Abstract
L-arginine and its decarboxylated product, agmatine are important mediators of NO production and vascular relaxation. However, the underlying mechanisms of their action are not understood. We have investigated the role of arginine and agmatine in resistance vessel relaxation of Sprague-Dawley (SD) and Dahl salt-sensitive hypertensive rats. Second or 3rd-order mesenteric arterioles were cannulated in an organ chamber, pressurized and equilibrated before perfusing intraluminally with agonists. The vessel diameters were measured after mounting on the stage of a microscope fitted with a video camera. The gene expression in Dahl rat vessel homogenates was ascertained by real-time PCR. L-arginine initiated relaxations (EC50, 5.8 ± 0.7 mM; n = 9) were inhibited by arginine decarboxylase (ADC) inhibitor, difluoromethylarginine (DFMA) (EC50, 18.3 ± 1.3 mM; n = 5) suggesting that arginine-induced vessel relaxation was mediated by agmatine formation. Agmatine relaxed the SD rat vessels at significantly lower concentrations (EC50, 138.7 ± 12.1 μM; n = 22), which was compromised by L-NAME (L-NG-Nitroarginine methyl ester, an eNOS inhibitor), RX821002 (α-2 AR antagonist) and pertussis toxin (G-protein inhibitor). The agmatine-mediated vessel relaxation from high salt Dahl rats was abolished as compared to that from normal salt rats (EC50, 143.9 ± 23.4 μM; n = 5). The α-2A AR, α-2B AR and eNOS mRNA expression was downregulated in mesenteric arterioles of high-salt treated Dahl hypertensive rats. These findings demonstrate that agmatine facilitated the relaxation via activation of α-2 adrenergic G-protein coupled receptor and NO synthesis, and this pathway is compromised in salt-sensitive hypertension.
Keywords: agmatine, arginine decarboxylase, L-arginine, nitric oxide, α-2 adreno receptor, hypertension
1. Introduction
Over the past 2 decades there has been a significant interest in understanding the role of NO in vascular relaxation. L-arginine serves as a substrate for different nitric oxide synthases (NOS). The potential use of exogenous L-arginine as a therapeutic agent has been widely suggested in various physiological and pathophysiological processes such as wound healing [1; 2; 3], protein synthesis and muscle building [4], endocrine metabolism [5], erectile dysfunction [6], and a variety of cardiovascular functions [7; 8; 9]. Supplying arginine to the endothelial cells and vessels contributes to the enhanced NO synthesis and vessel relaxation despite its saturating cellular levels. Different mechanisms are hypothesized to explain this arginine paradox including endogenous NOS inhibitors and compartmentalization of intracellular arginine [10; 11; 12; 13]. However, there still exists an ambiguity in understanding how exogenous L-arginine mediates NO-dependent relaxation [14]. The apparent benefits of L-arginine supplementation are difficult to reconcile with a purely substrate based mechanism for NO synthesis. An alternative proposed by us explains arginine’s NOS substrate independent actions via the activation of α-2 adrenergic receptor (α-2 AR) as demonstrated in cultured endothelial cells [15]. Agmatine [4-(aminobutyl) guanidine] is produced endogenously via decarboxylation of L-arginine by the endothelial arginine decarboxylase (ADC) [16; 17] and it does not act as a substrate for NOS. A biological function for agmatine was suggested based on the observation that ADC activity transiently increased 7 fold during cerebral ischemia [18]. In addition, the importance of agmatine has been highlighted by its discovery as a novel neurotransmitter [19; 20; 21] demonstrating its potential to affect multiple biological targets. The presence of agmatine in serum [22] suggests a physiological role in the vasculature. Agmatine was shown to serve as a ligand for imidazoline and/or α-2 AR [23] and α-2 AR agonists mediate endothelium-dependent relaxation in mouse and rat aorta [24]. α-2 ARs (G-protein coupled receptors) play a pivotal role in the cardiovascular system and influence vascular tone at multiple points. These receptors are targets for antihypertensive therapy and their stimulation produces long lasting drop in systemic blood pressure [25]. However, the signaling mechanisms participating in agmatine-initiated NO synthesis [15; 26] and regulation of vascular tone is little understood, and the contribution of α-2 ARs is implicated in this process [15]. Compromised NO synthesis and endothelial dysfunction has been reported in the hypertensive vasculature including salt-sensitive hypertension [27; 28]. However, the factors that are responsible for its impaired synthesis are varied and not clearly understood. Impaired α-2 AR function has been documented in several models of hypertension [29; 30; 31]. However, whether this impairment is a cause or an effect of hypertension remains to be elucidated. Here we show that arginine-mediated arteriolar relaxations are due to agmatine produced by the actions of ADC and signaling via GPCR in rat microcirculation. Evidence is also presented documenting attenuated agmatine-mediated relaxation in Dahl salt-sensitive hypertensive rat mesenteric resistant arterioles, which correlates with reduced α-2 AR gene expression.
