Abstract
The tear film coats the cornea and conjunctiva and serves several important functions. It provides lubrication, prevents drying of the ocular surface epithelia, helps provide a smooth surface for refracting light, supplies oxygen and is an important component of the innate defense system of the eye providing protection against a range of potential pathogens. This review describes both classic antimicrobial compounds found in tears such as lysozyme and some more recently identified such as members of the cationic antimicrobial peptide family and surfactant protein-D as well as potential new candidate molecules that may contribute to antimicrobial protection. As is readily evident from the literature review herein, tears, like all mucosal fluids, contain a plethora of molecules with known antimicrobial effects. That all of these are active in vivo is debatable as many are present in low concentrations, may be influenced by other tear components such as the ionic environment, and antimicrobial action may be only one of several activities ascribed to the molecule. However, there are many studies showing synergistic/additive interactions between several of the tear antimicrobials and it is highly likely that cooperativity between molecules is the primary way tears are able to afford significant antimicrobial protection to the ocular surface in vivo. In addition to effects on pathogen growth and survival some tear components prevent epithelial cell invasion and promote the epithelial expression of innate defense molecules. Given the protective role of tears a number of scenarios can be envisaged that may affect the amount and/or activity of tear antimicrobials and hence compromise tear immunity. Two such situations, dry eye disease and contact lens wear, are discussed here.
Keywords: Antimicrobial, tears, dry eye, contact lens
1. Introduction
The tear film coats the cornea and bulbar and palpebral conjunctiva and is a complex structure composed of an outer, anterior-most lipid component that prevents evaporation, an aqueous component that has ions, soluble mucins, enzymes and a wide range of other proteins and closest to the epithelial surface is a thick mucous, primarily composed of the gel-forming mucin MUC5Ac (Mantelli and Argueso, 2008). The lipid component is produced primarily by the meibomian (tarsal) glands in the eyelid, whereas the enzymes and other proteins originate mostly in the main lacrimal glands. Some of the proteins also come from the cornea and conjunctival epithelial cells, serum exudates and from neutrophils that can be found in the tears particularly upon awakening (Sack et al., 2001). MUC5Ac of the inner mucous component is from conjunctival goblet cells and together with membrane bound mucins and other components of the glycocalyx of the epithelia helps spread the tears over the hydrophobic ocular surface. The tear film is necessary for lubrication, it protects the underlying epithelia from desiccation, helps create a smooth surface for refraction of light, provides oxygen to the avascular cornea and provides protection from noxious chemicals and pathogens.
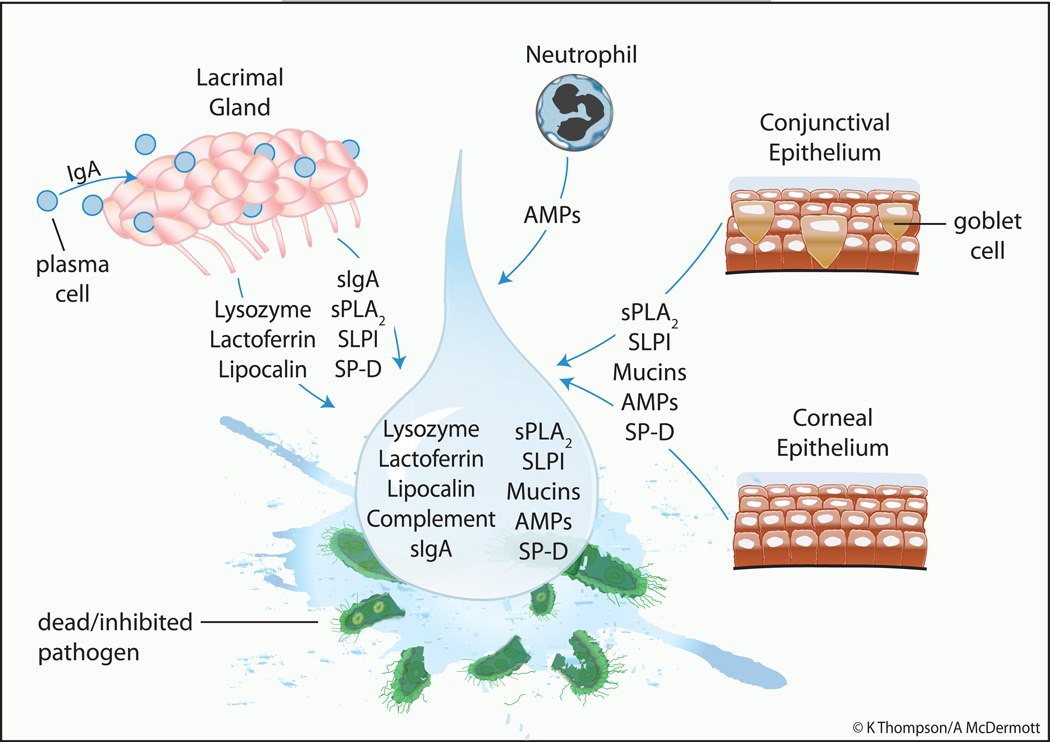
A range of microorgansims can infect the cornea and conjunctiva. The most common bacterial pathogens of the ocular surface include Pseudomonas (P.) aeruginosa, Serratia (S.) marcescens, Staphylococcus (S.) aureus, Staphylococcus (S.) epidermidis and Streptococcus pneumonia (Karsten et al., 2012). Herpes simplex virus is the most common culprit for viral infection, and Aspergillus, Candida and Fusarium species are common causes of fungal infection (Farooq and Shukla, 2012; Kalkanci and Ozdek, 2011). Acanthamoeba keratitis, as with corneal infection caused by P. aeruginosa and Fusarium (F.) Solani, is associated with contact lens wear (Lorenzo-Morales et al., 2013). As it is in immediate contact with the external environment the tear film provides the first barrier to such pathogens trying to breach the physical barrier created by the ocular surface epithelia. In vitro and in vivo studies have confirmed the antimicrobial activity of tears at the ocular surface (Fleiszig et al., 2003; Kwong et al., 2007; McNamara et al., 2005) and multiple mechanisms are involved. In conjunction with blinking and reflex tearing the tear film helps washout invading pathogens and contains a plethora of antimicrobial molecules to facilitate pathogen killing or at least inhibit the replication of microorganisms. Also tear components can help prevent ocular surface epithelial cell invasion by bacteria and modulate epithelial innate responses to enhance protection. Figure 1 provides an overview of the major antimicrobial components in tears and their primary sites of origin.
Figure 1. Known Tear Components with Antimicrobial Activity and their Major Source.
The central drop representing the tear film contains several antimicrobial molecules which are derived primarily from the lacrimal gland and ocular surface (corneal and conjunctival) epithelial cells. An additional source depicted is the neutrophil, which can be found in tears particularly on eye opening. AMPs = antimicrobial peptides; sIgA = secretory Immunoglobulin A; sPLA2 = secretory phospholipase A2; SLPI = secretory leukocyte protease inhibitor; SP-D = surfactant protein D.
2. “Classic” Tear Antimicrobial Components
Tear components long recognized to have antimicrobial function include lysozyme, lactoferrin, lipocalin, secretory immunoglobulin A (IgA) and complement. Lysozyme was shown to be present in human tears and to kill Gram-positive bacteria by Alexander Fleming (Fleming, 1922). This enzyme, which is secreted by the main and accessory lacrimal glands, accounts for up to 20–30% of total protein in both basal and reflex tears (Aho et al., 1996; Albert DM, 2008; Sack et al., 2001). Lysozyme catalyzes the hydrolysis of 1,4-beta-linkages between N-acetylmuramic acid and N-acetyl-D-glucosamine in the peptidoglycan backbone of bacterial cell walls. It is also able to cleave chitodextrins in fungal cell walls and has been reported to have anti-HIV activity (Lee-Huang et al., 1999). The compromised cell wall is then no longer able to maintain a stable osmotic environment and lysis of the organism ensues. Lactoferrin also represents some 20–30% of the total protein in basal and reflex tears and is secreted by lacrimal gland acinar cells (Janssen and van Bijsterveld, 1983; Sack et al., 2001). Lactoferrin has a high capacity to bind divalent cations including iron thus depriving bacteria of this essential nutrient for growth and production of some toxins, although iron-independent antimicrobial actions have also been described (Farnaud and Evans, 2003). Furthermore, a highly basic sequence at the N-terminus (referred to as lactoferricin) allows lactoferrin to act as a cationic detergent and disrupt the cell membrane of some bacteria, fungi and viruses (Farnaud and Evans, 2003). It is believed that in general lactoferrin has a microbiostatic role in biological fluids such as tears rather than a microbicidal one (Alexander et al., 2012).
Tear lipocalin (formally referred to as tear specific prealbumin, (Redl, 2000) represents approximately 25% of the reflex tear protein and is produced by the acinar cells of the main lacrimal gland (Albert DM, 2008; Sack et al., 2001). Lipocalin, which is detected as several isoforms in tears, has been shown to be capable of binding siderophores produced by a range of bacteria and fungi. Siderophores are chelating compounds that transport iron in to microorganisms. Thus, like lactoferrin, lipocalin exerts a bacteriostatic effect by interfering with the ability of pathogens to take up iron (Dartt, 2011; Fluckinger et al., 2004). Some isoforms of lipocalin have a putative protease inhibitory domain and may also help protect the ocular surface from cysteine proteases of microbial origin (van't Hof et al., 1997).
