Abstract
Recent research suggests that exercise can be effective in reducing pain in animals and humans with neuropathic pain. To investigate mechanisms in which exercise may improve hyperalgesia associated with prediabetes, C57Bl/6 mice were fed either standard chow or a high-fat diet for 12 weeks and were provided access to running wheels (exercised) or without access (sedentary). The high-fat diet induced a number of prediabetic symptoms, including increased weight, blood glucose, and insulin levels. Exercise reduced but did not restore these metabolic abnormalities to normal levels. In addition, mice fed a high-fat diet developed significant cutaneous and visceral hyperalgesia, similar to mice that develop neuropathy associated with diabetes. Finally, a high-fat diet significantly modulated neurotrophin protein expression in peripheral tissues and altered the composition of epidermal innervation. Over time, mice that exercised normalized with regards to their behavioral hypersensitivity, neurotrophin levels, and epidermal innervation. These results confirm that elevated hypersensitivity and associated neuropathic changes can be induced by a high-fat diet and exercise may alleviate these neuropathic symptoms. These findings suggest that exercise intervention could significantly improve aspects of neuropathy and pain associated with obesity and diabetes. Additionally, this work could potentially help clinicians determine those patients which will develop painful versus insensate neuropathy using intraepidermal nerve fiber quantification.
Keywords: Prediabetes, nociception, neurotrophins, nerve growth factor, exercise, diabetic neuropathy
Introduction
Diabetic neuropathy (DN) occurs in up to 60-70% of diabetes patients. Distal symmetric DN is the most common neuropathy associated with diabetes and may present with either positive (pain, burning or tingling), or negative symptoms (numbness or altered proprioception) [45]. Neuropathy symptoms can precede diagnosis of diabetes and may develop in the initial stages of glucose dysregulation, or prediabetes [14; 40], with prediabetes being defined as impaired fasting glucose and/or impaired glucose tolerance [3]. While neuropathy associated with prediabetes is usually less severe than neuropathy in overt diabetic patients [51], it is still a devastating complication of the disease. Unfortunately, painful symptoms are the predominant feature in prediabetes patients. Current treatment options for patients with painful diabetic neuropathy (PDN) are rarely effective and less than 30% of patients achieve satisfactory pain relief [4].
Recent studies have demonstrated that cutaneous nerve growth factor (NGF) is increased in hindpaw skin of rodent models of type 1 and type 2 diabetes [9; 15]. It has been suggested that increased NGF may play a significant role in the development of PDN. Additionally, NGF is known to be critical in the development and maintenance of chronic pain, especially inflammatory pain [30; 43]. In fact, cutaneous injections of NGF result in thermal and mechanical hyperalgesia in both animals and humans [33]. Besides NGF, additional neurotrophins that play a role in pain sensation include brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF). Like NGF, BDNF is believed to play a role in the development and maintenance of pain states, as BDNF is upregulated in the dorsal root ganglion (DRG) in inflammatory conditions and in models of neuropathic pain [32]. Furthermore, delivery of antibodies against BDNF reduced pain related behaviors in rat [60] and mouse [59]. While NGF and BDNF are important for the development and maintenance of pain, GDNF is believed to play an antinociceptive role. Previous studies have shown that administration of exogenous GDNF results in analgesia in various models of neuropathic pain [1; 6].
Exercise training has long been suggested to reduce pain and improve functional outcomes[29; 53]. In fact, a recent study demonstrated that aerobic and strength training had positive effects on diabetic peripheral neuropathy patients, improving pain and other neuropathic symptoms [28]. Furthermore, studies have shown exercise increases neurotrophin expression [25; 35; 36] and increased neurotrophins can promote neuronal healing [5; 17; 23]. Additionally, these exercise-induced neurotrophin alterations are associated with analgesia [50]. Previous studies have begun to investigate potential therapeutic roles of neurotrophins in diabetic neuropathy using type 1 and type 2 models [2; 9; 22]. In the current study, we investigated how a high-fat diet alters metabolic and neural features normally associated with DN, as well as how exercise modulates these neuropathy phenotypes. We demonstrate that a high-fat diet induces mechanical allodynia and visceral hyperalgesia, and that exercise reverses these behavioral changes. The exercise-induced modulation of behavior was associated with normalization of neurotrophin levels and epidermal fiber density.
Materials and Methods
Animals and Diet
Seven week-old male C57Bl/6 mice were purchased from Charles River (Wilmington, Mass) and maintained on a 12:12h light/dark cycle in the research support facility at the University of Kansas Medical Center. All mice were given ad libitum access to food and water and were fed either a standard diet (8604; Harlan Teklad, Madison, Wisconsin; 14% kcals from fat, 32% protein, and 54% carbohydrate) or a high-fat diet (07011; Harlan Teklad; 54% kcals from lard and corn oil fat, 21% protein, and 24% carbohydrate). All animals were fed the standard diet through all baseline tests. After baseline tests, the animals were separated according to diet. All animal use was in accordance with NIH guidelines and conformed to the principles specified in a protocol approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee.
Energy Intake
Daily food intake was measured by monitoring the weight of the remaining food after an initial food bolus. New food boluses were given every 3-4 days. Energy intake was calculated by:
Standard diet energy was calculated by multiplying the intake per day by 3.0kcal/g, while high-fat diet energy was calculated by multiplying intake per day by 4.9kcal/g. The combined mean energy intake from each mouse was used to calculate the group means.
Voluntary Exercise
Following baseline testing, animals were separated into either sedentary controls or exercise groups. Exercise animals were housed individually in cages designed to hold stainless-steel running wheels (Mini Mitter; Bend, OR) and given free access to run 24/7. VitalView (Mini Mitter) software measured total wheel revolutions for each mouse during the course of the study. Sedentary animals were housed one to two per cage at the suggestion of the veterinarians in the animal facility. Mice were started on diet and running wheels simultaneously following baseline behavior testing. Treatment groups are identified throughout the study as: standard diet sedentary (Std-Sed), standard diet exercise (Std-Ex), high-fat diet sedentary (HF-Sed) and high-fat diet exercise (HF-Ex).
Blood Chemistry
Animal weight, blood glucose (glucose diagnostic reagents; Sigma, St. Louis, MO), and serum insulin (mouse insulin ELISA; Alpco, Salem, NH) were measured biweekly. Hemoglobin A1c levels (A1CNow+; Bayer, Sunnyvale, CA) were measured at 0, 6, and 12 weeks following high-fat diet and exercise initiation. All mice were fasted three hours prior to blood collection for all blood chemistry panels, with the exception of the glucose tolerance test.
After 12 weeks, an intraperitoneal glucose tolerance test (IPGTT) was performed after a 6 hour fast. Animals were given an intraperitoneal (IP) injection of glucose at 2g glucose/kg body weight. Blood glucose levels were measured via tail clip immediately before glucose injection and 15, 30, 60 and 120 minutes thereafter.
Behavior Testing
Behavior testing to assess signs of diabetic neuropathy was carried out at baseline and biweekly time points. For all behavioral tests, animals were allowed to acclimate to the testing equipment in two separate sessions prior to the initial testing day. Before each behavior test, animals were allowed to acclimate to the behavior testing room for 30 minutes followed by a 30-minute acclimation to the testing equipment.
Mechanical Sensitivity
Mice were placed in individual clear plastic cages on a wire mesh table 55 cm above the table. von Frey monofilaments (0.07-4.0 g) were applied perpendicularly to the plantar surface of the hindpaw until the filament bent. Testing began with the 0.6 g filament. If the animals withdrew their paw, it was counted as a positive withdrawal and the next lowest filament was applied. If the animal did not respond, the next larger filament was applied. Filaments were applied until there was an initial change in response followed by four additional filament applications. The 50% withdrawal threshold was calculated using the formula from the up-down method previously described [8].
