Abstract
Purpose
To determine the effect of PepT1 on the absorption and disposition of cefadroxil, including the potential for saturable intestinal uptake, after escalating oral doses of drug.
Methods
The absorption and disposition kinetics of [3H]cefadroxil was determined in wild-type and PepT1 knockout mice after 44.5, 89.1, 178, and 356 nmol/g oral doses of drug. The pharmacokinetics of [3H]cefadroxil was also determined in both genotypes after 44.5 nmol/g intravenous bolus doses.
Results
PepT1 deletion reduced the area under the plasma concentration-time profile (AUC0-120) of cefadroxil by 10-fold, the maximum plasma concentration (Cmax) by 17.5-fold, and increased the time to reach a maximum plasma concentration (Tmax) by 3-fold. There was no evidence of nonlinear intestinal absorption since AUC0-120 and Cmax values changed in a dose-proportional manner. Moreover, the pharmacokinetics of cefadroxil was not different between genotypes after intravenous bolus doses, indicating that PepT1 did not affect drug disposition. Finally, no differences were observed in the peripheral tissue distribution of cefadroxil (i.e., outside gastrointestinal tract) once these tissues were corrected for differences in perfusing blood concentrations.
Conclusions
The findings demonstrate convincingly the critical role of intestinal PepT1 in both the rate and extent of oral administration for cefadroxil and potentially other aminocephalosporin drugs.
Keywords: cefadroxil, oral absorption, tissue distribution, knockout mice, PepT1
INTRODUCTION
At present, the proton-coupled oligopeptide transporter family is comprised of four mammalian members, namely PepT1 (SLC15A1), PepT2 (SLC15A2), PhT1 (SLC15A4) and PhT2 (SLC15A3). All of these membrane proteins transport di- and tri-peptides across biological membranes in the body using an electrochemical proton gradient (1-3). Whereas PepT1 and PepT2 can transport di- and tri-peptides, PhT1 and PhT2 can also transport the amino acid L-histidine (4,5). Moreover, peptide transporters can deliver pharmacologically active (peptide-like) compounds such as the antiviral drug valacyclovir, the angiotensin-converting enzyme inhibitor captopril, the anticancer agent bestatin, and the β-lactam antibiotic cefadroxil (6-8).
Cefadroxil is a broad-spectrum, first generation, aminocephalosporin used to treat skin, upper respiratory, and urinary tract infections caused by both gram-positive and gram-negative bacteria (9). This antibiotic is almost completely absorbed from the gastrointestinal tract, is not metabolized, and is excreted in the urine unchanged by renal glomerular filtration, active tubular secretion, and active tubular reabsorption (10,11). Having low lipid solubility, and being completely ionized in the gastrointestinal tract, the absorption of cefadroxil (and other aminocephalosporin antibiotics) was considered to occur by some “specialized” transport mechanism (12). With the advent of molecular cloning, we now realize that the intestinal absorption of cefadroxil is a coordinated process in which cellular uptake from the lumen occurs via PepT1 and cellular efflux into the blood occurs, in part, via the ATP-binding cassette (ABC) transporters Mrp3 (Abcc3) and Mrp4 (Abcc4) (13).
Cefadroxil is a substrate for several transporters that are expressed in polarized epithelia of the intestines, kidneys and brain. For example, PepT1 is located at the apical membrane of small intestinal and renal epithelia (14), PepT2 is located at the apical membrane of renal and brain choroid plexus epithelia (14,15), MRP3 is located at the basolateral membrane of enterocytes (16), MRP4 is located preferentially at the basolateral membrane of enterocytes (17) and at the apical membrane of renal proximal tubule cells (18), and the organic anion transporters OAT1-3 (SLC22A6-8) are located at the basolateral membrane of renal epithelia (19). Given the multitude of transporter proteins that can translocate cefadroxil across important biological membranes for drug absorption (i.e., small intestine), distribution (i.e., kidney and brain) and elimination (i.e., kidney), it is possible that this β-lactam antibiotic may display nonlinear pharmacokinetics, especially in the small intestine where high concentrations of drug after oral dosing may saturate the efficient absorption of cefadroxil by luminally-expressed PepT1.
Studies in rat and human have suggested that cefadroxil exhibits nonlinear intestinal absorption kinetics. In one study (20), in situ perfusions of rat proximal jejunum over a 1,000-fold range of initial concentrations (≈ 0.03-30 mM) showed a nonlinear transport of cefadroxil that was characterized by a Michaelis constant (Km) of 6.5-7.0 mM. In another study (21), a dose-dependent reduction in the absorption rate constant (Ka) was observed in healthy male volunteers as the oral dose increased from 5 to 30 mg/kg. However, an analysis of these results (and others) is complicated by possible dose-dependent changes in cefadroxil disposition because of saturation of active renal tubular secretion and reabsorption mechanisms (22).
To better understand the impact of intestinal PepT1 on the absorption mechanism of cefadroxil, we recently reported on the in situ intestinal permeability of this antibiotic in wild-type and PepT1 knockout mice (23). However, only a preliminary analysis was performed on the in vivo absorption and disposition of cefadroxil in which a small number of animals were studied (n=3) after a single 44.5 nmol/g oral dose. Moreover, the tissue distribution of cefadroxil was not examined so the impact of PepT1 on systemic tissue pharmacokinetics is not known. As a result, the primary objective of this study was to determine the oral absorption properties of cefadroxil, including the potential for saturable PepT1-mediated intestinal uptake, after escalating oral doses of drug. The secondary objective was to characterize the role of PepT1 on cefadroxil tissue distribution.