Secretory immunoglobulin A (sIgA) is the major antibody present in the tear film. sIgA is produced by plasma cells (terminally differentiated B lymphocytes) residing in the lacrimal gland, accessory lacrimal glands and conjunctival associated lymphoid tissue (Franklin and Remus, 1984; Knop and Knop, 2005; Wieczorek et al., 1988). The secreted IgA is taken up by lacrimal gland acinar cells or conjunctival epithelial cells by receptor mediated endocytosis and traverses the cell by transcytosis. The IgA is then released bound to a protein called secretory component, which is actually a remnant of the receptor to which the antibody was bound during its passage through the cells. Secretory component helps stabilize the IgA and masks proteolytic sites so making it less vulnerable to the effects of both host and pathogen proteases. sIgA, which exists in tears as a dimer, is present at greater concentrations in the morning than in the afternoon (Puinhas et al., 2013). In contrast to some other isotypes sIgA is not an efficient activator of complement nor is it a good opsonin, but analogous to its role in the intestinal mucosa, at the ocular surface IgA promotes the clearance of pathogens (Mantis et al., 2011). Via its specific antigen binding sites sIgA is able to neutralize pathogens so preventing their attachment to host cells. It can also bind to lectin-like adhesin molecules on pathogens resulting in their aggregation, entrapment within the tear film and subsequent removal. Thus sIgA is of great importance as it facilitates removal of pathogens right at the point of entry at the ocular surface. sIgA has also been shown to be chemotactic for phagocytic neutrophils (Lan et al., 1998) and to prevent the binding of P. aeruginosa and Acanthamoeba polyphaga to contact lenses (Campos-Rodriguez et al., 2004; Lan et al., 1999).
Low levels of functionally active complement factors have also been detected in tears (Willcox et al., 1997). The relative amounts of different components, namely abundant C3 and factor B, but less C1q, suggests that activation via the alternative pathway (i.e. spontaneous hydrolysis of C3) is the predominant mechanism. Activation of the complement pathway generates fragments involved in acute inflammatory responses, fragments that act as opsonins, which facilitate target recognition by neutrophils and results in the formation of membrane attack complexes that can lyse pathogens. As the concentration of the various complement components is increased in closed-eye tears, the pathway is believed to be most active during sleep when the eyes are closed (Willcox et al., 1997). Possible sources of the various complement factors in tears include leakage of plasma through the conjunctival vessels during sleep, infiltrating neutrophils, and local synthesis by corneal and conjunctival epithelial cells. To prevent unnecessary activation and hence tissue damage from pro-inflammatory components, the complement pathway is regulated by a number of factors including decay-accelerating factor (CD55, inhibits activation of C3), membrane cofactor protein (CD46, regulates activation of C3) and membrane inhibitor of reactive lysis (CD59, prevents formation of membrane attack complex), all of which have been detected in tears (Cocuzzi et al., 2001; Hara et al., 1992; Szczotka et al., 2000; Willcox et al., 1997). Notably the complement pathway is not active in reflex tears and both lysozyme and lactoferrin have also been found to inhibit the pathway (Kijlstra, 1990; Ogundele, 1999; Willcox et al., 1997).
3. Other Identified Tear Antimicrobials and Potential Candidates
There are many other examples of tear components with antimicrobial properties although it should be noted that several of these have other (often multiple-other) activities and antimicrobial effects may not be their primary function is tears.
The enzyme secretory phospholipase A2 (sPLA2) has been identified as the major tear protein active against Gram-positive bacteria, although it has no activity on its own against Gram-negatives in the normal ionic environment of tears (Qu and Lehrer, 1998). sPLA2 is produced by lacrimal gland as well as corneal and conjunctival epithelial cells (Turner et al., 2007; Wei et al., 2012). It is found at much lower levels in tears than lysozyme, is reduced in reflex compared to basal tears and has been reported to show diurnal variation (Aho et al., 2002; Aho et al., 2003; Saari et al., 2001). Secretory PLA2 binds to the anionic bacterial surface due to its cationic nature and kills via its lipolytic enzymatic activity. Specifically it hydrolyses the sn-2-fatty acyl moiety from phospholipids, in particular phosphatidylglycerol, which is abundant on bacterial cell membranes (Buckland et al., 2000; Nevalainen et al., 2008).
Secretory leukocyte protease inhibitor (SLPI) is a member of the whey acidic protein (WAP) family of molecules which have conserved cysteine-rich regions known as 4-disulphide core domains (Sallenave, 2010). SLPI was first identified for its anti-protease activity but is known to have both anti-inflammatory and antimicrobial properties. SLPI is active against both Gram positive and negative bacteria, fungi and HIV and, similar to the “antimicrobial peptide” (AMP) family discussed below, this may be related to its high cationic charge (Sallenave, 2010). SLPI is produced by lacrimal gland and ocular surface epithelial cells, is present in reflex tears and at much higher levels in closed-eye tears (Franken et al., 1989; Sathe et al., 1998). It is also known to inhibit neutrophil elastase so may help protect the ocular surface cells from the damaging effects of this degradative enzyme. Elafin is another member of the WAP family of molecules found in tears, although at a much lower concentration that SPLI (Sathe et al., 1998). Like the latter, elafin it is multifunctional being both anti-inflammatory and directly antimicrobial (Sallenave, 2010). Bactericidal/permeability-increasing protein (BPI), which is known to be stored in neutrophils, to bind LPS and kill Gram-negative bacteria, has also been detected in tears (Holweg et al., 2011; Peuravuori et al., 2006). This protein could be detected in lacrimal gland and conjunctival epithelial cells which could therefore be an additional source to that from neutrophils in the tears. It was also found that the level of BPI decreased as the tear flow rate increased (Peuravuori et al., 2006). Ford et al. (Ford et al., 1976) reported the presence of an antimicrobial factor called β-lysin in tears. This protein, which is distinct from lysozyme and is found in platelets and serum, targets the bacterial cell membrane of Gram-positive bacteria and has been reported to inhibit bacterial catalase and peroxidase and enhance phagocytosis (Bukharin and Suleimanov, 1997; Donaldson and Tew, 1977). It should be noted that the presence of β-lysin in tears has been disputed (Janssen et al., 1984; Selsted and Martinez, 1982) also, presuming it does exist, it is not clear from the literature if it has been “re-discovered” more recently and given another name. Notably its characteristics: small, cationic, membrane targeting, are reminiscent of the AMP family, which is discussed below.
As briefly mentioned in the introduction mucins are an important component of tears. Mucins are high-molecular weight glycoproteins with extensive O-glycosylation and the major ones present in tears are shed membrane bound MUC1, MUC4 and MUC16 and gel forming MUC5Ac, low amounts of secreted mucin MUC2 have also been reported (Mantelli and Argueso, 2008; Paulsen and Berry, 2006; Spurr-Michaud et al., 2007). In addition to providing lubrication and a means by which aqueous tears are held on the hydrophobic ocular surface, mucins are known to provide antimicrobial protection through several mechanisms. MUC5Ac has what has been described as a “janitorial” function in that it can trap pathogens helping to move them to the lacrimal drainage pathway so facilitating their removal from the surface (Mantelli and Argueso, 2008; Ramamoorthy and Nichols, 2008). There is also evidence that sIgA and positively charged proteins such as lysozyme and SLPI accumulate in the mucous layer coating the ocular surface epithelia so creating a reservoir of antimicrobial agents (Sack et al., 2001). Hence, mucins may trap microbes, which are then killed by accumulated antimicrobials or aggregated by sIgA and then cleared by blinking. Tear mucins have also been reported to prevent adherence of P. aeruginosa, but not that of Staphylococcus or Streptococcus, to the corneal epithelium (Fleiszig et al., 1994). It has been suggested that membrane bound MUC1 binds pathogens and then is cleaved from the epithelial cell surface and the pathogen-ectoderm domain complex is then removed via the lacrimal drainage system (Govindarajan and Gipson, 2010). However, experimental data for a role of MUC1 are equivocal, with one study reporting an increase in infection in MUC1 null mice and another no increase (Danjo et al., 2000; Kardon et al., 1999). MUC16 has been reported to prevent bacterial adherence to corneal epithelial cells (Blalock et al., 2008). This implies a direct interaction between MUC16 and bacterial cells, which may also occur with MUC16 shed in to the tears so facilitating pathogen removal.
Tears also contain Surfactant protein (SP) A and D which are members of the collectin family of C-type lectins and which are known to bind pathogens and regulate host defense (Awasthi, 2010). Both SP-A and SP-D are produced by lacrimal gland cells and also corneal and conjunctival epithelial cells (Brauer et al., 2007; Ni et al., 2005; Stahlman et al., 2002). C-type lectins bind to carbohydrates on microbial surfaces and to receptors on phagocytic cells so promoting microbial clearance. Therefore SP-A/D in tears may help facilitate pathogen removal in the presence of neutrophils for example. Tear SP-D has been shown to help protect corneal epithelial cells from invasion by P. aeruginosa (Ni et al., 2005). Although SP-D is known to inhibit the growth of certain Gram-negative bacteria (Wu et al., 2003), its corneal epithelial cytoprotective effect did not appear to be related to growth inhibition or effects on bacterial aggregation or motility but may be associated with its ability to bind LPS (Ni et al., 2005). SP-D has also recently been shown to be necessary for host defense against P. aeruginosa in vivo where it can help in bacterial clearance and block microbial traversal in the cornea, although bacterial elastase may compromise its activity (Alarcon et al., 2011; McCormick et al., 2007; Mun et al., 2009).
Antimicrobial peptides (AMPs), also referred to as host defense peptides due to their having immunomodulatory effects as well as direct antimicrobial actions, have also been detected in tears. Members of this large family are generally small (less than 50 amino acids) peptides with an overall positive charge that through electrostatic interaction cause disruption of microbial cell membranes leading to cell death (Choi et al., 2012). Low levels of the α-defensins Human neutrophil peptides −1,−2,−3 have been detected, with the primary source presumed to be neutrophils that can be found in the tears for example on eye opening (Haynes et al., 1999). The presence of β-defensins, human β-defensin (hBD)-2 and hBD-3 has also been reported for basal and reflex (only hBD-2) tears (Garreis et al., 2010). These defensins are presumed to be secreted in to tears by corneal and conjunctival epithelial cells as well as possibly lacrimal glands (Kolar and McDermott, 2011). The amounts of α and β-defensins in normal tears are less than would be needed for antimicrobial activity based on the results of in vitro studies but several of these peptides are inducible and potentially may reach effective levels in inflammation and infection as has been observed for patients under going ocular surgery and in a rabbit injury model (Zhou et al., 2007; Zhou et al., 2004). Other AMPs detected in tears include psoriasin (S100A7), which has highly potent activity against Escherichia coli and is produced by cornea, conjunctiva and lacrimal gland (Garreis et al., 2011). Also the presence of dermcidin (You et al., 2010) and the anti-fungal peptide histatin has also been reported (Steele et al., 2002).