Thermal Sensitivity
Mice were placed in individual clear plastic cages on a Hargreaves's apparatus and a 4.0 V radiant heat source was applied twice to each hind paw for a total of four tests. Time elapsed for each animal to withdraw the hind paw was counted as withdrawal latency (sec). Latencies from four applications were used to calculate the mean latency per animal and mean latencies were combined to calculate group means.
Visceral Sensitivity
At 12 weeks, electrode implantation and colorectal distension (CRD) were performed as described previously [11]. The electromyographic (EMG) activity of the abdominal musculature was amplified, filtered and recorded with Spike 2 software (Cambridge Electronic Design, Cambridge, UK) during each applied pressure 15, 30, 45, 60, and 75mmHg, in triplicate for 20 s with a 4-minute rest period in between. CRD responses were quantified by measuring the area under the curve for the entire distension period divided by the duration of the distension and expressed as a percent of baseline activity (10s prior to distension).
Nerve Conduction Velocity
At 12 weeks, immediately before sacrifice, animals were anesthetized with an IP injection of Avertin (1.25% v/v tribromoethanol [Sigma-Aldrich], 2.5% tert-amyl alcohol [Sigma-Aldrich], dH2O; 200 μL/10 g body weight) and motor and sensory nerve conduction velocities were recorded as previously described [20].
Intraepidermal Nerve Fiber (IENF) Measurement
Immediately following nerve conduction velocity measurements, animals were sacrificed at 12 weeks. Right hind foot pads were collected, immersed for one hour in Zamboni's fixative (3% paraformaldehyde, 15% picric acid in 0.1M phosphate buffer [PBS, pH 7.4]), rinsed overnight in 1% PBS, immersed in 30% sucrose in PBS overnight, cryoembedded in mounting media (OCT Compound; Sakura Finetek, Torrance, CA), and sectioned on a cryostat (Leica CM 1950; Leica Biosystems, Richmond, IL) at 30μm before immunohistochemistry.
After a 5-minute thaw at room temperature, sections were incubated with blocking solution (1.5% normal donkey serum, 0.5% porcine gelatin, 0.5% Triton X-100) at room temperature for 6 hours. Slides were then incubated overnight at 4° C in primary antibody diluted in blocking solution. Slides were then washed 2×10 minutes in PBST, followed by a 1-hour incubation with antibodies conjugated to different fluorophores. Sections were then washed 2×10 minutes in PBS, rinsed in deionized distilled water and coverslipped.
IENF quantification was performed using rabbit anti-PGP9.5 (1:400; Chemicon, Temecula, CA) to visualize all intraepidermal fibers and rat anti-Trk A (1:250; R&D Systems, Minneapolis, MN) to visualize peptidergic fiber types. AlexaFluor 488 and AlexaFluor 555 (1:2000; Molecular Probes, Eugene, OR) were used as fluorophore conjugated secondary antibodies. Fluorescent images were collected using a Nikon Eclipse 90i microscope using a 40× objective. NIH Image J software was used to measure each epidermal region. IENF density (IENFD) was expressed as number of fibers per millimeter of epidermis from a total of 9 images per mouse. The combined mean IENF density from each mouse was used to calculate the group means.
Growth Factor Quantification
Immediately following sacrifice, L4-L6 spinal cord, all dorsal root ganglion sciatic nerve, gastrocnemius, and hindpaw skin were dissected out, snap frozen in liquid nitrogen, and stored at -80° C. Frozen tissue samples were homogenized separately in lysis buffer (20mM Tris-HCL (pH 8.0), 137mM NaCl, 1% NP40, 1mM PMSF, 10% glycerol, 10 μg/mL aprotinin, 1μg/mL leupeptin, 0.5 mM sodium vanadate, and 4% Triton X-100), homogenates centrifuged and supernatants collected. Total protein concentration of each sample was measured using a protein assay based on the Bradford method (Bio-Rad protein reagent; Hercules, CA). Each protein was quantified using an ELISA kit (BDNF, GDNF, and NGF Emax ImmunoAssay Systems; Promega, Madison, WI) following the manufacturer's instructions. Equal amounts of each protein were loaded to quantify levels of BDNF, GDNF, and NGF.
Statistical Analysis
All data is presented as mean ± SEM. Data was analyzed using a two-factor ANOVA or repeated measures ANOVA with post hoc comparisons analyzed using Fisher's test of least square difference where appropriate. All statistics were run using SPSS Statistics 20. Statistical significance was defined as p≤0.05.
Results
Running Distances in Exercise-Grouped Mice
Std-Ex and HF-Ex mice began running the day following baseline behavior measures. Average daily distances per 2 weeks can be seen in Table 1. High-fat fed mice averaged significantly greater distances than standard diet fed mice through 6 weeks. Interestingly, both exercise groups decreased physical activity over the course of the study, with Std-Ex mice exhibiting a 56% decline and HF-Ex mice exhibiting a 70% decline in average distance ran over the course of 12 weeks.
Table 1. Average Daily Distances.
Average daily distances ran on running wheels by both exercise groups through 12 weeks of study. Data is presented as mean±SEM. Standard diet, n=9; High-fat diet, n=13.
| Average Daily Distance Ran (km) | ||||||
|---|---|---|---|---|---|---|
| Week 2 | Week 4 | Week 6 | Week 8 | Week 10 | Week 12 | |
| Standard Diet | 8.71±1.13 | 7.14±1.01 | 4.96±0.36 | 4.36±0.51 | 4.37±0.44 | 3.85±0.38 |
| High-fat Diet | 11.48±0.97** | 9.15±0.79* | 6.88±0.31* | 5.88±0.50 | 4.28±0.45 | 3.49±0.41 |
=p<0.05
=p<0.01
A High-fat Diet Induces Prediabetes
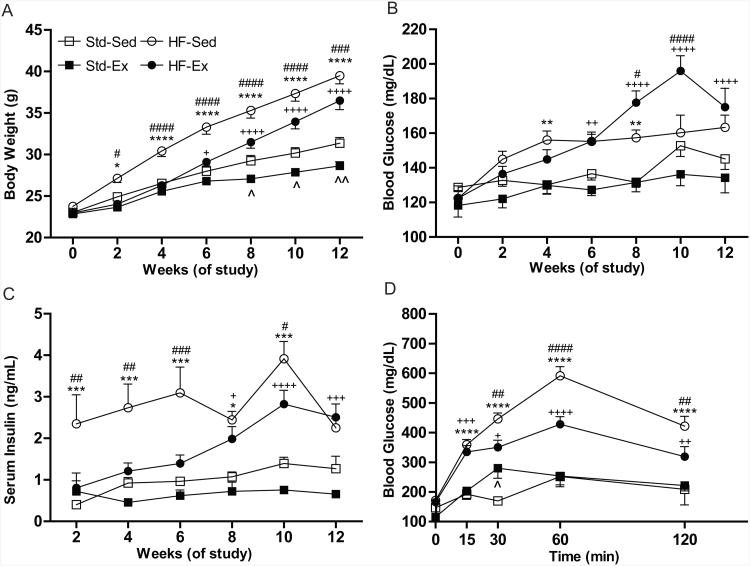
HF-Sed mice gained significantly more weight throughout the study than all other groups, beginning at two weeks (Fig. 1A). HF-Ex mice gained excess weight compared to Std-Ex mice; however, HF-Ex mice weighed significantly less than HF-Sed mice throughout the study. While HF-fed mice gained more weight than Std diet mice, energy intake between the groups was not significantly different (Std-Sed 2.48±0.40, n=7; Std-Ex 3.53±0.43, n=12; HF-Sed 2.59±0.35, n=12; HF-Ex 3.24±0.35, n=16 kcal/day).