MATERIALS AND METHODS
Chemicals
[3H]Cefadroxil (0.8 Ci/mmol) and [14C]dextran-carboxyl 70,000 (1.1 mCi/g) were obtained from Moravek Biochemicals and Radiochemicals (Brea, CA). Hyamine hydroxide was purchased from ICN Radiochemicals (Irvine, CA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Animals
All experiments were performed in 6-8 week old gender-matched wild-type (PepT1+/+) and PepT1 knockout (PepT1−/−) mice (24). The mice were maintained in a temperature-controlled room with 12-hr light and dark cycles, and were fed a standard diet with access to water ad libitum. All of the procedures were approved by the University of Michigan Committee on Use and Care of Animals (UCUCA), and were carried out in accordance with the Guide for Use of Laboratory Animals as adopted and promulgated by the US National Institutes of Health (NIH publication No. 85-23, revised in 1985).
Oral Administration of Cefadroxil
Wild-type and PepT1 knockout mice were fasted overnight (about 14 hr) before the start of each experiment. Cefadroxil was dissolved in 200-250 μL of water and administered to the mice by oral gavage using a 20 G needle. Oral doses in mice (44.5, 89.1, 178 and 356 nmol/g) were scaled from relevant human doses using a surface area adjustment (25). A 0.5 μCi/g aliquot of [3H]cefadroxil was administered along with the oral doses of unlabeled drug. Plasma was harvested from blood samples (15-20 μL), collected by tail nicks, at 5, 10, 15, 20, 30, 45, 60, 90 and 120 min after dosing. Blood was collected in a PCR tube containing 1 μl of 7.5% EDTA and centrifuged at 3,000 g, room temperature, for 3 min. A 5-μL portion of plasma was then placed in a scintillation vial containing 6 mL of CytoScint scintillation fluid (MP Biomedicals, Solon, OH), and radioactivity was measured by a dual-channel liquid scintillation counter (Beckman LS 6000 SC; Beckman Coulter Inc., Fullerton, CA). Mice had free access to water during the whole experiment.
Systemic Administration of Cefadroxil
Wild-type and PepT1 knockout mice were given a 44.5 nmol/g dose of unlabeled cefadroxil (dissolved in saline solution), administered by tail vein injection, using a 27 G needle. A 0.5 μCi/g aliquot of [3H]cefadroxil was administered along with the intravenous dose of unlabeled drug. Blood samples (15-20 μL) were collected at 0.5, 2, 5, 15, 30, 45, 60, 90 and 120 min after intravenous dosing, via tail nicks, and the plasma harvested. A 5 μL aliquot of plasma was added to 6 mL of scintillation fluid and the sample measured for radioactivity, as described before. Mice had free access to water during the duration of experimentation.
Tissue Distribution after Oral Administration of Cefadroxil
Wild-type and PepT1 knockout mice were fasted overnight (about 14 hr) and then given 178 nmol/g cefadroxil (dissolved in water), along with 0.5 μCi/g [3H]cefadroxil, by oral gavage with a 20 G needle. After 15 min post-dose, 100 μL of [14C]dextran 70,000 was injected via the tail vein and, after another 5 min (i.e., 20 min post-dose), the animal was euthanized. Whole blood and selected tissues were collected and incubated overnight at 37°C with 330 μL of 1 M hyamine hydroxide. A 40-μL volume of 30% hydrogen peroxide was added, followed by 6 mL of scintillation fluid, and the sample measured for radioactivity, as described before.
Data Analysis
The plasma concentration-time profiles of oral and intravenous cefadroxil were analyzed in both genotypes by noncompartmental analysis using Phoenix WinNonlin v5.3 (Pharsight, Sunnyvale, CA). Pharmacokinetic parameters included the area under the plasma concentration-time curve from time zero to 120 min (AUC0-120), partial cumulative AUC from time zero to t min (AUC0-t), maximum plasma concentration (Cmax) and time to reach the maximum plasma concentration (Tmax) after oral dosing. After intravenous bolus dosing, the pharmacokinetic parameters included AUC0-120, total clearance (CL), mean residence time from zero to time infinity (MTR0-inf), terminal half-life (T1/2) and volume of distribution steady-state (Vdss).
Tissue concentrations of cefadroxil were calculated as: Ctiss,corr = Ctiss – DS • Cb where Ctiss,corr is the corrected concentration of cefadroxil in each tissue (nmol/g of wet tissue), DS is the blood vascular volume in the tissue (mL/g) calculated using [14C]dextran, and Cb is the concentration of cefadroxil in blood (nmol/mL).
Statistical Analysis
Data are reported as mean ± SE. A two-sample t-test was used to examine whether or not statistically significant differences occurred between wild-type and PepT1 knockout mice. An analysis of variance was used to test for statistical differences between multiple treatment groups, followed by either a Dunnett's test for pairwise comparisons with the control group or a Tukey test for multiple comparisons. Quality of fit for the linear models was judged by standard error of parameter estimates, by visual inspection of residual plots, and by coefficient of determination (r2). All statistical analyses were performed with Prism v5.0 (GraphPad, La Jolla, CA). A p value ≤ 0.05 was considered statistically significant.