Molecules with potential for a role as a tear antimicrobial include lacritin. This protein is secreted primarily by acinar cells of the main lacrimal gland, but also by the meibomian glands in the eyelids. It is described as a prosecretory mitogen owing to its being able to stimulate tear production and corneal epithelial cell proliferation in vitro, an effect mediated via interaction with syndecan-1 (Ma et al., 2008). In addition, preliminary studies have show that lacritin, which shows some sequence alignment with the AMP dermcidin, has antimicrobial activity against Gram-positive and negative organisms (McKown et al., 2008). Lacritin has also shown potential as a topical treatment for bacterial keratitis (Hosseini et al., 2012). Other possible candidates include various molecules with antimicrobial activity produced by the larcimal gland and/or ocular surface epithelia and which may then enter the tear film. Examples include cytokeratins (Tam et al., 2012), and various chemokines such as CCL20 (Cole et al., 2001; Huang et al., 2007a; Shirane et al., 2004; Yang et al., 2003). Reports appear regularly in the literature describing the identification of new antimicrobial molecules or assigning antimicrobial properties to a previously identified entity. Given that tears contain some 500 or more different proteins sourced primarily from the lacrimal glands, but also ocular surface epithelial cells, neutrophils and leakage of serum it will not be surprising to find that some of these newly identified antimicrobial compounds are also present in tears.
While this discussion has focused primarily on tear components with direct antimicrobial activity it is important to remember that some tear components may play an equally important role by modulating the defense response of the ocular surface epithelial cells. For example Mun et al. (Mun et al., 2011) recently showed that tears induced corneal epithelial expression of RNase7 (known to have potent antimicrobial activity) and ST-2, which protected the cells against P. aeruginosa invasion. The effects of tears on RNAse7 and ST-2 were subsequently shown to be mediated by modulation of expression of specific microRNAs (Mun et al., 2013).
Tears are one of several mucosal fluids in the body. Hu et al. (Hu et al., 2006) reviewed the literature on proteome analysis of several body fluids and concluded there was minimal overlapping of identified proteins, hence the proteomic content in each body fluid is distinct. Indeed this is logical based on the location and function of the tissue, which the particular fluid bathes. However in keeping with the protective role of body fluids, one group of proteins that are commonly found are antimicrobials. Given the similarities between lacrimal and salivary glands it is not surprising to find that saliva contains many of same antimicrobials as tears including lysozyme, lactoferrin, SLPI, mucins, AMPs (Dale and Fredericks, 2005). However, while some antimicrobials are common to several body fluids their absolute concentrations may vary markedly. For example a study in which the activity of lysozyme was compared among several body fluids showed that tears had the highest content and that this was some 120 times higher than that found in serum and saliva (Hankiewicz and Swierczek, 1974). Tears have also been reported to contain higher levels of sPLA2 than most other body fluids including serum (Aho et al., 2002; Mochizuki et al., 2008; Qu and Lehrer, 1998). AMPs such as defensins are commonly found in mucosal and other body fluids but some have a more limited distribution including the anti-fungal histatins, which have been detected in tears but which are found at much higher concentrations in saliva (Dale and Fredericks, 2005; Steele et al., 2002). These differences presumably reflect the range of organisms that tend to colonize and infect the tissue a particular fluid is bathing as well as different working conditions such as the ionic environment, which may favor the activity of some antimicrobial molecules but not others.
4. Functionality
The preceding discussion shows that the tears contain a plethora of molecules with documented antimicrobial activity, at least in vitro. It should be noted that many of these molecules have been found to have other actions, and indeed in some case the non-antimicrobial activity may actually be the primary functional role for a particular molecules – for example lipocalin is the principal lipid binding protein in tears (Redl, 2000). The question then is which of the tear antimicrobial components are important for defense against pathogens in vivo?
The antimicrobial action of some AMPs is salt sensitive and compromised by salt and mucins in the tear film (Huang et al., 2007a; Huang et al., 2006; Huang et al., 2007b), calling into question their role as antimicrobial agents at the ocular surface. However, the expression of some is inducible by infection and inflammation and the ensuing elevated levels may go some way to compensate for these detrimental effects (Huang et al., 2007a). Animal models of bacterial and fungal keratitis have shown that AMPs such as defensins and cathelicidin are important in reducing severity of infection indicating that they are indeed functional in vivo although of course this cannot be solely attributed to their presence in tears (Gao et al., 2011; Huang et al., 2007c; Wu et al., 2009a; Wu et al., 2009b). The fact that keratitis studies are typically performed on an ocular surface compromised by a scratch and which may therefore circumvent some tear effects also needs to be considered. SP-D deficient mice had more severe Pseudomonas keratitis than wild type mice indicating a functional role for this protein (McCormick et al., 2007) however as noted previously results from keratitis studies in MUC1 deficient mice were equivocal (Danjo et al., 2000; Kardon et al., 1999). Interestingly, a polymorphism of the lactoferrin gene has recently been linked to susceptibility to herpes simplex keratitis (Keijser et al., 2008).
sPLA2 which is found at high concentrations in basal tears has been described as the only tear antimicrobial that is bactericidal to Gram-positive bacteria in this isotonic environment (Qu and Lehrer, 1998). While sPLA2 may indeed provide important antimicrobial protection it cannot be the major/only player as the commonly used C57BL/6 mouse does not express this gene and is resistant to Staphylococcal keratitis (Hume et al., 2005; Kennedy et al., 1995). Thus, in vivo the likely scenario is that no one single molecule is the primary antimicrobial factor rather that several tear antimicrobials interact together. This is borne out by numerous literature examples of synergistic/additive interactions between various tear antimicrobial molecules. For example lysozyme and lactoferrin interact synergistically against some Gram-negative bacteria as well as Staphylococcus (Ellison and Giehl, 1991; Leitch and Willcox, 1998) . Lactoferrin has also been found to increase the susceptibility of S. epidermidis biofilms to lysozyme and the antibiotic vancomycin (Leitch and Willcox, 1999). Lipocalin has been reported to enhance the activity of lysozyme (Josephson and Wald, 1969) and spectroscopy studies not only confirmed the interaction with lysozyme but also revealed an interaction with lactoferrin (Gasymov et al., 1999). Synergistic or additive interactions have also been reported for combinations of lysozyme, lactoferrin, SLPI and AMPs (Singh et al., 2000). sPLA2 can also kill Gram-negative bacteria with the help of additional antibacterial compounds, such as BPI (Buckland et al., 2000; Nevalainen et al., 2008). A common theme in several of these interactions is that an antimicrobial factor acting on the microbial cell wall facilitates access to other sites, for example the cell membrane, for the second antimicrobial molecule to act.
5. Modulation of Tear Antimicrobials
A number of situations may be envisaged that could possibly compromise the antimicrobial properties of tears and so predispose to infection. Two such scenarios, namely the very common ocular surface disease dry eye and a popular form of vision correction - contact lens wear, are discussed below.
5.1 Dry Eye and Tear Antimicrobials
As defined by the 2007 Dry Eye Workshop report, dry eye is a multifactorial disease that is caused by a decrease in tear production or an increased in tear evaporation and is associated with elevated tear osmolarity and symptoms of ocular irritation (2007a). Prevalence is more common in women and older populations with rates of 5–30% reported for people greater than 50 years of age (2007b). Symptoms which include ocular burning, itching, redness may have significant impact on visual function and overall quality of life (2007b; Abetz et al., 2011; Friedman, 2010; Pouyeh et al., 2012). The disrupted ocular surface of the dry eye patient would be predicted to increase the risk of infection yet a recent review by Narayanan et al. (Narayanan et al., 2013) concluded that there was minimal evidence to support a link between dry eye and microbial keratitis. In reviewing the literature Narayanan et al. (Narayanan et al., 2013) show that tear antimicrobials lysozyme, lactoferrin and lipocalin were decreased (or lysozyme was unchanged in some studies) while sPLA2 was increased in dry eye patients compared to normal controls. Three studies on sIgA gave equivocal results while MUC1 and MUC5AC were increased and decreased respectively. Overall, losses to the tear film armamentarium in dry eye appeared to be counterbalanced by other innate defenses (such as epithelial AMPs) being unchanged or enhanced which can account, at least in part, for the lack of association between dry eye and microbial keratitis in the patient population.
5.2 Contact Lens Wear and Tear Antimicrobials
Contact lens wear is a popular choice for refractive error correction but has long been recognized as a risk factor for microbial keratitis, a potentially vision threatening disease. P. aeruginosa remains the most common cause of contact lens related microbial keratitis (Robertson, 2013) but recent world wide epidemics caused by fungi (F. solani) and acanthamoeba are reminders that other organisms may also take advantage of the ocular surface compromised by contact lens wear (Patel and Hammersmith, 2008; Yoder et al., 2012). Microbial keratitis associated with contact lens wear and its accompanying use of care solutions is a multifactorial process (Evans and Fleiszig, 2013). Contributing factors, some associated with hypoxia, include reduced corneal epithelial turnover allowing for more contact time with potential pathogens, enhanced ability of corneal epithelial cells to bind and internalize (via lipid raft-formation) P. aeruginosa, and adaptation of the organism to the contact-lens modulated ocular surface environment (Evans and Fleiszig, 2013; Ladage, 2004; Robertson, 2013). As recently reviewed by Evans and Fleiszig (Evans and Fleiszig, 2013), contact lens wear may affect tear-mediated defenses in a number of ways. The postlens tear film volume may be affected by the lens material resulting in possible reduction in concentration of essential antimicrobial compounds. The lens material may also directly affect tear film composition through chemical absorption/adsorption, inactivation of components or compartmentalization so separating an enzyme from its substrate for example.