Figure 1. A High-Fat Diet Induces Symptoms of Prediabetes.
(A) A high-fat diet causes increased weight gain in both the HF-Sed (n=21) and HF-Ex (n=24) compared to their standard diet controls (Std-Sed, n=14; Std-Ex, n=16). (B) Glucose levels are slightly elevated in high-fat fed animals, though overt hyperglycemia does not develop in either sedentary or exercise mice. (C) High-fat sedentary mice develop early hyperinsulinemia which continues throughout the study. High-fat exercise mice develop hyperinsulinemia, but not until 8 weeks. (D) A glucose tolerance test performed at 12 weeks shows significantly elevated blood glucose levels in both HF-Sed and HF-Ex mice. All data presented as mean±SEM. *Std-Sed vs HF-Sed; +Std-Ex vs HF-Ex; #HF-Sed vs HF-Ex; ˆStd-Sed vs Std-Ex., *=p<0.05, **=p<0.01, ***=p<0.001, ****=p<0.0001.
HF-Sed and HF-Ex mice had significantly elevated blood glucose levels compared to their Std-fed counterparts, beginning at 4 and 6 weeks, respectively (Fig. 1B); however their blood glucose levels remained below the threshold of diabetes (250mg/dl) throughout the study. Furthermore, hemoglobin A1c levels measured at 12 weeks were not significantly different between groups (Std-Sed 4.50%±0.11 [26mmol/mol], Std-Ex 4.48%±0.09 [25mmol/mol], HF-Sed 4.44%±0.11 [25mmol/mol], HF-Ex 4.64% ±0.08 [27mmol/mol]).
HF-Sed mice displayed significantly elevated insulin levels compared to all other groups, as early as two weeks post-diet change, whereas the onset of significantly elevated serum insulin in HF-Ex mice was not observed until 8 weeks (Fig. 1C). An IPGTT was also performed at 12 weeks, and both groups of HF-fed mice displayed significantly increased blood glucose levels over the course of the experiment (Fig. 1D). While blood glucose levels of HF-Ex mice were significantly higher than those of Std-Ex mice, they were also significantly lower than those of the HF-Sed group (Fig, 1D). Collectively, these data suggest that mice fed a high-fat diet develop a condition similar to prediabetes beginning two weeks following diet intervention that was partially attenuated by exercise.
High-fat Diet Induced-Hypersensitivity is Attenuated by Exercise
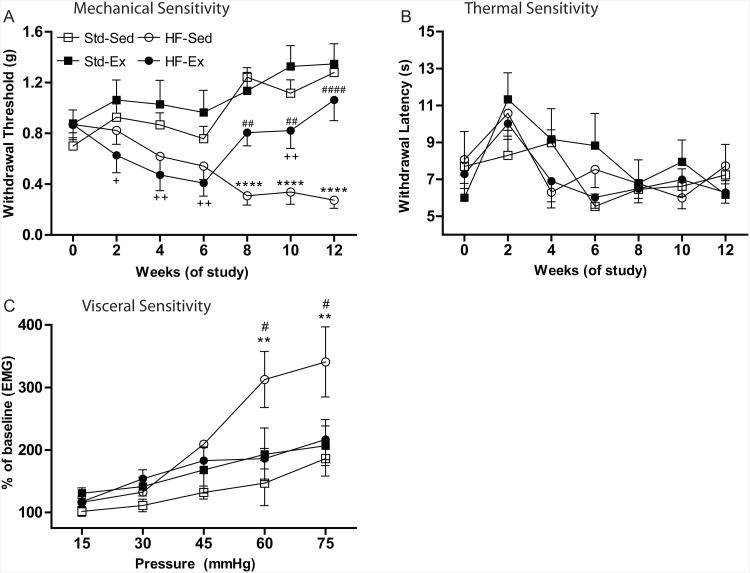
Cutaneous sensitivity was assessed by determining mechanical and thermal withdrawal thresholds. At baseline, there were no significant differences between mice in either mechanical or thermal thresholds. By week 2, the HF-Ex mice had a significantly lower mechanical withdrawal threshold compared to the Std-Ex group (Fig. 2A). By week 4, HF-Ex mice had significantly lower withdrawal thresholds than either Std diet group. In addition, the HF-Sed group was also significantly more sensitive than the Std-Ex group at this time point. Interestingly, at 8 weeks, HF-Ex withdrawal thresholds returned to baseline levels and were significantly increased compared to the HF-Sed mice, yet at 10 weeks, were still significantly lower than Std-Ex mice (Fig. 2A). By the end of 12 weeks, the mechanical thresholds of HF-Ex mice were no longer significantly different from either Std-Sed or Std-Ex mice, but were significantly higher than HF-Sed mice (Fig.2A). No effect of diet or exercise was observed on hindpaw thermal sensitivity (Fig. 2B).
Figure 2. A High-Fat Diet Induces Mechanical and Visceral Hypersensitivity and Is Reversed by Exercise.
(A) von Frey mechanical sensitivity testing shows allodynia in both HF-Ex (n=13) and HF-Sed (n=13) compared to control (Std-Ex, n=10; Std-Sed, n=10) animals. (B) Hargreave's thermal withdrawal latencies were unchanged based on diet or exercise. Std-Sed ( n=4), Std-Ex (n=6), HF-Sed (n=8) HF-Ex (n=11). (C) Visceral hypersensitivity is significantly increased in HF-Sed (n=3) animals at the two highest pressues, but is normal in HF-Ex (n=8) animals compared to controls (Std-Sed, n=4; Std-Ex, n=3). All data presented as mean±SEM. *Std-Sed vs HF-Sed; +Std-Ex vs HF-Ex; #HF-Sed vs HF-Ex; ˆStd-Sed vs Std-Ex. *=p<0.05, **=p<0.01, ***=p<0.001, ****=p<0.0001.
In addition to assessing changes in hindpaw sensitivity, we measured visceromotor response (VMR) during colorectal distension at 12 weeks to determine whether HF diet influenced visceral hypersensitivity. All four groups of mice displayed an increased VMR with increasing intraballoon pressure (Fig. 2C). Although an overall significant effect of diet-exercise was not observed (p=0.0954), posthoc analysis revealed that HF-Sed mice had a significantly higher VMR than both HF-Ex and Std-Sed mice at the two highest intraballoon pressures applied (Fig. 2C).
Nerve Conduction Velocity and Intraepidermal Innervation
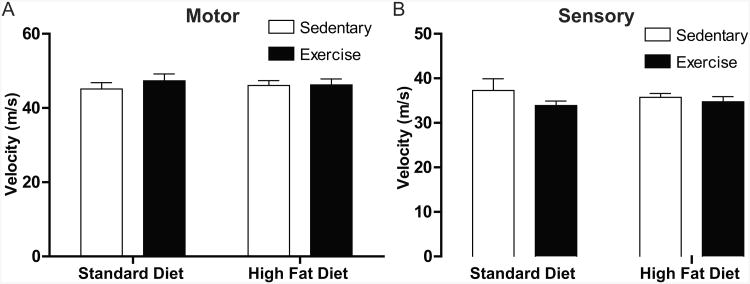
Neither a high-fat diet nor exercise, alone or in combination, altered motor or sensory nerve conduction velocities following 12 weeks of study (Figs. 3A-B).
Figure 3. Nerve Conduction Velocities Are Not Altered.