RESULTS
Oral Administration of Cefadroxil
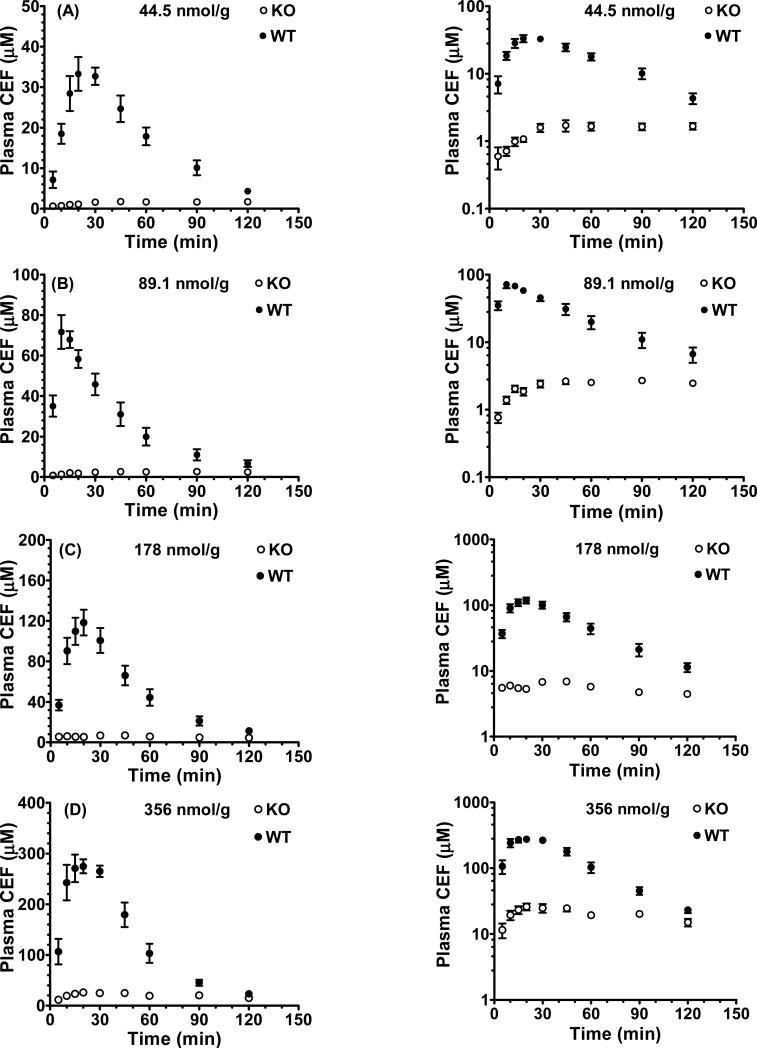
As observed in Fig. 1 (panels A-D), the plasma concentration-time profiles of oral cefadroxil were markedly different between wild-type and PepT1 mice. In wild-type mice, the plasma concentrations increased rapidly after oral administration, had a time of maximum concentration (Tmax) of about 20 min, and then decreased rapidly with time. In contrast, the plasma concentrations in PepT1 knockout mice increased very slowly over time and plateaued at about 60 min. Moreover, the plasma concentration-time curves (AUC0-120) and maximum plasma concentrations (Cmax) of cefadroxil were substantially lower in PepT1 knockout mice as compared to wild-type animals. As shown in Table I, the AUC0-120 and Cmax values were about 90% and 95% lower, respectively, in PepT1 knockout mice and the Tmax 2- to 5-fold higher. These findings suggest that the rate and extent for oral absorption of cefadroxil are highly dependent upon the expression of intestinal PepT1.
Fig. 1.
Plasma concentration-time profiles of [3H]cefadroxil (CEF) in wild-type (WT) and PepT1 knockout (KO) mice after 44.5 nmol/g (A), 89.1 nmol/g (B), 178 nmol/g (C), and 356 nmol/g (D) oral doses of drug. Data are presented as mean ± SE (n=6-8) in which the y-axis is displayed on a linear scale (left panel) and on a logarithmic scale (right panel).
Table I.
Pharmacokinetic Parameters of [3H]Cefadroxil in Wild-Type and PepT1 Knockout Mice after Escalating Oral Doses of Drug
| Dose (nmol/g) | Wild-Type (WT) | PepT1 Knockout (KO) | KO/WT |
|---|---|---|---|
| AUC0-120 (min•μM) | |||
| 44.5 | 2041 ± 145 | 176 ± 22** | 0.086 |
| 89.1 | 3166 ± 340 | 279 ± 19** | 0.088 |
| 178 | 6032 ± 608 | 634 ± 42** | 0.105 |
| 356 | 15063 ± 733 | 1634 ± 281** | 0.109 |
| Cmax (μM) | |||
| 44.5 | 36.6 ± 3.6 | 2.0 ± 0.3*** | 0.055 |
| 89.1 | 77.4 ± 6.4 | 2.9 ± 0.2*** | 0.038 |
| 178 | 125 ± 14 | 8.9 ± 0.9*** | 0.071 |
| 356 | 300 ± 16 | 19.6 ± 3.6*** | 0.065 |
| Tmax (min) | |||
| 44.5 | 27.5 ± 4.0 | 82.5 ± 17.2* | 3.00 |
| 89.1 | 15.0 ± 5.4 | 70.0 ± 14.3* | 4.67 |
| 178 | 21.7 ± 2.8 | 42.0 ± 5.6* | 1.94 |
| 356 | 22.5 ± 3.4 | 47.5 ± 10.6* | 2.11 |
Data are presented as mean ± SE (n=6-8).
p < 0.05
p < 0.01
p < 0.001, as compared to wild-type mice.