A number of studies have addressed the effects of contact lens wear on various tear antimicrobial compounds. Lysozyme has been reported not to change with various types of wear modality in most studies (Carney et al., 1997; Farris, 1985; Stuchell et al., 1981; Vinding et al., 1987). However some studies have shown increased tear lysozyme (Kijlstra et al., 1992; Sariri and Khamedi, 2007; Temel et al., 1991a) and one showed this enzyme to be decreased with both soft and RGP lens wear (Kramann et al., 2011). Tear lactoferrin levels are not affected by contact lens wear (Carney et al., 1997; Farris, 1985; Kijlstra et al., 1992; Stuchell et al., 1981). Tear sIgA levels have been found to be decreased in some studies (Lan et al., 1999; Pearce et al., 1999; Vinding et al., 1987), unchanged (Temel et al., 1991b) and increased with RGP wear, although this normalized by one year of lens wear (Kijlstra et al., 1992; Temel et al., 1991b). Notably in one study, Pseudomonas specific IgA was lower in contact lens wearers although the levels of total sIgA were not different between lens wearers and controls (Cheng et al., 1996). Two studies reported no change in tear sPLA2 (Hume et al., 2004; Yamada et al., 2006) whereas a third reported a decrease with lens wear (Aho et al., 2003). These differing results may be related to the diurnal variation in sPLA2 levels, which could confound interpreting results depending on the time of tear collection. It should also be noted that tear collection and analytical method have been shown to impact the proteins detected in tears thus direct comparison between studies is not always appropriate (Green-Church et al., 2008). Overall the majority of studies indicate that levels of lysozyme, lactoferrin and sPLA2 are unchanged with contact lens wear. However the preponderance of studies suggest a decrease in sIgA with contact lens wear. This is of significance as this antibody has been shown to reduce binding of P. aeruginosa to contact lenses and be chemoattractive for phagocytic neutrophils (Lan et al., 1999; Lan et al., 1998).
As noted above (section 3) tear mucins have roles in defence against pathogens. As reviewed in detail by Ramamoorthy and Nichols (Ramamoorthy and Nichols, 2008) a number of studies have addressed the amount of mucin in tears and expression by ocular surface cells in contact lens wear however the results are conflicting. For example studies examining total mucins showed reduced amounts in tears with lens wear (Garcher et al., 1998; Yasueda et al., 2005). Whereas a study by Hori et al. (Hori et al., 2006), did not find any differences in conjunctival expression or tear content of MUC1, 4, 5Ac or 16. A more recent study by Corrales et al. (Corrales et al., 2009) did find some significant lens wear related changes (primarily an increase) in conjunctival mRNA expression of various mucins but protein analysis was not performed so the significance of the observations is not clear. Corneal epithelial cell exposure to contact lens care solutions has also been shown to reduce amounts of protective mucins, particularly MUC16, and this was associated with increased internalization of P. aeruginosa (Gordon et al., 2011; Imayasu et al., 2010; Tchedre et al., 2011). A study in an in vitro model of contact lens wear showed that corneal epithelial cells exposed to contact lenses failed to upregulate hBD-2 in response to P. aeruginosa suggesting that contact lens wear may compromise the innate ability of the corneal epithelium to respond adequately in some cases and consequently lead to reduced antimicrobials in the tear film (Maltseva et al., 2007). How other tear antimicrobials such as SLPI and SP-D are affected by lens wear has yet to be determined.
It is well known that contact lenses bind tear film proteins and that this varies with the lens material, the care solutions used for cleaning/disinfection, as well as the individual (Luensmann and Jones, 2012; Omali et al., 2013). Several of the aforementioned tear antimicrobials including lysozyme, lactoferrin, sPLA2 and mucins are known to bind to contact lenses (Berry et al., 2012; Boone et al., 2009; Green-Church and Nichols, 2008; Mochizuki et al., 2008; Omali et al., 2013). Binding does not necessarily significantly deplete the tears of these antimicrobials as suggested by several studies revealing no change in tear levels with lens wear (Aho et al., 2003; Carney et al., 1997; Hume et al., 2004; Yamada et al., 2006). However binding of these antimicrobial (and other) proteins to a lens often enhances bacterial adhesion and is typically associated with some degree of loss of activity, usually through denaturation (Dutta et al., 2012; Mannucci et al., 1985; Vijay et al., 2012). These effects vary with lens material and pathogen (Willcox, 2013). For example in a recent study by Subbaraman et al. (Subbaraman et al., 2011) lysozyme coating increased binding of a S. aureus strain but not that of P. aeruginosa. However the lysozyme coating did not cause significant death of the adhered Staphylococcus strain. Lactoferrin, increased binding of both Staphyloccus and Pseudomonas, and while unable to kill the former, did reduced viable counts of adhered Pseudomonas. Recent studies with care solutions containing protein-stabilization agents show that they are able to prevent denaturation of lysozyme and lactoferrin hence may be beneficial in preventing loss of antimicrobial activity when these proteins bind to lenses (Barniak et al., 2010; Wright et al., 2012).
A major problem is that after adhering to surfaces bacteria tend to form biofilms which renders them more resistant to antimicrobial substances including chemical disinfectants, antibiotics and host defense molecules (Donlan and Costerton, 2002). Therefore while tears contain a plethora of antimicrobial molecules few/none may be effective against organisms in a biofilm on a contact lens placed on to the eye. In keeping with this S.marcescens biofilms on etafilcon A lenses were resistant to phagocytosis by neutrophils (Hume et al., 2003) and some P. aeruginosa strains show enhanced biofilm formation in the presence of neutrophils (Burnham et al., 2012).
6. Summary and Conclusions
As discussed here analytical studies have shown that tears contain a variety of molecules with antimicrobial activity that can directly kill or prevent the growth of a range of pathogenic organisms. While there is some redundancy in that there is overlap in spectrum of activity, having a large number of antimicrobials is a common feature of all biological fluids and reflects the complexity of the flora to which the body is constantly exposed. Having multiple antimicrobials with differing mechanisms of action helps to ensure eradication of a pathogen, which may just happen to be resistant to a specific compound. Also it permits synergistic/additive interactions between two or perhaps more molecules, which can reduce the amounts needed and lower the risk of toxic effects to ocular surface cells. In addition to direct action on microbial growth and survival, reflex tearing and some tear antimicrobials such as mucins and sIgA which bind pathogens facilitate their removal via the lacrimal drainage system. Others, such as SP-D help prevent ocular surface cell invasion and yet to be identified tear components induce epithelial expression of innate defense molecules. Contact lens wear appears to suppress tear antimicrobial effects in a number of ways which contributes to the increased risk of microbial keratitis that is associated with this modality of refractive error correction. On the other hand, in dry eye, although the ocular surface is compromised and there are reported decreases in some tear antimicrobials, others are increased as are epithelial cell innate defenses, which affords sufficient protection to maintain an infection free environment.
Highlights.