(A) Motor and (B) sensory nerve conduction velocities are not altered by either a high-fat diet or exercise. All data presented as mean±SEM. Std-Sed (n=4), Std-Ex (n=6), HF-Sed (n=8), HF-Ex (n=11).
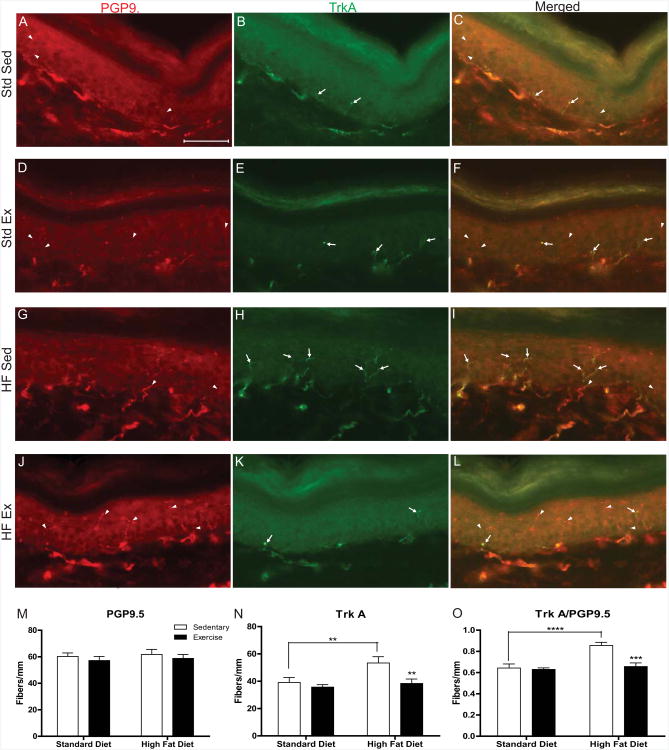
To determine if nerve fiber densities were altered by diet or exercise after 12 weeks, epidermal innervation of the hindpaw skin was examined using PGP9.5 antibody, a pan-neuronal marker, and a TrkA antibody, the high affinity receptor for NGF. Intraepidermal nerve fibers were either TrkA-positive (Fig. 4, arrows) or TrkA-negative (Fig. 4, arrowheads) and colocalization allowed for quantification of the intraepidermal nerve fiber phenotype. Representative images in Fig. 4 illustrate the presence of both PGP9.5 and TrkA in all groups including Std-Sed (Fig. 4A-C), Std-Ex (Figure 4D-F), HF-Std (Fig. 4G-I), and HF-Ex (Fig. 4J-L). Quantification of IENFD showed that neither a high-fat diet nor exercise significantly altered the number of PGP9.5-immunopositive fibers (Fig. 4M). However, HF-Sed mice had a 1.36-fold increase in the number of TrkA positive fibers compared to Std-Sed mice (Fig. 4N). HF-Ex mice did not display this same increase in TrkA fiber density. In addition, the fiber density of HF-Ex TrkA was not significantly different from Std-Ex, but was 1.39-fold lower than HF-Sed TrkA (Fig. 4N). To confirm that the increase in TrkA fiber counts was not due to an overall increase in total fibers, we calculated the percentages of TrkA-positive/PGP9.5-positive fibers in each animal and normalized the data to the means of Std-Sed for each group (Fig. 4O). We found a 1.4-fold increase in the ratio of HF-Sed mice to Std-Sed mice compared to only a 1.1-fold increase in the HF-Ex mice.
Figure 4. Intraepidermal Nerve Fiber Phenotype is Altered by a High-Fat Diet.
Increased TrkA receptor expression in the hind paw skin of high-fat mice. Representative images of double immunofluorescent staining for the pan-neuronal marker PGP9.5 ([A] Std-Sed; [D] Std-Ex; [G] HF-Sed; [J] HF-Ex), NGF receptor TrkA ([B] Std-Sed; [E] Std-Ex; [H] HF-Sed; [K] HF-Ex) or merged images ([C] Std-Sed; [F] Std-Ex; [I] HF-Sed; [L] HF-Ex). Arrowheads indicate TrkA-/PGP9.5+ neurons; Arrows indicate TrkA+/PGP9.5 neurons. IENF quantification for PGP9.5 (M) shows no change in overall fiber count due to diet or exercise. However, quatification of TrkA+ neurons (N) show an increase in HF-Sed mice and a recovery in HF-Ex mice. Additionally, the ratio of TrkA/PGP9.5 shows HF-Sed mice retain this increase and reversal due to exercise (O). Scale bar equals 50μm. Data presented as mean±SEM, n=6 animals/group. *=p<0.05, **=p<0.01, ***=p<0.001, ****=p<0.0001.
Neurotrophins Expression is Altered by Both Diet and Exercise
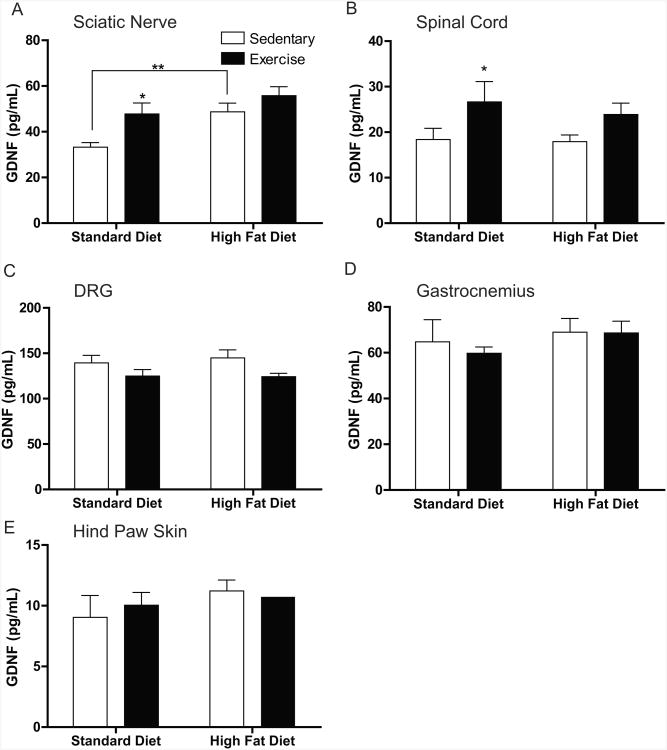
In the current study, we wanted to investigate if a high-fat diet, exercise, or a combination of both can alter neurotrophin protein expression in neural and non-neuronal tissues. Our results demonstrate a 1.4-fold increase in GDNF levels in both the sciatic nerve (Fig. 5A) and spinal cord (Fig. 5B) of Std-Ex compared to Std-Sed mice. Additionally, GDNF was increased 1.5-fold in the sciatic nerve of HF-Sed mice compared to the Std-Sed mice (Fig. 5A). In the DRG, HF-Ex animals appeared to have a decreased concentration of GDNF in comparison to HF-Sed animals, yet it was not significant (p=0.0501; Fig. 5C). In non-neuronal tissues, the gastrocnemius muscle and hind paw footpad, there were no significant alterations in GDNF levels in any of the animal cohorts (Figs. 5D,E respectively).
Figure 5. GDNF Protein Content in Neural and Non-Neuronal Tissues.
Quantification of glial cell-line derived neurotrophic factor (GDNF) in (A) sciatic nerve, (B) spinal cord, (C) dorsal root ganglia (DRG), (D) gastrocnemius, and (E) hindpaw skin. The only changes in GDNF content was seen in the spinal cord (A) where exercise in the standard diet group was increased and in the sciatic nerve (C) where GDNF was increased in both Std-Ex and HF-Sed mice compared to control mice. Data are presented as means±SEM where n=9-11 per group per tissue. *=p<0.05, **=p<0.01.