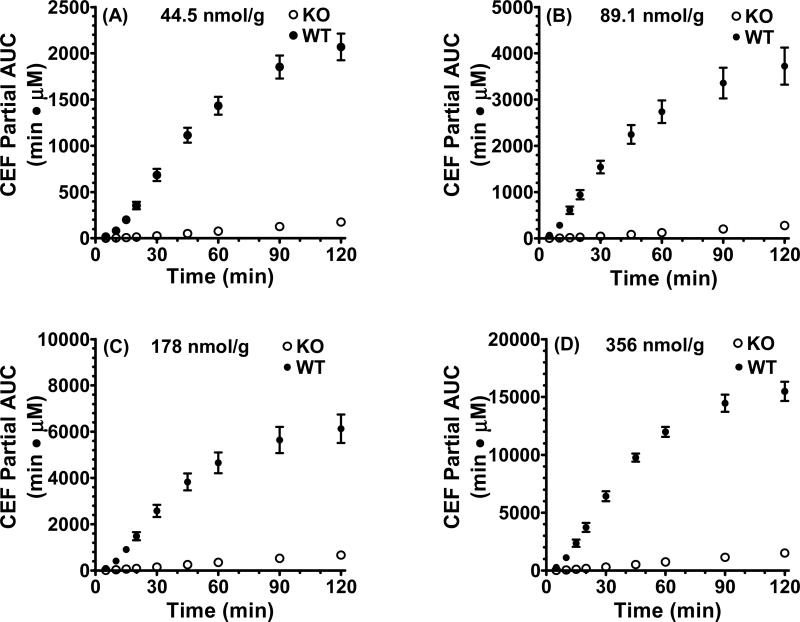
To better characterize the differences in cefadroxil absorption rate between wild-type and PepT1 knockout mice, we performed an analysis of partial cumulative AUC versus time after the four escalating oral doses of drug (Fig. 2, panels A-D). As shown in these panels, the initial slopes (i.e., from 10-30 min) in wild-type mice were much steeper than that observed in PepT1 knockout mice. This difference was further highlighted in Table II where the initial slopes in wild-type mice were 19- to 31-fold greater than in PepT1 knockout animals.
Fig. 2.
Partial cumulative area under the plasma concentration-time curve (AUC) versus time of [3H]cefadroxil (CEF) in wild-type (WT) and PepT1 knockout (KO) mice after 44.5nmol/g (A), 89.1nmol/g (B), 178 nmol/g (C), and 356 nmol/g (D) oral doses of drug. Data are presented as mean ± SE (n=6-8).
Table II.
Initial Slopes (10-30 min) of Partial Cumulative AUC versus Time Plots of [3H]Cefadroxil in Wild-Type and PepT1 Knockout Mice after Escalating Oral Doses of Drug
| Dose (nmol/g) | Wild-Type (WT) | PepT1 Knockout (KO) | KO/WT |
|---|---|---|---|
| Initial Slope | |||
| 44.5 | 30.5 ± 2.7 | 1.18 ± 0.13*** | 0.039 |
| 89.1 | 62.9 ± 6.1 | 2.00 ± 0.22*** | 0.032 |
| 178 | 109 ± 11 | 5.79 ± 0.94*** | 0.053 |
| 356 | 267 ± 22 | 12.8 ± 2.8*** | 0.048 |
Data are presented as mean ± SE (n=6-8).
* p < 0.05,
** p < 0.01 and
p < 0.001, as compared to wild-type mice.
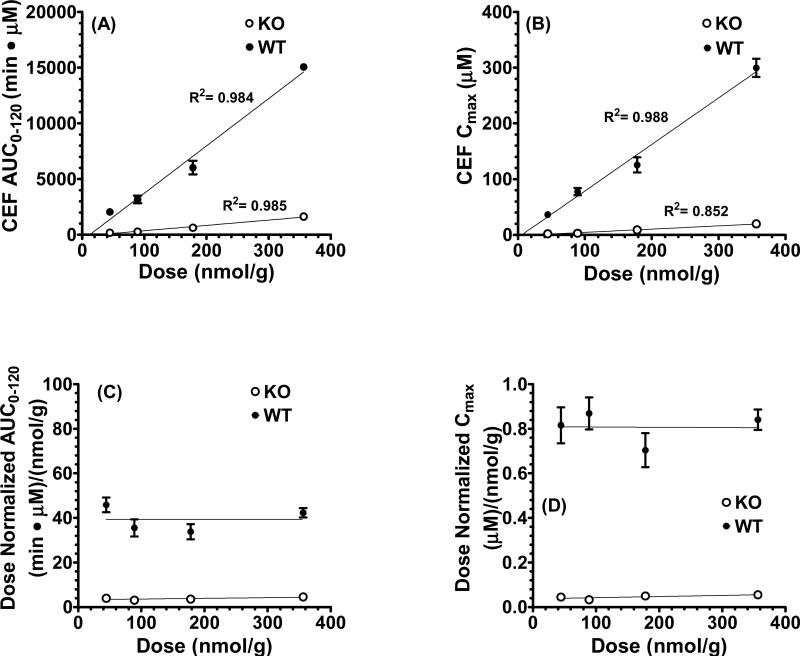
To test for the potential saturation of PepT1-mediated uptake, AUC0-120 versus dose and Cmax versus dose relationships were evaluated for orally administered cefadroxil. Fig. 3 (panels A and B) shows that, regardless of genotype, all four slopes were linear and intercepted the origin. Moreover, the dose-corrected AUC0-120 and Cmax relationships had slopes that were horizontal when correlated with dose, and were not different than zero (Fig. 3, panels C and D). Thus, it appears that the intestinal absorption and systemic exposure of cefadroxil are linear after oral administration over the 44.5 - 356 nmol/g dose range.
Fig. 3.
Relationship between AUC0-120 versus dose (A), Cmax versus dose (B), AUC0-120/dose versus dose (C), and Cmax/dose versus dose (D) of [3H]cefadroxil (CEF) in wild-type and PepT1 knockout mice after 44.5nmol/g, 89.1nmol/g, 178 nmol/g, and 356 nmol/g oral doses of drug. Data are presented as mean ± SE (n=6-8).
