.05% sodium-azide and stored at 4°C.
Double labeling TIP39 and CTB
Brain sections of animals injected with CTB were processed for double labeling with CTB and TIP39. Every fourth free-floating section was first stained for TIP39 by using FITC-tyramide amplification fluorescent immunocytochemistry. An affinity-purified antiserum from a rabbit immunized with rat TIP39, which can be absorbed with synthetic TIP39 (Dobolyi et al., 2002; Dobolyi et al., 2003), and labels cell bodies with the same distribution as observed by in situ hybridization histochemistry (Dobolyi et al., 2003; Dobolyi et al., 2006a), was used as primary antiserum. This antiserum (1:3000) was applied for 48h at room temperature, followed by incubation of the sections in biotinylated donkey anti-rabbit secondary antibody (1:1000 dilution; Jackson ImmunoResearch), then in ABC complex (1:500; Vector Laboratories) for 2h. Sections were subsequently incubated with FITC-tyramide (1:8000) and H2O2 in Tris hydrochloride buffer (0.1 M, pH 8.0) for 6 min. Sections were then incubated overnight in goat anti-CTB (1:10,000; product #703, lot #7032AA, List Biological Laboratories) at room temperature. Following application of the primary antibody, sections were incubated in donkey Alexa Fluor 594 anti-goat secondary antibody (Life Technologies, Grand Island, NY) for 2h. After washes, sections were mounted and coverslipped with antifade medium (Prolong Antifade Kit; Molecular Probes, Eugene, OR).
Fos activation study
Pup exposure of mother rats
Rat dams (n=15) were deprived of pups on postpartum day 8-9 at 13:00. During separation, the litter was held together in a cage that was kept warm by a lamp. The following day at 9:00, pups were returned to the cages of 5 mother rats. All 5 mothers accepted the pups and suckling started within 5 min. Pups were returned to another 5 mothers in a way that prevented physical contact but allowed the dams to see, hear, and smell the litter through metal bars (about 3 cm distance). Control dams were not united with their litters. All rat dams were sacrificed 22h after the pups had been removed, which in relevant cases was 2h after pups were returned to their mothers. Animals were perfused transcardially and processed for Fos and TIP39 immunohistochemistry.
Fos immunohistochemistry
In each group of five brains, every fourth free-floating section was immunolabeled for Fos with DAB immunoperoxidase labeling using a rabbit anti-Fos primary antiserum (1:30000; c-Fos (4) sc-52; Santa Cruz Biotechnology, Delaware, CA). The sections were incubated in biotin-conjugated donkey anti-rabbit secondary antibody at 1:1000 (Jackson ImmunoResearch, West Grove, PA) for 1h and then in ABC complex (1:500; Vector Laboratories) for 2h and incubated in 0.02% 3,3-diaminobenzidine (DAB; Sigma), 0.08% nickel (II) sulfate, and 0.003% hydrogen peroxide in PB. Finally, the sections were mounted, dehydrated and coverslipped with Cytoseal 60 (Stephens Scientific, Riverdale, NJ).
Double immunolabeling of Fos and TIP39
One set of every fourth free-floating section from the 15 rat dams used for single-labeling of Fos was immunolabeled for TIP39 using FITC-tyramide amplification immunofluorescence, as described above. Sections were then placed in rabbit anti-Fos primary antiserum (1:10000) for 48h at room temperature and visualized with Alexa Fluor 594 donkey anti-rabbit secondary antibody, as described above. Amplification allowed the use of a dilution of the TIP39 antibody (1:3000) that could not be visualized with the Alexa Fluor 594 donkey anti-rabbit secondary antibody, as previously described (Hunyady et al., 1996).
Analysis of double immunolabeling for TIP39 and Fos in the PIL
After identifying the PIL section with the most TIP39-ir neurons in each of the 15 animals double-labeled for TIP39 and Fos, we counted all TIP39-ir neurons with an identifiable cell nucleus and all double-labeled cells. Counts were obtained using an Olympus BX60 light microscope with a 20x objective, fluorescent epi-illumination, and a filter that allows for simultaneous green and red visualization. The number of single-labeled Fos-ir cells was subsequently calculated in the area of the TIP39 neurons.
For the statistical analysis of neuronal activation in the PIL, the number of Fos-ir neurons, the number of TIP39 neurons, and the number of double labeled neurons were compared between the 3 groups (suckled dams, dams exposed to pups without physical contact, control dams not exposed to pups) using one-way ANOVA tests followed by Bonferroni Post-Tests for posthoc comparisons.
Triple-immunolabeling of Fos, TIP39, and Kv2.1
Preoptic area sections from 3 suckling mothers were double-immunolabeled for Fos and TIP39 as described above, then incubated in mouse anti-rat Kv2.1 α-subunit (1:300; catalog #75-014C9848, clone K89/34, lot #444-1LC-27, NeuroMab, Davis, CA). Then, these sections were incubated in Cy5 anti-mouse secondary antibody, mounted, and coverslipped.
In situ hybridization histochemistry for TIP39
Brains of five primiparous female rats were dissected and frozen at each of the following times: during pregnancy, at day 21; postpartum, at days 1, 9, and 23; and 1 week after weaning at postpartum day 23. In situ hybridization histochemistry was performed, as described previously (Dobolyi et al., 2002; Dobolyi et al., 2003). Briefly, serial coronal sections (12 μm) were cut, immediately mounted on positively charged slides (Superfrost Plus, Fisher Scientific, Pittsburgh, PA, USA), dried, and stored at −80°C until use. A region of the rat TIP39 cDNA sequence, corresponding to amino acids −55 to 37, where amino acid 1 is the first residue of mature TIP39, subcloned into a TOPO TA vector (Life Technologies) containing a T7 RNA polymerase recognition site was used to generate [35S]UTP-labeled riboprobes, with a MAXIscript transcription kit (Ambion, Austin, TX).
Tissue was prepared using an mRNA-locator Kit (Ambion) according to manufacturer's instructions. For hybridization, we used 80 μl hybridization buffer and 1 million DPM of labeled probe per slide. Washing procedures included a 30 min incubation in RNase A, followed by decreasing concentrations of sodium-citrate buffer (pH = 7.4) at room temperature, and then at 65°C. After drying, slides were dipped in NTB nuclear track emulsion (Eastman Kodak, Rochester, NY), stored for 3 weeks at 4°C for autoradiography, developed with Kodak Dektol developer, fixed with Kodak fixer, counterstained with Giemsa, and coverslipped.
