Abstract
Human immunodeficiency virus type-1 (HIV) causes mild or severe neurological problems, termed HIV-associated neurocognitive disorder (HAND), even when HIV patients receive antiretroviral therapy. Thus, novel adjunctive therapies are necessary to reduce or abolish the neurotoxic effect of HIV. However, new therapies require a better understanding of the molecular and cellular mechanisms of HIV-induced neurotoxicity. HAND subjects are characterized by being profoundly depressed, and they experience deficits in memory, learning and movements. Experimental evidence has also shown that HIV reduces neurogenesis. These deficits resemble those occurring in premature brain aging or in a brain with impaired neural repair properties. Thus, it appears that HIV diminishes neuronal survival, along with reduced neuronal connections. These two phenomena should not occur in the adult and developing brain when synaptic plasticity is promoted by neurotrophic factors, polypeptides that are present in adult synapses. This review will outline experimental evidence as well as present emerging concepts for the use of neurotrophic factors and in particular brain-derived neurotrophic factor as an adjunct therapy to prevent HIV-mediated neuronal degeneration and restore the loss of synaptic connections.
Keywords: BDNF, FGFs, GDNF, gp120, HIV, PDGF, TrkB
Introduction
Human immunodeficiency virus type-1 (HIV) infects the central nervous system (CNS) and promotes neurological problems in more than 50% of patients who do not receive antiretroviral therapy (Gonzalez-Scarano and Martin-Garcia, 2005; Price and Spudich, 2008). Symptoms include profound motor and behavioral/psychosocial abnormalities that disrupt work or other activities of daily living. These deficits can be mild, which are referred to as HIV-associated neurocognitive disorders (HAND), or severe, which are referred to as HIV associated dementia (HAD). HIV does not spare children because they can develop abnormalities manifested as attention deficit disorders (Cohen et al., 1991) and neuronal loss (Gelbard and Epstein, 1995).
HIV does not infect neurons; nevertheless, postmortem brains of HAD subjects have shown neuronal loss accompanied by synaptic simplifications (Everall et al., 2005). In addition, HIV impairs adult neurogenesis (Tran and Miller, 2005; Okamoto et al., 2007), the process by which new neurons are created even in the adult CNS. Experimental and clinical research has revealed that the loss of adult neurogenesis in the hippocampus can lead to depression, one of the cardinal symptoms seen in HIV positive subjects (Eriksson et al., 1998). In addition, there is a strong correlation between the delay onset of antidepressant activity and the time required for neurogenesis (Malberg et al., 2000). Thus, alternative adjunct therapies against HAND must take into account the multiple neurotoxic effects of HIV in addition to restoring the innate ability of the CNS to promote neurogenesis, survival, and adaptation to injury. Ideally, this therapy should use physiological compounds utilized by the CNS to prevent neuronal injury and promote survival of cells (biological therapies). These include neurotrophic factors, naturally occurring diffusible polypeptides that stimulate survival of a variety of CNS cells after injury as well as to induce and maintain the differentiation of surviving neurons to their mature phenotype. Moreover, these peptides, and in particular brain-derived neurotrophic factor (BDNF), have been shown to promote neurogenesis, and are equally vital for neuronal plasticity. This article will introduce new emerging concepts and principles in the use of neurotrophic factors as an adjunct therapy to prevent HIV-mediated neuronal degeneration and restore the loss of synaptic connections.
HIV and synaptic plasticity
The search for drugs that reduce neuronal injury requires a better understanding of the pathogenic mechanisms and the numerous steps involved in the pathogenic cascade. HAND/HAD pathology includes loss of both synaptic connections and neuronal differentiation (Ellis et al., 2007). In addition, HAD subjects exhibit depression and mania (Grant, 1990; Pumpradit et al., 2010) combined with cognitive and motor impairments. In fact, the clinical manifestations of HAD include tremor, gait ataxia, loss of fine motor movement, mental slowing, forgetfulness, poor concentration and behavioral abnormalities (McArthur et al., 2010). Neuroimaging studies of HAD patients have revealed generalized white matter reduction, with additional grey matter loss particularly in the basal ganglia and posterior cortex (Dal Pan et al., 1992; Aylward et al., 1995). Neuronal loss has been confirmed in postmortem brains in the basal ganglia and other regions of the brain including the hippocampus and frontal cortex (Davies et al., 1998; Everall et al., 2005). Pathological alterations of the basal ganglia in HAD include neuronal loss in the putamen (Everall et al., 2005) and the globus pallidus (Fox et al., 1997), degeneration of nigro-striatal dopamine (DA) neurons (Reyes et al., 1991; Itoh et al., 2000) (Itoh et al., 2000) and dysfunctional DAergic transport (Wang et al., 2004).
Higher cognitive functions depend on a highly complex synaptodendritic network in the frontal cortex. Damages to this network result in abnormal output, measured as deficiencies in cognitive skills and behavior. Cognitive impairments (moderate to severe) and frontocortical atrophy in HAD are similar to those observed in Alzheimer’s disease (AD) (Thompson et al., 2007). This disease is categorized by a reduced number of neurons and connections in the cortex and hippocampus as well as impaired short-term memory (Scheff and Price, 2006). Granted that memory and attention alterations could be also due to the high prevalence of CNS comorbidities in HIV populations (e.g. drug abuse), it is important to keep in mind that these cognitive alterations correlate with the loss of synaptodendritic networks in the cortex and hippocampus. Moreover, neuroimaging studies have revealed microstructural abnormalities in the cerebral white matter (Lopez-Villegas et al., 1997), supporting the theory of synaptodendritic pathology as the main cause of neuronal loss.
Neurotrophic factors as biological therapies for HAND
Neurotrophic factors have been shown to reduce synaptic and axonal degeneration mediated by a number of neurotoxins and to interfere with the fundamental mechanism of apoptotic cell death in numerous neurodegenerative diseases. In addition, neurotrophic factors promote axonal growth when an appropriate growth-promoting substrate is present (Horner and Gage, 2000). Thus, HAND syndrome and pathology are excellent targets for a therapy based on neurotrophic factors. In addition, some neurotrophic factors are naturally implemented to promote neuronal differentiation and neurogenesis. The important question is which neurotrophic factors are more suitable to treat HAND pathology. The answer does not appear to be straightforward because there are many neurotrophic factors each exhibiting neurotrophic activity on either overlapping or distinct neuronal and glial populations. Indeed, “trophic ligands” influence neurons and glial cells by binding to specific membrane-associated receptors. These receptors are not necessarily equally distributed on all neuronal subtypes. Moreover, the levels of neurotrophic factors and/or their receptors could be decreased in the presence of axonal and synaptic pathology, which is a characteristic of many neurological disorders including HAND. Nevertheless, the remaining synapses may be influenced to release more neurotrophic factors. Alternatively, the delivery of recombinant neurotrophic factors may stimulate neuronal plasticity and promote neurogenesis which may help replace neurons and synapses that otherwise will be lost.
HIV neurotoxicity is promoted by HIV viral proteins, common agents released after HIV infection in the CNS. Thus, another intervention for HAND would be to block or eliminate the biological effects of these proteins. Viral proteins may interfere with neuronal survival by a number of mechanisms including production of free radicals, nitric oxide, and release of glutamate or other excitotoxins (i.e. quinolinic acid) or inflammatory cytokines (Kaul et al., 2001). The neurotoxic effects of these compounds can be blocked by several trophic factors delivered at pharmacological concentrations. Thus, there are several polypeptides that could exert trophic function on neurons and their synapses, yet only four have been experimentally tested as therapeutic compounds for neuroAIDS in animal models. These include fibroblast growth factor (FGF) (Sanders et al., 2000), brain-derived neurotrophic factor (BDNF) (Bachis et al., 2003), glial cell derived neurotrophic factor (GDNF) (Nosheny et al., 2006) and platelet-derived neurotrophic factor (PDGF) (Peng et al., 2008). To better comprehend the potential use of polypeptides as therapeutics, a brief description of their biological activity is necessary. BDNF will be reviewed as a separate neurotrophic factor because of its strong axonal growth properties.
FGFs
There are at least 10 members of the FGF family of growth factors that are expressed in the CNS (Turner et al., 2006). However, FGF2 (or basic FGF) and FGF1 (or acidic FGF) appear to be the most abundant in the adult CNS (Wilcox and Unnerstall, 1991; Gomez-Pinilla et al., 1992). There are several neurotrophic properties of FGFs that may be useful in a therapy for HAND. In fact, FGFs promote gliogenesis and neurogenesis when added to cultures of precursor cells from various brain areas (Vescovi et al., 1993; Qian et al., 1997) or in vivo in developing rats (Raballo et al., 2000) and adult rats (Shihabuddin et al., 1997; Yoshimura et al., 2001). In addition, FGFs enhance the survival of neurons following toxin-induced cell death (Frim et al., 1993; Zechel et al., 2010). FGF2 is also important because it is one of the most potent growth factor that is used to inhibit excitotoxicity (Fernandez-Sanchez and Novelli, 1993; Kirschner et al., 1995; Brandoli et al., 1998) as well as reduce inflammatory responses and improve recovery of function after mechanical injury or stroke (Peterson et al., 1996; Kawamata et al., 1997; Teng et al., 1999). Given the fact that glutamate and inflammation play a key role in neuroAIDS, the FGFs approach will lead to amelioration of the pathology and symptoms that arise from excessive glutamate.