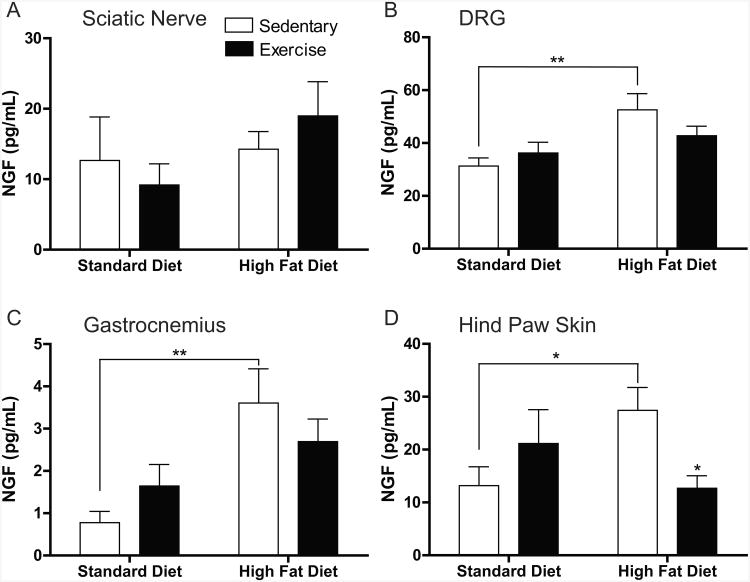
In contrast to GDNF, NGF protein expression was mostly altered in the non-neuronal tissues as opposed to the neural tissues. In the neural compartments, there were no changes in the sciatic nerve (Fig. 6A) as was seen with GDNF protein, yet NGF levels were increased in HF-Sed mice versus Std-Sed mice in the DRG (Fig. 6B). Peripherally, NGF was increased in HF-Sed mice compared to Std-Sed mice in both gastrocnemius muscle (Fig. 6C) and hind paw footpad (Fig. 6D) (4.6- and 2.1-fold increase, respectively). Additionally, our results show that exercise significantly decreased NGF levels 2.2-fold in the footpad of high-fat fed mice. The protein values were below the detection limit of the NGF ELISA for the spinal cord.
Figure 6. NGF Protein is Upregulated in Non-Neuronal Tissues by a High-Fat Diet.
Quantification of nerve growth factor (NGF) in (A) sciatic nerve, (B) DRG, (C) gastrocnemius, and (D) hindpaw skin. NGF levels were below the limit of detection in the spinal cord. A high-fat diet caused an increase in NGF in the DRG (A), gastrocnemius muscle (B), and hindpaw skin. While it appears that exercise decreases NGF closer to baseline levels in high-fat diet mice in the DRG and gastrocnemius, the only place where there was a significant decline in NGF content from HF-Sed to HF-Ex was in the hindpaw skin. Data are presented as means±SEM where n=6-11 per group per tissue. *=p<0.05, **=p<0.01.
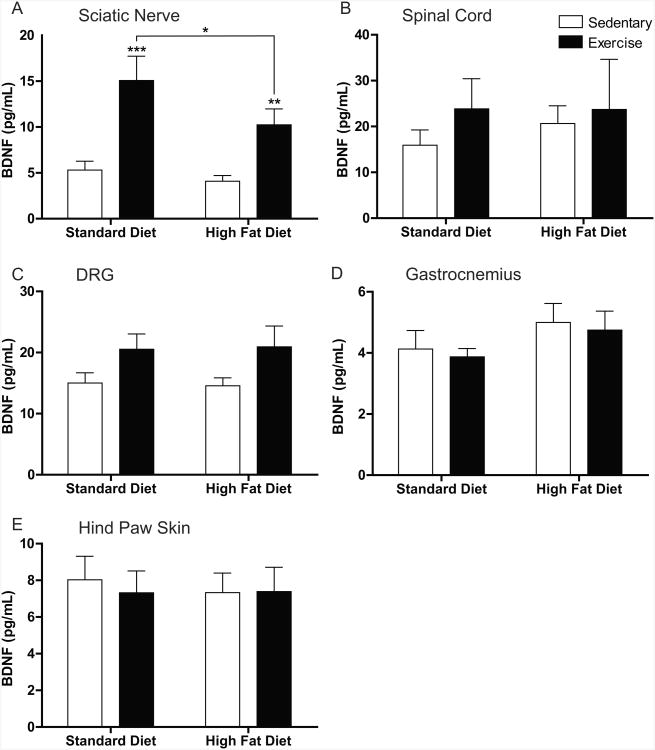
Finally, we investigated changes in BDNF protein expression in non-neuronal and neural tissues. In all tissues except sciatic nerve (Fig. 7B-E), we saw no alterations to BDNF levels associated with a high-fat diet or with exercise. However, in the sciatic nerve (Fig. 7A), significant increases in BDNF levels were evident following exercise in both the standard diet and high-fat diet groups. While exercise acted to increase BDNF levels, a high-fat diet in combination with exercise appeared to slightly restore BDNF expression to control levels.
Figure 7. BDNF Protein Alteration is Restricted to the Sciatic Nerve.
Brain derived neurotrophic factor (BDNF) protein quantification in (A) sciatic nerve, (B) spinal cord, (C) dorsal root ganglia (DRG), (D) gastrocnemius, and (E) hindpaw skin. BDNF protein expression was altered only in the sciatic nerve (C) where exercise significantly increased expression in both exercise groups. HF-Ex mice had significantly decreased BDNF expression compared to Std-Ex mice. Other tissues did not display any alterations in BDNF protein expression. Data are presented as means±SEM where n=8-11 per group per tissue.
Discussion
Using a high-fat diet to induce prediabetes, we demonstrate that C57Bl/6 mice develop cutaneous and visceral hyperalgesia, alterations in neurotrophin protein levels, and changes in the composition of epidermal innervation. Our results reveal that voluntary exercise can reverse cutaneous and visceral hypersensitivity, as well as normalize peripheral NGF levels and epidermal innervation. These findings provide evidence of how neurotrophin levels and epidermal innervation can change in a setting of painful prediabetes, and how exercise may be beneficial in correcting these changes.
A High-fat Diet and Prediabetes
Mice fed a high-fat diet develop accelerated weight gain, mild hyperglycemia, hyperinsulinemia, and abnormal responsiveness to glucose. Exercise delayed several metabolic features, including weight gain, hyperinsulinemia and glucose intolerance. Exercise was unable to prevent or reverse the metabolic features, suggesting that exercise-mediated improvements in sensory symptoms may occur independently from metabolic status. Interestingly, both exercise groups decreased their activity over the 12-week study, and the reason(s) why mice decline their running wheel activity remains unclear. Moreover, as HF- Ex mice decreased exercise levels, their prediabetic-related metabolic parameters worsen. Furthermore, while many of the metabolic parameters worsen by 8 weeks, HF-Ex mice are still exercising far more than their HF-Sed counterparts, suggesting that the high-fat diet may overwhelm the ability of exercise to keep metabolic parameters in check.