2. Material and Methods
2.1 Isolated mesenteric arteriole preparation
Resistance mesenteric arterioles of the 2nd or 3rd order (resting diameter ≤ 150 μm) were isolated from male Sprague-Dawley (SD) and Dahl rats (250-300 g), cleaned of the surrounding tissue and cannulated at both ends on glass cannula. The organ chamber was maintained constant at 37°C by superfusion with a modified Krebs-Ringer solution containing (mM): NaCl 145, KCl 5, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, HEPES 20, and Glucose 10.1, pH 7.4 [32; 33]. Cannulated vessels were axially pre-stretched to remove any bents and to simulate physiological stretch conditions and were pressurized at 50 mmHg and allowed to equilibrate for 60 min before initiating the experiment. To establish a concentration that gave submaximal constriction, we constructed a dose-response curve to norepinephrine (NE). The vessels were preconstricted with continuous superfusion of NE and those that retained a constant pressure and a consistent constriction to NE (Fig. S1.a and b), and fully responded to the acetylcholine (Fig. S3.a) were included in the study. The presence of functional endothelium was assessed by the ability of acetylcholine (10 μM) to induce more than 90% relaxation. The vessel reactivity study was carried out by intraluminal perfusion with various agonist concentrations. This was achieved by an automated solenoid valve controlled pressure driven perfusion system. Diameter measurements were tracked in real time by mounting the perfusion chamber on the stage of an inverted microscope (Olympus, Center Valley, PA) fitted with a CCD camera (QImaging, Surrey, Canada). Post analysis was performed with IPLAB (BioVision Technologies, Exton, PA) and MATLAB (MathWorks, Natick, MA) software. Chemicals NE, L-NAME (L-NG-Nitroarginine methyl ester), RX821002, agmatine, L-arginine were obtained from Sigma-Aldrich Co. (St. Louis, MO) and Pertussis toxin (PTx) were obtained from Tocris Bioscience (Ellisville, MO) and SPERNO from Cayman Chemicals (Ann Arbor, MI).
2.2 Animal Model
Male SD and Dahl salt-sensitive (SS/JrHsd) rats and their diet were purchased from Harlan Laboratories (Madison, WI). Sprague Dawley rats were maintained on standard pellet chow (2018 Teklad Global) rodent diet whereas the Dahl salt-sensitive rats were fed 0.49% NaCl diet (Harlan Cat. #TD 96208) or 4% NaCl diet (Harlan Cat. #TD 92034). The animals were housed in temperature and humidity controlled rooms with 12 hour on/off light cycle at the animal care facility. All animal studies were performed following Institutional Animal Care and Use approved procedures.
After acclimatization for 1 week, 6-weeks old Dahl salt-sensitive rats were separated into 2 diet groups; normal salt (NS), fed 0.49% NaCl diet and high salt (HS), fed 4% NaCl diet for 5 weeks. Systolic blood pressure was measured weekly by the tail-cuff method [34] and HS rats consistently demonstrated sustained hypertension (BP > 200 mmHg) while NS rats remained normotensive (BP < 160 mmHg) (Fig. S2). The rats were euthanized by CO2 inhalation and vascular reactivity assessed on isolated, cannulated and pressurized mesenteric arterioles as described above.
2.3 Determination of plasma nitrite
Blood derived from rats was centrifuged immediately at 5,000g for 5 min and plasma collected. The nitrite analysis was carried out using iodine/iodide in glacial acetic acid supplemented with 1% v/v antifoam SE-15 (Sigma Aldrich) using an ozone based chemiluminescence analyzer (Sievers, model 280i) as described [35].
2.4 Real Time-Polymerase Chain Reaction (RT-PCR)
RT-PCR was carried out on mesenteric tissue from Dahl rats [36], cleaned of fat and stabilized with RNAlater (Qiagen, Valencia, CA). The tissue was homogenized (~30 mg) with a sonicator in RLT buffer (Qiagen), the lysate centrifuged (10,000g) and total RNA purified with reagents from RNeasy® Fibrous Tissue Mini Kit (Qiagen). A first strand cDNA synthesis was performed using purified mRNA by Superscript III RT (Invitrogen, Grand Island, NY) in a thermocycler (MJ Research). The new cDNA strand was purified with QIAquick® PCR Purification Kit (Qiagen). Pure cDNA (~10 ng) was reacted with Power SYBR Green PCR Master Mix reagent (Applied Biosystems, Mountain View, CA) in RNase-free water in a StepOne RT-PCR system (Applied Biosystems). The relative expression of α-2A, α-2B AR and eNOS was determined using β-actin as a housekeeping gene. The primers used were: α-2A; TTT GCA CGT CGT CCA TAG TG (forward) and CAG TGA CAA TGA TGG CCT TG (reverse). α-2B; AAA CAC TGC CAG CAT CTC CT (forward) and CTG GCA ACT CCC ACA TTC TT (reverse). eNOS; CAA CGCTAC CAC GAG GAC ATT (forward) and CTC CTG CAA AGA AAA GCT CTG G (reverse). β-actin; TCC TAG CAC CAT GAA GAT C (forward) and AAA CGC AGCTCA GTA ACA G (reverse). Standard curves (initial amount of cDNA versus Ct values) were tested for each set of primers, demonstrating that for the similar range of total cDNA amplification the efficiency of target genes and housekeeping gene (β-actin) were equal. ‘No reverse transcription control’ was used where the PCR reaction was run in the absence of reverse transcriptase. Expression of the gene of interest was divided by the housekeeping gene and expressed as fold-change compared with the corresponding normal-salt rat group.
2.5 Data analysis
Relaxations were expressed as percentage of NE (2 μM) induced contraction. The vasodilation was studied by obtaining the maximal response and EC50 values were then calculated by fitting the concentration-response relationship to a logistic function. Amplified transcripts from RT-PCR were quantified using the comparative threshold cycle method (2−ΔΔCt) with β-actin as a normalizer and the corresponding sample from the normal salt fed rat mesentery as internal control.
All data were expressed as mean ± SEM with n representing independent rat experiments. Statistical significance was tested using a paired t-test with P<0.05 considered significant.