FGF2 also plays a key role in depression and in the mechanism of action of antidepressants. Indeed, alterations in FGF2 expression are seen in cortical brain regions in individuals with major depression compared to control subjects (Evans et al., 2004). Moreover, antidepressants such as desipramine (Mallei et al., 2002), electroshock (Follesa et al., 1994) or fluoxetine (Maragnoli et al., 2004) increase the synthesis of FGF2. This induction of FGF2 expression may explain the ability of antidepressants to increase neurogenesis. However, the neurogenic activity of antidepressant cannot rely solely on FGF2 because antidepressant agents have been shown to increase other trophic factors (Nibuya et al., 1996). Nevertheless, FGF2 may help blocking the outgoing pathology of depression in HAND subjects.
A direct link between FGFs as a therapy in the pathology of HAND has been established in animal models. In fact, both FGF2 and FGF1 reduce the expression of CXCR4, a chemokine receptor that mediates the entry of T-tropic HIV (Dittmar et al., 1997) as well as the neurotoxic effect of its viral protein gp120 (Meucci et al., 1998; Zheng et al., 1999). Indeed, FGFs reduce the neurotoxic effect of gp120 (Sanders et al., 2000; Everall et al., 2001). Moreover, FGFs levels are inversely correlated with CXCR4 expression in postmortem AIDS brains (Sanders et al., 2000). Therefore, inhibitors of the expression/signaling of this receptor may have an important therapeutic property against HIV-mediated neurological impairment caused by CXCR4 activation. Nevertheless, the therapeutic potentials of FGFs in the treatment of HAND patients need scrutiny and caution because these growth factors have been shown to promote/support tumor growth and angiogenesis (Czubayko et al., 1994).
GDNF
The GDNF family of trophic factors consists of four members: GDNF, Neurturin, Artemin, and Persephin. This family has been shown to play a role in a number of biological processes including cell survival, neurite outgrowth, cell differentiation and cell migration. In particular, GDNF promotes the survival of rat dopaminergic neurons of the nigro-striatal system (Lin et al., 1993; Tomac et al., 1995), one of the major pathways involved in the control of motor activity. Moreover, Neurturin protects non-human primate DA neurons from MPTP, a toxin that has been known to cause Parkinson in humans. Therefore, GDNF and Neurturin have been suggested to be used in Parkinson’s disease (PD). In addition, GDNF rescues motor neurons from axotomy-mediated cell death (Yan et al., 1995). This property is also shared by at least two more growth factors, ciliary neurotrophic factor (CNTF) and BDNF (Sendtner et al., 1990; Sendtner et al., 1992).
The trophic effect of GDNF and the related family members are mediated by a receptor complex formed by two subunits, RET, a transmembrane receptor tyrosine kinase, and glycosyl-phosphotidylinositol-anchored co-receptor GDNF receptor α (Paratcha and Ledda, 2008). Activation of this complex triggers different pathways, including the Ras-MAPK (mitogen-activated protein kinase), the phosphatidylinositol-3 kinase (PI3K)-Akt, the PLC-γ and the Src signaling pathway and consequently increases intracellular Ca2+. These signaling pathways may not explain the trophic effect of GDNF. In fact, a new mechanism of GDNF signaling through RET has been proposed by showing that GDNF evokes phosphorylation of specific protocadherin proteins which, in turn, promote interaction with RET (Schalm et al., 2010). Protocadherins are found in synapses and have been implicated in neuronal survival, synaptic development (Katori et al., 2009), and learning and memory (Fukuda et al., 2008). The association of protocadherins with RET plays a key role in the receptor stability and ensures a delay in the degradation of RET. Any delay in the degradation of this receptor allows neurons to survive longer in the presence of GDNF (Tsui and Pierchala, 2010).
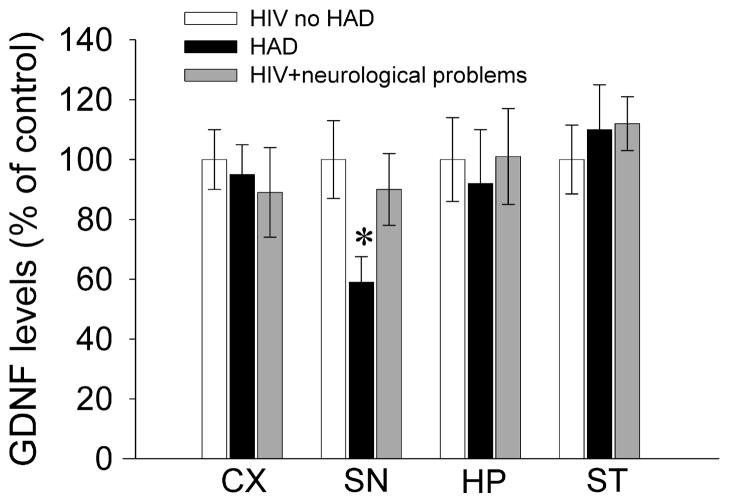
The trophic property of GDNF could also be of a particular importance for HAND, especially in the late phase or HAD. During this stage, clinical features in HAD resemble those found in PD, such as the postural instability, involuntary movements, bradykinesia, and impairment in fine motor skills (Berger and Nath, 1997). In addition, the substantia nigra (SN) of HAD subjects exhibit lower expression of GDNF than HIV subjects without dementia (Fig. 1). Granted that results obtained from postmortem human brains may reflect secondary effects of the primary pathological events, both animals and human studies suggest that GDNF and Neurturin could play a role in protecting and/or enhancing the survival of SN neurons in HIV positive subjects.
Figure 1. GDNF levels are decreased in the brain of HAD subjects.
GDNF levels were measured by ELISA in human cerebral cortex (CX), substantia nigra (SN), hippocampus (HP), and striatum (ST). Brain tissues of either sex were obtained from the National NeuroAIDS Tissue Consortium (NNTC). Samples were from: HIV negative subjects (control, n=4), HIV+ with normal neurocognitive diagnosis (HIV no HAD, n=7), HIV+ subjects with HAD (n=8), and HIV+ with neurological problems, caused by opportunistic infections, such as cytomegalovirus (HIV + neurological problems, n=7). Data are the mean ± SEM. More details about these subjects are given in Bachis et al., 2012. *p<0.01 vs HIV negative subjects (one-way ANOVA and Sheffe’s test).
The therapeutic application of GDNF in HAD requires more experimental studies. To the extent that we accept viral protein-mediated neuronal loss as a necessary animal model of HAD, studies have shown a profound degeneration of the SN of rats injected with gp120 in the striatum which inversely correlated with expression of GDNF. In fact, gp120 damages striatal neurons and initiates the degeneration of nigrostriatal DAergic fibers, thus causing a functional disconnect between the SN and the striatum (Nosheny et al., 2006). In these animals a decrease in GDNF expression in the SN precedes the degeneration of DA neurons and the increase in the number of apoptotic neurons. These results may invite some modifications of the classical views of HAD and support the neurotrophic factor hypothesis stating that lack or loss of trophic support could be one of the main causes of neuronal degeneration evoked by HIV. Most importantly these observations indicate the need for examining the trophic environment in HAD because lower trophic levels or activity could be a common risk factor for losing neurons and the development of neurological impairments. This issue rarely receives the attention it deserves, a problem that may have a negative impact on the success of future clinical trials.
PDGFs
PDGFs are a family of growth factors that have a broad spectrum of biological activity on cell growth and division (Farooqi et al., 2011). This family is composed of four different isoforms encoded by four different genes, PDGF-A, -B, -C and -D. PDGFs have a common structure (described mostly as dimeric glycoproteins) composed of two A (-AA), B (-BB), C (-CC) or D (-DD) chains or a combination of A and B (-AB). These distinct isoforms bind with different affinities to two structurally related receptor types, α and β. These receptors contain an intracellular tyrosine kinase domain that upon activation and dimerization of the receptor promotes tyrosine phosphorylation.
Members of the PDGF family are expressed in several tissues including the brain (Fredriksson et al., 2004). Nevertheless, while the mitogen activity of PDGFs for many cell types has been established (Li et al., 2003) their functional role in the brain is still under investigation. PDGF-A is a potent mitogen for glial cells, and in particular oligodendrocytes (Betsholtz, 2004); consequently, PDGF-A null mice display severe myelination deficiency in some brain areas (Fruttiger et al., 1999). Thus, PDGFs may have a therapeutic property for HAND because proliferation of cells and re-myelination could be a positive phenomenon in the neurodegenerative brain. Most importantly, experimental data have suggested that PDGFs have a role in neurogenesis, which, as mentioned above, is impaired in HAND. In particular, PDGF-BB has been shown to stimulate the growth of neuronal processes (Smits et al., 1991) as well as to promote the maturation of progenitor/stem cells in vitro (Pringle et al., 1992). Stem cells are capable of differentiation into neurons and glial cells. The neurotrophic activity of PDGF-BB is not limited to an in vitro model. In fact, this growth factor enhances neurogenesis in vivo after chemical lesion (Mohapel et al., 2005). Moreover, a more recent study (Yao et al., 2012) has shown that PDGF-BB increases proliferation of neuronal stem cells in an animal model of HAND. Thus, PDGFs may have a therapeutic property for HIV subjects since enhancing neurogenesis can have a broad impact on improving neurological symptoms and mood-related behavior in these subjects.