Tears contain multiple antimicrobial compounds
In addition to direct effects on microbial growth/survival tears can modulate epithelial innate responses
Effects on tear antimicrobials contributes to the increased risk of infection with contact lens wear
Acknowledgements
Thanks to Kim Thompson of the University of Houston College of Optometry Audio-Visual department for drawing the figure. The author’s work on the role of antimicrobial peptides at the ocular surface is supported by NIH grant EY13175.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) The ocular surface. 2007a;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007) The ocular surface. 2007b;5:93–107. doi: 10.1016/s1542-0124(12)70082-4. [DOI] [PubMed] [Google Scholar]
- Abetz L, Rajagopalan K, Mertzanis P, Begley C, Barnes R, Chalmers R. Development and validation of the impact of dry eye on everyday life (IDEEL) questionnaire, a patient-reported outcomes (PRO) measure for the assessment of the burden of dry eye on patients. Health and quality of life outcomes. 2011;9:111. doi: 10.1186/1477-7525-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aho HJ, Saari KM, Kallajoki M, Nevalainen TJ. Synthesis of group II phospholipase A2 and lysozyme in lacrimal glands. Investigative ophthalmology & visual science. 1996;37:1826–1832. [PubMed] [Google Scholar]
- Aho VV, Nevalainen TJ, Saari KM. Group IIA phospholipase A2 content of basal, nonstimulated and reflex tears. Current eye research. 2002;24:224–227. doi: 10.1076/ceyr.24.3.224.8299. [DOI] [PubMed] [Google Scholar]
- Aho VV, Paavilainen V, Nevalainen TJ, Peuravuori H, Saari KM. Diurnal variation in group IIa phospholipase A2 content in tears of contact lens wearers and normal controls. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2003;241:85–88. doi: 10.1007/s00417-002-0607-3. [DOI] [PubMed] [Google Scholar]
- Alarcon I, Tam C, Mun JJ, LeDue J, Evans DJ, Fleiszig SM. Factors impacting corneal epithelial barrier function against Pseudomonas aeruginosa traversal. Investigative ophthalmology & visual science. 2011;52:1368–1377. doi: 10.1167/iovs.10-6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert DM, M J, Azar DT, Blodi BA. Alberts & Jakobiec’s Principles and Practice of Ophthalmology. Saunders; 2008. [Google Scholar]
- Alexander DB, Iigo M, Yamauchi K, Suzui M, Tsuda H. Lactoferrin: an alternative view of its role in human biological fluids. Biochemistry and cell biology = Biochimie et biologie cellulaire. 2012;90:279–306. doi: 10.1139/o2012-013. [DOI] [PubMed] [Google Scholar]
- Awasthi S. Surfactant protein (SP)-A and SP-D as antimicrobial and immunotherapeutic agents. Recent patents on anti-infective drug discovery. 2010;5:115–123. doi: 10.2174/157489110791233559. [DOI] [PubMed] [Google Scholar]
- Barniak VL, Burke SE, Venkatesh S. Comparative evaluation of multipurpose solutions in the stabilization of tear lysozyme. Contact lens & anterior eye : the journal of the British Contact Lens Association. 2010;33(Suppl 1):S7–s11. doi: 10.1016/j.clae.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Berry M, Purslow C, Murphy PJ, Pult H. Contact lens materials, mucin fragmentation and relation to symptoms. Cornea. 2012;31:770–776. doi: 10.1097/ICO.0b013e3182254009. [DOI] [PubMed] [Google Scholar]
- Blalock TD, Spurr-Michaud SJ, Tisdale AS, Gipson IK. Release of membrane-associated mucins from ocular surface epithelia. Investigative ophthalmology & visual science. 2008;49:1864–1871. doi: 10.1167/iovs.07-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone A, Heynen M, Joyce E, Varikooty J, Jones L. Ex vivo protein deposition on bi-weekly silicone hydrogel contact lenses. Optometry and vision science : official publication of the American Academy of Optometry. 2009;86:1241–1249. doi: 10.1097/OPX.0b013e3181bbc1b3. [DOI] [PubMed] [Google Scholar]
- Brauer L, Kindler C, Jager K, Sel S, Nolle B, Pleyer U, Ochs M, Paulsen FP. Detection of surfactant proteins A and D in human tear fluid and the human lacrimal system. Investigative ophthalmology & visual science. 2007;48:3945–3953. doi: 10.1167/iovs.07-0201. [DOI] [PubMed] [Google Scholar]
- Buckland AG, Heeley EL, Wilton DC. Bacterial cell membrane hydrolysis by secreted phospholipases A(2): a major physiological role of human group IIa sPLA(2) involving both bacterial cell wall penetration and interfacial catalysis. Biochimica et biophysica acta. 2000;1484:195–206. doi: 10.1016/s1388-1981(00)00018-4. [DOI] [PubMed] [Google Scholar]
- Bukharin OV, Suleimanov KG. [The role of the thrombocytic cationic protein (beta-lysin) in anti-infectious protection] Zhurnal mikrobiologii, epidemiologii, i immunobiologii. 1997:3–6. [PubMed] [Google Scholar]
- Burnham GW, Cavanagh HD, Robertson DM. The impact of cellular debris on Pseudomonas aeruginosa adherence to silicone hydrogel contact lenses and contact lens storage cases. Eye & contact lens. 2012;38:7–15. doi: 10.1097/ICL.0b013e31823bad0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Rodriguez R, Oliver-Aguillon G, Vega-Perez LM, Jarillo-Luna A, Hernandez-Martinez D, Rojas-Hernandez S, Rodriguez-Monroy MA, Rivera-Aguilar V, Gonzalez-Robles A. Human IgA inhibits adherence of Acanthamoeba polyphaga to epithelial cells and contact lenses. Canadian journal of microbiology. 2004;50:711–718. doi: 10.1139/w04-057. [DOI] [PubMed] [Google Scholar]
- Carney FP, Morris CA, Willcox MD. Effect of hydrogel lens wear on the major tear proteins during extended wear. Australian and New Zealand journal of ophthalmology. 1997;25(Suppl 1):S36–s38. doi: 10.1111/j.1442-9071.1997.tb01752.x. [DOI] [PubMed] [Google Scholar]
- Cheng KH, Spanjaard L, Rutten H, Dankert J, Polak BC, Kijlstra A. Immunoglobulin A antibodies against Pseudomonas aeruginosa in the tear fluid of contact lens wearers. Investigative ophthalmology & visual science. 1996;37:2081–2088. [PubMed] [Google Scholar]
- Choi KY, Chow LN, Mookherjee N. Cationic host defence peptides: multifaceted role in immune modulation and inflammation. Journal of innate immunity. 2012;4:361–370. doi: 10.1159/000336630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocuzzi E, Szczotka LB, Brodbeck WG, Bardenstein DS, Wei T, Medof ME. Tears contain the complement regulator CD59 as well as decay-accelerating factor (DAF) Clinical and experimental immunology. 2001;123:188–195. doi: 10.1046/j.1365-2249.2001.01408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole AM, Ganz T, Liese AM, Burdick MD, Liu L, Strieter RM. Cutting edge: IFN-inducible ELR- CXC chemokines display defensin-like antimicrobial activity. J Immunol. 2001;167:623–627. doi: 10.4049/jimmunol.167.2.623. [DOI] [PubMed] [Google Scholar]
- Corrales RM, Galarreta D, Herreras JM, Saez V, Arranz I, Gonzalez MJ, Mayo A, Calonge M, Chaves FJ. Conjunctival mucin mRNA expression in contact lens wear. Optometry and vision science : official publication of the American Academy of Optometry. 2009;86:1051–1058. doi: 10.1097/OPX.0b013e3181b4f02e. [DOI] [PubMed] [Google Scholar]
- Dale BA, Fredericks LP. Antimicrobial peptides in the oral environment: expression and function in health and disease. Current issues in molecular biology. 2005;7:119–133. doi: 10.1093/jac/dki103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danjo Y, Hazlett LD, Gipson IK. C57BL/6 mice lacking Muc1 show no ocular surface phenotype. Investigative ophthalmology & visual science. 2000;41:4080–4084. [PubMed] [Google Scholar]
- Dartt DA. Tear lipocalin: structure and function. The ocular surface. 2011;9:126–138. doi: 10.1016/s1542-0124(11)70022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson DM, Tew JG. beta-Lysin of platelet origin. Bacteriological reviews. 1977;41:501–513. doi: 10.1128/br.41.2.501-513.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clinical microbiology reviews. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta D, Cole N, Willcox M. Factors influencing bacterial adhesion to contact lenses. Molecular vision. 2012;18:14–21. [PMC free article] [PubMed] [Google Scholar]
- Ellison RT, 3rd., Giehl TJ. Killing of gram-negative bacteria by lactoferrin and lysozyme. The Journal of clinical investigation. 1991;88:1080–1091. doi: 10.1172/JCI115407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DJ, Fleiszig SM. Microbial keratitis: could contact lens material affect disease pathogenesis? Eye & contact lens. 2013;39:73–78. doi: 10.1097/ICL.0b013e318275b473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnaud S, Evans RW. Lactoferrin--a multifunctional protein with antimicrobial properties. Molecular immunology. 2003;40:395–405. doi: 10.1016/s0161-5890(03)00152-4. [DOI] [PubMed] [Google Scholar]
- Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Survey of ophthalmology. 2012;57:448–462. doi: 10.1016/j.survophthal.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris RL. Tear analysis in contact lens wearers. Transactions of the American Ophthalmological Society. 1985;83:501–545. [PMC free article] [PubMed] [Google Scholar]
- Fleiszig SM, Kwong MS, Evans DJ. Modification of Pseudomonas aeruginosa interactions with corneal epithelial cells by human tear fluid. Infection and immunity. 2003;71:3866–3874. doi: 10.1128/IAI.71.7.3866-3874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig SM, Zaidi TS, Ramphal R, Pier GB. Modulation of Pseudomonas aeruginosa adherence to the corneal surface by mucus. Infection and immunity. 1994;62:1799–1804. doi: 10.1128/iai.62.5.1799-1804.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming A. On a remarkable bacteriolytic element found in tissues and secretions. Proc R Soc London B. 1922;21:463–480. [Google Scholar]