Behavioral Changes Associated with a High-fat Diet
Our studies agree with previous reports that a high-fat diet induces prediabetic-like symptoms and alters nociceptive behaviors in rodents [20; 38]. However, our results differ from other studies in that no alterations in thermal sensitivity or nerve conduction velocities were observed [13; 38] and may be due to the younger age of our mice and/or the reduced length of our study. Notably, we demonstrate that in addition to cutaneous hypersensitivity, high-fat fed mice also display visceral hypersensitivity. Visceral hypersensitivity has been reported previously in streptozotocin-treated rats, and our studies extend this finding to mice fed a high-fat diet [18]. These findings are relevant, as diabetic patients with autonomic neuropathy suffer from gastroporesis, limited esophageal motility and incontinence [16], and visceral pain [37]. Our data suggests that hypersensitivity of visceral organs may reflect problems associated with visceral afferent nociception. Importantly, exercised mice displayed normalized cutaneous mechanical and visceral sensitivity, consistent with a role for exercise to normalize abnormal sensation [29].
Phenotype Alterations in Epidermal Innervation
In the current study, mice fed a high-fat diet did not develop reductions in total epidermal innervation. These findings agree with previous reports that prediabetic obese mice do not have altered intraepidermal innervation following 16 weeks of dietary intervention [38]. Additionally, the role of intraepidermal nerve fibers in human prediabetic patients related to pain status need additional study, as conflicting results have been reported [44; 52].
However, while total epidermal innervation was not reduced in high-fat fed mice, there were significant increases in TrkA-positive axons. High-fat fed mice displayed an increase in TrkA axons compared to mice on the standard diet. These findings are important because of the integral role of NGF in pain. TrkA is the high affinity receptor for NGF, and TrkA-expressing fibers are small, peptidergic fibers that express key nociceptive neuropeptides [27]. Our findings of increased epidermal TrkA fibers in concert with mechanical allodynia in mice fed a high-fat diet are consistent with studies in type 2 mice that report increases in both PGP9.5 and TrkA epidermal fibers in mice displaying mechanical allodynia [9; 10]. This finding is also supported by a complimentary study in which type 1 diabetic mice developed mechanical hypoalgesia when peptidergic epidermal fibers were selectively lost [26]. These findings highlight the strong relationship between peptidergic fiber innervation and nociceptive input.
In addition to NGF-responsive axons, remaining epidermal fibers are nonpeptidergic and sensitive to GDNF. While peptidergic neurons convey nociceptive signals, nonpeptidergic neurons may have more complex functions, including analgesia. Both local [1] and intrathecal [6] administration of GDNF can reduce mechanical and thermal sensitivities. All cutaneous axons arise developmentally as NGF-sensitive fibers, but divide postnatally to form peptidergic and nonpeptidergic axonal populations [34]. An increase in TrkA-expressing fibers could arise from de novo TrkA expression in nonpeptidergic fibers, leading to a change in axonal phenotype. It should be noted that nonpeptidergic fibers were not identifiably labeled, so we cannot conclusively state that nonpeptidergic fibers were lost at the expense of peptidergic fibers. It should also be noted that, although not quantified, TrkA did not appear to be increased in keratinocytes of high-fat animals, as reported in human keratinocytes in pre-diabetic states [7; 56]. Our findings that a high-fat diet can increase the TrkA expressing fibers may suggest that the balance of fibers, i.e. peptidergic versus nonpeptidergic, could be key in regulating cutaneous nociceptive thresholds. Our results also suggest that exercise can correct the phenotypic change and abnormal behavioral sensitivity. Together, these findings suggest that alterations in subsets of epidermal axons may be sufficient to regulate cutaneous sensation and could be a key step that initiates pain or sensory loss in diabetes. Alterations in axonal phenotypes as an underlying mechanism could help explain settings where sensory symptoms are evident in patients with normal IENF levels.
Neurotrophic Changes in Neural and Peripheral Tissues
In the current study, GDNF protein levels were increased by exercise in the spinal cord and sciatic nerve of standard diet-fed mice. GDNF is known to be a potent analgesic in several rodent pain models [6; 55; 57], including type 1 diabetes (Wright, unpublished observations). It is not clear why GDNF is elevated in these neural compartments following exercise and it will be important to determine the consequences of elevated GDNF, particularly as axonal phenotypes change.
NGF has seemingly opposing actions, acting as both pro- and antinociceptive. NGF has been reported to be effective in reducing pain in models of chronic constriction injury (CCI) [46] and diabetic neuropathy [12] as well as neuropathic pain in patients [48]. In contrast, NGF can elicit hyperalgesia [30] and modulate inflammatory pain [47; 58]. Here, NGF protein concentration was significantly increased in high-fat fed mice in the DRG, gastrocnemius muscle and hind paw skin. While we observed normalized NGF protein content in the DRG and gastrocnemius in HF-Ex animals, these changes were not significant. However, this decrease of NGF in hindpaw skin from HF-Sed animals to HF-Ex animals was significant. This data suggests that a high-fat diet causes an increase in NGF content and that exercise reverses this abnormality. Thus, the increased NGF content may be related to diet-induced inflammation in the periphery that is responsive to exercise intervention.
Obesity can induce an insulin-resistant state and prediabetes. Furthermore, high-fat-diet-induced obese mice display signs of chronic low-grade inflammation [19; 39], including increased proinflammatory macrophages that secrete cytokines such as tumor necrosis factor α (TNF-α), macrophage migration inhibitory factor (MIF), interleukin-1β (Il-1β) and Il-6 [41]. Increases in proinflammatory cytokines can directly lead to lowered insulin sensitivity [39]. Additionally, IL-1β and TNF-α can increase NGF release from cultured fibroblasts [21] and sciatic nerve [31]. Since a high-fat diet is known to induce prediabetes and increased inflammation, elevated levels of NGF may be associated with these inflammatory changes. Our results reveal that HF-Sed mice have elevated NGF in the gastrocnemius muscle and hind paw skin, while HF-Ex mice have levels similar to control mice. Interestingly, along with the increase in NGF protein seen in the hindpaw skin in the HF-Sed group, our study revealed an increase in TrkA-expressing epidermal axons. Previous groups have shown that overexpression of NGF can lead to hyperinnvervation in the bladder [49], can cause Aδ fibers to become responsive to substance P [24], and can promote the sprouting of TrkA-expressing nociceptors, resulting in hyperinnervation of the skin [54]. Taken together, our results suggest that prediabetes is associated with increased levels of NGF, and possibly peripheral inflammation, leading to an increase in epidermal axons bearing a nociceptive TrkA phenotype.
Another key finding in this study is that mice that exercised had normal levels of NGF in the skin, and normal ratios of TrkA- and non-TrkA-positive axons. Here, exercise may act to decrease elevated NGF associated with the high-fat diet, leading to normalized TrkA-positive axonal innervation. Exercise has anti-inflammatory actions [42] and physical activity can increase systemic anti-inflammatory cytokines. Following exercise, Il-6 is increased by up to 100-fold and then followed by an increase in Il-1 receptor antagonist (Il-1ra) and Il-10, all cytokines with anti-inflammatory effects [42]. We suggest that a while a high-fat diet may induce systemic inflammation and increase NGF, exercise may counteract rising inflammation and normalize NGF levels.
Conclusion
The current study demonstrates that mice fed a high-fat diet develop prediabetes symptoms, visceral hypersensitivity and mechanical allodynia. The high-fat diet also changes the composition of peptidergic and nonpeptidergic phenotypes of epidermal axons, while not changing the overall number of epidermal fibers. These findings suggest that alterations in cutaneous sensitivity may be related axonal phenotypes innervating the epidermis. In addition, this study demonstrates that exercise can correct many of the abnormalities, including visceral hypersensitivity and mechanical allodynia.
Acknowledgments
The authors of the manuscript would like to thank the Kansas Intellectual and Developmental Disabilities Research Center P30 NICHD HD 002528 for their contribution to this work. In addition, the authors would like to thank Michelle Winter for her work on the serum insulin data.