Neurotrophins
Another family of neurotrophic factors with strong trophic activity is the neurotrophins. In mammals, this family includes nerve growth factor (NGF) (Levi-Montalcini, 1987), brain-derived neurotrophic factor (BDNF) (Barde et al., 1982), neurotrophin-3 (NT-3) and NT-4 (Maisonpierre et al., 1990). NGF was the first to be isolated, characterized and cloned. However, NGF has limited activity in the brain and only a few populations of cholinergic neurons of the basal forebrain appear to be sensitive to NGF. On the other hand, BDNF exerts multiple neurotrophic activities on a variety of neurons. Neurotrophic effects of BDNF include modulation of dendritic branching and spines in the cortex, and long-term potentiation in the hippocampus (Patterson et al., 1996; Zakharenko et al., 2003). Through these properties, BDNF plays a critical role in learning and memory and preservation of cortical circuits. Conversely, a reduction of BDNF secretion/activity is responsible for the loss of cortical and hippocampal synapses and fear learning. This has been demonstrated not only in animals but also in humans. In fact, impaired learning and memory have been observed in both human subjects (Egan et al., 2003) and mice (Soliman et al., 2010) in which the regulated BDNF secretion is reduced due to a single nucleotide polymorphism in the BDNF gene that encodes a valine (Val) to methionine (Met) substitution at codon 66 (Val66Met). In addition, correlative studies of human chronic neurodegenerative diseases characterized by reduced BDNF levels/activity have confirmed the role of BDNF in adult plasticity. For instance, a deficiency in BDNF synthesis has been described in postmortem brains (Phillips et al., 1991) or cerebrospinal fluid (Laske et al., 2007) of patients with AD.
BDNF treatment has the potential to reduce or abolish neuronal death in several brain areas. Moreover, BDNF and NT-3 prevent degeneration of motor descending pathways (Diener and Bregman, 1994; Schnell et al., 1994). Indeed, early experimental studies in animal models have shown a remarkable neuroprotective effect of BDNF against various neurotoxins that mimic human chronic neurodegenerative diseases such as PD and Huntington’s disease (Hyman et al., 1991; Frim et al., 1994; Alberch et al., 2002). Similar neuroprotection has been observed in rodents and non-human primates after the lesion of the perforant path, an animal model of AD (Nagahara et al., 2009). BDNF can also prevent synaptic simplifications of neurons exposed to gp120 (Bachis et al., 2012). These and other experimental evidence have prompted the suggestion that BDNF could be a potential therapeutic agent for the treatment of these and other diseases characterized by loss of synaptic plasticity, including HAND. Overall, BDNF remains one of the best understood agents against neurodegeneration and thus it deserves a critical analysis of its biological effect. We will describe some experimental evidence that BDNF can be used as biological therapy against HAND.
BDNF and HIV Dementia, Basic Tenets
Very little is known about a possible role of BDNF in HAD, which as presented above exhibits certain clinical signs similar to AD and PD. Due to the similarity of AD, PD and HAD in terms of neuronal loss, one may suggest that BDNF treatment could be a beneficial therapeutic approach in these diseases. Moreover, it can be predicted that HIV may promote neuronal degeneration by lowering BDNF levels. This hypothesis has recently been tested in HIV positive subjects. It was found that BDNF levels are significantly lower in HIV positive individuals than in HIV negative subjects in both the serum (Avdoshina et al., 2011) and brain (Bachis et al., 2012). These data pose the question as to whether HIV promotes synaptic simplification by reducing BDNF along with the other neurotrophic factors whose physiological role is to support and maintain synapses. This theory was first developed for sensory and sympathetic neurons the density of which is regulated by postsynaptic targets producing NGF (Johnson et al., 1980; Levi-Montalcini, 1987). Nevertheless, such a concept can also be applied to the CNS. Indeed, low levels of BDNF have been shown to impair the innervation of the cortex by serotonergic fibers (Lyons et al., 1999). Similarly, gp120-mediated reduction of BDNF levels appears to cause neuronal degeneration even in the absence of inflammation (Nosheny et al., 2004). These observations suggest that an environment characterized by low BDNF (or other trophic factors) levels or activity could be a common risk factor for the development of neurological diseases. This issue rarely receives the attention it deserves, a problem that may have a negative impact on the success of future clinical trials.
BDNF and Neuroprotection
The biological activity of the neurotrophins begins when they bind to a receptor complex composed of two different receptors. The first neurotrophin receptor that was characterized was a member of the tumor necrosis factor receptor family that was named p75 NGF (Johnson et al., 1986; Radeke et al., 1987). Since p75 binds to all neurotrophins with a similar affinity (Rodriguez-Tebar et al., 1990) it was subsequently renamed p75NTR. The other component of the neurotrophin receptor complex is the proto-oncogene Trk. This is a receptor tyrosine kinase which, like other tyrosine kinase receptors, is activated by ligand-induced formation of non-covalently associated receptor dimers (Kaplan et al., 1991). There are three structurally related Trks and each neurotrophin binds selectively to a respective Trk: BDNF binds to TrkB, NGF to TrkA and NT-3 to TrkC (Chao, 2003); however, at high concentrations BDNF can also bind to TrkC (Klein et al., 1991). Both Trk and p75NTR are necessary to confer high affinity binding to the neurotrophins and to influence their biological activities (Hempstead et al., 1991). When activation of p75NTR occurs without a concomitant activation of Trk, p75NTR promotes death of oligodendrocytes (Gu et al., 1999) as well as axonal degeneration in both peripheral nerves (Kenchappa et al., 2006) and CNS (Park et al., 2010). Thus, p75NTR exhibits an opposite action on oligodendrocyte survival than PDGF.
The brain expresses also a truncated isoform of TrkB or TrkB.T1. This receptor does not signal through a canonical tyrosine kinase domain. However, at physiological levels Trk.T1 negatively regulates full length TrkB (TrkB.FL) signaling so that it acts as a dominant/negative or BDNF-sequestering trophic activity (Carim-Todd et al., 2009). For instance, BDNF exerts strong pro-survival effects on injured motoneurons; however, in clinical trials BDNF has failed to benefit patients with amyotrophic lateral sclerosis (ALS). It was discovered that motoneurons express the TrkB.FL receptor but also high levels of TrkB.T1 receptor isoform, which, in turn, limited BDNF pro-survival effect. Indeed, deletion of TrkB.T1 in an ALS mouse model (Gurney et al., 1994), significantly slowed the onset of motor neuron degeneration (Yanpallewar et al., 2012). In addition, TrkB.T1 has been shown to promote the production of nitric oxide from astrocytes, which could cause neuronal degeneration (Colombo et al., 2012). Thus, expression of Trk.T1 on neuronal and glial populations must be considered when approaching the use of BDNF for human neurological diseases.
BDNF and gp120
TrkB mediates the neuroprotective effect of BDNF against gp120. In fact, TrkB-mediated activation of the extracellular-signal-regulated kinase (ERK) pathway blocks the pro-apoptotic effect of gp120 (Mocchetti and Bachis, 2004). Conversely, BDNF does not prevent gp120 toxicity in neurons that do not express TrkB (Ahmed et al., 2008). One of the key mechanisms that may explain the neuroprotective effect of BDNF is its ability to down-regulate CXCR4. This receptor is abundant in neurons and areas of the CNS that also express TrkB, such as the cortex, hippocampus and striatum (Ahmed et al., 2008). CXCR4 is a G-protein coupled receptor that can be desensitized by tyrosine kinase intracellular signal crosstalk similar to that induced by TrkB (Daub et al., 1996). Evidence that neuronal CXCR4 is a BDNF target is overwhelming. Indeed, in vitro and in vivo data have shown that BDNF decreases the expression of CXCR4 (Bachis et al., 2003; Nosheny et al., 2007). Conversely, BDNF heterozygous animals exhibit increased levels of CXCR4 mRNA in the cortex, hippocampus and striatum when compared to wild type controls (Ahmed et al., 2008). Moreover, in BDNF heterozygous mice, increased CXCR4 correlates with a more robust neurotoxic effect of gp120 (Nosheny et al., 2004). Thus, from a pharmacological point of view, BDNF is particularly important as a neuroprotective compound against HIV or gp120 neurotoxicity that occurs through CXCR4 receptors.