3. Results
3.1 L-arginine-mediated relaxation is dependent upon ADC activity
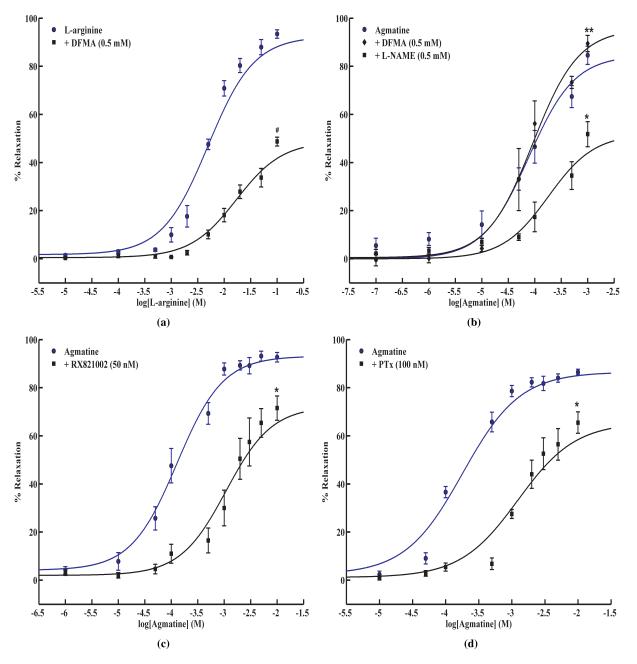
As shown in Fig. 1a, L-arginine dose-dependently relaxed the vessel with an EC50 value of 5.8 ± 0.7 mM (n = 9). The requirement of mM levels of arginine prompted us to hypothesize that the actions of arginine may be mediated via its metabolism to agmatine by ADC, which is shown to be localized in the endothelium. The relaxations to arginine were significantly inhibited in the presence of ADC inhibitor, DFMA (Fig. 1a: EC50, 18.3 ± 1.3 mM; n = 5). The EC50 value for L-arginine increased several fold in the presence of ADC inhibitor. DFMA is a specific inhibitor of ADC [37] and its specificity in our system was verified by the absence of any effect on agmatine-mediated vessel relaxation (Fig. 1b). Thus, these experiments demonstrated that the arginine’s actions are mediated at least in part by the formation of agmatine.
Figure 1. Concentration dependant relaxation responses to L-arginine and agmatine.
(a). Dose-response to intraluminal perfusion of L-arginine (n = 9) in SD rat mesenteric arterioles and after pre-treatment with ADC blocker, DFMA (0.5 mM)(n = 5); #P < 0.05 vs. L-arginine. (b). Concentration dependent dose response curve to intraluminal perfusion of agmatine in rat mesenteric arterioles in the presence and absence of an eNOS blocker, L-NAME (0.5 mM)(n = 4) and ADC blocker, DFMA (0.5 mM)(n = 3). Values are mean ± SE with; *P < 0.05 vs. agmatine; **P > 0.05 vs. agmatine. (c). Dose response to agmatine in SD rat vessels was obtained in the presence and absence of an α-2 AR antagonist, RX821002 (50 nM) (n = 6); *P < 0.05 vs. agmatine. (d). Agmatine relaxation response in the absence and presence of G-protein inhibitor, PTx (100 nM). Values are mean ± SE (n = 4); *P < 0.05 vs. agmatine.
3.2 Agmatine-induced vessel relaxation
To examine the effect of agmatine treatment on vessel tone, the isolated mesenteric arterioles were subjected to increasing agmatine concentrations by intraluminal perfusion. Agmatine dose-dependently relaxed the vessel with an EC50 of 138.7 ± 12.1 μM (Fig.1b; n = 22). Thus, significantly less agmatine was required as compared to arginine for arteriolar relaxation. As illustrated in Fig 1b, the agmatine-mediated relaxation was partially NO dependent as eNOS inhibitor, L-NAME (0.5 mM) did not completely attenuate the relaxation (EC50, 346.0 ± 19.4 μM; n = 4).
3.3 α-2 AR activity in agmatine-mediated relaxation
It has been previously reported that agmatine acts as an α-2 AR ligand [23]. To validate if the agmatine-induced relaxation is mediated via α-2 AR, we treated the vessels with agmatine in the presence and absence of RX821002, a specific antagonist of α-2 AR [38; 39]. As shown in Fig 1c, it partly attenuated agmatine-mediated relaxation (EC50, 1498.0 ± 294.0 μM; n = 6) indicating that α-2 AR may be participating in the relaxation process. These data corroborate our earlier observation in cultured HUVECs using another selective α-2 AR antagonist, rauwolscine, where it attenuated arginine-induced cellular NO synthesis [15].
3.4 Inhibition of agmatine-mediated relaxation by pertussis toxin
To narrow down the downstream mechanisms that may be participating in the agmatine-induced relaxation, we examined the possible role of G-proteins. We inhibited the G-proteins by pretreating the vessel with PTx (100 nM) for 60 min at 37°C. As shown in Fig 1d, we observed a significantly reduced relaxation as opposed to that due to agmatine alone while the EC50 value increased to 1300 ± 91 μM (n = 4). These data indicated that G-proteins may mediate agmatine-induced vessel relaxation. Similar inhibition with PTx was observed in our experiments with cultured HUVECs [15].
3.5 Agmatine and arginine-mediated vessel relaxation attenuated in DS rats
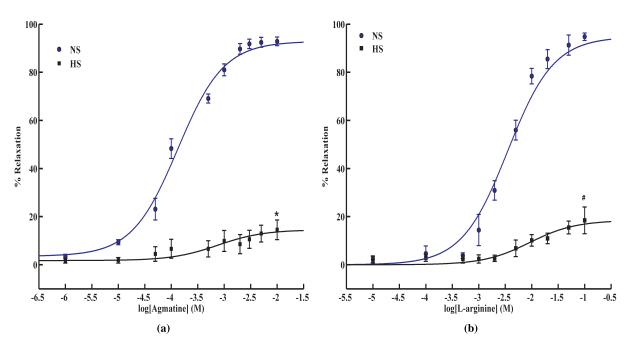
Accumulating evidence indicates that endothelium-dependent relaxation is impaired in salt-sensitive hypertension [40; 41; 42]. However, the underlying mechanisms have not been clearly delineated. We investigated if the agmatine-induced arterial relaxation is affected in Dahl salt-sensitive rats. 6-week old rats were placed on a diet containing either 0.49% NaCl (normal salt) or 4% NaCl (high salt) for 5 weeks and isolated mesenteric arterial relaxations were recorded in response to agmatine dose. The agmatine-induced relaxation was greatly inhibited in high-salt as compared to normal salt conditions (Fig 2a; % max. relaxation, 90.4 ± 1.7% (NS); 19.8 ± 2.4% (HS)) with normal salt rats exhibiting relaxation to physiological agmatine concentration (EC50, 143.9 ± 23.4 μM; n = 5). Similarly, L-arginine-mediated relaxation was impaired in high-salt diet rats (Fig. 2b). Ach mediated relaxation was also impaired in vessels from high salt Dahl rats (Fig. S3.b) and thus verifying the presence of endothelial dysfunction. However, relaxation due to an NO donor, SPER-NO was not affected, thereby indicating an undiminished signaling pathway downstream of NO synthesis in Dahl hypertensive rats (Fig. S4). These data illustrate that agmatine-mediated signal transduction pathway eliciting vascular relaxation is severely impaired in salt-induced hypertension.