Microscopy and image processing
Sections were examined using an Olympus BX60 light microscope equipped with fluorescent epi-illumination and a dark-field condenser. Images were captured at 2048 × 2048 pixel resolution with a SPOT Xplorer digital CCD camera (Diagnostic Instruments, Sterling Heights, MI) using 4-40 × objectives. Confocal images were acquired with a Nikon Eclipse E800 confocal microscope equipped with a BioRad Radiance 2100 Laser Scanninig System using a 20-60 × objectives at an optical thickness of 1-3 μm. Images were adjusted using the “levels” and “sharpness” commands in Adobe Photoshop CS 8.0. Full resolution of the images was maintained until the final versions, which were adjusted to a resolution of 300 dpi.
Densitometric analysis of in situ hybridization histochemistry
Dark-field photomicrographs were taken of the sections where the TIP39 signal was the highest in the PIL using a 10x objective. Each image was divided into 2 halves with identical size, such that one half contained all the observed TIP39 autoradiography signals, while the other half served as background control. The pixel number of white area (lighter than an arbitrary grayness used for all the images) was calculated for both halves of the images using ImageJ 1.47v (National Institutes of Health, USA) software. The difference between the 2 values (the half picture containing TIP39-expressing cells – the half picture containing only background autoradiography signal) was used to quantify the TIP39 mRNA level. TIP39 mRNA levels at the 5 different time points were compared using one-way ANOVA followed by Bonferroni's multiple comparison test.
The analysis of TIP39 innervation of preoptic neurons
Sections triple labeled for Fos, TIP39, and Kv2.1 potassium channel from 3 mothers were used for the analysis. Analysis was performed on serial confocal images collected at an optical thickness of 1 μm were analyzed for 32 neurons in the medial preoptic nucleus and 32 neurons in the ventral subdivision of the bed nucleus of the stria terminalis that were labeled by Kv2.1. The fraction of Fos-expressing neurons that were closely apposed by TIP39 fibers were assessed in both the medial preoptic nucleus and the ventral subdivision of the bed nucleus of the stria terminalis.
RESULTS
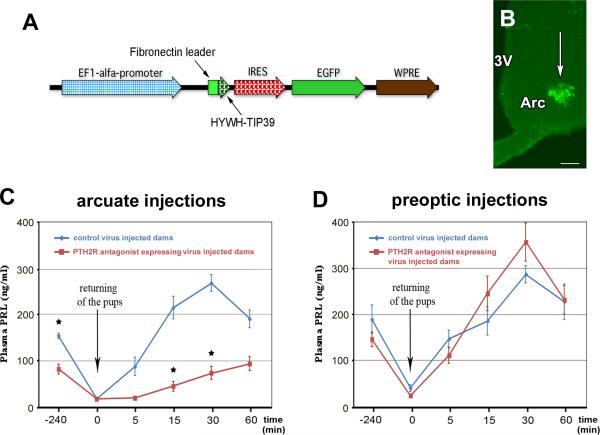
The effect of the PTH2 receptor block on the plasma prolactin level
To evaluate a potential causal relationship between TIP39 signaling and prolactin level we infected cells in the mediobasal hypothalamus near the arcuate nucleus with a virus encoding a secreted PTH2-receptor antagonist (HYWH-TIP39) and enhanced GFP (Fig. 1A). At least 10 infected cells per the injection site were seen in the most densely infected section of the animals as illustrated in Fig. 1B. Basal plasma prolactin levels in the mother rats expressing HYWH-TIP39 were significantly lower than that in the control dams (82.8±20.8 vs. 153.8±11.1 ng/ml; p<0.05). After 4h of separation from their pups, levels of plasma prolactin in the two groups of mothers had fallen to similar levels (HYWH-TIP39, 18.1±7.9, control virus 18.5±7.3 ng/ml). When pups were returned after the 4h separation period, attachment and suckling began within less than 5 min for all animals. The control virus and the PTH2 receptor antagonist-expressing virus injected animals had a significantly different prolactin response as determined using two-way repeated-measures ANOVA (F=9.962). Plasma prolactin levels in the control dams reached 215±48 ng/ml at 15 min, and 267±36 ng/ml at 30 min after reunion with their pups. In the antagonist expressing dams, the increase in plasma prolactin was significantly less, reaching only 46±21 ng/ml at 15 min and 74±28 ng/ml at 30 min after reunion (p<0.001 for both time points; Fig. 1C). In contrast, virus injected into the preoptic area did not significantly change either the basal plasma prolactin level (190±61 ng/ml in control vs. 146±31 ng/ml in the antagonist expressing rats) or the suckling-induced release of prolactin. At 15 and 30 min after returning the pups to the mothers, the concentrations (ng/ml) of prolactin were 186±62 and 287±77 in controls, and 245±75 and 357±83 in antagonist-expressing dams (Fig. 1D).
Fig. 1.
Effect of virus encoding a peptide PTH2 receptor antagonist on prolactin release. A: Structure of the viral construct expressing HYWH-TIP39, an antagonist of the PTH2 receptor. B: Hypothalamic virus injection site. The white arrow indicates the infected cells visualized with EGFP. The injection site is located just lateral to the arcuate nucleus. C: Basal and suckling-induced plasma prolactin levels in mother rats injected with the PTH2 receptor antagonist expressing virus were significantly lower than control mothers when the injections were targeted to the arcuate nucleus but not when targeted to the medial preoptic area (D). Abbreviations: Arc – arcuate nucleus, 3V – third ventricle. Scale bar = 100 μm.