BDNF and the immune system
There are other effects of BDNF that can be beneficial for HIV positive subjects. For instance, in the immune system BDNF decreases apoptosis of T cells (Maroder et al., 1996; De Santi et al., 2009). These cells are depleted in AIDS. Thus, one may envision the use of BDNF in conjunction with combination antiretroviral therapy (cART) to maintain the appropriate number of immune cells and to delay AIDS. However, this effect also has a broader implication for the CNS function. In fact, progressive neurological deficits occur after the onset of severe immunodeficiency. Thus, keeping healthy immune cells will reduce “inflammation” of the immune system that can amplify nervous system damage via inflammatory cytokines. These may enter the CNS by infected cells or be produced locally by microglia. For example, in a state of chronic inflammation induced in a non-human primate by infection with simian immunodeficiency virus, the brain was positive for peripheral circulating monocytes trafficking from bone marrow (Burdo et al., 2010). This event correlates with the severity of encephalitis. Unusual activation of cytokine and chemokine receptors in the context of HIV infection results in dendritic beading and loss of dendritic spines (Suzumura et al., 2006). These changes are accompanied by failure of long term potentiation (LTP), which might underlie impaired learning and memory. Given the well-known property of BDNF in promoting dendritic branching and spine morphology (Horch and Katz, 2002; Tanaka et al., 2008) which are key for the BDNF-mediated LTP (Figurov et al., 1996), BDNF could be used to prevent atrophy of dendrite branching and memory loss. Of course such treatment will require an early diagnosis of cognitive impairments.
p75NTR: the death receptor?
BDNF can avidly be transported in axons (Conner et al., 1998) and released upon activation of neuronal activity (Marini et al., 1998). When such transport is interrupted, the release diminishes and, consequently, neurons do degenerate as BDNF profoundly affects neuronal homeostasis and the ability of neurons to counteract naturally occurring neuro-inflammatory responses. This hypothesis has been supported in animal models of diseases. For instance, reduced BDNF release/levels have been associated with depression (Duman, 2004) and Huntington’s disease (Zuccato and Cattaneo, 2009). Given the tight relation between BDNF and neuronal survival, one may suggest that gp120 neurotoxicity includes a reduction of BDNF and other neurotrophic factors at the synapses. In vivo studies have shown that gp120 decreases the levels of BDNF in cortico-striatal terminals without affecting BDNF in the cell bodies (Nosheny et al., 2004), suggesting that gp120 modifies the anterograde transport of BDNF. A reduced availability of BDNF in the axonal terminal will culminate in a decreased release of BDNF which will profoundly affect neuronal homeostasis and the ability of neurons to counteract the inflammatory responses and production of cytokines that are caused by HIV infection (Kraft-Terry et al., 2010). However, transport alone cannot dictate how much BDNF is released. In fact, one way of reducing BDNF release/levels is to affect the processing of BDNF from its precursor pro-BDNF (Fig. 2). Pro-BDNF, like other pro-neurotrophins, is cleaved into mature BDNF in the endoplasmic reticulum by the proconvertase furin (Seidah et al., 1996) or extracellularly by proteases such as plasmin and matrix metalloproteases (Pang et al., 2004). Pro-BDNF, which is found in human brains (Fahnestock et al., 2001) can be stored in synaptic vesicles and released from neurons (Yang et al., 2009). Conversion of pro-BDNF to mature BDNF is an important process for synaptic plasticity. In fact, pro-BDNF can bind with high affinity to p75NTR (Greenberg et al., 2009) and promote neuronal loss (Teng et al., 2005). This issue deserves more attention and further investigation because HAD brains exhibit higher levels of pro-BDNF and lower levels of BDNF than control and non-demented HIV subjects (Bachis et al., 2012). An altered mBDNF/proBDNF ratio in HAND could compromise synaptic connections and neuronal survival (Fig. 2). This scenario suggests that the neurotoxic effects of HIV may encompass a reduction of the neurotrophic factor environment. Understanding how HIV inhibits the availability of BDNF and other neurotrophic factors is crucial for the development of new therapies.
Figure 2. Neurotrophic factors hypothesis of HIV neurotoxicity.
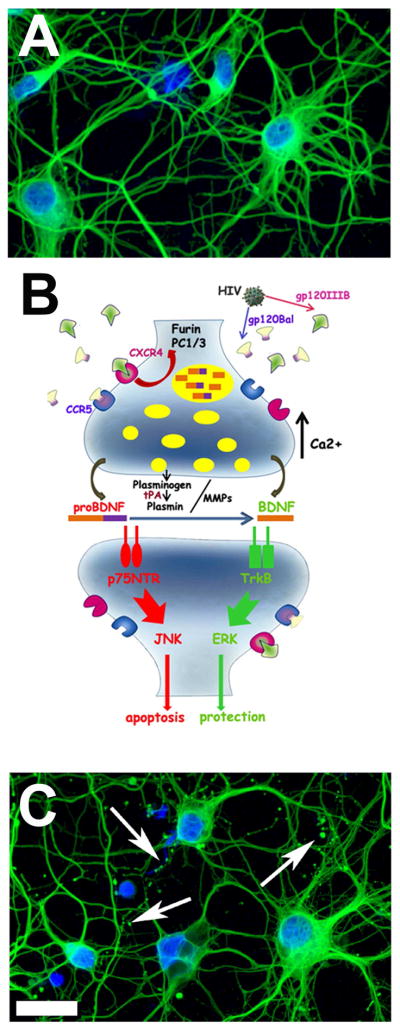
HIV, through gp120 binding to chemokine receptors, reduces neurotrophic factor levels. Many mechanisms can account for this effect. The example described in this figure pertains to BDNF. (A) Rat cortical neurons without HIV are healthy and exhibit numerous processes. (B) HIV, through gp120, decreases the processing of mature BDNF from pro-BDNF. This phenomenon would result in less BDNF and more pro-BDNF available to neurons whose axons degenerate through a p75NTR-mediated mechanism. (C) Examples of HIV-mediated degeneration showing synaptodendritic damage (arrows) of cortical neurons. Green=class III β– tubulin; Blue=DAPI. Bar = 20 μm.
Therapeutic use of neurotrophic factors
Gene delivery
Neurotrophic factors are large hydrophilic molecules that do not cross the blood brain barrier. Therefore, these molecules need to be delivered to the CNS, which requires surgery and chronic instrumentations and therefore lowers the enthusiasm for clinical application. In addition, when infused into the ventricular system, growth factors have limited diffusion. In recent years new techniques to increase trophic factors into the human brain have been developed. One promising although still under experimentation is the delivery of viral vectors including adeno-associated viral vectors (AAV) (Nagahara and Tuszynski, 2011). Using appropriate promoters, these vectors permit stable, long term expression of transgene in brain neurons after a single injection with little or no discernible toxicity. One major advantage of this system is that it is reliant on neurotrophic factors that are endogenously synthesized, modified and released for a long period of time without causing any known neuropathology. For instance, the recombinant AAV serotype 2 vector expressing BDNF in the rat SN has been shown to reduce 6-hydroxydopamine induced degeneration of nigral DA neurons for up to nine months (Klein et al., 1999). The same vector injected into the striatum has been shown to reduce the neurotoxic effect of gp120 on DA neurons (Nosheny et al., 2007). Similarly, gene delivery of GDNF or Neurturin has been used successfully to reduce the degeneration of DA in rats (Gasmi et al., 2007) and non-human primates (Kordower et al., 2000).
Although promising, gene delivery in humans has been limited to few cases. There could be several reasons why the clinical application of gene delivery has not yet been widely used. For instance, some patients have suffered from intracranial hemorrhage (Christine et al., 2009). Another concern is that an abnormal overproduction of BDNF and other neurotrophic factors may cause side effects that may be intolerable over a long period of time. Such effects may be seen locally (fiber spouting around the site of BDNF production) or systemically such as weight loss (Cao et al., 2009). Clinical trials of gene delivery of AAV-neurturin to the SN of PD patients (Bartus et al., 2013) or to the striatum of non-human primates (Bartus et al., 2011) have shown no adverse effects of this growth factor even after several months. On the other hand, abnormal growing of cells has also observed in PD patients with AAV-neurturin (Marks et al., 2010). Thus, additional experimental “strategies” must be examined and tested.
Pharmacological regulation of neurotrophic factors
Ongoing research has demonstrated safer and more reliable methods to increase endogenous BDNF and other trophic factors to consequently influence neuronal survival. This could replace the need for delivering neurotrophic factors by invasive approaches. For instance, physical exercise has been reported to increase BDNF levels in the hippocampus (Cotman and Berchtold, 2002) which, in turn, promotes hippocampus-based learning, synaptic plasticity and neurogenesis (Vaynman et al., 2004). Antidepressant drugs, which are safely used in humans, also increase BDNF synthesis (Nibuya et al., 1996; Hashimoto et al., 2002).
There are also compounds that promote the endogenous release of the neurotrophins. Such compounds are gangliosides. Gangliosides are endogenous compounds that are classified as acidic glycosphingolipids because they contain sialic acid linked to an oligoglycosyl backbone attached to a ceramide base (Ledeen and Yu, 1982). Gangliosides are lypophilic and cross the blood brain barrier. Different laboratories have independently shown that GM1, the prototype of ganglioside, activates tyrosine phosphorylation of Trks (Ferrari et al., 1995; Mutoh et al., 1995; Rabin and Mocchetti, 1995) via the release of the neurotrophins (Rabin et al., 2002). Intriguingly, LIGA20, a semisynthetic derivative of GM1, has been shown to prevent gp210 neurotoxicity in vitro (Bachis and Mocchetti, 2006). These experimental data encourage further research into the use of small molecules to promote trophic activity. This is not important only for HAD but also for other neurological disorders. Thus, there are alternatives to minimize the use of chronic instrumentation.