Systemic Administration of Cefadroxil
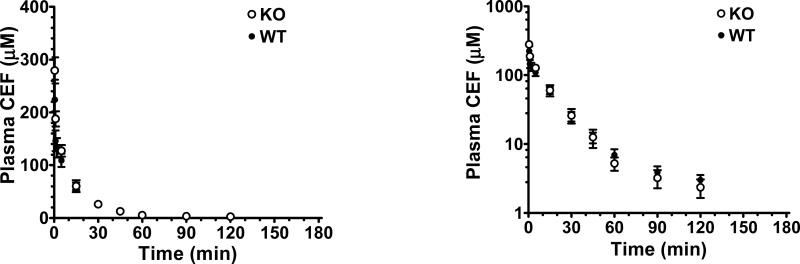
As shown in Fig. 4, wild-type and PepT1 knockout mice had very similar plasma concentration-time profiles following an intravenous bolus injection of 44.5 nmol/g cefadroxil. In fact, the pharmacokinetics of cefadroxil were not significantly different between genotypes (Table III), and deviated by less than 8% for clearance, mean residence time and volume of distribution steady-state. This finding indicates that once cefadroxil appears in the systemic circulation, its disposition is not affected by the expression of PepT1 in peripheral tissues. More importantly, any changes in the pharmacokinetic profile of oral cefadroxil during PepT1 ablation must be due to changes in intestinal absorption rather than systemic clearance.
Fig. 4.
Plasma concentration-time profiles of [3H]cefadroxil (CEF) in wild-type (WT) and PepT1 knockout (KO) mice after 44.5 nmol/g intravenous bolus doses of drug. Data are presented as mean ± SE (n=6-8) in which the y-axis is displayed on a linear scale (left panel) and on a logarithmic scale (right panel).
Table III.
Pharmacokinetic Parameters of [3H]Cefadroxil in Wild-Type and PepT1 Knockout Mice after 44.5 nmol/g Intravenous Bolus Doses of Drug.
| Parameter | Wild-Type (WT) | PepT1 Knockout (KO) | KO/WT |
|---|---|---|---|
| AUC0-120 (min•μM) | 3224 ± 312 | 3363 ± 488 | 1.04 |
| CL (mL/min) | 0.288 ± 0.039 | 0.292 ± 0.034 | 1.01 |
| MRT0-inf (min) | 27.0 ± 1.9 | 27.2 ± 1.6 | 1.01 |
| T1/2 (min) | 32.6 ± 1.9 | 27.2 ± 2.5 | 0.83 |
| Vdss (mL) | 10.8 ± 1.2 | 10.0 ± 2.0 | 0.93 |
Data are presented as mean ± SE (n=6-8).
There are no significant differences between genotypes in the pharmacokinetic parameters of cefadroxil after intravenous bolus doses of drug.
Tissue Distribution of Oral Cefadroxil
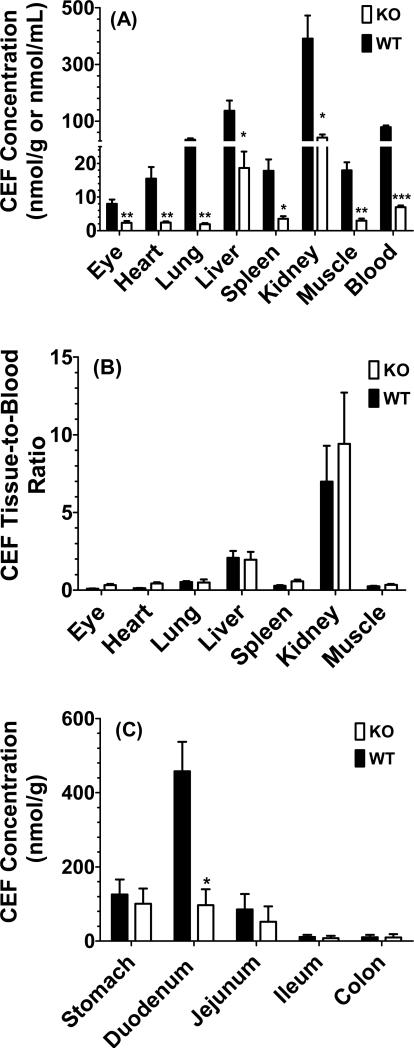
A tissue distribution study of cefadroxil was performed 20 min after oral dosing since this time best represented the Tmax of drug in wild-type mice. As shown in Fig. 5 (panel A), there were significant differences between genotypes in the eye, heart, lung, liver, spleen, kidney, muscle and blood. However, when these same tissues were corrected for differences in blood concentration, which was 12-fold higher in wild-type mice as compared to PepT1 knockout animals, the tissue-to-blood ratios were not different for all seven tissues (Fig. 5, panel B). The regional distribution of cefadroxil was also examined along the gastrointestinal tract after oral dosing and, as demonstrated in Fig. 5 (panel C), the largest concentrations of drug were observed in the duodenum with lower levels in the stomach and jejunum, and very low levels in the ileum and colon when sampled 20 min after dosing. In this analysis, differences between the two genotypes were only observed for the duodenum in which cefadroxil concentrations were, on average, about 4.5-fold higher in wild-type mice.
Fig. 5.
Tissue distribution (A) and tissue-to-blood concentration ratios (B) of [3H]cefadroxil (CEF) in peripheral tissues, and tissue distribution of [3H]cefadroxil (CEF) in gastrointestinal tract (C) of wild-type (WT) and PepT1 knockout (KO) mice 20 min after 178 nmol/g oral doses of drug. Data are presented as mean ± SE (n=6). *p < 0.05, **p < 0.01 and ***p < 0.001, as compared to wild-type mice.
DISCUSSION
This study presents new findings on the in vivo intestinal absorption, disposition and tissue distribution of cefadroxil in wild-type and PepT1 knockout mice after escalating oral doses. We report for the first time that: 1) PepT1 deletion reduced the systemic exposure of cefadroxil by 90% after oral administration of drug; 2) the Cmax of cefadroxil was reduced by 15- to 20-fold in PepT1 knockout mice; 3) there was “apparent” dose linearity in the AUC and Cmax of cefadroxil for both genotypes over the 44.5-356 nmol/g oral dose range; 4) the systemic disposition of intravenously administered cefadroxil was unchanged by PepT1 ablation; and 6) PepT1 had no effect on the peripheral tissue distribution of cefadroxil after oral dosing once adjusted for differences in the blood concentrations of drug perfusing these tissues; in contrast, there was a difference in duodenal concentrations of cefadroxil between the two genotypes 20 min after dosing.