Evaluation of maternal motivation after preoptic antagonism of the PTH2 receptor
Preoptic area virus injections resulted in a number of infected cells similar to the mediobasal hypothalamic injections. The behavior of mother rats that received virus injections into the preoptic area was analyzed using a place preference test. Defining a preferred cage as one that animals spend at least 20% more time in than the non-preferred cage, 10 out of 11 control dams preferred the pup associated cage. Dams injected in the preoptic area with the PTH2 receptor antagonist expressing virus had a significantly different cage preference (X2=8.023, p<0.05) as only 5 out of 13 rats preferred the pup-associated cage evaluated in this way, while 4 rats showed preference for the control cage (Fig. 2A). The amount of time spent in the different compartments was also analyzed. The control virus injected mothers did not spend an equal amount of time in the different compartments (F=3.84). Rather, they spent significantly more time in the pup-associated cage than in the control cage (25.9±1.9 vs. 15.7±2.1 min; p<0.05). In contrast, the time spent in the different compartments did not differ for the rats infected with the antagonist producing virus (F=0.783). When the time spent in the different cages were compared between the control animals and the animals expressing the PTH2 receptor antagonist expressing virus we found a significant difference (F=9.83). The mothers expressing the PTH2 receptor antagonist spent less time in the pup-associated cage than control mothers (19.1±1.7 vs. 25.9±1.9 min; p<0.05). This data is illustrated in Fig 2B as a preference index (100 * (time spent in the pup associated cage – time spent in the control cage) / (time spent in the pup associated cage + time spent in the control cage)). The preference index for control virus injected dams was significantly greater than for the antagonist injected dams (25.7±7.6 vs. 3.4±5.5; p<0.05). The latter group did not show preference as the index value was not significantly different from 0. The nulliparous females did not show significant cage preference either: the index values for the groups injected with the control, and the antagonist-expressing virus were 0.9±11.6 and −7.3±4.6, respectively. In fact, the antagonist injected nulliparous females exhibited a tendency to prefer the white cage (the control cage for mothers) indicated by the negative preference index value.
Fig. 2.
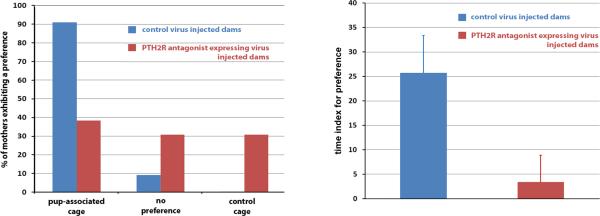
A: Mother rats with medial preoptic area control virus injection deprived of their pups for 2h demonstrate significant preference for a cage visually similar to one in which they were housed with their litter. In contrast, rats injected with a virus expressing the PTH2 receptor antagonist do not show preference for the pup associated cage. B: The time animals spent in the pup-associated and control cage is expressed as a time index for preference: 100 * (time spent in the pup associated cage – time spent in the control cage) / (time spent in the pup associated cage + time spent in the control cage). The control virus injected mother rats spend significantly more time in the preferred pup-associated cage than PTH2 receptor antagonist expressing animals. The latter group does not have preference for the pup-associated cage.
Pup retrieval time did not differ between the mothers injected in the preoptic area with the PTH2 receptor antagonist expressing and the control virus. The time required for the mothers to bring the first, second, and third pup to the nest was (in seconds) 43±17 vs. 37±18, 74±18 vs. 81±20, and 111±14 vs. 123±24 for the PTH2 receptor antagonist-expressing, and the control animals, respectively.
Association of PIL TIP39 neurons with suckling-activated preoptic neurons
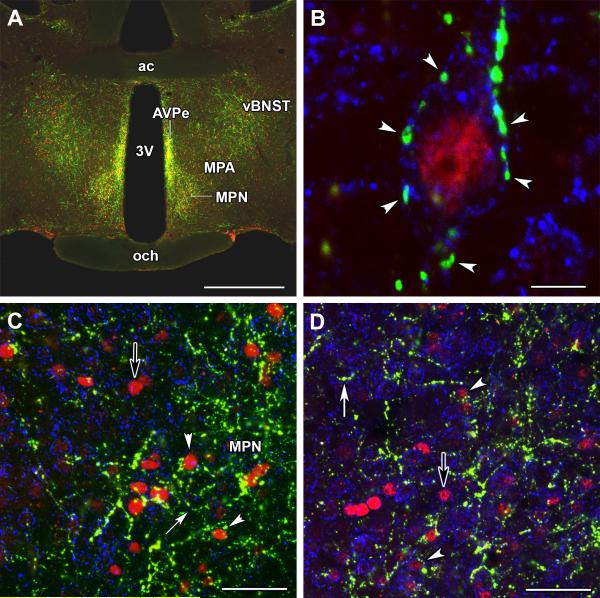
At the preoptic level, the anteroventral periventricular nucleus, the medial preoptic nucleus, the medial preoptic area, and the ventral subdivision of the bed nucleus of the stria terminalis all contained a high density of Fos-expressing neurons following suckling. The distribution pattern of Fos-expressing neurons was very similar to the distribution patterns of TIP39 labeled fibers and terminals observed in the area (Fig. 3A). Using immunreactivity for the potassium channel Kv2.1 to help define the plasma membrane, TIP39 containing fibers appeared to closely appose Fos-expressing neurons (Fig. 3B) in all regions of the preoptic area that contain Fos-expressing neurons following suckling. Fos-expressing neurons closely apposed by TIP39 fibers were abundant in both the medial preoptic nucleus (Fig. 3C), and the ventral subdivision of the bed nucleus of the stria terminalis (Fig. 3D). The percentage of Fos-expressing neurons that were closely apposed by TIP39 fibers was 78% in the former and 85% in the latter brain region.
Fig. 3.
The distribution of TIP39 fibers (green) around activated neurons (red) in the maternal preoptic area. A: The similarity of the distribution of TIP39 fibers and Fos-ir neurons is demonstrated in pup exposed mother rats in the anteroventral periventricular nucleus (AvPe), the medial preoptic nucleus (MPN), medial preoptic area (MPA), and in the ventral subdivision of the bed nucleus of the stria terminalis (vBNST). B: A high magnification confocal image of a section triple labeled with TIP39, Fos, and Kv2.1 potassium channel (blue) demonstrates that TIP39 terminals closely appose Fos-expressing neurons, as they appear to contact the cell surface as indicated by Kv2.1 immunoreactivity. C, D: Low magnification confocal images of the preoptic area that includes part of the MPN (C) and the vBNST (D), respectively. A number of Fos-activated cells that are closely apposed by TIP39 fibers are demonstrated (white arrowheads). There are also a few Fos-positive neurons that may not be innervated by TIP39 (black arrows). In addition, some Fos-negative neurons seem to be surrounded by TIP39 fibers, too (white arrows). Scale bars = 1 mm for A, 10 μm for B, and 50 μm for C and D.
