Abstract
Microelectrode recordings of cortical activity in primates performing working memory tasks reveal some cortical neurons exhibiting sustained or graded persistent elevations in firing rate during the period in which sensory information is actively maintained in short-term memory. These neurons are called “memory cells”. Imaging and transcranial magnetic stimulation studies indicate that memory cells may arise from distributed cortical networks. Depending on the sensory modality of the memorandum in working memory tasks, neurons exhibiting memory-correlated patterns of firing have been detected in different association cortices including prefrontal cortex, and primary sensory cortices as well.
Here we elaborate on neurophysiological experiments that lead to our understanding of the neuromechanisms of working memory, and mainly discuss findings on widely distributed cortical networks involved in tactile working memory.
1. Distributed cortical network of working memory in primates
Short-term memory functions were first hypothesized to be related to the cortical surface of the frontal lobe by early lesion studies (Jacobsen, 1936; Milner, 1963). This hypothesis was strengthened when it was discovered that in the principal sulcus (area 46), there were cells that continued to fire during the delay period of a delayed-response task (Fuster and Alexander, 1971). In the task, a trial began with the cue period when a piece of food was placed in one of two wells in front of the monkey. These two wells were then covered with two identical test objects. During the subsequent delay period (memorization period), the monkey's view of objects was blocked. At the end of the delay, the monkey could see the objects again and was allowed to remove only one of them. Memory cells were characterized by sustained elevated discharge (significantly above the spontaneous inter-trial baseline) during the whole or part of the forced delay period between the cue and the motor response. Mock trials failed to elicit delay activation, and when the animal no longer needed to memorize the cue, the discharge of the cell returned to the inter-trial baseline level. Short-term memory cells were also investigated by another pioneer study (Kubota and Niki, 1971) in which Kubota and Niki recorded single units in the prefrontal cortex (PFC) when monkeys performed a delay-alternation task. These two findings give the first insight into the neuronal basis of short-term memory.
The term “working memory” evolved from the earlier concept of short-term memory, proposed by the psychologist Baddeley and Hitch in 1974 (Baddeley and Hitch, 1974). In their theory, there are three components in the model of working memory: an attentional control system and two storage systems for phonological and visual-spatial information. In 1980s, the concept of working memory was first introduced into neuroscience by a series of outstanding electrophysiological studies (Goldman-Rakic, 1995). Among these studies, Funahashi and Goldman-Rakic (Funahashi et al., 1989) developed an oculomotor delay-response task in which the monkey was trained to make a saccade to a remembered target location at the end of the delay period, and found that PFC neurons exhibited directional delay-period activity, as revealed in single-unit recordings. Based on these results, Funahashi and his colleagues proposed that those PFC neurons have mnemonic receptive fields (memory fields) regarding visual cues in the visual field (Funahashi et al., 1989; Rainer et al., 1998). Memory cells in the PFC have also been reported by other studies on monkeys (Miller et al., 1996; Niki, 1974; Rao et al., 1997; Romo et al., 1999). From the results of those studies, it appears that other than those cells showing sustained firing during the whole or part of the delay, in general there exist two other types of delay cells in PFC (Fuster, 1995). One is the “sensory-coupled” cue cell, the discharge of which tends to diminish from the peak to the baseline level through the delay period. The other is the “motor-coupled" cell; its discharge tends to increase as time for expected movement approaches. These two types of cells may participate in two complementary processes: sensory-coupled cells are involved in the retention of sensory information, and motor-coupled cells engage in preparation for motor action responding to that information. It is apparent that PFC plays a critical role in temporal organization of behavior by integrating motor action with recent sensory information through cooperation of its two basic cognitive functions: short-term memory and preparatory set (Fuster, 1997, 2001). In PFC, memory cells appear to be components in neuronal networks that encode a large variety of sensory stimuli associated with impending action. These similar types of cells have also been found in inferior temporal cortex by Miyashita and his colleagues, in which they termed them as “cue-holding” and “target-recall” cells(Takeda et al., 2005).
Over the last decade, great progress has been achieved in understanding the organization of working memory networks and the functional specialization of brain areas that constitute the networks. Neurons with memory-related responses have been reported in multiple brain regions, such as the prefrontal cortex (D'Esposito et al., 1995; D'Esposito et al., 1999; D'Esposito et al., 2000; Fuster, 1973; Fuster and Alexander, 1971; Hernandez et al., 2002; Miller et al., 1996; Petrides et al., 1995; Romo et al., 1999), the inferior temporal cortex (Fiebach et al., 2006; Fuster and Jervey, 1982; Miller et al., 1993; Miyashita and Chang, 1988) and the posterior parietal cortex (PPC) (Champod and Petrides, 2007; Constantinidis and Steinmetz, 1996; Curtis et al., 2004; Joelving et al., 2007; Koch and Fuster, 1989; Pierrot-Deseilligny et al., 2005; Zhou et al., 2007). These distributed cortical areas play a critical role in working memory networks. Especially, neural synchronization between PFC and PPC has been proposed to be the representation of task-specific information in visual working memory (Salazar et al., 2012), and other cognitive processes (Buschman and Miller, 2007; Pesaran et al., 2008). Recently, there is increasing evidence that elemental sensory dimensions, such as tactile information in the somatosensory system (Hernandez et al., 2000; Zhou and Fuster, 1996), are stored by segregated feature-selective systems that include not only the associative cortex, but also the sensory cortex that carries out early-stage processing(Gottlieb et al., 1989; Super et al., 2001). Neural circuits in these cortical areas seem to have a dual function: the precise sensory encoding and the short-term storage of the encoded information(Pasternak and Greenlee, 2005).