Small molecules
Neurotrophic factors activate selectively different class of receptors. For instance, the site of BDNF binding has been located to the fifth extracellular domain of the Trk receptor, with this region regulating both the affinity and specificity of TrkB for BDNF (142, 143). Thus, it is possible to design a small peptide that crosses the blood brain barrier and binds specifically to the binding site and activate a given neurotrophic factor receptor. This strategy would mimic the activity of endogenous neurotrophic factors without the limitation of delivering them through invasive methods. In the last decade a number of attempts have been made to synthetize small ligands. A small peptide (16 aminoacids) identified form the TrkB crystal structure and containing the SRRGE motif crucial for binding to TrkB has been shown to mimic most of the neurotrophic activity of BDNF in vitro (Williams et al., 2005). More recently, a non-peptidergic compound derived from the loop II region of BDNF has shown to activate TrkB in vitro and in vivo (Massa et al., 2010). Thus, there are encouraging data that allow suggesting using small molecules to restore neurotrophic activity. It remains to be established whether small molecules could be administered chronically without side effects.
CONCLUSIONS
The dividing line between viral and host-mediated neurotoxicity in HIV infection is neither precise nor rigid. An effective therapy aimed at treating HIV-induced CNS diseases will require both cART and adjunctive therapies that include neuroprotective and neuro-regenerative agents. Besides their traditional role as molecules promoting survival and regeneration, neurotrophic factors are seen as potent mediators of synaptic plasticity and neurogenesis in the adult CNS. These effects could play a role in limiting AIDS-mediated neuronal degeneration and stimulating neuronal function. Although still tested only in animal models of HAD, the powerful properties of neurotrophic factors provide incentive for further research and raise hope for the therapeutic possibilities in the near future.
Table 1.
Neurotrophic activity of trophic factors in animal models of neurological diseases
| Neurotrophic factors trials | Target neurons | Animal models | Clinical |
|---|---|---|---|
| BDNF, GDNF, CNTF | Motor neurons | ALS | BDNF |
| NGF, BDNF, FGF2 | Basal forebrain Cholinergic, hippocampal and cortical neurons | AD | NGF, BDNF |
| BDNF, GDNF/Neurturin, FGF2 | Dopaminergic neurons | PD | GDNF, BDNF |
| BDNF, NT-4, FGF2 | Striatal neurons | Huntington’s disease | |
| BDNF, NT-3, FGFs | Descending motor pathways | spinal cord injury | |
| BDNF, FGFs, PDGF | striatal, nigral, cortical, | HAND |
The activity of neurotrophic factors described here refers to prevention of cell death in vivo. Clinical trials of BDNF in ALS have shown modest or no effect. References are given in text.
Acknowledgments
This work was supported by grants from the US Public Health Service, DA026174, NS074916 and NS079172. Special thanks to the National NeuroAIDS Tissue Consortium for human brain tissue.
Footnotes
Conflict of interest statement. The authors declare that they have no conflict of interest.
References
- Ahmed F, Tessarollo L, Thiele C, Mocchetti I. Brain-derived neurotrophic factor modulates expression of chemokine receptors in the brain. Brain Res. 2008;1227:1–11. doi: 10.1016/j.brainres.2008.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberch J, Perez-Navarro E, Canals JM. Neuroprotection by neurotrophins and GDNF family members in the excitotoxic model of Huntington’s disease. Brain Res Bull. 2002;57:817–822. doi: 10.1016/s0361-9230(01)00775-4. [DOI] [PubMed] [Google Scholar]
- Avdoshina V, Garzino-Demo A, Bachis A, Monaco MC, Maki PM, Tractenberg RE, Liu C, Young MA, Mocchetti I. HIV-1 decreases the levels of neurotrophins in human lymphocytes. AIDS. 2011;25:1126–1128. doi: 10.1097/QAD.0b013e32834671b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward EH, Brettschneider PD, McArthur JC, Harris GJ, Schlaepfer TE, Henderer JD, Barta PE, Tien AY, Pearlson GD. Magnetic resonance imaging measurement of gray matter volume reductions in HIV dementia. Am J Psychiatry. 1995;152:987–994. doi: 10.1176/ajp.152.7.987. [DOI] [PubMed] [Google Scholar]
- Bachis A, Mocchetti I. Semisynthetic sphingoglycolipid LIGA20 is neuroprotective against human immunodeficiency virus-gp120-mediated apoptosis. J Neurosci Res. 2006;83:890–896. doi: 10.1002/jnr.20780. [DOI] [PubMed] [Google Scholar]
- Bachis A, Major EO, Mocchetti I. Brain-derived neurotrophic factor inhibits human immunodeficiency virus-1/gp120-mediated cerebellar granule cell death by preventing gp120 internalization. J Neurosci. 2003;23:5715–5722. doi: 10.1523/JNEUROSCI.23-13-05715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Avdoshina V, Zecca L, Parsadanian M, Mocchetti I. Human immunodeficiency virus type 1 alters brain-derived neurotrophic factor processing in neurons. J Neurosci. 2012;32:9477–9484. doi: 10.1523/JNEUROSCI.0865-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. Embo J. 1982;1:549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Herzog CD, Chu Y, Wilson A, Brown L, Siffert J, Johnson EM, Jr, Olanow CW, Mufson EJ, Kordower JH. Bioactivity of AAV2-neurturin gene therapy (CERE-120): differences between Parkinson’s disease and nonhuman primate brains. Mov Disord. 2011;26:27–36. doi: 10.1002/mds.23442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Baumann TL, Siffert J, Herzog CD, Alterman R, Boulis N, Turner DA, Stacy M, Lang AE, Lozano AM, Olanow CW. Safety/feasibility of targeting the substantia nigra with AAV2-neurturin in Parkinson patients. Neurology. 2013;80:1698–1701. doi: 10.1212/WNL.0b013e3182904faa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger JR, Nath A. HIV dementia and the basal ganglia. Intervirology. 1997;40:122–131. doi: 10.1159/000150539. [DOI] [PubMed] [Google Scholar]
- Betsholtz C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 2004;15:215–228. doi: 10.1016/j.cytogfr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Brandoli C, Sanna A, De Bernardi MA, Follesa P, Brooker G, Mocchetti I. Brain-derived neurotrophic factor and basic fibroblast growth factor downregulate NMDA receptor function in cerebellar granule cells. J Neurosci. 1998;18:7953–7961. doi: 10.1523/JNEUROSCI.18-19-07953.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdo TH, Soulas C, Orzechowski K, Button J, Krishnan A, Sugimoto C, Alvarez X, Kuroda MJ, Williams KC. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 2010;6:e1000842. doi: 10.1371/journal.ppat.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Lin EJ, Cahill MC, Wang C, Liu X, During MJ. Molecular therapy of obesity and diabetes by a physiological autoregulatory approach. Nat Med. 2009;15:447–454. doi: 10.1038/nm.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carim-Todd L, Bath KG, Fulgenzi G, Yanpallewar S, Jing D, Barrick CA, Becker J, Buckley H, Dorsey SG, Lee FS, Tessarollo L. Endogenous truncated TrkB.T1 receptor regulates neuronal complexity and TrkB kinase receptor function in vivo. J Neurosci. 2009;29:678–685. doi: 10.1523/JNEUROSCI.5060-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Christine CW, Starr PA, Larson PS, Eberling JL, Jagust WJ, Hawkins RA, VanBrocklin HF, Wright JF, Bankiewicz KS, Aminoff MJ. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology. 2009;73:1662–1669. doi: 10.1212/WNL.0b013e3181c29356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SE, Mundy T, Karassik B, Lieb L, Ludwig DD, Ward J. Neuropsychological functioning in human immunodeficiency virus type 1 seropositive children infected through neonatal blood transfusion. Pediatrics. 1991;88:58–68. [PubMed] [Google Scholar]
- Colombo E, Cordiglieri C, Melli G, Newcombe J, Krumbholz M, Parada LF, Medico E, Hohlfeld R, Meinl E, Farina C. Stimulation of the neurotrophin receptor TrkB on astrocytes drives nitric oxide production and neurodegeneration. J Exp Med. 2012;209:521–535. doi: 10.1084/jem.20110698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Gall CM. Anterograde transport of neurotrophin proteins in the CNS--a reassessment of the neurotrophic hypothesis. Rev Neurosci. 1998;9:91–103. doi: 10.1515/revneuro.1998.9.2.91. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Czubayko F, Smith RV, Chung HC, Wellstein A. Tumor growth and angiogenesis induced by a secreted binding protein for fibroblast growth factors. J Biol Chem. 1994;269:28243–28248. [PubMed] [Google Scholar]
- Dal Pan GJ, McArthur JH, Aylward E, Selnes OA, Nance-Sproson TE, Kumar AJ, Mellits ED, McArthur JC. Patterns of cerebral atrophy in HIV-1-infected individuals: results of a quantitative MRI analysis. Neurology. 1992;42:2125–2130. doi: 10.1212/wnl.42.11.2125. [DOI] [PubMed] [Google Scholar]