Retrograde labeling following injections into the arcuate nucleus and the medial preoptic area
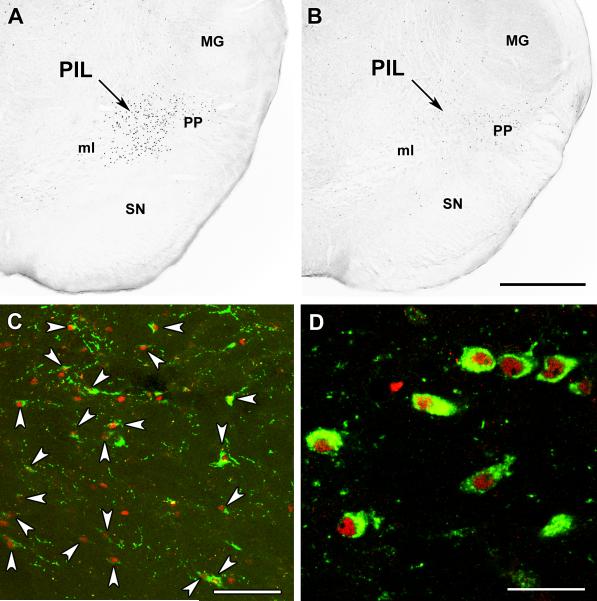
The retrograde tracer CTB was injected into the medial preoptic and the arcuate nuclei, unilaterally. The tracer deposition overlapped with TIP39 terminal fields and separate injections into both sites resulted in a predominantly ipsilateral labeling of cells that were distributed uniformly throughout the PIL. Following the medial preoptic nucleus injection (Fig. 4A), the majority of TIP39 neurons in the PIL were labeled with the retrograde tracer (Fig. 4B) and a relatively large number of CTB-labeled but TIP39-negative cells were also visible. Following injections into the arcuate nucleus (Fig. 4C), there were also some CTB-labeled TIP39 neurons in the PIL (Fig. 4D). Cells in brain regions adjacent to the PIL did not contain label following either CTB injection. TIP39 neurons located in the periventricular gray of the thalamus and the medial paralemniscal nucleus were also not labeled with CTB. Altogether, these data suggest that PIL TIP39 neurons project both to the medial preoptic area and the arcuate nucleus (Fig. 4E).
Fig. 4.
Projections of the PIL into the medial preoptic and arcuate nuclei. Cholera toxin beta subunit (CTB) is shown in red and TIP39 in green. A: A site of CTB injection into the medial preoptic nucleus (MPN) is shown in relation to TIP39 fibers. B: In the PIL, the majority of TIP39 neurons are labeled with CTB following medial preoptic CTB injection (yellow; white arrowheads). In addition, a number of TIP-negative CTB-labeled neurons are also present. C: A site of CTB injection into the arcuate nucleus (Arc). D: A portion of the PIL TIP39 neurons are labeled with CTB (white arrowheads) following its injection into the arcuate nucleus. E: A drawing prepared by modifications of panels from a rat brain atlas (Paxinos and Watson, 2007) shows the schematics of the PIL-hypothalamic projections. Large green dots in the PIL represent TIP39 cell bodies while small green dots represent TIP39 fiber terminals. The arrows show the projections from the PIL to the medial preoptic area and the arcuate nucleus, respectively. Additional abbreviations: ac – anterior commissure, cc – corpus callosum; CP – caudate putamen; DM – dorsomedial nucleus, f – fornix, ic – internal capsule; Hipp – hippocampus; MG – medial geniculate body, MPA – medial preoptic area, mt – mamillothalamic tract, och – optic chiasm, PAG – periaqueductal gray; pc – posterior commissure; SN – substantia nigra, vBNST – ventral subdivision of the bed nucleus of the stria terminalis, 3V – third ventricle. Scale bar = 1 mm for C, and 500 μm for D.
Levels of TIP39 mRNA in the PIL of pregnant females and postpartum rat dams
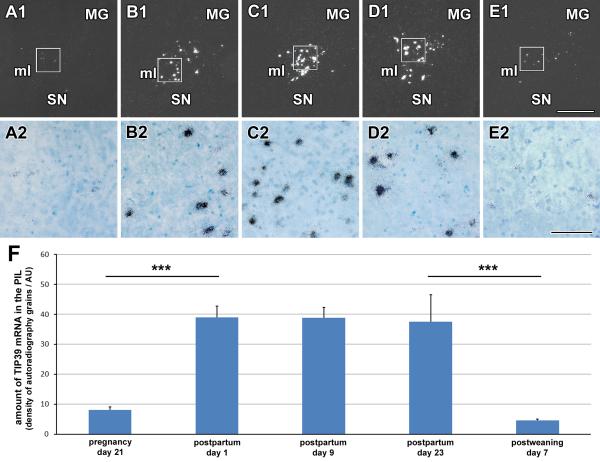
The level of TIP39 mRNA was highly significantly increased in mothers in the postpartum period (F=24.46; p<0.0001; Fig. 5). On day 21 of pregnancy, PIL neurons contained a very little TIP39 mRNA. The few mRNA-expressing cells observed contained only a small number of autoradiographic grains, and the intensity of the TIP39 mRNA signal was similar to that reported in virgin female rats (Cservenak et al., 2010). After parturition, however, both the number of TIP39 mRNA-expressing neurons and the number of autoradiographic grains per neuron increased dramatically (p<0.0001). At one day after delivery, a large number of TIP39-expressing neurons appeared in the PIL, and their autoradiographic signal was intense. At 9 and 23 days after parturition, TIP39 expression was increased over pre-partum dams similarly to at day one postpartum. These findings indicate that TIP39 mRNA levels remain elevated throughout the lactation period. Neurons demonstrating increased TIP39 mRNA levels were relatively evenly dispersed in the PIL (Fig. 5). On the 7th day after pups were weaned, the dams’ expression of TIP39 was markedly reduced as compared to the lactating mothers (p<0.0001) and had returned to the basal level.
Fig. 5.
Variation in TIP39 mRNA level during the reproductive cycle. A1-E1: Dark-field images of coronal brain sections of mother rats show TIP39 mRNA in the PIL detected by in situ hybridization histochemistry. A2-E2: Higher magnification bright-field images of the framed area in the corresponding image in A1-E1, in which black grain clusters mark cells that express TIP39 mRNA. Sections are shown from the 21st day of pregnancy, which is 1 day before the expected day of delivery (A1,2), 1 day after parturition (B1,2), from the 9th (C1,2), and 23rd postpartum day (D1,2), and from the 7th day after weaning of the pups (E1,2) to demonstrate that elevated TIP39 expression is confined to the lactation period. F: Quantitative measurement of the amount of TIP39 mRNA by the density of autoradiography grains indicates a significant increase (***: p<0.0001) around parturition and a significant decrease after weaning (***: p<0.0001). Abbreviations: MG – medial geniculate body, ml – medial lemniscus, SN – substantia nigra. Scale bar = 500 μm for E1, and 100 μm for E2.