2. Tactile working memory in monkeys
Tactile working memory in early cortical somatosensory areas
Memory cells have been further revealed in primary sensory cortices(Super et al., 2001; Zhou and Fuster, 1996). Findings of memory cells in primary sensory cortices indicate that the same neurons are involved in both sensory processing and temporary storage of sensory information, and agree with the idea that memory is stored as changes in the same neural systems that participate in perception, analysis, and processing of sensory information (Squire, 1986; Squire and Zola-Morgan, 1991; Thompson, 1986). Our studies(Zhou and Fuster, 1996) have shown that memory cells exist in the primary somatosensory cortex (SI) of monkeys performing a tactile unimodal delayed matching-to-sample task (Figure 1 & 2). As the neural correlate of the sample stimulus, the delay activity is possibly the neural representation of stimulus information in cortical memory networks, and carries this information for the animal's corresponding behavioral action. Recently, our studies (Wang et al., 2012a) have further shown that this persistent delay activity appears to be an intrinsic property of SI neurons and suggests that there exist neural networks in SI subserving tactile memory. SI cortex may also participate in decision-making during the haptic choice in the haptic delay task (Wang et al., 2012b). The study has indicated that SI cells (Figure 1&3) show the significant differential neural activity only when the monkey has to make a choice between two different haptic objects, and such differential activity diminishes significantly in incorrect-response trials.