- Daub H, Ulrich Weiss F, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- Davies J, Everall IP, Weich S, Glass J, Sharer LR, Cho ES, Bell JE, Majteny C, Gray F, Scaravilli F, Lantos PL. HIV-associated brain pathology: a comparative international study. Neuropathol Appl Neurobiol. 1998;24:118–124. doi: 10.1046/j.1365-2990.1998.00096.x. [DOI] [PubMed] [Google Scholar]
- De Santi L, Cantalupo L, Tassi M, Raspadori D, Cioni C, Annunziata P. Higher expression of BDNF receptor gp145trkB is associated with lower apoptosis intensity in T cell lines in multiple sclerosis. J Neurol Sci. 2009;277:65–70. doi: 10.1016/j.jns.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Diener PS, Bregman BS. Neurotrophic factors prevent the death of CNS neurons after spinal cord lesions in newborn rats. Neuroreport. 1994;5:1913–1917. doi: 10.1097/00001756-199410000-00018. [DOI] [PubMed] [Google Scholar]
- Dittmar MT, McKnight A, Simmons G, Clapham PR, Weiss RA, Simmonds P. HIV-1 tropism and co-receptor use. Nature. 1997;385:495–496. doi: 10.1038/385495a0. [DOI] [PubMed] [Google Scholar]
- Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 2004;5:11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Evans SJ, Choudary PV, Neal CR, Li JZ, Vawter MP, Tomita H, Lopez JF, Thompson RC, Meng F, Stead JD, Walsh DM, Myers RM, Bunney WE, Watson SJ, Jones EG, Akil H. Dysregulation of the fibroblast growth factor system in major depression. Proc Natl Acad Sci USA. 2004;101:15506–15511. doi: 10.1073/pnas.0406788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall IP, Hansen LA, Masliah E. The shifting patterns of HIV encephalitis neuropathology. Neurotox Res. 2005;8:51–61. doi: 10.1007/BF03033819. [DOI] [PubMed] [Google Scholar]
- Everall IP, Trillo-Pazos G, Bell C, Mallory M, Sanders V, Masliah E. Amelioration of neurotoxic effects of HIV envelope protein gp120 by fibroblast growth factor: a strategy for neuroprotection. J Neuropathol Exp Neurol. 2001;60:293–301. doi: 10.1093/jnen/60.3.293. [DOI] [PubMed] [Google Scholar]
- Farooqi AA, Waseem S, Riaz AM, Dilawar BA, Mukhtar S, Minhaj S, Waseem MS, Daniel S, Malik BA, Nawaz A, Bhatti S. PDGF: the nuts and bolts of signalling toolbox. Tumour Biol. 2011;32:1057–1070. doi: 10.1007/s13277-011-0212-3. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sanchez MT, Novelli A. Basic fibroblast growth factor protects cerebellar neurons in primary culture from NMDA and non-NMDA receptor mediated neurotoxicity. FEBS Lett. 1993;335:124–131. doi: 10.1016/0014-5793(93)80453-2. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Anderson BL, Stephens RM, Kaplan DR, Greene LA. Prevention of apoptotic neuronal death by GM1 ganglioside. Involvement of Trk neurotrophin receptors. J Biol Chem. 1995;270:3074–3080. doi: 10.1074/jbc.270.7.3074. [DOI] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Follesa P, Gale K, Mocchetti I. Regional and temporal pattern of expression of nerve growth factor and basic fibroblast growth factor mRNA in rat brain following electroconvulsive shock. Exp Neurol. 1994;127:37–44. doi: 10.1006/exnr.1994.1077. [DOI] [PubMed] [Google Scholar]
- Fox L, Alford M, Achim C, Mallory M, Masliah E. Neurodegeneration of somatostatin-immunoreactive neurons in HIV encephalitis. J Neuropathol Exp Neurol. 1997;56:360–368. doi: 10.1097/00005072-199704000-00004. [DOI] [PubMed] [Google Scholar]
- Fredriksson L, Li H, Eriksson U. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004;15:197–204. doi: 10.1016/j.cytogfr.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Frim D, Uhler T, Galpern W, Beal M, Breakefield X, Isacson O. Implanted fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevent 1-methyl-4-phenylpyridinium toxicity to dopaminergic neurons in the rat. Proc Natl Acad Sci USA. 1994;24:5104–5108. doi: 10.1073/pnas.91.11.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frim DM, Uhler TA, Short MP, Ezzedine ZD, Klagsbrun M, Breakefield XO, Isacson O. Effects of biologically delivered NGF, BDNF and bFGF on striatal excitotoxic lesions. Neuroreport. 1993;4:367–370. doi: 10.1097/00001756-199304000-00006. [DOI] [PubMed] [Google Scholar]
- Fruttiger M, Karlsson L, Hall AC, Abramsson A, Calver AR, Bostrom H, Willetts K, Bertold CH, Heath JK, Betsholtz C, Richardson WD. Defective oligodendrocyte development and severe hypomyelination in PDGF-A knockout mice. Development. 1999;126:457–467. doi: 10.1242/dev.126.3.457. [DOI] [PubMed] [Google Scholar]
- Fukuda E, Hamada S, Hasegawa S, Katori S, Sanbo M, Miyakawa T, Yamamoto T, Yamamoto H, Hirabayashi T, Yagi T. Down-regulation of protocadherin-alpha A isoforms in mice changes contextual fear conditioning and spatial working memory. Eur J Neurosci. 2008;28:1362–1376. doi: 10.1111/j.1460-9568.2008.06428.x. [DOI] [PubMed] [Google Scholar]
- Gasmi M, Brandon EP, Herzog CD, Wilson A, Bishop KM, Hofer EK, Cunningham JJ, Printz MA, Kordower JH, Bartus RT. AAV2-mediated delivery of human neurturin to the rat nigrostriatal system: long-term efficacy and tolerability of CERE-120 for Parkinson’s disease. Neurobiol Dis. 2007;27:67–76. doi: 10.1016/j.nbd.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Gelbard HA, Epstein LG. HIV-1 encephalopathy in children. Curr Opin Pediatr. 1995;7:655–662. doi: 10.1097/00008480-199512000-00005. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Lee JW, Cotman CW. Basic FGF in adult rat brain: cellular distribution and response to entorhinal lesion and fimbria-fornix transection. J Neurosci. 1992;12:345–355. doi: 10.1523/JNEUROSCI.12-01-00345.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Grant I. The neuropsychiatry of human immunodeficiency virus. Semin Neurol. 1990;10:267–275. doi: 10.1055/s-2008-1041278. [DOI] [PubMed] [Google Scholar]
- Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: Implications in CNS function. J Neurosci. 2009;29:12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Casaccia-Bonnefil P, Srinivasan A, Chao MV. Oligodendrocyte apoptosis mediated by caspase activation. J Neurosci. 1999;19:3043–3049. doi: 10.1523/JNEUROSCI.19-08-03043.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Takei N, Shimazu K, Christ L, Lu B, Chuang D-M. Lithium induces brain-derived neurotrophic factor and activates TrkB in rodent cortical neurons: An essential step for neuroprotection against glutamate excitotoxicity. Neuropharmacology. 2002;43:1173. doi: 10.1016/s0028-3908(02)00217-4. [DOI] [PubMed] [Google Scholar]
- Hempstead BL, Martin-Zanca D, Kaplan DR, Parada LF, Chao MV. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991;350:678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Horch HW, Katz LC. BDNF release from single cells elicits local dendritic growth in nearby neurons. Nat Neurosci. 2002;5:1177–1184. doi: 10.1038/nn927. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Gage FH. Regenerating the damaged central nervous system. Nature. 2000;407:963–970. doi: 10.1038/35039559. [DOI] [PubMed] [Google Scholar]
- Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Itoh K, Mehraein P, Weis S. Neuronal damage of the substantia nigra in HIV-1 infected brains. Acta Neuropathol (Berl) 2000;99:376–384. doi: 10.1007/s004010051139. [DOI] [PubMed] [Google Scholar]
- Johnson D, Lanahan A, Buck CR, Sehgal A, Morgan C, Mercer E, Bothwell M, Chao M. Expression and structure of the human NGF receptor. Cell. 1986;47:545–554. doi: 10.1016/0092-8674(86)90619-7. [DOI] [PubMed] [Google Scholar]
- Johnson EM, Jr, Gorin PD, Brandeis LD, Pearson J. Dorsal root ganglion neurons are destroyed by exposure in utero to maternal antibody to nerve growth factor. Science. 1980;210:916–918. doi: 10.1126/science.7192014. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Hempstead BL, Martin-Zanca D, Chao MV, Parada LF. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 1991;252:554–558. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- Katori S, Hamada S, Noguchi Y, Fukuda E, Yamamoto T, Yamamoto H, Hasegawa S, Yagi T. Protocadherin-alpha family is required for serotonergic projections to appropriately innervate target brain areas. J Neurosci. 2009;29:9137–9147. doi: 10.1523/JNEUROSCI.5478-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kawamata T, Dietrich WD, Schallert T, Gotts JE, Cocke RR, Benowitz LI, Finklestein SP. Intracisternal basic fibroblast growth factor enhances functional recovery and up-regulates the expression of a molecular marker of neuronal sprouting following focal cerebral infarction. Proc Natl Acad Sci U S A. 1997;94:8179–8184. doi: 10.1073/pnas.94.15.8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenchappa RS, Zampieri N, Chao MV, Barker PA, Teng HK, Hempstead BL, Carter BD. Ligand-dependent cleavage of the P75 neurotrophin receptor is necessary for NRIF nuclear translocation and apoptosis in sympathetic neurons. Neuron. 2006;50:219–232. doi: 10.1016/j.neuron.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Kirschner PB, Henshaw R, Weise J, Trubetskoy V, Finklestein S, Schulz JB, Beal MF. Basic fibroblast growth factor protects against excitotoxicity and chemical hypoxia in both neonatal and adult rats. J Cereb Blood Flow Metab. 1995;15:619–623. doi: 10.1038/jcbfm.1995.76. [DOI] [PubMed] [Google Scholar]