Assessment of c-Fos activation in the PIL of lactating dams
When pups were returned to their mothers after a 20h separation, the dams all began care for them immediately, and suckling started within 5 min. Following pup return, c-Fos-expressing (c-Fos-ir) neurons appeared in a number of regions in the dams’ brains (including the PIL, lateral septal nucleus, anteroventral periventricular nucleus, medial preoptic nucleus, medial preoptic area, the ventral subdivision of the bed nucleus of the stria terminalis, some parts of the periaqueductal gray, and the medial paralemniscal nucleus). While c-Fos-ir neurons were evenly distributed within the PIL, none appeared in adjacent regions, except in the peripeduncular area lateral to the PIL (Fig. 6A).
Fig. 6.
Fos activation in the PIL of mother rats in response to suckling. A: A high density of Fos-ir cell nuclei (black dots) can be seen in the PIL of mother rats that were deprived of their pups for 22h, and then reunited with them for 2h. B: The PIL contained only a small number of Fos-ir neurons in mothers who were deprived of their pups for 22h and then reunited for 2h in such a way that suckling was prevented. Some Fos-ir cells appear in the peripeduncular area (PP) lateral to the PIL, in both groups. C: The majority of TIP39 neurons (green) contain Fos (red) in the PIL (white arrowheads), in response to suckling. D: A high-magnification confocal image demonstrates Fos labeling of TIP39 neurons. Additional abbreviations: MG – medial geniculate body, ml – medial lemniscus, SN – substantia nigra. Scale bar = 1 mm for C, and 100 μm for D.
In the PIL section with the largest number of labeled cells, the number of Fos-ir neurons changed significantly with pup exposure (F=82.95). In suckling dams, it was 161±10, which was significantly greater (p<0.001) than the 27±8 labeled cells following pup exposure without any physical contact (Fig. 6B). This number was somewhat greater (p<0.05) than the number of Fos-ir neurons (8±6) in the control group, dams whose pups were removed and not returned (Table 1).
Table 1.
The number of neurons activated in the PIL of mother rats in response to pups. The cells were counted in the sections, which contained the largest number of labeled cells (n=5 per group).
| Number of cells per PIL section | Suckled dams | Pup exposure without suckling | Dams following 20h pup deprivation |
|---|---|---|---|
| Fos-positive neurons | 161±10 | 27±8 | 8±6 |
| TIP39-positive neurons | 40±6 | 44±5 | 41±3 |
| Double labeled neurons | 35±6 | 6±2 | 0 |
While the number of TIP39 labeled cells in the PIL did not differ between the three groups and was an average of 42±5 per side in the densest section, the number of Fos-expressing TIP39 neurons was changed significantly by pup exposure (F=34.17). The number of double labeled cells was greater in suckling rat dams than in those exposed to pups without physical contact (35±6 vs. 6±2; p<0.001; Table 1), while the number of double-labeled cells did not significantly differ between dams without physical pup contact and dams whose pups were not returned (Fig. 6C,D).
DISCUSSION
Neuroanatomical evidence is presented addressing the participation of TIP39 neurons in ascending sensory pathways that relay effects of suckling to hypothalamic centers. In addition, neuroendocrinological and behavioral evidence are presented that suggest a role of TIP39 in stimulating maternal motivation and prolactin release. The results indicate the emergence of a novel neuropeptide regulator in postpartum maternal adaptations.
TIP39 neurons in the posterior intralaminar complex of the thalamus (PIL)
The PIL, defined by the area containing TIP39 neurons, includes the posterior intralaminar thalamic nucleus, the parvicellular subparafascicular nucleus, and some parts of the caudal subdivision of the zona incerta (Dobolyi et al., 2003). Some of the projections of PIL neurons have been described in previous studies, including the medial preoptic area (Simerly and Swanson, 1986), the paraventricular hypothalamic nucleus (Campeau and Watson, 2000), the arcuate nucleus (Li et al., 1999; Szabo et al., 2010), and the amygdaloid nuclei (LeDoux et al., 1990). Our tracer studies suggest that TIP39 neurons in the PIL project to the arcuate nucleus and the preoptic area in the hypothalamus and that neurons projecting to these brain regions are confined to the PIL within the posterior thalamus. The distribution of TIP39 neurons projecting to both hypothalamic sites were evenly distributed within the PIL. These results support the idea that the PIL constitutes a topographical unit, although it does not correspond to an obvious cytoarchitectonically defined nucleus. In addition, the lack of retrograde labeling in other TIP39 cell groups suggest that TIP39 fibers in the the arcuate nucleus and the preoptic area originate exclusively from the PIL.
Viral injections of a PTH2 receptor antagonist to establish TIP39 actions
Methodological considerations
We used a lentivirus to produce and constitutively secrete the selective PTH2 receptor antagonist HYWH-TIP39 (Kuo and Usdin, 2007) from infected cells. This method allows the direct identification of infected cells but not the precise spread of the peptide antagonist in the tissue. Since peptides are known to be able diffuse significant distances in the neural tissue, we expect that the PTH2 receptor antagonist HYWH-TIP39 reached PTH2 receptors abundant in the arcuate nucleus and the PTH2 receptor-expressing regions in the preoptic area, following mediobasal and preoptic injections, respectively. The exact location of PTH2 receptor-expressing neurons of interest was not targeted in order to avoid infection of these cells and potential changes in their function as a result of viral infection. Comparison of the effects of the HYWH-virus on prolactin secretion to our previous observations of the effect on prolactin secretion of intracerebroventricular injection of the same PTH2 receptor antagonist (Cservenak et al., 2010) allows some indirect estimation of the released HYWH-TIP39. The inhibition of prolactin release was somewhat less using the virus than that after the direct injection of 0.075 mg (approximately 17 nmol) HYWH-TIP39 into the lateral ventricle. Consequently, less than 17 nmol HYWH-TIP39 is expected to be released from the infected cells during the period of the experiment even though acute lateral ventricular injections and continuous cellular release cannot be directly compared. On the other hand, a similar amount of HYWH-TIP39 is expected to be released from preoptic neurons, which was not sufficient to inhibit prolactin secretion, suggesting that only a small portion of the preoptically released antagonist reached the arcuate nucleus, the established site of the regulation of prolactin release.