This publication was supported by NIH RO1NS43314 (D.E.W.). In addition, support was provided by the NIH Grant P20 GM103418 from the Kansas Idea Network of Biomedical Research Excellence (INBRE) program of the National Institute of General Medical Sciences.
Footnotes
No potential conflicts of interest relevant to this article were reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adler JE, Nico L, VandeVord P, Skoff AM. Modulation of neuropathic pain by a glial-derived factor. Pain Med. 2009;10(7):1229–1236. doi: 10.1111/j.1526-4637.2009.00708.x. [DOI] [PubMed] [Google Scholar]
- 2.Anand P, Terenghi G, Warner G, Kopelman P, Williams-Chestnut RE, Sinicropi DV. The role of endogenous nerve growth factor in human diabetic neuropathy. Nature medicine. 1996;2(6):703–707. doi: 10.1038/nm0696-703. [DOI] [PubMed] [Google Scholar]
- 3.Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29(Suppl 1):S43–48. [PubMed] [Google Scholar]
- 4.Barrett AM, Lucero MA, Le T, Robinson RL, Dworkin RH, Chappell AS. Epidemiology, public health burden, and treatment of diabetic peripheral neuropathic pain: a review. Pain Med. 2007;8(2):S50–62. doi: 10.1111/j.1526-4637.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 5.Bobinski F, Martins DF, Bratti T, Mazzardo-Martins L, Winkelmann-Duarte EC, Guglielmo LG, Santos AR. Neuroprotective and neuroregenerative effects of low-intensity aerobic exercise on sciatic nerve crush injury in mice. Neuroscience. 2011;194:337–348. doi: 10.1016/j.neuroscience.2011.07.075. [DOI] [PubMed] [Google Scholar]
- 6.Boucher TJ, Okuse K, Bennett DL, Munson JB, Wood JN, McMahon SB. Potent analgesic effects of GDNF in neuropathic pain states. Science. 2000;290(5489):124–127. doi: 10.1126/science.290.5489.124. [DOI] [PubMed] [Google Scholar]
- 7.Bronzetti E, Ciriaco E, Germana G, Vega JA. Immunohistochemical localization of neurotrophin receptor proteins in human skin. Italian journal of anatomy and embryology = Archivio italiano di anatomia ed embriologia. 1995;100(1):565–571. [PubMed] [Google Scholar]
- 8.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 9.Cheng HT, Dauch JR, Hayes JM, Hong Y, Feldman EL. Nerve growth factor mediates mechanical allodynia in a mouse model of type 2 diabetes. J Neuropathol Exp Neurol. 2009;68(11):1229–1243. doi: 10.1097/NEN.0b013e3181bef710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng HT, Dauch JR, Hayes JM, Yanik BM, Feldman EL. Nerve growth factor/p38 signaling increases intraepidermal nerve fiber densities in painful neuropathy of type 2 diabetes. Neurobiol Dis. 2011;45(1):280–287. doi: 10.1016/j.nbd.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christianson JA, Gebhart GF. Assessment of colon sensitivity by luminal distension in mice. Nat Protoc. 2007;2(10):2624–2631. doi: 10.1038/nprot.2007.392. [DOI] [PubMed] [Google Scholar]
- 12.Christianson JA, Riekhof JT, Wright DE. Restorative effects of neurotrophin treatment on diabetes-induced cutaneous axon loss in mice. Exp Neurol. 2003;179(2):188–199. doi: 10.1016/s0014-4886(02)00017-1. [DOI] [PubMed] [Google Scholar]
- 13.Davidson E, Coppey L, Lu B, Arballo V, Calcutt NA, Gerard C, Yorek M. The roles of streptozotocin neurotoxicity and neutral endopeptidase in murine experimental diabetic neuropathy. Experimental diabetes research. 2009;2009:431980. doi: 10.1155/2009/431980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dyck PJ, Dyck PJ, Klein CJ, Weigand SD. Does impaired glucose metabolism cause polyneuropathy? Review of previous studies and design of a prospective controlled population-based study. Muscle Nerve. 2007;36(4):536–541. doi: 10.1002/mus.20846. [DOI] [PubMed] [Google Scholar]
- 15.Evans L, Andrew D, Robinson P, Boissonade F, Loescher A. Increased cutaneous NGF and CGRP-labelled trkA-positive intra-epidermal nerve fibres in rat diabetic skin. Neuroscience letters. 2012;506(1):59–63. doi: 10.1016/j.neulet.2011.10.049. [DOI] [PubMed] [Google Scholar]
- 16.Gatopoulou A, Papanas N, Maltezos E. Diabetic gastrointestinal autonomic neuropathy: Current status and new achievements for everyday clinical practice. Eur J Intern Med. 2012;23(6):499–505. doi: 10.1016/j.ejim.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Pinilla F, Ying Z, Opazo P, Roy RR, Edgerton VR. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. The European journal of neuroscience. 2001;13(6):1078–1084. doi: 10.1046/j.0953-816x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- 18.Grabauskas G, Heldsinger A, Wu X, Xu D, Zhou S, Owyang C. Diabetic visceral hypersensitivity is associated with activation of mitogen-activated kinase in rat dorsal root ganglia. Diabetes. 2011;60(6):1743–1751. doi: 10.2337/db10-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 20.Guilford BL, Ryals JM, Wright DE. Phenotypic changes in diabetic neuropathy induced by a high-fat diet in diabetic C57BL/6 mice. Experimental diabetes research. 2011;2011:848307. doi: 10.1155/2011/848307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hattori A, Tanaka E, Murase K, Ishida N, Chatani Y, Tsujimoto M, Hayashi K, Kohno M. Tumor necrosis factor stimulates the synthesis and secretion of biologically active nerve growth factor in non-neuronal cells. The Journal of biological chemistry. 1993;268(4):2577–2582. [PubMed] [Google Scholar]
- 22.Hellweg R, Hartung HD. Endogenous levels of nerve growth factor (NGF) are altered in experimental diabetes mellitus: a possible role for NGF in the pathogenesis of diabetic neuropathy. Journal of neuroscience research. 1990;26(2):258–267. doi: 10.1002/jnr.490260217. [DOI] [PubMed] [Google Scholar]
- 23.Houle JD, Cote MP. Axon regeneration and exercise-dependent plasticity after spinal cord injury. Annals of the New York Academy of Sciences. 2013;1279:154–163. doi: 10.1111/nyas.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunter DD, Myers AC, Undem BJ. Nerve growth factor-induced phenotypic switch in guinea pig airway sensory neurons. Am J Respir Crit Care Med. 2000;161(6):1985–1990. doi: 10.1164/ajrccm.161.6.9908051. [DOI] [PubMed] [Google Scholar]
- 25.Hutchinson KJ, Gomez-Pinilla F, Crowe MJ, Ying Z, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127(Pt 6):1403–1414. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- 26.Johnson MS, Ryals JM, Wright DE. Early loss of peptidergic intraepidermal nerve fibers in an STZ-induced mouse model of insensate diabetic neuropathy. Pain. 2008;140(1):35–47. doi: 10.1016/j.pain.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kashiba H, Ueda Y, Senba E. Coexpression of preprotachykinin-A, alpha-calcitonin gene-related peptide, somatostatin, and neurotrophin receptor family messenger RNAs in rat dorsal root ganglion neurons. Neuroscience. 1996;70(1):179–189. doi: 10.1016/0306-4522(95)00334-f. [DOI] [PubMed] [Google Scholar]