The in vivo reduction (by 90%) in systemic exposure of cefadroxil after oral dosing in PepT1 knockout mice was in excellent agreement with previous results from our laboratory during in situ intestinal perfusions of cefadroxil in mice (23). This finding was not that surprising because of the abundant expression of PepT1 protein in the duodenal, jejunal and ileal, but not colonic, segments of the intestines (26). The linear oral absorption profile of cefadroxil demonstrated a lack of intestinal PepT1 saturation that was also consistent with a dose escalation study of glycylsarcosine in wild-type and PepT1 knockout mice (27). It should be appreciated that the oral doses of cefadroxil administered to mice (i.e., 44.5 to 356 nmol/g) were representative of the doses used clinically in 70 kg patients (i.e., 0.125 to 1.0 g) and resulted in peak plasma concentrations of 37 to 300 μM in wild-type animals, values also observed in humans (10,21,28). Given a stomach fluid volume of 0.4 mL (29), a 20 g mouse would have small intestinal concentrations approximating 18 mM, values in excess of cefadroxil's Km of 2-4 mM as determined by in situ jejunal perfusions in wild-type mice (23). Thus, the dose-proportionality observed for cefadroxil was an unexpected finding. Although it is possible that the small intestine's residual length and residence times might compensate for reduced absorption at higher doses, this scenario is unlikely since a more distal absorption of cefadroxil should be accompanied by longer Tmax values. Alternatively, if the Km value of cefadroxil in vivo was larger than the value estimated by in situ perfusions, then dose-linearity could be preserved at the doses tested in this study.
The short Tmax of cefadroxil in wild-type mice (i.e., 20 min) suggested that the absorption of this drug was quite fast and occurred mostly in the upper portion of the intestinal tract. This belief was supported by the tissue distribution studies (Fig. 5, panel C) in which the concentration of cefadroxil in duodenum was several-fold higher than that of other tissues. On the other hand, the shallow accumulation of cefadroxil in plasma and uniform drug concentrations over 1-2 hr after oral dosing in PepT1 knockout mice suggested that drug was being absorbed slowly throughout the entire length of the small intestine. This phenomenon was also observed following 25 nmol/g oral doses of the antiviral prodrug valacyclovir in wild-type and PepT1 knockout mice, in which Tmax values of 25 and 70 min, respectively, were reported (30). There were no differences in small intestinal transit between genotypes, as shown before using a charcoal meal (31) and, therefore, this potential confounding factor could be ruled out.
The absorption and disposition of cefadroxil are complex in that capacity-limited kinetics has been reported in the literature following both oral and intravenous administrations of drug in which transporters were involved in its intestinal uptake, renal tubular secretion and renal tubular reabsorption. For example, studies have reported nonlinear intestinal absorption kinetics of cefadroxil following in situ jejunal perfusions in rat (20), a reduced absorption rate constant following increasing oral doses of cefadroxil in human (21), a saturable active tubular secretion of cefadroxil in human resulting in non-proportional increases in AUC following increasing oral doses of drug (28), and a saturable active tubular reabsorption of cefadroxil in human (21) and rat (32) resulting in non-proportional decreases in AUC in the latter study following both oral and intravenous doses of drug. In contrast, other studies have reported dose-proportional increases in the AUC and Cmax of cefadroxil in human during 250-1500 mg (33) and 250-1000 mg (34) oral doses of drug. These studies illustrate the conflicting results observed in cefadroxil pharmacokinetics and may reflect the varying extent to which saturation in intestinal absorption, renal tubular secretion and/or renal tubular reabsorption processes can change the directionality of drug exposure. Thus, as reported before in wild-type and PepT2 null mice (22), the concentration-dependent saturation of renal tubular secretion by OATs and renal tubular reabsorption by PepT2 can affect the systemic exposure of cefadroxil in opposite directions.
In the present study, we observed dose-proportional increases in the AUC0-120 and Cmax of cefadroxil in mice at oral doses (i.e., 44.5 to 356 nmol/g) that resulted in plasma concentrations of antibiotic that were clinically relevant in human. Based on this observation, we concluded that the intestinal absorption of cefadroxil by PepT1 was not capacity-limited (or nonlinear). Because AUC/Dose = F/CL, it is also possible that changes in bioavailability (F) and systemic clearance (CL), of the same magnitude and direction, can result in no change in the dose-normalized value of AUC. This scenario is unlikely, though, since changes in clearance would also affect the Tmax of cefadroxil in wild-type mice, which was not observed.
No change was observed between wild-type and PepT1 knockout mice in the peripheral tissue distribution of cefadroxil once normalized for the different blood concentrations of drug perfusing that tissue (Fig. 5, panel B). Thus, once cefadroxil was absorbed into the systemic circulation, PepT1 expression outside the intestines had no significant effect on the extent of drug distribution in the body. This finding was supported by our results following the intravenous administration of cefadroxil in which drug disposition was virtually identical between genotypes (Fig. 4). Moreover, the results corroborated our previous oral and intravenous dosing studies with 10 nmol/g glycylsarcosine in wild-type and PepT1 knockout mice (27). Since renally expressed PepT1 only accounted for 5% and 14% of the tubular reabsorption of cefadroxil (22) and glycylsarcosine (35) in mice, respectively, its absence would not be expected to markedly change the systemic pharmacokinetics of either substrate. In contrast, there were significant differences between wild-type and PepT1 knockout mice in the concentrations of GlySar in ileum and colon (27), a difference not observed with cefadroxil in the current study. We believe the disparity between studies might reflect differences in sampling times in which tissues were obtained at 1 hour for GlySar and at 20 min for cefadroxil, the latter being a time in which drug would be confined to the upper gastrointestinal tract (31).