The role of TIP39 in the regulation of prolactin release
Suckling is the most potent known stimulus of the maternal increase in serum prolactin levels, however, the mechanisms are only partially understood. The PTH2 receptor antagonist released around the mediobasal hypothalamus reduced the basal prolactin levels in lactating mothers and markedly inhibited the suckling-induced elevation of plasma prolactin. PTH2 receptor-expressing neurons and PTH2 receptor-containing nerve terminals are absent from the pituitary but are abundant in the arcuate nucleus (Dobolyi et al., 2006a; Faber et al., 2007), which is therefore the likely site of action of the antagonist secreted from the virus-infected cells in the mediobasal hypothalamus.
The role of TIP39 in maternal motivation
During the early postpartum period, pup suckling is more rewarding than cocaine (Ferris et al., 2005). The medial preoptic area has been shown to be critically important for maternal motivation (Arrati et al., 2006; Pereira and Morrell, 2011) through its projections to the nucleus accumbens and the ventral tegmental area (Numan et al., 2005). In the present study, we not only confirmed the role of the medial preoptic area in maternal motivation but also provided evidence for the involvement of the TIP39-PTH2 receptor system there. We demonstrated that fibers of TIP39 neurons projecting to the preoptic area from the PIL have a distribution similar to that of the neurons expressing expressing Fos in response to pup exposure in areas that include the medial preoptic nucleus, other parts of the topographical medial preoptic area, and the ventral subdivision of the bed nucleus of the stria terminalis. This is a characteristic pattern in the medial preoptic region, often referred to as the medial preoptic area in which Fos-expressing neurons have been implicated in pup attachment (Lonstein et al., 1998; Stack and Numan, 2000). In turn, we also provided morphological evidence that TIP39-containing terminals innervate the Fos-expressing neurons. Most importantly, however, the presence of the PTH2 receptor antagonist reduced the number of dams demonstrating preference for the pup-associated cage in a place preference test, and also the amount of time the dams spent in the pup-associated cage, but did not affect the time control females spent in the different cages of the test apparatus. The conditioned place preference test, used regularly in the study of addiction (Schwarz and Bilbo, 2013) and food intake regulation (Labouebe et al., 2013), is a particularly sensitive way to assess maternal motivation (Seip and Morrell, 2009). It can differentiate even between otherwise behaviorally identical postpartum maternal rats when both the number of dams demonstrating preference for the pup-associated cage and the time the dams spend in the pup-associated cage are analyzed (Mattson et al., 2003), as we also evaluated the conditioned place preference test data in this study. It is also important to note that preoptic injection of the virus expressing the PTH2 receptor antagonist did not affect plasma prolactin levels. Therefore, an indirect mechanism of action on maternal motivation via prolactin can be excluded even though prolactin can itself stimulate maternal behavior (Bridges et al., 1990). The finding that pup retrieval did not differ in the presence of the PTH2 receptor antagonist can be explained by the higher sensitivity of the conditioned place preference test. Alternatively, the TIP39-PTH2 receptor system may be more involved in the motivational aspects of maternal behaviors than the actual behavior towards the young.
The proposed function of TIP39 neurons in the PIL
Based on previous studies on the afferent neuronal connections of the parvicellular subparafascicular nucleus in male rats, neurons in the PIL receive input from the spinal cord. These ascending inputs were implicated in the processing of sensory information related to mating and ejaculation (Coolen et al., 2004). In mothers, these afferent connections are candidates to convey suckling information from the nipples to the PIL. Previous electrophysiological mapping suggested that the ascending pathway carrying suckling information reaches the diencephalon through the peripeduncular area ventral to the medial geniculate body (Tindal and Knaggs, 1977). TIP39 neurons in the PIL could also process auditory information, e.g. pup ultrasonic vocalization, which has a role in nursing (Febo et al., 2008), as they were shown to be activated by high-intensity auditory input (Palkovits et al., 2009). In this study, however, suckling information is the likely source of PIL neuron activation because Fos expression in the PIL was significant following suckling but much less if the pups were returned to their mothers without allowing physical contact. Additional lines of evidence also suggest that the PIL may be a relay nucleus that conveys suckling information towards the forebrain centers for maternal behavior. A majority of TIP39-positive neurons in the PIL express Fos in response to suckling. In addition, TIP39 expression is enhanced during the postpartum period, as reported on previously for the 9-10th postpartum day (Cservenak et al., 2010). Significantly, we showed in the present study that TIP39 expression is also markedly up-regulated on the 1st, 9th and 23rd postpartum day but not on the last day of pregnancy or after weaning, which further supports the idea that elevated activity of these neurons is specific for the period of lactation. In turn, PIL neurons provide projections to the medial preoptic area, the arcuate nucleus and potentially to other limbic and hypothalamic areas and nuclei that might also be involved in the processing of suckling information, e.g. the release of oxytocin, the altered stress response in mothers, and lactational anoestrus. In particular, we provided functional evidence that TIP39 affects prolactin release through thalamo-arcuate projections and maternal motivation through thalamo-preoptic projections.
In conclusion, the data obtained suggest that TIP39 neurons in the PIL convey suckling information towards the hypothalamus. TIP39 may contribute to prolactin release from the pituitary and to the maintenance of maternal motivation via its receptor, the PTH2 receptor. Because the TIP39-PTH2 receptor system is neuroanatomically similar in humans and rodents (Bago et al., 2009), the results may be relevant to human breastfeeding.
ACKNOWLEDGEMENTS
Grant support was provided by the Bolyai János Fellowship Award of the Hungarian Academy of Sciences, an OTKA K100319 research grant and the KTIA NAP Program for AD, and NIMH IRP for TBU. The authors also thank Prof. Zoltán Nusser (Institute of Experimental Medicine, Budapest) for his advice on using the potassium channel Kv2.1 as a cell surface marker and providing a sample antibody. The technical assistance of Jonathan Kuo in producing the virus as well as the general technical assistance of Nikolett Hanák, Viktória Dellaszéga-Lábas, and Szilvia Deák is also acknowledged. We also appreciate the editing service by Elizabeth Sherman (National Institute of Mental Health).