Figure 2. Relaxation response to agmatine and L-arginine in Dahl salt-sensitive hypertension.
(a) Agmatine and (b) L-arginine-mediated relaxation observed in salt-sensitive Dahl rats maintained on high salt, HS (4% NaCl) and normal salt, NS (0.49% NaCl) for 5 weeks, (a: n = 5, b: n = 3). Values are mean ± SE, #P < 0.05 vs. L-arginine, *P < 0.05 vs. agmatine.
3.6 Attenuated NO synthesis in Dahl rats
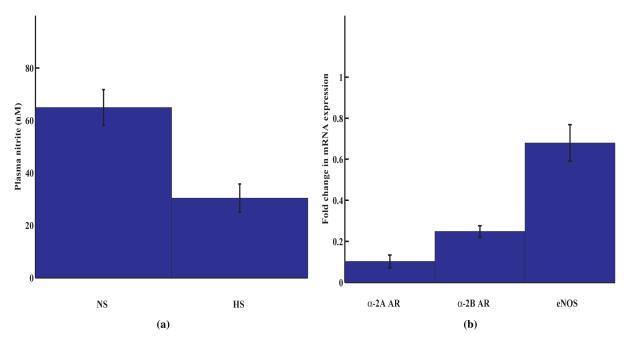
Plasma nitrite levels, as a measure of endogenous NO synthesis, were analyzed by ozone based chemiluminescence analyzer. The results indicated that NO synthesis was significantly attenuated in high salt rats as compared to normal salt rats (Fig. 3a). These data are supported by the similar observations made by Fujii et al [43].
Figure 3. Compromised nitric oxide activity in Dahl salt-sensitive hypertension.
(a) Plasma nitrite concentrations in HS and NS fed Dahl rats (n = 3). (b) Real Time PCR data for α-2A AR, α-2B AR and eNOS mRNA expression. Mesenteric artery tissue was harvested and total RNA isolated from NS and HS rats. The semi-quantitative RT-PCR was carried out using SYBR Green technology in a StepOne RT-PCR system. Values are mean ± SE and expressed as fold change in mRNA expression in HS rats as compared to that in NS rats (n = 4).
3.7 Down-regulation of mesenteric artery α-2 AR mRNA expression in Dahl rats
Since agmatine is proposed to act via α-2 AR binding, we carried out mRNA expression analysis of α-2 AR and eNOS in Dahl rats by real time RT-PCR. The mRNA expression of α-2A, α-2B AR and eNOS were attenuated in high-salt Dahl rat mesenteric arteries as compared to normal-salt arterioles (Fig. 3b) indicating that mRNA expression levels of these genes were reversed during the development of salt-sensitive hypertension. Among the 3 genes analyzed, there was a 10 fold under-expression of α-2A AR mRNA.
4. Discussion
Although the beneficial effects of arginine in vascular relaxation are well documented and appreciated, the mechanism of its action is not clearly understood. Arginine serves a major role by acting as a substrate for NOS and synthesizing NO, which in turn participates in signal transduction of vascular relaxation. Since millimolar amounts of arginine are required to produce measurable NO levels [44], it was speculated that arginine’s metabolic products could be contributing to its observed effects including vasodilation [15]. We have proposed that some of the observed effects of arginine could be attributed to its conversion to agmatine by ADC.