CONCLUSIONS
In conclusion, the results from these studies are unique in characterizing, for the first time, the in vivo pharmacokinetics of a therapeutically relevant PepT1 substrate, the β-lactam antibiotic, cefadroxil, in wild-type and PepT1 knockout mice after oral dose escalation. In particular, we demonstrate the high-capacity nonsaturable properties of intestinal PepT1 as judged by the dose proportional increase in systemic exposure of cefadroxil with increasing oral doses of 44.5 to 356 nmol/g. Moreover, the AUC0-120 and Cmax values are substantially reduced, by about 10-fold and 17.5-fold, respectively, in PepT1 knockout mice as compared to wild-type animals, and the Tmax increased by 3-fold. The findings demonstrate convincingly the critical role of intestinal PepT1 in both the rate and extent of oral absorption for this aminocephalosporin drug and potentially others in its class.
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant R01-GM035498] (to D.E.S.).
REFERENCES
- 1.Brandsch M, Knutter I, Bosse-Doenecke E. Pharmaceutical and pharmacological importance of peptide transporters. J Pharm Pharmacol. 2008;60:543–85. doi: 10.1211/jpp.60.5.0002. [DOI] [PubMed] [Google Scholar]
- 2.Rubio-Aliaga I, Daniel H. Peptide transporters and their roles in physiological processes and drug disposition. Xenobiotica. 2008;38:1022–42. doi: 10.1080/00498250701875254. [DOI] [PubMed] [Google Scholar]
- 3.Smith DE, Clemençon B, Hediger MA. Proton-coupled oligopeptide transporter family SLC15: Physiological, pharmacological and pathological implications. Mol Aspects Med. 2013;34:323–36. doi: 10.1016/j.mam.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamashita T, Shimada S, Guo W, Sato K, Kohmura E, Hayakawa T, Takagi T, Tohyama M. Cloning and functional expression of a brain peptide/histidine transporter. J Biol Chem. 1997;272:10205–11. doi: 10.1074/jbc.272.15.10205. [DOI] [PubMed] [Google Scholar]
- 5.Sakata K, Yamashita T, Maeda M, Moriyama Y, Shimada S, Tohyama M. Cloning of a lymphatic peptide/histidine transporter. Biochem J. 2001;356(Pt 1):53–60. doi: 10.1042/0264-6021:3560053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han H, de Vrueh RL, Rhie JK, Covitz KM, Smith PL, Lee CP, Oh DM, Sadée W, Amidon GL. 5'-Amino acid esters of antiviral nucleosides, acyclovir, and AZT are absorbed by the intestinal PEPT1 peptide transporter. Pharm Res. 1998;15:1154–9. doi: 10.1023/a:1011919319810. [DOI] [PubMed] [Google Scholar]
- 7.Bretschneider B, Brandsch M, Neubert R. Intestinal transport of beta-lactam antibiotics: analysis of the affinity at the H+/peptide symporter (PEPT1), the uptake into Caco-2 cell monolayers and the transepithelial flux. Pharm Res. 1999;16:55–61. doi: 10.1023/a:1018814627484. [DOI] [PubMed] [Google Scholar]
- 8.Rubio-Aliaga I, Daniel H. Mammalian peptide transporters as targets for drug delivery. Trends Pharmacol Sci. 2002;23:434–40. doi: 10.1016/s0165-6147(02)02072-2. [DOI] [PubMed] [Google Scholar]
- 9.Buck RE, Price KE. Cefadroxil, a new broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1977;11:324–30. doi: 10.1128/aac.11.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanrisever B, Santella PJ. Cefadroxil: A review of its antibacterial, pharmacokinetic and therapeutic properties in comparison with cephalexin and cephradine. Drugs. 1986;32(Suppl 3):1–16. doi: 10.2165/00003495-198600323-00003. [DOI] [PubMed] [Google Scholar]
- 11.Marino EL, Dominguez-Gil A, Muriel C. Influence of dosage form and administration route on the pharmacokinetic parameters of cefadroxil. Int J Clin Pharmacol Ther Toxicol. 1982;20:73–7. [PubMed] [Google Scholar]
- 12.Tsuji A, Nakashima E, Kagami I, Yamana T. Intestinal absorption mechanism of amphoteric beta-lactam antibiotics I: Comparative absorption and evidence for saturable transport of amino-beta-lactam antibiotics by in situ rat small intestine. J Pharm Sci. 1981;70:768–72. doi: 10.1002/jps.2600700714. [DOI] [PubMed] [Google Scholar]
- 13.de Waart DR, van de Wetering K, Kunne C, Duijst S, Paulusma CC, Oude Elferink RP. Oral availability of cefadroxil depends on ABCC3 and ABCC4. Drug Metab Dispos. 2012;40:515–21. doi: 10.1124/dmd.111.041731. [DOI] [PubMed] [Google Scholar]
- 14.Shen H, Smith DE, Yang T, Huang YG, Schnermann JB, Brosius FC., 3rd Localization of PEPT1 and PEPT2 proton-coupled oligopeptide transporter mRNA and protein in rat kidney. Am J Physiol. 1999;276:F658–65. doi: 10.1152/ajprenal.1999.276.5.F658. [DOI] [PubMed] [Google Scholar]
- 15.Shen H, Smith DE, Keep RF, Brosius FC., 3rd Immunolocalization of the proton-coupled oligopeptide transporter PEPT2 in developing rat brain. Mol Pharm. 2004;1:248–56. doi: 10.1021/mp049944b. [DOI] [PubMed] [Google Scholar]