FUNDING SOURCES, THE ROLE OF FUNDING SOURCES
The Funding sources are all governmental. The agencies supported the science by providing funds but did not contribute to the manuscript in any other way and did not influence the authors in any way.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors:
Melinda Cservenák performed all experiments and data analysis.
Éva R. Szabó performed histological techniques in the paper and contributed to the behavioral studies.
Ibolya Bodnár performed blood sampling and prolactin measurement.
András Lékó contributed to some of the double labeling experiments.
Miklós Palkovits participated in the design of the tracer studies and contributed to writing of the manuscript.
György M. Nagy participated in the design of suckling-induced prolactin measurement.
Ted B. Usdin produced the antagonist-expressing virus, contributed to some of the data analysis and the writing of the manuscript.
Arpád Dobolyi designed the experiments, evaluated the results and performed statistical analyses, and wrote the first draft of the original and the revised manuscript.
All authors contributed to and have approved the final manuscript.
DECLARATION OF CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Arrati PG, Carmona C, Dominguez G, Beyer C, Rosenblatt JS. GABA receptor agonists in the medial preoptic area and maternal behavior in lactating rats. Physiol Behav. 2006;87:51–65. doi: 10.1016/j.physbeh.2005.08.048. [DOI] [PubMed] [Google Scholar]
- Bago AG, Dimitrov E, Saunders R, Seress L, Palkovits M, Usdin TB, Dobolyi A. Parathyroid hormone 2 receptor and its endogenous ligand tuberoinfundibular peptide of 39 residues are concentrated in endocrine, viscerosensory and auditory brain regions in macaque and human. Neuroscience. 2009;162:128–147. doi: 10.1016/j.neuroscience.2009.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar I, Banky Z, Nagy GM, Halasz B. Non-NMDA glutamate receptor antagonist injected into the hypothalamic paraventricular nucleus blocks the suckling stimulus-induced release of prolactin. Brain Res Bull. 2005;65:163–168. doi: 10.1016/j.brainresbull.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm Behav. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Brenner D, Bago AG, Gallatz K, Palkovits M, Usdin TB, Dobolyi A. Tuberoinfundibular peptide of 39 residues in the embryonic and early postnatal rat brain. J Chem Neuroanat. 2008;36:59–68. doi: 10.1016/j.jchemneu.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RS, Numan M, Ronsheim PM, Mann PE, Lupini CE. Central prolactin infusions stimulate maternal behavior in steroid-treated, nulliparous female rats. Proc Natl Acad Sci U S A. 1990;87:8003–8007. doi: 10.1073/pnas.87.20.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA. The expectant brain: adapting for motherhood. Nat Rev Neurosci. 2008;9:11–25. doi: 10.1038/nrn2280. [DOI] [PubMed] [Google Scholar]
- Campeau S, Watson SJ., Jr. Connections of some auditory-responsive posterior thalamic nuclei putatively involved in activation of the hypothalamo-pituitary-adrenocortical axis in response to audiogenic stress in rats: an anterograde and retrograde tract tracing study combined with Fos expression. J Comp Neurol. 2000;423:474–491. [PubMed] [Google Scholar]
- Coolen LM, Allard J, Truitt WA, McKenna KE. Central regulation of ejaculation. Physiol Behav. 2004;83:203–215. doi: 10.1016/j.physbeh.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Coutellier L, Logemann A, Rusnak M, Usdin TB. Maternal absence of the parathyroid hormone 2 receptor affects postnatal pup development. J Neuroendocrinol. 2011;23:612–619. doi: 10.1111/j.1365-2826.2011.02149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenak M, Bodnar I, Usdin TB, Palkovits M, Nagy GM, Dobolyi A. Tuberoinfundibular peptide of 39 residues is activated during lactation and participates in the suckling-induced prolactin release in rat. Endocrinology. 2010;151:5830–5840. doi: 10.1210/en.2010-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov EL, Kuo J, Kohno K, Usdin TB. Neuropathic and inflammatory pain are modulated by tuberoinfundibular peptide of 39 residues. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1306342110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobolyi A, Irwin S, Wang J, Usdin TB. The distribution and neurochemistry of the parathyroid hormone 2 receptor in the rat hypothalamus. Neurochem Res. 2006a;31:227–236. doi: 10.1007/s11064-005-9011-9. [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Palkovits M, Usdin TB. Expression and distribution of tuberoinfundibular peptide of 39 residues in the rat central nervous system. J Comp Neurol. 2003;455:547–566. doi: 10.1002/cne.10515. [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Palkovits M, Usdin TB. The TIP39-PTH2 receptor system: unique peptidergic cell groups in the brainstem and their interactions with central regulatory mechanisms. Prog Neurobiol. 2010;90:29–59. doi: 10.1016/j.pneurobio.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobolyi A, Ueda H, Uchida H, Palkovits M, Usdin TB. Anatomical and physiological evidence for involvement of tuberoinfundibular peptide of 39 residues in nociception. Proc Natl Acad Sci U S A. 2002;99:1651–1656. doi: 10.1073/pnas.042416199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobolyi A, Wang J, Irwin S, Usdin TB. Postnatal development and gender-dependent expression of TIP39 in the rat brain. J Comp Neurol. 2006b;498:375–389. doi: 10.1002/cne.21063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber CA, Dobolyi A, Sleeman M, Usdin TB. Distribution of tuberoinfundibular peptide of 39 residues and its receptor, parathyroid hormone 2 receptor, in the mouse brain. J Comp Neurol. 2007;502:563–583. doi: 10.1002/cne.21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Stolberg TL, Numan M, Bridges RS, Kulkarni P, Ferris CF. Nursing stimulation is more than tactile sensation: It is a multisensory experience. Horm Behav. 2008;54:330–339. doi: 10.1016/j.yhbeh.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Kulkarni P, Sullivan JM, Jr., Harder JA, Messenger TL, Febo M. Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J Neurosci. 2005;25:149–156. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- Grattan DR, Steyn FJ, Kokay IC, Anderson GM, Bunn SJ. Pregnancy-induced adaptation in the neuroendocrine control of prolactin secretion. J Neuroendocrinol. 2008;20:497–507. doi: 10.1111/j.1365-2826.2008.01661.x. [DOI] [PubMed] [Google Scholar]