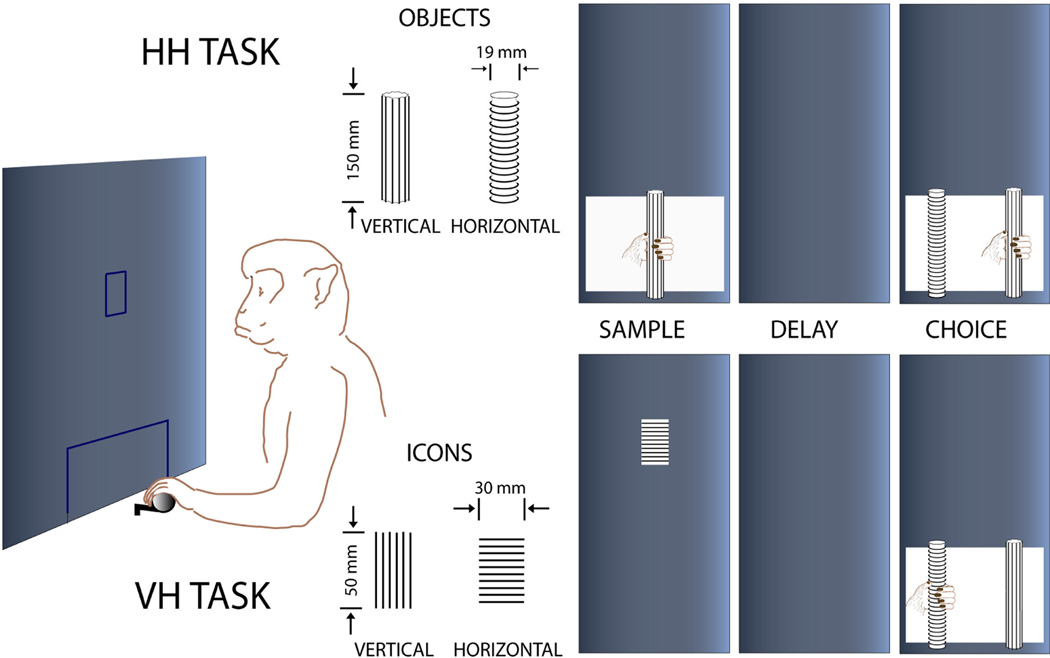
Figure 1.
Diagram of two working memory tasks. In a trial of the hapti-chaptic (HH) task (top), the monkey touches the tactile sample (without seeing it), one of two objects (rods) differing by a surface feature (orientation of parallel ridges, vertical vs. horizontal). At the end of the delay period that follows the sample touch, the animal is presented with the two rods simultaneously and must choose by touching and pulling the one that matches the sample to get a certain amount of fluid as a reward. In a trial of the visual-haptic (VH) task (bottom), the animal first views an icon of vertical or horizontal stripes, and then at the end of the delay, the animal makes a behavioral choice by touching and pulling one of the two rods with ridges oriented in the same direction as the stripes of the sample icon. (The figure is adapted from Zhou et al. 2007, Cerebral Cortex)
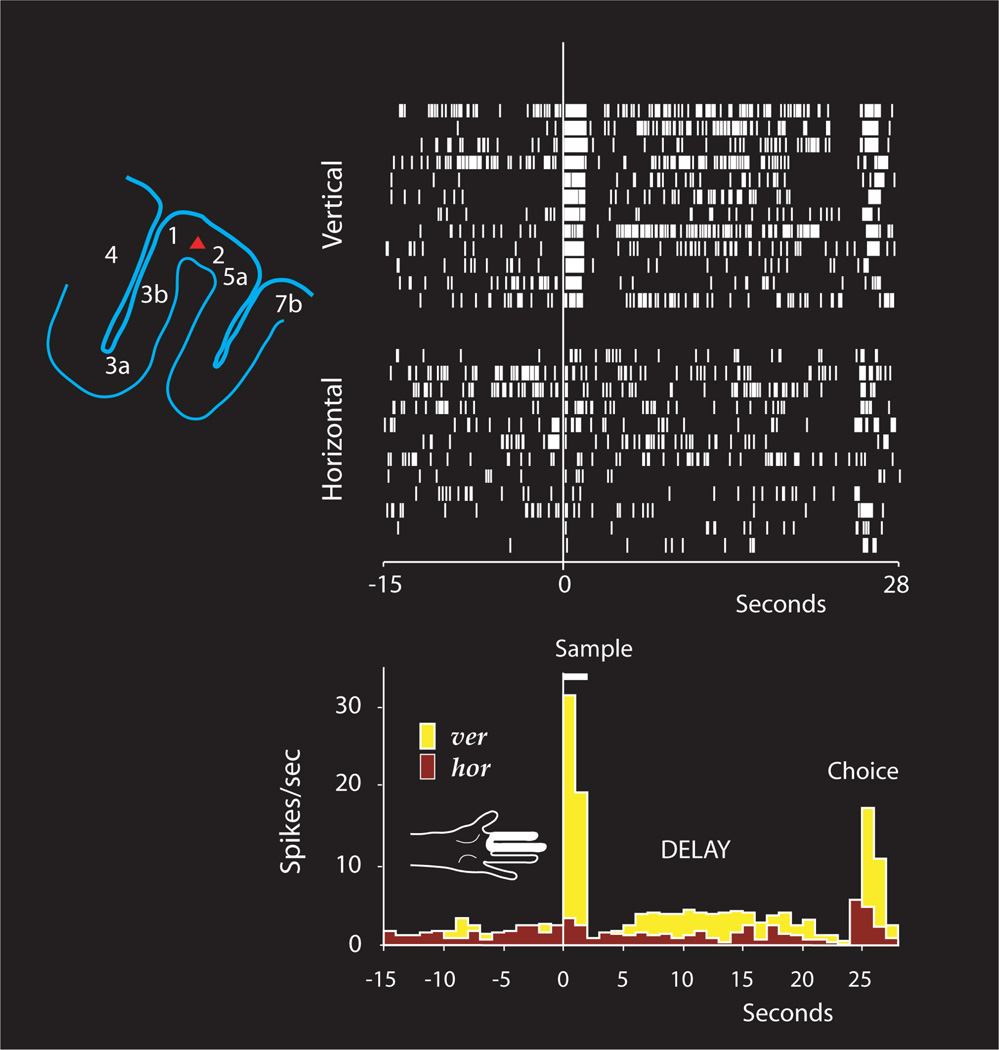
Figure 2.
Spike rasters and histograms from a sample- and delay-differential unit in SI cortex through horizontal (hor)- and vertical (ver)-sample trials. The unit's receptive field is marked (white) on the hand diagram, the unit's position (red triangle) on the outlined parietal section on upper left. (The figure is adapted from Zhou et al. 2007, Cerebral Cortex)
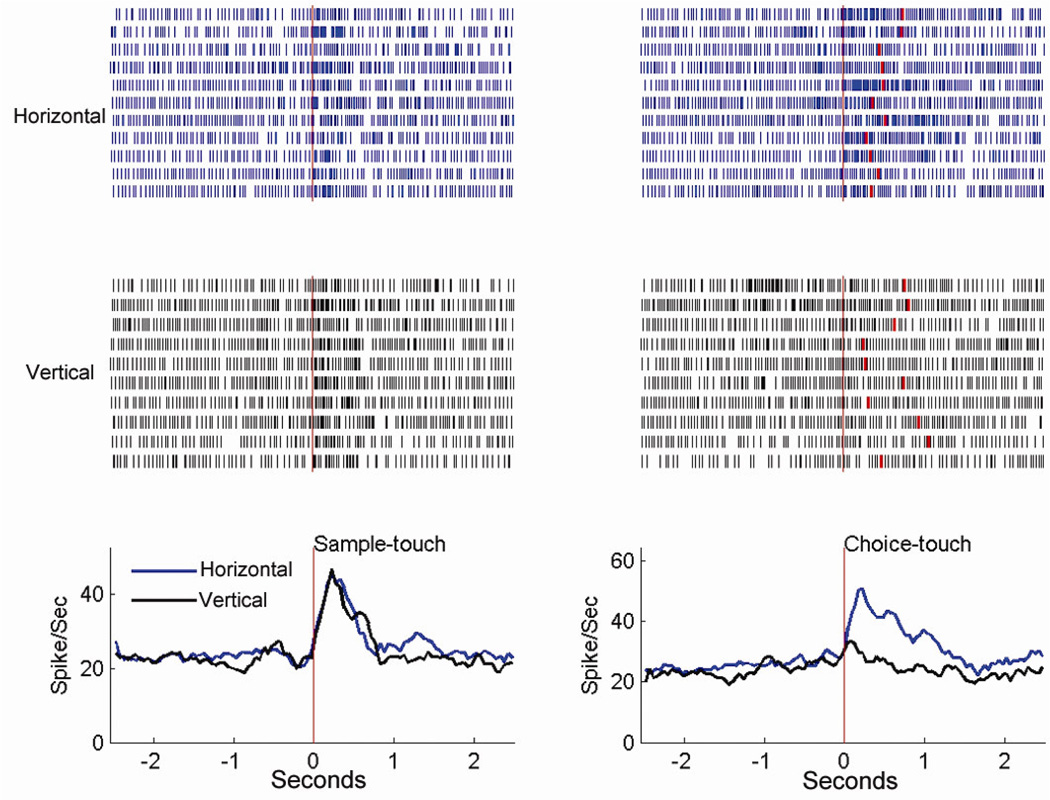
Figure 3.