- Fluckinger M, Haas H, Merschak P, Glasgow BJ, Redl B. Human tear lipocalin exhibits antimicrobial activity by scavenging microbial siderophores. Antimicrobial agents and chemotherapy. 2004;48:3367–3372. doi: 10.1128/AAC.48.9.3367-3372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford LC, DeLange RJ, Petty RW. Identification of a nonlysozymal bactericidal factor (beta lysin) in human tears and aqueous humor. American journal of ophthalmology. 1976;81:30–33. doi: 10.1016/0002-9394(76)90188-4. [DOI] [PubMed] [Google Scholar]
- Franken C, Meijer CJ, Dijkman JH. Tissue distribution of antileukoprotease and lysozyme in humans. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1989;37:493–498. doi: 10.1177/37.4.2926127. [DOI] [PubMed] [Google Scholar]
- Franklin RM, Remus LE. Conjunctival-associated lymphoid tissue: evidence for a role in the secretory immune system. Investigative ophthalmology & visual science. 1984;25:181–187. [PubMed] [Google Scholar]
- Friedman NJ. Impact of dry eye disease and treatment on quality of life. Current opinion in ophthalmology. 2010;21:310–316. doi: 10.1097/ICU.0b013e32833a8c15. [DOI] [PubMed] [Google Scholar]
- Gao N, Kumar A, Guo H, Wu X, Wheater M, Yu FS. Topical flagellin-mediated innate defense against Candida albicans keratitis. Investigative ophthalmology & visual science. 2011;52:3074–3082. doi: 10.1167/iovs.10-5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcher C, Bron A, Baudouin C, Bildstein L, Bara J. CA 19–9 ELISA test: a new method for studying mucus changes in tears. The British journal of ophthalmology. 1998;82:88–90. doi: 10.1136/bjo.82.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garreis F, Gottschalt M, Schlorf T, Glaser R, Harder J, Worlitzsch D, Paulsen FP. Expression and regulation of antimicrobial peptide psoriasin (S100A7) at the ocular surface and in the lacrimal apparatus. Investigative ophthalmology & visual science. 2011;52:4914–4922. doi: 10.1167/iovs.10-6598. [DOI] [PubMed] [Google Scholar]
- Garreis F, Schlorf T, Worlitzsch D, Steven P, Brauer L, Jager K, Paulsen FP. Roles of human beta-defensins in innate immune defense at the ocular surface: arming and alarming corneal and conjunctival epithelial cells. Histochemistry and cell biology. 2010;134:59–73. doi: 10.1007/s00418-010-0713-y. [DOI] [PubMed] [Google Scholar]
- Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Interaction of tear lipocalin with lysozyme and lactoferrin. Biochemical and biophysical research communications. 1999;265:322–325. doi: 10.1006/bbrc.1999.1668. [DOI] [PubMed] [Google Scholar]
- Gordon GM, Moradshahi N, Jeong S, Lane C, Fini ME. A novel mechanism of increased infections in contact lens wearers. Investigative ophthalmology & visual science. 2011;52:9188–9194. doi: 10.1167/iovs.11-7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan B, Gipson IK. Membrane-tethered mucins have multiple functions on the ocular surface. Experimental eye research. 2010;90:655–663. doi: 10.1016/j.exer.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green-Church KB, Nichols JJ. Mass spectrometry-based proteomic analyses of contact lens deposition. Molecular vision. 2008;14:291–297. [PMC free article] [PubMed] [Google Scholar]
- Green-Church KB, Nichols KK, Kleinholz NM, Zhang L, Nichols JJ. Investigation of the human tear film proteome using multiple proteomic approaches. Molecular vision. 2008;14:456–470. [PMC free article] [PubMed] [Google Scholar]
- Hankiewicz J, Swierczek E. Lysozyme in human body fluids. Clinica chimica acta; international journal of clinical chemistry. 1974;57:205–209. doi: 10.1016/0009-8981(74)90398-2. [DOI] [PubMed] [Google Scholar]
- Hara T, Kuriyama S, Kiyohara H, Nagase Y, Matsumoto M, Seya T. Soluble forms of membrane cofactor protein (CD46, MCP) are present in plasma, tears, and seminal fluid in normal subjects. Clinical and experimental immunology. 1992;89:490–494. doi: 10.1111/j.1365-2249.1992.tb06986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes RJ, Tighe PJ, Dua HS. Antimicrobial defensin peptides of the human ocular surface. The British journal of ophthalmology. 1999;83:737–741. doi: 10.1136/bjo.83.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holweg A, Schnare M, Gessner A. The bactericidal/permeability-increasing protein (BPI) in the innate defence of the lower airways. Biochemical Society transactions. 2011;39:1045–1050. doi: 10.1042/BST0391045. [DOI] [PubMed] [Google Scholar]
- Hori Y, Argueso P, Spurr-Michaud S, Gipson IK. Mucins and contact lens wear. Cornea. 2006;25:176–181. doi: 10.1097/01.ico.0000177838.38873.2f. [DOI] [PubMed] [Google Scholar]
- Hosseini A, Lattanzio FA, Samudre SS, Sheppard JD, Laurie GW, McKown RL, Williams PB. Lacritin, a novel tear glycoprotein, is an effective topical antimicrobial agent in an animal model. Investigative ophthalmology & visual science. 2012;53 doi: 10.1167/iovs.10-6220. E-absract 6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Loo JA, Wong DT. Human body fluid proteome analysis. Proteomics. 2006;6:6326–6353. doi: 10.1002/pmic.200600284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LC, Jean D, Proske RJ, Reins RY, McDermott AM. Ocular surface expression and in vitro activity of antimicrobial peptides. Current eye research. 2007a;32:595–609. doi: 10.1080/02713680701446653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LC, Petkova TD, Reins RY, Proske RJ, McDermott AM. Multifunctional roles of human cathelicidin (LL-37) at the ocular surface. Investigative ophthalmology & visual science. 2006;47:2369–2380. doi: 10.1167/iovs.05-1649. [DOI] [PubMed] [Google Scholar]
- Huang LC, Redfern RL, Narayanan S, Reins RY, McDermott AM. In vitro activity of human beta-defensin 2 against Pseudomonas aeruginosa in the presence of tear fluid. Antimicrobial agents and chemotherapy. 2007b;51:3853–3860. doi: 10.1128/AAC.01317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LC, Reins RY, Gallo RL, McDermott AM. Cathelicidin-deficient (Cnlp −/−) mice show increased susceptibility to Pseudomonas aeruginosa keratitis. Investigative ophthalmology & visual science. 2007c;48:4498–4508. doi: 10.1167/iovs.07-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume EB, Cole N, Khan S, Garthwaite LL, Aliwarga Y, Schubert TL, Willcox MD. A Staphylococcus aureus mouse keratitis topical infection model: cytokine balance in different strains of mice. Immunology and cell biology. 2005;83:294–300. doi: 10.1111/j.1440-1711.2005.01326.x. [DOI] [PubMed] [Google Scholar]
- Hume EB, Cole N, Parmar A, Tan ME, Aliwarga Y, Schubert T, Holden BA, Willcox MD. Secretory phospholipase A2 deposition on contact lenses and its effect on bacterial adhesion. Investigative ophthalmology & visual science. 2004;45:3161–3164. doi: 10.1167/iovs.03-1242. [DOI] [PubMed] [Google Scholar]
- Hume EB, Stapleton F, Willcox MD. Evasion of cellular ocular defenses by contact lens isolates of Serratia marcescens. Eye & contact lens. 2003;29:108–112. doi: 10.1097/01.ICL.0000062461.24391.7F. [DOI] [PubMed] [Google Scholar]
- Imayasu M, Hori Y, Cavanagh HD. Effects of multipurpose contact lens care solutions and their ingredients on membrane-associated mucins of human corneal epithelial cells. Eye & contact lens. 2010;36:361–366. doi: 10.1097/ICL.0b013e3181faa43e. [DOI] [PubMed] [Google Scholar]
- Janssen PT, Muytjens HL, van Bijsterveld OP. Nonlysozyme antibacterial factor in human tears. Fact or fiction? Investigative ophthalmology & visual science. 1984;25:1156–1160. [PubMed] [Google Scholar]
- Janssen PT, van Bijsterveld OP. Origin and biosynthesis of human tear fluid proteins. Investigative ophthalmology & visual science. 1983;24:623–630. [PubMed] [Google Scholar]
- Josephson AS, Wald A. Enhancement of lysozyme activity by anodal tear protein. Proc Soc Exp Biol Med. 1969;131:677–679. doi: 10.3181/00379727-131-33951. [DOI] [PubMed] [Google Scholar]
- Kalkanci A, Ozdek S. Ocular fungal infections. Current eye research. 2011;36:179–189. doi: 10.3109/02713683.2010.533810. [DOI] [PubMed] [Google Scholar]
- Kardon R, Price RE, Julian J, Lagow E, Tseng SC, Gendler SJ, Carson DD. Bacterial conjunctivitis in Muc1 null mice. Investigative ophthalmology & visual science. 1999;40:1328–1335. [PubMed] [Google Scholar]
- Karsten E, Watson SL, Foster LJ. Diversity of microbial species implicated in keratitis: a review. The open ophthalmology journal. 2012;6:110–124. doi: 10.2174/1874364101206010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keijser S, Jager MJ, Dogterom-Ballering HC, Schoonderwoerd DT, de Keizer RJ, Krose CJ, Houwing-Duistermaat JJ, van der Plas MJ, van Dissel JT, Nibbering PH. Lactoferrin Glu561Asp polymorphism is associated with susceptibility to herpes simplex keratitis. Experimental eye research. 2008;86:105–109. doi: 10.1016/j.exer.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Kennedy BP, Payette P, Mudgett J, Vadas P, Pruzanski W, Kwan M, Tang C, Rancourt DE, Cromlish WA. A natural disruption of the secretory group II phospholipase A2 gene in inbred mouse strains. The Journal of biological chemistry. 1995;270:22378–22385. doi: 10.1074/jbc.270.38.22378. [DOI] [PubMed] [Google Scholar]
- Kijlstra A. The role of lactoferrin in the nonspecific immune response on the ocular surface. Regional immunology. 1990;3:193–197. [PubMed] [Google Scholar]
- Kijlstra A, Polak BC, Luyendijk L. Transient decrease of secretory IgA in tears during rigid gas permeable contact lens wear. Current eye research. 1992;11:123–126. doi: 10.3109/02713689209000062. [DOI] [PubMed] [Google Scholar]