- 16.Scheffer GL, Kool M, de Haas M, de Vree JM, Pijnenborg AC, Bosman DK, Elferink RP, van der Valk P, Borst P, Scheper RJ. Tissue distribution and induction of human multidrug resistant protein 3. Lab Invest. 2002;82:193–201. doi: 10.1038/labinvest.3780411. [DOI] [PubMed] [Google Scholar]
- 17.Ming X, Thakker DR. Role of basolateral efflux transporter MRP4 in the intestinal absorption of the antiviral drug adefovir dipivoxil. Biochem Pharmacol. 2010;79:455–62. doi: 10.1016/j.bcp.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 18.Russel FG, Koenderink JB, Masereeuw R. Multidrug resistance protein 4 (MRP4/ABCC4): a versatile efflux transporter for drugs and signaling molecules. Trends Pharmacol Sci. 2008;29:200–7. doi: 10.1016/j.tips.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 19.International Transporter Consortium. Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–36. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sánchez-Picó A, Peris-Ribera JE, Toledano C, Torres-Molina F, Casabó VG, Martín-Villodre A, Plá-Delfina JM. Non-linear intestinal absorption kinetics of cefadroxil in the rat. J Pharm Pharmacol. 1989;41:179–85. doi: 10.1111/j.2042-7158.1989.tb06425.x. [DOI] [PubMed] [Google Scholar]
- 21.Garrigues TM, Martin U, Peris-Ribera JE, Prescott LF. Dose-dependent absorption and elimination of cefadroxil in man. Eur J Clin Pharmacol. 1991;41:179–83. doi: 10.1007/BF00265914. [DOI] [PubMed] [Google Scholar]
- 22.Shen H, Ocheltree SM, Hu Y, Keep RF, Smith DE. Impact of genetic knockout of PEPT2 on cefadroxil pharmacokinetics, renal tubular reabsorption, and brain penetration in mice. Drug Metab Dispos. 2007;35:1209–16. doi: 10.1124/dmd.107.015263. [DOI] [PubMed] [Google Scholar]
- 23.Posada MM, Smith DE. Relevance of PepT1 in the intestinal permeability and oral absorption of cefadroxil. Pharm Res. 2013;30:1017–25. doi: 10.1007/s11095-012-0937-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu Y, Smith DE, Ma K, Jappar D, Thomas W, Hillgren KM. Targeted disruption of peptide transporter Pept1 gene in mice significantly reduces dipeptide absorption in intestine. Mol Pharm. 2008;5:1122–30. doi: 10.1021/mp8001655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rowland M, Tozer TN. Clinical pharmacokinetics and pharmacodynamics: concepts and applications. Wolters Kluwer; Philadelphia: 2011. [Google Scholar]
- 26.Jappar D, Wu SP, Hu Y, Smith DE. Significance and regional dependency of peptide transporter (PEPT) 1 in the intestinal permeability of glycylsarcosine: in situ single-pass perfusion studies in wild-type and Pept1 knockout mice. Drug Metab Dispos. 2010;38:1740–6. doi: 10.1124/dmd.110.034025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jappar D, Hu Y, Smith DE. Effect of dose escalation on the in vivo oral absorption and disposition of glycylsarcosine in wild-type and Pept1 knockout mice. Drug Metab Dispos. 2011;39:2250–7. doi: 10.1124/dmd.111.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.La Rosa F, Ripa S, Prenna M, Ghezzi A, Pfeffer M. Pharmacokinetics of cefadroxil after oral administration in humans. Antimicrob Agents Chemother. 1982;21:320–2. doi: 10.1128/aac.21.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McConnell EL, Basit AW, Murdan S. Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in-vivo experiments. J Pharm Pharmacol. 2008;60:63–70. doi: 10.1211/jpp.60.1.0008. [DOI] [PubMed] [Google Scholar]
- 30.Yang B, Smith DE. Significance of peptide transporter 1 in the intestinal permeability of valacyclovir in wild-type and PepT1 knockout mice. Drug Metab Dispos. 2013;41:608–14. doi: 10.1124/dmd.112.049239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma K, Hu Y, Smith DE. Influence of fed-fasted state on intestinal PEPT1 expression and in vivo pharmacokinetics of glycylsarcosine in wild-type and Pept1 knockout mice. Pharm Res. 2012;29:535–45. doi: 10.1007/s11095-011-0580-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.García-Carbonell MC, Granero L, Torres-Molina F, Aristorena JC, Chesa-Jiménez J, Plá-Delfina JM, Peris-Ribera JE. Nonlinear pharmacokinetics of cefadroxil in the rat. Drug Metab Dispos. 1993;21:215–7. [PubMed] [Google Scholar]
- 33.Mariño EL, Dominguez-Gil A. Influence of dose on the pharmacokinetics of cefadroxil. Eur J Clin Pharmacol. 1980;18:505–9. doi: 10.1007/BF00874664. [DOI] [PubMed] [Google Scholar]
- 34.Barbhaiya RH. A pharmacokinetic comparison of cefadroxil and cephalexin after administration of 250, 500 and 1000 mg solution doses. Biopharm Drug Dispos. 1996;17:319–30. doi: 10.1002/(SICI)1099-081X(199605)17:4<319::AID-BDD957>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 35.Ocheltree SM, Shen H, Hu Y, Keep RF, Smith DE. Role and relevance of peptide transporter 2 (PEPT2) in the kidney and choroid plexus: in vivo studies with glycylsarcosine in wild-type and PEPT2 knockout mice. J Pharmacol Exp Ther. 2005;315:240–7. doi: 10.1124/jpet.105.089359. [DOI] [PubMed] [Google Scholar]