- Hunyady B, Krempels K, Harta G, Mezey E. Immunohistochemical signal amplification by catalyzed reporter deposition and its application in double immunostaining. J Histochem Cytochem. 1996;44:1353–1362. doi: 10.1177/44.12.8985127. [DOI] [PubMed] [Google Scholar]
- Kuo J, Usdin TB. Development of a rat parathyroid hormone 2 receptor antagonist. Peptides. 2007;28:887–892. doi: 10.1016/j.peptides.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutner RH, Zhang XY, Reiser J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat Protoc. 2009;4:495–505. doi: 10.1038/nprot.2009.22. [DOI] [PubMed] [Google Scholar]
- Labouebe G, Liu S, Dias C, Zou H, Wong JC, Karunakaran S, Clee SM, Phillips AG, Boutrel B, Borgland SL. Insulin induces long-term depression of ventral tegmental area dopamine neurons via endocannabinoids. Nat Neurosci. 2013 doi: 10.1038/nn.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Farb C, Ruggiero DA. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. J Neurosci. 1990;10:1043–1054. doi: 10.1523/JNEUROSCI.10-04-01043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Chen P, Smith MS. Identification of neuronal input to the arcuate nucleus (ARH) activated during lactation: implications in the activation of neuropeptide Y neurons. Brain Res. 1999;824:267–276. doi: 10.1016/s0006-8993(99)01217-2. [DOI] [PubMed] [Google Scholar]
- Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Simmons DA, Swann JM, Stern JM. Forebrain expression of c-fos due to active maternal behaviour in lactating rats. Neuroscience. 1998;82:267–281. doi: 10.1016/s0306-4522(97)00283-2. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Williams SE, Rosenblatt JS, Morrell JI. Preferences for cocaine- or pup-associated chambers differentiates otherwise behaviorally identical postpartum maternal rats. Psychopharmacology (Berl) 2003;167:1–8. doi: 10.1007/s00213-002-1351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville MC. Lactation and Its Hormonal Control. In: Neill JD, editor. Physiology of Reproduction. Academic Press; Amsterdam: 2006. pp. 2993–3054. [Google Scholar]
- Numan M, Fleming AS, Levy F. Maternal Behavior. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. Academic Press; Oxford: 2006. pp. 1729–2058. [Google Scholar]
- Numan M, Numan MJ, Schwarz JM, Neuner CM, Flood TF, Smith CD. Medial preoptic area interactions with the nucleus accumbens-ventral pallidum circuit and maternal behavior in rats. Behav Brain Res. 2005;158:53–68. doi: 10.1016/j.bbr.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Numan M, Woodside B. Maternity: neural mechanisms, motivational processes, and physiological adaptations. Behav Neurosci. 2010;124:715–741. doi: 10.1037/a0021548. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Kalinichev M, Morrell JI, Rosenblatt JS. MPOA cytotoxic lesions and maternal behavior in the rat: effects of midpubertal lesions on maternal behavior and the role of ovarian hormones in maturation of MPOA control of maternal behavior. Horm Behav. 2002;41:126–138. doi: 10.1006/hbeh.2001.1753. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Helfferich F, Dobolyi A, Usdin TB. Acoustic stress activates tuberoinfundibular peptide of 39 residues neurons in the rat brain. Brain Struct Funct. 2009;214:15–23. doi: 10.1007/s00429-009-0233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 2007. [Google Scholar]
- Pereira M, Morrell JI. Functional mapping of the neural circuitry of rat maternal motivation: effects of site-specific transient neural inactivation. J Neuroendocrinol. 2011;23:1020–1035. doi: 10.1111/j.1365-2826.2011.02200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA, Douglas AJ, Ingram CD. Brain preparations for maternity--adaptive changes in behavioral and neuroendocrine systems during pregnancy and lactation. An overview. Prog Brain Res. 2001;133:1–38. doi: 10.1016/s0079-6123(01)33002-9. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Bilbo SD. Adolescent morphine exposure affects long-term microglial function and later-life relapse liability in a model of addiction. J Neurosci. 2013;33:961–971. doi: 10.1523/JNEUROSCI.2516-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seip KM, Morrell JI. Transient inactivation of the ventral tegmental area selectively disrupts the expression of conditioned place preference for pup- but not cocaine-paired contexts. Behav Neurosci. 2009;123:1325–1338. doi: 10.1037/a0017666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. The organization of neural inputs to the medial preoptic nucleus of the rat. J Comp Neurol. 1986;246:312–342. doi: 10.1002/cne.902460304. [DOI] [PubMed] [Google Scholar]
- Stack EC, Numan M. The temporal course of expression of c-Fos and Fos B within the medial preoptic area and other brain regions of postpartum female rats during prolonged mother--young interactions. Behav Neurosci. 2000;114:609–622. doi: 10.1037//0735-7044.114.3.609. [DOI] [PubMed] [Google Scholar]
- Stern JM, Lonstein JS. Neural mediation of nursing and related maternal behaviors. Prog Brain Res. 2001;133:263–278. doi: 10.1016/s0079-6123(01)33020-0. [DOI] [PubMed] [Google Scholar]
- Szabo FK, Snyder N, Usdin TB, Hoffman GE. A direct neuronal connection between the subparafascicular and ventrolateral arcuate nuclei in non-lactating female rats. Could this pathway play a role in the suckling-induced prolactin release? Endocrine. 2010;37:62–70. doi: 10.1007/s12020-009-9266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindal JS, Knaggs GS. Pathways in the forebrain of the rat concerned with the release of prolactin. Brain Res. 1977;119:211–221. doi: 10.1016/0006-8993(77)90101-9. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Hoare SR, Wang T, Mezey E, Kowalak JA. TIP39: a new neuropeptide and PTH2-receptor agonist from hypothalamus. Nat Neurosci. 1999;2:941–943. doi: 10.1038/14724. [DOI] [PubMed] [Google Scholar]
- Varga T, Mogyorodi B, Bago AG, Cservenak M, Domokos D, Renner E, Gallatz K, Usdin TB, Palkovits M, Dobolyi A. Paralemniscal TIP39 is induced in rat dams and may participate in maternal functions. Brain Struct Funct. 2012;217:323–335. doi: 10.1007/s00429-011-0357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodside B. Prolactin and the hyperphagia of lactation. Physiol Behav. 2007;91:375–382. doi: 10.1016/j.physbeh.2007.04.015. [DOI] [PubMed] [Google Scholar]