Rasters and perievent histograms (bin size = 50 msec) of a cell recorded in the hand area of SI cortex in the HH task, showing the activity in both sample and choice periods. Left: The time-locking event for histograms is the first touch of the sample. The average firing rate (non-differential) for both rods in the sample period increases significantly from the baseline level. Right: The time-locking event for histograms is the onset of the last touch prior to the pull of the chosen rod. The red raster in each trial indicates the onset of the choice (the pull). The firing rate of the cell is significantly higher in touch of the horizontal rod (p < 0.001). This cell shows the choice-only differential activity. (The figure is adapted from Wang et al. 2012, Journal of Cognitive Neuroscience)
In the cortical dynamics of the perception-action cycle (Fuster, 2001), interactions are supposed to occur between cortical stations at various levels of the two hierarchies, including those between two lowest stations, MI (primary motor cortex) and SI. Evidence from past studies (Jiang et al., 1991; Lebedev et al., 1994; Nelson and Douglas, 1989) indicates that cortical neuronal signals may arrive in SI prior to movement onset. In a recent study, Peterson and his colleagues (Matyas et al., 2010) have found that SI directly drives whisker retraction, providing a rapid negative feedback signal for sensorimotor integration. Those studies indicate the existence of dynamic interactions between SI and MI. The close anatomical relationship between those two cortices (Jones et al., 1979; Stepniewska et al., 1993) may provide a neural basis for the timely reciprocal information exchange from one to the other during active tactile exploration. This exchange may play an essential role in the matching between a tactile memory trace and the present haptic stimulus represented by neural networks in SI cortex (Zhou and Fuster, 1996). Further, this timely information exchange may be critical for fine hand movements during tactile exploration leading to proper decisions(Wang et al., 2012b).
A recent functional magnetic resonance imaging (fMRI) study (Kaas et al., 2012) has specifically shown that the effect of tactile working memory load can be observed in the hand- and modality-specific regions, BA2 (SI) and the secondary somatosensory cortex (SII), indicating the involvement of these regions in tactile encoding and maintenance of fine surface textures. The involvement of SI in the short-term retention of tactile information has also been shown in a human working memory study (Harris et al., 2002). The study has shown that the SI cortex temporarily retains (< 1 sec) information of a tactile vibration frequency in a retention period of a task for later comparison between two frequencies. Performance of the task is significantly disrupted when a pulse of transcranial magnetic stimulation (TMS) is delivered early in the retention period to SI cortex contralateral to the tactile stimulus.
In addition, SI cells in monkeys have been observed to respond to a visual stimulus that has been associated with a tactile stimulus in a visuo-tactile crossmodal task, and they may also be involved in retention of tactile information that visual stimulus represents for a later tactile choice (Zhou and Fuster, 1997, 2000). In those studies, the firing rate of certain SI cells in the delay period of the task depends on the visual stimulus presented before, and some other cells show similar responses to the visual stimulus and its corresponding tactile stimulus (Figure 4). The presence of such cross-modal cells in SI suggests that associated non-tactile stimuli activate SI cells via cross-modal cortical associations shaped through the task-specific animal training, and therefore the modality-specific primary sensory cortex contributes to associations between stimuli from different sensory modalities. This notion has also been suggested by a human TMS study (Bolognini et al., 2011), in which authors have found that rTMS (repetitive TMS) over SI selectively impairs subject performance for interpreting whether a contralateral visual tactile stimulus contains a tactile event.
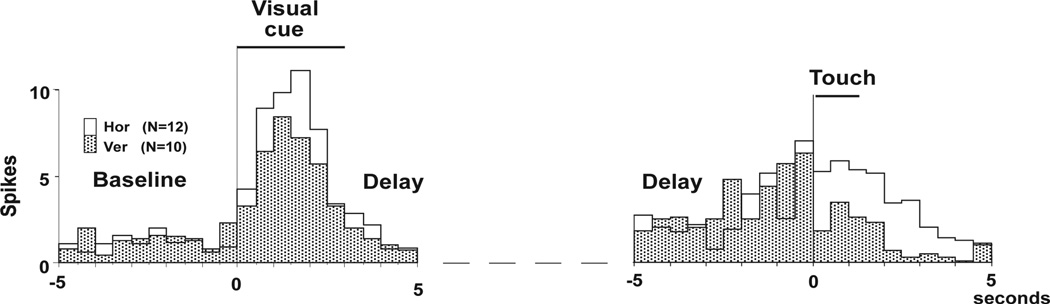
Figure 4.
Average frequency histograms (bin size 500 ms) from an SI cell showing differential activity in two periods, cue and choice. The locking event for the left histograms is the onset of the visual cue, and for the right histograms it is the first contact of the matching object. The middle part of the delay is omitted from the histograms (dashed line). The cell favors the horizontal ridges at the choice (P < 0.01). Accordingly, it favors the horizontal visual cue (P < 0.05). (The figure is adapted from Zhou and Fuster 2000, PNAS)
SII cortex has reciprocal connections with all areas of SI and via insular projections to frontal and temporal limbic structures, and is probably a higher-order station in the somatosensory processing hierarchy that proceeds from SI and SII to other cortical areas related to tactile learning and memory (Burton et al., 1995; Cipolloni and Pandya, 1999; Friedman et al., 1986; Kaas, 1995; Mesulam and Mufson, 1982; Murray and Mishkin, 1984). Results of a recent study have shown that in performance of a tactile working memory task, cells in SII of monkeys are involved in retention of information of a vibration frequency (base stimulus) for a later comparison with another vibration frequency (comparison stimulus) (Salinas et al., 2000). Many SII cells in this study show stimulus frequency-specific firing modulation during the period between the base and the comparison stimuli. When the animal does not need to remember the stimulus, the frequency-specific modulation is diminished significantly.
From the studies discussed above, it is apparent that in working memory, sensory information is temporally maintained in widely distributed cortical neural networks including those in sensory cortical areas, the sensory modality-specific cortical stage of a sensory system.