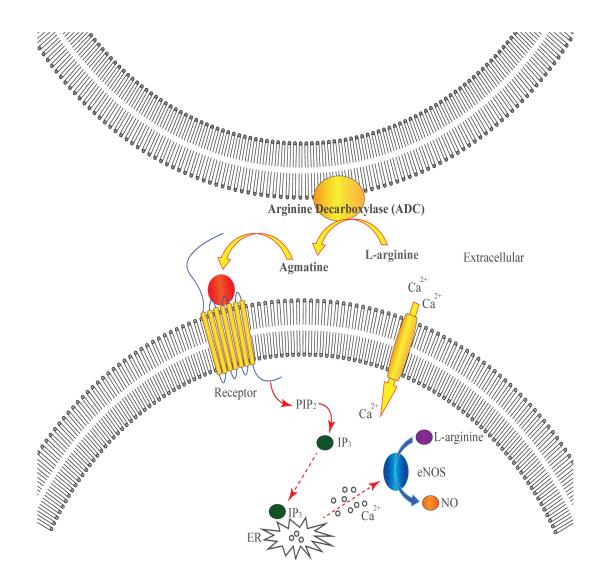
Our important finding that the inhibition of ADC with DFMA significantly attenuated arginine-initiated mesenteric arterial dilation, thereby pointing towards an arginine-toagmatine conversion for the observed relaxation. The endothelium is known to possess ADC activity [16], which was documented to mediate an increased vasodilation and glomerular filtration rate following arginine infusion into animals [45; 46]. In macrophages under inflammatory conditions, ADC was shown to modulate agmatine levels, which in turn down-regulated iNOS catalyzed NO synthesis [47]. Higher levels of ADC are present in the brain [48]and hence it can be speculated that agmatine synthesized may have biological role in the distant organs in particular the vascular function. We observed that agmatine initiated the relaxation at a significantly lower concentration (EC50, 138.7 ± 12.1 μM; n = 22) as compared to arginine. The increased vessel relaxation with μM agmatine is physiologically relevant since the plasma agmatine levels have been reported in the μM range [49; 50]. Agmatine was shown to activate NO synthesis in endothelial cells but the mechanism of activation was not elucidated [26; 51]. The partial attenuation of agmatine-initiated relaxation by L-NAME indicated that agmatine partly triggered the relaxation via NO independent, possibly EDHF mediated pathway. Binding of agmatine to imidazoline or α-2 AR is well documented in the mammalian system. Our hypothesis that agmatine acts by receptor binding, possibly via α-2 AR (Fig. 4), was examined by using an α-2 AR antagonist. The observation that RX821002 did not completely attenuate agmatine-triggered relaxation implied that agmatine affecting relaxation partly via α-AR independent pathway. Recently, agmatine’s relaxing effects in rat aorta are documented to be via small conductance Ca2+-activated K+ channel and ATP-sensitive inward rectifying K+ channels, and idazoxan failed to inhibit these relaxations [52]. It appears that rat aorta is devoid of I-receptors, as Musgrave et al [53] have reported the absence of I-receptor antagonist effects, and thus it is likely that agmatine is using alternative pathways of aortic relaxation.
Figure 4.
Schematic representation of the proposed receptor-mediated hypothesis for arginine/agmatine stimulation of vascular NO synthesis.
Since α-2 AR belongs to GPCR family, we tested the effects of G-protein inhibition. PTx, a potent G-protein inhibitor, considerably attenuated agmatine-initiated relaxation. The inhibitory effects of α-2 AR antagonist (RX821002) and G-protein inhibitor (PTx) together provide stronger evidence for the possible participation of GPCR in the vasodilatory actions of agmatine. Using 2 knockout mouse models, Shafaroudi et al. [24] have demonstrated the mediation of endothelial α-2A AR in vasodilation via NO-dependent mechanisms. These receptors may play important physiological functions as they’re activated by the endogenous ligand NE. In addition, there is evidence for their participation in various pathophysiological conditions. For example, blood pressure was elevated in transgenic α-2A AR knockout mice on a normal salt diet [54], showing that α-2A AR activation with specific agonists may serve to alleviate hypertensive conditions. Dahl hypertensive rats are reported to exhibit endothelial dysfunction [55] and agmatine-mediated vascular relaxation could be impaired in this model. Small size arterioles (100-500 μm diameter) are proposed to contribute significantly to the vascular resistance [56]. Our measurements of agmatine-initiated vessel relaxation showed that relaxation was severely impaired in high salt as compared to normal salt diet fed Dahl rats. This observation supports our postulation that α-2A AR activity and associated signaling pathway is down-regulated in high salt treated rats leading to impairment of agmatine-mediated relaxation. Secondly, mRNA analysis by real time PCR showed suppression of α-2A and α-2B AR expression in the mesenteric artery isolated from high salt fed Dahl rats as compared those from normal salt fed rats. There was noticeably a robust 10 fold down-regulation of α-2A AR, which could be the predominant factor responsible for the observed abolishment of vessel relaxation in HS rats. Hirano et al [57] have also observed attenuated pre- and post-synaptic α-2A AR reactivity in Dahl rats fed high salt diet. The attenuated L-arginine and agmatine-mediated relaxation could be also due to the observed down-regulation of eNOS expression. Similarly, down-regulation of eNOS protein expression was observed in kidney and aorta from Dahl salt-sensitive rats [58]. Our finding that substantial depletion in plasma nitrite levels in hypertensive rats could be the result of either decreased L-arginine/agmatine levels and/or reduced α-2A AR and eNOS expression. Further investigations are warranted to delineate the impaired agmatine pathway as the underlying cause of endothelial dysfunction in hypertension. In summary, we have demonstrated the mediation of ADC in the arginine-initiated stimulation of rat mesenteric artery relaxation. Physiological concentrations of agmatine activated vessel relaxation via α-2 AR and G-proteins and this was partially dependent on vascular NO synthesis. Agmatine relaxed the vessels at 100 times lower concentration as compared to arginine and thus making it a physiologically relevant agonist at α-2 AR binding site. Most importantly, our investigations have provided a novel mechanistic explanation for the observed beneficial effects of L-arginine-initiated vessel relaxation via agmatine formation. Lastly, α-2 AR activity, mRNA expression, and agmatine-mediated relaxations are impaired in vessels isolated from high salt loaded Dahl hypertensive rats. These findings may help define fundamental aspects of cardiovascular physiology and pathophysiology by demonstrating that many effects of arginine may occur due to its metabolism to agmatine.
Supplementary Material
Highlights.
▶ Agmatine induced relaxation in isolated rat mesenteric arterioles.
▶The relaxations were mediated by G-proteins, α-2 AR and eNOS.
▶ L-arginine relaxed at much higher concentrations via arginine decarboxylase.
▶The relaxation to agmatine was abolished in high salt treated Dahl rats.
▶ α-2A AR, α-2B AR and eNOS mRNA expressions were down regulated in high salt Dahl rats.
Acknowledgment
This work was supported by the National Institutes of Health grant (NMT). We wish to thank Deepak Balasubramanian and Kalai Mathee for their valuable help in carrying out RT-PCR experiments and analysis of data.