- Knop E, Knop N. The role of eye-associated lymphoid tissue in corneal immune protection. Journal of anatomy. 2005;206:271–285. doi: 10.1111/j.1469-7580.2005.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolar SS, McDermott AM. Role of host-defence peptides in eye diseases. Cellular and molecular life sciences : CMLS. 2011;68:2201–2213. doi: 10.1007/s00018-011-0713-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramann C, Boehm N, Lorenz K, Wehrwein N, Stoffelns BM, Pfeiffer N, Grus FH. Effect of contact lenses on the protein composition in tear film: a ProteinChip study. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2011;249:233–243. doi: 10.1007/s00417-010-1456-0. [DOI] [PubMed] [Google Scholar]
- Kwong MS, Evans DJ, Ni M, Cowell BA, Fleiszig SM. Human tear fluid protects against Pseudomonas aeruginosa keratitis in a murine experimental model. Infection and immunity. 2007;75:2325–2332. doi: 10.1128/IAI.01404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladage PM. What does overnight lens wear do to the corneal epithelium?: is corneal refractive therapy different? Eye & contact lens. 2004;30:194–197. doi: 10.1097/01.icl.0000140224.70483.2f. discussion 205-196. [DOI] [PubMed] [Google Scholar]
- Lan J, Willcox MD, Jackson GD. Effect of tear-specific immunoglobulin A on the adhesion of Pseudomonas aeruginosa I to contact lenses. Australian and New Zealand journal of ophthalmology. 1999;27:218–220. doi: 10.1046/j.1440-1606.1999.00208.x. [DOI] [PubMed] [Google Scholar]
- Lan JX, Willcox MD, Jackson GD, Thakur A. Effect of tear secretory IgA on chemotaxis of polymorphonuclear leucocytes. Australian and New Zealand journal of ophthalmology. 1998;26(Suppl 1):S36–s39. doi: 10.1111/j.1442-9071.1998.tb01367.x. [DOI] [PubMed] [Google Scholar]
- Lee-Huang S, Huang PL, Sun Y, Huang PL, Kung HF, Blithe DL, Chen HC. Lysozyme and RNases as anti-HIV components in beta-core preparations of human chorionic gonadotropin. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:2678–2681. doi: 10.1073/pnas.96.6.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch EC, Willcox MD. Synergic antistaphylococcal properties of lactoferrin and lysozyme. Journal of medical microbiology. 1998;47:837–842. doi: 10.1099/00222615-47-9-837. [DOI] [PubMed] [Google Scholar]
- Leitch EC, Willcox MD. Lactoferrin increases the susceptibility of S. epidermidis biofilms to lysozyme and vancomycin. Current eye research. 1999;19:12–19. doi: 10.1076/ceyr.19.1.12.5342. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Morales J, Martin-Navarro CM, Lopez-Arencibia A, Arnalich-Montiel F, Pinero JE, Valladares B. Acanthamoeba keratitis: an emerging disease gathering importance worldwide? Trends in parasitology. 2013;29:181–187. doi: 10.1016/j.pt.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Luensmann D, Jones L. Protein deposition on contact lenses: the past, the present, and the future. Contact lens & anterior eye : the journal of the British Contact Lens Association. 2012;35:53–64. doi: 10.1016/j.clae.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Ma P, Wang N, McKown RL, Raab RW, Laurie GW. Focus on molecules: lacritin. Experimental eye research. 2008;86:457–458. doi: 10.1016/j.exer.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltseva IA, Fleiszig SM, Evans DJ, Kerr S, Sidhu SS, McNamara NA, Basbaum C. Exposure of human corneal epithelial cells to contact lenses in vitro suppresses the upregulation of human beta-defensin-2 in response to antigens of Pseudomonas aeruginosa. Experimental eye research. 2007;85:142–153. doi: 10.1016/j.exer.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Mannucci LL, Moro F, Cosani A, Palumbo M. Conformational state of lacrimal proteins adsorbed on contact lenses. Current eye research. 1985;4:734–736. doi: 10.3109/02713688509017675. [DOI] [PubMed] [Google Scholar]
- Mantelli F, Argueso P. Functions of ocular surface mucins in health and disease. Current opinion in allergy and clinical immunology. 2008;8:477–483. doi: 10.1097/ACI.0b013e32830e6b04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantis NJ, Rol N, Corthesy B. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal immunology. 2011;4:603–611. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CC, Hobden JA, Balzli CL, Reed JM, Caballero AR, Denard BS, Tang A, O'Callaghan RJ. Surfactant protein D in Pseudomonas aeruginosa keratitis. Ocular immunology and inflammation. 2007;15:371–379. doi: 10.1080/09273940701486423. [DOI] [PubMed] [Google Scholar]
- McKown RL, Coleman EV, Crawley EE, Genero CJ, Raab RW, White CA, Laurie GW. Antimicrobial activity in recombinant variants of prosecretory mitogen lacritin. Investigative ophthalmology & visual science. 2008;49 E-abstract 5287. [Google Scholar]
- McNamara NA, Andika R, Kwong M, Sack RA, Fleiszig SM. Interaction of Pseudomonas aeruginosa with human tear fluid components. Current eye research. 2005;30:517–525. doi: 10.1080/02713680590969456. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Yamada M, Hatou S, Kawashima M, Hata S. Deposition of lipid, protein, and secretory phospholipase A2 on hydrophilic contact lenses. Eye & contact lens. 2008;34:46–49. doi: 10.1097/ICL.0b013e3180676d5d. [DOI] [PubMed] [Google Scholar]
- Mun J, Tam C, Chan G, Kim JH, Evans D, Fleiszig S. MicroRNA-762 Is Upregulated in Human Corneal Epithelial Cells in Response to Tear Fluid and Pseudomonas aeruginosa Antigens and Negatively Regulates the Expression of Host Defense Genes Encoding RNase7 and ST2. PloS one. 2013;8:e57850. doi: 10.1371/journal.pone.0057850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun JJ, Tam C, Evans DJ, Fleiszig SM. Modulation of epithelial immunity by mucosal fluid. Scientific reports. 2011;1:8. doi: 10.1038/srep00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun JJ, Tam C, Kowbel D, Hawgood S, Barnett MJ, Evans DJ, Fleiszig SM. Clearance of Pseudomonas aeruginosa from a healthy ocular surface involves surfactant protein D and is compromised by bacterial elastase in a murine null-infection model. Infection and immunity. 2009;77:2392–2398. doi: 10.1128/IAI.00173-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan S, Redfern RL, Miller W, Nichols KK, McDermott AM. Drye Eye Disease and Microbial Keratitis: Is There a Connection? The ocular surface In press; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevalainen TJ, Graham GG, Scott KF. Antibacterial actions of secreted phospholipases A2. Review. Biochimica et biophysica acta. 2008;1781:1–9. doi: 10.1016/j.bbalip.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Ni M, Evans DJ, Hawgood S, Anders EM, Sack RA, Fleiszig SM. Surfactant protein D is present in human tear fluid and the cornea and inhibits epithelial cell invasion by Pseudomonas aeruginosa. Infection and immunity. 2005;73:2147–2156. doi: 10.1128/IAI.73.4.2147-2156.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogundele MO. Inhibitors of complement activity in human breast-milk: a proposed hypothesis of their physiological significance. Mediators of inflammation. 1999;8:69–75. doi: 10.1080/09629359990559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omali NB, Zhao Z, Zhu H, Tilia D, Willcox MD. Quantification of individual proteins in silicone hydrogel contact lens deposits. Molecular vision. 2013;19:390–399. [PMC free article] [PubMed] [Google Scholar]
- Patel A, Hammersmith K. Contact lens-related microbial keratitis: recent outbreaks. Current opinion in ophthalmology. 2008;19:302–306. doi: 10.1097/ICU.0b013e3283045e74. [DOI] [PubMed] [Google Scholar]
- Paulsen FP, Berry MS. Mucins and TFF peptides of the tear film and lacrimal apparatus. Progress in histochemistry and cytochemistry. 2006;41:1–53. doi: 10.1016/j.proghi.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Pearce DJ, Demirci G, Willcox MD. Secretory IgA epitopes in basal tears of extended-wear soft contact lens wearers and in non-lens wearers. Australian and New Zealand journal of ophthalmology. 1999;27:221–223. doi: 10.1046/j.1440-1606.1999.00206.x. [DOI] [PubMed] [Google Scholar]
- Peuravuori H, Aho VV, Aho HJ, Collan Y, Saari KM. Bactericidal/permeability-increasing protein in lacrimal gland and in tears of healthy subjects. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2006;244:143–148. doi: 10.1007/s00417-005-0062-z. [DOI] [PubMed] [Google Scholar]
- Pouyeh B, Viteri E, Feuer W, Lee DJ, Florez H, Fabian JA, Perez VL, Galor A. Impact of ocular surface symptoms on quality of life in a United States veterans affairs population. American journal of ophthalmology. 2012;153:1061–1066. doi: 10.1016/j.ajo.2011.11.030. e1063. [DOI] [PubMed] [Google Scholar]
- Puinhas A, Sampaio P, Castanheira EM, Real Oliveira ME, Lira M. Comparison of IgA, TNF-alpha and surface tension of the tear film in two different times of the day. Contact lens & anterior eye : the journal of the British Contact Lens Association. 2013 doi: 10.1016/j.clae.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Qu XD, Lehrer RI. Secretory phospholipase A2 is the principal bactericide for staphylococci and other gram-positive bacteria in human tears. Infection and immunity. 1998;66:2791–2797. doi: 10.1128/iai.66.6.2791-2797.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy P, Nichols JJ. Mucins in contact lens wear and dry eye conditions. Optometry and vision science : official publication of the American Academy of Optometry. 2008;85:631–642. doi: 10.1097/OPX.0b013e3181819f25. [DOI] [PubMed] [Google Scholar]
- Redl B. Human tear lipocalin. Biochimica et biophysica acta. 2000;1482:241–248. doi: 10.1016/s0167-4838(00)00142-4. [DOI] [PubMed] [Google Scholar]