Tactile working memory in association areas (PPC and PFC)
Memory cells have been recorded in PPC of monkeys during performance of a haptic delayed matching-to-sample task (Koch and Fuster, 1989). The discharge of some cells is elevated through most or all of the delay period, in some cases selectively with regard to tactile samples (cube or sphere) perceived by active touch. These memory cells are mostly located in area 5 of parietal association cortex. Tactile memory cells are commonly excited not only by the touch or task-required retention of a tactile cue but also by the movement of arm and hand while the animal reaches and palpates that cue. These movement-related tactile memory cells may function in both sensorimotor integration and active tactile short-term memory. The neural activity related to maintenance of tactile information about the object’s surface texture has also been observed in the superior parietal lobule in an fMRI study(Kaas et al., 2012), in which subjects are asked to answer whether a probe stimulus (sandpaper) matches the memory array in a delay task.
In a study on PFC neural firing in working memory, Romo and his colleagues (Romo et al., 1999) have found that the firing rate of PFC neurons in the delay period is monotonically correlated with the vibration frequency of the tactile stimulus when a monkey performs a vibration frequency discrimination task. In this task, two vibrating stimuli are sequentially presented in each trial. The monkey has to memorize the frequency of the first stimulus during the delay and then make a behavioral choice at the end of the delay by comparing the frequency of the first stimulus with that of the second stimulus. The work indicates that monotonic encodings in PFC neurons are the basic representation of sensory magnitude continua in working memory, and are likely derived from inputs from cortical somatosensory areas (Romo and Salinas, 2003). This paper shows clear evidence of involvement of PFC neural networks in tactile working memory.
fMRI imaging studies have also demonstrated that PFC plays an important role in tactile working memory (Fiehler et al., 2008; Kaas et al., 2012; Kostopoulos et al., 2007; Preuschhof et al., 2006). A recent fMRI study (Kaas et al., 2007) has shown that haptic sensory traces, maintained in contralateral sensorimotor cortex, are transformed into more abstract hapticospatial representations in the early delay stages. Maintenance of these representations engages anterior PFC and parietal-occipital cortex.
Previous electrophysiological and imaging studies have shown that both dorsal and ventral parts of PFC are intensively involved in the neural processing of visual and auditory working memory(Bodner et al., 1996; Constantinidis and Procyk, 2004; D'Esposito et al., 2000; Miller et al., 1996; Quintana and Fuster, 1999; Romanski, 2007). However, the contribution of different prefrontal areas to tactile working memory is still unclear. Both human fMRI and primate electrophysiology studies have shown that the ventral-lateral prefrontal region is involved in the tactile (vibration) working memory (Kostopoulos et al., 2007; Romo et al., 1999). We have recently investigated whether the dorsal-lateral prefrontal cortex (area 46d/8) participates in haptic working memory. Cells have been recorded in the dorsal-lateral PFC in two monkeys performing a haptic-haptic unimodal working memory task (Figure 1). Persistent delay activity, preferring to either horizontal or vertical tactile information, has been observed (see Figure 5, unpublished data). This result indicates that the dorsal-lateral prefrontal cortex may possess information of the haptic stimuli selectively.
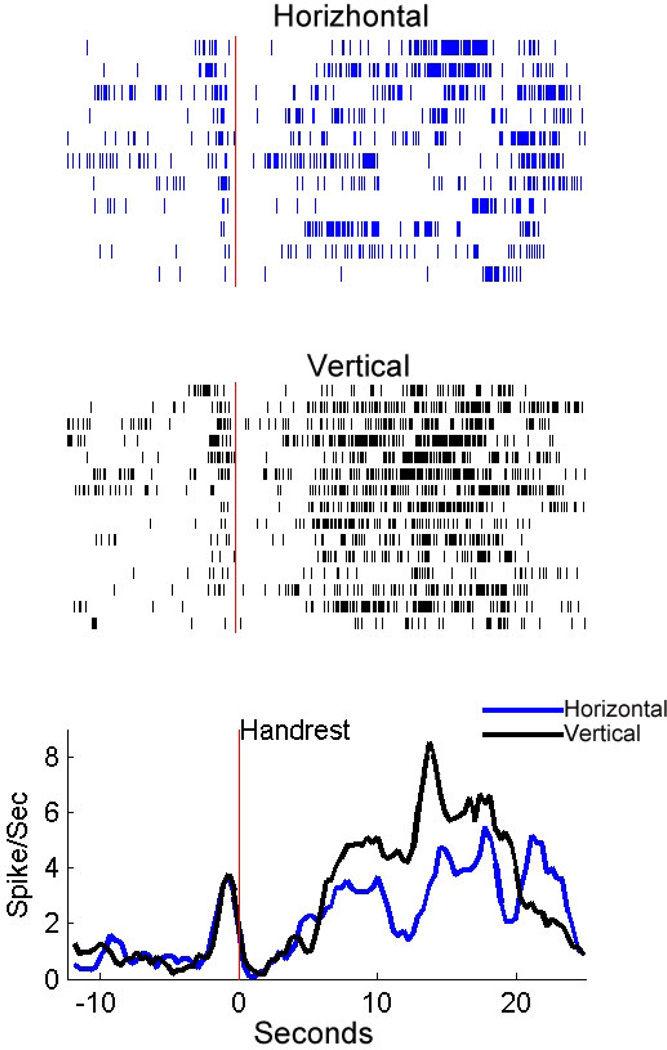
Figure 5.
The differential delay activity of a frontal cell in the HH task. Rasters and histograms are aligned to the beginning of the delay. Blue rasters and histograms indicate the neural activity in horizontal trials, and black ones, in vertical trials. Only correct trials are included.