- Klein R, Nanduri V, Jing SA, Lamballe F, Tapley P, Bryant S, Cordon-Cardo C, Jones KR, Reichardt LF, Barbacid M. The trkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell. 1991;66:395–403. doi: 10.1016/0092-8674(91)90628-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, Lewis MH, Muzyczka N, Meyer EM. Prevention of 6-hydroxydopamine-induced rotational behavior by BDNF somatic gene transfer. Brain Res. 1999;847:314–320. doi: 10.1016/s0006-8993(99)02116-2. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen E-Y, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Deglon N, Aebischer P. Neurodegeneration Prevented by Lentiviral Vector Delivery of GDNF in Primate Models of Parkinson’s Disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- Kraft-Terry SD, Stothert AR, Buch S, Gendelman HE. HIV-1 neuroimmunity in the era of antiretroviral therapy. Neurobiol Dis. 2010;37:542–548. doi: 10.1016/j.nbd.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laske C, Stransky E, Leyhe T, Eschweiler GW, Maetzler W, Wittorf A, Soekadar S, Richartz E, Koehler N, Bartels M, Buchkremer G, Schott K. BDNF serum and CSF concentrations in Alzheimer’s disease, normal pressure hydrocephalus and healthy controls. J Psychiatr Res. 2007;41:387–394. doi: 10.1016/j.jpsychires.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Ledeen RW, Yu RK. Gangliosides: structure, isolation, and analysis. Methods Enzymol. 1982;83:139–191. doi: 10.1016/0076-6879(82)83012-7. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Li H, Fredriksson L, Li X, Eriksson U. PDGF-D is a potent transforming and angiogenic growth factor. Oncogene. 2003;22:1501–1510. doi: 10.1038/sj.onc.1206223. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Lopez-Villegas D, Lenkinski RE, Frank I. Biochemical changes in the frontal lobe of HIV-infected individuals detected by magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 1997;94:9854–9859. doi: 10.1073/pnas.94.18.9854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre PC, Belluscio L, Squinto S, Ip NY, Furth ME, Lindsay RM, Yancopoulos GD. Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science. 1990;247:1446–1451. doi: 10.1126/science.247.4949.1446. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallei A, Shi B, Mocchetti I. Antidepressant treatments induce the expression of basic fibroblast growth factor in cortical and hippocampal neurons. Mol Pharmacol. 2002;61:1017–1024. doi: 10.1124/mol.61.5.1017. [DOI] [PubMed] [Google Scholar]
- Maragnoli ME, Fumagalli F, Gennarelli M, Racagni G, Riva MA. Fluoxetine and olanzapine have synergistic effects in the modulation of fibroblast growth factor 2 expression within the rat brain. Biol Psychiatry. 2004;55:1095–1102. doi: 10.1016/j.biopsych.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Marini AM, Rabin SJ, Lipsky RH, Mocchetti I. Activity-dependent release of brain-derived neurotrophic factor underlies the neuroprotective effect of N-methyl-D-aspartate. J Biol Chem. 1998;273:29394–29399. doi: 10.1074/jbc.273.45.29394. [DOI] [PubMed] [Google Scholar]
- Marks WJ, Jr, et al. Gene delivery of AAV2-neurturin for Parkinson’s disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9:1164–1172. doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- Maroder M, Bellavia D, Meco D, Napolitano M, Stigliano A, Alesse E, Vacca A, Giannini G, Frati L, Gulino A, Screpanti I. Expression of trKB neurotrophin receptor during T cell development. Role of brain derived neurotrophic factor in immature thymocyte survival. J Immunol. 1996;157:2864–2872. [PubMed] [Google Scholar]
- Massa SM, Yang T, Xie Y, Shi J, Bilgen M, Joyce JN, Nehama D, Rajadas J, Longo FM. Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents. J Clin Invest. 2010;120:1774–1785. doi: 10.1172/JCI41356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC, Steiner JP, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocchetti I, Bachis A. Brain-derived neurotrophic factor activation of TrkB protects neurons from HIV-1/gp120-induced cell death. Crit Rev Neurobiol. 2004;16:51–57. doi: 10.1615/critrevneurobiol.v16.i12.50. [DOI] [PubMed] [Google Scholar]
- Mohapel P, Frielingsdorf H, Haggblad J, Zachrisson O, Brundin P. Platelet-derived growth factor (PDGF-BB) and brain-derived neurotrophic factor (BDNF) induce striatal neurogenesis in adult rats with 6-hydroxydopamine lesions. Neuroscience. 2005;132:767–776. doi: 10.1016/j.neuroscience.2004.11.056. [DOI] [PubMed] [Google Scholar]
- Mutoh T, Tokuda A, Miyadai T, Hamaguchi M, Fujiki N. Ganglioside GM1 binds to the Trk protein and regulates receptor function. Proc Natl Acad Sci USA. 1995;92:5087–5091. doi: 10.1073/pnas.92.11.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011;10:209–219. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, Chao MV, Koo EH, Geschwind D, Masliah E, Chiba AA, Tuszynski MH. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat Med. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosheny RL, Bachis A, Acquas E, Mocchetti I. Human immunodeficiency virus type 1 glycoprotein gp120 reduces the levels of brain-derived neurotrophic factor in vivo: potential implication for neuronal cell death. Eur J Neurosci. 2004;20:2857–2864. doi: 10.1111/j.1460-9568.2004.03764.x. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Bachis A, Aden SA, De Bernardi MA, Mocchetti I. Intrastriatal administration of human immunodeficiency virus-1 glycoprotein 120 reduces glial cell-line derived neurotrophic factor levels and causes apoptosis in the substantia nigra. J Neurobiol. 2006;66:1311–1321. doi: 10.1002/neu.20288. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Amhed F, Yakovlev AG, Meyer EM, Ren K, Tessarollo L, Mocchetti I. Brain-derived neurotrophic factor prevents the nigrostriatal degeneration induced by human immunodeficiency virus-1 glycoprotein 120 in vivo. Eur J Neurosci. 2007;25:2275–2284. doi: 10.1111/j.1460-9568.2007.05506.x. [DOI] [PubMed] [Google Scholar]
- Okamoto S-i, Kang Y-J, Brechtel CW, Siviglia E, Russo R, Clemente A, Harrop A, McKercher S, Kaul M, Lipton SA. HIV/gp120 Decreases Adult Neural Progenitor Cell Proliferation via Checkpoint Kinase-Mediated Cell-Cycle Withdrawal and G1 Arrest. Cell Stem Cell. 2007;1:230–236. doi: 10.1016/j.stem.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Paratcha G, Ledda F. GDNF and GFRalpha: a versatile molecular complex for developing neurons. Trends Neurosci. 2008;31:384–391. doi: 10.1016/j.tins.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Park KJ, Grosso CA, Aubert I, Kaplan DR, Miller FD. p75NTR-dependent, myelin-mediated axonal degeneration regulates neural connectivity in the adult brain. Nat Neurosci. 2010;13:559–566. doi: 10.1038/nn.2513. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Peng F, Dhillon NK, Yao H, Zhu X, Williams R, Buch S. Mechanisms of platelet-derived growth factor-mediated neuroprotection--implications in HIV dementia. Eur J Neurosci. 2008;28:1255–1264. doi: 10.1111/j.1460-9568.2008.06444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DA, Lucidi-Phillipi CA, Murphy DP, Ray J, Gage FH. Fibroblast growth factor-2 protects entorhinal layer II glutamatergic neurons from axotomy-induced death. J Neurosci. 1996;16:886–898. doi: 10.1523/JNEUROSCI.16-03-00886.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- Price RW, Spudich S. Antiretroviral therapy and central nervous system HIV type 1 infection. J Infect Dis. 2008;197(Suppl 3):S294–306. doi: 10.1086/533419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle NP, Mudhar HS, Collarini EJ, Richardson WD. PDGF receptors in the rat CNS: during late neurogenesis, PDGF alpha-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development. 1992;115:535–551. doi: 10.1242/dev.115.2.535. [DOI] [PubMed] [Google Scholar]
- Pumpradit W, Ananworanich J, Lolak S, Shikuma C, Paul R, Siangphoe U, Chaoniti N, Kaew-On P, Paris R, Ruxrungtham K, Valcour V. Neurocognitive impairment and psychiatric comorbidity in well-controlled human immunodeficiency virus-infected Thais from the 2NN Cohort Study. J Neurovirol. 2010;16:76–82. doi: 10.3109/13550280903493914. [DOI] [PubMed] [Google Scholar]