Abbreviations
- ADC
arginine decarboxylase
- DFMA
difluoromethylarginine
- eNOS
endothelial nitric oxide synthases
- L-NAME
L-NG-Nitroarginine methyl ester
- AR
adrenergic receptor
- PTx
Pertussis toxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Stechmiller JK, Childress B, Cowan L. Arginine Supplementation and Wound Healing. Nutrition in Clinical Practice. 2005;20:52–61. doi: 10.1177/011542650502000152. [DOI] [PubMed] [Google Scholar]
- [2].Angele MK, Nitsch SM, Hatz RA, Angele P, Hernandez-Richter T, Wichmann MW, Chaudry IH, Schildberg FW. L-arginine: a unique amino acid for improving depressed wound immune function following hemorrhage. Eur Surg Res. 2002;34:53–60. doi: 10.1159/000048888. [DOI] [PubMed] [Google Scholar]
- [3].Molderings GJ, Kribben B, Heinen A, Schröder D, Brüss M, Göthert M. Intestinal tumor and agmatine (decarboxylated arginine) Cancer. 2004;101:858–868. doi: 10.1002/cncr.20407. [DOI] [PubMed] [Google Scholar]
- [4].Morris SM. Arginine: beyond protein. The American Journal of Clinical Nutrition. 2006;83:508S–512S. doi: 10.1093/ajcn/83.2.508S. [DOI] [PubMed] [Google Scholar]
- [5].Mocchegiani E, Nistico G, Santarelli L, Fabris N. Effect of L-arginine on thymic function. Possible role of L-arginine: Nitric oxide (no) pathway. Archives of Gerontology and Geriatrics. 1994;19:163–170. doi: 10.1016/s0167-4943(05)80061-5. [DOI] [PubMed] [Google Scholar]
- [6].Carson CC., 3rd Erectile dysfunction: diagnosis and management with newer oral agents. Proc (Bayl Univ Med Cent) 2000;13:356–60. doi: 10.1080/08998280.2000.11927705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wu G, Bazer FW, Davis TA, Kim SW, Li P, Marc Rhoads J, Carey Satterfield M, Smith SB, Spencer TE, Yin Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009;37:153–68. doi: 10.1007/s00726-008-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Appleton J. Arginine: clinical potential of a semi-essential amino acid. (Arginine) Alternative Medicine Review. 2002;7:512–11. [PubMed] [Google Scholar]
- [9].Bode-Böger SM, Scalera F, Ignarro LJ. The l-arginine paradox: Importance of the larginine/asymmetrical dimethylarginine ratio. Pharmacology & Therapeutics. 2007;114:295–306. doi: 10.1016/j.pharmthera.2007.03.002. [DOI] [PubMed] [Google Scholar]
- [10].Wei LH, Jacobs AT, Morris SM, Jr., Ignarro LJ. IL-4 and IL-13 upregulate arginase I expression by cAMP and JAK/STAT6 pathways in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2000;279:C248–56. doi: 10.1152/ajpcell.2000.279.1.C248. [DOI] [PubMed] [Google Scholar]
- [11].García-Cardeña G, Oh P, Liu J, Schnitzer JE, Sessa WC. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McDonald KK, Zharikov S, Block ER, Kilberg MS. A Caveolar Complex between the Cationic Amino Acid Transporter 1 and Endothelial Nitric-oxide Synthase May Explain the “Arginine Paradox”. Journal of Biological Chemistry. 1997;272:31213–31216. doi: 10.1074/jbc.272.50.31213. [DOI] [PubMed] [Google Scholar]
- [13].Nagaraja S, Kapela A, Tran CH, Welsh DG, Tsoukias NM. Role of microprojections in myoendothelial feedback: a theoretical study. The Journal of Physiology. 2013 doi: 10.1113/jphysiol.2012.248948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kapela A, Bezerianos A, Tsoukias NM. A mathematical model of Ca2+ dynamics in rat mesenteric smooth muscle cell: Agonist and NO stimulation. Journal of Theoretical Biology. 2008;253:238–260. doi: 10.1016/j.jtbi.2008.03.004. [DOI] [PubMed] [Google Scholar]
- [15].Joshi MS, Ferguson TB, Jr., Johnson FK, Johnson RA, Parthasarathy S, Lancaster JR., Jr. Receptor-mediated activation of nitric oxide synthesis by arginine in endothelial cells. Proc Natl Acad Sci U S A. 2007;104:9982–7. doi: 10.1073/pnas.0506824104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Regunathan S, Youngson C, Raasch W, Wang H, Reis DJ. Imidazoline receptors and agmatine in blood vessels: a novel system inhibiting vascular smooth muscle proliferation. Journal of Pharmacology & Experimental Therapeutics. 1996;276:1272–1282. [PubMed] [Google Scholar]
- [17].Zhu MY, Iyo A, Piletz JE, Regunathan S. Expression of human arginine decarboxylase, the biosynthetic enzyme for agmatine. Biochim Biophys Acta. 2004;1670:156–64. doi: 10.1016/j.bbagen.2003.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gilad GM, Gilad VH, Rabey JM. Arginine and ornithine decarboxylation in rodent brain: coincidental changes during development and after ischemia. Neurosci Lett. 1996;216:33–6. doi: 10.1016/0304-3940(96)12996-7. [DOI] [PubMed] [Google Scholar]
- [19].Reis DJ, Regunathan S. Agmatine: An Endogenous Ligand at Imidazoline Receptors Is a Novel Neurotransmittera. Annals of the New York Academy of Sciences. 1999;881:65–80. doi: 10.1111/j.1749-6632.1999.tb09343.x. [DOI] [PubMed] [Google Scholar]
- [20].Donald J Reis SR. Is agmatine a novel neurotransmitter in brain? Trends in Pharmacological Sciences. 2000;21:187–193. doi: 10.1016/s0165-6147(00)01460-7. [DOI] [PubMed] [Google Scholar]