- Robertson DM. The effects of silicone hydrogel lens wear on the corneal epithelium and risk for microbial keratitis. Eye & contact lens. 2013;39:67–72. doi: 10.1097/ICL.0b013e31827c5b73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saari KM, Aho V, Paavilainen V, Nevalainen TJ. Group II PLA(2) content of tears in normal subjects. Investigative ophthalmology & visual science. 2001;42:318–320. [PubMed] [Google Scholar]
- Sack RA, Nunes I, Beaton A, Morris C. Host-defense mechanism of the ocular surfaces. Bioscience reports. 2001;21:463–480. doi: 10.1023/a:1017943826684. [DOI] [PubMed] [Google Scholar]
- Sallenave JM. Secretory leukocyte protease inhibitor and elafin/trappin-2: versatile mucosal antimicrobials and regulators of immunity. American journal of respiratory cell and molecular biology. 2010;42:635–643. doi: 10.1165/rcmb.2010-0095RT. [DOI] [PubMed] [Google Scholar]
- Sariri R, Khamedi A. Variations in electrophoretic tear protein pattern due to contact lens wear. Journal of chromatography. A. 2007;1161:64–66. doi: 10.1016/j.chroma.2007.05.108. [DOI] [PubMed] [Google Scholar]
- Sathe S, Sakata M, Beaton AR, Sack RA. Identification, origins and the diurnal role of the principal serine protease inhibitors in human tear fluid. Current eye research. 1998;17:348–362. doi: 10.1080/02713689808951215. [DOI] [PubMed] [Google Scholar]
- Selsted ME, Martinez RJ. Isolation and purification of bactericides from human tears. Experimental eye research. 1982;34:305–318. doi: 10.1016/0014-4835(82)90079-3. [DOI] [PubMed] [Google Scholar]
- Shirane J, Nakayama T, Nagakubo D, Izawa D, Hieshima K, Shimomura Y, Yoshie O. Corneal epithelial cells and stromal keratocytes efficently produce CC chemokine-ligand 20 (CCL20) and attract cells expressing its receptor CCR6 in mouse herpetic stromal keratitis. Current eye research. 2004;28:297–306. doi: 10.1076/ceyr.28.5.297.28682. [DOI] [PubMed] [Google Scholar]
- Singh PK, Tack BF, McCray PB, Jr., Welsh MJ. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. American journal of physiology. Lung cellular and molecular physiology. 2000;279:L799–L805. doi: 10.1152/ajplung.2000.279.5.L799. [DOI] [PubMed] [Google Scholar]
- Spurr-Michaud S, Argueso P, Gipson I. Assay of mucins in human tear fluid. Experimental eye research. 2007;84:939–950. doi: 10.1016/j.exer.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlman MT, Gray ME, Hull WM, Whitsett JA. Immunolocalization of surfactant protein-D (SP-D) in human fetal, newborn, and adult tissues. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2002;50:651–660. doi: 10.1177/002215540205000506. [DOI] [PubMed] [Google Scholar]
- Steele PS, Jumblatt MM, Smith NB, Pierce WM. Detection of Histatin 5 in normal human Schirmer strip samples by mass spectroscopy. Investigative ophthalmology & visual science. 2002;43 e-abstract 98. [Google Scholar]
- Stuchell RN, Farris RL, Mandel ID. Basal and reflex human tear analysis. II. Chemical analysis: lactoferrin and lysozyme. Ophthalmology. 1981;88:858–861. doi: 10.1016/s0161-6420(81)34938-0. [DOI] [PubMed] [Google Scholar]
- Subbaraman LN, Borazjani R, Zhu H, Zhao Z, Jones L, Willcox MD. Influence of protein deposition on bacterial adhesion to contact lenses. Optometry and vision science : official publication of the American Academy of Optometry. 2011;88:959–966. doi: 10.1097/OPX.0b013e31821ffccb. [DOI] [PubMed] [Google Scholar]
- Szczotka LB, Cocuzzi E, Medof ME. Decay-accelerating factor in tears of contact lens wearers and patients with contact lens-associated complications. Optometry and vision science : official publication of the American Academy of Optometry. 2000;77:586–591. doi: 10.1097/00006324-200011000-00008. [DOI] [PubMed] [Google Scholar]
- Tam C, Mun JJ, Evans DJ, Fleiszig SM. Cytokeratins mediate epithelial innate defense through their antimicrobial properties. The Journal of clinical investigation. 2012;122:3665–3677. doi: 10.1172/JCI64416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchedre KT, Imayasu M, Hori Y, Cavanagh HD. Assessment of effects of multipurpose contact lens care solutions on human corneal epithelial cells. Eye & contact lens. 2011;37:328–330. doi: 10.1097/ICL.0b013e31822c36c2. [DOI] [PubMed] [Google Scholar]
- Temel A, Kazokoglu H, Taga Y. Tear lysozyme levels in contact lens wearers. Annals of ophthalmology. 1991a;23:191–194. [PubMed] [Google Scholar]
- Temel A, Kazokoglu H, Taga Y, Orkan AL. The effect of contact lens wear on tear immunoglobulins. The CLAO journal : official publication of the Contact Lens Association of Ophthalmologists, Inc. 1991b;17:69–71. [PubMed] [Google Scholar]
- Turner HC, Budak MT, Akinci MA, Wolosin JM. Comparative analysis of human conjunctival and corneal epithelial gene expression with oligonucleotide microarrays. Investigative ophthalmology & visual science. 2007;48:2050–2061. doi: 10.1167/iovs.06-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van't Hof W, Blankenvoorde MF, Veerman EC, Amerongen AV. The salivary lipocalin von Ebner's gland protein is a cysteine proteinase inhibitor. The Journal of biological chemistry. 1997;272:1837–1841. doi: 10.1074/jbc.272.3.1837. [DOI] [PubMed] [Google Scholar]
- Vijay AK, Zhu H, Ozkan J, Wu D, Masoudi S, Bandara R, Borazjani RN, Willcox MD. Bacterial adhesion to unworn and worn silicone hydrogel lenses. Optometry and vision science : official publication of the American Academy of Optometry. 2012;89:1095–1106. doi: 10.1097/OPX.0b013e318264f4dc. [DOI] [PubMed] [Google Scholar]
- Vinding T, Eriksen JS, Nielsen NV. The concentration of lysozyme and secretory IgA in tears from healthy persons with and without contact lens use. Acta ophthalmologica. 1987;65:23–26. doi: 10.1111/j.1755-3768.1987.tb08485.x. [DOI] [PubMed] [Google Scholar]
- Wei Y, Pinhas A, Liu Y, Epstein S, Wang J, Asbell P. Isoforms of secretory group two phospholipase A (sPLA2) in mouse ocular surface epithelia and lacrimal glands. Investigative ophthalmology & visual science. 2012;53:2845–2855. doi: 10.1167/iovs.11-8684. [DOI] [PubMed] [Google Scholar]
- Wieczorek R, Jakobiec FA, Sacks EH, Knowles DM. The immunoarchitecture of the normal human lacrimal gland. Relevancy for understanding pathologic conditions. Ophthalmology. 1988;95:100–109. doi: 10.1016/s0161-6420(88)33228-8. [DOI] [PubMed] [Google Scholar]
- Willcox MD. Microbial adhesion to silicone hydrogel lenses: a review. Eye & contact lens. 2013;39:61–66. doi: 10.1097/ICL.0b013e318275e284. [DOI] [PubMed] [Google Scholar]
- Willcox MD, Morris CA, Thakur A, Sack RA, Wickson J, Boey W. Complement and complement regulatory proteins in human tears. Investigative ophthalmology & visual science. 1997;38:1–8. [PubMed] [Google Scholar]
- Wright EA, Payne KA, Jowitt TA, Howard M, Morgan PB, Maldonado-Codina C, Dobson CB. Preservation of human tear protein structure and function by a novel contact lens multipurpose solution containing protein-stabilizing agents. Eye & contact lens. 2012;38:36–42. doi: 10.1097/ICL.0b013e31823fdb2a. [DOI] [PubMed] [Google Scholar]
- Wu H, Kuzmenko A, Wan S, Schaffer L, Weiss A, Fisher JH, Kim KS, McCormack FX. Surfactant proteins A and D inhibit the growth of Gram-negative bacteria by increasing membrane permeability. The Journal of clinical investigation. 2003;111:1589–1602. doi: 10.1172/JCI16889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, McClellan SA, Barrett RP, Hazlett LD. Beta-defensin-2 promotes resistance against infection with P. aeruginosa. J Immunol. 2009a;182:1609–1616. doi: 10.4049/jimmunol.182.3.1609. [DOI] [PubMed] [Google Scholar]
- Wu M, McClellan SA, Barrett RP, Zhang Y, Hazlett LD. Beta-defensins 2 and 3 together promote resistance to Pseudomonas aeruginosa keratitis. J Immunol. 2009b;183:8054–8060. doi: 10.4049/jimmunol.0902140. [DOI] [PubMed] [Google Scholar]
- Yamada M, Mochizuki H, Kawashima M, Hata S. Phospholipids and their degrading enzyme in the tears of soft contact lens wearers. Cornea. 2006;25:S68–S72. doi: 10.1097/01.ico.0000247217.16510.f2. [DOI] [PubMed] [Google Scholar]
- Yang D, Chen Q, Hoover DM, Staley P, Tucker KD, Lubkowski J, Oppenheim JJ. Many chemokines including CCL20/MIP-3alpha display antimicrobial activity. Journal of leukocyte biology. 2003;74:448–455. doi: 10.1189/jlb.0103024. [DOI] [PubMed] [Google Scholar]
- Yasueda S, Yamakawa K, Nakanishi Y, Kinoshita M, Kakehi K. Decreased mucin concentrations in tear fluids of contact lens wearers. Journal of pharmaceutical and biomedical analysis. 2005;39:187–195. doi: 10.1016/j.jpba.2005.02.039. [DOI] [PubMed] [Google Scholar]
- Yoder JS, Verani J, Heidman N, Hoppe-Bauer J, Alfonso EC, Miller D, Jones DB, Bruckner D, Langston R, Jeng BH, Joslin CE, Tu E, Colby K, Vetter E, Ritterband D, Mathers W, Kowalski RP, Acharya NR, Limaye AP, Leiter C, Roy S, Lorick S, Roberts J, Beach MJ. Acanthamoeba keratitis: the persistence of cases following a multistate outbreak. Ophthalmic epidemiology. 2012;19:221–225. doi: 10.3109/09286586.2012.681336. [DOI] [PubMed] [Google Scholar]
- You J, Fitzgerald A, Cozzi PJ, Zhao Z, Graham P, Russell PJ, Walsh BJ, Willcox M, Zhong L, Wasinger V, Li Y. Post-translation modification of proteins in tears. Electrophoresis. 2010;31:1853–1861. doi: 10.1002/elps.200900755. [DOI] [PubMed] [Google Scholar]
- Zhou L, Beuerman RW, Huang L, Barathi A, Foo YH, Li SF, Chew FT, Tan D. Proteomic analysis of rabbit tear fluid: Defensin levels after an experimental corneal wound are correlated to wound closure. Proteomics. 2007;7:3194–3206. doi: 10.1002/pmic.200700137. [DOI] [PubMed] [Google Scholar]
- Zhou L, Huang LQ, Beuerman RW, Grigg ME, Li SF, Chew FT, Ang L, Stern ME, Tan D. Proteomic analysis of human tears: defensin expression after ocular surface surgery. Journal of proteome research. 2004;3:410–416. doi: 10.1021/pr034065n. [DOI] [PubMed] [Google Scholar]