- 28.Kluding PM, Pasnoor M, Singh R, Jernigan S, Farmer K, Rucker J, Sharma NK, Wright DE. The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. Journal of diabetes and its complications. 2012;26(5):424–429. doi: 10.1016/j.jdiacomp.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuphal KE, Fibuch EE, Taylor BK. Extended swimming exercise reduces inflammatory and peripheral neuropathic pain in rodents. The journal of pain : official journal of the American Pain Society. 2007;8(12):989–997. doi: 10.1016/j.jpain.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Lewin GR, Ritter AM, Mendell LM. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J Neurosci. 1993;13(5):2136–2148. doi: 10.1523/JNEUROSCI.13-05-02136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindholm D, Heumann R, Meyer M, Thoenen H. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature. 1987;330(6149):658–659. doi: 10.1038/330658a0. [DOI] [PubMed] [Google Scholar]
- 32.McMahon SB. NGF as a mediator of inflammatory pain. Philos Trans R Soc Lond B Biol Sci. 1996;351(1338):431–440. doi: 10.1098/rstb.1996.0039. [DOI] [PubMed] [Google Scholar]
- 33.McMahon SB, Bennett D, Bevan S. Inflammatory mediators and modulators of pain. Wall and Melzack's Textbook of Pain. 2006;5:49–72. [Google Scholar]
- 34.Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19(4):849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- 35.Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373(6510):109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 36.Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain research. 1996;726(1-2):49–56. [PubMed] [Google Scholar]
- 37.O'Connor RC, Andary MT, Russo RB, DeLano M. Thoracic radiculopathy. Phys Med Rehabil Clin N Am. 2002;13(3):623–644. doi: 10.1016/s1047-9651(02)00018-9. viii. [DOI] [PubMed] [Google Scholar]
- 38.Obrosova IG, Ilnytska O, Lyzogubov VV, Pavlov IA, Mashtalir N, Nadler JL, Drel VR. High-fat diet induced neuropathy of pre-diabetes and obesity: effects of “healthy” diet and aldose reductase inhibition. Diabetes. 2007;56(10):2598–2608. doi: 10.2337/db06-1176. [DOI] [PubMed] [Google Scholar]
- 39.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 40.Papanas N, Vinik AI, Ziegler D. Neuropathy in prediabetes: does the clock start ticking early? Nat Rev Endocrinol. 2011;7(11):682–690. doi: 10.1038/nrendo.2011.113. [DOI] [PubMed] [Google Scholar]
- 41.Park S, Park NY, Valacchi G, Lim Y. Calorie restriction with a high-fat diet effectively attenuated inflammatory response and oxidative stress-related markers in obese tissues of the high diet fed rats. Mediators of inflammation. 2012;2012:984643. doi: 10.1155/2012/984643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedersen BK. The anti-inflammatory effect of exercise: its role in diabetes and cardiovascular disease control. Essays Biochem. 2006;42:105–117. doi: 10.1042/bse0420105. [DOI] [PubMed] [Google Scholar]
- 43.Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 44.Polydefkis M, Griffin JW, McArthur J. New insights into diabetic polyneuropathy. JAMA : the journal of the American Medical Association. 2003;290(10):1371–1376. doi: 10.1001/jama.290.10.1371. [DOI] [PubMed] [Google Scholar]
- 45.Prevention CfDCa. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. CfDCaP Department of Health and Human Services editor; Atlanta, GA: U.S: 2011. [Google Scholar]
- 46.Ren K, Thomas DA, Dubner R. Nerve growth factor alleviates a painful peripheral neuropathy in rats. Brain research. 1995;699(2):286–292. doi: 10.1016/0006-8993(95)00920-l. [DOI] [PubMed] [Google Scholar]
- 47.Saade NE, Nasr IW, Massaad CA, Safieh-Garabedian B, Jabbur SJ, Kanaan SA. Modulation of ultraviolet-induced hyperalgesia and cytokine upregulation by interleukins 10 and 13. Br J Pharmacol. 2000;131(7):1317–1324. doi: 10.1038/sj.bjp.0703699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schifitto G, Yiannoutsos C, Simpson DM, Adornato BT, Singer EJ, Hollander H, Marra CM, Rubin M, Cohen BA, Tucker T, Koralnik IJ, Katzenstein D, Haidich B, Smith ME, Shriver S, Millar L, Clifford DB, McArthur JC, Team ACTG. Long-term treatment with recombinant nerve growth factor for HIV-associated sensory neuropathy. Neurology. 2001;57(7):1313–1316. doi: 10.1212/wnl.57.7.1313. [DOI] [PubMed] [Google Scholar]
- 49.Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford AP, Vizzard MA, Cockayne DA. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol. 2010;298(3):R534–547. doi: 10.1152/ajpregu.00367.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma NK, Ryals JM, Gajewski BJ, Wright DE. Aerobic exercise alters analgesia and neurotrophin-3 synthesis in an animal model of chronic widespread pain. Physical therapy. 2010;90(5):714–725. doi: 10.2522/ptj.20090168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singleton JR, Smith AG, Bromberg MB. Increased prevalence of impaired glucose tolerance in patients with painful sensory neuropathy. Diabetes Care. 2001;24(8):1448–1453. doi: 10.2337/diacare.24.8.1448. [DOI] [PubMed] [Google Scholar]
- 52.Smith AG, Ramachandran P, Tripp S, Singleton JR. Epidermal nerve innervation in impaired glucose tolerance and diabetes-associated neuropathy. Neurology. 2001;57(9):1701–1704. doi: 10.1212/wnl.57.9.1701. [DOI] [PubMed] [Google Scholar]
- 53.Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, Philip Malan T., Jr Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiology. 2011;114(4):940–948. doi: 10.1097/ALN.0b013e318210f880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stucky CL, Koltzenburg M, Schneider M, Engle MG, Albers KM, Davis BM. Overexpression of nerve growth factor in skin selectively affects the survival and functional properties of nociceptors. J Neurosci. 1999;19(19):8509–8516. doi: 10.1523/JNEUROSCI.19-19-08509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takasu K, Sakai A, Hanawa H, Shimada T, Suzuki H. Overexpression of GDNF in the uninjured DRG exerts analgesic effects on neuropathic pain following segmental spinal nerve ligation in mice. The journal of pain : official journal of the American Pain Society. 2011;12(11):1130–1139. doi: 10.1016/j.jpain.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 56.Terenghi G, Mann D, Kopelman PG, Anand P. trkA and trkC expression is increased in human diabetic skin. Neuroscience letters. 1997;228(1):33–36. doi: 10.1016/s0304-3940(97)00350-9. [DOI] [PubMed] [Google Scholar]
- 57.Wang R, Guo W, Ossipov MH, Vanderah TW, Porreca F, Lai J. Glial cell line-derived neurotrophic factor normalizes neurochemical changes in injured dorsal root ganglion neurons and prevents the expression of experimental neuropathic pain. Neuroscience. 2003;121(3):815–824. doi: 10.1016/s0306-4522(03)00491-3. [DOI] [PubMed] [Google Scholar]
- 58.Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol. 1997;121(3):417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yajima Y, Narita M, Usui A, Kaneko C, Miyatake M, Narita M, Yamaguchi T, Tamaki H, Wachi H, Seyama Y, Suzuki T. Direct evidence for the involvement of brain-derived neurotrophic factor in the development of a neuropathic pain-like state in mice. Journal of neurochemistry. 2005;93(3):584–594. doi: 10.1111/j.1471-4159.2005.03045.x. [DOI] [PubMed] [Google Scholar]
- 60.Zhou XF, Deng YS, Xian CJ, Zhong JH. Neurotrophins from dorsal root ganglia trigger allodynia after spinal nerve injury in rats. The European journal of neuroscience. 2000;12(1):100–105. doi: 10.1046/j.1460-9568.2000.00884.x. [DOI] [PubMed] [Google Scholar]