In the somatosensory system, information is processed by SI, and relayed to SII and higher order association cortical areas, including areas 5 and 7b in the parietal lobe and the insular cortex. The lateral frontal cortical regions, including both ventral and dorsal parts, receive information through anatomical connections with parietal lobe regions and SII (Cavada and Goldman-Rakic, 1989; Mesulam and Mufson, 1982; Murray and Mishkin, 1984). We propose that the haptic information flows through different stations in the somatosensory cortical hierarchy up into dorsal lateral prefrontal cortex, and manipulated and integrated there for later action.
3. Conclusion
Working memory appears to engage widely distributed cortical areas including primary sensory cortices, although certain cortical areas may be more selectively activated depending on the sensory modality of the memorandum involved in the working memory task. In the somatosensory system, cortical areas involved in tactile working memory include not only association areas, such as prefrontal cortex and posterior parietal cortex, but also sensory areas, such as SI and SII--the modality-specific cortical stage of the system. This notion is supported by the recording of cortical activity in monkeys performing tactile unimodal and cross-modal working memory tasks and imaging studies in humans.
Due to the dual functions in SI (sensory encoding and short-term storage of sensory information), it seems necessary to understand the dynamics of the local neural circuit in this cortex, which subserves working memory. Multi-contact laminar electrode recording may provide a valuable tool in future research to investigate the circuit by exploring the laminar structures of SI cortex. The involvement of PFC in crossmodal working memory is also a potentially important topic as PFC has been thought to be the center for crossmodal transfer of information from one sensory modality to the other (Fuster et al., 2000)
Highlights.
Working memory cells may arise from distributed cortical networks.
Primary sensory cortex may have a dual function: sensory coding and short-term memory storage.
Dorsal-lateral prefrontal cortex may possess information of the haptic stimuli selectively.
Acknowledgment
This work was supported by the National Key Basic Research Program of China (973 Program No: 2013CB32950X), an NINDS Grant NS-44919 and a research fund from the M.I.N.D. Research Institute, California to Y. –D. Zhou. This work was also supported by the National Science Foundation of China 31200834 and Shanghai Science Foundation 12ZR1443100 to L-P. Wang.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baddeley AD, Hitch GJL. In: Working Memory The psychology of learning and motivation: advances in research and theory. B, editor. New York: Academic Press, New York; 1974. pp. 47–89. [Google Scholar]
- Bodner M, Kroger J, Fuster JM. Auditory memory cells in dorsolateral prefrontal cortex. Neuroreport. 1996;7(12):1905–1908. doi: 10.1097/00001756-199608120-00006. [DOI] [PubMed] [Google Scholar]
- Bolognini N, Rossetti A, Maravita A, Miniussi C. Seeing touch in the somatosensory cortex: a TMS study of the visual perception of touch. Hum Brain Mapp. 2011;32(12):2104–2114. doi: 10.1002/hbm.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Fabri M, Alloway K. Cortical areas within the lateral sulcus connected to cutaneous representations in areas 3b and 1: a revised interpretation of the second somatosensory area in macaque monkeys. The Journal of comparative neurology. 1995;355(4):539–562. doi: 10.1002/cne.903550405. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315(5820):1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. The Journal of comparative neurology. 1989;287(4):393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Champod AS, Petrides M. Dissociable roles of the posterior parietal and the prefrontal cortex in manipulation and monitoring processes. Proc Natl Acad Sci U S A. 2007;104(37):14837–14842. doi: 10.1073/pnas.0607101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolloni PB, Pandya DN. Cortical connections of the frontoparietal opercular areas in the rhesus monkey. The Journal of comparative neurology. 1999;403(4):431–458. [PubMed] [Google Scholar]
- Constantinidis C, Procyk E. The primate working memory networks. Cogn Affect Behav Neurosci. 2004;4(4):444–465. doi: 10.3758/cabn.4.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidis C, Steinmetz MA. Neuronal activity in posterior parietal area 7a during the delay periods of a spatial memory task. J Neurophysiol. 1996;76(2):1352–1355. doi: 10.1152/jn.1996.76.2.1352. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Rao VY, D'Esposito M. Maintenance of spatial and motor codes during oculomotor delayed response tasks. J Neurosci. 2004;24(16):3944–3952. doi: 10.1523/JNEUROSCI.5640-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;378(6554):279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Jonides J, Smith EE. The neural substrate and temporal dynamics of interference effects in working memory as revealed by event-related functional MRI. Proc Natl Acad Sci U S A. 1999;96(13):7514–7519. doi: 10.1073/pnas.96.13.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Exp Brain Res. 2000;133(1):3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Rissman J, D'Esposito M. Modulation of Inferotemporal Cortex Activation during Verbal Working Memory Maintenance. Neuron. 2006;51(2):251–261. doi: 10.1016/j.neuron.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiehler K, Burke M, Engel A, Bien S, Rosier F. Kinesthetic working memory and action control within the dorsal stream. Cereb Cortex. 2008;18(2):243–253. doi: 10.1093/cercor/bhm071. [DOI] [PubMed] [Google Scholar]