- [21].Regunathan S, Reis DJ. Characterization of Arginine Decarboxylase in Rat Brain and Liver. Journal of Neurochemistry. 2000;74:2201–2208. doi: 10.1046/j.1471-4159.2000.0742201.x. [DOI] [PubMed] [Google Scholar]
- [22].Raasch W, Regunathan S, Li G, Reis DJ. Agmatine, the bacterial amine, is widely distributed in mammalian tissues. Life Sciences. 1995;56:2319–2330. doi: 10.1016/0024-3205(95)00226-v. [DOI] [PubMed] [Google Scholar]
- [23].Li G, Regunathan S, Barrow CJ, Eshraghi J, Cooper R, Reis DJ. Agmatine: an endogenous clonidine-displacing substance in the brain. Science. 1994;263:966–969. doi: 10.1126/science.7906055. [DOI] [PubMed] [Google Scholar]
- [24].Shafaroudi MM, McBride M, Deighan C, Wokoma A, Macmillan J, Daly CJ, McGrath JC. Two “knockout” mouse models demonstrate that aortic vasodilatation is mediated via alpha2aadrenoceptors located on the endothelium. J Pharmacol Exp Ther. 2005;314:804–10. doi: 10.1124/jpet.105.085944. [DOI] [PubMed] [Google Scholar]
- [25].Hieble JP, Kolpak DC. Mediation of the hypotensive action of systemic clonidine in the rat by alpha 2-adrenoceptors. Br J Pharmacol. 1993;110:1635–9. doi: 10.1111/j.1476-5381.1993.tb14012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Morrissey JJ, Klahr S. Agmatine activation of nitric oxide synthase in endothelial cells. Proceedings of the Association of American Physicians. 1997;109:51–57. [PubMed] [Google Scholar]
- [27].Satoh M, Haruna Y, Fujimoto S, Sasaki T, Kashihara N. Telmisartan improves endothelial dysfunction and renal autoregulation in Dahl salt-sensitive rats. Hypertens Res. 2010;33:135–42. doi: 10.1038/hr.2009.190. [DOI] [PubMed] [Google Scholar]
- [28].Zhou M-S, Jaimes EA, Raij L. Atorvastatin Prevents End-Organ Injury in Salt-Sensitive Hypertension: Role of eNOS and Oxidant Stress. Hypertension. 2004;44:186–190. doi: 10.1161/01.HYP.0000136395.06810.cf. [DOI] [PubMed] [Google Scholar]
- [29].Moura E, Pinto CE, Serrao MP, Afonso J, Vieira-Coelho MA. Adrenal alpha(2)-adrenergic receptors in the aging normotensive and spontaneously hypertensive rat. Neurobiol Aging. 2012;33:969–78. doi: 10.1016/j.neurobiolaging.2010.06.021. [DOI] [PubMed] [Google Scholar]
- [30].Park J, Galligan JJ, Fink GD, Swain GM. Alterations in sympathetic neuroeffector transmission to mesenteric arteries but not veins in DOCA-salt hypertension. Auton Neurosci. 2010;152:11–20. doi: 10.1016/j.autneu.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dohi Y, Thiel MA, Buhler FR, Luscher TF. Activation of endothelial L-arginine pathway in resistance arteries. Effect of age and hypertension, Hypertension. 1990;16:170–9. doi: 10.1161/01.hyp.16.2.170. [DOI] [PubMed] [Google Scholar]
- [32].Duling BR, Gore RW, Dacey RG, Jr., Damon DN. Methods for isolation, cannulation, and in vitro study of single microvessels. Am J Physiol. 1981;241:H108–116. doi: 10.1152/ajpheart.1981.241.1.H108. [DOI] [PubMed] [Google Scholar]
- [33].Knot HJ, Lounsbury KM, Brayden JE, Nelson MT. Gender differences in coronary artery diameter reflect changes in both endothelial Ca2+ and ecNOS activity. Am J Physiol. 1999;276:H961–9. doi: 10.1152/ajpheart.1999.276.3.H961. [DOI] [PubMed] [Google Scholar]
- [34].Feng M, Whitesall S, Zhang Y, Beibel M, D’Alecy L, DiPetrillo K. Validation of volume-pressure recording tail-cuff blood pressure measurements. Am J Hypertens. 2008;21:1288–91. doi: 10.1038/ajh.2008.301. [DOI] [PubMed] [Google Scholar]
- [35].Pelletier MM, Kleinbongard P, Ringwood L, Hito R, Hunter CJ, Schechter AN, Gladwin MT, Dejam A. The measurement of blood and plasma nitrite by chemiluminescence: pitfalls and solutions. Free Radic Biol Med. 2006;41:541–8. doi: 10.1016/j.freeradbiomed.2006.05.001. [DOI] [PubMed] [Google Scholar]
- [36].Taylor S, Wakem M, Dijkman G, Alsarraj M, Nguyen M. A practical approach to RT-qPCR—Publishing data that conform to the MIQE guidelines. Methods. 2010;50:S1–S5. doi: 10.1016/j.ymeth.2010.01.005. [DOI] [PubMed] [Google Scholar]
- [37].Yarlett N, Waters WR, Harp JA, Wannemuehler MJ, Morada M, Bellcastro J, Upton SJ, Marton LJ, Frydman BJ. Activities of dl-α-Difluoromethylarginine and Polyamine Analogues against Cryptosporidium parvum Infection in a T-Cell Receptor Alpha-Deficient Mouse Model. Antimicrobial Agents and Chemotherapy. 2007;51:1234–1239. doi: 10.1128/AAC.01040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Callado LF, Gabilondo AM, Meana JJ. [3H]RX821002 (2-methoxyidazoxan) binds to α2-adrenoceptor subtypes and a non-adrenoceptor imidazoline binding site in rat kidney. European Journal of Pharmacology. 1996;316:359–368. doi: 10.1016/s0014-2999(96)00692-9. [DOI] [PubMed] [Google Scholar]
- [39].O’Rourke MF, Blaxall HS, Iversen LJ, Bylund DB. Characterization of [3H]RX821002 binding to alpha-2 adrenergic receptor subtypes. J Pharmacol Exp Ther. 1994;268:1362–7. [PubMed] [Google Scholar]