- Qian X, Davis AA, Goderie SK, Temple S. FGF2 concentration regulates the generation of neurons and glia from multipotent cortical stem cells. Neuron. 1997;18:81–93. doi: 10.1016/s0896-6273(01)80048-9. [DOI] [PubMed] [Google Scholar]
- Raballo R, Rhee J, Lyn-Cook R, Leckman JF, Schwartz ML, Vaccarino FM. Basic fibroblast growth factor (Fgf2) is necessary for cell proliferation and neurogenesis in the developing cerebral cortex. J Neurosci. 2000;20:5012–5023. doi: 10.1523/JNEUROSCI.20-13-05012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin SJ, Mocchetti I. GM1 ganglioside activates the high-affinity nerve growth factor receptor trkA. J Neurochem. 1995;65:347–354. doi: 10.1046/j.1471-4159.1995.65010347.x. [DOI] [PubMed] [Google Scholar]
- Rabin SJ, Bachis A, Mocchetti I. Gangliosides activate Trk receptors by inducing the release of neurotrophins. J Biol Chem. 2002;277:49466–49472. doi: 10.1074/jbc.M203240200. [DOI] [PubMed] [Google Scholar]
- Radeke MJ, Misko TP, Hsu C, Herzenberg LA, Shooter EM. Gene transfer and molecular cloning of the rat nerve growth factor receptor. Nature. 1987;325:593–597. doi: 10.1038/325593a0. [DOI] [PubMed] [Google Scholar]
- Reyes MG, Faraldi F, Senseng CS, Flowers C, Fariello R. Nigral degeneration in acquired immune deficiency syndrome (AIDS) Acta Neuropathol (Berl) 1991;82:39–44. doi: 10.1007/BF00310921. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Tebar A, Dechant G, Barder Y. Binding of brain derived neurotrophic factor to the nerve growth factor receptor. Neuron. 1990;4:487–492. doi: 10.1016/0896-6273(90)90107-q. [DOI] [PubMed] [Google Scholar]
- Sanders VJ, Everall IP, Johnson RW, Masliah E. Fibroblast growth factor modulates HIV coreceptor CXCR4 expression by neural cells. HNRC Group. J Neurosci Res. 2000;59:671–679. doi: 10.1002/(SICI)1097-4547(20000301)59:5<671::AID-JNR10>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Schalm SS, Ballif BA, Buchanan SM, Phillips GR, Maniatis T. Phosphorylation of protocadherin proteins by the receptor tyrosine kinase Ret. Proc Natl Acad Sci U S A. 2010;107:13894–13899. doi: 10.1073/pnas.1007182107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Price DA. Alzheimer’s disease-related alterations in synaptic density: neocortex and hippocampus. J Alzheimers Dis. 2006;9:101–115. doi: 10.3233/jad-2006-9s312. [DOI] [PubMed] [Google Scholar]
- Schnell L, Schneider R, Kolbeck R, Barde YA, Schwab ME. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature. 1994;367:170–173. doi: 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]
- Seidah NG, Benjannet S, Pareek S, Chretien M, Murphy RA. Cellular processing of the neurotrophin precursors of NT3 and BDNF by the mammalian proprotein convertases. FEBS Lett. 1996;379:247–250. doi: 10.1016/0014-5793(95)01520-5. [DOI] [PubMed] [Google Scholar]
- Sendtner M, Kreutzberg GW, Thoenen H. Ciliary neurotrophic factor prevents the degeneration of motor neurons after axotomy. Nature. 1990;345:440–441. doi: 10.1038/345440a0. [DOI] [PubMed] [Google Scholar]
- Sendtner M, Holtmann B, Kolbeck R, Thoenen H, Barde YA. Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature. 1992;360:757–759. doi: 10.1038/360757a0. [DOI] [PubMed] [Google Scholar]
- Shihabuddin LS, Ray J, Gage FH. FGF-2 is sufficient to isolate progenitors found in the adult mammalian spinal cord. Exp Neurol. 1997;148:577–586. doi: 10.1006/exnr.1997.6697. [DOI] [PubMed] [Google Scholar]
- Smits A, Kato M, Westermark B, Nister M, Heldin CH, Funa K. Neurotrophic activity of platelet-derived growth factor (PDGF): Rat neuronal cells possess functional PDGF beta-type receptors and respond to PDGF. Proc Natl Acad Sci U S A. 1991;88:8159–8163. doi: 10.1073/pnas.88.18.8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, Jing D, Tottenham N, Amso D, Somerville LH, Voss HU, Glover G, Ballon DJ, Liston C, Teslovich T, Van Kempen T, Lee FS, Casey BJ. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzumura A, Takeuchi H, Zhang G, Kuno R, Mizuno T. Roles of glia-derived cytokines on neuronal degeneration and regeneration. Ann N Y Acad Sci. 2006;1088:219–229. doi: 10.1196/annals.1366.012. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Horiike Y, Matsuzaki M, Miyazaki T, Ellis-Davies GC, Kasai H. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science. 2008;319:1683–1687. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YD, Mocchetti I, Taveira-DaSilva AM, Gillis RA, Wrathall JR. Basic fibroblast growth factor increases long-term survival of spinal motor neurons and improves respiratory function after experimental spinal cord injury. J Neurosci. 1999;19:7037–7047. doi: 10.1523/JNEUROSCI.19-16-07037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Dutton RA, Chiang MC, Leow AD, Sowell ER, De Zubicaray G, Becker JT, Lopez OL, Aizenstein HJ, Toga AW. Tracking Alzheimer’s disease. Ann N Y Acad Sci. 2007;1097:183–214. doi: 10.1196/annals.1379.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ, Olson L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;273:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- Tran PB, Miller RJ. HIV-1, chemokines and neurogenesis. Neurotox Res. 2005;8:149–158. doi: 10.1007/BF03033826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui CC, Pierchala BA. The differential axonal degradation of Ret accounts for cell-type-specific function of glial cell line-derived neurotrophic factor as a retrograde survival factor. J Neurosci. 2010;30:5149–5158. doi: 10.1523/JNEUROSCI.5246-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Akil H, Watson SJ, Evans SJ. The fibroblast growth factor system and mood disorders. Biol Psychiatry. 2006;59:1128–1135. doi: 10.1016/j.biopsych.2006.02.026. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Vescovi AL, Reynolds BA, Fraser DD, Weiss S. bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGF-generated CNS progenitor cells. Neuron. 1993;11:951–966. doi: 10.1016/0896-6273(93)90124-a. [DOI] [PubMed] [Google Scholar]
- Wang G-J, Chang L, Volkow ND, Telang F, Logan J, Ernst T, Fowler JS. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004;127:2452–2458. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- Wilcox BJ, Unnerstall JR. Expression of acidic fibroblast growth factor mRNA in the developing and adult rat brain. Neuron. 1991;6:397–409. doi: 10.1016/0896-6273(91)90248-x. [DOI] [PubMed] [Google Scholar]
- Williams G, Williams E-J, Maison P, Pangalos MN, Walsh FS, Doherty P. Overcoming the Inhibitors of Myelin with a Novel Neurotrophin Strategy. J Biol Chem. 2005;280:5862–5869. doi: 10.1074/jbc.M411121200. [DOI] [PubMed] [Google Scholar]
- Yan Q, Matheson C, Lopez OT. In vivo neurotrophic effects of GDNF on neonatal and adult facial motor neurons. Nature. 1995;373:341–344. doi: 10.1038/373341a0. [DOI] [PubMed] [Google Scholar]
- Yang J, Siao CJ, Nagappan G, Marinic T, Jing D, McGrath K, Chen ZY, Mark W, Tessarollo L, Lee FS, Lu B, Hempstead BL. Neuronal release of proBDNF. Nat Neurosci. 2009;12:113–115. doi: 10.1038/nn.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanpallewar SU, Barrick CA, Buckley H, Becker J, Tessarollo L. Deletion of the BDNF truncated receptor TrkB.T1 delays disease onset in a mouse model of amyotrophic lateral sclerosis. PLoS One. 2012;7:e39946. doi: 10.1371/journal.pone.0039946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Duan M, Yang L, Buch S. Platelet-derived growth factor-BB restores human immunodeficiency virus Tat-cocaine-mediated impairment of neurogenesis: role of TRPC1 channels. J Neurosci. 2012;32:9835–9847. doi: 10.1523/JNEUROSCI.0638-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S, Takagi Y, Harada J, Teramoto T, Thomas SS, Waeber C, Bakowska JC, Breakefield XO, Moskowitz MA. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc Natl Acad Sci U S A. 2001;98:5874–5879. doi: 10.1073/pnas.101034998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharenko SS, Patterson SL, Dragatsis I, Zeitlin SO, Siegelbaum SA, Kandel ER, Morozov A. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron. 2003;39:975–990. doi: 10.1016/s0896-6273(03)00543-9. [DOI] [PubMed] [Google Scholar]
- Zechel S, Werner S, Unsicker K, von Bohlen und Halbach O. Expression and functions of fibroblast growth factor 2 (FGF-2) in hippocampal formation. Neuroscientist. 2010;16:357–373. doi: 10.1177/1073858410371513. [DOI] [PubMed] [Google Scholar]
- Zheng J, Thylin MR, Ghorpade A, Xiong H, Persidsky Y, Cotter R, Niemann D, Che M, Zeng YC, Gelbard HA, Shepard RB, Swartz JM, Gendelman HE. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J Neuroimmunol. 1999;98:185–200. doi: 10.1016/s0165-5728(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol. 2009;5:311–322. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]