- Friedman DP, Murray EA, O'Neill JB, Mishkin M. Cortical connections of the somatosensory fields of the lateral sulcus of macaques: evidence for a corticolimbic pathway for touch. The Journal of comparative neurology. 1986;252(3):323–347. doi: 10.1002/cne.902520304. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1989;61(2):331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Unit activity in prefrontal cortex during delayed-response performance: neuronal correlates of transient memory. J Neurophysiol. 1973;36(1):61–78. doi: 10.1152/jn.1973.36.1.61. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Memory in the Cerebral Cortex. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- Fuster JM. The Prefrontal Cortex. 3rd Edition. New York: Raven Press; 1997. [Google Scholar]
- Fuster JM. The prefrontal cortex--an update: time is of the essence. Neuron. 2001;30(2):319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173(997):652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Bodner M, Kroger JK. Cross-modal and cross-temporal association in neurons of frontal cortex. Nature. 2000;405(6784):347–351. doi: 10.1038/35012613. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Jervey JP. Neuronal firing in the inferotemporal cortex of the monkey in a visual memory task. J Neurosci. 1982;2(3):361–375. doi: 10.1523/JNEUROSCI.02-03-00361.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14(3):477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Gottlieb Y, Vaadia E, Abeles M. Single unit activity in the auditory cortex of a monkey performing a short term memory task. Exp Brain Res. 1989;74(1):139–148. doi: 10.1007/BF00248287. [DOI] [PubMed] [Google Scholar]
- Harris JA, Miniussi C, Harris IM, Diamond ME. Transient storage of a tactile memory trace in primary somatosensory cortex. J Neurosci. 2002;22(19):8720–8725. doi: 10.1523/JNEUROSCI.22-19-08720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A, Zainos A, Romo R. Neuronal correlates of sensory discrimination in the somatosensory cortex. Proc Natl Acad Sci USA. 2000;97(11):6191–6196. doi: 10.1073/pnas.120018597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A, Zainos A, Romo R. Temporal evolution of a decision-making process in medial premotor cortex. Neuron. 2002;33(6):959–972. doi: 10.1016/s0896-6273(02)00613-x. [DOI] [PubMed] [Google Scholar]
- Jacobsen CF. Studies of cerebral function in primates. Comparative Psychology Monogaphs. 1936;13:1–68. [Google Scholar]
- Jiang W, Chapman CE, Lamarre Y. Modulation of the cutaneous responsiveness of neurones in the primary somatosensory cortex during conditioned arm movements in the monkey. Exp Brain Res. 1991;84(2):342–354. doi: 10.1007/BF00231455. [DOI] [PubMed] [Google Scholar]
- Joelving FC, Compte A, Constantinidis C. Temporal properties of posterior parietal neuron discharges during working memory and passive viewing. J Neurophysiol. 2007;97(3):2254–2266. doi: 10.1152/jn.00977.2006. [DOI] [PubMed] [Google Scholar]
- Jones EG, Coulter JD, Wise SP. Commissural columns in the sensory-motor cortex of monkeys. The Journal of comparative neurology. 1979;188(1):113–135. doi: 10.1002/cne.901880110. [DOI] [PubMed] [Google Scholar]
- Kaas AL, van Mier H, Goebel R. The neural correlates of human working memory for haptically explored object orientations. Cereb Cortex. 2007;17(7):1637–1649. doi: 10.1093/cercor/bhl074. [DOI] [PubMed] [Google Scholar]
- Kaas AL, van Mier H, Visser M, Goebel R. The neural substrate for working memory of tactile surface texture. Hum Brain Mapp. 2012 doi: 10.1002/hbm.21500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH. The evolution of isocortex. Brain Behav Evol. 1995;46(4–5):187–196. doi: 10.1159/000113273. [DOI] [PubMed] [Google Scholar]
- Koch KW, Fuster JM. Unit activity in monkey parietal cortex related to haptic perception and temporary memory. Exp Brain Res. 1989;76(2):292–306. doi: 10.1007/BF00247889. [DOI] [PubMed] [Google Scholar]
- Kostopoulos P, Albanese MC, Petrides M. Ventrolateral prefrontal cortex and tactile memory disambiguation in the human brain. Proc Natl Acad Sci USA. 2007;104(24):10223–10228. doi: 10.1073/pnas.0700253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota K, Niki H. Prefrontal cortical unit activity and delayed alternation performance in monkeys. J Neurophysiol. 1971;34(3):337–347. doi: 10.1152/jn.1971.34.3.337. [DOI] [PubMed] [Google Scholar]
- Lebedev MA, Denton JM, Nelson RJ. Vibration-entrained and premovement activity in monkey primary somatosensory cortex. J Neurophysiol. 1994;72(4):1654–1673. doi: 10.1152/jn.1994.72.4.1654. [DOI] [PubMed] [Google Scholar]
- Matyas F, Sreenivasan V, Marbach F, Wacongne C, Barsy B, Mateo C, Aronoff R, Petersen CC. Motor control by sensory cortex. Science. 2010;330(6008):1240–1243. doi: 10.1126/science.1195797. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. The Journal of comparative neurology. 1982;212(1):1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci. 1996;16(16):5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Li L, Desimone R. Activity of neurons in anterior inferior temporal cortex during a short-term memory task. J Neurosci. 1993;13(4):1460–1478. doi: 10.1523/JNEUROSCI.13-04-01460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B. Effects of different brain lesions on card sorting. Archives of Neurology. 1963;9:100–110. [Google Scholar]
- Miyashita Y, Chang HS. Neuronal Correlate of Pictorial Short-Term-Memory in the Primate Temporal Cortex. Nature. 1988;331(6151):68–70. doi: 10.1038/331068a0. [DOI] [PubMed] [Google Scholar]