- [40].Ozawa Y, Hayashi K, Kanda T, Homma K, Takamatsu I, Tatematsu S, Yoshioka K, Kumagai H, Wakino S, Saruta T. Impaired nitric oxide- and endothelium-derived hyperpolarizing factor-dependent dilation of renal afferent arteriole in Dahl salt-sensitive rats. Nephrology (Carlton) 2004;9:272–7. doi: 10.1111/j.1440-1797.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- [41].Hermann M, Camici G, Fratton A, Hurlimann D, Tanner FC, Hellermann JP, Fiedler M, Thiery J, Neidhart M, Gay RE, Gay S, Luscher TF, Ruschitzka F. Differential effects of selective cyclooxygenase-2 inhibitors on endothelial function in salt-induced hypertension. Circulation. 2003;108:2308–11. doi: 10.1161/01.CIR.0000101683.30157.0B. [DOI] [PubMed] [Google Scholar]
- [42].Payne JA, Alexander BT, Khalil RA. Decreased endothelium-dependent NO-cGMP vascular relaxation and hypertension in growth-restricted rats on a high-salt diet. Hypertension. 2004;43:420–7. doi: 10.1161/01.HYP.0000111832.47667.13. [DOI] [PubMed] [Google Scholar]
- [43].Fujii S, Zhang L, Igarashi J, Kosaka H. L-arginine reverses p47phox and gp91phox expression induced by high salt in Dahl rats. Hypertension. 2003;42:1014–20. doi: 10.1161/01.HYP.0000094557.36656.D0. [DOI] [PubMed] [Google Scholar]
- [44].Hall CN, Garthwaite J. What is the real physiological NO concentration in vivo? Nitric Oxide. 2009;21:92–103. doi: 10.1016/j.niox.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Blantz RC, Satriano J, Gabbai F, Kelly C. Biological effects of arginine metabolites. Acta Physiol Scand. 2000;168:21–5. doi: 10.1046/j.1365-201x.2000.00646.x. [DOI] [PubMed] [Google Scholar]
- [46].Thomson SC, Deng A, Bao D, Satriano J, Blantz RC, Vallon V. Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes. The Journal of Clinical Investigation. 2001;107:217–224. doi: 10.1172/JCI10963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Regunathan S, Piletz JE. Regulation of inducible nitric oxide synthase and agmatine synthesis in macrophages and astrocytes. Ann N Y Acad Sci. 2003;1009:20–9. doi: 10.1196/annals.1304.002. [DOI] [PubMed] [Google Scholar]
- [48].Li G, Regunathan S, Barrow C, Eshraghi J, Cooper R, Reis D. Agmatine: an endogenous clonidine-displacing substance in the brain. Science. 1994;263:966–969. doi: 10.1126/science.7906055. [DOI] [PubMed] [Google Scholar]
- [49].Lortie MJ, Novotny WF, Peterson OW, Vallon V, Malvey K, Mendonca M, Satriano J, Insel P, Thomson SC, Blantz RC. Agmatine, a bioactive metabolite of arginine. Production, degradation, and functional effects in the kidney of the rat. Journal of Clinical Investigation. 1996;97:413–420. doi: 10.1172/JCI118430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cox TT, Boeker EA. Analysis of enzyme kinetics by using integrated rate equations. Arginine decarboxylase. Biochem J. 1987;245:59–65. doi: 10.1042/bj2450059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Coleman CS, Hu G, Pegg AE. Putrescine biosynthesis in mammalian tissues. Biochem J. 2004;379:849–55. doi: 10.1042/BJ20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Santhanam AVR, Viswanathan S, Dikshit M. Activation of protein kinase B/Akt and endothelial nitric oxide synthase mediates agmatine-induced endothelium-dependent relaxation. European Journal of Pharmacology. 2007;572:189–196. doi: 10.1016/j.ejphar.2007.06.031. [DOI] [PubMed] [Google Scholar]
- [53].Musgrave IF, Van Der Zypp A, Grigg M, Barrow CJ. Endogenous imidazoline receptor ligands relax rat aorta by an endothelium-dependent mechanism. Ann N Y Acad Sci. 2003;1009:222–7. doi: 10.1196/annals.1304.027. [DOI] [PubMed] [Google Scholar]
- [54].Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, Costentin J, Heath JK, Vassart G, Parmentier M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–8. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- [55].Johnson FK, Johnson RA, Peyton KJ, Durante W. Arginase inhibition restores arteriolar endothelial function in Dahl rats with salt-induced hypertension. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1057–62. doi: 10.1152/ajpregu.00758.2004. [DOI] [PubMed] [Google Scholar]
- [56].Christensen KL, Mulvany MJ. Perindopril changes the mesenteric pressure profile of conscious hypertensive and normotensive rats. Hypertension. 1994;23:325–8. doi: 10.1161/01.hyp.23.3.325. [DOI] [PubMed] [Google Scholar]
- [57].Hirano Y, Tsunoda M, Shimosawa T, Matsui H, Fujita T, Funatsu T. Suppression of catechol-O-methyltransferase activity through blunting of alpha2-adrenoceptor can explain hypertension in Dahl salt-sensitive rats. Hypertens Res. 2007;30:269–78. doi: 10.1291/hypres.30.269. [DOI] [PubMed] [Google Scholar]
- [58].Ni Z, Oveisi F, Vaziri ND. Nitric oxide synthase isotype expression in salt-sensitive and salt-resistant Dahl rats. Hypertension. 1999;34:552–7. doi: 10.1161/01.hyp.34.4.552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.