- Murray EA, Mishkin M. Relative contributions of SII and area 5 to tactile discrimination in monkeys. Behav Brain Res. 1984;11(1):67–83. doi: 10.1016/0166-4328(84)90009-3. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Douglas VD. Changes in premovement activity in primary somatosensory cortex differ when monkeys make hand movements in response to visual vs vibratory cues. Brain Res. 1989;484(1–2):43–56. doi: 10.1016/0006-8993(89)90346-6. [DOI] [PubMed] [Google Scholar]
- Niki H. Differential activity of prefrontal units during right and left delayed response trials. Brain Res. 1974;70(2):346–349. doi: 10.1016/0006-8993(74)90324-2. [DOI] [PubMed] [Google Scholar]
- Pasternak T, Greenlee MW. Working memory in primate sensory systems. Nat Rev Neurosci. 2005;6(2):97–107. doi: 10.1038/nrn1603. [DOI] [PubMed] [Google Scholar]
- Pesaran B, Nelson MJ, Andersen RA. Free choice activates a decision circuit between frontal and parietal cortex. Nature. 2008;453(7193):406–409. doi: 10.1038/nature06849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Evans AC. Functional Activation of the Human Ventrolateral Frontal-Cortex during Mnemonic Retrieval of Verbal Information. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(13):5803–5807. doi: 10.1073/pnas.92.13.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Muri RM, Nyffeler T, Milea D. The role of the human dorsolateral prefrontal cortex in ocular motor behavior. Ann N Y Acad Sci. 2005;1039:239–251. doi: 10.1196/annals.1325.023. [DOI] [PubMed] [Google Scholar]
- Preuschhof C, Heekeren HR, Taskin B, Schubert T, Villringer A. Neural correlates of vibrotactile working memory in the human brain. J Neurosci. 2006;26(51):13231–13239. doi: 10.1523/JNEUROSCI.2767-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana J, Fuster JM. From perception to action: temporal integrative functions of prefrontal and parietal neurons. Cereb Cortex. 1999;9(3):213–221. doi: 10.1093/cercor/9.3.213. [DOI] [PubMed] [Google Scholar]
- Rainer G, Asaad WF, Miller EK. Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature. 1998;393(6685):577–579. doi: 10.1038/31235. [DOI] [PubMed] [Google Scholar]
- Rao SC, Rainer G, Miller EK. Integration of what and where in the primate prefrontal cortex. Science. 1997;276(5313):821–824. doi: 10.1126/science.276.5313.821. [DOI] [PubMed] [Google Scholar]
- Romanski LM. Representation and integration of auditory and visual stimuli in the primate ventral lateral prefrontal cortex. Cereb Cortex. 2007;17(Suppl 1):i61–i69. doi: 10.1093/cercor/bhm099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo R, Brody CD, Hernandez A, Lemus L. Neuronal correlates of parametric working memory in the prefrontal cortex. Nature. 1999;399(6735):470–473. doi: 10.1038/20939. [DOI] [PubMed] [Google Scholar]
- Romo R, Salinas E. Flutter discrimination: neural codes, perception, memory and decision making. Nat Rev Neurosci. 2003;4(3):203–218. doi: 10.1038/nrn1058. [DOI] [PubMed] [Google Scholar]
- Salazar RF, Dotson NM, Bressler SL, Gray CM. Content-specific fronto- parietal synchronization during visual working memory. Science. 2012;338(6110):1097–1100. doi: 10.1126/science.1224000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas E, Hernandez A, Zainos A, Romo R. Periodicity and firing rate as candidate neural codes for the frequency of vibrotactile stimuli. J Neurosci. 2000;20(14):5503–5515. doi: 10.1523/JNEUROSCI.20-14-05503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Mechanisms of memory. Science. 1986;232(4758):1612–1619. doi: 10.1126/science.3086978. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253(5026):1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Preuss TM, Kaas JH. Architectonics, somatotopic organization, and ipsilateral cortical connections of the primary motor area (Ml) of owl monkeys. The Journal of comparative neurology. 1993;330(2):238–271. doi: 10.1002/cne.903300207. [DOI] [PubMed] [Google Scholar]
- Super H, Spekreijse H, Lamme VA. A neural correlate of working memory in the monkey primary visual cortex. Science. 2001;293(5527):120–124. doi: 10.1126/science.1060496. [DOI] [PubMed] [Google Scholar]
- Takeda M, Naya Y, Fujimichi R, Takeuchi D, Miyashita Y. Active maintenance of associative mnemonic signal in monkey inferior temporal cortex. Neuron. 2005;48(5):839–848. doi: 10.1016/j.neuron.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Thompson RF. The neurobiology of learning and memory. Science. 1986;233(4767):941–947. doi: 10.1126/science.3738519. [DOI] [PubMed] [Google Scholar]
- Wang L, Li X, Hsiao SS, Bodner M, Lenz F, Zhou YD. Persistent neuronal firing in primary somatosensory cortex in the absence of working memory of trial-specific features of the sample stimuli in a haptic working memory task. J Cogn Neurosci. 2012a;24(3):664–676. doi: 10.1162/jocn_a_00169. [DOI] [PubMed] [Google Scholar]
- Wang L, Li X, Hsiao SS, Bodner M, Lenz F, Zhou YD. Behavioral choice-related neuronal activity in monkey primary somatosensory cortex in a A haptic delay task. J Cogn Neurosci. 2012b;24(7):1634–1644. doi: 10.1162/jocn_a_00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YD, Ardestani A, Fuster JM. Distributed and associative working memory. Cereb Cortex. 2007;17(Suppl 1):i77–i87. doi: 10.1093/cercor/bhm106. [DOI] [PubMed] [Google Scholar]
- Zhou YD, Fuster JM. Mnemonic neuronal activity in somatosensory cortex. Proc Natl Acad Sci U S A. 1996;93(19):10533–10537. doi: 10.1073/pnas.93.19.10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YD, Fuster JM. Neuronal activity of somatosensory cortex in a cross-modal (visuo-haptic) memory task. Exp Brain Res. 1997;116(3):551–555. doi: 10.1007/pl00005783. [DOI] [PubMed] [Google Scholar]
- Zhou YD, Fuster JM. Visuo-tactile cross-modal associations in cortical somatosensory cells. Proc Natl Acad Sci U S A. 2000;97(17):9777–9782. doi: 10.1073/pnas.97.17.9777. [DOI] [PMC free article] [PubMed] [Google Scholar]